Abstract
Objective
To isolate and characterize novel high-affinity llama single-domain antibodies against human HER2.
Results
We immunized a llama with human HER2, constructed a phage-displayed VHH library from the lymphocytes of the animal, and isolated six unique HER2-specific VHHs by panning. All six VHHs were unique at the amino acid level and were clonally unrelated, as reflected by their distinct CDR3 lengths. All six VHHs recognized recombinant human HER2 ectodomain with monovalent affinities ranging from 1 to 51 nM, had comparable affinities for cynomolgus monkey HER2, and bound HER2+ SKOV3 cells by flow cytometry. Three of the VHHs recognized recombinant murine HER2 with no loss of affinity compared with human and cynomolgus monkey HER2. The VHHs recognized three major epitopes on HER2 (including one conserved across the human, simian and murine orthologues), all of which were distinct from that of trastuzumab. These VHHs may be useful in the design of modular cancer immunotherapeutics.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3955-8) contains supplementary material, which is available to authorized users.
Keywords: Single-domain antibody, VHH, HER2, Cancer
Introduction
HER2/neu (ERBB2, CD340) is a receptor tyrosine kinase of the epidermal growth factor receptor family that is frequently amplified and/or overexpressed in solid tumors [1]. Antibodies (Abs) and Ab-drug conjugates (ADCs) against HER2, exemplified by trastuzumab (Herceptin), pertuzumab (Perjeta) and trastuzumab emtansine (Kadcyla), play an important role in the diagnosis and treatment of breast cancer [2]. Multiple other anti-HER2 Abs and Ab fragments are in development as naked antibodies, ADCs, bispecific Abs and radioimmunotherapeutics for breast cancer and other indications [3]. A camelid single-domain Ab (sdAb or VHH) against HER2 is currently in clinical trials for breast cancer imaging [4].
Here, we report the generation and preliminary characterization of a panel of novel llama VHHs directed against human HER2. Several of the VHHs have attractive properties that may make them useful components of modular cancer immunotherapeutics.
Main text
We immunized a male llama (Lama glama) with recombinant human HER2 ectodomain (Cat. No. HE2-H5225; ACROBiosystems, Beijing, China) as previously described [5–7]. Briefly, the animal was immunized subcutaneously five times with 200 µg of human HER2 (days 0, 21, 28, 35 and 42). The priming immunization was adjuvanted with complete Freund’s adjuvant and boost immunizations were adjuvanted with incomplete Freund’s adjuvant. Blood samples were collected on days 35 and 49, from which serum was obtained after clotting and peripheral blood mononuclear cells were purified by density gradient centrifugation. Interestingly, serum ELISA and western blotting indicated that although HER2 immunization elicited polyclonal Abs against the immunizing antigen, immune sera from unrelated animals showed similar degrees of HER2 reactivity (see Additional file 1). We speculate that serum polyreactivity against this recombinant HER2 ectodomain reflects some degree of unfolding and/or aggregation, although binding by trastuzumab and other antibodies indicated that some proportion was also folded correctly.
We constructed a phage-displayed VHH library from the peripheral blood lymphocytes of the HER2-immunized llama as previously described [5–7]. Briefly, total RNA was extracted in 16 replicates from peripheral blood mononuclear cells (eight samples each from the day 35 and 49 bleeds, each containing ~ 1 × 107 cells) using the PureLink™ RNA Mini Kit (Thermo Fisher, Waltham, MA). Approximately 1–2 µg of total RNA was reverse transcribed using qScript® cDNA SuperMix (Quantabio, Beverly, MA) and then rearranged VHH exons were amplified using semi-nested PCR and cloned into the pMED1 phagemid vector. The final library size was ~ 8 × 107 independent transformants with an insert rate of ~ 92%. VHH-displaying phages were rescued from library phagemid-bearing Escherichia coli TG1 cells using M13KO7 helper phage (New England Biolabs, Ipswich, MA), panned for four rounds against human HER2 directly immobilized in wells of microtiter plates, and eluted with triethylamine as previously described [5–7]. At the conclusion of four rounds of panning, 96 individual clones (48 each from rounds 3 and 4) were tested for binding to human HER2 by ELISA, yielding six unique and clonally unrelated VHH sequences (Table 1).
Table 1.
