Abstract
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. Advancing age is a major risk factor for developing cardiovascular disease because of the lifelong exposure to cardiovascular risk factors and specific alterations affecting the heart and the vasculature during ageing. Indeed, the ageing heart is characterized by structural and functional changes that are caused by alterations in fundamental cardiomyocyte functions. In particular, the myocardium is heavily dependent on mitochondrial oxidative metabolism and is especially susceptible to mitochondrial dysfunction. Indeed, primary alterations in mitochondrial function, which are subsequently amplified by defective quality control mechanisms, are considered to be major contributing factors to cardiac senescence. In this Review, we discuss the mechanisms linking defective mitochondrial quality control mechanisms (that is, proteostasis, biogenesis, dynamics, and autophagy) to organelle dysfunction in the context of cardiac ageing. We also illustrate relevant molecular pathways that might be exploited for the prevention and treatment of age-related heart dysfunction
Cardiovascular disease is the main cause of morbidity and mortality in the general population and is especially prevalent among older adults1. Approximately 20% of people aged ≥80 years are at risk of heart failure, and those aged ≥65 years are at high risk of atrial fibrillation and related stroke1. Indeed, advancing age is associated with the progressive degeneration of blood vessels and the heart, making them more vulnerable to stressors and contributing to increased morbidity and mortality2. In particular, the aged heart has increased mass, ventricular wall thickness, and cardiomyocyte cross-sectional area despite decreased cell number3–5.
Aged cardiomyocytes show abnormalities in mitochondrial structure (enlarged organelles, matrix derangement, and loss of cristae) and increased generation of reactive oxygen species (ROS)6. These modifications are, in turn, associated with functional impairments at the organ and whole-body levels, including diastolic dysfunction, left ventricular hypertrophy, increased risk of atrial fibrillation, valvular degeneration, and decreased exercise capacity7. As such, alterations in mitochondrial function, amplified by dysfunctional quality control mechanisms, are considered to be major contributing factors to heart senescence8.
In this Review, we discuss the mechanisms linking defective mitochondrial quality control (MQC) (FIG. 1) to organelle dysfunction in the context of cardiac ageing. Relevant molecular pathways that might be exploited for the prevention and treatment of age-related heart dysfunction are also summarized.
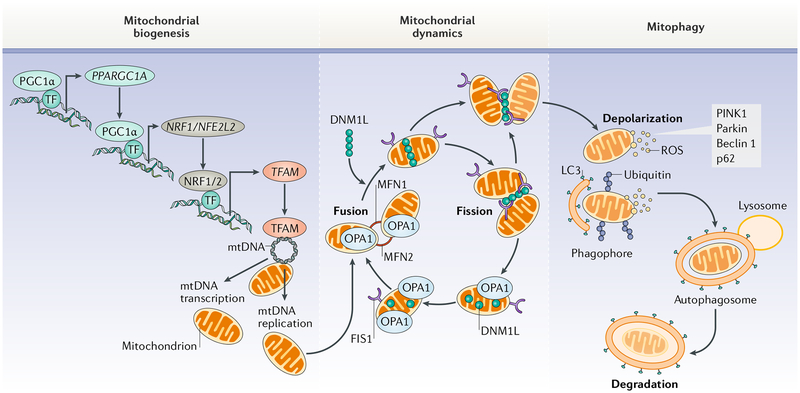
Fig. 1 |. Mitochondrial quality control pathways.
