Abstract
Objective
The World Maternal Antifibrinolytic (WOMAN) Trial was the first in the UK to use the option of waiver of informed consent at the time of an obstetric emergency. This qualitative study aimed to investigate participants’ views of the acceptability of the recruitment methods used.
Design
Qualitative study using in‐depth interviews with women who did and did not give consent at the time of their recruitment to the WOMAN Trial.
Setting
Highest UK recruitment site for the WOMAN Trial (129/569). Interviews were conducted in participants’ homes.
Population
About 40 of the 129 women who were recruited to the WOMAN Trial at one UK site were invited to take part, 15 women were interviewed.
Methods
Qualitative, interview study.
Main outcome measures
Facilitators and barriers to successful recruitment during obstetric emergencies. Guidance for future researchers.
Results
Findings revealed that what is important is not so much the consent process used or a signature on a form, but the way in which consent is obtained. Clinicians who successfully negotiate consent to research during childbirth emergencies engage in a ‘humane choreography’ of words and actions. This emphasises the importance of prompt decision‐making and treatment, while respecting the woman's personal situation and experience.
Conclusions
Our findings do not support a single pathway to consent in the context of an obstetric emergency. Women understand that consent to research in an emergency is complex. Clinicians’ skills in considering the clinical, ethical, and emotional aspects within the context of the clinical emergency can hamper or promote women's satisfaction.
Tweetable abstract
Study reports on women's views of consent to research in an obstetric emergency.
Plain Language Summary
Why and how was the study carried out?
We undertook this study to find out what women thought about being included in a research study called the WOMAN Trial at the time they were being treated for heavy bleeding after giving birth. Some women had been asked if they wanted to be a part of the research at the time they were bleeding. Others were asked later, after they had recovered. We conducted interviews with 15 women who had been involved and asked what they thought about the way they had been asked, their preferences and ideas for improvements in future similar studies
What were the main findings?
Women understood how difficult it was for their doctors and midwives to ask them about the research study. They were pleased to have been included in the research and were mostly happy with the way they gave consent. Women's views were similar whether they were asked about the research at the time of the bleeding or after they had recovered. The most important thing was that doctors and midwives carefully thought about the situation the woman found herself in and how this might make her feel, so they could tailor their approach accordingly.
What are the limitations of the work?
This study only involved women from one hospital. The WOMAN Trial included women from many areas of the UK and other countries around the world. We do not know how their experiences or views may differ.
What is the implication for professionals?
Careful use of actions and words by birth attendants was the difference between a good or bad experience for the woman and her family. This is an important skill that could be developed as part of professional training.
Keywords: Consent, obstetric emergency, research, women's views
Tweetable abstract
Study reports on women's views of consent to research in an obstetric emergency.
Introduction
Debate about consent to research during the vulnerable time of childbirth and childbirth emergencies is long‐standing.1, 2, 3, 4, 5 Guidelines for the conduct of maternity research where time is critical recognise how informing all women about potential emergencies in advance may create unnecessary anxiety.4 However, giving information and gaining consent at the time can delay potentially lifesaving treatments.5 The ideal of valid, informed consent becomes unworkable in some obstetric emergencies, and the developments of flexible research protocols that acknowledge this are welcomed. Understanding the views and experiences of those directly involved is paramount. Deferred consent precedents have been set and evaluated in the context of emergency medicine6, 7, 8 and paediatric trials.9, 10 However, in obstetrics, deferred consent had only been explored hypothetically.11 The use of a verbal consent within emergency peripartum trials is associated with professional anxiety.12 The completion of the World Maternal Antifibrinolytic (WOMAN) Trial presented a unique opportunity to investigate the views of women who had lived through this experience.
The WOMAN Trial showed that tranexamic acid, compared to placebo, reduced the risk of death from postpartum haemorrhage (PPH) by 20%.13 The trial faced an important challenge in terms of consent, as the treatment being studied needed to be given at the time women were experiencing a PPH. The trial design included a range of consent approaches, depending on the woman's condition (Figure 1). Consent was obtained from women if their physical and mental capacity allowed (as judged by the treating clinician). If a woman was unable to give consent, proxy consent was obtained from a relative or representative. If a proxy was unavailable or unable to consent, consent was deferred and the woman was informed about the trial as soon as possible, written consent was requested later for data collection. Trial procedures were compliant with international guidelines and legislative frameworks relating to consent to emergency research.13, 14, 15, 16, 17, 18 The UK Clinical Trials Regulations Amendment 219 and the updated Declaration of Helsinki.20 In the UK, 569 women were randomised at seven maternity facilities. Five hundred and six of the 569 women were randomised without prior written consent, and 501 women gave retrospective written consent to continue.
