Abstract
Background
Calmodulin (CaM) is an important calcium sensor protein that transduces Ca2+ signals in plant stress signaling pathways. A previous study has revealed that transgenic rice over-expressing the calmodulin gene OsCam1–1 (LOC_Os03g20370) is more tolerant to salt stress than wild type. To elucidate the role of OsCam1–1 in the salt stress response mechanism, downstream components of the OsCam1–1-mediated response were identified and investigated by transcriptome profiling and target identification.
Results
Transcriptome profiling of transgenic ‘Khao Dawk Mali 105’ rice over-expressing OsCam1–1 and wild type rice showed that overexpression of OsCam1–1 widely affected the expression of genes involved in several cellular processes under salt stress, including signaling, hormone-mediated regulation, transcription, lipid metabolism, carbohydrate metabolism, secondary metabolism, photosynthesis, glycolysis, tricarboxylic acid (TCA) cycle and glyoxylate cycle. Under salt stress, the photosynthesis rate in the transgenic rice was slightly lower than in wild type, while sucrose and starch contents were higher, suggesting that energy and carbon metabolism were affected by OsCam1–1 overexpression. Additionally, four known and six novel CaM-interacting proteins were identified by cDNA expression library screening with the recombinant OsCaM1. GO terms enriched in their associated proteins that matched those of the differentially expressed genes affected by OsCam1–1 overexpression revealed various downstream cellular processes that could potentially be regulated by OsCaM1 through their actions.
Conclusions
The diverse cellular processes affected by OsCam1–1 overexpression and possessed by the identified CaM1-interacting proteins corroborate the notion that CaM signal transduction pathways compose a complex network of downstream components involved in several cellular processes. These findings suggest that under salt stress, CaM activity elevates metabolic enzymes involved in central energy pathways, which promote or at least maintain the production of energy under the limitation of photosynthesis.
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1538-4) contains supplementary material, which is available to authorized users.
Keywords: Calmodulin, CaM, Rice, Salt stress, Transcriptome
Background
Salinity stress is a major abiotic stress that affects plant growth, resulting in a loss of crop yield, especially rice, which is one of the most salt-sensitive plants in comparison to other cereals [1]. Salt stress affects plants via both osmotic and ionic effects. Osmotic effects result in a reduction of water absorption ability such that the effects are similar to drought stress. Ionic stress causes Na+ toxicity, which disrupts photosynthesis, protein synthesis, and enzyme activity [2, 3]. Numerous reports have shown negative effects of salt stress on rice growth and productivity based on the total chlorophyll content, protein concentration [4], photosynthetic CO2 fixation, stomatal conductance, transpiration [5], shoot dry weight, tiller number per plant, spikelets per panicle, and grain yield [6].
Ca2+ is a crucial second messenger consisting of a transient elevation of cytosolic [Ca2+]. The Ca2+ signals are transduced and decoded via Ca2+ binding protein, and then the information is relayed to downstream responses. The signals are mainly transduced through kinases mediating the phosphorylation cascade, resulting in downstream response regulation, including changes in gene expression through the regulation of transcription factors [7]. Calcium signaling is used to respond to environmental stimuli, as well as to coordinate growth and development in plants. In the plant calcium signal transduction process, calcium sensors, including calmodulin (CaM), calcineurin B-like (CBL) protein and Ca2+-dependent protein kinase (CPK), play important roles in the transduction of various stimuli [8, 9].
CaM is a protein that contains characteristic EF-hand motifs that bind Ca2+ ions with high affinity and specificity [10]. CaM binding to Ca2+ leads to the exposure of hydrophobic regions on the molecule surface and subsequent interactions with target proteins or nucleic acids [11]. Rice carries 5 CaM-encoding genes: OsCam1–1, OsCam1–2, OsCam1–3, OsCam2 and OsCam3 [12]. The expression of OsCam1–1 increases to a great extent in response to NaCl, mannitol and wounding treatment [13]. Several lines of evidence have revealed that calcium sensors are involved with an enhanced abiotic tolerance capacity in plants [14]. Evidence has shown that the constitutive expression of bovine calmodulin in tobacco results in a shortened germination time of transgenic tobacco seeds under salt stress (120–160 mM NaCl) [15]. Arabidopsis overexpressing GmCaM4 (Glycine max calmodulin) exhibit increased expression of AtMYB2-regulated genes, including proline-synthesizing enzymes, suggesting that this feature confers salt tolerance to the transgenic Arabidopsis by enabling the accumulation of proline [16]. Our previous report have shown that transgenic rice over-expressing OsCam1–1 grow better under salt stress than wild type [17]. Wu H. and colleagues have found that the biphasic Ca2+ signal and enhancement of OsCam1–1 expression in rice cause heat stress-mediated expression of downstream heat shock-related genes, and OsCam1–1 overexpression Arabidopsis are more tolerant to heat stress than its wild type [18]. In another report, AtCam3 knockout mutant Arabidopsis showed a clear reduction of thermotolerance after heat treatment at 45 °C, and when AtCam3 was overexpressed in mutant and wild type Arabidopsis, the thermotolerant ability was rescued and increased, respectively. Moreover, co-expression of some heat shock protein genes with AtCaM3 suggested that AtCam3 plays a key role in the Ca2+-CaM heat shock transduction pathway [19]. The versatile functions of CaM are interesting, especially the role in the regulation of gene expression. CaM proteins directly modulate transcription factors (TFs), and some of these TFs have been verified to play roles in stress signaling pathways; however, the Ca2+ and Ca2+/CaM-regulating TF mechanisms remain incompletely understood and require further investigation [20, 21]. Transcriptomics analysis can lead to the discovery of genes or processes that respond to such factors. The aim of the present study was to investigate the downstream effects of OsCam1–1 overexpression on gene expression regulation in rice under salt stress using a transcriptomic approach and to identify the interacting proteins to elucidate the role of OsCam1–1 in the salt stress response mechanism.
Results
RNA-Seq of Rice overexpressing OsCam1–1 and differential gene expression analysis
CaM is a multifunctional protein that regulates the activities of numerous target proteins. Genome-wide analysis techniques such as transcriptome profiling are particularly suitable for identifying the downstream components that are potentially regulated by CaM. In our previous report [17], rice overexpressing OsCam1–1 showed a significantly higher relative growth rate than wild type when grown under salt stress. Here, transcriptome profiling of the 3-week-old rice leaves of transgenic rice over-expressing OsCam1–1 (L1) and its wild type (WT) under normal condition (NS) and salt stress (150 mM NaCl) conditions (S) for 4 h was conducted. More than 185 million reads from eight libraries from single-end RNA-Seq by Illumina Hi-Seq 2000 were obtained, with a total read of each library between 22 and 25 million reads. The reads were processed by POPE [22], which provided a total clean read per library of more than 99% of the total reads. At least 93% of the clean reads were mapped to the rice genome reference, Michigan State University rice annotation project’s MSU7 [23] and less than 11% of the clean reads were multiple alignment reads (Table 1).
Table 1.
RNA-Seq read count information
| Samplea | Raw Input Reads | Clean Reads | % Clean Reads | Mapped Reads | % Mapped Readsb | Multiple Alignment Reads | %Multiple Alignment Readsb |
|---|---|---|---|---|---|---|---|
| WTNS R1 | 24,019,397 | 23,961,896 | 99.76 | 22,973,989 | 95.88 | 1,952,214 | 8.15 |
| WTNS R2 | 22,859,782 | 22,779,097 | 99.65 | 21,709,287 | 95.30 | 2,029,217 | 8.91 |
| WTS R1 | 23,050,208 | 22,990,113 | 99.74 | 21,979,140 | 95.60 | 1,746,186 | 7.60 |
| WTS R2 | 23,437,864 | 23,362,805 | 99.68 | 22,119,198 | 94.68 | 2,102,270 | 9.00 |
| L1NS R1 | 23,259,980 | 23,234,303 | 99.89 | 22,207,203 | 95.58 | 2,048,681 | 8.82 |
| L1NS R2 | 23,944,162 | 23,859,454 | 99.65 | 22,673,161 | 95.03 | 2,110,311 | 8.84 |
| L1S R1 | 22,358,313 | 22,282,400 | 99.66 | 21,248,337 | 95.36 | 2,332,891 | 10.47 |
| L1S R2 | 22,980,550 | 22,928,854 | 99.78 | 21,527,676 | 93.89 | 1,739,326 | 7.59 |
a R1 and R2 indicate biological replicates
bThe % mapped reads and % multiple alignment reads were calculated using the clean reads as a denominator
To compare the transcriptome profiles of the rice, differential gene expression analysis of the transcriptome data using DESeq [24] was carried out, which provided the number of differentially expressed genes (DEGs) summarized in Table 2. Analysis of the wild type identified 12,184 DEGs (p < 0.05) between the transcriptome profile under normal and salt stress conditions (WTNSWTS), in which 5842 and 6342 genes were up-regulated and down-regulated, respectively. For transgenic rice over-expressing OsCam1–1, comparisons between normal and salt stress conditions (L1NSL1S) revealed a total of 13,259 DEGs with 6434 and 6825 up-regulated and down-regulated genes, respectively. Furthermore, the transcriptome profiles of the transgenic rice were compared with those of the wild type. Under normal conditions (WTNSL1NS), 2022 DEGs were identified, with 892 and 1130 DEGs expressed at higher or lower levels in the transgenic rice, respectively. Under salt stress, comparisons of transgenic rice with wild type rice (WTSL1S) revealed 1677 DEGs, with 957 and 720 DEGs expressed at higher or lower levels in the transgenic rice, respectively. The scatterplots showed quantitative overview of the four transcriptome profile comparisons (Fig. 1). OsCam1–1 was found to be highly expressed in transgenic rice under both normal and stress condition, with an average RPKM of 1758.67 and 1644.62, while the average RPKM of wild type under normal and stress conditions was 91.94 and 97.84, respectively. The expression of OsCam1–1 in the wild type was not induced at 4 h after salt stress (150 mM), in good agreement with a previous study. According to a gene expression study conducted by Chinpongpanich et al. [25], the transcript level of OsCam1–1 determined by qRT-PCR was highly induced at 1 h after 150 mM NaCl treatment and then sharply decreased after 1 h. This result validated the overexpression of OsCam1–1 in transgenic rice with an approximately 18-fold change in RPKM compared with wild type. Based on a differential transcriptome analysis, the gene expression levels of those 2022 and 1677 DEGs were thus likely affected by OsCam1–1 overexpression.
Table 2.
Differential gene expression analysis results showing the number of significantly differentially expressed genes comparing each rice line and/or condition
| Comparison of rice line and/or condition | Total number of differentially expressed genes (p < 0.05) | Number of differentially up-regulated genes (p < 0.05) | Number of differentially down-regulated genes (p < 0.05) |
|---|---|---|---|
| WTNSWTS | 12,184 | 5842 | 6342 |
| L1NSL1S | 13,259 | 6434 | 6825 |
| WTNSL1NS | 2022 | 892 | 1130 |
| WTSL1S | 1677 | 957 | 720 |
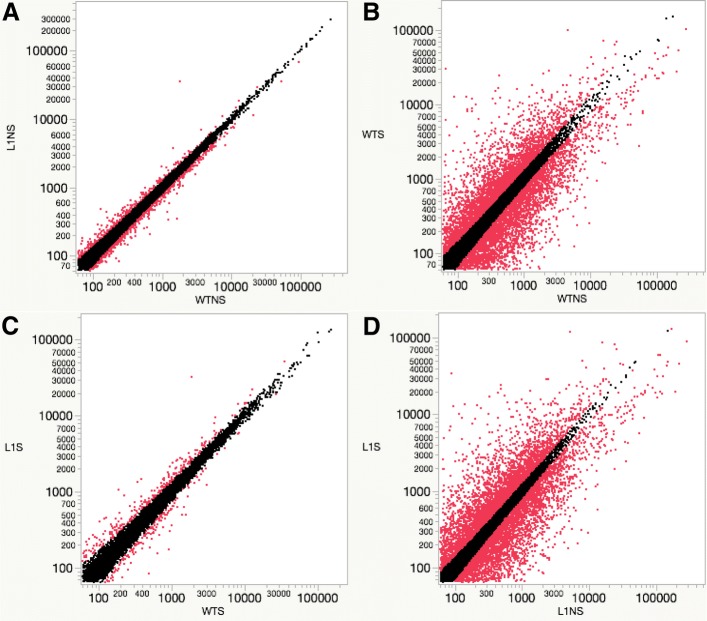
Fig. 1.
Scatter plot showing significantly differential gene expression (p < .05) comparing different rice lines and/or conditions. a Comparison of wild type under normal condition (WTNS) and 35S-OsCam1–1 under normal conditions (L1NS), b wild type under normal conditions (WTNS) and wild type under stress conditions (WTS). c wild type under stress conditions (WTS) and 35S-OsCam1–1 under stress conditions (L1S). d 35S-OsCam1–1 under normal conditions (L1NS) and 35S-OsCam1–1 under stress conditions (L1S). The X and Y axes represent the base mean for the RNA-seq data
qRT-PCR verification of the transcriptome data
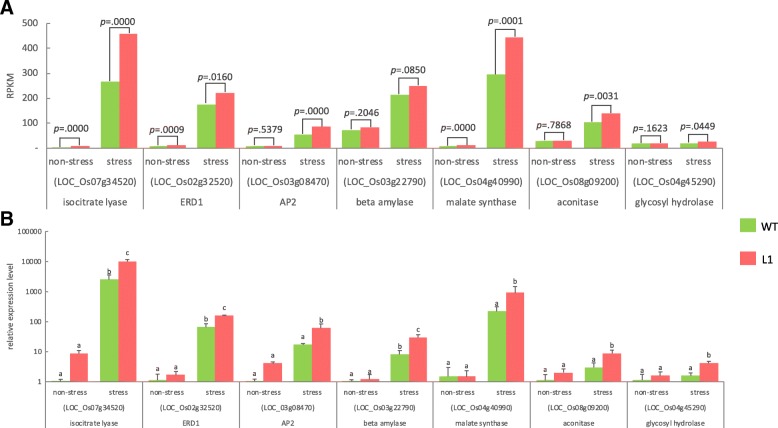
To verify the reliability of the transcriptome data, nine salt-responsive genes, β-amylase (LOC_Os03g22790), isocitrate lyase (LOC_Os07g34520), malate synthase (LOC_Os04g40990), aconitase (LOC_Os08g09200), glycosyl hydrolase (LOC_Os04g45290), ERD1 (LOC_Os02g32520), AP2 (LOC_Os03g08470), isocitrate dehydrogenase (LOC_Os05g49760) and pyruvate decarboxylase (LOC_Os03g18220), were selected for qRT-PCR. Figure 2 shows the qRT-PCR results for seven genes, which agreed well with the transcriptome data. Compared with wild type, they all exhibited higher levels in transgenic rice, demonstrating a statistically significant difference under salt stress. In contrast, the expression of the other two genes examined by qRT-PCR did not agree well with the transcriptome data (data not shown), potentially because of their low expression levels.
