Abstract Abstract
The authors established the taxonomic status of endophytic fungi associated with leaves of Pandanaceae collected from southern Thailand. Morphotypes were initially identified based on their characteristics in culture and species level identification was done based on both morphological characteristics and phylogenetic analyses of DNA sequence data. Twenty-two isolates from healthy leaves were categorised into eight morphotypes. Appropriate universal primers were used to amplify specific gene regions and phylogenetic analyses were performed to identify these endophytes and established relationships with extant fungi. The authors identified both ascomycete and basidiomycete species, including one new genus, seven new species and nine known species. Morphological descriptions, colour plates and phylogenies are given for each taxon.
Keywords: Ascomycetes, Basidiomycota, biodiversity, phylogenetic analysis
Introduction
Endophytic fungi are beneficial to their host plants and have the ability to produce bioactive compounds that have applied uses (Fisher et al. 1994; Strobel et al. 2004; Gunatilaka 2006; Arnold et al. 2007; Saikkonen et al. 2010; Aly et al. 2010; Lin et al. 2010; Rajulu et al. 2011; Chowdhary et al. 2015). Research on endophytic fungi began approximately 30 years ago and has intensified over the past 20 years (Thomson et al. 1997; Arnold et al. 2000; Stone et al. 2000; Hyde and Soytong 2008; Lumyong et al. 2009). This rising interest in endophytic fungi dates back to Bills’ 1996 novel concept that mycelia sterilia isolates could be assigned to groups based on their degree of similarity in colony surface texture (Rodrigues 1994; Fisher et al. 1995; Lodge et al. 1996; Brown et al. 1998; Taylor et al. 1999; Umali et al. 1999; Fróhlich et al. 2000). Lacap et al. (2003) used molecular data to demonstrate the reliability of Bill’s 1996 concept based on the cultural approach. Guo et al. (2000, 2003) found that morphological characteristics were insufficient to identify most endophyte isolates, especially when they do not sporulate and so DNA sequence data were used for identification of these taxa. Although this has been followed by numerous authors using ITS sequence data analysis, the use of ITS alone is not accurate (Promputtha et al. 2005). Subsequent studies have shown that multi-gene analyses are needed to identify endophytes (Ko et al. 2011).
Endophytic fungal strains have been isolated from many different plants including trees, vegetables, fruits, cereal grains and other crops (Rosenblueth and Martinez-Romero 2006). Dickinson (1976) published the first study of endophyte - leaf associations. However, there has been less research on the endophytic fungi associated with the leaves of tropical plants (Promputtha et al. 2007). The high species diversity of endophytic fungi makes their study a pressing research area. Globally, endophytic fungi were estimated to comprise 7 % of the 1.5 million species of fungi (Hawksworth 2001; Chowdhary et al. 2015). The actual numbers may be far higher. Recently, Hawksworth and Lucking (2017) estimated that there are 2.2 to 3.8 million fungal taxa. Endophytes are expected to be numerous because their host-specificity will drive diversification and they can occupy several niches, including that of pathogens and saprobes (Zhou and Hyde 2001). Several studies have investigated the relationships between endophytes and saprotrophs and also between endophytes and pathogens (Petrini 1991; Yanna and Hyde 2002; Ghimire and Hyde 2004; Photita et al. 2004; Hyde et al. 2006).
The authors have been investigating saprobic and endophytic fungi associated with Pandanaceae (Tibpromma et al. 2016a, b, c, 2017a, b) and, in this study, taxonomic details are presented regarding the endophytic fungi that were isolated. Pandanaceae are monocotyledonous plants. Their associated endophytic fungi were first studied by McKenzie et al. (2002), with further research conducted by Thongkantha et al. (2008), Bungihan et al. (2011), Ariffin (2013), Bungihan et al. (2013) and Eskandarighadikolaii et al. (2015).
The objectives of the present study were to establish the endophytic fungal community on selected Pandanaceae collected in southern Thailand. The authors isolated 22 endophytic isolates and sorted them in morphotypes and identified the taxa based on DNA sequence analyses. Both ascomycete and basidiomycete genera were identified, including one new genus, seven new species and nine known species. The recommendations of Jeewon and Hyde (2016) were followed when introducing the new species based on molecular data.
Materials and methods
Sample collection and fungal isolation
Healthy mature leaves of Pandanus and Freycinetia species (Pandanaceae, Figure 1) were collected from Chumphon (10°57'38.2"N 99°29'21.8"E) and Ranong (9°55'15.9"N, 98°38'30.7"E) provinces of southern Thailand during the rainy season (December) of 2016. Leaves with physical damage or showing signs of pathogenic infection were excluded from the study. In total, more than 100 healthy leaves were placed in Ziploc plastic bags, preserved with ice and transported to the laboratory. Leaves were randomly cut into 0.5 cm size pieces (10 pieces/leaf) using a hole puncher under aseptic conditions. These sections were soaked in 95 % ethanol for 1 minute, then in 3 % sodium hypochlorite solution for 3 minutes and finally in 95 % ethanol for 30 seconds. All samples were rinsed with sterile distilled water and dried on sterile tissue paper. Leaf sections were placed in Malt Extract Agar (MEA), Potato Dextrose Agar (PDA) and Water Agar (WA). They were incubated at room temperature (25-30 °C) for 1-3 days. If hyphal tips of any fungal colony appeared during incubation, the colony was transferred to new PDA plates and incubated to obtain pure cultures.
Figure 1.
Habitats of the host plants: a, b Pandanus spp. c, d Freycinetia spp.
Cultures and identification
The above methods resulted in 22 isolates which were separated into morphotypes based on visual assessment of the similarity of the cultures (Bills 1996; Umali et al. 1999; Fróhlich et al. 2000; Lacap et al. 2003). All of these cultures were grown on Potato Dextrose Agar (PDA). Growth rate measurements are shown in Table 1 with colony colour defined with the Methuen Handbook of Colour (Kornerup and Wanscher 1967). New taxa were examined in pure culture, allowing photographs, records of morphological characteristics and descriptions to be recorded. Herbarium specimens were prepared from cultures that were dried in silica gel. The holotypes were deposited in the Mae Fah Luang University Herbarium (Herb. MFLU), Chiang Rai, Thailand and in the Kunming Institute of Botany Academia Sinica (HKAS), Kunming, China. The ex-types cultures were deposited in the Mae Fah Luang University Culture Collection (MFLUCC) with duplicates deposited in the BIOTEC Culture Collection Laboratory (BCC) and the Kunming Institute of Botany Culture (KMUCC). New taxa were registered in Facesoffungi (FoF) (Jayasiri et al. 2015) and MycoBank (Crous et al. 2004).
Table 1.
Details of genes/loci with PCR primers and protocols.
| Gene/Loci | PCR primers (Forward/Reverse) | References |
|---|---|---|
| LSU | LROR/LR5 | Vilgalys and Hester 1990 |
| ITS | ITS5/ITS4 | White et al. 1990 |
| SSU | NS1/NS4 | White et al. 1990 |
| TEF1 | 983F/2218 | Rehner 2001 |
| 728F/986R | Carbone and Kohn 1999 | |
| RPB2 | fRPB2-5f/fRPB2–7cR | Liu et al. 1999 |
| β-tubulin | BT2a/BT2b | Glass and Donaldson 1995 |
| T1/T2 | O’Donnell and Cigelnik 1997 | |
| Actin | 512F/783R | Carbone and Kohn 1999 |
| CHS-1 | 79F/354R | Carbone and Kohn 1999 |
| GADPH | Gpd1/Gpd2 | Myllys et al. 2002 |
| GDF/GDR | Templeton et al. 1992 |
DNA extraction, PCR amplification, and sequencing
Genomic DNA was extracted from pure fungal cultures using Biospin Fungal Genomic DNA extraction Kit–BSC14S1 (BioFlux, P.R. China). Polymerase chain reaction (PCR) was used to amplify partial gene regions of Internal Transcribed Spacers (ITS), 28S ribosomal RNA (LSU), 18S ribosomal RNA (SSU), RNA polymerase II second largest subunit (RPB2), β-tubulin (Tub2), Actin (ACT), Glyceraldehyde-3- Phosphate Dehydrogenase (GADPH), Chitin synthase 1 (CHS-1) and Translation Elongation Factor 1-alpha (TEF1) using primers as shown in Table 1. The total volume of PCR mixtures for amplifications were 25 μl containing 8.5 μl ddH2O, 12.5 μl 2× Easy Taq PCR Super Mix (mixture of Easy Taq TM DNA Polymerase, dNTPs and optimised buffer (Beijing Trans Gen Biotech Co., Chaoyang District, Beijing, PR China), 2 μl of DNA template, 1 μl of each forward and reverse primers (10 pM). The quality of PCR products was checked on 1 % agarose gel electrophoresis stained with 4S green nucleic acid (Life Science Products & Services, Cat. No: A616694). Purification and sequencing of PCR products were carried out by Sangon Biotech Co., Shanghai, China.
Phylogenetic analysis
The sequence data generated during this study were the subject of BLAST searches in the nucleotide database of GenBank (www http://blast.ncbi.nlm.nih.gov/) to determine their most probable closely related taxa. Sequence data were retrieved from GenBank based on recent publications. Raw forward and reverse sequences were assembled using Geneious Pro.v4.8.5. Sequence alignments were carried out with MAFFT v.6.864b (Katoh and Standley 2016) and alignments were manually improved where necessary. The sequence datasets were combined using BioEdit v.7.2.5 (Hall 2004). Maximum Likelihood (ML) and Bayesian Inference (BI) analyses were performed for the sequence dataset. The phylogenetic trees were configured in FigureTree v. 1.4 (Rambaut and Drummond 2008) and edited using Microsoft Office PowerPoint 2007 and Adobe Illustrator CS3 (Adobe Systems Inc., USA).
Results and discussion
Identification of morphotypes
Twenty-two fungal isolates from Pandanus and Freycinetia species were recovered and these mycelia sterilia were separated into eight morphotypes based on the similarity of their culture characteristics, as summarised in Table 2 (Bills 1996; Umali et al. 1999; Fróhlich et al. 2000; Lacap et al. 2003).
Table 2.
Culture characteristics of the 22 strains (8 morphotypes) of mycelia sterilia on PDA.
| Morpho-types | Isolate code | Host | Size (cm) of colony | Shape | Colour | Mycelium | Edge | ||
|---|---|---|---|---|---|---|---|---|---|
| 3 days | 7 days | Above | Reverse | ||||||
| 1 | PE05 | Pandanus sp. | 4.6 | >A | Circular | 4A1 | 4A2 | Aerial | Undulate |
| PE09 | Pandanus sp. | 4.6 | >A | Circular | 6D3 | 6B3 | Aerial | Entire | |
| PE15 | Pandanus sp. | >A | >A | Circular | 5B2 | 5B3 | Flat | Entire | |
| 2 | PE10 | Pandanus sp. | 1.1 | 3.2 | Irregular | 4C1 | 4A3 | Aerial | Undulate |
| PE60 | Pandanus sp. | 1.6 | 3.8 | Irregular | 4B1 | 4A3 | Aerial | Undulate | |
| FE46 | Freycinetia sp. | 2.1 | 5.6 | Irregular | 5B2 | 5A2 | Aerial | Undulate | |
| FE42 | Freycinetia sp. | 1.5 | 5 | Irregular | 4A1 | 4A3 | Aerial | Undulate | |
| FE43 | Freycinetia sp. | 1.4 | 4.2 | Irregular | 5D4 | 5C4 | Flat | Undulate | |
| PE75 | Pandanus sp. | 1.6 | 5 | Circular | 6A1/6D3 | 6A1/6F5 | Aerial | Undulate | |
| PE84 | Pandanus sp. | 1.5 | 3.8 | Circular | 5F4 | 5F7 | Aerial | Curled | |
| FE98 | Freycinetia sp. | 1.3 | 3.1 | Irregular | 5B2 | 5D5 | Flat | Filamentous | |
| 3 | PE25 | Pandanus sp. | >A | >A | Circular | 5E1 | 5F2 | Aerial | Entire |
| 4 | PE26 | Pandanus sp. | 3.1 | 7.2 | Irregular | 5B3 | 5B5 | Aerial | Undulate |
| PE52 | Pandanus sp. | 1.2 | 2.9 | Circular | 5A2 | 5A3 | Aerial | Undulate | |
| 5 | PE35 | Pandanus sp. | 1.1 | 2.7 | Filamentous | 8E2 | 8F2 | Aerial | Filamentous |
| 6 | PE92 | Pandanus sp. | 5.1 | >A | Irregular | 4B1 | 4A6 | Aerial | Curled |
| PE37 | Pandanus sp. | 2.3 | 7.9 | Circular | 4A1 | 4B3 | Aerial | Curled | |
| FE88 | Freycinetia sp. | 2.9 | 6.2 | Circular | 5D3 | 5B2 | Flat | Undulate | |
| PE77 | Pandanus sp. | 4.2 | 7.1 | Irregular | 6B1/6E1 | 6B2 | Aerial | Undulate | |
| FE41 | Freycinetia sp. | >A | >A | Irregular | 4D2 | 4F6 | Flat | Filamentous | |
| 7 | PE58 | Pandanus sp. | <B | 1.7 | Circular | 4F2 | 4F8 | Aerial | Entire |
| 8 | FE101 | Freycinetia sp. | <B | 2 | Circular | 4B2 | 4A3 | Aerial | Entire |
Notes: >A Completely covering plate, <B Less than 1 cm
Phylogenetic analysis
Based on phylogenetic analysis, 22 fungal isolates were identified for 16 species. These include one new genus, seven new species and nine known species. All sequences obtained from this study are summarised in Table 3.
Table 3.