Figure 1.
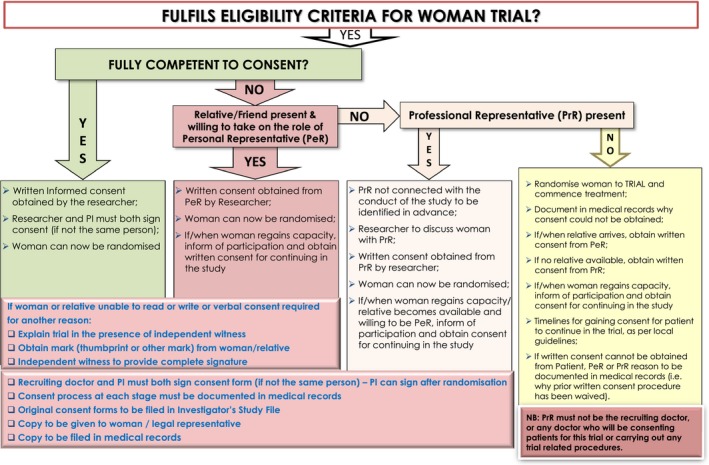
Flow chart: guidance for obtaining informed consent for the WOMAN trial.
This study aimed to investigate the views of a cohort of the participants in the WOMAN Trial to identify preferred method(s) of consent, assess the acceptability of waiver of prior consent and inform future guidance.
Methods
The study is reported following the consolidated criteria for reporting qualitative research (COREQ) guidelines.21 An interpretative qualitative methodology using in‐depth interviews was used to investigate women's views.
Participants were recruited from the UK site where the highest number of WOMAN Trial participants were recruited (n = 129 of 569). Purposive sampling ensured maximum variation of interviewees based on the method of consent used22 (Figure 2). Forty potential participants were identified from the randomisation log. Sixteen gave consent while their PPH was ongoing. Two had prior consent waived and subsequently declined to give written consent. There were 111 women who had consent waived and gave consent subsequently. Every fifth woman was invited, this ensured representation across the Trial's duration (n = 22). Written consent by relatives at the time of the emergency was not obtained for any of the participants. Trial recruitment occurred at the site between October 2011 and July 2013. This study was conducted once recruitment to the WOMAN Trial in the UK was completed and international recruitment remained ongoing. Interviews commenced following ethical approval in March 2015, with the intention that the findings would be available soon after the results of the WOMAN Trial became available.
Figure 2.
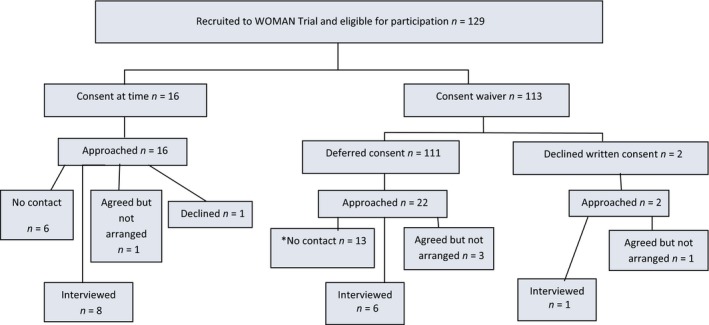
Study algorithm and sample characteristics. *It is noteworthy that more women in the waiver group were not contactable. Some women initially seemed keen to be included; however, interviews were not arranged for a range of reasons: no further telephone contact made, moved out of area, work/childcare commitments.
Women were sent an Invitation and Information Sheet and then contacted by telephone. There were opportunities to ask questions before written consent. Interviews were audio‐recorded and conducted using an interview schedule (Appendix S1). All participants preferred to be interviewed at home. Family members and children were present during some interviews. Data saturation was reached after 15 interviews; evidenced during the final interviews and confirmed during initial coding. Participants consented to information collection from their records (Table 1).
Table 1.