Fig. 2.
Real time RT-PCR verification of RNA-Seq. a RNA-Seq results with p values from DESeq analysis, b real-time RT-PCR results calculated using the 2-(ΔΔCT) method. Data are shown as the mean + 1 SD, and are derived from four independent biological replicates. For each gene, means with a different letter are significantly different (p < 0.05)
Gene ontology enrichment analysis
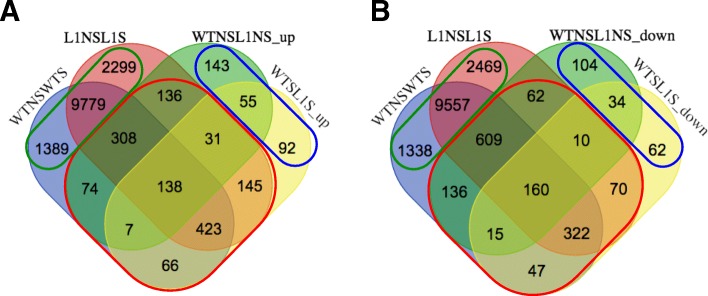
To categorize the DEGs, Venn diagrams were constructed from the four comparisons. The first Venn diagram was constructed from the salt-responsive DEGs from either wild type (WTNSWTS, blue colored circle) or transgenic rice (L1NSL1S, red colored circle) and the DEGs that were expressed at higher levels in the transgenic rice either under normal (WTNSL1NS_up, green colored circle) or salt stress (WTSL1S_up, yellow colored circle) conditions (Fig. 3a). In contrast, the second diagram was constructed from the salt-responsive DEGs and the DEGs that were expressed at lower levels in transgenic rice either under normal (WTNSL1NS_down, green colored circle) or salt stress (WTSL1S_down, yellow colored circle) conditions (Fig. 3b). According to the Venn diagrams, we identified 1328 salt-responsive DEGs with higher expression levels in transgenic rice, which will be referred to as HT salt-responsive DEGs (Fig. 3a, red line circle), and 1431 salt-responsive DEGs with lower expression levels in transgenic rice, which will be referred to as LT salt-responsive DEGs (Fig. 3b, red line circle). For those with unaffected expression levels by salt stress, 290 genes or 200 genes showed higher (Fig. 3a, blue line circle) or lower (Fig. 3b, blue line circle) expression levels in transgenic rice, which will be referred to as HT DEGs or LT DEGs, respectively. In both Venn diagrams, the largest of number DEGs with expression levels that did not differ between transgenic rice and wild type (green circles) were salt-responsive DEGs.
Fig. 3.
Venn diagram showing (a) the number of significantly (p < 0.05) HT salt-responsive DEGs (red circle), HT DEGs (blue circle) and salt-responsive DEGs (green circle), while (b) shows the number of LT salt-responsive DEGs (red circle), LT DEGs (blue circle) and salt-responsive DEGs (green circle)
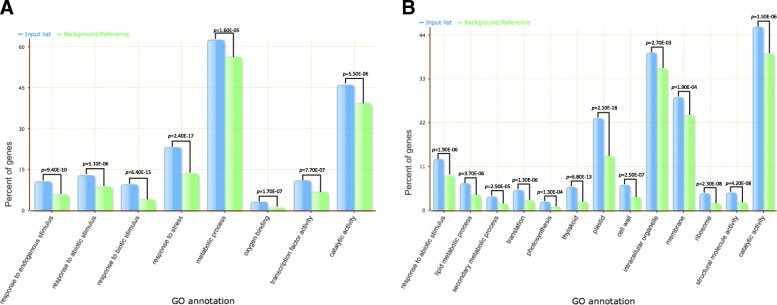
Gene enrichment analysis was performed using those 1328 HT salt-responsive DEGs. The results showed that, in terms of biological process, the terms of response to endogenous stimulus (GO:0009719), response to abiotic stimulus (GO:0009628), response to biotic stimulus (GO:0009607), response to stress (GO:0006950) and metabolic process (GO:0008152), were enriched, while for the term of molecular function, the terms of oxygen binding (GO:0019825), transcription factor activity (GO:0003700) and catalytic activity (GO:0003824) were overrepresented (Fig. 4a). For those 1431 LT salt-responsive DEGs, in terms of biological process, the terms of response to abiotic stimulus (GO:0009628), lipid metabolic process (GO:0006629), secondary metabolic process (GO:0019748), translation (GO:0006412), and photosynthesis (GO:0015979) were enriched, while in terms of the cellular compartment, the enriched terms included the thylakoid (GO:0009579), plastid (GO:0009536), cell wall (GO:0005618), intracellular organelles (GO:0043229), membrane (GO:0016020) and ribosome (GO:0005840), and in terms of molecular function, those of structural molecule activity (GO:0005198) and catalytic activity (GO:0003824) were overrepresented (Fig. 4b). Based on these results, we observed that the set of genes involving photosynthetic process were uniquely allocated in the LT salt-responsive DEGs, while genes involving response to stimuli and metabolic process were distributed in both HT and LT salt-responsive DEGs.
Fig. 4.
GO enrichment analysis results of (a) the salt-responsive DEGs with higher expression levels in transgenic rice and (b) the salt-responsive DEGs with lower expression levels in transgenic rice
Functional identification of OsCam1–1 regulated DEGs
Table 3 summarizes the number of OsCam1–1-regulated DEGs in each functional category according to GO terms. For the categories of HT and LT DEGs with expression levels that remained unchanged under salt stress, a small number of genes with diverse functions were found, which were involved in RNA regulation, protein metabolism, signaling, development, transport, hormone, and stress. However, the high-fold-change-DEGs were mainly identified as unknown proteins (see Additional file 1). Nonetheless, some known genes were annotated in the genome database including ankyrin repeat containing protein (LOC_Os01g09384), hexose carrier protein (LOC_Os11g38160), ATPase/hydrogen-translocating pyrophosphatase (LOC_Os01g23580), UDP glucosyltransferase (LOC_Os01g49240), C3HC4-type RING finger (LOC_Os10g32760), cleavage and polyadenylation specificity factor 100 kDa subunit (LOC_Os02g06940).
Table 3.
Number of OsCam1–1-regulated DEGs in each functional category according to GO terms
| GO Term | Number of | ||||
|---|---|---|---|---|---|
| HT salt-responsive DEG | LT salt-responsive DEG | HT DEG | LT DEG | salt responsive DEG | |
| photosynthesis | 2 | 31 | 0 | 1 | 111 |
| cell wall | 26 | 52 | 0 | 2 | 178 |
| lipid metabolism | 30 | 55 | 1 | 3 | 233 |
| N metabolism | 4 | 2 | 0 | 0 | 15 |
| amino acid metabolism | 17 | 21 | 2 | 4 | 155 |
| S assimilation | 2 | 1 | 0 | 0 | 3 |
| metal handling | 5 | 6 | 2 | 1 | 31 |
| secondary metabolism | 35 | 50 | 9 | 3 | 185 |
| hormone | 51 | 27 | 13 | 6 | 227 |
| co-factor and vitamin metabolism | 2 | 5 | 0 | 1 | 35 |
| tetrapyrrole synthesis | 0 | 13 | 1 | 0 | 19 |
| major CHO metabolism | 7 | 7 | 2 | 0 | 61 |
| stress | 66 | 54 | 14 | 7 | 420 |
| redox | 6 | 16 | 1 | 2 | 116 |
| polyamine metabolism | 2 | 0 | 0 | 0 | 8 |
| nucleotide metabolism | 8 | 9 | 0 | 0 | 82 |
| biodegradation of xenobiotics | 2 | 1 | 0 | 0 | 44 |
| C1-metabolism | 0 | 3 | 0 | 1 | 14 |
| miscellaneous | 138 | 145 | 22 | 15 | 750 |
| RNA regulation | 126 | 113 | 26 | 15 | 1318 |
| DNA synthesis | 9 | 25 | 2 | 8 | 224 |
| protein metabolism | 130 | 164 | 28 | 17 | 1575 |
| minor CHO metabolism | 11 | 6 | 1 | 2 | 65 |
| signaling | 71 | 53 | 18 | 10 | 656 |
| cell division | 13 | 48 | 8 | 5 | 329 |
| development | 53 | 36 | 10 | 6 | 330 |
| transport | 68 | 67 | 11 | 6 | 576 |
| not assigned | 430 | 410 | 118 | 85 | 4151 |
| glycolysis | 2 | 0 | 0 | 0 | 29 |
| fermentation | 2 | 5 | 0 | 0 | 11 |
| gluconeogenesis | 3 | 1 | 0 | 0 | 7 |
| OPP | 2 | 1 | 0 | 0 | 15 |
| TCA | 2 | 3 | 0 | 0 | 52 |
| electron transport chain | 3 | 1 | 1 | 0 | 50 |
| micro RNA | 0 | 0 | 0 | 0 | 1 |
| Total | 1328 | 1431 | 290 | 200 | 12,076 |
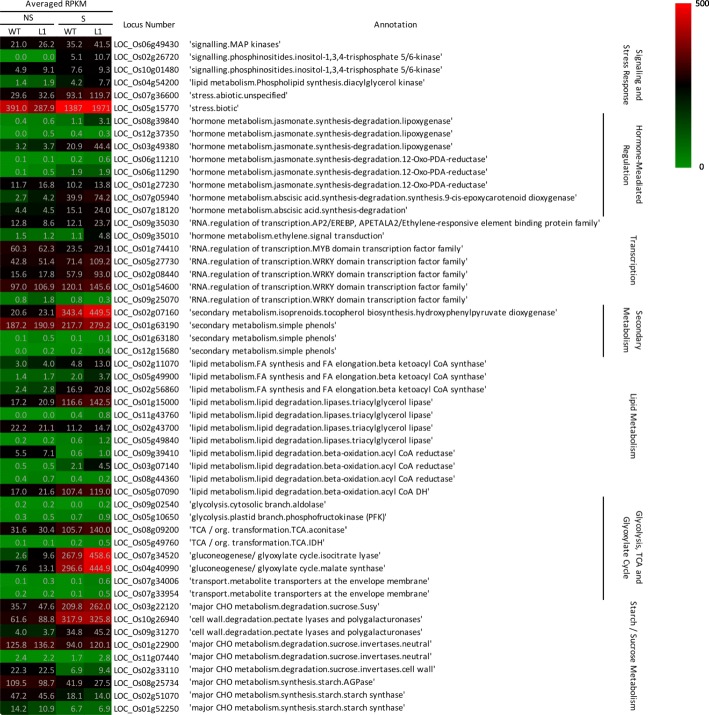
Overall, under salt stress, 1434 salt-responsive genes exhibited different expression levels between the wild type and transgenic rice. Figure 5 shows salt-responsive DEGs that encode potential downstream components of OsCaM1 in salt stress response. These DEGs are involved in several major cellular processes, including signaling and stress responses, hormone-mediate regulation, transcription, secondary metabolism, lipid metabolism, glycolysis, TCA cycle, glyoxylate cycle, photosynthesis, and carbohydrate metabolism. In signaling, the HT salt-responsive DEGs include LOC_Os06g49430, which encodes BWMK1, a rice MAP kinase; LOC_Os02g26720 and LOC_Os10g01480, which encode inositol 1,3,4-trisphosphate 5/6-kinase (IPTK); and LOC_Os04g54200, which encodes diacylglycerol kinase (DGK). Involved in stress response, the transcriptome results showed that the expression of 46 biotic and 19 abiotic stress DEGs was allocated in the HT salt-responsive DEG category (see Additional file 1). These include a universal stress protein (USP) (LOC_Os07g36600), and a xylanase inhibitor protein gene (OsXIP2) (LOC_Os05g15770). For those involved in hormone-mediated regulation, we have identified three lipoxygenase (LOX) genes (LOC_Os08g39840, LOC_Os12g37350, and LOC_Os03g49380) and three 12-oxo-PDA-reductase (OPR) genes (e.g. LOC_Os06g11210, LOC_Os06g11290, and LOC_Os01g27230), which encode enzymes in the jasmonate (JA) biosynthesis pathway. In addition, the genes encoding key enzymes in the ABA biosynthesis pathway, 9-cis-epoxycarotenoid dioxygenase (NCED) (LOC_Os07g05940) and abscisic aldehyde oxidase (AAO) (LOC_Os07g18120), were identified as HT salt-responsive DEGs, which were up-regulated approximately 1.9-fold and 1.6-fold, respectively in transgenic rice compared with wild type rice under salt stress.
Fig. 5.
Salt-responsive DEGs that encode potential downstream components of OsCaM1 in salt stress response. Expression levels of each gene by RPKM from wild type and transgenic rice under normal and salt stress conditions were presented as heat map
According to the transcriptome results, thirteen APETALA2/ethylene-responsive element binding protein (AP2/EREBP) genes were identified as HT salt-responsive DEGs (e.g. LOC_Os09g35030, LOC_Os09g35010), while five AP2/EREBP genes were identified as LT salt-responsive DEGs (see Additional file 1). In addition, eight MYB genes were found allocated in the category of HT salt-responsive DEGs (e.g. LOC_Os01g74410) and 16 HT salt-responsive DEGs were WRKY, which is a large TF family that responds to plant stress (e.g. LOC_Os05g27730, LOC_Os02g08440, LOC_Os01g54600, LOC_Os09g25070). In secondary metabolism, 35 DEGs were identified as HT salt-responsive genes (see Additional file 1) such as a hydroxyphenylpyruvate dioxygenase (HPPD) gene (LOC_Os02g07160) and five laccase genes (e.g. LOC_Os12g15680, LOC_Os01g63180, LOC_Os01g63190).