Mitochondrial homeostasis is ensured through the coordination of mitochondrial biogenesis, dynamics, and autophagy. After appropriate stimuli (such as exercise stimulus), the upregulation of proliferator-activated receptor- γ co-activator 1α (PGC1α) and other transcription factors (TFs) activates the transcription of nuclear genes encoding mitochondrial proteins such as mitochondrial transcription factor A (TFAM). TFAM is then imported into mitochondria by the protein import machinery and reaches its final destination on mitochondrial DNA (mtDNA). Here, TFAM upregulates the expression of genes encoding electron transport chain subunits, resulting in increased oxygen consumption, ATP synthesis, and mitochondrial content. Changes in mitochondrial morphology are under the control of fusion (mitofusin 1 (MFN1), MFN2, and mitochondrial dynamin-like 120 kDa protein (OPA1)) and fission (dynamin 1-like protein (DNM1L) and mitochondrial fission 1 protein (FIS1)) proteins. These factors regulate mitochondrial turnover by facilitating the dilution and clearance of damaged organelles. Mitochondrial components are eventually recycled through a specialized autophagic pathway, known as mitophagy. LC3,microtubule-associated proteins 1A/1B light chain 3; NRF1, nuclear respiratory factor 1; NRF2, nuclear factor erythroid 2-related factor 2; p62, sequestosome 1; parkin, E3 ubiquitin-protein ligase parkin; PINK1, serine/threonine-protein kinase PINK1; ROS, reactive oxygen species.
Cellular senescence and cardiac ageing
An increase in the number of cardiac, muscular, endothelial, and endothelial progenitor senescent cells occurs in several disease conditions associated with cardiovascular dysfunction, including hypertension, atherosclerosis, heart failure, and stroke9. Cellular senescence is characterized by genome instability (that is, nuclear DNA damage), telomere attrition, and mitochondrial dysfunction8. In particular, genome instability and telomere attrition are underlying causes of molecular damage and are indicated as primary hallmarks of cell senescence8 (BOX 1). Conversely, antagonistic hallmarks in the ageing heart include mitochondrial dysfunction and cellular senescence, which exert beneficial or protective effects at low levels but are deleterious at high levels8. Finally, when the accumulating damage cannot be compensated for by homeostatic mechanisms, stem-cell exhaustion and altered intercellular communication occur, contributing to ageing (integrative hallmarks)8 (BOX 1).
Box 1 |. Hallmarks of senescence in cardiac ageing.
Primary hallmarks of ageing (the cause of molecular damage):
Genome instability
Telomere shortening
Secondary hallmarks of ageing (which arise as a response to damage to mitigate the insult, but their persistence has detrimental effects):
Mitochondrial dysfunction
Oxidative stress
Systemic inflammation
Mitochondrial dysfunction is a core feature of ageing and forms the crossroads for several pathways related to senescence10. For instance, genomic instability causes metabolic disarrangements that favour cellular senescence and organismal ageing through a complex response to persistent DNA damage11. This response affects pathways that regulate bioenergetic metabolism and block cell anabolism induced by insulin, insulin-like growth factor I (IGF1), and somatotropin12.
Similarly, telomere attrition promotes activation of cellular tumour antigen p53 and inhibits m itochondrial biogenesis and function in mice deficient in either telomerase reverse transcriptase (Tert−/−) or telomerase RNA component (Terc−/−)13. Therefore, telomere shortening might limit mitochondrial turnover despite age-related metabolic perturbations. In humans, leukocyte telomere length negatively correlates with γ-glutamyltyrosine and γ-glutamylphenylalanine, two markers of oxidative stress14. Conversely, lysolipids have been found to be associated with phospholipase A2 expression, which might indicate poor membrane fluidity14. Furthermore, deficiency in the recycling autophagic machinery contributes to cell senescence through the accumulation of intracellular toxic waste15.
Mitochondrial-derived oxidative stress is thought to have a pivotal role in the development of age-related conditions through irreversible damage to macromolecules and bioenergetic failure16,17. In addition, the activation of redox-sensitive mediators, including nuclear factor-κB (NF-κB), modulates the transcription of several pro-inflammatory cytokines18. Under normal conditions, transient NF-κB activation in response to oxidative stimuli ceases with resolution of the inflammatory reaction18. However, long-term exposure to high levels of oxidants, as occurs during ageing, results in chronic activation of the NF-κB-mediated inflammatory response and cellular damage18. A set of NF-κB-responsive cytokines and chemokines has been identified as part of the senescence-associated secretory phenotype (SASP)19 (FIG. 2). An increase in inflammatory mediators leads to the expression of inflammatory proteins involved in extracellular matrix remodelling and of pro-inflammatory cytokines (such as tumour necrosis factor, IL-1α, IL-1β, and IL-6). SASP mediators or their products (such as inducible nitric oxide synthase, cytochrome c oxidase, and prostaglandin E2) are prominent sources of ROS20,21. Indeed, endothelial damage, vascular smooth muscle cell proliferation, and extracellular matrix remodelling are associated with the genesis and progression of atherosclerosis and hypertension22,23.