Species of endophytes obtained in this study.
| No. | Original code | Species name | Culture collection no. |
|---|---|---|---|
| 1 | PE26 | Alternaria burnsii | MFLUCC 17-0582 |
| 2 | PE58 | Cladosporium endophyticum | MFLUCC 17-0599 |
| 3 | PE09 | Colletotrichum pandanicola | MFLUCC 17-0571 |
| 4 | FE88 | Colletotrichum fructicola | MFLUCC 17-0555 |
| PE84 | MFLUCC 17-0613 | ||
| 5 | PE77 | Diaporthe pandanicola | MFLUCC 17-0607 |
| 6 | PE37 | Diaporthe siamensis | MFLUCC 17-0591 |
| 7 | FE41 | Endomelanconiopsis freycinetiae | MFLUCC 17-0547 |
| 8 | FE42 | Endopandanicola thailandica | MFLUCC 17-0548 |
| FE43 | MFLUCC 17-0549 | ||
| FE46 | MFLUCC 17-0551 | ||
| PE10 | MFLUCC 17-0572 | ||
| PE60 | MFLUCC 17-0600 | ||
| 9 | PE25 | Lasiodiplodia theobromae | MFLUCC 17-0581 |
| 10 | PE52 | Massarina pandanicola | MFLUCC 17-0596 |
| 11 | FE98 | Meyerozyma caribbica | MFLUCC 17-0556 |
| PE75 | MFLUCC 17-0606 | ||
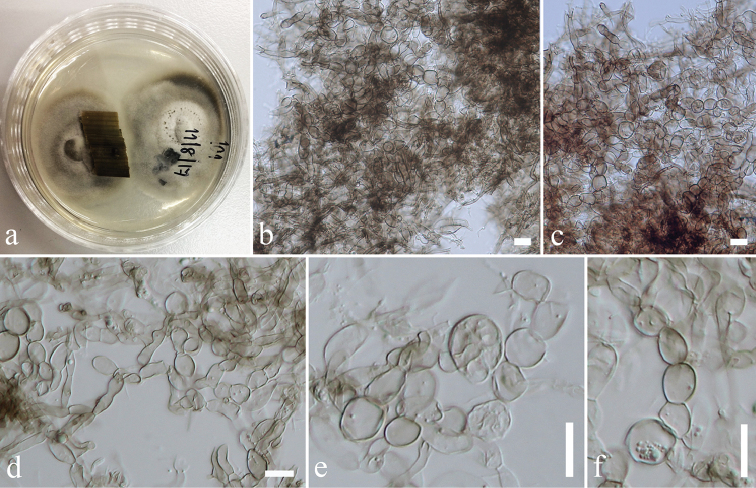
| 12 | FE101 | Mycoleptodiscus endophytica | MFLUCC 17-0545 |
| 13 | PE05 | Pestalotiopsis jiangxiensis | MFLUCC 17-0567 |
| 14 | PE92 | Pestalotiopsis microspora | MFLUCC 17-0619 |
| 15 | PE15 | Phanerochaete chrysosporium | MFLUCC 17-0575 |
| 16 | PE35 | Phyllosticta capitalensis | MFLUCC 17-0589 |
Basidiomycota R.T. Moore
Agaricomycetes Doweld
Polyporales Gäum., 1926
Polyporaceae
Fr. ex Corda
Remarks.
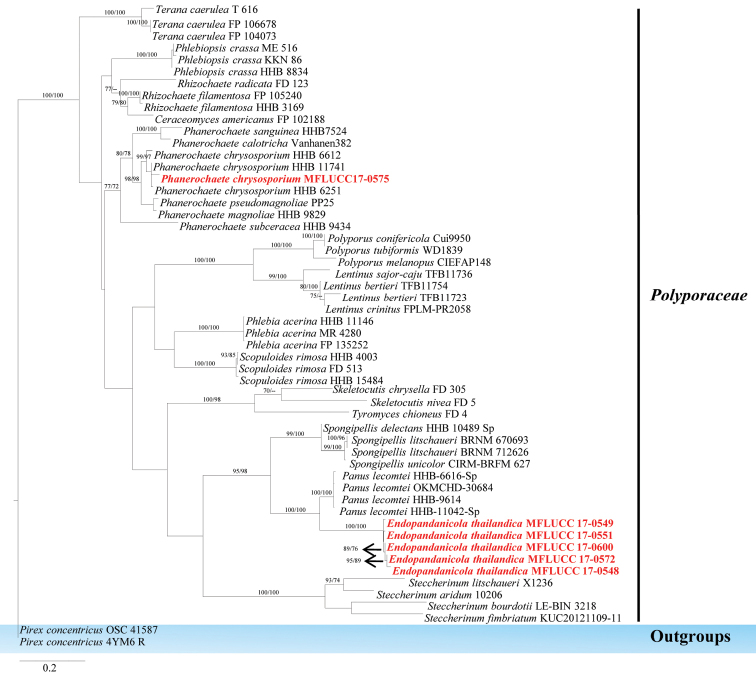
The family Polyporaceae was introduced by Fr. ex Corda (1839) and includes 92 genera and 636 species (Kirk et al. 2008). According to Cannon and Kirk (2007), the species in this family are characterised by poroid, irregular or lamellate hymenophores and are saprobes. Recent phylogenetic analyses of Polyporaceae are by Binder et al. (2013) and Hyde et al. (2017). In this study, a new endophytic genus, Endopandanicola with En. thailandica as the type species was discovered. In addition to the new genus, Phanerochaete chrysosporium was also identified.
Endopandanicola
Tibpromma & K.D. Hyde gen. nov.
MB823835
Etymology.
Named after its habitat as an endophyte of Pandanus.
Type species.
Endopandanicola thailandica Tibpromma & K.D. Hyde
Culture characteristics.
Colonies on PDA (PE60), superficial, initially white, later becoming yellow-white, smooth at the surface, irregular, with undulate margin, flossy to velvety; reverse white to yellow-white. Generative hyphae simple-septate, branched, sub-hyaline, thin-walled.
Notes.
Endopandanicola formed a single, well-supported clade (100 % in ML, 100 % in MP), which is distinct as compared to other genera in Polyporaceae (Figure 3). This genus comprises resupinate or crust polypores that live inside leaves or wood as endophytes and do not form fruiting bodies (sexual morph), but form flat mycelia. More collections of Pandanus are needed in the future to locate the sexual morph of Endopandanicola.
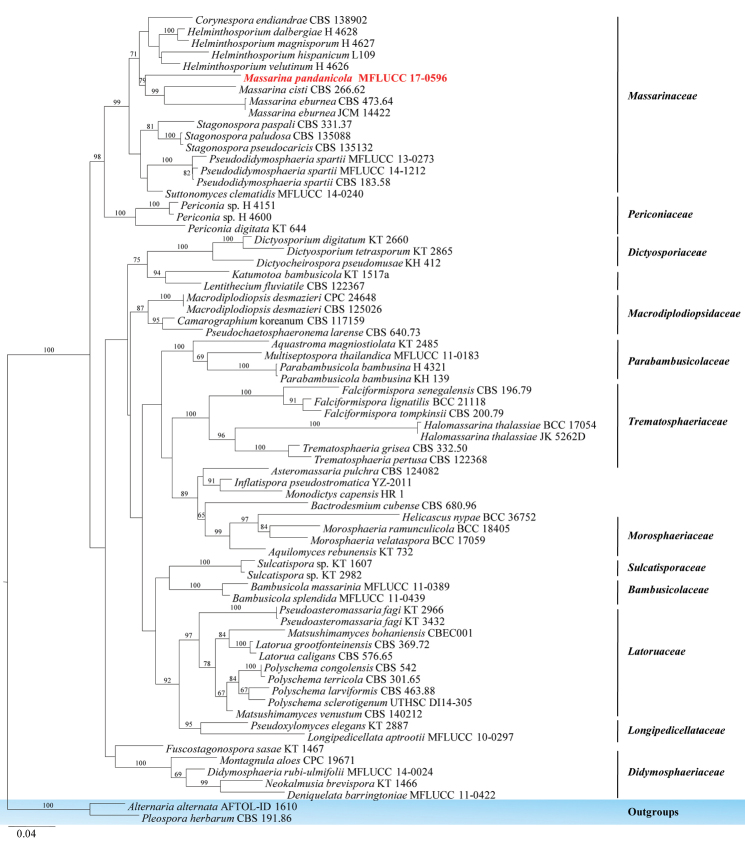
Figure 3.
Phylogram generated from maximum likelihood analysis based on ITS sequence data. Maximum parsimony (left) and maximum likelihood (right) bootstrap support values are given above/below the nodes. The newly generated sequences are in red text. The tree is rooted with Pirex concentricus.
Endopandanicola thailandica
Tibpromma & K.D. Hyde sp. nov.
MB823836
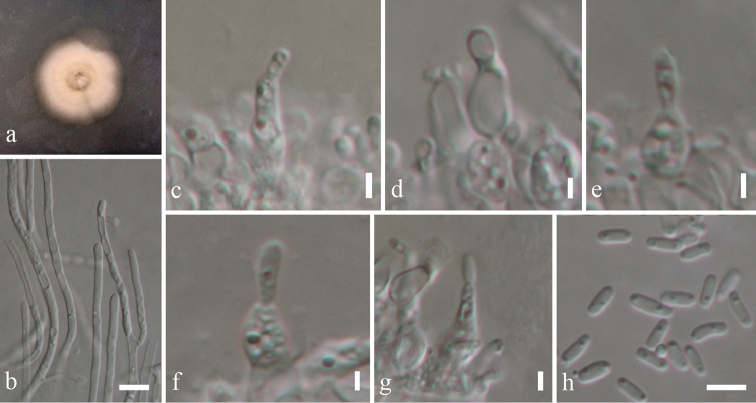
Figure 4.
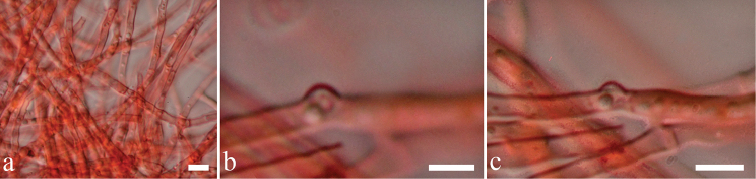
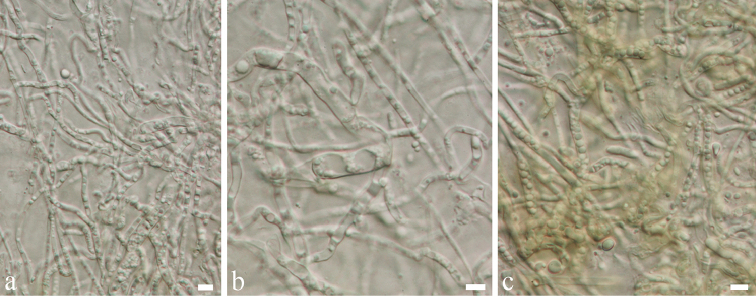
Endopandanicola thailandica (MFLU 18-0021, holotype). a Mycelia masses b, c Clamp connections. Scale bars: 10 μm (a), 5 μm (b, c).
Etymology.
named after Thailand, the country where the fungus was first discovered.
Holotype.
MFLU 18-0021
Culture characteristics.
Colonies on PDA (Figure 2 PE10, FE42, FE43, FE46 and PE60), superficial, initially white, later becoming yellow-white, irregular, with undulate margin, smooth with flossy to velvety; reverse white to yellow-white. Generative hyphae simple-septate, branched, with clamp connections, sub-hyaline, thin-walled, 1.5–3.5 µm wide.
Figure 2.
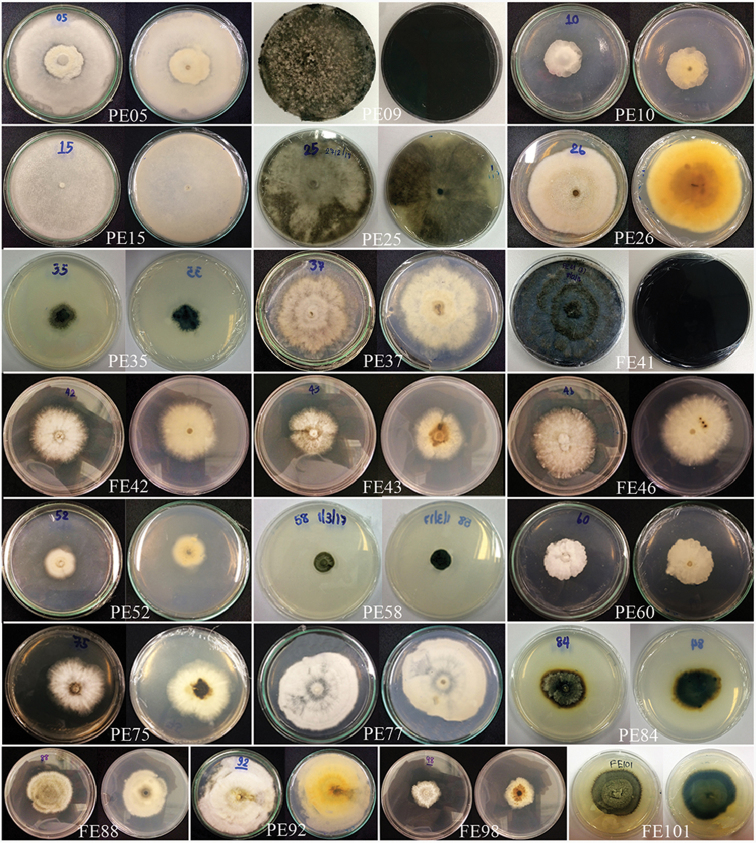
All cultures from this study are grown on PDA at room temperature after 7 days (original codes are written at the bottom of each picture).
Material examined.
THAILAND, Chumphon, Pathio District, on healthy leaves of Pandanus sp. (Pandanaceae), 1 December 2016, S. Tibpromma PE60 (MFLU 18-0021, holotype); HKAS100856, paratype, ex-type living cultures, MFLUCC 17-0600 = KUMCC 17-0295; Chumphon, Pathio District, 1 December 2016, S. Tibpromma PE10, living culture, MFLUCC 17-0572; Ranong, Muang, Muang District, 3 December 2016, S. Tibpromma FE42, living culture, MFLUCC 17-0548; FE43, living culture, MFLUCC 17-0549 = KUMCC 17-0264; FE46, living culture, MFLUCC 17-0551 = KUMCC 17-0265.