Functions of several HT salt-responsive DEGs involve in the energy metabolism. These included 30 DEGs in lipid metabolism (see Additional file 1) with examples including three 3-ketoacyl-CoA synthase genes (LOC_Os02g11070, LOC_Os05g49900 and LOC_Os02g56860), four class III lipase genes (LOC_Os01g15000, LOC_Os11g43760, LOC_Os02g43700 and LOC_Os05g49840), and four genes encoding enzymes involving beta oxidation (LOC_Os09g39410, LOC_Os03g07140, LOC_Os08g44360 and LOC_Os05g07090). Several HT salt-responsive DEGs are also involved in carbohydrate metabolism including a fructose bisphosphate aldolase (FBP) gene (LOC_Os09g02540) and a phosphofructokinase (PFK) gene (LOC_Os05g10650) in the glycolysis pathway; and an aconitase gene (LOC_Os08g09200) and an isocitrate dehydrogenase (IDH) gene (LOC_Os05g49760) in the TCA cycle. In addition, two genes encoding key enzymes in the glyoxylate cycle shuttling the TCA cycle pathway, isocitrate lyase (ICL) (LOC_Os07g34520) and malate synthase (MLS) (LOC_Os04g40990) were up-regulated approximately 1.7-fold and 1.5-fold, respectively in transgenic rice compared with wild type rice under salt stress. Additionally, the DEGs included two glucose-6-phosphate transporter genes, LOC_Os07g34006 and LOC_Os07g33954, both with higher expression levels in transgenic rice under salt stress.
Finally, seven DEGs involved in sucrose and starch metabolism were allocated in the category of HT salt-responsive DEGs. Among these DEGs, sucrose synthase (LOC_Os03g22120) was up-regulated in both wild type or transgenic rice under salt stress, with greater up-regulation in transgenic rice. The transcriptome data also revealed three invertase genes (LOC_Os01g22900, LOC_Os11g07440, LOC_Os02g33110) and seven cell wall degradation DEGs, which were expressed at higher levels in transgenic rice (see Additional file 1) including two DEGs encoding polygalacturonase (LOC_Os10g26940 and LOC_Os09g31270).
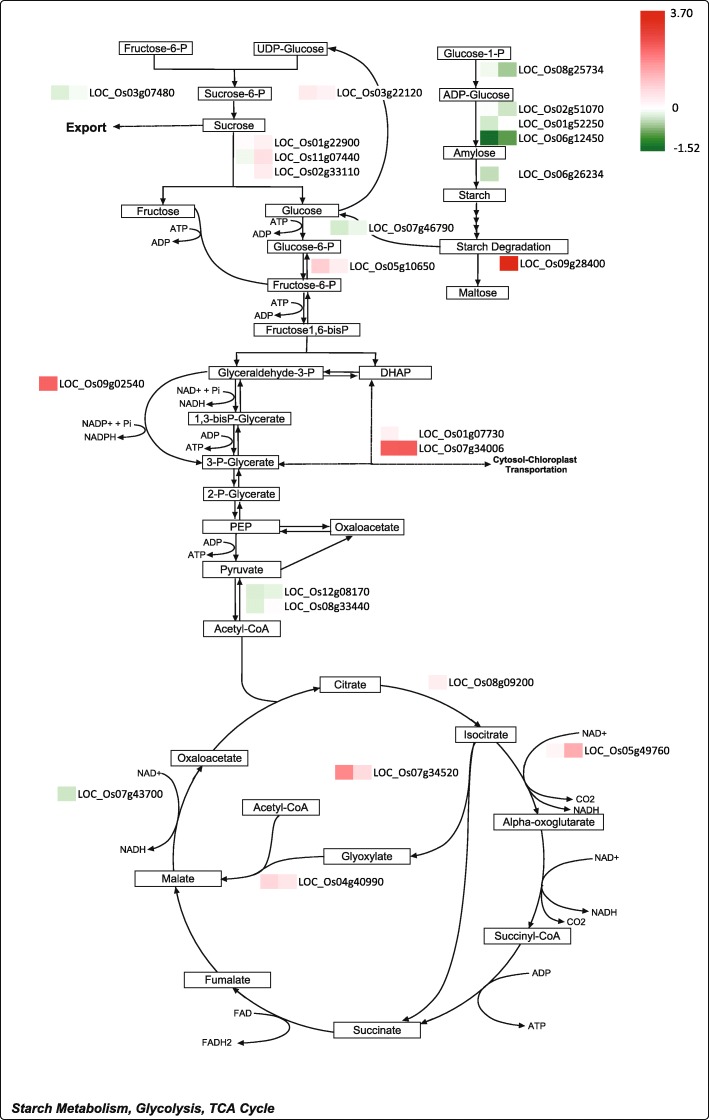
When these salt-responsive DEGs were mapped onto metabolic pathways, several genes with consistent changes in their expression levels within certain pathways were observed, including the light reactions and Calvin cycle of the photosynthetic process, sucrose and starch metabolism, and central energy pathways. In Fig. 6 and Fig. 7, gene expression ratios between the transgenic rice and the wild type rice both under normal and salt stress conditions are presented for each corresponding step of these pathways. Overall, expression levels of 31 out of 33 DEGs in the photosynthetic process (e.g., chlorophyll a/b binding protein, protein subunit in photosystem I and II, ferredoxin, plastoquinone dehydrogenase complex, ribulose-bisphosphate carboxylase, which were repressed by salt stress) were lower in the transgenic rice overexpressing OsCam1–1 (Fig. 6) (see Additional file 2). In Fig. 7, the expression levels of several genes involved in sucrose degradation (e.g., LOC_Os03g22120 encoding sucrose synthase, which was highly induced by salt stress; LOC_Os01g22900 and LOC_Os02g33110 encoding invertase, which were repressed by salt stress) were higher in transgenic rice, especially under salt stress, while those of genes in the starch biosynthetic pathway (e.g., LOC_Os08g25734 encoding glucose-1-phosphate adenylyltransferase; LOC_Os02g51070 and Os01g52250 encoding starch synthase, which were repressed by salt stress) were lower in transgenic rice. In addition, genes involved in glycolysis and the TCA cycle (e.g., LOC_Os05g10650 encoding phosphofructokinase; and LOC_Os05g49760 encoding isocitrate dehydrogenase, which were induced by salt stress) were expressed at higher levels in transgenic rice. Remarkably, three genes in the glyoxylate cycle: LOC_Os08g09200, LOC_Os07g34520, and LOC_Os04g40990 encoding aconitase, isocitrate lyase, and malate synthase, respectively, which were all highly induced by salt stress, were expressed at higher levels in transgenic rice both under normal and salt stress conditions (Fig. 7).
Fig. 6.
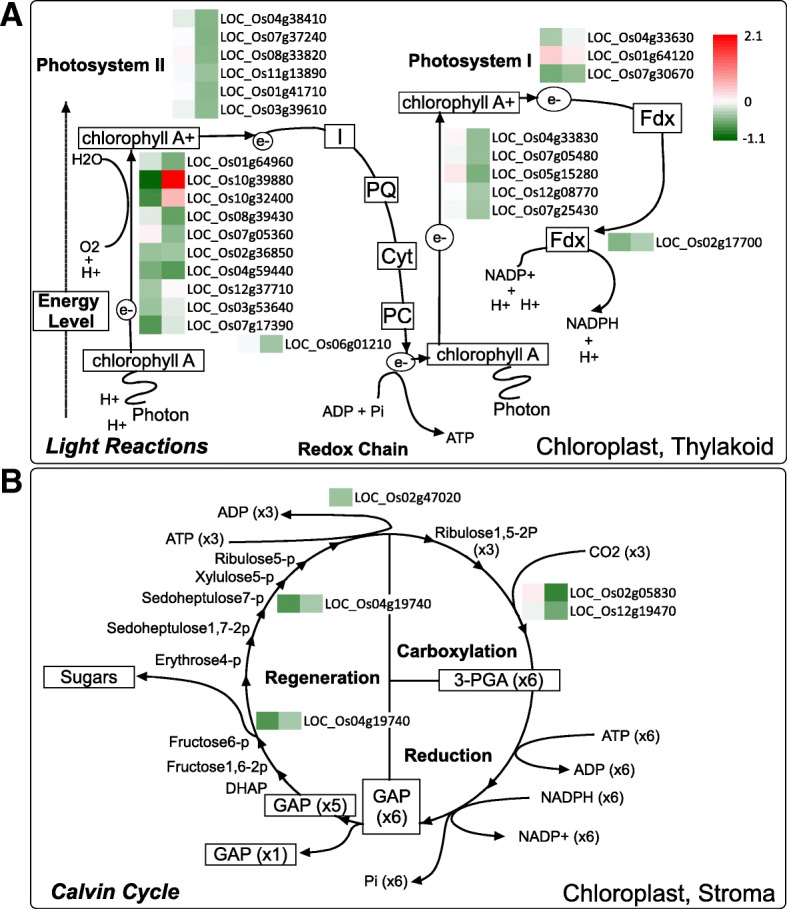
Photosynthetic pathway showing the expression level and role(s) of the genes in the light reaction (a) and Calvin cycle. b The left box shows the log2-fold change in comparisons of WT and transgenic plants under normal conditions, and the right box represents the log2-fold change under salt stress conditions
Fig. 7.
Carbohydrate and energy metabolism pathway consisting of sucrose-starch metabolism, glycolysis and the TCA cycle show the gene expression level and function in metabolism. The left box shows the log2-fold change of comparisons of WT and the transgenic plants under normal conditions, and the right box represents the log2-fold change under salt stress conditions
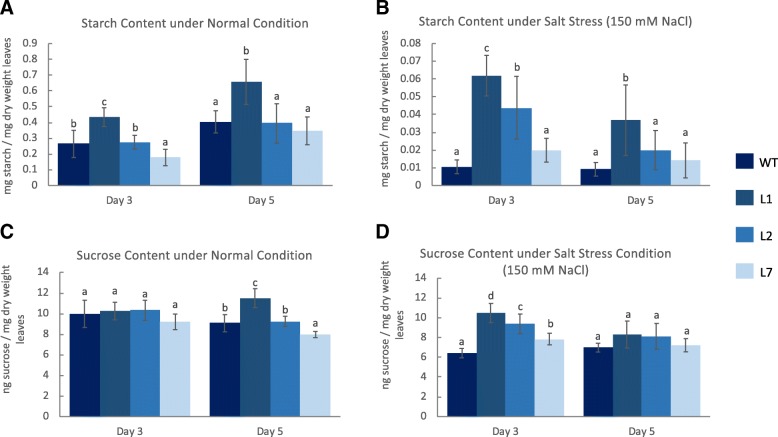
Rice overexpressing OsCam1–1 exhibited higher sucrose and starch contents under salt stress
In our previous report [17], OsCam1–1-overexpressing lines showed a significantly higher relative growth rate than wild type when grown under salt stress. Based on the genes identified herein, among which several were involved in central energy pathways, sucrose and starch levels were determined in the three independent lines (L1, L2, L7) under normal and salt stress (150 mM NaCl) conditions at day 3 and 5 after treatment. Salt stress led to a significant reduction of the starch level and slightly decreased sucrose levels in both wild type and transgenic rice lines. Noticeably, at day 3, the transgenic lines could maintain the sucrose and starch levels better than the wild type under salt stress conditions. At day 5, the trends observed for sucrose and starch levels in transgenic rice under salt stress conditions were similar to those in wild type (Fig. 8).
Fig. 8.
Starch and sucrose contents in the three lines of transgenic rice overexpressing OsCam1–1 (L1, L2, L7) comparing wild type (WT) at days 3 and 5 exposed to 150 mM NaCl salt stress treatment from five independent biological replicates. a starch content under normal conditions, b starch content under salt stress conditions, c sucrose content under normal conditions, and (d) sucrose content under salt stress conditions
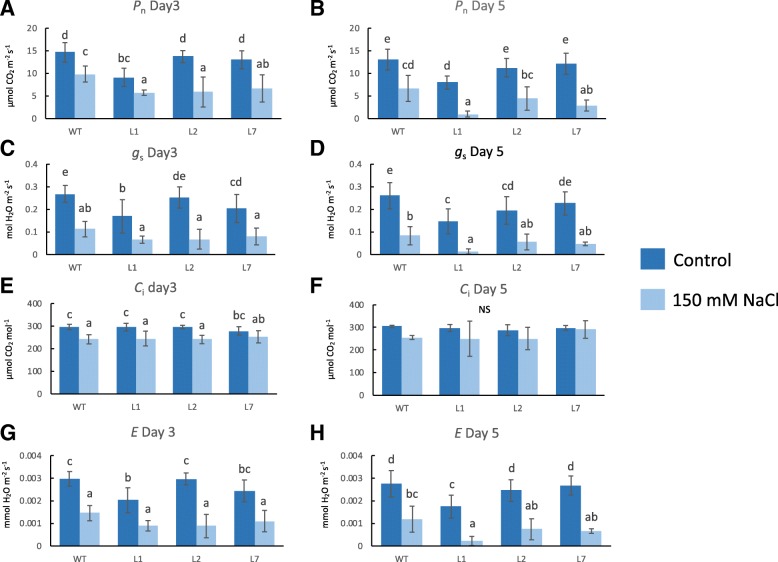
In addition, the photosynthesis rate (Pn), stomatal conductance (gs), intercellular carbon dioxide (Ci) and transpiration rate (E) were examined in the transgenic rice over-expressing OsCam1–1. Under salt stress, Pn, gs and E decreased at both day 3 and day 5, while Ci decreased slightly at day 3 of treatment. Interestingly, transgenic rice had slightly lower Pn values than wild type rice at both day 3 and day 5 and tended to have lower gs and E values at day 5 of salt stress treatment. In contrast, the Ci measurements did not reveal significant difference between the transgenic and wild type (Fig. 9). For FV′/FM′, which reflects the maximum efficiency of photosystem II [26], no change was observed under the given salt stress conditions, and the transgenic rice did not exhibit difference either under normal or salt stress conditions compared with the wild type (see Additional file 3).