Properties of HER2-specific VHHs isolated in this study
| VHH | CDR3 Length (aa)a |
Human HER2 | Cynomolgus HER2 | Mouse HER2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kon (M−1s−1) |
koff (s−1) |
K
D
(nM) |
kon (M−1s−1) |
koff (s−1) |
K
D
(nM) |
kon (M−1s−1) |
koff (s−1) |
K
D
(nM) |
||
| NRC-sdAb034 | 12 | 5.4 × 105 | 3.4 × 10−3 | 6.2 | 5.8 × 105 | 2.7 × 10−3 | 4.6 | 5.0 × 105 | 2.9 × 10−3 | 5.8 |
| NRC-sdAb035 | 15 | 8.5 × 105 | 4.4 × 10−2 | 51 | 1.0 × 106 | 4.3 × 10−2 | 41 | n.b. | ||
| NRC-sdAb036 | 19 | 3.9 × 105 | 5.6 × 10−3 | 14 | 4.8 × 105 | 5.4 × 10−3 | 11 | 4.4 × 105 | 5.8 × 10−3 | 13 |
| NRC-sdAb037 | 16 | 4.3 × 105 | 1.7 × 10−2 | 39 | 5.1 × 105 | 5.6 × 10−2 | 110 | n.b. | ||
| NRC-sdAb038 | 10 | 1.1 × 106 | 1.6 × 10−3 | 1.4 | 1.0 × 106 | 1.4 × 10−3 | 1.3 | 1.1 × 106 | 1.0 × 10−3 | 0.9 |
| NRC-sdAb039 | 18 | 1.4 × 106 | 4.5 × 10−3 | 3.3 | 1.7 × 106 | 6.0 × 10−3 | 3.6 | n.b. | ||
n.b. no binding
aIMGT numbering
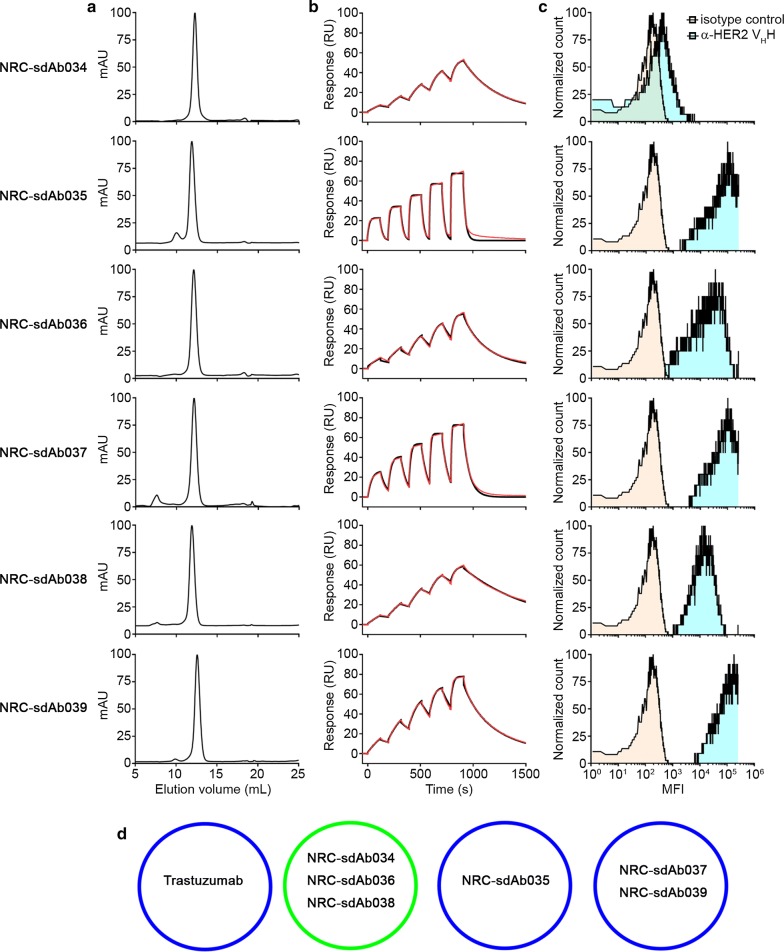
The DNA sequences encoding the six VHHs were cloned into the pSJF2H expression vector [8]. C-terminally c-Myc- and His6-tagged VHHs were expressed in 200 mL overnight cultures of E. coli TG1 under IPTG induction and purified by Ni2+ affinity chromatography as previously described [5–7]. All six VHHs were primarily monomeric by size exclusion chromatography, although trace aggregates or impurities were observed for NRC-sdAb035 and NRC-sdAb037 (Fig. 1a). We immobilized human (ACROBiosystems HE2-H5225), cynomolgus (ACROBiosystems HE2-C52Hb) and murine HER2 (ACROBiosystems ER2-M5220) ectodomains on adjacent flow cells of a CM5 Series S sensor chip (GE Healthcare, Piscataway, NJ) by amine coupling and analyzed binding of the VHHs to each surface using single-cycle kinetics on a Biacore T200 surface plasmon resonance (SPR) instrument (GE Healthcare). All six VHHs showed high-affinity binding to HER2 (KD range 1–51 nM), with nearly equivalent kinetic and affinity parameters observed for human and cynomolgus HER2 (Fig. 1b, Table 1 and Additional file 2); moreover, three of the VHHs also cross-reacted with murine HER2 with no apparent loss of binding affinity. All six VHHs bound to HER2+ SKOV3 cells by flow cytometry, although staining by NRC-sdAb034 was weak (Fig. 1c). Epitope binning experiments indicated that despite their unique amino acid sequences, all three cross-reactive VHHs (NRC-sdAb034, NRC-sdAb036 and NRC-sdAb038) targeted a nearly identical epitope (Fig. 1d and Additional file 3); the epitopes of NRC-sdAb037 and NRC-sdAb039 also showed a high degree of overlap, while NRC-sdAb035’s epitope was distinct. The epitopes of all six VHHs were distinct from the trastuzumab epitope.