Demographics of participants
| Participant | Age | Parity | Blood loss | Blood transfusion | Hba | Induction | Length of labour | Pain relief in labour | Mode of birth | Loss of consciousness | Surgical intervention to control bleedingb | Additional anaesthetic | HDU | Believed intervention worked for themc | Believed life threatened and savedc | Debriefed at 6 weeks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent at time | ||||||||||||||||
| C01 | 33 | G3 P2 | 900 ml | No | 9.5 | No | 10 hours 18 minutes | Epidural | Rotational Forceps | No | No | No | No | Unclear | No | No |
| C02 | 25 | G3 P0 | 4750 ml | No | 8.3 | Yes | 13 hours 5 minutes | Diamorphine and spinal | Emerg. CS | No | No | No | Yes | Unclear | No | No |
|
C04 Baby 4.9 kg |
31 | G3 P2 | 500 ml | No | 8.6 | Yes | 6 hours 44 minutes | Diamorphine | Spontaneous | No | No | No | No | Unclear | No | No |
|
C05 Placenta praevia |
35 | G5P2 | 2100 ml | Yes | 11.1 | N/A | N/A | Spinal |
Emerg. CS No labour |
No | No | No | No | Unclear | Yes | No |
| C08 | 35 | G1 P0 | 900 ml | No | 6.7 | Yes | 10 hours 38 minutes | Diamorphine | Spontaneous | No | Considered | No | No | Yes | Yes | No |
| C09 | 34 | G1 P0 | 1300 ml | No | 8.3 | Yes | 3 hours 15 minutes | None | Spontaneous | No | Yes | Spinal | No | Yes | Unclear | Yes |
|
C10 Pregnancy loss |
29 | G1 P0 | 2000 ml | Yes | 9.6 | No | 3 hours 15 minutes | None | Spontaneous | No | Yes | Spinal | Yes | Unclear | Unclear | No |
| C13 | 35 | G2 P1 | 900 ml | Yes | 8.9 | No | 2 hours 5 minutes | Entonox | Spontaneous | Partial | Yes | Yes | No | Yes | Yes | No |
| Consent waiver | ||||||||||||||||
| Waiver 01 Hysterectomy | 30 | G3 P2 | 5000 ml | Yes | 10.0 | N/A | N/A | Spinal at CS | Elective repeat CS | Yes | Yes | GA | Yes | Yes | Yes | Yes |
| Waiver 02 | 31 | G2 P1 | 4000 ml | Yes | 9.7 | Yes | 17 hours 25 minutes | Diamorphine | Spontaneous | Yes | Yes | GA | Yes | Unclear | Yes | Yes |
| Waiver 13 | 40 | G3 P2 | 2000 ml | No | 9.6 | No | 8 hours 5 minutes | Diamorphine and Spinal | Rotational Forceps | Yes | Yes | GA | Yes | Unclear | No | Yes |
| Waiver 16 CK2 | 32 | G2 P0 | 1000 ml | No | 9.6 | Yes | 15 hours 43 minutes | Epidural | Simpsons Forceps | No | No | No | No | Yes | Yes | No |
|
Waiver 20 Shoulder dystocia |
28 | G1 P0 | 2500 ml | Yes | 10.1 | Yes | 24 hours 48 minutes | Diamorphine epidural and spinal | Rotational Forceps | Yes | Yes | No | Yes | Yes | Yes | No |
| Waiver 22 | 24 | G3 P2 | 1000 ml | No | 10.1 | Yes | 1 hour 36 minutes | Entonox | Spontaneous | Yes | No | No | No | Yes | Yes | No |
| Waiver and declined retrospective written consent | ||||||||||||||||
|
Waiver—Declined Twins |
28 | G2 P1 | 1000 ml | No | 8.6 | No | 10 hours | Epidural | T1 Spontaneous T2 Simpsons Forceps | No | No | No | No | Yes | Yes | No |
On discharge.
Surgical intervention includes examination under anaesthetic, Bakri Balloon, B‐Lynch suture, vaginal pack, manual removal of products, hysterectomy.
As reported by woman.