Fig. 9.
Gas exchange measurements in the leaves of three lines of transgenic rice overexpressing OsCam1–1 (L1, L2, L7) comparing wild type (WT) of 150 mM NaCl salt stress treatment from five independent biological replicates. (a) the photosynthesis rate (Pn) at day 3 and (b) day 5, (c) stomatal conductance (gs) at day 3 and (d) day 5, (e) intercellular carbon dioxide (Ci) at day 3 and (f) day 5, and (g) transpiration rate (E) at day 3 and (h) day 5 of salt stress treatment
In a previous study, the northern blot results showed the highest expression levels of OsCam1–1 in transgenic rice line L1 among the three transgenic rice lines [17]. Under both normal and salt stress conditions, the sucrose and starch content correlated with the expression level of OsCam1–1 in those transgenic rice lines.
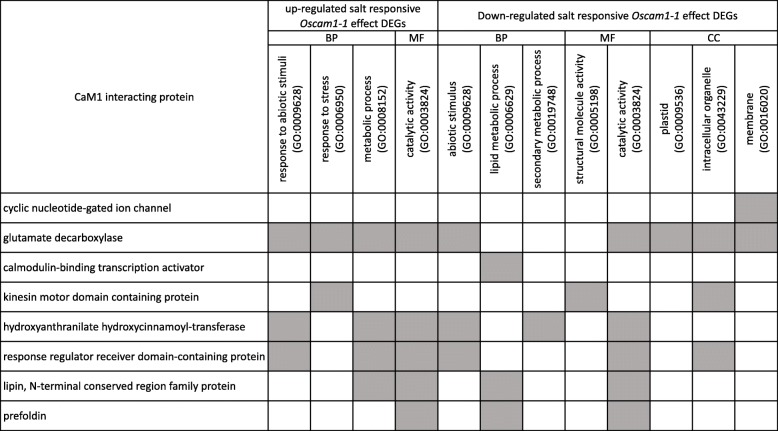
Identification of OsCaM1-interacting proteins
CaM does not possess functional domains other than EF hand motifs, so it functions by binding to and altering the activities of various interacting proteins. To understand how CaM1 mediates Ca2+-signal responses, its specific interacting proteins were identified using a cDNA expression library with 35S-labeled rOsCaM1 protein as the probe. The purity of the prepared 35S-labeled rOsCaM1 protein was examined by SDS-PAGE (see Additional file 4: Figure S3A). To test its specificity, PVDF membrane spotted with various amounts of CaMKII peptide [27], calcineurin [28], and BSA was incubated with the probe. The autoradiograph (see Additional file 4: Figure S3B) showed that the probe only interacted with CaMKII peptide and calcineurin but not BSA, and the intensity of the signal on the X-ray film was dose-dependent. The results indicated that the 35S-labeled rOsCaM1 protein could specifically bind to well-known target proteins in the presence of Ca2+.
After screening the cDNA library, the purified clones from the tertiary screening were titered before performing single-clone excision. As a result, 10 distinct positive cDNA clones were obtained. All unique pBluescript SK(−) plasmids obtained from the single-clone excision were sequenced to determine the cloned cDNA insert sequences. The resulting sequences were BLAST-searched against the Rice Genome Annotation Project (MSU-RGAP) and the Rice Annotation Project (RAP) databases [29]. The functions of 8 OsCaM1 targets were identified (Table 4), which were diverse and potentially involved in various cellular processes, including metabolism, transcription, movement of organelles and vesicles, membrane transport, and signal transduction. Four known CaM-binding proteins previously identified in other plants were obtained from this screening, which included a cyclic nucleotide-gated ion channel [30] (LOC_Os06g33570), a glutamate decarboxylase [31] (LOC_Os03g51080), a CaM-binding transcription activator (CAMTA) [32] (LOC_Os04g31900), and a kinesin motor domain-containing protein [33] (LOC_Os04g57140). The six identified putative novel CaM1-binding proteins comprised a transferase family protein (LOC_Os02g39850), a response regulator receiver domain-containing protein (LOC_Os09g36220), a lipin (LOC_Os05g38710), a myosin heavy chain-containing protein (LOC_Os12g17310), and two proteins with unknown function, LOC_Os08g34060 and LOC_Os02g13060. Interaction of eight putative target proteins (Table 4) with OsCaM1 was confirmed by protein blot analysis (see Additional file 5).
Table 4.
OsCaM1 target gene list obtained by cDNA expression library screening
| Locus | Gene Annotation | Chromosome | ORF (bp) | Protein (aa residues) | Protein Blot Confirmation |
|---|---|---|---|---|---|
| LOC_Os06g33570 | cyclic nucleotide-gated ion channel | 6 | 2085 | 694 | + |
| LOC_Os03g51080 | glutamate decarboxylase | 3 | 1533 | 510 | + |
| LOC_Os04g31900 | calmodulin-binding transcription activator | 4 | 3012 | 1003 | + |
| LOC_Os04g57140 | kinesin motor domain containing protein | 4 | 3588 | 1195 | + |
| LOC_Os02g39850 | hydroxyanthranilate hydroxycinnamoyltransferase | 2 | 1329 | 442 | + |
| LOC_Os09g36220 | response regulator receiver domain-containing protein | 9 | 1872 | 623 | – |
| LOC_Os05g38710 | lipin, N-terminal conserved region family protein | 5 | 2655 | 884 | + |
| LOC_Os12g17310 | myosin heavy chain-containing protein | 12 | 1944 | 647 | + |
| LOC_Os08g34060 | DUF1336 domain containing protein, expressed | 8 | 2292 | 763 | aND |
| LOC_Os02g13060 | expressed protein | 2 | 699 | 232 | + |
aND represents not determine
Statistical verification of OsCam1–1 affected DEGs and OsCaM1 targets
Overall, by RNA Seq, 3249 genes were found to be differentially expressed between OsCam1–1-overexpressing rice and wild type. To confirm the validity of this gene list, a statistical approach was employed using Fisher’s exact test to determine the statistical confidence of the data as being true. Of the 55,986 rice genes according to the MSU7 rice genome database (http://rice.plantbiology.msu.edu/analyses_facts.shtml) [23], 60 rice genes were co-expressed with OsCam1–1 by co-expression analysis using the web-based tool STRING (https://string-db.org/) [34], which were used as a reference list of known OsCam1–1-affected genes (see Additional file 6). Within this supposed known gene set, 30 genes were found in the list of 3249 OsCam1–1-affected genes based on the RNA-Seq results (see Additional file 7). By Fisher’s exact test, genes in the known gene set were significantly over-presented in our list of 3249 OsCam1–1-affected genes with a calculated p-value of 1.52 × 10− 21.
Similarly, to confirm the validity of the ten putative OsCaM1 targets identified in this study, 60 rice homologs of known Arabidopsis CaM target proteins [35] were identified and used as a reference list of known OsCaM1 targets in rice (see Additional file 8). Within this supposed known protein set, 4 proteins were found in the present study list of 10 OsCaM targets identified by cDNA expression library screening. By Fisher’s exact test, OsCaM1 targets in the known protein set were significantly over-represented in our list of 10 putative OsCaM1 target proteins with a calculated p-value of 2.49 × 10− 10.
Discussion
Based on the transcriptomics analysis, OsCam1–1 overexpression affects genes in several cellular processes, potentially contributing to rice salt tolerance. As the expression level of OsCam1–1 in transgenic rice was much higher than that in wild type, even under normal conditions, numerous DEGs exhibited altered expression levels in the transgenic rice (WTNSL1NS, green colored circles), suggesting that their functions likely confer advantages to plants in coping with future salt stress. Approximately 80% of these genes were salt-responsive, indicating a likely effect on the salt stress response of OsCam1–1 overexpression. Under salt stress, approximately 18.4% of the salt-responsive genes differentially expressed between the transgenic and wild type rice (Table 3), which are further discussed below in detail. However, it should be noted that DEGs with expression levels that remained unchanged under salt stress, categorized as HT DEGs and LT DEGs, might also contribute to the salt tolerance of transgenic rice. However, the high-fold-change-DEGs were mainly identified as unknown proteins (see Additional file 6). Nonetheless, some known genes, which were annotated in the genome database, were found to be involved in salt stress or at least in abiotic stress responses, including ankyrin repeat containing protein (LOC_Os01g09384), hexose carrier protein (LOC_Os11g38160), ATPase/hydrogen-translocating pyrophosphatase (LOC_Os01g23580), UDP glucosyltransferase (LOC_Os01g49240), C3HC4-type RING finger (LOC_Os10g32760), cleavage and polyadenylation specificity factor 100 kDa subunit (LOC_Os02g06940).
Signaling and stress responses
The mitogen-activated protein kinase (MAPK/MPK) cascade is a highly conserved central regulator of diverse cellular processes [36]. CaM plays role in the MAPK/MPK cascade by binding to mitogen-activated protein kinase (MPK) and/or mitogen-activated protein kinase phosphatase (MKP) [37, 38]. A rice MAPK, BWMK1 encoded by an HT salt-responsive DEG, could phosphorylate the OsEREBP1 transcription factor for binding to the GCC box element (AGCCGCC), which is a basic component of several pathogenesis-related gene promoters [39]. Inositol 1,3,4-trisphosphate 5/6-kinase (IPTK) encoded by two HT salt-responsive DEGs, phosphorylates inositol 1,3,4-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4,6, tetrakisphosphate, which are ultimately converted to inositol hexaphosphate (IP6) and play roles in plant growth and development [40]. In rice, the T-DNA mutant of an IPTK gene showed reduced osmolyte accumulation and growth under drought conditions, and some genes involved in osmotic adjustment and reactive oxygen species scavenging were down-regulated. In addition, overexpression of DSM3 (OsITPK2) resulted in a decrease in inositol trisphosphate (IP3), and the phenotypes were similar to the mutant under salt and drought stress conditions. These findings suggested that DSM3 might play a role in fine-tune balancing the inositol phosphate level when plants are exposed to stress or during development [41]. Diacylglycerol kinase (DGK) encoded by an HT salt-responsive DEG, catalyzes the conversion of diacylglycerol (DAG) to phosphatidic acid (PA) [42], and PA plays a role in the stress signaling pathway, including the MAPK/MPK cascade [43]. A report has shown that the expression of OsBIDK1 encoding rice DGK is induced by benzothiadiazole and fungal infection. Moreover, transgenic tobacco constitutively expressing OsBIDK1 was more tolerant to plant pathogenic virus and fungi [44]. These findings suggest that several genes in the signaling process might be enhanced by OsCam1–1 under salt stress.
Interestingly, a universal stress protein (USP) gene was identified as an HT salt-responsive DEG. Evidence has shown that the expression of tomato USP (spUSP) is induced by drought, salt, oxidative stress and ABA, and overexpression of spUSP improves tomato drought tolerance via interactions with annexin, leading to the accumulation of ABA [45]. In addition, a xylanase inhibitor protein gene (OsXIP2), was highly expressed and induced by salt stress and OsCam1–1 overexpression. A previous report has shown that OsXIP can be induced by methyl jasmonate and wounding, so it was suggested that OsXIP may play a role in pathogen defense [46]. As many OsCam1–1 and/or salt stress affecting DEGs involve both biotic and abiotic stresses, OsCam1–1 may be a component that mediates the crosstalk of biotic stress and abiotic stress responses.
Hormone-mediated regulation
Plant hormones play a crucial role in acclimation to abiotic stress and regulate the growth and development and often alter gene expression [47, 48]. Our study revealed that the expression of several genes involved with hormones were changed due to the impact of OsCam1–1 overexpression under salt stress. Lipoxygenase (LOX) encoded by three HT salt-responsive DEGs including homologs of AtLOX2 and AtLOX5, is the enzyme in the early step of the jasmonate (JA) biosynthesis pathway [49]. JA plays a role in the physiological response in plants under biotic and abiotic stress [50, 51]. An earlier report has shown that the absence of AtLOX2 expression results in no change under normal conditions, but JA accumulation induced by wounding is absent and the expression of vsp, a wound-JA-induced gene, is also suppressed [52]. In addition, three HT salt-responsive 12-oxo-PDA-reductase (OPR) genes identified encode JA precursor-catalyzed enzyme that catalyzes the cis-12-oxophytodienoic acid (OPDA) reduction reaction [53]. These findings suggest that the JA content might be enhanced by the overexpression of OsCam1–1 under salt stress by enhancing the production of enzymes in the JA biosynthesis pathway. In addition, the expression of NCED and AAO genes participating in ABA biosynthesis [54] was altered by the influence of OsCam1–1 overexpression under salt stress. ABA biosynthesis is activated by abiotic stress through Ca2+ signaling and the phosphorylation cascade [55]. Our previous report has shown that the expression levels of NCED and AAO, and ABA content are enhanced in transgenic rice over-expressing OsCam1–1 under salt stress in comparison to wild type [17].
Collectively, the transcriptome indicates that OsCam1–1 overexpression likely has effects across biotic and abiotic stresses via plant hormonal regulation through JA and ABA. It has been suggested that biotic-abiotic stress crosstalk may occur via the MAPK/MPK cascade to regulate the plant hormone response to stress [56].
Transcription
Transcription factors (TFs) play roles as master regulators controlling clusters of genes [57] in the plant regulation of the stress response [58]. AP2/EREBP encoded by several HT salt-responsive DEGs, is in a large gene family of TFs that function in plant growth, primary and secondary metabolism, and response to hormones and environmental stimuli [59, 60]. Two AP2/EREBP DEGs were identified as OsDREB1A and OsDREB1B [61], and a previous report has shown that OsDREB1A-overexpressing transgenic Arabidopsis exhibit induced expression of target stress-inducible genes of Arabidopsis DREB1A and increased tolerance to drought, high salt and freezing stress, as compared with wild type [62].