GenBank numbers.
ITS; MFLUCC 17-0545=MG646961, MFLUCC 17-0548=MG646964, MFLUCC 17-0549=MG646963, MFLUCC 17-0551=MG646962, MFLUCC 17-0572=MG646959, MFLUCC 17-0600=MG646960.
Notes.
Endopandanicola is introduced and typified by En. thailandica which is represented by six isolates and is described as a novel species based on its asexual morph. The phylogenetic analysis of ITS sequence data showed that this species clustered together with Panus, but there is a high level of statistical support for its separation (100% in ML, 100% in MP) (Figure 3).
Phanerochaete chrysosporium
Burds., in Burdsall & Eslyn, Mycotaxon 1(2): 124 (1974)
Culture characteristics.
Colonies on PDA (Figure 2, PE15), superficial, white, surface smooth with flat media surface, circular, with entire edge; reverse white to yellow-white.
GenBank numbers.
ITS=MG646957.
Notes.
Burdsall and Eslyn (1974) introduced Phanerochaete chrysosporium which was collected on dead wood of Platanus wrightii in the USA. Phylogenetic analysis of ITS sequence data shows this taxon groups with Phanerochaete chrysosporium (sequences obtained from GenBank) that had been collected from different hosts. The phylogenetic placement of this species is shown in Figure 3.
Ascomycota Whittaker
Dothideomycetes O.E. Erikss. & Winka
Botryosphaeriales
C.L. Schoch, Crous & Shoemaker
Remarks.
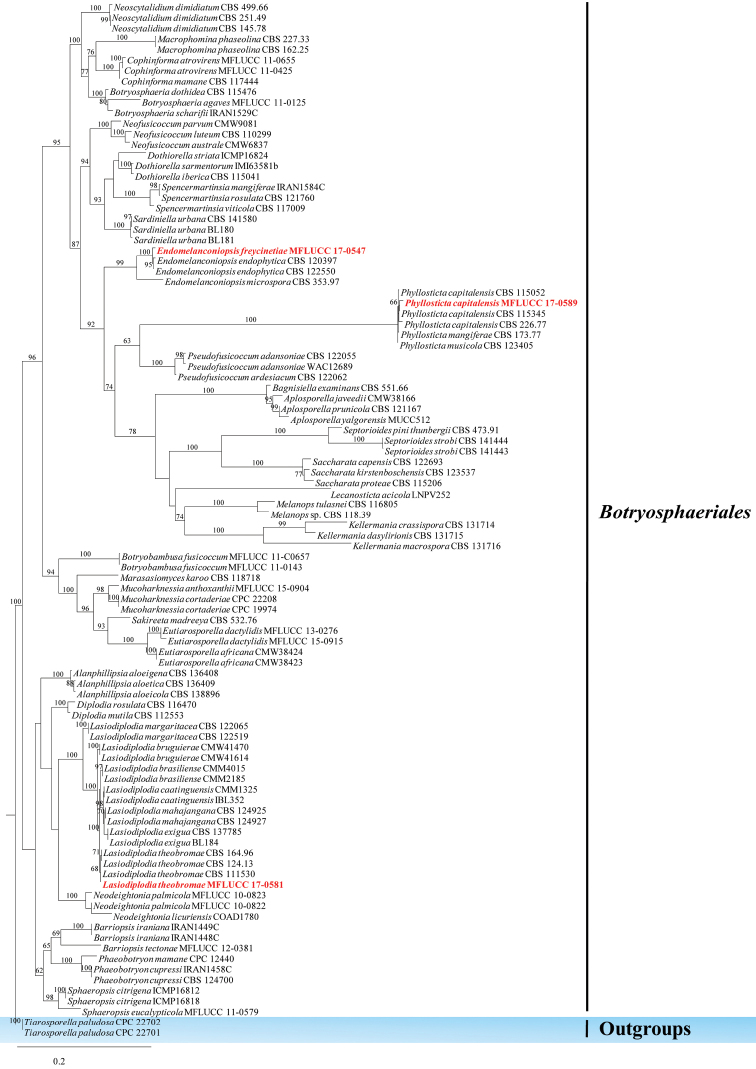
The order Botryosphaeriales was introduced by Schoch et al. (2006) with Botryosphaeriaceae as the type family. Botryosphaeriales is a diverse order with a worldwide distribution, comprising species that vary from endophytes to pathogens (Slippers and Wingfield 2007; Phillips et al. 2013; Chethana et al. 2016; Daranagama et al. 2016; Dissanayake et al. 2016; Konta et al. 2016a, b; Linaldeddu et al. 2016a, b, c; Manawasinghe et al. 2016; Zhang et al. 2017). Currently, nine families are recognised, namely, Aplosporellaceae, Botryosphaeriaceae, Endomelanconiopsisaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae, Pseudofusicoccumaceae, Saccharataceae and Septorioideaceae (Schoch et al. 2006; Minnis et al. 2012; Wikee et al. 2013; Slippers et al. 2013; Wyka and Broders 2016; Dissanayake et al. 2016; Yang et al. 2017). In this study, Endomelanconiopsis freycinetiae is introduced as a new species and reports are provided on Phyllosticta capitalensis and Lasiodiplodia theobromae.
Endomelanconiopsis freycinetiae
Tibpromma & K.D. Hyde sp. nov.
MB823837
Figure 6.
Endomelanconiopsis freycinetiae (MFLU 18-0002, holotype). a–d Mycelia masses. Scale bars: 20 μm (a–c), 10 μm (d).
Etymology.
name referring to the host genus on which the fungus was found (Freycinetia).
Holotype.
MFLU 18-0002
Culture characteristics.
Colonies on PDA (Figure 2, FE41), superficial, initially white-grey with flat mycelium on media with dark centre, later becoming dark olivaceous with circular rings and flossy at the margin; reverse dark olivaceous. Generative hyphae simple-septate, branched, sub-hyaline to brown, cylindrical, guttulate, thick-walled. Not sporulating in culture (Figure 6).
Material examined.
THAILAND, Ranong, Muang, on healthy leaves of Freycinetia sp. (Pandanaceae), 3 December 2016, S. Tibpromma FE41 (MFLU 18-0002, holotype); HKAS100853, paratype, ex-type living cultures, MFLUCC 17-0547 = KUMCC 17-0292.
GenBank numbers.
ITS=MG646955, LSU=MG646948, TEF1=MG646983, β-tubulin=MG646924.
Notes.
Endomelanconiopsis freycinetiae is closely related to the endophytic fungus En. endophytica. Therefore, the culture characteristics of these two taxa were compared and it was found that, in En. endophytica, at first the hyphae are colourless, immersed, later becoming olivaceous in the centre with irregular concentric rings; aerial mycelia are dark olivaceous or grey when dense; shiny black when the aerial mycelia are loose (Rojas et al. 2008) whereas aerial mycelia of En. freycinetiae has dark olivaceous, circular rings and flossy surface (Figure 2, FE41). Nucleotide base pairs of ITS and TEF1 were also compared and it was found that there are differences (ITS 3 bp, TEF1 8 bp).
Phyllosticta capitalensis
Henn., Hedwigia 48: 13 (1908)
Culture characteristics.
Colonies on PDA (Figure 2, PE35), superficial, dark olivaceous with filamentous hyphae and raised edge; reverse dark olivaceous. Sporulating in culture after 2 months.
GenBank numbers.
Notes.
Phyllosticta capitalensis (Hennings 1908) is known as an endophytic taxon and a minor plant pathogen. It has a worldwide distribution and has been recorded on 70 plant families (Baayen et al. 2002; Okane et al. 2003; Motohashi et al. 2009; Wikee et al. 2013). The isolate recovered herein clusters with reasonable ML bootstrap support with other P. capitalensis isolates (Figure 5). Morphological examination also depicts similar morphs and hence it is identified as P. capitalensis.
Figure 5.
Phylogram generated from maximum likelihood analysis based on ITS, LSU and TEF1 sequenced data. Maximum likelihood bootstrap values are given above/below the nodes. The newly generated sequences are in red bold. The tree is rooted with Tiarosporella paludosa.
Lasiodiplodia theobromae
(Pat.) Griffon & Maubl., Bull. Soc. Mycol. Fr. 25: 57 (1909)
Culture characteristics.
Colonies on PDA (Figure 2, PE25), superficial, initially white with flat mycelium on media, later becoming dark, circular, flossy and velvety; reverse dark. Not sporulating in culture.
GenBank numbers.
Notes.
Morphological and phylogenetic data supported placement of this isolate as Lasiodiplodia theobromae. The phylogenetic analysis showed the isolate groups with Lasiodiplodia theobromae. Nucleotide base pairs of published sequences of Lasiodiplodia theobromae (strain EucN188, CBS 111530, PHLO9, CDFA145) were also compared with the sequence and found that the nucleotide base pairs of the ITS gene are 100% similar.
Capnodiales Woron., 1925
Cladosporiaceae Castell. & R.G. Archibald
Cladosporium
Link, 1816
Remarks.
The genus Cladosporium (Cladosporiaceae, Capnodiales) is a large genus of the Ascomycota (Wijayawardene et al. 2017). The genus comprises species that are saprobes, endophytes and pathogens. A few species have been documented as being etiologic agents in vertebrate hosts (David 1997; Bensch et al. 2012, 2015; Crous et al. 2014). In this study, a new species of Cladosporium is described, with high bootstrap support in the phylogenetic analysis (Figure 7).
Figure 7.
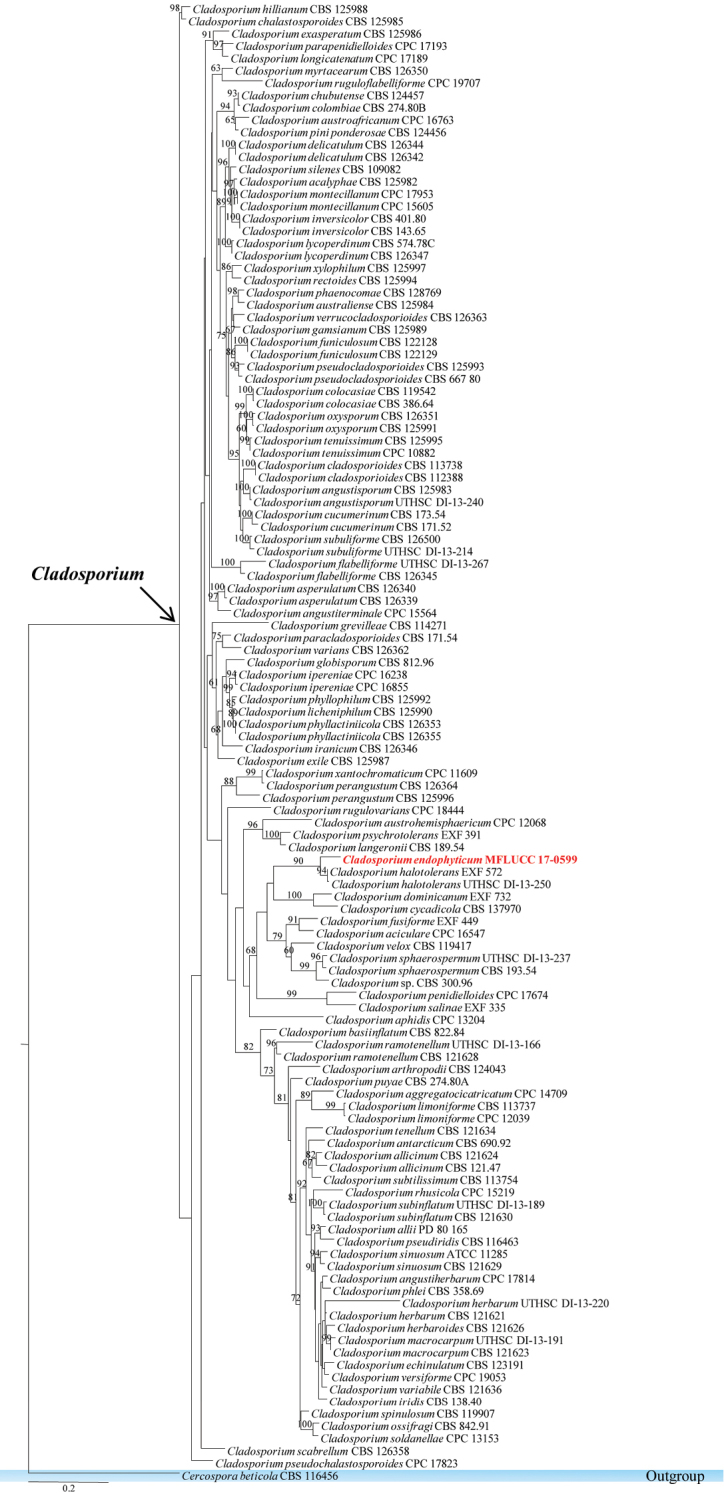
Phylogram generated from maximum likelihood analysis based on ITS, TEF1 and Actin sequenced data. Maximum likelihood bootstrap is given above/below the nodes. The newly generated sequences in red bold. The tree is rooted with Cercospora beticola.
Cladosporium endophyticum
Tibpromma & K.D. Hyde sp. nov.
MB823838
Figure 8.
Cladosporium endophyticum (MFLU 18-0005, holotype). a Colony on MEA media b Mycelium masses c–e Conidia and conidiogenous cells f, g Conidia h Conidia and conidiogenous cells. Scale bars: 5 µm (b–h), 10 µm (h).
Etymology.
named after its status as an endophytic fungus.
Holotype.
MFLU 18-0005
Description.