Fig. 1.
Characterization of anti-HER2 llama VHHs. a Size exclusion chromatography profiles of anti-HER2 VHHs. Approximately 0.5 mg of each VHH was injected over a Superdex™ 75 GL column (GE Healthcare) connected to an ÄKTA FPLC protein purification system (GE Healthcare) in a mobile phase consisting of HBS-EP + (10 mM HEPES, pH 7.4, containing 150 mM NaCl, 3 mM EDTA and 0.05% surfactant P20). Maximum A280 values were normalized to 100 for each VHH. b Single-cycle kinetic analysis of VHHs binding to human HER2 by SPR. All VHHs were purified by preparative size exclusion chromatography prior to analysis. Approximately 1323 response units (RUs) of human HER2 were immobilized on adjacent flow cells of a CM5 Series S sensor chip in 10 mM acetate, pH 4.0, using an amine coupling kit (GE Healthcare). An ethanolamine-blocked flow cell served as the reference. Monomeric VHHs at concentrations ranging from 1–400 nM were injected over the surfaces in HBS-EP+ buffer at a flow rate of 40 µL min−1. The contact time was 120 s and the dissociation time was 600 s. The surfaces were regenerated using 10 mM glycine, pH 1.5. Data were analyzed using Biacore T200 Software v3.0 (GE Healthcare) and fitted to a 1:1 binding model (black lines show data and red lines show fits). Affinity and kinetic parameters (25 °C) are shown in Table 1, and sensorgrams showing binding to cynomolgus and murine HER2 are shown in Additional file 2. c Binding of VHHs to HER2+ SKOV3 cells by flow cytometry. SKOV3 cells were grown to 70–80% confluency at 37 °C in a humidified 5% CO2 atmosphere in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, 100 µg mL−1 streptomycin and 250 ng mL−1 amphotericin B. Cells were dissociated from flasks using Accutase® solution, washed in PBS and then resuspended in PBS containing 1% bovine serum albumin. Approximately 1 × 105 cells were stained sequentially on ice for 30 min with: (i) 10 µg mL−1 of each VHH, (ii) 5 µg mL−1 of mouse anti-c-Myc IgG (clone 9E10), and (iii) 5 µg mL−1 of APC-conjugated goat anti-mouse IgG (Thermo-Fisher). The cells were washed with PBS in between each staining step, and after the final wash, data (10,000 events) were acquired on a BD FACSCanto™ instrument (BD Biosciences, San Jose, CA). d Summary of epitope binning of anti-HER2 VHHs by SPR. HER2 was immobilized as described in B. In the first injection, each VHH at a concentration equivalent to 20 × KD or trastuzumab (20 nM) was injected at a flow rate of 20 µL s−1 for 300 s contact time to saturate the HER2 surface. The second injection consisted of the same VHH along with a second VHH (both at 20 × their respective KDs). All co injection experiments were performed in both orientations. Blue circles represent distinct epitopes conserved between human and cynomolgus HER2, and green circle represents a distinct epitope conserved across human, cynomolgus and mouse HER2
In summary, we have reported the isolation and preliminary characterization of six novel anti-HER2 llama VHHs. Interestingly, despite their unique and clonally unrelated sequences, the VHHs targeted only three major epitopes on HER2, including an apparently immunodominant epitope conserved across the human, simian and murine orthologues. The nearly identical binding of the VHHs to cynomolgus HER2 would permit toxicity assessment and, for mouse cross-reactive VHHs, evaluation in syngeneic tumor models in combination with immunomodulatory agents. Several other groups have described anti-HER2 VHHs and characterized them in some detail [9], but in most cases cross-reactivity with murine HER2 was not assessed. Moreover, targeting of a non-trastuzumab epitope is clearly an advantage for imaging of patients being dosed with Herceptin/Kadcyla [10]. The other major potential advantage of these VHHs in comparison with conventional antibodies is their modularity, which would permit facile incorporation into multifunctional biologics.