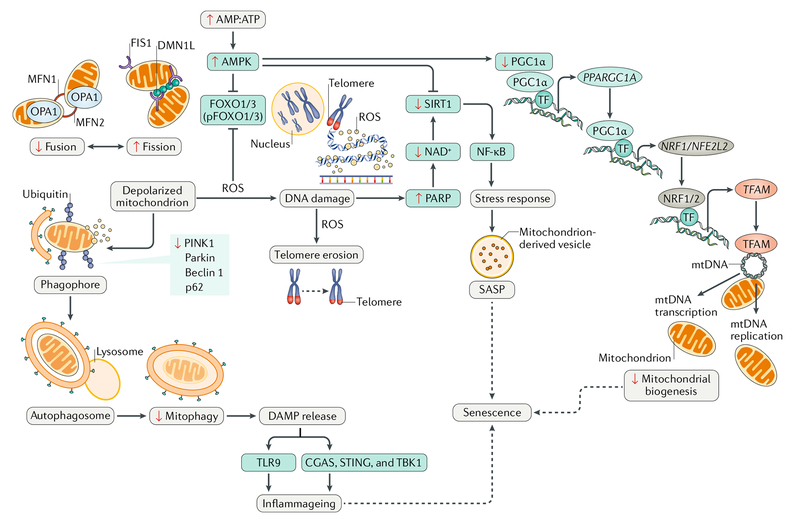
Fig. 2 |. Nucleus–mitochondrion crosstalk during cardiac ageing.
The age-related surge in generation of reactive oxygen species (ROS) arising primarily from the accumulation of dysfunctional mitochondria causes nuclear DNA damage and activates 5′-AMP-activated protein kinase (AMPK) signalling. The latter, in turn, inhibits NAD-dependent protein deacetylase sirtuin 1 (SIRT1) activity. The decrease in NAD+ levels in the setting of oxidative stress further affects SIRT1, resulting in decreased levels of proliferator-activated receptor- γ co-activator 1α (PGC1α), leading to a decline in mitochondrial biogenesis; upregulation of nuclear factor- κB (NF- κB), leading to inflammation; and decreased expression and phosphorylation of forkhead box protein O1 (FOXO1) and FOXO3, transcription factors (TFs) that participate in cytoprotection. This multi-pathway derangement leads to cellular stress, induction of the senescence-associated secretory pathway (SASP), and senescence. CGAS, cGMP-AMP synthase; DAMP, damage-associated molecular pattern; DNM1L, dynamin 1-like protein; FIS1, mitochondrial fission 1 protein; MFN, mitofusin; mtDNA, mitochondrial DNA; NRF1, nuclear respiratory factor 1; NRF2, nuclear factor erythroid 2-related factor 2; OPA1, mitochondrial dynamin-like 120 kDa protein; p62, sequestosome 1; PARP, poly[ADP-ribose] polymerase; parkin, E3 ubiquitin-protein ligase parkin; PINK1, serine/threonine-protein kinase PINK1; STING, stimulator of interferon genes protein; TBK1, serine/threonine-protein kinase TBK1; TFAM, mitochondrial transcription factor A; TLR9, Toll-like receptor 9.