Colonies on PDA attaining 9 cm diam. in six weeks at room temperature, slow growing, dark olivaceous. Mycelium superficial and immersed composed of septate, branched, 2.3–4.5 µm wide, sub-hyaline, with smooth and thick-walled hyphae. Sexual morph Undetermined. Asexual morph Conidiophores 6–10 µm high, 3–4 µm diam. (x̄ = 8.24 × 3.52 µm, n = 10), terminal and intercalary, cylindrical or sub-cylindrical, darkened conidiogenous loci. Conidia 3–6 × 2–4 µm (x̄ = 3.64 × 2.75 µm, n = 30), forming long branched chains, hyaline to pale-olivaceous, smooth and thin-walled, aseptate, globose to ovoid with rounded ends.
Culture characteristics.
Colonies on PDA (Figure 2, PE58), superficial, dark olivaceous with dark-grey centre, irregular, undulate with wrinkled and raised on surface media; reverse dark olivaceous. Generative hyphae simple-septate, branched, sub-hyaline, guttules, thick-walled (Figure 8).
Material examined.
THAILAND, Chumphon, Pathio District, on healthy leaves of Pandanus sp. (Pandanaceae), 1 December 2016, S. Tibpromma PE58 (MFLU 18-0005, holotype); HKAS100855, paratype, ex-type living cultures, MFLUCC 17-0599 = KUMCC 17-0294.
GenBank numbers.
Notes.
Cladosporium endophyticum was isolated as an endophyte from Pandanus sp. in Thailand. In the phylogenetic analysis of combined gene sequence data of ITS, LSU, SSU and TEF1, the new taxon Cladosporium endophyticum is sister to C. halotolerans (Figure 7), but well-separated with high bootstrap support (90% in ML). Moreover, the morphology of this new taxon was compared with Cladosporium halotolerans which has brown to dark brown, subglobose to globose with verrucose, less often short-ovoid conidia, narrower at both ends (Zalar et al. 2007), while C. endophyticum has globose to ovoid, hyaline to pale-olivaceous conidia with rounded ends. Here, the authors introduce the new species C. endophyticum and provide an updated phylogenetic tree for the genus Cladosporium.
Pleosporales Luttr. ex M.E. Barr, 1987
Massarinaceae
Munk.
Remarks.
The family Massarinaceae was introduced by Munk (1956) under Pleosporales together with Cucurbitariaceae and Didymosphaeriaceae. Later, Barr (1987) segregated Massarinaceae under Lophiostomataceae based on morphology, while based on multigene phylogenetic analysis Schoch et al. (2009) also showed Massarinaceae is a distinct family in order Pleosporales. Recently, Zhang et al. (2009, 2012) recognised Massarinaceae as a distinct lineage based on both morphology and molecular phylogeny. In this study, a new species of endophytic Massarina, based on morphological and phylogenetic support, is introduced from Pandanus sp. in Thailand.
Massarina pandanicola
Tibpromma & K.D. Hyde sp. nov.
MB823839
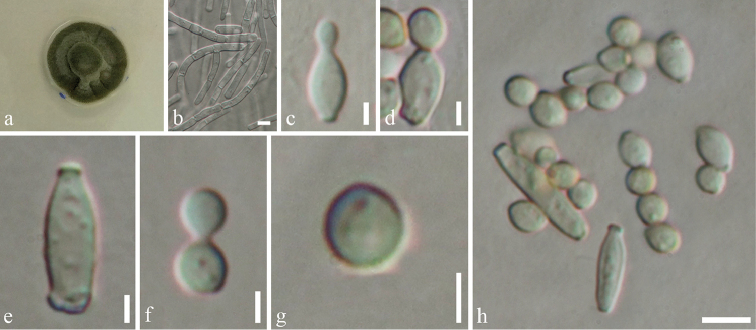
Figure 10.
Massarina pandanicola (MFLU 18-0004, holotype). a Colony on MEA media b Mycelium masses c–g Conidia and conidiogenous cells h Conidia. Scale bars: 20 μm (b), 2 μm (c–g), 5 μm (h).
Etymology.
name referring to the host genus of the plant on which the fungus was first discovered (Pandanus).
Holotype.
MFLU 18-0004
Description.
Colonies on PDA attaining 9 cm diam. in 4 weeks at room temperature, slow growing, white to yellow-white. Mycelium superficial and immersed composed of septate, branched, 2.5–7 µm wide, sub-hyaline, with smooth and thick-walled hyphae. Sexual morph Undetermined. Asexual morph Conidiophores 12–25 µm high, 8–14 µm diam. (x̄ = 15.12 × 10.45 µm, n = 10), enteroblastic, phialidic, cylindrical or sub-cylindrical, sub-hyaline. Conidia 3–5 × 1–3 µm (x̄ = 4.34 × 1.75 µm, n = 30), cylindrical, hyaline, smooth and thin-walled, aseptate, rounded ends, guttulate, without sheet or appendages.
Culture characteristics.
Colonies on PDA (Figure 2, PE52), superficial, white to yellow-white, irregular, undulate with smooth and raised on surface media; reverse yellow-white. Generative hyphae simple-septate, branched, sub-hyaline, with guttulate cells, thin-walled. Sporulating in culture within 3 months (Figure 10).
Material examined.
THAILAND, Chumphon, Pathio District, on healthy leaves of Pandanus sp. (Pandanaceae), 1 December 2016, S. Tibpromma PE52 (MFLU 18-0004, holotype); HKAS100854, paratype, ex-type living cultures, MFLUCC 17-0596 = KUMCC 17-0293.
Genbank numbers.
Notes.
The genus Massarina has been known as a phylogenetically diverse group in the order Pleosporales based on molecular data (Liew et al. 2002) and most members in Massarina except for the type species (M. eburnea) are morphologically variable. The taxon, Massarina pandanicola collected from Pandanus sp. in Thailand is introduced here as a new species with both morphology and phylogeny support. The morphology of the taxon showed similar conidia with Massarina eburnean (Tanaka et al. 2015), but based on phylogenetic analysis of combined ITS, LSU, SSU and TEF1 gene sequence data, the new taxon M. pandanicola is well-separated from other species in Massarina (Figure 9) with high bootstrap support (79 % in ML). This is the first record of Massarina from Pandanus sp.
Figure 9.
Phylogram generated from maximum likelihood analysis based on ITS, TEF1, SSU, LSU and RPB2 sequenced data. Maximum likelihood bootstrap values are given above/below the nodes. The newly generated sequences in red bold. The tree is rooted with Alternaria alternata and Pleospora herbarum.
Pleosporaceae
Nitschke
Remarks.
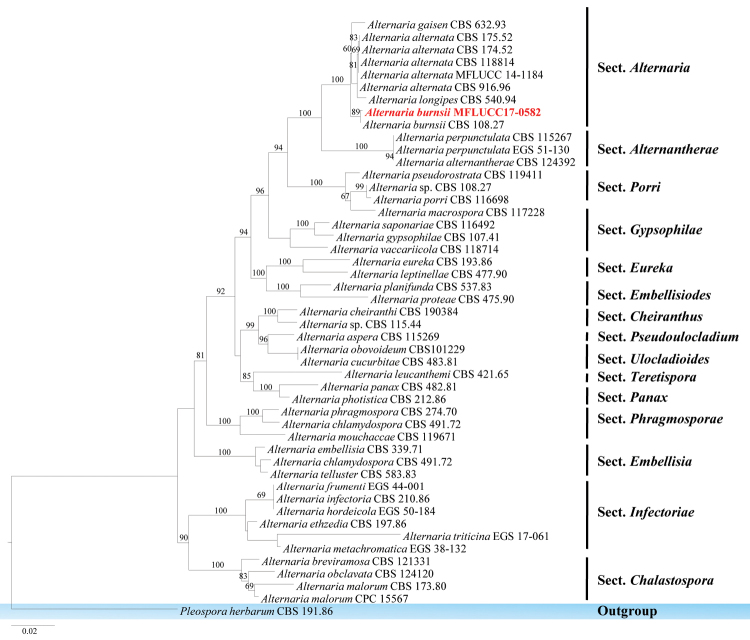
The family Pleosporaceae was introduced by Nitschke (1869) and is the largest family of the order Pleosporales (Hyde et al. 2013; Ariyawansa et al. 2015b; Liu et al. 2017). Members of this family can be endophytes, aquatic or terrestrial saprobes, plant pathogens or opportunistic animal pathogens (Sivanesan 1984; Carter and Boudreaux 2004). A backbone tree for Pleosporaceae was provided by Ariyawansa et al. (2015a). In this study, Alternaria burnsii is reported from a Pandanus sp. host in Thailand.
Alternaria burnsii
Uppal, Patel & Kamat, Indian J. Agric. Sci. 8: 49 (1938)
Culture characteristics.
Colonies on PDA (Figure 2, PE26), superficial, white-orange to cream, circular, entire edge, smooth, flossy, velvety and raised on surface media; reverse yellow-white at the margin and yellow-brown in centre. Not sporulating in culture.
GenBank numbers.
Notes.
Alternaria burnsii was introduced by Uppal et al. (1938) from India on Cumnium cyminum. This species has a close phylogenetic relationship with Alternaria tomato and A. jacinthicola (Woudenberg et al. 2015). Results from phylogenetic analysis show that the authors’ collection belongs to Alternaria burnsii with a relatively high bootstrap support (89% in ML) (Figure 11). Nucleotides across the ITS regions of Alternaria burnsii CBS 108.27 and the isolates were compared and the authors noted that they are identical.
Figure 11.
Phylogram generated from maximum likelihood analysis based on ITS, TEF1, LSU and RPB2 sequence data. Maximum likelihood bootstrap values are given above/below the nodes. The newly generated sequences are in red bold. The tree is rooted with Pleospora herbarum.
Sordariomycetes O.E. Erikss. & Winka
Diaporthales Nannf.
Diaporthaceae
Höhn. ex Wehm.
Remarks.
The family Diaporthaceae was introduced by von Höhnel (1917) and was placed in the order Diaporthales. This family comprised two Diaporthe genera (Phomopsis and Mazzantia) (Wehmeyer 1975; Castlebury et al. 2002). Later, Diaporthaceae was given the synonym Valsaceae (Barr 1978). Based on DNA sequence data, some other genera have been placed in Diaporthaceae (Dai et al. 2014; Voglmayr and Jaklitsch 2014). Recently, Maharachchikumbura et al. (2015) and Senanayake et al. (2017) listed further genera that belong to Diaporthaceae. In this study, a new and a known species of Diaporthe from Pandanaceae hosts in Thailand is reported.
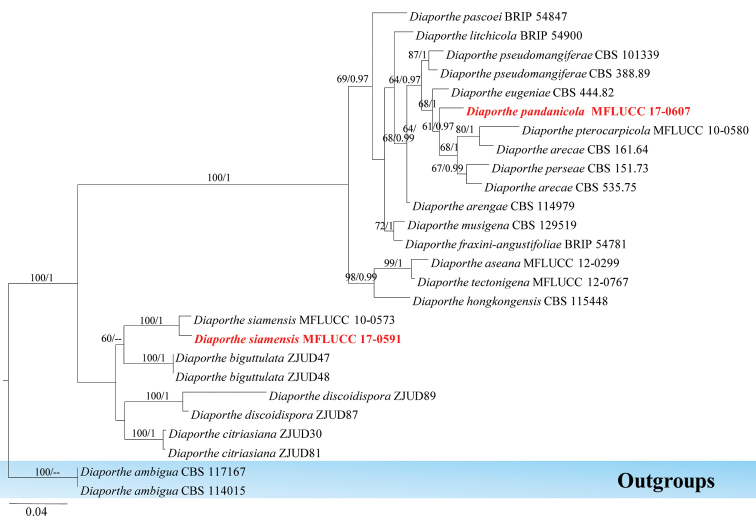
Diaporthe pandanicola
Tibpromma & K.D. Hyde sp. nov.
MB823840
Figure 13.
Diaporthe pandanicola (MFLU 18-0006, holotype). a–c Mycelia masses. Scale bars: 5 µm (a–c).
Etymology.
Name referring to the host genus on which the fungus was first discovered (Pandanus).
Holotype.
MFLU 18-0006
Culture characteristics.
Colonies on PDA (Figure 2, PE77), superficial, white, circular with entire edge, smooth and raised on surface media, flossy and velvety; reverse yellow-white, 9 cm diam. in 10 days. Generative hyphae simple-septate, branched, sub-hyaline, cells with guttules, thin-walled, 1.5–7 µm wide. Not sporulating in culture (Figure 13).
Figure 12.
Phylogram generated from maximum likelihood analysis based on ITS, TEF1 and β-tubulin sequenced data. Maximum likelihood (left) and Bayesian inference (right) bootstrap values are given above/below the nodes. The newly generated sequences are in red bold. The tree is rooted with Diaporthe ambigua.
Material examined.
THAILAND, Chumphon, Pathio District, on healthy leaves of Pandanus sp. (Pandanaceae), 1 December 2016, S. Tibpromma PE77 (MFLU 18-0006, holotype); HKAS100858, paratype, ex-type living cultures, MFLUCC 17-0607 = KUMCC 17-0297.
GenBank numbers.
Notes.
Diaporthe species are plant pathogens, endophytes or saprobes (Carroll 1986; Garcia-Reyne et al. 2011; Udayanga et al. 2011, 2012, 2014, Hyde et al. 2014). Here, a new species Diaporthe pandanicola is introduced based on phylogeny support. Based on phylogenetic analysis, the new species was well-separated from closely related species of Diaporthe (61% in ML, 0.97 in PP). However, this isolate is an endophytic fungus and did not sporulate in culture during 5 months (Figure 13).
Diaporthe siamensis
Udayanga, X.Z. Liu & K.D. Hyde, 2012
Culture characteristics.
Colonies on PDA (Figure 2, PE37), superficial, white to yellow-white, irregular, curled and raised on media surface, flossy; under surfaceyellow-white.
GenBank numbers.
ITS=MG646975, TEF1=MG646989, β-tubulin=MG646925, ACT=MG646940.
Notes.