Limitations
At the current time, we have not been able to explore whether these VHHs have anti-cancer activity. We do not know: (i) the precise locations of their epitopes on HER2, (ii) whether any of the VHHs inhibit HER2 signaling or inhibit receptor dimerization, permitting their development as naked Ab therapeutics, (iii) whether any of the VHHs internalize into HER2+ tumor cells, permitting their development as ADCs or radioimmunotherapeutics, or (iv) whether their sequences can be humanized without loss of stability or binding affinity. Moreover, we are unable to disclose the amino acid sequences of these VHHs for intellectual property reasons. In future studies, we hope to comprehensively investigate the roles of molecular size, valency and serum half-life on tumor uptake using anti-HER2 VHH-based biologics as a model system.
Additional files
Additional file 1: Figure S1. Polyclonal antibody responses after immunization with human HER2 ectodomain.
Additional file 2: Figure S2. Complete sensorgrams for single-cycle kinetic analysis of VHHs binding to HER2 by SPR.
Additional file 3: Figure S3. Complete sensorgrams for epitope binning SPR co-injection experiments.
Authors’ contributions
KAH designed the study, KAH and MJL isolated the VHHs, SR and GH performed and analyzed SPR experiments. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge Qingling Yang for technical assistance.
Competing interests
The authors declare that have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Experiments involving animals were conducted using protocols approved by the National Research Council Canada Animal Care Committee and in accordance with the guidelines set out in the OMAFRA Animals for Research Act, R.S.O. 1990, c. A.22.
Funding
This work was funded by the National Research Council Canada.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Ab
antibody
- ADC
antibody-drug conjugate
- sdAb or VHH
single-domain antibody
- SPR
surface plasmon resonance
Contributor Information
Greg Hussack, Email: gregory.hussack@nrc-cnrc.gc.ca.
Shalini Raphael, Email: shalini.raphael@nrc-cnrc.gc.ca.
Michael J. Lowden, Email: michael.lowden@nrc-cnrc.gc.ca
Kevin A. Henry, Phone: +1-613-998-3373, Email: kevin.henry@nrc-cnrc.gc.ca
References
- 1.Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Liu Q, Han X, Qin S, Zhao W, Li A, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, et al. Phase I study of 68GaHER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 5.Baral TN, MacKenzie R, Arbabi Ghahroudi M. Single-domain antibodies and their utility. Curr Protoc Immunol. 2013;103:2–17. doi: 10.1002/0471142735.im0217s103. [DOI] [PubMed] [Google Scholar]
- 6.Henry KA, Hussack G, Collins C, Zwaagstra JC, Tanha J, MacKenzie CR. Isolation of TGF-β-neutralizing single-domain antibodies of predetermined epitope specificity using next-generation DNA sequencing. Protein Eng Des Sel. 2016;29:439–443. doi: 10.1093/protein/gzw043. [DOI] [PubMed] [Google Scholar]
- 7.Henry KA, Tanha J, Hussack G. Identification of cross-reactive single-domain antibodies against serum albumin using next-generation DNA sequencing. Protein Eng Des Sel. 2015;28:379–383. doi: 10.1093/protein/gzv039. [DOI] [PubMed] [Google Scholar]
- 8.Arbabi-Ghahroudi M, To R, Gaudette N, Hirama T, Ding W, MacKenzie R, et al. Aggregation-resistant VHs selected by in vitro evolution tend to have disulfide-bonded loops and acidic isoelectric points. Protein Eng Des Sel. 2009;22:59–66. doi: 10.1093/protein/gzn071. [DOI] [PubMed] [Google Scholar]
- 9.Vaneycken I, Devoogdt N, Van Gassen N, Vincke C, Xavier C, Wernery U, et al. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011;25:2433–2446. doi: 10.1096/fj.10-180331. [DOI] [PubMed] [Google Scholar]
- 10.Pruszynski M, D’Huyvetter M, Bruchertseifer F, Morgenstern A, Lahoutte T. Evaluation of an anti-HER2 nanobody labeled with 225Ac for targeted α-particle therapy of cancer. Mol Pharm. 2018;15:1457–1466. doi: 10.1021/acs.molpharmaceut.7b00985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Polyclonal antibody responses after immunization with human HER2 ectodomain.
Additional file 2: Figure S2. Complete sensorgrams for single-cycle kinetic analysis of VHHs binding to HER2 by SPR.
Additional file 3: Figure S3. Complete sensorgrams for epitope binning SPR co-injection experiments.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional files.