MYB, which was found encoded by several HT salt-responsive DEGs, is an important gene family of TFs, and several Arabidopsis MYB genes respond to hormone(s) or stress [63]. A previous report has shown that overexpression of OsMYB48–1, which is a member of those DEGs, resulted in enhanced salt and drought tolerance in rice. Furthermore, OsMYB48–1 also controlled ABA biosynthesis by regulating the expression of OsNCED4 and OsNCED5 in response to drought stress [64].
WRKY is a large TF family that responds to plant stress by regulating the plant hormone signal transduction pathway and is also involved in the biosynthesis of carbohydrate and secondary metabolites, senescence, and development [65]. According to several reports, WRKY genes identified here as HT salt-responsive DEGs are involved in the biotic stress response. The evidence shows that OsWRKY53 can bind to mitogen-activated protein kinases, OsMPK3 and OsMPK6, and inhibit their activity, resulting in a reduction of JA, jasmonoyl-isoleucine and ethylene production and causing a suppression of herbivore defense ability [66]. The expression of OsWRKY71 was induced by salicylic acid (SA), JA, and 1-aminocyclo-propane-1-carboxylic acid (ACC). Overexpression of OsWRKY71 affected the induction of OsNPR1 and OsPR1b expression, which are defense signaling genes, resulting in an enhancement of bacterial plant pathogen resistance [67]. WRKY13 has been shown to regulate crosstalk between abiotic and biotic stress by suppressing the SNAC1 and WRKY45–1 genes, which are involved in drought and bacterial infection, by binding to W-like-type cis-elements on their gene promoters [68]. OsWRKY62, which was down-regulated by effect of OsCam1–1 overexpression and salt stress, was found in two splicing forms, short and full-length forms. Overexpression of the full-length form of OsWRKY62 resulted in the suppression of blast fungus resistance. In contrast, the knockout Oswrky62 line showed an enhanced defense-related gene expression level and accumulation of phytoalexins [69].
Based on the transcriptome profiles, OsCam1–1 overexpression clearly affected the expression of transcription factors that are well-known to regulate both biotic and abiotic stress responses. Therefore, OsCam1–1 likely functions through the activity of these transcription factors in mediating biotic-abiotic crosstalk regulation via diverse mechanisms. According to our transcriptomics data analysis, plant hormones might mediate the regulation of these TFs, leading to the downstream acclimated phenotypes in response to diverse stresses.
Secondary metabolism
Secondary metabolites play important roles in acclimating the plant to the environment and stress conditions [70]. A hydroxyphenylpyruvate dioxygenase (HPPD), which participates in the first committed reaction in the vitamin E biosynthesis pathway [71], was highly expressed and enhanced by the effect of either OsCam1–1 overexpression or salt stress. Previous evidence has shown that the expression of HPPD responded to oxidative stress in barley leaf because it was induced by senescence, methyl jasmonate, ethylene, hydrogen peroxide and herbicide; paraquat and 3-(3,4-dichlorophenyl)-1,1-dimethylurea [72]. Furthermore, a report on the expression of two rice laccase (LAC) genes (LOC_Os01g63180 and LOC_Os12g15680), which were identified as HT salt-responsive DEG here, in yeast cells suggested that the laccases played roles in atrazine and isoproturon herbicide detoxification [73]. In Arabidopsis, atlac1 and atlac2 mutants exhibited deficient root elongation under polyethylene glycol (PEG) treatment, while the atlac8 mutant showed early flowering and the atlac15 mutant showed abnormal seed color. In addition, the evidence revealed that the expression level of AtLAC2 was enhanced by salt and PEG treatment [74].
Lipid metabolism
Previous evidence has shown that 3-ketoacyl-CoA synthase encoded by three HT salt-responsive DEGs, plays a role in wax biosynthesis. The kcs1–1 mutant exhibited reduced wax content, a thin seedling stem and low moisture sensitivity [75]. Another report has shown that the expression of KCS20 and KCS2/DAISY, two other Arabidopsis 3-ketoacyl-CoA synthase genes, is induced by salt, ABA and drought conditions and that these genes play roles in cuticular wax and suberin biosynthesis in root [76]. In addition, previous evidence has shown that Arabidopsis genes encoding class III triacylglycerol lipase encoded by four HT salt-responsive DEGs, are involved in many processes. At4g16070, which is orthologous to one of HT salt-responsive DEGs, was predicted to be a gene involved in stress or the Ca2+ signaling pathway. At4g16820 and At4g18550, which are orthologous to other rice DEGs are involved in seed germination, senescence or the stress response. At1g02660, which is orthologous to another rice DEG, is involved in the plant defense response signaling pathway [77]. Finally, an early report showed that acyl-CoA dehydrogenase, which was identified encoded by another salt-responsive DEG, functions in mitochondrial β-oxidation in maze root tip under glucose starvation conditions [78]. Based on these results, the activity of OsCam1–1 might affect lipid metabolism and possibly be linked to energy metabolism during salt stress.
Glycolysis, TCA and Glyoxylate cycle
Glycolysis and TCA cycle are essential in the respiratory pathway to generate energy [79]. Earlier comparative proteomic reports comparing salt-sensitive and salt-tolerant rice strains showed that the expression of FBP, which was identified as an HT salt-responsive DEG, was induced by salt stress in a salt-sensitive rice cultivar [80] and in either salt-sensitive or salt-tolerant strains of barley [81], while the activity of PFK encoded by another HT salt-responsive DEG, was increased under NaCl treatment along with that of pyruvate kinase and phosphoenolpyruvate carboxylase, resulting in an increase in respiratory O2 uptake and drastic changes in the levels of glycolytic metabolites in Bruguiera sexangula cell cultures [82]. The aconitase gene, which encodes the enzyme that isomerizes citrate to isocitrate in the early step of the TCA cycle, and the isocitrate dehydrogenase (IDH) gene, which encodes the enzyme that catalyzes the oxidative decarboxylation of isocitrate in the TCA cycle were also found in the HT salt-responsive DEG category. Previous studies have shown that in addition to its other function as an RNA binding protein, aconitase mediates resistance to oxidative stress in plants [83] and overexpression of maize IDH in Arabidopsis enhances salt tolerance in Arabidopsis [84].
In addition, early reports suggested that ICL and MLS, which were identified as HT salt-responsive DEGs here, play a role in converting lipids to sugar using an acetyl unit from β-oxidation to generate the substrate of gluconeogenesis, and this process is important during the post-germination stage in Arabidopsis [85, 86]. Additionally, the HT salt-responsive DEGs included two glucose-6-phosphate transporter genes. A previous study has demonstrated that the transcript level of glucose-6-phosphate/phosphate translocator (GPT2) in Arabidopsis correlates with the sugar level in leaf [87], and another study has suggested that GPT2 functions as a plastid anti-porter transporting glucose-6-phosphate into the plastid to support starch biosynthesis [88]. Recently, a proteomic study has found that cucumber seed germination is enhanced by melatonin under high salt conditions via regulated energy metabolism and the up-regulation of proteins involved in glycolysis, the TCA cycle and the glyoxylate cycle [89]. The transcriptome results herein illustrated possible changes in the cellular respiratory pathway in transgenic rice under salt stress. These lines of evidence suggest that OsCam1–1 may confer salt tolerance by regulating central energy metabolism.
Starch and sucrose metabolism
Salt stress inhibited the activity of granule-bound starch synthase (GSSB) and suppressed the expression of GSSBI and GSSBII, resulting in a decrease in starch content in rice leaf [90]. An earlier report has shown that Pokkali, the standard salt-tolerant rice, shows significantly higher starch concentration under salt stress than KDML 105, which was identified as a salt-sensitive cultivar [91]. The transgenic KDML105 rice examined herein exhibited significant decrease in starch levels, but to a lesser extent than the wild type, while it showed improved maintenance of sucrose levels under salt stress, which probably reflected the higher salt tolerance ability. The transcriptome results revealed that several sucrose and starch degradation genes were up-regulated. An early report revealed that sucrose synthase, which its gene expression level was up-regulated to a higher level in transgenic rice, plays a role in starch synthesis by generating ADP-glucose or UDP-glucose through the cleavage of sucrose, which can be used for starch polymerization. Moreover, the findings showed that sucrose synthase activity correlated with starch and ADP-glucose accumulation in developing barley seed [92] and that transgenic potato plants with a disrupted sucrose synthase gene were defective in starch accumulation [93]. However, the transcriptome results herein showed that lower expression levels of several genes in the starch biosynthetic pathways in transgenic compared with wild type rice. These findings suggested that starch metabolism in higher plant might be regulated by several mechanisms: post-translational modifications such as redox modulation and protein phosphorylation, or allosteric modulation by metabolites, which is related to the metabolic flux [94]. Additionally, three invertase genes, encoding a sucrose-digesting enzyme, were identified as HT salt-responsive DEGs. A double mutant of two isoforms of Arabidopsis neutral invertase genes, inv1/inv2, has been shown to exhibit severe growth defects, and therefore the authors suggested that cytosolic invertase may play role in supplying sucrose to Arabidopsis non-photosynthetic cells [95].
Together with the altered expression levels of these genes, our results for increased starch and sucrose levels in transgenic rice suggest that starch and sucrose metabolism are likely downstream components that are regulated by OsCam1–1 under salt stress. The down-regulation of photosynthetic genes due to the impact of OsCam1–1 and up-regulation of genes involved in lipid metabolism suggests that transgenic rice may balance carbon and energy metabolism under salt stress by obtaining monosaccharide units through the mobilization of lipids, which might be converted to sugar via the glyoxylate cycle and gluconeogenesis, as in previous discussions and/or the cell wall, which is a large carbon reservoir in the cell.
OsCaM1 targets elucidating OsCaM1 downstream components
Four known CaM-interacting proteins (CIPs) previously identified in other plants were obtained from the rice cDNA expression library screening indicating specific CaM target identification. Cyclic nucleotide-gated channels (CNGCs), one of the four known CIPs [30], are activated by binding cyclic nucleotide monophosphates, which play roles in ion-homeostasis control, development, and biotic or abiotic stress defense [96]. In Arabidopsis, AtCNGC10 functions in cation uptake in root, and AtCNGC10 antisense Arabidopsis lines are more salt-sensitive than wild type [97]. Another well-known CIP identified herein, glutamate decarboxylase (GAD), is the enzyme that converts L-glutamate to γ-amino butyric acid (GABA), which is involved in amino acid metabolism. CaM binds to GAD and regulates its activity, resulting in a balance of glutamate-GABA metabolism. Transgenic tobacco expressing petunia GAD lacking the CaM-binding domain exhibits a severely abnormal morphology associated with a high level of GABA but low level of glutamate [98].
Calmodulin-binding transcription activators (CAMTAs) are found in several species of multicellular organisms [32]. Herein, an OsCAMTA was confirmed to be a CaM target, even though the transcriptome showed that overexpression of OsCam1–1 and salt stress did not significantly affect the expression levels of its gene. In Arabidopsis, CAMTA3 regulates a set of biotic stress-responsive genes, and the camta3 Arabidopsis mutant showed enhanced biotic stress tolerance [99]. A rice kinesin motor domain-containing protein [33] was also confirmed as a CaM target in this study. Some evidence has revealed that kinesin motor domain-containing protein plays a role in cell developmental processes. In late anaphase, the amino-terminal motor kinesin (AtPAKRP1) is accumulated along the microtubule toward the spindle mid-zone and then localized to microtubules near the future cell plate area, suggesting that AtPAKRP1 may play a role in the maintenance or establishment of the phragmoplast microtubule array [100]. Another report has revealed that AtPAKRP2 first exhibits a punctate pattern in late anaphase, and then is concentrated at the division site following the appearance of the phragmoplast microtubule array in the mirror pair. Treatment with brefeldin A, which inhibits protein transportation from the endoplasmic reticulum to the Golgi apparatus, resulted in the alteration of AtPAKRP2 localization, so the authors suggested that AtPAKRP2 functions in Golgi-derived vesicles transportation in the phragmoplast [101].
Here, six CaM-interacting proteins that have not been found in other plant species were identified in rice. In Arabidopsis, hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase (HCT) (AT5G48930), a homolog of LOC_Os02g39850, which was identified as CIP herein, contains acyltransferase activity capable of catalyzing the conversion p-coumaroyl-CoA to caffeoyl-CoA, which plays a role in the lignin biosynthesis pathway. Silencing of this acyltransferase gene in Arabidopsis results in a dwarf phenotype and change in lignin composition [102].
LOC_Os09g36220 was identified as OsPRR95, a pseudo response regulator, which takes part in circadian systems by binding a core oscillator to define rhythm to adapt to the daily changing environment [103]. OsPRR95 corresponds to Arabidopsis PRR, AtPRR5 or AtPRR9 [104]. A report has revealed that triple mutant prr9–11 prr7–10 prr5–10 Arabidopsis exhibit better salt, drought and cold tolerance than wild type, and thus suggested that PRR5, PRR7 and PRR9 are involved in the diurnal cold stress-initiating stress response by mediating the cyclic expression of stress response genes, including DREB1/CBF [105]. Additionally, Mesembryanthemum crystallinum (ice plant) CSP1, which is a class of pseudo-response regulator-like proteins, co-localizes with calcium-dependent protein kinase (McCDPK1) in the nucleus of NaCl-stressed ice plants, suggesting that it may be regulated by McCDPK1 through reversible phosphorylation [106].
According to the MSU7 database, LOC_Os05g38710, the novel CaM1 target, is annotated as lipin, and the mRNA sequence of LOC_Os05g38710 is annotated as phosphatidate phosphatase (PAH1) [107]. A report has demonstrated that the N- and C-terminal regions of mammalian lipin protein share sequence similarity to yeast PAH1 [108]. Phosphatidate phosphatase is the enzyme that converts phosphatidic acid to diacylglycerol and Pi [109]. In Phaseolus vulgaris cotyledons, phosphatidate phosphatase is stimulated by Ca2+ or CaM with Ca2+, and a possible role of Ca2+-second-messenger in membrane-lipid degradation initiation has been suggested [110]. Therefore, its identification as a CaM-interacting protein herein suggests that Ca2+/CaM stimulates phosphatidate phosphatase via direct binding.