Interviews were transcribed verbatim to create transcripts for thematic network analysis.23 This method has parallels with the basic components of grounded theory, which organises data into concepts, categories, and propositions. GH and CK undertook the analysis. In stage one, following data familiarisation, a coding framework was devised, first independently, and then agreed by consensus. MAXQDA11 was used to dissect the text into coded segments. Four a priori codes were assigned, and 19 were grounded in the data (Appendix S2). GH and CK then abstracted and refined themes from coded segments, arranging them into nine basic themes and three organising themes, from which the global theme was deduced. The initial thematic network was verified and refined by constant comparative reflection and discussion. In stage two, GH and CK described and explored the thematic networks further, before summarising them. In stage three, GH and CK brought the network summaries together with existing theories, original research questions, and the interests underpinning them. Figure 3 was produced in this final stage.
Figure 3.
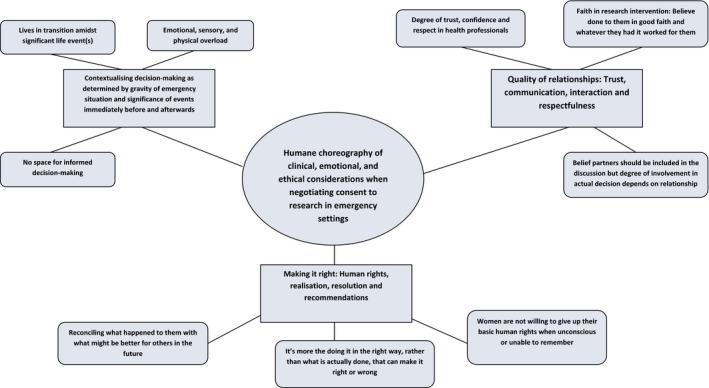
Thematic network illustrating basic, organising, and global themes.
GH and MD are practicing midwives. CK is a sociologist and maternity researcher. ZA is an obstetrician and researcher. GH, MD, and ZA were collaborators in the WOMAN Trial. HS was lead investigator in the WOMAN Trial. The ethical dilemmas raised by the unprecedented use of the waiver of prior consent provided the impetus for this study. Although there was nothing to suggest that women were concerned about the consent processes used in the Trial in terms of complaints and declining continuation, the research team were reluctant to assume this equated to unanimous acceptance. GH, CK, and MD conducted the interviews. As GH and MD were responsible for recruitment to the WOMAN trial, the trial logs were checked to ensure that GH and MD did not approach or interview women they had met in Trial activities.
Results
Fifteen women participated; eight gave consent to participate in the WOMAN trial while their PPH was ongoing; for seven, consent was waived (including one of two women who declined written consent retrospectively). The study algorithm and sample characteristics are illustrated in Figure 2. Table 1 reports demographic and clinical characteristics. Figure 3 outlines the thematic structure of the findings. Interviews lasted 20 minutes to 1½ hours. All transcripts conveyed the global theme ‘humane choreography of clinical, emotional, and ethical considerations when negotiating consent to research’, underpinned by the three organising themes (1) Too much to process, (2) Quality of relationships, and (3) Making it right. Figure 3 illustrates the interconnectivity between themes.
Theme 1 Women's experiences: too much to process
Thirteen of the fifteen women experienced labour; two had an elective caesarean section; fourteen gave birth to a live baby. Women explained how their ability to process information and make decisions was compromised by having just given birth and experienced a potentially life‐threatening event. A series of undistinguishable interactions with professionals were described. All women who signed a consent form around the time of Trial entry recalled being spoken to by professionals who were concerned about bleeding. However, none could remember clearly which conversations related to clinical care and which were about research: ‘I think he [the Doctor] explained that it was a trial to do with stemming blood loss, but that was all a bit hazy. I was sobbing. I actually remember saying am I going to die? I didn't really know at the time what I was saying yes to’ (C13).
As expected, the consent waiver was used most commonly when a woman's consciousness was affected. This meant some women remembered very little. Six participants signed consent for continued participation in the hours or days after recruitment. Few recalled these discussions or signing the form. Some recalled more when prompted.
Can you remember talking to anybody about taking part in any research?
No.
Not at all?
I can't remember that at all.
The interviewer then showed the ‘Alert Card’ given to all WOMAN Trial participants
So this is the research that you took part in?
Oh. Right, OK. I have got one of these.
Long pause.
So I have been involved in it then haven't I? (W13)
Although we expected the consent waiver to be used when a woman's consciousness was impaired, we did not anticipate how similar the interviews with women recruited using the three methods would be. Six women lost consciousness, many more described an altered state of consciousness where they were unable to think or remember clearly.