In the phylogenetic analysis, the authors’ collection grouped with Diaporthe siamensis MFLUCC 10-0573 with high statistical values of 100% in ML and 1.00 in PP. Diaporthe siamensis is an endophytic fungus collected from a Pandanaceae host in Thailand.
Glomerellales Chadef. ex Réblová et al.
Glomerellaceae
Locq. ex Seifert & W. Gams, in Zhang et al. (2007)
Remarks.
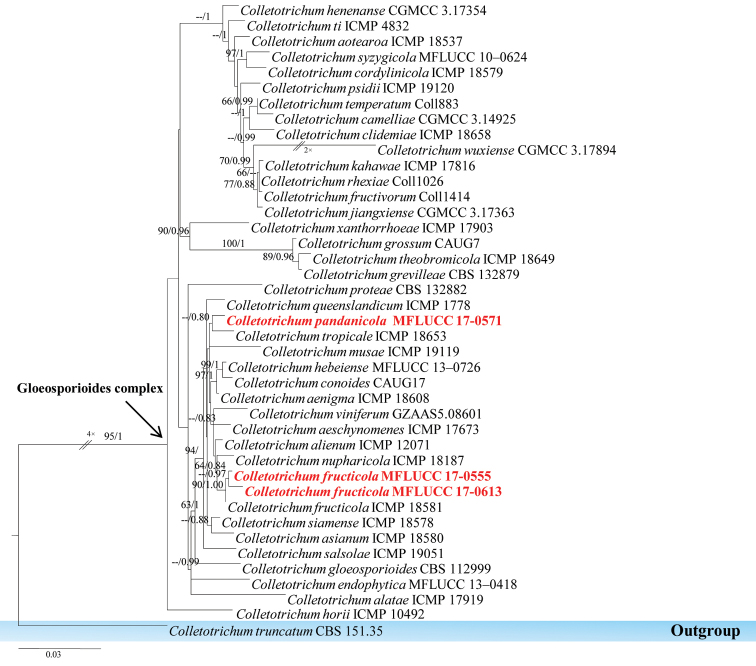
The family Glomerellaceae was introduced by Locquin (1984), but was invalidly published. To date, most Glomerellaceae have been recorded to be pathogens (Maharachchikumbura et al. 2016b). Earlier studies reported that the position of the family Glomerellaceae was not stable (Zhang et al. 2006; Kirk et al. 2001; Kirk et al. 2008). Réblová et al. (2011) resolved the placement of Glomerellaceae by using phylogenetic analysis of combined ITS, LSU, SSU and RPB2 sequence data. Recently, the family Glomerellaceae was established based on the genus Glomerella (Zhang et al. 2006), which had been given a synonym under its asexual morph Colletotrichum (Maharachchikumbura et al. 2015). Recently, Jayawardena et al. (2016) provided notes on currently accepted species of Colletotrichum. In this study, the authors introduce a new endophytic Colletotrichum species and report a known species of endophytic Colletotrichum from gloeosporioides species complex based on morphology and phylogenetic analysis.
Colletotrichum fructicola
Prihast., L. Cai & K.D. Hyde, 2009
Culture characteristics.
Colonies on PDA (Figure 2, PE84, 88), superficial, white to olivaceous in the beginning and later become olivaceous to dark-olivaceous, circular, entire edge, smooth, dense and raised on surface media; reverse dark-olivaceous. Sporulating in culture after 1 month.
GenBank numbers.
MFLUCC 17-0613 ITS=MG646968, β-tubulin=MG646927, GAPDH=MG646932, CHS-1=MG646937, ACT=MG646939. MFLUCC 17-0555 ITS=MG646969, β-tubulin=MG646928, GADPH=MG646933, CHS-1=MG646936, ACT=MG646944.
Notes.
The gloeosporioides species complex is mainly plant pathogens (Weir et al. 2012) and some species are endophytes (Liu et al. 2015). Colletotrichum fructicola has a wide host range (Weir et al. 2012) and was originally reported from coffee berries in Thailand (Prihastuti et al. 2009). In this study, the authors followed Jayawardena et al. (2016) and identify the collection as Colletotrichum fructicola which was isolated from a Pandanaceae host. Based on phylogenetic analysis, this taxon grouped with Colletotrichum fructicola with 90 % in ML and 1.00 in PP. The ITS, β-tubulin, GAPDH, CHS-1 and ACT DNA nucleotide comparison showed that the taxon and other strains of Colletotrichum fructicola Prihast., L. Cai & K.D. Hyde have 100% similarity.
Colletotrichum pandanicola
Tibpromma & K.D. Hyde sp. nov.
MB823841
Figure 15.
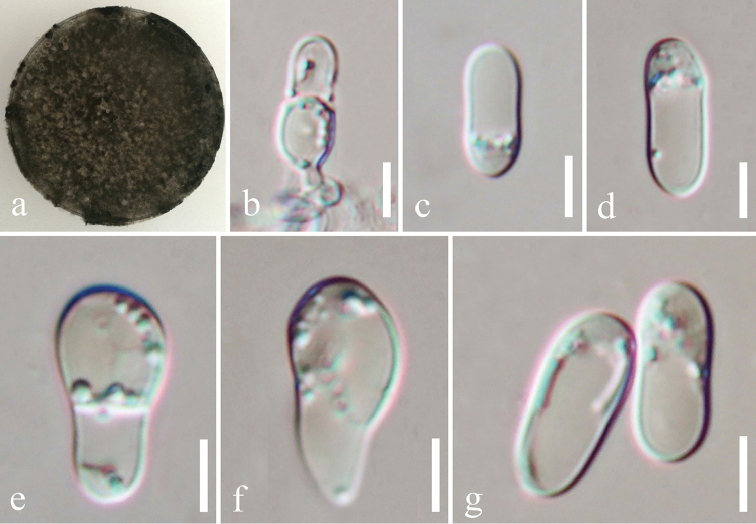
Colletotrichum pandanicola (MFLU 18-0003, holotype). a Colony on PDA media b Conidia and conidiogenous cells c–g Conidia on PDA culture. Scale bars: 5 μm (b), 2 μm (c–g).
Etymology.
name referring to the host genus (Freycinetia).
Holotype.
MFLU 18-0003
Description.
Colonies on PDA attaining 9 cm diam. in 7 days at room temperature, dark-grey. Sexual morph Undetermined. Asexual morph Conidiophores hyaline, smooth-walled, cylindrical to slightly inflated. Conidia 9–18 µm high, 4–8 µm diam. (= 13.39 × 5.35 µm, n = 20), hyaline, cylindrical with rounded ends tapering slightly towards the base, smooth, septate, guttulate.
Culture characteristics.
Colonies on PDA (Figure 2, PE09), superficial, white in the beginning and later becoming dark-grey, circular, entire edge, smooth, flossy, velvety and raised on surface media; reverse dark. Sporulating in culture after 1 month.
Material examined.
THAILAND, Chumphon, Pathio District, on healthy leaves of Pandanus sp. (Pandanaceae), 1 December 2016, S. Tibpromma PE09 (MFLU 18-0003, holotype); GZAAS 16-0145, paratype, ex-type living cultures, MFLUCC 17-0571.
GenBank numbers.
ITS=MG646967, β-tubulin=MG646926, GAPDH= MG646931, CHS-1=MG646935, ACT=MG646938.
Notes.
Colletotrichum pandanicola is introduced here as a new species in the gloeosporioides species complex based on morphological and phylogenetic data. The phylogenetic analysis shows that this new taxon is well-separated from other known Colletotrichum species (Figure 14). The authors also compared nucleotides of β-tubulin, GAPDH, CHS-1 and ACT and found that there are differences between Colletotrichum tropicale and this new species (β-tubulin 7 bp, GAPDH 11 bp, CHS-1 7 bp and ACT 3 bp).
Figure 14.
Phylogram generated from maximum likelihood analysis based on combined ITS, Actin, β-tubulin, GADPH and CHS-1 sequenced data. Maximum likelihood (left) and Bayesian inference (right) bootstrap values are given above/below the nodes. The newly generated sequences are in red text. The tree is rooted with Colletotrichum truncatum.
Magnaporthaceae
P.F. Cannon
Remarks.
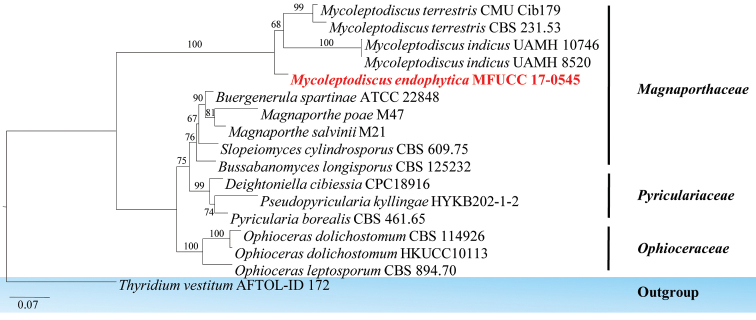
The family Magnaporthaceae was introduced by Cannon (1994) and was placed as a family within the class Sordariomycetes (Kirk et al. 2001; Lumbsch and Huhndorf 2007). According to Thongkantha et al. (2009), the placement of the taxa Magnaporthaceae has long been problematic due to a lack of convincing morphological characteristics and inconclusive molecular data. Thongkantha et al. (2009) established a new order, Magnaporthales, to accommodate Magnaporthaceae, based on a combination of morphological characteristics and the phylogenetic analysis of combined sequence data. Maharachchikumbura et al. (2015) provided an updated outline of the family Magnaporthaceae with 20 genera, which included both sexual and asexual morphs. In this study, Mycoleptodiscus endophyticus is introduced as a new species.
Mycoleptodiscus endophyticus
Tibpromma & K.D. Hyde sp. nov.
MB823842
Figure 17.
Mycoleptodiscus endophyticus (MFLU 18-0001, holotype). a Colony on MEA media b, c Mycelia masses d–f Vegetative hyphae in culture. Scale bars: 10 μm (b–d), 5 μm (e, f).
Etymology.
Named after its original habitat as an endophytic fungus.
Holotype.
MFLU 18-0001
Culture characteristics.
Colonies on PDA (Figure 2, FE101), superficial, dark olivaceous with circular rings with filiform edge and rough and raised on media surface; reverse dark olivaceous. Mycelium composed of branched, pale-brown to dark-brown, thick-walled, guttulate, hyphae, with cells sub-globose to ovoid in shape. Not sporulating in culture.
Material examined.
THAILAND, Ranong, Muang, on healthy leaves of Freycinetia sp. (Pandanaceae), 3 December 2016, S. Tibpromma FE101 (MFLU 18-0001, holotype); HKAS100847, paratype, ex-type living cultures, MFLUCC 17-0545 = KUMCC 17-0263.
GenBank numbers.
Notes.
Mycoleptodiscus Ostaz. (1968) belongs to Magnaporthaceae, Magnaporthales. Since 1968, there have been 17 records of Mycoleptodiscus in Index Fungorum. Most of these species were described without molecular data. In this study, a new species Mycoleptodiscus endophyticus is introduced, based on culture characteristics and phylogenetic analysis (100 % in ML). Mycoleptodiscus endophyticus was found as an endophytic fungus on leaves of Freycinetia sp; Mycoleptodiscus freycinetiae Whitton, K.D. Hyde & McKenzie was found as a saprobic fungus on the same host but there was no molecular data available to confirm this identification. The authors were unable to compare the morphological differences between the new taxon and Mycoleptodiscus freycinetiae, because only culture characteristics are presented here for this new taxon (Fig. 17).
Figure 16.
Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, SSU and TEF1 sequenced data. Maximum parsimony bootstrap values are given above/below the nodes. The newly generated sequences are in red bold. The tree is rooted with Thyridium vestitum.
Sporocadaceae
Corda, 1842
Remarks.
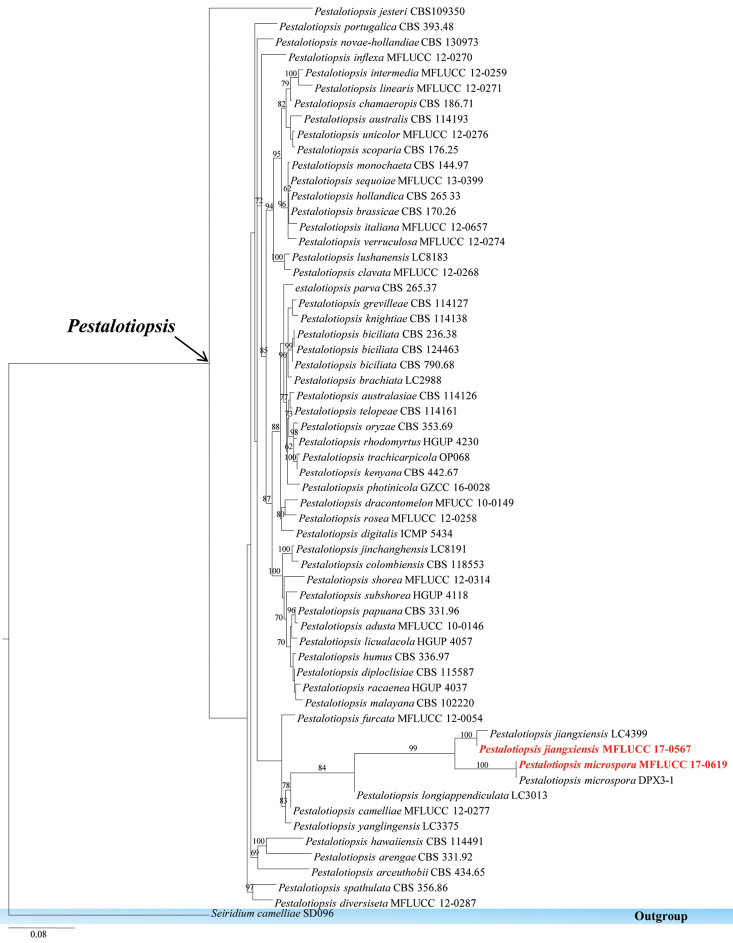
Sporocadaceae was introduced by Corda (1842) with Pestalotiopsis-like asexual morphs and confirmed by Senanayake et al. (2015). Members of Sporocadaceae are saprobes, endophytes or foliar pathogens in tropical and temperate regions (Jeewon et al. 2004; Tanaka et al. 2011). Pestalotiopsis can be found as saprobes or pathogens worldwide (Jeewon et al. 2002, 2003; Maharachchikumbura et al. 2011, 2012, 2013, 2014a, b, 2016a, c). Recently, Chen et al. (2017) provided updates for this genus based on morphology and phylogeny. In this study, two known species of Pestalotiopsis from Pandanaceae hosts were isolated.