Increases in pro-inflammatory mediators are necessary for senescent cell removal. However, accumulation of senescent cells is paralleled by stem-cell exhaustion and loss of function of regenerative cell lineages, which contribute to ageing24,25. The senescent state is under the control of two complex pathways (p53–p27 (cyclin-dependent kinase inhibitor 1) and p16INK4A (cyclin-dependent kinase inhibitor 2A)–retinoblastoma-related protein)24,25, but their role in the cardiovascular system is currently undetermined.
A role for crosstalk between the endoplasmic reticulum (ER) and mitochondria has been proposed in the context of mitochondrial dysfunction (reviewed previously26). A major mediator in this crosstalk is Ca2+, which is mainly stored in the ER. Following stress, Ca2+ is released from the ER, enters the mitochondria, and increases both oxidative phosphorylation27 and ROS generation28. ROS themselves can alter Ca2+ homeostasis by regulating the function of inositol 1,4,5-trisphosphate receptors and ryanodine receptors29,30.
Oxidative stress is also modulated by steroidogenesis in the adrenal glands and by energy metabolism in the heart and brown adipose tissue. This control is exerted through the concerted activity of the antioxidant enzymes sulfiredoxin 1 and mitochondrial thioredoxin-dependent peroxide reductase (also known as peroxiredoxin III; PRDX3)31. PRDX3 is the most abundant and efficient mitochondrial hydrogen peroxide (H2O2) scavenger in these tissues, and mitochondrial H2O2 is buffered within the organelle with concomitant accumulation of its oxidized form, PRDX3-SO2H. When PRDX3 antioxidant capacity is overwhelmed, H2O2 is delivered into the cytosol and triggers the phosphorylation of mitogen-activated protein kinase (MAPK) 11–14, collectively known as p38 MAPK, resulting in downregulation of cortisone production31. The levels of both PRDX3-SO2H and phosphorylated p38 MAPK have a daily oscillation in several tissues, including the heart. This variation suggests that mitochondrial H2O2 is released into the cytosol in a rhythmic manner and supports the concept of a link between mitochondrial biology and circadian rhythms31,32.
Oxidative stress and mtDNA mutations
Reactive oxygen species.
According to the ROS theory of ageing, mitochondria are the primary source and the immediate target of ROS within the cell33. However, the extent to which oxidative stress is involved in heart senescence remains to be conclusively established.
Increased mitochondrial size, reduced mitochondrial respiratory capacity, and higher ROS production have been reported in cardiac ageing34. Because of their postmitotic nature, cardiomyocytes are devoid of replicative dilution of damage and are, therefore, particularly vulnerable to ROS-mediated lesions. Indeed, defective electron transport chain (ETC) subunits, including sites in complexes I and III, contribute to ROS generation35,36. However, a causative role for ROS in mitochondrial dysfunction is debated. Although ROS have been linked to mitochondrial DNA (mtDNA) damage (FIG. 2), a number of findings indicate mtDNA mutations primarily arising from errors during mtDNA replication as a major factor in cardiac ageing37,38. Indeed, mice expressing a proofreading-deficient mtDNA polymerase- γ (mtDNA mutator mice) have increased rates of mtDNA mutation and several features of ageing, including increased risk of cardiac disease39–41. Furthermore, long-lived naked mole rats reach very old age despite oxidative stress through uncertain cytoprotective mechanisms42. Of note, antiageing and ROS-limiting calorie restriction interventions alone are not sufficient to rescue the ageing phenotype of mutator mice, which questions the idea that oxidative stress is the primary mechanism determining ageing phenotypes in this experimental model43. Collectively, these findings indicate that accumulation of mtDNA mutations during ageing might compromise cell signalling and promote cell impairment independently of oxidative stress.
Most of the concerns raised against this hypothesis pertain to the extent to which increased ROS production causes mtDNA damage40. Deep sequencing and PCR-based mutation-detection methods have not identified a mutation spectrum consistent with ROS-mediated mutagenesis in mtDNA from flies and humans37,38. In this regard, a 50% reduction in manganese superoxide dismutase, one of the main mitochondrial antioxidant enzymes, in older mice did not affect mitochondrial function44.