Views on providing information and obtaining informed written consent to research at the time of an emergency varied from hypothetically desirable to an inappropriate inconvenience. All women understood the need for prompt action and how delays could compromise any possible benefit the research may offer. One who gave prior consent said ‘They could have given me a piece of paper to say I was signing my mortgage away. The signing thing, it's just it seems quite pointless really’ (C08). A woman, for whom the consent waiver was used, said ‘You couldn't discuss something like that at that point. It had to be done by someone else’ (W02). Another from the waiver group stressed the immediacy of the intervention: ‘I think you should go ahead if you think it is going to help’ (W16). All but one participant recruited using the consent waiver felt that the process was acceptable. Her consciousness appears to have been affected very briefly, and she felt there were missed opportunities for discussion.
Among women who provided written consent, some were initially shocked to learn others had been entered into the Trial without; ‘I don't think I would have been happy’ (C04). Others disagreed; ‘I think when you are in a critical situation, conscious or not, I'd have been happy for them to waiver consent’ (C09). The woman who declined to sign a consent form retrospectively was not negative ‘It needs to be done there and then. Just to go straight to it, in case any more damage happens’ (D02). Her reason for not signing was related to early hospital discharge.
Women's ability to process information was affected at the time of trial entry and in the days and weeks afterwards. Women were asked if they looked at Trial information later: ‘Not really, you get given all these things, the pack, little red book and you have got this baby in your arms. When I get five minutes to myself I will read the leaflets’ (W16). Overall, women appeared to have little capacity for research activities in their life‐changed, postbirth, post‐PPH, world; for most, the invitation to participate in this study was the first time they had found time to give the WOMAN Trial a thought.
Tables [Link], [Link], [Link] provide more quotes to support the three organising themes.
Theme 2 Women's views: quality of relationships
With one exception, interviewees demonstrated immense trust in professional expertise. The degree of trust reflected participant's perceptions of the quality of the relationships that developed within clinical scenarios. Many recalled interactions where trust and respect were built or lost. ‘I remember these two (doctors) being really excited about the trial. I remember a senior doctor telling them off. I mostly felt at that time that (wife) was a bit of a guinea pig’ (Partner of C08).
Participants understood the challenges associated with conducting research during emergencies and were happy for the obstetrician to carry this burden. Participants appeared to understand that a placebo was used, interestingly many firmly believed their clinical situation had been improved by the Trial medication. ‘In my eyes it worked. Whether it was water, medication, orange juice, whatever’ (W01).
The woman who was not satisfied felt that her doctor failed to acknowledge her previous experiences of motherhood; ‘The placenta got stuck. I said to her (Doctor) it's stuck and she said no it's not. I said it is. This is number 3 not number 1’ (W22). Women's views on whether their birth partner should be involved in decision‐making varied, some recognised how this might be compromised by their own birth experience ‘I think they would be in a state at the time’ (C05). Partner's involvement was viewed as a courtesy rather than a necessity.
Theme 3 Women's needs: making it right
While most participants were ‘fine’ with the recruitment process, many suggested improvements. During the WOMAN Trial, a brief information leaflet was provided in clinics. Increasing opportunities for giving information was important; obtaining a signature on a form was not. Women articulated the difficulties clinicians face in providing balanced information during pregnancy and labour ‘I suppose do you wanna scare people by telling them all the things that could go wrong?’ (C08). Most women felt an individualised approach was best, and the complexity of doing this well was acknowledged ‘I don't know whether there is a right way. You've just got to do what you can in the situation at that time’ (W02).
Providing explanations and answering questions at an appropriate time were crucial. Professional awareness of the impact that childbirth, particularly a traumatic experience, can have upon cognitive ability was critical. ‘They could've come the day after when I was more alert, more aware, and I didn't have 20 people coming in and out’ (W02). C04 initially appeared against the idea of retrospective consent; however on reflection, she describes how the explanation was all important. ‘Because it was explained properly, you go, well I accept that and thanks for taking the time to go into it and you know sort of do the right thing.’
Many women expressed a positive view of research and verbalised altruism towards other women and society ‘I think it's a very good idea because how else are we meant to learn for other people for the future’ (W01).