Figure 18.
Phylogram generated from maximum likelihood analysis based on the combination of ITS, β-tubulin and TEF1 sequenced data. Maximum parsimony bootstrap is given above/below the nodes. The newly generated sequences are in red bold. The tree is rooted with Seiridium camelliae.
Pestalotiopsis jiangxiensis
F. Liu & L. Cai, 2017
Culture characteristics.
Colonies on PDA (Figure 2, PE05), superficial, white at the margin with yellow-white in the centre, with circular to undulate at the edge and raised and dense aerial mycelia on surface; reverse yellow-white. Sporulating in culture after 2 months.
GenBank numbers.
ITS=MG646966, ACT=MG646942, GAPDH=MG646934, β-tubulin=MG646929.
Notes.
The authors’ collection from Pandanaceae host in Thailand was identified as Pestalotiopsis jiangxiensis. This taxon grouped with Pestalotiopsis jiangxiensis LC4399 which is collected from Eurya sp., with high bootstrap support of 100% in ML.
Pestalotiopsis microspora
(Speg.) G.C. Zhao & N. Li, 1995
Culture characteristics.
Colonies on PDA (Figure 2, PE92), superficial, white to yellow-white, edge irregular, flossy and velvety; under surface yellow-white to yellow. Sporulating in culture after 2 months.
GenBank numbers.
Notes.
Pestalotiopsis microspora was isolated from a Pandanaceae host in Thailand. This strain clusters with Pestalotiopsis microspora DPX3-1 with a strong bootstrap support.
Saccharomycetes
Debaryomycetaceae
Kurtzman & M. Suzuki
Remarks.
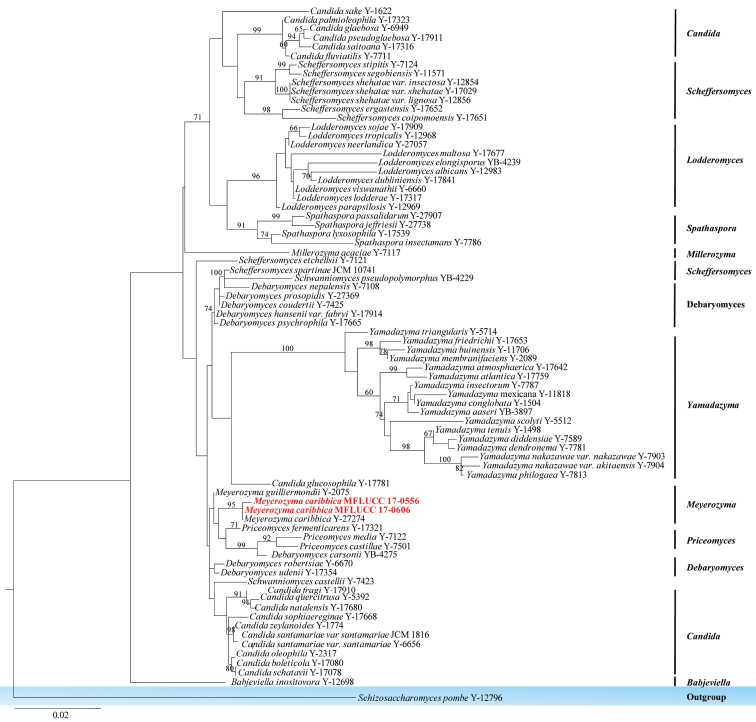
Debaryomycetaceae was introduced by Kurtzman and Suzuki in 2010 and was typified by Debaryomyces Klöcker. Meyerozyma belongs to family Debaryomycetaceae and was detailed in Kurtzman and Suzuki (2010). In this study, Meyerozyma caribbica was found on a Pandanaceae host as an endophytic fungus. Species identification was confirmed by DNA sequence data.
Figure 19.
Phylogram generated from maximum likelihood analysis based on combined LSU and SSU sequence data. Maximum parsimony bootstrap is given above/below the nodes. The newly generated sequences are in red text. The tree is rooted with Schizosaccharomyces pombe.
Meyerozyma caribbica
(Vaughan-Mart., Kurtzman, S.A. Mey. & E.B. O’Neill) Kurtzman & M. Suzuki, Mycoscience 51(1): 8 (2010)
Culture characteristics.
Colonies on PDA (Figure 2, PE75, 98), superficial, white to yellow-white, rings with irregular, undulate edge and curled, raised on the surface media; reverse yellow-white to yellow at the margin and dark-brown at the centre. Sporulating in culture after 2 months.
GenBank numbers.
MFLUCC 17-0556 ITS=MG646971, LSU=MG646950, SSU=MG646977. MFLUCC 17-0606 ITS=MG646972, LSU=MG646951, SSU=MG646980.
Notes.
Meyerozyma caribbica collected in this study is represented by two endophytic isolates from Pandanaceae. Phylogenetic analysis also supported the identification of this sample as Meyerozyma caribbica.
Conclusion
In this study on fungal endophytes found on leaves of Pandanaceae, it was found that the taxa belonged to both Ascomycota and Basidiomycota. The majority of the taxa were Ascomycota, as found in most previous endophytic studies (Crozier et al. 2006; Selim et al. 2017). In classical mycology, most endophytic fungi were described based on their morphological features (Barseghyan and Wasser 2010). However, there are difficulties in identifying ascomycetes to the species level based only on morphological features (Lu et al. 2012), because they have only a small set of morphological characteristics and exhibit homoplasy (Barseghyan and Wasser 2010).
The 22 endophytic fungal strains found in this study were chiefly identified using their microscopic characteristics and DNA sequence data and holotype materials in the form of dried cultures. Future studies are however needed to recollect the taxa which are sporulating to describe sexual and asexual characteristics (sensu Lacap et al. 2003). In this study, 22 endophytes were isolated and sorted into eight morphotype based on colony characteristics. The authors, however, subjected all isolates to phylogenetic analysis and found they belong to 16 different taxa. The taxa were sorted roughly into morphotypes, but they did not reflect the actual species. Several isolates of this study did not sporulate, but are introduced as new species because DNA sequence comparison and multi-gene phylogenetic analyses provided sufficient evidence to show that they are distinct taxa (Jeewon and Hyde 2016). The new taxa are, however, code compliant, as they are provided with MycoBank numbers, full descriptions, colour photographss and illustrations.
The species composition of endophytic microorganisms is likely to depend on the plant age, genotype, sampled tissue, host type and season of isolation (Rosenblueth and Martinez-Romero 2006). Promputtha et al. (2007) showed that endophytic species can change their ecological strategies and adopt a saprotrophic lifestyle. However, it was found that for the cultures of some endophytic fungal species, mycelia are the only visible morphological structures. According to these conclusions, the authors agree with Petrini (1991), Yanna and Hyde (2002), Ghimire and Hyde (2004) and Hyde et al. (2006) regarding the relationships between fungal endophytes and saprobic fungi. However, the use of next-generation sequencing (NGS) (Shendure and Ji 2008) is another option for identification of fungal species that cannot be cultured in vitro and has now become popular. These methods have also been applied to large-scale culture-independent molecular biological methods (Zoll et al. 2016). Future developments in technology are likely to produce further novel methods that mycologists could apply to the field of taxonomy (e.g. Hawksworth and Lucking 2017).
Supplementary Material
Acknowledgements
We would like to thank Molecular Biology Experimental Centre at Kunming Institute of Botany for their help with sequencing work. Saowaluck Tibpromma thanks the Mushroom Research Foundation (MRF), Chiang Rai, Thailand for financial support. Kevin D. Hyde thanks Mae Fah Luang University for the grant “Biodiversity, phylogeny and role of fungal endophytes of Pandanaceae” (Grant number: 592010200112) and the Chinese Academy of Sciences (project number 2013T2S0030), for the award of Visiting Professorship for Senior International Scientists, at Kunming Institute of Botany and Chiang Mai University. Andrew Stevenson, Fiona Worthy, Sajeewa Maharachchikumbura, Danushka Sandaruwan, Chada Norphanphoun, Asha Dissanayake, Ruvishika Jayawardena and Kasun Thambugala are thanked for their help and valuable suggestions. Samantha C. Karunarathna thanks Yunnan Provincial Department of Human Resources and Social Security funded postdoctoral project (number 179122) and National Science Foundation of China (NSFC) project code 31750110478. Peter E. Mortimer thanks the National Science Foundation of China (NSFC) project codes 41761144055 and 41771063. Fiona Worthy in the World Agroforestry Centre (ICRAF), Kunming Institute of Botany, China is thanked for English editing.
Citation
Tibpromma S, Hyde KD, Bhat JD, Mortimer PE, Xu J, Promputtha I, Doilom M, Jun-Bo Yang, Tang AMC, Karunarathna SC (2018) Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 33: 25–67. https://doi.org/10.3897/mycokeys.33.23670
Funding Statement
Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, People’s Republic of China
References
- Aly AH, Debbab A, Kjer J, Proksch P. (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Diversity 41: 1–16. 10.1007/s13225-010-0034-4 [DOI] [Google Scholar]
- Ariffin SA. (2013) The Antitumour Properties of Endophytic Fungi from Marine Plants in Malaysia. Doctoral dissertation, University of Otago.
- Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen YY, Monika CD, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Moncada S, Parnmen S, de Azevedo Santiago ALCM, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH. (2015a) Fungal diversity notes 111–252: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75: 1–248. 10.1007/s13225-015-0346-5 [DOI] [Google Scholar]
- Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Boonmee S, Wanasinghe DN, Phookamsak R, Hongsanan S, Singtripop C, Chukeatirote E, Kang JC, Jones EBG, Hyde KD. (2015b) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Diversity 71: 85–139. 10.1007/s13225-015-0323-z [DOI] [Google Scholar]
- Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA. (2000) Are tropical fungal endophytes hyperdiverse? Ecology letters 3: 267–274. 10.1046/j.1461-0248.2000.00159.x [DOI]
- Arnold AE, Lutzoni F. (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88: 541–549. 10.1890/05-1459 [DOI] [PubMed]
- Baayen RP, Bonants PJM, Verkley G, Carroll GC, Van Der Aa HA, De Weerdt M, Van Brouwershaven IR, Schutte GC, Maccheroni Jr W, de Blanco CG, Azevedo JL. (2002) Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology 92: 464–477. 10.1094/PHYTO.2002.92.5.464 [DOI] [PubMed] [Google Scholar]
- Barr ME. (1978) The Diaporthales of North America. Mycol. Mem. 7: 1–232. [Google Scholar]
- Barr ME. (1987) Prodromus to class Loculoascomycetes. University of Massachusetts, Amherst.
- Barseghyan GS, Wasser SP. (2010) Species diversity of hypogeous ascomycetes in Israel. Mycobiology 38: 159–165. 10.4489/MYCO.2010.38.3.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, Crous PW. (2012) The genus Cladosporium. Studies in Mycology 72: 1–401. 10.1016/S0166-0616(14)60070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Braun U, Dijksterhuis J, de Jesús Yáñez-Morales M, Crous PW. (2015) Common but different: The expanding realm of Cladosporium. Studies in Mycology 82: 23–74. 10.1016/j.simyco.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills GF. (1996) Isolation and analysis of endophytic fungal communities from woody plants. In: Redlin SC, Carris LM, St Paul MN. (Eds) Endophytic Fungi in Grasses and Woody Plants. APS Press USA, 31–65.
- Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH. (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105: 1350–1373. 10.3852/13-003 [DOI] [PubMed] [Google Scholar]
- Brown KB, Hyde KD, Guest DI. (1998) Preliminary studies on endophytic fungal communities of Musa acuminata species complex in Hong Kong and Australia. Fungal Diversity 1: 27–51. [Google Scholar]
- Bungihan ME, Tan MA, Kitajima M, Kogure N, Franzblau SG, dela Cruz TEE, Takayama H, Nonato MG. (2011) Bioactive metabolites of Diaporthe sp. P133, an endophytic fungus isolated from Pandanus amaryllifolius. Journal of Natural Medicines 65: 606–609. 10.1007/s11418-011-0518-x [DOI] [PubMed] [Google Scholar]
- Bungihan ME, Tan MA, Takayama H, Cruz DE, Nonato GM. (2013) A new macrolide isolated from the endophytic fungus Colletotrichum sp. Philippine. Science Letters 6: 57–73. [Google Scholar]
- Cannon PF. (1994) The newly recognized family Magnaporthaceae and its interrelationships. Systema Ascomycetum 13: 25–42. [Google Scholar]
- Cannon PF, Kirk PM. (2007) Fungal families world, 7th edn. CAB International, Wallingford. 10.1079/9780851998275.0000 [DOI]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes Mycologia: 553–556. 10.2307/3761358 [DOI]
- Carter E, Boudreaux C. (2004) Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. Journal of Clinical Microbiology 42: 5419–5423. 10.1128/JCM.42.11.5419-5423.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll GC. (1986) The biology of endophytism in plants with particular reference to woody perennials. In: Fokkema NJ, van den Heuvel J. (Eds) Microbiology of the Phyllosphere. Cambridge University Press, Cambridge, 205–222.