Nevertheless, several findings still support the ROS theory of ageing. The implementation of redox-sensitive mass spectrometry approaches allowed the detection of increased production of ROS in mutator mice and indicated the existence of a mitochondrial redox signalling cascade in response to chronic exposure to ROS40. Such a signalling system would operate through the generation of a pro-inflammatory environment and is hypothesized to contribute to the accelerated ageing of mutator mice45. Indeed, expression of the antioxidant enzyme catalase rescues some of the age-related features in this model (such as heart enlargement and cardiomyopathy)39,41. Furthermore, superoxide production correlates with ageing and damage to mitochondrial lipids and proteins3.
In further support of a role for ROS-mediated damage and mitochondrial decline in cardiac ageing is the finding that supplementation with the natural polyamine spermidine counteracts age-related declines in the expression and function of ETC complex I in mice46. Although the mechanism of action of spermidine might be multifactorial, current evidence indicates that spermidine acts as an antioxidant46.
Additional studies showed that B2 bradykinin receptor deficiency exacerbates cardiac dysfunction in aged mice via increased p53 expression, reduced mitochondrial biogenesis through lower proliferator-activated receptor- γ co-activator 1 α (PG C 1α) expression, increased inflammation, and increased oxidative stress47.
The equivocal results about the role of ROS in mitochondrial dysfunction during ageing might be explained, at least in part, by the experimental approaches followed by different studies. For instance, variability in time points of measurements, degree of mitochondrial purity, assays and techniques used, data analyses, and samples used to determine ROS concentrations might all affect the results. Nevertheless, although low levels of mitochondrial-derived ROS potentially promote autophagy signalling and organelle clearing, high and sustained levels of ROS clearly cause mitochondrial and cellular toxicity48. Therefore, low levels of ROS are beneficial and instrumental for normal cellular function and cardioprotection through a hormetic response49–51. In particular, the leakage of ROS outside mitochondria activates a H2O2-mediated cell-warning system for oxidative stress that acts as a retrograde signal to nuclear-targeted cytosolic pathways52. When intracellular ROS concentrations overwhelm antioxidant defences, the resulting oxidative stress can induce loss of ROS signal localization and disruption of cell homeostasis53. However, the relationship between endogenous levels of ROS and ‘healthy’ cardiac ageing is an area that requires further clarification for therapeutic exploitation.
mtDNA mutations.
Cells have hundreds to thousands of copies of mtDNA. The accrual of mtDNA mutations exceeding a certain threshold (that is, >60–80% heteroplasmy based on the type of mutation) results in the synthesis of a critical mass of defective ETC components54. This eventually leads to mitochondrial dysfunction and phenotypic expression54. mtDNA mutations can be present in only a subset of genome copies (heteroplasmy) or in all copies (homoplasmy). These mutations can be either inherited or formed de novo in the oocyte or embryo. Unless point mutations affect regions that are relevant to regulating mtDNA replication55, negative selection does not seem to occur against low levels of heteroplasmic mtDNA point mutations. Under these circumstances, clonal expansion of mtDNA mutations in somatic tissues follows the neutral drift principle56,57. As subsequent cycles of mtDNA replication occur, levels of mutated mtDNA can either increase or decrease. For a mutation to cause oxidative phosphorylation dysfunction, its relative levels must exceed a certain threshold that is dependent on the type of mutation and the energy demand of the affected cell type58.
In the setting of cardiac ageing, the accumulation of heteroplasmic mtDNA mutations can result in a mosaic of cells that can reach a critical threshold of mtDNA mutations59. mtDNA deletions accumulate steadily in cardiomyocytes of Twnk (encoding twinkle mtDNA helicase) mutant mice during ageing53. Notably, the mutational load is paralleled by mitochondrial deficiency, as measured by cytochrome c oxidase staining, and is associated with arrhythmias4. These findings support the ideas that foci of dysfunctional mitochondria in cardiomyocytes are sufficient for inducing whole-organ dysfunction and that mtDNA damage contributes to cardiac ageing.