By protein functional association analysis of each of these CIPs, the GO terms enriched in each set of resulting associated proteins that matched those from OsCam1–1 affected salt-responsive DEGs are presented in Fig. 10. Matched GO terms revealed interacting protein candidates that potentially regulate various cellular processes represented by each enriched GO term of the OsCam1–1 affected salt-responsive DEGs.
Fig. 10.
The matched GO term of either up- or down-regulated OsCam1–1-effect genes obtaining from RNA-seq versus genes interacting with OsCaM1 targets from the STRING database
Conclusion
Transcriptome profiling revealed that 18.4% of salt-responsive DEGs are affected by OsCam1–1 overexpression, which has been previously found to confer salt stress tolerance to transgenic rice [17]. GSEA showed that the DEGs are mainly enriched in the terms of stress response and metabolic process, suggesting that overexpression of OsCam1–1 confers tolerance by regulating a wide range of processes, including signaling and stress response, hormone-mediated regulation, transcription, secondary metabolism, lipid metabolism, photosynthesis, carbohydrate and energy metabolism. The transcriptome data suggest that CaM action results in an enhancement of metabolic enzymes involved in central energy pathways, which may lead to the mobilization of carbon sources that benefits plant acclimation under salt stress during periods of decreased photosynthesis. The CaM1 target screening results consolidated knowledge that CaM1 downstream components are diverse, involving several cell systems and constituting a complex network of downstream components, as represented by the OsCam1–1 affected salt-responsive DEGs involved in CaM-mediated salt stress response mechanisms in rice.
Methods
Plant materials and stress treatment
The rice Oryza sativa L. “Khao Dawk Mali 105” (KDML 105) and transgenic KDML 105 rice over-expressing OsCam1–1, generated in a previous work [17], were grown in a growth chamber (Humanlab, South Korea) with Yoshida’s solution [111] for 1 week and then in the greenhouse for 2 weeks using a completely randomized design (CRD) with two biological replicates. Subsequently, the rice was treated with Yoshida’s solution containing 150 mM NaCl. The pooled tissues of leaf blade and leaf sheet were collected, rapidly frozen in liquid nitrogen and stored at − 80 °C for further analyses.
RNA isolation
The 3-week-old rice was treated with 150 mM NaCl for 4 h, and then the leaves were collected and ground with liquid nitrogen using a chilled mortar and pestle. Subsequently, total RNA was isolated from the tissue using TRI Reagent® (MRC, USA) following the manufacturer’s instruction. After the RNA was precipitated and dissolved in DEPC-treated water, the concentration of RNA was determined using a spectrophotometer (Eppendorf, Germany) based on A260/A280, and the quality of the RNA was determined by agarose-gel electrophoresis.
RNA-seq
cDNA library preparation was initiated by isolating mRNA from the prepared total RNA using Dynabeads® (Ambion™, USA), and then first-strand cDNA was synthesized from the mRNA template using Superscript III® reverse transcriptase (Invitrogen™, USA). The mRNA template was eliminated by RNase H (Thermo Fisher Scientific, USA), and second-strand cDNA was synthesized using DNA polymerase I (Thermo Fisher Scientific, USA). The cDNA was fragmented, and the fragments were modified into blunt end fragments using End Repair Enzyme (New England Biolabs, USA). Deoxyadenosines was added to the 3’end of the fragments by Klenow exo- (New England Biolabs, USA), and then the adapters were ligated to the end of the fragments that would later benefit the sample identification process, as each sample was ligated to a different adapter. The ligated fragments were amplified by PCR using Phusion® High-Fidelity DNA Polymerase (New England Biolabs, USA) with adapter primers, and the quality of the PCR products was checked by gel electrophoresis. Every step was followed by purification using AMPure (Beckman Coulter, USA). The cDNA libraries were subjected to single-read sequencing using an Illumina HiSeq 2000 (Illumina, USA).
Data analysis
The mRNA sequence data obtained from the genome analyzer were demultiplexed and mapped to MSU rice genome version 7 [23] using the computer command sets, Pipeline of Parentally Biased Expression (POPE) [22]. The gene expression data for the wild type and the transgenic rice under normal and salt stress condition were compared using DESeq [24]. Downstream data analyses, Venn diagram construction by the web-based software at http://bioinformatics.psb.ugent.be/webtools/Venn/, and gene enrichment analysis by agriGO [112] were performed. Pathway analysis was conducted to illustrate genes in the pathway using MapMan software [113].
Real-time RT-PCR
Rice(s) growing, stress treatment and total RNA isolation were repeated with 4 biological replications. The isolated RNA was treated with DNaseI (Thermo Scientific™, USA) and converted to cDNA using the iScript™ cDNA synthesis kit (Bio-Rad, USA). Real-time RT-PCR was performed using SsoFast EvaGreen Supermix (Bio-Rad, USA) with the CFX96™ Real-Time PCR machine (Bio-Rad, USA) with EF-1-α as an internal control. The DNA primers used in this experiment were as follows: EF-1-α (LOC_Os03g08010) F-5′ATGGTTGTGGAGACCTTC3′, R-5′TCACCTTGGCACCGGTTG3′, aconitase (LOC_Os08g09200) F-5′CATCCTCCCATACGTCATCC3′, R-5′TGTCTCCTGCGGCTTTATTT3′, isocitrate lyase (LOC_Os07g34520) F-5′AGAGCAGCAGCCATGTTCTT3′, R-5′CGTGCGTGCTGTAGTTCAGT3′, malate synthase (LOC_Os04g40990) F-5′CGTACAACCTCATCGTGGTG3′, R-5′CGGAGAAGTTACACGGAGAGA3′, AP2 (LOC_Os03g08470) F-5′CTGTGGAGCTTCGACGACTT3′, R-5′ACAAACACAAACACCGCAAT3′, ERD1 (LOC_Os02g32520) F-5′GAGCCACCTGAATGAGAAGG3′, R-5′TTATATGCCCGAACGAATCC3′, glycosyl hydrolase (LOC_Os04g45290) F-5′GCTCAGGTGTGTGTGGTACA3′, R-5′CGACAGCACACATCCTCTGT3′, β-amylase (LOC_Os03g22790) F-5′ATGGATGATGCCCCCTGT3′, R-5′TTGGGGTACACGTCTCATGT3′, isocitrate dehydrogenase (LOC_Os05g49760) F-5′ TCGAGTCTGGGAAGATGACC3′, R-5′AGTGCATCGGATCACATCAA3′ and pyruvate decarboxylase (LOC_Os03g18220) F-5′AGGACGACACCAGCAAAGAG3′, R-5′ GAGGGAATGGACACAAGGAA3′.
Sucrose and starch determination
The 3-week-old rice grown in a CRD with five replicates was treated with 150 mM NaCl for 3 and 5 days, and then leaves were collected, lyophilized, ground and weighed. Sucrose extraction was performed according to Cowan A.K. et al. [114], and starch was extracted according to Alison and Samuel [115]. The samples for sucrose measurement were then treated with invertase (Sigma, USA) and those for starch measurement with α- and β-amylase (Sigma, USA), followed by incubation at 37 °C for 2 h. The amounts of sucrose and starch were determined based on glucose using the D-Fructose/D-Glucose test kit (Megazyme, Ireland). Potato starch (Sigma, USA) and sucrose (Sigma, USA) were used to construct standard curves.
Gas exchange and fluorescence measurements
The photosynthesis rate (Pn), stomatal conductance (gs), intercellular carbon dioxide (Ci), transpiration rate (E) and FV′/FM′ of 3-week-old transgenic and wild type rice, which were hydroponically grown under natural light in a CRD with five replicates, were measured using the LI-6400XT portable photosynthesis system (LI-COR®, USA) at day 3 and 5 of 150 mM NaCl treatment. The same parameters were also measured in untreated plants at each time point, and the data were compared.
Statistical analysis
Data from real-time RT-PCR, sucrose and starch determination, and gas exchange and fluorescence measurements were compared using analysis of variance (ANOVA), and then the means were compared with Duncan’s multiple range test, with significance accepted at the p < 0.05 level. Fisher’s exact test was used to verify that genes in the known gene set were significantly over-presented in the list of OsCam1–1 affected DEGs or OsCaM1 targets, with significance accepted at the p < 0.05 level.
Preparation of 35S-labeled rOsCaM1 protein
The rOsCaM1 protein [116] was prepared according to Fromm and Chua [117] to be used as a probe for cDNA library expression screening. Production of the recombinant protein in 50 ml cell culture of BL21(DE3) cells harboring pET-21a(+) containing a complete OsCam1–1 ORF was induced by IPTG. After 15 min of IPTG induction, 1 mCi or Tran 35S Label™ (MP Biomedicals, USA) was added to the culture, and the incubation was continued for 4 h. After centrifugation, the pelleted cells were resuspended in 4 ml B-PER reagent containing 1 U/ml DNase I and 20 μg/ml lysozyme. The homogenate was heated in boiling water for 5 min and centrifuged at 15,000 xg for 10 min. The supernatant was collected, and the 35S-labeled rOsCaM1 protein was purified by phenyl-sepharose chromatography in the presence of Ca2+ according to Liao and Zielinski [118].
cDNA expression library construction
The cDNA library was constructed by RNA isolation of 100 mM NaCl-treated hydroponically grown 1-week-old KDML105 rice using TRI Reagent® (MRC, USA) and mRNA purification using the Illustra™ Quick Prep Micro mRNA Purification Kit (GE Healthcare, UK). cDNA synthesis and cDNA library construction were carried out using the Uni-ZAP XR vector system (Stratagene, USA). The cDNA was ligated to XhoI-EcoRI double-digested Uni-ZAP XR vector, and then the ligated mixture was packaged using Gigapack III packaging extract (Stratagene, USA). The packaging was performed using XL1-Blue MRF’ E. coli cells, and the cells were cultured on NZY agar plates overlaid with NZY agar containing IPTG and X-gal for blue-white colony selection. The libraries were propagated in XL1-Blue MRF′ E. coli, and the propagated libraries were stored in 0.3% (v/v) chloroform at 4 °C.
cDNA expression library screening
Primary screening was carried out using the propagated library according to Chinpongpanich et al. [119]. The 600-μl aliquots of XL1-Bule MRF2 cell suspension were mixed with the equivalent of 25,000 pfu of the amplified library and incubated at 37 °C for 15 min before plating as top agar with the addition of IPTG onto an LB agar plate. A prewetted nitrocellulose membrane was then placed onto each agar plate, followed by incubation at 37 °C overnight. The membranes were lifted, washed with 1X TBS buffer containing 0.05% Tween-20 (TTBS), and incubated in Blocking Buffer (1X TBS buffer containing 0.05% (v/v) Tween-20, 5 mM CaCl2 and 3% (w/v) skim milk). They were then washed twice in TTBS with 5 mM CaCl2 and incubated with 35S-labeled rOsCaM1 probe solution for 1–4 h. The membranes were washed twice in TTBS with 5 mM CaCl2 and air-dried overnight. Probe binding on the membranes was then detected by autoradiography, and positive plaques were selected for secondary and tertiary screening. The cDNA-containing pBluescript phagemid was excised from the Uni-ZAP XR vector using ExAssist helper phage with the E. coli SOLR strain, and the pBluescript phagemid was isolated and then sequenced using M13F and M13R universal primers.
Protein blot analysis
The E. coli strain SOLR cells harboring the cloned cDNA insert-containing pBluescript SK(−) plasmids were cultured and induced by IPTG addition at room temperature overnight. The OsCaM1-binding ability of the positive clones was examined using the 35S-labeled rOsCaM1 probe. The duplicate blots were incubated in the probe solution and then separately washed in 1X TTBS containing CaCl2 or EDTA. Probe binding on the membrane was detected by autoradiography.
Additional files
The DEG lists grouped by HT salt-responsive DEG, LT salt-responsive DEG, HT DEG, LT DEG and salt-responsive DEG. This list contains locus number and annotation of the genes. (XLSX 2043 kb)
The bar-chart showing expression level (RPKM) of photosynthetic DEGs in the rice(s) under both stress and normal condition. Bar-chart showing expression level of photosynthetic DEGs from RNA-seq data comparing between the transgenic rice overexpressing OsCam1–1 under stress (150 mM NaCl) condition (L1S), wild type under stress (WTS), transgenic under non-stress (L1NS) and wild type under non-stress (WTNS), and the Y axis represent RPKM. (PDF 171 kb)
The bar-chart showing FV’/FM’ of the rice(s) under either normal or salt-stress condition. Fluorescence measurement in leaf of the transgenic rice overexpressing OsCam1–1 (L1, L2, L7) and wild type (WT) under normal and salt stress (150 mM NaCl) condition (A) at day 3 and (B) day 5 of treatment. (PDF 31 kb)
The reliable testing of the 35S-labeled rOsCaM1 binding for testing accuracy and specificity. Examination of the 35S-labeled rOsCaM1 protein, A) 12% SDS-PAGE of the 35S-labeled rOsCaM1 elutes. Lane M: Protein molecular weight marker. Lanes 1–4: Fractions 1–4 of the 35S-labeled rOsCaM1 elute, respectively, and B) Autoradiograph of the blot spotted with various amounts (as written above each spot) of positive (CaMKII peptide and calcineurin) or negative (BSA) control. (PDF 1805 kb)
Autoradiographs of far western analysis of 11 putative OsCaM1-binding proteins. Lane M: protein molecular weight markers (negative control); Lane P: calcineurin (positive control); Lane N: crude protein extract from the SOLR cells harboring the pBluescript SK(−) plasmid (negative control); Numbers indicated above the lanes are clone No. assigned when these clones were isolated from the primary screening as following: 2, Cyclic nucleotide-gated ion channel (CNGC); 5, glutamate decarboxylase (GAD); 10, Hydroxyanthranilate hydroxyl cinnamoyltransferase (HHT); 12, CaM-binding transcription activator (CAMTA); 19, Kinesin motor domain- containing protein (KCBP); 21, Kinesin motor domain- containing protein (KCBP); 23, Myosin heavy chain; 24, unknown expressed protein, while 18, 26 and 31 which represent response regulator receiver domain-containing protein (PRR), lipin, N-terminal conserved region family protein, and another clone of Kinesin motor domain- containing protein (KCBP) respectively, were unclear. (PDF 8517 kb)
The list of OsCam1–1 co-expressed DEGs obtaining from STRING (Excel file). This list contains locus number and annotation of the genes. (XLSX 17 kb)
The list of shared genes between OsCam1–1 co-expressed gene obtaining from STRING and DEGs affected by OsCam1–1 overexpression from RNA-Seq result. This list contains locus number and annotation of the genes. (XLSX 12 kb)
The list of rice homologous CaM target genes using known Arabidopsis CaM target gene as references. This list contains locus number and annotation of the genes. (XLSX 23 kb)
Acknowledgments
We would like to sincerely thank Ms. Thammaporn Kojonna and Mr. Chakkree Lekklar for kind assistance with the gas exchange and fluorescence measurement experiments, and Ms. Srivilai Phean-o-pas with the cDNA expression screening experiments.