- Chen YY, Maharachchikumbura SSN, Liu JK, Hyde KD, Nanayakkara RR, Zhu GS, Liu ZY. (2017) Fungi from Asian Karst formations I. Pestalotiopsis photinicola sp. nov., causing leaf spots of Photinia serrulata. Mycosphere 8: 103–110. 10.5943/mycosphere/8/1/9 [DOI] [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN. (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. 10.1080/15572536.2003.11833157 [DOI] [PubMed] [Google Scholar]
- Chethana KWT, Phillips AJL, Zhang W, Chen Z, Hao YY, Hyde KD, Li XH, Yan JY. (2016) Mycosphere Essays 5: Is it important to name species of Botryosphaeriaceae? Mycosphere 7: 870–882. 10.5943/mycosphere/si/1b/3 [DOI]
- Chowdhary LK, Kaushik N. (2015) Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS One 10: 1–25. 10.1371/journal.pone.0141444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, Van der Bank M, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, Braun U. (2014) Fungal Planet description sheets: 214–280. Persoonia: Molecular Phylogeny and Evolution of Fungi 32: 184. 10.3767/003158514X682395 [DOI] [PMC free article] [PubMed]
- Crozier J, Thomas SE, Aime MC, Evans HC, Holmes KA. (2006) Molecular characterization of fungal endophytic morphospecies isolated from stems and pods of Theobroma cacao. Plant Pathology 55: 783–791. 10.1111/j.1365-3059.2006.01446.x [DOI] [Google Scholar]
- Dai DQ, Wijayawardene NN, Bhat DJ, Chukeatirote E, Bahkali AH, Zhao RL, Xu JC, Hyde KD. (2014) Pustulomyces gen. nov. accommodated in Diaporthaceae, Diaporthales, as revealed by morphology and molecular analyses. Cryptogamie Mycologie 35: 63–72. 10.7872/crym.v35.iss1.2014.63 [DOI] [Google Scholar]
- Daranagama DA, Thambugala KM, Campino B, Alves A, Bulgakov TS, Phillips AJL, Liu XZ, Hyde KD. (2016) Phaeobotryon negundinis sp. nov. (Botryosphaeriales) from Russia. Mycosphere 7: 933–941. 10.5943/mycosphere/si/1b/2 [DOI] [Google Scholar]
- David JC. (1997) A contribution to the systematics of Cladosporium. CABI International.
- Dickinson CH. (1976) Fungi on the aerial surfaces of higher plants. In: Preece TF, Dickinson CH. (Eds) Microbiology of Aerial Plant Surfaces. Academic Press, London, 293–324. 10.1016/B978-0-12-215050-0.50016-3 [DOI]
- Dissanayake AJ, Phillips AJL, Li XH, Hyde KD. (2016) Botryosphaeriaceae: Current status of genera and species. Mycosphere 7: 1001–1073. 10.5943/mycosphere/si/1b/13 [DOI] [Google Scholar]
- Eskandarighadikolaii S, Tee DC, Bungihan M. (2015) Antioxidant properties of fungal endophytes associated with the three medicinal plants Gliricidia sepium, Canna indica and Gardenia jasminoides. Journal of Scientific Research and Reports 6: 217–226. 10.9734/JSRR/2015/16272 [DOI] [Google Scholar]
- Feller IC. (1995) Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecological Monographs 65: 477–505. 10.2307/2963499 [DOI] [Google Scholar]
- Fisher PJ, Petrini O, Petrini LE, Sutton BC. (1994) Fungal endophytes from the leaves and twigs of Quercus ilex L. from England, Majorca and Switzerland. New Phytologist 127: 133–137. 10.1111/j.1469-8137.1994.tb04267.x [DOI] [PubMed] [Google Scholar]
- Fróhlich J, Hyde KD, Petrini O. (2000) Endophytic fungi associated with palms. Mycological Research 104: 1202–1212. 10.1017/S095375620000263X [DOI] [Google Scholar]
- Garcia-Reyne A, Lopez-Medrano F, Morales JM, Esteban CG, Martin I. (2011) Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: first report of human infection by this fungus. Transplant Infectious Disease 13: 204–207. 10.1111/j.1399-3062.2010.00570.x [DOI] [PubMed] [Google Scholar]
- Ghimire SR, Hyde KD. (2004) Fungal endophytes. In: Varma A, Abbott L, Werner D, Hampp R. (Eds) Plant Surface Microbiology. Springer-Verlag, Berlin, 281–292.
- Glass NL, Donaldson GC. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunatilaka AL. (2006) natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. Journal of Natural Products 69: 509–526. 10.1021/np058128n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LD, Hyde KD, Liew EC. (2000) Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytologist 147: 617–630. 10.1046/j.1469-8137.2000.00716.x [DOI] [PubMed] [Google Scholar]
- Guo LD, Huang GR, Wang Y, He WH, Zheng WH, Hyde KD. (2003) Molecular identification of white morphotype strains of endophytic fungi from Pinus tabulaeformis. Mycological Research 107: 680–688. 10.1017/S0953756203007834 [DOI] [PubMed] [Google Scholar]
- Hall T. (2004) Bioedit version 6.0.7. http://www.mbio.-ncsu.edu/bioedit/bioedit.html
- Hawksworth DL. (2000) The magnitude of fungal diversity: the 1.5 million species estimate revisited, Paper presented at the Asian Mycological Congress 2000 (AMC 2000), incorporating the 2nd Asia-Pacific Mycological Congress on Biodiversity and Biotechnology, and held at the University of Hong Kong on 9-13 July 2000. Mycological Research 105: 1422–1432. 10.1017/S0953756201004725 [DOI] [Google Scholar]
- Hawksworth DL, Lucking R. (2017) Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiology spectrum 5. 10.1128/microbiolspec.FUNK-0052-2016 [DOI] [PMC free article] [PubMed]
- Hennings P. (1908) Fungi S. Paulenses IV a cl. Puttmans collecti. Hedwigia 48: 13
- Ho WH, Hyde KD. (2002) Fungal succession on fronds of Phoenix hanceana in Hong Kong. Fungal Diversity 10: 185–211. [Google Scholar]
- Hyde KD, Bussaban B, Paulus B, Crous PW, Lee S, McKenzie EHC, Photita W, Lumyong S. (2006) Biodiversity of saprobic fungi. Biodiversity and Conservation 16: 7–35. 10.1007/s10531-006-9119-5 [DOI] [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monka J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Begoña AH, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukea E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JK, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, Mckenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zang M. (2013) Families of Dothideomycetes. Fungal Diversity 63: 1–313. 10.1007/s13225-013-0263-4 [DOI] [Google Scholar]
- Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li XH, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N. (2014) One stop shop:backbones trees for important phytopathogenic genera: I. Fungal Diversity 67: 21–125. 10.1007/s13225-014-0298-1 [DOI] [Google Scholar]
- Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo1 A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka1 AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kusan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, de Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castaneda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones EBG, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz Jr. HL, Lumyong S, Maharachchikumbura SSN, Matocec N, McKenzie EHC, Mesic A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AL, Ryvarden L, Su HY, Suetrong S, Tkalcec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE. (2017) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Diversity 87: 1–235. 10.1007/s13225-017-0391-3 [DOI] [Google Scholar]
- Hyde KD, Soytong K. (2008) The fungal endophyte dilemma. Fungal Diversity 33: 163–173. [Google Scholar]
- Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang KA, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q, Kang JC, Promputtha I. (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Diversity 74: 3–18. 10.1007/s13225-015-0351-8 [DOI] [Google Scholar]
- Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY. (2016) Notes on currently accepted species of Colletotrichum. Mycosphere 7: 1192–1260. 10.5943/mycosphere/si/2c/9 [DOI] [Google Scholar]
- Jeewon R, Liew ECY, Hyde KD. (2002) Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Molecular Phylogenetics and Evolution 25: 378–392. 10.1016/S1055-7903(02)00422-0 [DOI] [PubMed] [Google Scholar]
- Jeewon R, Liew ECY, Simpson JA, Hodgkiss IJ, Hyde KD. (2003) Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution 27: 372–383. 10.1016/S1055-7903(03)00010-1 [DOI] [PubMed] [Google Scholar]
- Jeewon R, Liew ECY, Hyde KD. (2004) Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity 17: 39–55. [Google Scholar]
- Jeewon R, Hyde KD. (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7(11): 1669–1677. 10.5943/mycosphere/7/11/4 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2016) A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 32: 1933–1942. 10.1093/bioinformatics/btw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. (2001) Ainsworth and Bisby’s dictionary of the fungi (No. Ed. 9). CABI publishing.
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. (2008) Dictionary of the Fungi. 10th edition. CABI, Wallingford: 1–771.
- Ko TWK, Stephenson SL, Bahkali AH, Hyde KD. (2011) From morphology to molecular biology: can we use sequence data to identify fungal endophytes? Fungal Diversity 50: 113–120. 10.1007/s13225-011-0130-0 [DOI]
- Konta S, Phillips AJL, Bahkali AH, Jones EBG, Eungwanichayapant DP, Hyde KD, Boonmee S. (2016a) Botryosphaeriaceae from palms in Thailand- Barriopsis archontophoenicis sp. nov, from Archontophoenix alexandrae. Mycosphere 7: 921–932. 10.5943/mycosphere/si/1b/1 [DOI] [Google Scholar]
- Konta S, Hongsanan S, Phillips AJ, Jones EBG, Boonmee S, Hyde KD. (2016b) Botryosphaeriaceae from palms in Thailand II-two new species of Neodeightonia, N. rattanica and N. rattanicola from Calamus (rattan palm). Mycosphere 7: 950–961. 10.5943/mycosphere/si/1b/6 [DOI] [Google Scholar]
- Kornerup A, Wanscher JH. (1967) Methuen Handbook of Colour, 2nd edn. Methuen & Co. London, England.
- Kurtzman CP, Suzuki M. (2010) Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 51: 2–14. 10.1007/S10267-009-0011-5 [DOI] [Google Scholar]
- Lacap DC, Hyde KD, Liew ECY. (2003) An evaluation of the fungal ‘morphotype’ concept based on ribosomal DNA sequences. Fungal Diversity 12: 53–66. [Google Scholar]
- Liew ECY, Aptroot A, Hyde KD. (2002) An evaluation of the monophyly of Massarina based on ribosomal DNA sequences. Mycologia 94: 803–813. 10.1080/15572536.2003.11833174 [DOI] [PubMed] [Google Scholar]
- Lin X, Huang YJ, Zheng ZH, Su WJ, Qian XM, Shen YM. (2010) Endophytes from the pharmaceutical plant, Annona squamosa: isolation, bioactivity, identification and diversity of its polyketide synthase gene. Fungal Diversity 41: 41–51. 10.1007/s13225-010-0017-5 [DOI] [Google Scholar]
- Linaldeddu BT, Deidda A, Scanu B, Franceschini A, Alves A, Abdollahzadeh J, Phillips AJL. (2016a) Phylogeny, morphology and pathogenicity of Botryosphaeriaceae, Diatrypaceae and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). European Journal of Plant Pathology 146: 259–279. 10.1007/s10658-016-0912-z [DOI] [Google Scholar]
- Linaldeddu BT, Alves A, Phillips AJL. (2016b) Sardiniella urbana gen. et sp. nov., a new member of the Botryosphaeriaceae isolated from declining Celtis australis trees in Sardinian streetscapes. Mycosphere 7: 893–905. 10.5943/mycosphere/si/1b/5 [DOI] [Google Scholar]
- Linaldeddu BT, Maddau L, Franceschini A, Alves A, Phillips AJL. (2016c) Botryosphaeriaceae species associated with lentisk dieback in Italy and description of Diplodia insularis sp. nov. Mycosphere 7: 962–977. 10.5943/mycosphere/si/1b/8 [DOI] [Google Scholar]
- Liu F, Weir BS, Damm U, Crous PW, Wang Y, Liu B, Wang M, Zhang M, Cai L. (2015) Unravelling Colletotrichum species associated with Camellia: employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 35: 63–86. 10.1016/j.funbio.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jeewon R, Phillips AJ, Maharachchikumbura SS, Ryberg M, Liu ZY, Zhao Q. (2017) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Diversity 84: 75–99. 10.1007/s13225-017-0385-1 [DOI] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 6: 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Fisher PJ, Sutton BC. (1996) Endophytic fungi of Manilkara bidentata leaves in Puerto Rico. Mycologia 88: 733–738. 10.2307/3760967 [DOI] [Google Scholar]
- Lu Y, Chen C, Chen H, Zhang J, Chen W. (2012) Isolation and identification of endophytic fungi from Actinidia macrosperma and investigation of their bioactivities. Evidence-Based Complementary and Alternative Medicine Article ID 382742. 10.1155/2012/382742 [DOI] [PMC free article] [PubMed]
- Lumbsch HT, Huhndorf SM. (2007) Outline of ascomycota–2007. Myconet 13: 1–58. [Google Scholar]
- Lumyong S, Techa W, Lumyong P, McKenzie EHC, Hyde KD. (2009) Endophytic fungi from Calamus kerrianus and Wallichia caryotoides (Arecaceae) at Doi Suthep-Pui National Park, Thailand. Chiang Mai Journal Science 36: 158–167. [Google Scholar]
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD. (2011) Pestalotiopsis–morphology, phylogeny, biochemistry and diversity. Fungal Diversity 50: 167–187. 10.1007/s13225-011-0125-x [DOI] [Google Scholar]
- Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, McKenzie EHC, Bahkali AH, Hyde KD. (2012) A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Diversity 56: 95–129. 10.1007/s13225-012-0198-1 [DOI] [Google Scholar]
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, McKenzie EHC, Hyde KD. (2013) A destructive new disease of Syzygium samarangense in Thailand caused by the new species Pestalotiopsis samarangensis. Tropical Plant Pathology 38: 227–235. 10.1590/S1982-56762013005000002 [DOI] [Google Scholar]
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, Hyde KD. (2014a) Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycological Progress 13: 617–624. 10.1007/s11557-013-0944-0 [DOI] [Google Scholar]
- Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW. (2014b) Pestalotiopsis revisited. Studies in Mycology 79: 121–186. 10.1016/j.simyco.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharachchikumbura SS, Hyde KD, Jones EG, McKenzie EH, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S. (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Diversity 72: 199–301. 10.1007/s13225-015-0331-z [DOI] [Google Scholar]
- Maharachchikumbura SSN, Guo LD, Liu ZY, Hyde KD. (2016a) Pseudopestalotiopsis ignota and Ps. camelliae spp. nov. associated with grey blight disease of tea in China. Mycological Progress 15: 22. 10.1007/s11557-016-1162-3 [DOI]
- Maharachchikumbura SS, Hyde KD, Jones EG, McKenzie EH, Bhat JD, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ. (2016b) Families of Sordariomycetes. Fungal Diversity 79: 1–317. 10.1007/s13225-016-0369-6 [DOI] [Google Scholar]
- Maharachchikumbura SSN, Larignon P, Hyde KD, Al-Sadi AM, Liu YZ. (2016c) Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathologia Mediterranea 55: 380–390. 10.14601/Phytopathol_Mediterr-18298 [DOI] [Google Scholar]
- Manawasinghe IS, Phillips AJL, Hyde KD, Chethana KWT, Zhang W, Zhao WS, Yan JY, Li XH. (2016) Mycosphere Essays 14: Assessing the aggressiveness of plant pathogenic Botryosphaeriaceae. Mycosphere 7: 883–892. 10.5943/mycosphere/si/1b/7 [DOI] [Google Scholar]
- McKenzie EHC, Whitton SR, Hyde KD. (2002) The Pandanaceae-does it have a diverse and unique fungal biota. Tropical Mycology 2: 51–61. 10.1079/9780851995434.0051 [DOI] [Google Scholar]
- Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY. (2012) Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29: 11–28. 10.3767/003158512X658766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi K, Inaba S, Anzai K, Takamatsu S, Nakashima C. (2009) Phylogenetic analyses of Japanese species of Phyllosticta sensu stricto. Mycoscience 50: 291–302. 10.1007/S10267-009-0487-Z [DOI] [Google Scholar]
- Munk A. (1956) On Metasphaeria coccodes (Karst.) Sacc. and other fungi probably related to Massarina Sacc. (Massarinaceae n. fam.). Friesia 5: 303–308. [Google Scholar]
- Myllys L, Stenroos S, Thell A. (2002) New genes for phylogenetic studies of lichenized fungi: glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. Lichenologist 34: 237–246. 10.1006/lich.2002.0390 [DOI] [Google Scholar]
- Nitschke TRJ. (1869) Pleosporaceae. Verh. Naturhist. Ver Preuss Rheinl 26: 74.