Not missing opportunities for research was also important:
It doesn't mean that should you come across a lady in my situation at the time the emergency is going on that you can't ask her. (C04)
The global theme humane choreography of consent to research (‘how it's done’) encapsulates what really mattered. How consent was negotiated was judged by perceptions of respect and the quality of human interactions during care. Women expected every reasonable effort to be made to communicate with them; they appreciated why this was not always easy or achievable. From what first appeared as indistinguishable fragmented memories of giving birth, receiving treatment for PPH, and being approached regarding research, emerged the proposition that doing consent well involves a skilful balance and co‐ordination of important aspects amidst a plethora of human emotions. This evoked the metaphor of a complex dance, dynamic and humanely choreographed when done well; chaotic and disrespectful when not.
Discussion
Main findings
Participants favoured no particular WOMAN Trial consent procedure; instead, they valued a humane choreography of informed consent appropriate to their personal situation. This does not run contrary to the principles in the Declaration of Helsinki or more recent policy statements that highlight the importance of high‐quality respectful, humanised care.20, 24, 25 Women completely understood the complexity of issues at play and the associated challenges linked to consent. Participants were less concerned with procedures and paperwork, and more concerned with the quality of human interactions. This was indicative of feeling that professionals had done the right thing at a time when a decision could not be made fully by the woman herself. The WOMAN Trial research protocol acknowledged how the differing clinical scenarios of PPH and the clinical status of a woman would determine the consent procedure used. It was an unanticipated finding of this study just how similar participants’ experiences would be, irrespective of the severity of their PPH or the consent procedure used.
Strengths and limitations
This is the first study of the views of women who have experienced being included in a randomised controlled trial of treatment for an obstetric emergency trial where a waiver of consent was used. A key strength of this study is that it included women who gave their written consent before entry into the Trial and women where prior consent was waived. Opportunities to purposively sample women who declined were limited. Only women who took part in the WOMAN trial from one UK site were included in this study, including women from other sites may have resulted in more varied responses. As many of the women interviewed for this study did not remember the WOMAN Trial, there was a need to explain what had actually happened. Views expressed at interview may therefore have been influenced by the short time participants had to consider their feelings and thoughts. The interviews took place 1 year or more after participants were included in the WOMAN trial. Although existing research suggests that in the long term (1 year or more), women usually describe aspects of their labours and birth consistently,26 the effect of this time lapse on participants in this particular study is unknown.
Interpretation
Conducting emergency obstetric care trials to improve outcomes for women and negotiating consent to research in this emergency situation is a necessary component of medical care. Clinical trials are governed by European Legislation, which sets the framework for valid informed consent as the cornerstone of experimental research involving humans.18 The European Directives made no provision for consent in critical emergency situations. In 2008, UK legislation was introduced to enable researchers to seek consent after a person had been given an investigational drug or device when the following conditions are met: ‘(1) treatment is required urgently; (2) urgent action is required for the purposes of the trial; (3) it is not reasonably practicable to obtain consent prospectively; and (4) an ethics committee has given approval to the procedure under which the action is taken.’7 However, some clinicians remain very uncomfortable deferring written consent.12
All women in this study could not recall details of their involvement in the WOMAN Trial. Most were largely unaware that they had been part of a research study, until approached to participate in this study. This is similar to the experiences of parents whose children were entered into emergency research27 and existing studies of women's experiences of PPH.28 This loss of memory may, in part, reflect the response of the brain to perceived trauma.29 This recurrent finding does, however, raise an important question about the meaningfulness of informed consent in any spheres of clinical practice where psychological trauma may occur. Akkad et al.30 proposed that truly informed consent may be impossible to achieve within the context of clinical emergencies. Some of the women included in this study agree, viewing discussing consent at such a time as ‘pointless’. Snowdon et al.11 asked women to consider hypothetically what they would do in this situation. Interviewees rejected decision‐making before delivery, and by their partners/representative at the time of the emergency. Preferred options were antenatal decisions, followed by doctors making decisions at the time of the emergency. The views of women considering the hypothetical situation were, to an extent, supported in this study.
The principles of informed consent were of utmost importance, at the same time, women accepted the complexity of when, how, and by whom this is achievable. Vernon et al.1 previously described a pathway for consent that acknowledged the importance of considering women's individual situations. These findings go further in explaining why a ‘one size fits all’ consent process is inadequate. What is important is not so much the process, but the way in which it is undertaken. Hinton et al.'s study31 of near‐miss maternal morbidities supports the importance of the ‘little things’ (personal touches, flexibility, taking time to explain) in helping women make sense of complex situations and improving perceptions of care.