Funding
This work was supported by Thailand Research Fund (No. BRG5680019) to T.B. W.Y. was supported by the Royal Golden Jubilee Ph.D. Program-RGJ (PHD/0043/2556–4.C.CU/56/G.1.O.XX) from the Thailand Research Fund.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CaM
Calmodulin
- Ci
Intercellular carbon dioxide
- CIP
CaM-interacting protein
- CRD
Completely randomized design
- DEG
Differentially expressed gene
- E
Transpiration rate
- gs
Stomatal conductance
- HT
DEGs whose expression levels were higher in the transgenic rice
- KDML 105
Khao Dawk Mali 105
- L1
Transgenic KDML 105 rice overexpressing OsCam1–1 Line 1
- L2
Transgenic KDML 105 rice overexpressing OsCam1–1 Line 2
- L7
Transgenic KDML 105 rice overexpressing OsCam1–1 Line 7
- LT
DEGs whose expression levels were lower in the transgenic rice
- NS
Non-stress / normal condition
- Pn
Photosynthesis Rate
- POPE
Pipeline of Parentally Biased Expression
- RPKM
Read Per Kilobase of Transcript Per Million Mapped Reads
- S
Stress condition
- TF
Transcription Factor
- WT
Wild Type
- WTNS
WT under non-stress
- WTS
WT under stress
Authors’ contributions
WY and AC carried out the laboratory work in collaboration with LC and SC, WY and TB performed the data analysis and interpretation. WY prepared the figures and tables. WY and TB drafted the manuscript with commentary from LC and SC. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Worawat Yuenyong, Email: worawadh@gmail.com.
Aumnart Chinpongpanich, Email: art.aumnart@hotmail.com.
Luca Comai, Email: lcomai@ucdavis.edu.
Supachitra Chadchawan, Email: Supachitra.C@chula.ac.th.
Teerapong Buaboocha, Email: Teerapong.B@chula.ac.th.
References
- 1.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 2.Horie T, Karahara I, Katsuhara M. Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice. 2012;5(1):11. doi: 10.1186/1939-8433-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Läuchli A, Grattan SR. Plant growth and development under salinity stress. In: Jenks MA, Hasegawa PM, Jain SM, editors. Advances in molecular breeding toward drought and salt tolerant crops. Dordrecht: Springer Netherlands; 2007. pp. 1–32. [Google Scholar]
- 4.Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of Rice ( Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot. 1996;78(3):389–398. doi: 10.1006/anbo.1996.0134. [DOI] [Google Scholar]
- 5.Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS-Scavenging Systems to salt stress during seedling and reproductive stages in Rice. Ann Bot. 2007;99(6):1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng L, Shannon MC, Lesch SM. Timing of salinity stress affects rice growth and yield components. Agric Water Manag. 2001;48(3):191–206. doi: 10.1016/S0378-3774(00)00146-3. [DOI] [Google Scholar]
- 7.Kudla J, Batistič O, Hashimoto K. Calcium signals: the Lead currency of plant information processing. Plant Cell. 2010;22(3):541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight H. International Review of Cytology - a Survey of Cell Biology. Vol. 195. 2000. Calcium signaling during abiotic stress in plants; pp. 269–324. [DOI] [PubMed] [Google Scholar]
- 9.Yang T, Poovaiah BW. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003;8(10):505–512. doi: 10.1016/j.tplants.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Means AR, Dedman JR. Calmodulin an intracellular calcium receptor. Nature. 1980;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- 11.Day IS, Reddy VS, Shad Ali G, Reddy ASN. Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol. 2002;3(10):RESEARCH0056. doi: 10.1186/gb-2002-3-10-research0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonburapong B, Buaboocha T. Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol. 2007;7(1):4. doi: 10.1186/1471-2229-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phean-o-Pas S, Punteeranurak P, Buaboocha T. Calcium signaling-mediated and differential induction of calmodulin gene expression by stress in Oryza sativa L. J Biochem Mol Biol. 2005;38(4):432–439. doi: 10.5483/bmbrep.2005.38.4.432. [DOI] [PubMed] [Google Scholar]
- 14.Botella JR, Arteca RN. Differential expression of two calmodulin genes in response to physical and chemical stimuli. Plant Mol Biol. 1994;24(5):757–766. doi: 10.1007/BF00029857. [DOI] [PubMed] [Google Scholar]
- 15.Olsson P, Yilmaz JL, Sommarin M, Persson S, Bülow L. Expression of bovine calmodulin in tobacco plants confers faster germination on saline media. Plant Sci. 2004;166(6):1595–1604. doi: 10.1016/j.plantsci.2004.02.013. [DOI] [Google Scholar]
- 16.Yoo JH, Park CY, Kim JC, Do Heo W, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem. 2005;280(5):3697–3706. doi: 10.1074/jbc.M408237200. [DOI] [PubMed] [Google Scholar]
- 17.Saeng-ngam S, Takpirom W, Buaboocha T, Chadchawan S. The role of the OsCam1-1 salt stress sensor in ABA accumulation and salt tolerance in rice. J Plant Biol. 2012;55(3):198–208. doi: 10.1007/s12374-011-0154-8. [DOI] [Google Scholar]
- 18.WU HUI-CHEN, LUO DAN-LI, VIGNOLS FLORENCE, JINN TSUNG-LUO. Heat shock-induced biphasic Ca2+ signature and OsCaM1-1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.) Plant, Cell & Environment. 2012;35(9):1543–1557. doi: 10.1111/j.1365-3040.2012.02508.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Zhou R-G, Gao Y-J, Zheng S-Z, Xu P, Zhang S-Q, Sun D-Y. Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 2009;149(4):1773–1784. doi: 10.1104/pp.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi MS, Kim MC, Yoo JH, Moon BC, Koo SC, Park BO, Lee JH, Koo YD, Han HJ, Lee SY, et al. Isolation of a calmodulin-binding transcription Factor from Rice (Oryza sativa L.) J Biol Chem. 2005;280(49):40820–40831. doi: 10.1074/jbc.M504616200. [DOI] [PubMed] [Google Scholar]
- 21.Reddy ASN, Ali GS, Celesnik H, Day IS. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell. 2011;23(6):2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missirian V, Henry I, Comai L, Filkov V. POPE: pipeline of parentally-biased expression. In: Bleris L, Măndoiu I, Schwartz R, Berlin WJ, editors. Bioinformatics research and applications: 8th international symposium, ISBRA 2012, Dallas, TX, USA, may 21–23, 2012 proceedings. Heidelberg: Springer Berlin Heidelberg; 2012. pp. 177–188. [Google Scholar]
- 23.Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6(1):1–10. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinpongpanich A, Limruengroj K, Phean-o-pas S, Limpaseni T, Buaboocha T. Expression analysis of calmodulin and calmodulin-like genes from rice, Oryza sativa L. BMC Res Notes. 2012;5(1):625. doi: 10.1186/1756-0500-5-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59(1):89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 27.Basavappa S, Mangel AW, Scott L, Liddle RA. Activation of calcium channels by cAMP in STC-1 cells is dependent upon Ca2+calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1999;254(3):699–702. doi: 10.1006/bbrc.1998.9997. [DOI] [PubMed] [Google Scholar]
- 28.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci. 1979;76(12):6270. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohyanagi H, Tanaka T, Sakai H, Shigemoto Y, Yamaguchi K, Habara T, Fujii Y, Antonio BA, Nagamura Y, Imanishi T, et al. The Rice annotation project database (RAP-DB): hub for Oryza sativa ssp. japonica genome information. Nucleic Acids Res. 2006;34(suppl_1):D741–D744. doi: 10.1093/nar/gkj094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuurink RC, Shartzer SF, Fath A, Jones RL. Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc Natl Acad Sci. 1998;95(4):1944–1949. doi: 10.1073/pnas.95.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H. A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J Biol Chem. 1993;268(26):19610–19617. [PubMed] [Google Scholar]
- 32.Bouché N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J Biol Chem. 2002;277(24):21851–21861. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- 33.Reddy ASN, Safadi F, Narasimhulu SB, Golovkin M, Hu X. A novel plant calmodulin-binding protein with a kinesin heavy chain motor domain. J Biol Chem. 1996;271(12):7052–7060. doi: 10.1074/jbc.271.12.7052. [DOI] [PubMed] [Google Scholar]
- 34.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy VS, Ali GS, Reddy ASN. Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J Biol Chem. 2002;277(12):9840–9852. doi: 10.1074/jbc.M111626200. [DOI] [PubMed] [Google Scholar]
- 36.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9(4):436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Katou S, Kuroda K, Seo S, Yanagawa Y, Tsuge T, Yamazaki M, Miyao A, Hirochika H, Ohashi Y. A calmodulin-binding mitogen-activated protein kinase phosphatase is induced by wounding and regulates the activities of stress-related mitogen-activated protein kinases in Rice. Plant Cell Physiol. 2007;48(2):332–344. doi: 10.1093/pcp/pcm007. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell. 2011;41(6):649–660. doi: 10.1016/j.molcel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, et al. BWMK1, a Rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132(4):1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Tan S, Xue H. Arabidopsis inositol 1,3,4-trisphosphate 5/6 kinase 2 is required for seed coat development. Acta Biochim Biophys Sin. 2013;45(7):549–560. doi: 10.1093/abbs/gmt039. [DOI] [PubMed] [Google Scholar]
- 41.Du H, Liu L, You L, Yang M, He Y, Li X, Xiong L. Characterization of an inositol 1,3,4-trisphosphate 5/6-kinase gene that is essential for drought and salt stress responses in rice. Plant Mol Biol. 2011;77(6):547–563. doi: 10.1007/s11103-011-9830-9. [DOI] [PubMed] [Google Scholar]
- 42.Arisz SA, Testerink C, Munnik T. Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta. 2009;1791(9):869–875. doi: 10.1016/j.bbalip.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Canonne J, Froidure-Nicolas S, Rivas S. Phospholipases in action during plant defense signaling. Plant Signal Behav. 2011;6(1):13–18. doi: 10.4161/psb.6.1.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidong Z, Jie C, Huijuan Z, Fengming S. Overexpression of a Rice diacylglycerol kinase gene OsBIDK1 enhances disease resistance in transgenic tobacco. Mol Cells. 2008;26(3):258–264. [PubMed] [Google Scholar]
- 45.Loukehaich R, Wang T, Ouyang B, Ziaf K, Li H, Zhang J, Lu Y, Ye Z. SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J Exp Bot. 2012;63(15):5593–5606. doi: 10.1093/jxb/ers220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokunaga T, Esaka M. Induction of a novel XIP-type xylanase inhibitor by external ascorbic acid treatment and differential expression of XIP-family genes in Rice. Plant Cell Physiol. 2007;48(5):700–714. doi: 10.1093/pcp/pcm038. [DOI] [PubMed] [Google Scholar]
- 47.Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 2011;14(3):290–295. doi: 10.1016/j.pbi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Sah SK, Reddy KR, Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 2016;7:571. doi: 10.3389/fpls.2016.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feussner I, Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53(1):275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Kazi AM, Gucel S. Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci. 2016;7:813. doi: 10.3389/fpls.2016.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20(4):219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci U S A. 1995;92(19):8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobajima H, Takeda M, Sugimori M, Kobashi N, Kiribuchi K, Cho E-M, Akimoto C, Yamaguchi T, Minami E, Shibuya N, et al. Cloning and characterization of a jasmonic acid-responsive gene encoding 12-oxophytodienoic acid reductase in suspension-cultured rice cells. Planta. 2003;216(4):692–698. doi: 10.1007/s00425-002-0909-z. [DOI] [PubMed] [Google Scholar]
- 54.Eiji N, Annie M-P. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56(1):165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 55.Xiong L, Zhu J-K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003;133(1):29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CristinaRodriguez M, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61(1):621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009;149(1):88–95. doi: 10.1104/pp.108.129791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh KB, Foley RC, Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol. 2002;5(5):430–436. doi: 10.1016/S1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 59.GUO Y., HUANG R., DUAN L., WANG J. The APETALA2/ethylene-responsive factor transcription factor OsDERF2 negatively modulates drought stress in rice by repressing abscisic acid responsive genes. The Journal of Agricultural Science. 2017;155(06):966–977. doi: 10.1017/S0021859617000041. [DOI] [Google Scholar]
- 60.Licausi F, Ohme-Takagi M, Perata P. APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol. 2013;199(3):639–649. doi: 10.1111/nph.12291. [DOI] [PubMed] [Google Scholar]
- 61.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and Rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003;33(4):751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- 63.Yanhui C, Xiaoyuan Y, Kun H, Meihua L, Jigang L, Zhaofeng G, Zhiqiang L, Yunfei Z, Xiaoxiao W, Xiaoming Q, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the Rice MYB family. Plant Mol Biol. 2006;60(1):107–124. doi: 10.1007/s11103-005-2910-y. [DOI] [PubMed] [Google Scholar]