- O’Donnell K, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Okane I, Nakagiri A, Ito T, Lumyong S. (2003) Extensive host range of an endophytic fungus, Guignardia endophyllicola (anamorph: Phyllosticta capitalensis). Mycoscience 44: 353–363. 10.1007/S10267-003-0128-X [DOI] [Google Scholar]
- Petrini O. (1991) Fungal endophytes of tree leaves. Microbial ecology of leaves. Springer, New York, NY, 179–197. 10.1007/978-1-4612-3168-4_9 [DOI]
- Photita W, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD. (2004) Are some endophytes of Musa acuminata latent pathogens? Fungal Diversity 16: 131–140.
- Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW. (2013) The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51–167. 10.3114/sim0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihastuti H, Cai L, Chen H, McKenzie EH, Hyde KD. (2009) Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity 39: 89–109. [Google Scholar]
- Promputtha I, Jeewon R, Lumyong S, McKenzie EHC, Hyde KD. (2005) Ribosomal DNA fingerprinting in the identification of non sporulating endophytes from Magnolia liliifera (Magnoliaceae). Fungal Diversity 20: 167–186. [Google Scholar]
- Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EHC, Hyde KD, Jeewon R. (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microbial Ecology 53: 579–590. 10.1007/s00248-006-9117-x [DOI] [PubMed] [Google Scholar]
- Rajulu MB, Thirunavukkarasu N, Suryanarayanan TS, Ravishankar JP, El Gueddari NE, Moerschbacher BM. (2011) Chitinolytic enzymes from endophytic fungi. Fungal Diversity 47: 43–53. 10.1007/s13225-010-0071-z [DOI] [Google Scholar]
- Rambaut A, Drummond A. (2008) FigureTree: Tree Figures drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh.
- Réblová M, Gams W, Seifert KA. (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68: 163–191. 10.3114/sim.2011.68.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner SA. (2001) Primers for elongation factor 1-alpha (EF1-alpha). http://ocid.nacse.org/research/deephyphae/EF1primer.pdf
- Rodrigues KF. (1994) The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 86: 376–385. 10.2307/3760568 [DOI] [Google Scholar]
- Rojas EI, Herre EA, Mejia LC, Arnold AE, Chaverri P, Samuels GJ. (2008) Endomelanconiopsis, a new anamorph genus in the Botryosphaeriaceae. Mycologia 100: 760–775. 10.3852/07-207 [DOI] [PubMed] [Google Scholar]
- Rosenblueth M, Martinez-Romero E. (2006) Bacterial endophytes and their interactions with hosts. Acta Pharmacologica Sinica 19: 827–837. 10.1094/MPMI-19-0827 [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Saari S, Helander M. (2010) Defensive mutualism between plants and endophytic fungi? Fungal Diversity 41: 101–113. 10.1007/s13225-010-0023-7 [DOI]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. 10.1080/15572536.2006.11832632 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys Å, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW. (2009) A class–wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. 10.3114/sim.2009.64.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim KA, Nagia MM, Ghwas DEE. (2017) Endophytic fungi are multifunctional biosynthesizers: ecological role and chemical diversity. Endophytic fungi: diversity, characterization and biocontrol 39.
- Senanayake IC, Maharachchikumbura SS, Hyde KD, Bhat JD, Jones EG, McKenzie EH, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S. (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Diversity 73: 73–144. 10.1007/s13225-015-0340-y [DOI] [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SSN, Jeewon R, Phillips AJL, Bhat JD, Perera RH, Li QR, Li WJ, Tangthirasunun N. (2017) Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. 10.1016/j.simyco.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendure J, Ji H. (2008) Next-generation DNA sequencing. Nature Biotechnology 26: 1135–1145. 10.1038/nbt1486 [DOI] [PubMed] [Google Scholar]
- Sivanesan A. (1984) The Bitunicate Ascomycetes and their Anamorphs. Strauss and Cramer. Vaduz, Germany.
- Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess T, Crous PW. (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Studies in Mycology 76: 31–49. 10.3114/sim0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Wingfield MJ. (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews 21: 90–106. 10.1016/j.fbr.2007.06.002 [DOI] [Google Scholar]
- Stone JK, Bacon CW, White JF. (2000) An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF (Eds) Microbial endophytes, Marcel Dekker Inc., New York.
- Strobel G, Daisy B, Castillo U, Harper J. (2004) Natural products from endophytic microorganisms. Journal of Natural Products 67: 257–268. 10.1021/np030397v [DOI] [PubMed] [Google Scholar]
- Tanaka K, Endo M, Hirayama K, Okane I, Hosoya T, Sato T. (2011) Phylogeny of Discosia and Seimatosporium, and introduction of Adisciso and Immersidiscosia genera nova. Persoonia 26: 85–98. 10.3767/003158511X576666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T. (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in mycology 82: 75–136. 10.1016/j.simyco.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Hyde KD, Jones EBG. (1999) Endophytic fungi associated with the temperate palm Trachycarpus fortunei both within and outside of its natural geographic range. New Phytologist 142: 335–346. 10.1046/j.1469-8137.1999.00391.x [DOI] [Google Scholar]
- Templeton AR, Crandall KA, Sing CF. (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research 25: 4875–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongkantha S, Jeewon R, Vijaykrishna D, Lumyong S, McKenzie EHC, Hyde KD. (2009) Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species, Ophioceras chiangdaoensis from Dracaena loureiri in Thailand. Fungal Diversity 34: 157.
- Thongkantha S, Lumyong S, McKenzie EHC, Hyde KD. (2008) Fungal saprobes and pathogens occurring on tissues of Dracaena loureiri and Pandanus spp. in Thailand. Fungal Diversity 30: 149–169. [Google Scholar]
- Tibpromma S, Boonmee S, Wijayawardene NN, Maharachchikumbura SSN, McKenzie EHC, Bahkali AH, Jones EBG, Hyde KD, Itthayakorn P. (2016a) The holomorph of Parasarcopodium (Stachybotryaceae), introducing P. pandanicola sp. nov. on Pandanus sp. Phytotaxa 266: 250–260. 10.11646/phytotaxa.266.4.2 [DOI] [Google Scholar]
- Tibpromma S, Bhat J, Doilom M, Lumyong S, Nontachaiyapoom S, Yang JB, Hyde KD. (2016b) Three new Hermatomyces species (Lophiotremataceae) on Pandanus odorifer from Southern Thailand. Phytotaxa 275: 127–139. 10.11646/phytotaxa.275.2.4 [DOI] [Google Scholar]
- Tibpromma S, McKenzie EHC, Karunarathna SC, Mortimer PE, Xu J, Hyde KD, Hu DM. (2016c) Muyocopron garethjonesii sp. nov. (Muyocopronales, Dothideomycetes) on Pandanus sp. Mycosphere 7: 1480–1489. 10.5943/mycosphere/7/9/19 [DOI] [Google Scholar]
- Tibpromma S, Daranagama DA, Boonmee S, Promputtha I, Nontachaiyapoom S, Hyde KD. (2017a) Anthostomelloides krabiensis gen. et sp. nov. (Xylariaceae) from Pandanus odorifer (Pandanaceae). Turkish Journal of Botany 41: 107–116. 10.3906/bot-1606-45 [DOI] [Google Scholar]
- Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo Santiago ALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Mešić A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz Jr HL, Ariyawansa HA, Lee H, Kušan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšík M, Samarakoon MC, Wijayawardene NN, Kim NK, Matočec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Svetasheva STY, Nguyen TTT, Antonín V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsa-ard J, Xu JC, Yan J, Chuan KJ, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC. (2017b) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83: 1–261. 10.1007/s13225-017-0378-0 [DOI] [Google Scholar]
- Udayanga D, Liu X, McKenzie EHC, Chukeatirote E, Bahkali AH, Hyde KD. (2011) The genus Phomopsis: biology, applications, species concepts and names of common pathogens. Fungal Diversity 50: 189–225. 10.1007/s13225-011-0126-9 [DOI] [Google Scholar]
- Udayanga D, Liu XZ, Crous PW, McKenzie EHC, Chukeatirote E, Hyde KD. (2012) A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Diversity 56: 157–171. 10.1007/s13225-012-0190-9 [DOI] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD. (2014) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Diversity 67: 203–229. 10.1007/s13225-014-0297-2 [DOI] [Google Scholar]
- Umali TE, Quimio TH, Hyde KD. (1999) Endophytic fungi in leaves of Bambusa tuldoides. Fungal science 14: 11–18. [Google Scholar]
- Uppal BN, Patel MK, Kamat MN. (1938) Alternaria blight of Cumin. Indian Journal of Agricultural Science 8: 49–62. [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. (2014) Stilbosporaceae resurrected: generic reclassification and speciation. Persoonia 33: 61–82. 10.3767/003158514X684212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer LE. (1975) The Pyrenomycetous Fungi. Mycologia Memoirs 6: 1–250. [Google Scholar]
- Weir BS, Johnston PR, Damm U. (2012) The Colletotrichum gloeosporioides species complex. Studies in Mycology. 73: 115–180. 10.3114/sim0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lucking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castaneda-Ruiz RF, Bahkali AH, Pang KL, Tanaka K, Dai DQ, Sakayaroj J, Hujslova M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Caceres MES, Perez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel-Wahab MA, Takamatsu S, Bensch K, de Silva NI, Kesel AD, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera RH, Tang LZ, Phukhamsakda C, Hernandez-Restrepo M, Ma X, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC. (2017) Notes for genera: Ascomycota. Fungal Diversity 86: 1–594. 10.1007/s13225-017-0386-0 [DOI] [Google Scholar]
- Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EHC, Hyde KD, Crous PW. (2013) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Studies in Mycology 76: 1–29. 10.3114/sim0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg JHC, Seidl MF, Groenewald JZ, de Vries M, Stielow JB, Thomma BPHJ, Crous PW. (2015) Alternaria section Alternaria: Species, formae speciales or pathotypes? Studies in Mycology 82: 1–21. 10.1016/j.simyco.2015.07.001 [DOI] [PMC free article] [PubMed]
- Wyka SA, Broders KD. (2016) The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biology 120: 1030–1040. 10.1016/j.funbio.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Yang T, Groenewald JZ, Cheewangkoon R, Jami F, Abdollahzadeh J, Lombard L, Crous PW. (2017) Families, genera and species of Botryosphaeriales. Fungal Biology 121: 322–346. 10.1016/j.funbio.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Zalar P, de Hoog GS, Schroers HJ, Crous PW, Groenewald JZ, Gunde-Cimerman N. (2007) Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157–183. 10.3114/sim.2007.58.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, He W, Wu JR, Zhang Y. (2017) Two new species of Spencermartinsia (Botryosphaeriaceae, Botryosphaeriales) from China. Mycosphere 7: 942–949. [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH. (2006) An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1087. 10.1080/15572536.2006.11832635 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD. (2009) Multi–locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. 10.3114/sim.2009.64.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012) Pleosporales. Fungal Diversity 53: 1–221. 10.1007/s13225-011-0117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Hyde KD. (2001) Host-specificity, host-exclusivity, and host-recurrence in saprobic fungi. Mycological Research 105: 1449–1457. 10.1017/S0953756201004713 [DOI] [Google Scholar]
- Zoll J, Snelders E, Verweij PE, Melchers WJ. (2016) Next-generation sequencing in the mycology lab. Current Fungal Infection Reports 10: 37–42. 10.1007/s12281-016-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.