The conduct of the WOMAN Trial did not result in complaints; the absence of complaint is, however, a poor measure of acceptability. These findings offer detailed insight that can be used by researchers planning similar studies. Multiple pathways to consent, when used appropriately within a range of clinical scenarios, rather than waiver of consent waiver per se, appear to be acceptable. The women in this study clearly articulated why complacency is unacceptable and that efforts to improve consent processes should focus on the quality of human interactions, increasing opportunities to communicate courtesy and impart information.
Conclusion
The consent procedure in the WOMAN Trial used a variety of approaches dependent on the clinical scenario. Overall, all the consent procedures were acceptable, with no difference in the views of women who gave consent and those where consent was deferred. The current study has shown that professional concerns appear largely unfounded; interviews illustrated that women remember very little of the emergency or the research. Women understood that obtaining consent to research in an emergency is complex and they appreciated an approach that took their own personal situation into consideration. Care must be taken not to interpret this as consent is unimportant. Clinicians need to recognise the importance of a humane choreography of clinical, ethical, and emotional considerations and should focus on developing skills in respectfully obtaining consent in partnership with women and their families. Professionals could develop skills by practising research recruitment alongside scenario‐based emergency drills. It is essential that those responsible for designing future research trials acknowledge the views of these women.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
GH and CK designed the study with input from ZA and HS‐S. GH, CK, and MD conducted the interviews with women. As GH and MD were responsible for recruiting of women for the WOMAN trial and obtaining consent, care was taken to ensure that GH and MD did not approach or conduct interviews with women they had met in Trial activities. GH and CK undertook the data analysis. GH, CK, ZA, and HS‐S all contributed to writing the paper.
Details of ethics approval
Ethical approval was granted by the National Research Ethics Service North West Haydock REC reference 15/NW/0190 in April 2015.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Supporting information
Appendix S1. Interview schedule.
Appendix S2. A priori, codes grounded in the data, and basic, organising, and global theme.
Table S1. Theme 1 Too much to process.
Table S2. Theme 2 Quality of relationships.
Table S3. Theme 3 Making it right.
Acknowledgements
The authors would like to thank all the women and families who participated in the interviews.
Houghton G, Kingdon C, Dower M, Shakur‐Still H, Alfirevic Z. What women think about consent to research at the time of an obstetric emergency: a qualitative study of the views of a cohort of World Maternal Antifibrinolytic Trial participants. BJOG 2018; 125:1744–1753.
Linked article This article is commented on by D Lanz et al., p. 1754 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.15340. This article is also commented by N Moss, p. 1755 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.15449.
References
- 1. Vernon G, Alfirevic Z, Weeks A. Issues of informed consent for intrapartum trials: a suggested consent pathway from the experience of the release trial [ISRCTN13204258]. Trials 2006;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohamed Eltorki M, Uleryk E, Freedman S. Waiver of informed consent in pediatric resuscitation research: a systematic review. Acad Emerg Med 2013;20:822–34. [DOI] [PubMed] [Google Scholar]
- 3. Smyth RM, Jacoby A, Elbourne D. Deciding to join a perinatal randomised controlled trial: experiences and views of pregnant women enrolled in the Magpie Trial. Midwifery 2012;28:E478–85. [DOI] [PubMed] [Google Scholar]
- 4. RCOG . Obtaining valid consent to participate in perinatal research where consent is time critical. Clinical Governance Advice No. 6; 2016.