- 64.Xiong H, Li J, Liu P, Duan J, Zhao Y, Guo X, Li Y, Zhang H, Ali J, Li Z. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in Rice. PLoS One. 2014;9(3):e92913. doi: 10.1371/journal.pone.0092913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59(2):86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- 66.Hu L, Ye M, Li R, Zhang T, Zhou G, Wang Q, Lu J, Lou Y. The Rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol. 2015;169(4):2907–2921. doi: 10.1104/pp.15.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Bai X, Wang X, Chu C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol. 2007;164(8):969–979. doi: 10.1016/j.jplph.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Xiao J, Cheng H, Li X, Xiao J, Xu C, Wang S. Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiol. 2013;163(4):1868–1882. doi: 10.1104/pp.113.226019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Chen X, Liang X, Zhou X, Yang F, Liu J, He SY, Guo Z. Alternative splicing of Rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 2016;171(2):1427–1442. doi: 10.1104/pp.15.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajjawi I, Shintani D. Engineered plants with elevated vitamin E: a nutraceutical success story. Trends Biotechnol. 2004;22(3):104–107. doi: 10.1016/j.tibtech.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Falk Jon KN, Dähnhardt D, Krupinska K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. J Plant Physiol. 2002;159(11):1245–1253. doi: 10.1078/0176-1617-00804. [DOI] [Google Scholar]
- 73.Huang MT, Lu YC, Zhang S, Luo F, Yang H. Rice (Oryza sativa) laccases involved in modification and detoxification of herbicides atrazine and Isoproturon residues in plants. J Agric Food Chem. 2016;64(33):6397–6406. doi: 10.1021/acs.jafc.6b02187. [DOI] [PubMed] [Google Scholar]
- 74.Cai X, Davis EJ, Ballif J, Liang M, Bushman E, Haroldsen V, Torabinejad J, Wu Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J Exp Bot. 2006;57(11):2563–2569. doi: 10.1093/jxb/erl022. [DOI] [PubMed] [Google Scholar]
- 75.Todd J, Post-Beittenmiller D, Jaworski Jan G. KCS1encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis inArabidopsis thaliana. Plant J. 1999;17(2):119–130. doi: 10.1046/j.1365-313X.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 76.Lee S-B, Jung S-J, Go Y-S, Kim H-U, Kim J-K, Cho H-J, Park OK, Suh M-C. Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J. 2009;60(3):462–475. doi: 10.1111/j.1365-313X.2009.03973.x. [DOI] [PubMed] [Google Scholar]
- 77.Li W, Ling H, Zhang F, Yao H, Sun X, Tang K. Analysis of Arabidopsis genes encoding putative class III lipases. J Plant Biochem Biotechnol. 2012;21(2):261–267. doi: 10.1007/s13562-011-0103-0. [DOI] [Google Scholar]
- 78.Dieuaide M, Couée I, Pradet A, Raymond P. Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for mitochondrial fatty acid β-oxidation and acyl-CoA dehydrogenase activity in a higher plant. Biochem J. 1993;296(1):199. doi: 10.1042/bj2960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol. 2004;7(3):254–261. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Lee D-G, Woong Park K, Young An J, Geol Sohn Y, Ki Ha J, Yoon Kim H, Won Bae D, Hee Lee K, Jun Kang N, Lee B-H, et al. Proteomics analysis of salt-induced leaf proteins in two rice germplasms with different salt sensitivity. Can J Plant Sci. 2011;91(2):337–349. doi: 10.4141/CJPS10022. [DOI] [Google Scholar]
- 81.Rasoulnia A, Bihamta MR, Peyghambari SA, Alizadeh H, Rahnama A. Proteomic response of barley leaves to salinity. Mol Biol Rep. 2011;38(8):5055–5063. doi: 10.1007/s11033-010-0651-8. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki M, Hashioka A, Mimura T, Ashihara H. Salt stress and glycolytic regulation in suspension-cultured cells of the mangrove tree, Bruguiera sexangula. Physiol Plant. 2005;123(3):246–253. doi: 10.1111/j.1399-3054.2005.00456.x. [DOI] [Google Scholar]
- 83.Moeder W, del Pozo O, Navarre DA, Martin GB, Klessig DF. Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol Biol. 2007;63(2):273–287. doi: 10.1007/s11103-006-9087-x. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Shi Y, Song Y, Wang T, Li Y. Characterization of a stress-induced NADP-isocitrate dehydrogenase gene in maize confers salt tolerance in Arabidopsis. J Plant Biol. 2010;53(2):107–112. doi: 10.1007/s12374-009-9091-1. [DOI] [Google Scholar]
- 85.Cornah JE, Germain V, Ward JL, Beale MH, Smith SM. Lipid utilization, gluconeogenesis, and seedling growth in Arabidopsis mutants lacking the Glyoxylate cycle enzyme malate synthase. J Biol Chem. 2004;279(41):42916–42923. doi: 10.1074/jbc.M407380200. [DOI] [PubMed] [Google Scholar]
- 86.Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA. Postgerminative growth and lipid catabolism in oilseeds lacking the gloxylate cycle. Proc Natl Acad Sci U S A. 2000;97(10):5669–5674. doi: 10.1073/pnas.97.10.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kunz HH, Häusler RE, Fettke J, Herbst K, Niewiadomski P, Gierth M, Bell K, Steup M, Flügge UI, Schneider A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. 2010;12:115–128. doi: 10.1111/j.1438-8677.2010.00349.x. [DOI] [PubMed] [Google Scholar]
- 88.Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U-I. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell. 1998;10(1):105–117. doi: 10.1105/tpc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang N, Zhang H-J, Sun Q-Q, Cao Y-Y, Li X, Zhao B, Wu P, Guo Y-D. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci Rep. 2017;7(1):503. doi: 10.1038/s41598-017-00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H-J, Chen J-Y, Wang S-J. Molecular regulation of starch accumulation in rice seedling leaves in response to salt stress. Acta Physiol Plant. 2008;30(2):135–142. doi: 10.1007/s11738-007-0101-y. [DOI] [Google Scholar]
- 91.Pattanagul W, Thitisaksakul M. Effect of salinity stress on growth and carbohydrate metabolism in three Rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Biol. 2008;46(10):736–742. [PubMed] [Google Scholar]
- 92.Baroja-Fernández E, Muñoz FJ, Saikusa T, Rodríguez-López M, Akazawa T, Pozueta-Romero J. Sucrose synthase catalyzes the de novo production of ADPglucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol. 2003;44(5):500–509. doi: 10.1093/pcp/pcg062. [DOI] [PubMed] [Google Scholar]
- 93.Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.) Plant J. 1995;7(1):97–107. doi: 10.1046/j.1365-313X.1995.07010097.x. [DOI] [PubMed] [Google Scholar]
- 94.Tetlow IJ, Morell MK, Emes MJ. Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot. 2004;55(406):2131–2145. doi: 10.1093/jxb/erh248. [DOI] [PubMed] [Google Scholar]
- 95.Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci. 2009;106(31):13124–13129. doi: 10.1073/pnas.0900689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007;581(12):2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 97.Kun-Mei G, Olga B, CD A, Tamas B, Zed R. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol Plant. 2008;134(3):499–507. doi: 10.1111/j.1399-3054.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- 98.Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996;15(12):2988–2996. doi: 10.1002/j.1460-2075.1996.tb00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galon Y, Nave R, Boyce JM, Nachmias D, Knight MR, Fromm H. Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis. FEBS Lett. 2008;582(6):943–948. doi: 10.1016/j.febslet.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 100.Lee YRJ, Liu B. Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr Biol. 2000;10(13):797–800. doi: 10.1016/S0960-9822(00)00564-9. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y-RJ, Giang HM, Liu B. A novel plant kinesin-related protein specifically associates with the Phragmoplast organelles. Plant Cell. 2001;13(11):2427–2439. doi: 10.1105/tpc.13.11.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M. Silencing of Hydroxycinnamoyl-coenzyme a shikimate/Quinate Hydroxycinnamoyltransferase affects Phenylpropanoid biosynthesis. Plant Cell. 2004;16(6):1446–1465. doi: 10.1105/tpc.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campoli C, Shtaya M, Davis SJ, von Korff M. Expression conservation within the circadian clock of a monocot: natural variation at barley Ppd-H1affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant Biol. 2012;12(1):97. doi: 10.1186/1471-2229-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T. The evolutionarily conserved OsPRR quintet: Rice Pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol. 2003;44(11):1229–1236. doi: 10.1093/pcp/pcg135. [DOI] [PubMed] [Google Scholar]
- 105.Nakamichi N, Kusano M, Fukushima A, Kita M, Ito S, Yamashino T, Saito K, Sakakibara H, Mizuno T. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol. 2009;50(3):447–462. doi: 10.1093/pcp/pcp004. [DOI] [PubMed] [Google Scholar]
- 106.Rahul PO, CJ C. A stress-induced calcium-dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two-component pseudo-response regulator. Plant J. 2000;24(5):679–691. doi: 10.1046/j.1365-313x.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 107.Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, Kishimoto N, Yazaki J, Ishikawa M, Yamada H, Ooka H, et al. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica Rice. Science. 2003;301(5631):376–379. doi: 10.1126/science.1081288. [DOI] [PubMed] [Google Scholar]
- 108.Péterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 109.Smith SW, Weiss SB, Kennedy EP. The enzymatic Dephosphorylation of phosphatidic acids. J Biol Chem. 1957;228(2):915–922. [PubMed] [Google Scholar]
- 110.Paliyath G, Thompson JE. Calcium- and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida S, Forno DA, Cock JH. Laboratory manual for physiological studies of rice. 1971. [Google Scholar]
- 112.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38(suppl 2):W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M. A guide to using MapMan to visualize and compare omics data in plants: a case study in the crop species, maize. Plant Cell Environ. 2009;32(9):1211–1229. doi: 10.1111/j.1365-3040.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 114.Cowan AK, Freeman M, Björkman P-O, Nicander B, Sitbon F, Tillberg E. Effects of senescence-induced alteration in cytokinin metabolism on source-sink relationships and ontogenic and stress-induced transitions in tobacco. Planta. 2005;221(6):801–814. doi: 10.1007/s00425-005-1489-5. [DOI] [PubMed] [Google Scholar]
- 115.Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nat Protocols. 2006;1(3):1342–1345. doi: 10.1038/nprot.2006.232. [DOI] [PubMed] [Google Scholar]
- 116.Phean-o-pas S, Limpaseni T, Buaboocha T. Structure and expression analysis of the OsCam1-1 calmodulin gene from Oryza sativa L. BMB Rep. 2008;41(11):771–777. doi: 10.5483/BMBRep.2008.41.11.771. [DOI] [PubMed] [Google Scholar]
- 117.Fromm H, Chua N-H. Cloning of plant cDNAs encoding calmodulin-binding proteins using35S-labeled recombinant calmodulin as a probe. Plant Mol Biol Report. 1992;10(2):199–206. doi: 10.1007/BF02668347. [DOI] [Google Scholar]
- 118.Liao B, Zielinski RE: Production of recombinant plant calmodulin and its use to detect calmodulin-binding proteins. In: Galbraith DW, Bohnert HJ, Bourque DP, editors. Methods in Cell Biology. Massachusetts: Academic Press; 1995. p. 487–500. [DOI] [PubMed]
- 119.Chinpongpanich A, Phean-O-Pas S, Thongchuang M, Qu L-J, Buaboocha T. C-terminal extension of calmodulin-like 3 protein from Oryza sativa L.: interaction with a high mobility group target protein. Acta Biochim Biophys Sin. 2015;47(11):880–889. doi: 10.1093/abbs/gmv097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The DEG lists grouped by HT salt-responsive DEG, LT salt-responsive DEG, HT DEG, LT DEG and salt-responsive DEG. This list contains locus number and annotation of the genes. (XLSX 2043 kb)
The bar-chart showing expression level (RPKM) of photosynthetic DEGs in the rice(s) under both stress and normal condition. Bar-chart showing expression level of photosynthetic DEGs from RNA-seq data comparing between the transgenic rice overexpressing OsCam1–1 under stress (150 mM NaCl) condition (L1S), wild type under stress (WTS), transgenic under non-stress (L1NS) and wild type under non-stress (WTNS), and the Y axis represent RPKM. (PDF 171 kb)
The bar-chart showing FV’/FM’ of the rice(s) under either normal or salt-stress condition. Fluorescence measurement in leaf of the transgenic rice overexpressing OsCam1–1 (L1, L2, L7) and wild type (WT) under normal and salt stress (150 mM NaCl) condition (A) at day 3 and (B) day 5 of treatment. (PDF 31 kb)
The reliable testing of the 35S-labeled rOsCaM1 binding for testing accuracy and specificity. Examination of the 35S-labeled rOsCaM1 protein, A) 12% SDS-PAGE of the 35S-labeled rOsCaM1 elutes. Lane M: Protein molecular weight marker. Lanes 1–4: Fractions 1–4 of the 35S-labeled rOsCaM1 elute, respectively, and B) Autoradiograph of the blot spotted with various amounts (as written above each spot) of positive (CaMKII peptide and calcineurin) or negative (BSA) control. (PDF 1805 kb)
Autoradiographs of far western analysis of 11 putative OsCaM1-binding proteins. Lane M: protein molecular weight markers (negative control); Lane P: calcineurin (positive control); Lane N: crude protein extract from the SOLR cells harboring the pBluescript SK(−) plasmid (negative control); Numbers indicated above the lanes are clone No. assigned when these clones were isolated from the primary screening as following: 2, Cyclic nucleotide-gated ion channel (CNGC); 5, glutamate decarboxylase (GAD); 10, Hydroxyanthranilate hydroxyl cinnamoyltransferase (HHT); 12, CaM-binding transcription activator (CAMTA); 19, Kinesin motor domain- containing protein (KCBP); 21, Kinesin motor domain- containing protein (KCBP); 23, Myosin heavy chain; 24, unknown expressed protein, while 18, 26 and 31 which represent response regulator receiver domain-containing protein (PRR), lipin, N-terminal conserved region family protein, and another clone of Kinesin motor domain- containing protein (KCBP) respectively, were unclear. (PDF 8517 kb)
The list of OsCam1–1 co-expressed DEGs obtaining from STRING (Excel file). This list contains locus number and annotation of the genes. (XLSX 17 kb)
The list of shared genes between OsCam1–1 co-expressed gene obtaining from STRING and DEGs affected by OsCam1–1 overexpression from RNA-Seq result. This list contains locus number and annotation of the genes. (XLSX 12 kb)
The list of rice homologous CaM target genes using known Arabidopsis CaM target gene as references. This list contains locus number and annotation of the genes. (XLSX 23 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.