- 5. Roberts I, Prieto‐Merino D, Shakur H, Chalmers I, Nicholl J. Effect of consent rituals on mortality in emergency care research. Lancet 2011;377:1071–2. [DOI] [PubMed] [Google Scholar]
- 6. Edwards P, Arango M, Balica L, Cottingham R, El‐Sayed H, Farrell B, et al. Final results of MRC CRASH, a randomised placebo‐controlled trial of intravenous corticosteroid in adults with head injury‐outcomes at 6 months. Lancet 2005;365:1957–9. [DOI] [PubMed] [Google Scholar]
- 7. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH‐2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Mechanical versus manual chest compression for out‐of‐hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947–55. [DOI] [PubMed] [Google Scholar]
- 9. Gamble C, Nadel S, Snape D, McKay A, Hickey H, Williamson P, et al. What parents of children who have received emergency care think about deferring consent in randomised trials of emergency treatments: postal survey. PLoS One 2012;7:e35982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert R, Mok Q, Dwan K, Harron K, Moitt T, Millar M, et al. Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 2016;387:1732–42. [DOI] [PubMed] [Google Scholar]
- 11. Snowdon C, Elbourne D, Forsey M, Alfirevic Z. Views of emergency research (VERA): a qualitative study of women and their partners’ views of recruitment to trials in severe postpartum haemorrhage. Midwifery 2012;28:800–8. [DOI] [PubMed] [Google Scholar]
- 12. Lawton P, Hallowell N, Snowdon C, Normal JE, Caruthers K, Denison FC. Written versus verbal consent: a qualitative study of stakeholders views of consent procedures used at the time of a peripartum trail conducted in an emergency setting. BMC Med Ethics 2017;18:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WOMAN Trial Collaborators . Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet 2017;389:2105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Health Research Authority . Medicines for human use (clinical trials regulations) informed consent in clinical trials Version 3 dated 1 May 2008
- 15. International Conference on Harmonisation . ICH harmonised guideline for good clinical practice E6(R1) Step 4 version International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 1996
- 16. Department of Health . Research Governance Framework for Health and Social Care, 2nd edn London: DH, 2005. [Google Scholar]
- 17. Department of Health . Mental Capacity Act. London: HMSO, 2005. [http://www.legislation.gov.uk/ukpga/2005/9/contents]. Accessed 13 July 2018. [Google Scholar]
- 18. Medicines for human use (clinical trials) regulations. 10: S.I. 2004/1031 [http://www.legislation.gov.uk/uksi/2004/1031/contents/made]. Accessed 13 July 2018.
- 19. The medicines for human use (clinical trials) and blood safety and quality (amendment) regulations 2008. 941. 10. 2008 [http://www.legislation.gov.uk/uksi/2008/941/contents/made]. Accessed 13 July 2018.
- 20. World Medical Association . World Medical Association Declaration of Helsinki, ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 21. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. [DOI] [PubMed] [Google Scholar]
- 22. Shakur H, Elbourne D, Gülmezoglu M, Alfirevic Z, Ronsmans C, Allen E, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials 2010;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Attride‐Sirling J. Thematic networks: an analytic tool for qualitative research. Qual Res 2001;1:385–405. [Google Scholar]
- 24. Patel D, Nasir S, Elati A, Vernon G, Weeks AD. Historical trends in the timing of informed consent for research into intrapartum complications. BJOG 2012;119:361–5. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organisation Statement . The prevention and elimination of disrespect and abuse during facility‐based childbirth, 2014. [http://apps.who.int/iris/bitstream/10665/134588/1/WHO_RHR_14.23_eng.pdf?ua=1&ua=1]. Accessed 27 July 2017.
- 26. Simkin P. Just another day in a woman's life? Part II: nature and consistency of women's long‐term memories of their first birth experiences. Birth 1992;19:64–81. [DOI] [PubMed] [Google Scholar]
- 27. Woolfall K, Frith L, Gamble C, Gilbert R, Mok Q, Young B. How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 2015;5:e008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunning P, Harris J, Sandall J. Women and their birth partners’ experiences following a primary postpartum haemorrhage: a qualitative study. BMC Pregnancy Childbirth 2016;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry 2001;35:589–600. 12p. [DOI] [PubMed] [Google Scholar]
- 30. Akkad A, Jackson C, Kenyon S, Dixon‐Woods M, Taub N, Habibaa M. Informed consent for elective and emergency surgery: questionnaire study. BJOG 2004;111:1133–8. [DOI] [PubMed] [Google Scholar]
- 31. Hinton L, Locock L, Knight M. Experiences of the quality of care of women with near‐miss maternal morbidities in the UK. BJOG 2014;121(Suppl 4):20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Interview schedule.
Appendix S2. A priori, codes grounded in the data, and basic, organising, and global theme.
Table S1. Theme 1 Too much to process.
Table S2. Theme 2 Quality of relationships.
Table S3. Theme 3 Making it right.