However, several findings indicate that the abundance of mtDNA mutations rarely exceeds 1% in aged humans, which is well below the phenotypic expression threshold60,61. Moreover, the idea of mtDNA deletions causing ageing and age-related diseases is contradicted by the study of so-called Mito-mice that harbour a 4,696 bp mtDNA deletion uniformly distributed across various tissues. These mice, despite showing accumulation of up to 60% mtDNA deletion in different tissues, have no signs of mitochondrial dysfunction or disease manifestation, whereas tissues with >85% mtDNA deletion show mitochondrial dysfunction and disease phenotypes62,63.
Another study argued against a central role for mtDNA mutations in the ageing process by using a highly sensitive random mutation capture assay64. In this study, mtDNA mutation frequency in the brain and heart of aged, wild-type mice was approximately tenfold lower than previously reported. This finding is in contrast to the 500-fold higher mtDNA mutation load of heterozygous mutator mice, which have a normal lifespan and no signs of premature ageing64. However, a caveat of this study is that large-scale mtDNA deletions were not detected, which instead have been shown to accumulate in aged human tissues65. Furthermore, species-specific differences that might causally relate mtDNA mutations to ageing in humans could not be ruled out66,67. Therefore, further studies are needed to clarify species-specific differences in somatic mtDNA mutation accumulation during ageing and to determine whether the lower frequency of mtDNA mutation reported in aged mice might be functionally relevant to human ageing68.
Computational approaches support the idea that only mtDNA mutations occurring early in life have sufficient time to undergo clonal expansion and cause focal oxidative phosphorylation dysfunction during human ageing56,69. Indeed, focal oxidative phosphorylation dysfunction is observed over the age of 30 years, and the prevalence of oxidative phosphorylation-deficient cells in the affected tissues (for example, colonic epithelium and skeletal muscle) increases with age70.
Taken together, mitochondrial respiratory deficiency, ROS generation, and mtDNA damage are central to age-associated dysfunction of cardiomyocytes (FIG. 2). Mitochondrial-targeted or untargeted antioxidant therapies, activation of organelle-specific autophagy, particularly mitophagy, and additional strategies for the selective elimination of mtDNA mutations in the aged heart might, therefore, be promising areas of investigation for developing treatments against cardiovascular disease.
Mitochondrial quality control failure
The heart tissue includes cardiomyocytes, fibroblasts, smooth muscle cells, and endothelial cells, with a contractile component accounting for about 30–40% of these cells in number and 75% in volume71,72. Like neurons and skeletal myocytes, adult cardiomyocytes are postmitotic cells with very limited regenerative capacity73. Therefore, damaged organelle clearing to ensure organ integrity and maintenance relies on the recycling of cardiomyocyte components.
Cardiac muscle cells have a high energy demand and are, therefore, enriched in mitochondria (35% of cell volume). Most of the ATP generated through oxidative phosphorylation is required to sustain the Ca2+-dependent contraction of cardiomyocytes. Consequently, the maintenance of mitochondrial homeostasis is crucial to proper heart function. However, mitochondrial function is not limited to energy provision. This organelle is a hub for many other activities within the cell, including metabolic signalling, iron-sulfur cluster and haem biosynthesis, regulation of programmed cell death, and Ca2+ and iron buffering74. The intimate dependence of the heart on mitochondrial function highlights the vulnerability of cardiac tissue to mitochondrial dysfunction in ageing and disease39,40,75. MQC mechanisms are, therefore, crucial to preserving cardiomyocyte homeostasis by preventing the expansion of the primary mitochondrial defect(s). MQC involves the coordinated regulation of a hierarchical network of pathways acting sequentially from individual molecules to the whole organelle76. Antioxidant systems function as the primary line of defence to prevent mitochondrial molecular damage. When damage has occurred, a second battery of MQC pathways is engaged, which includes mitochondrial repair processes (that is, mtDNA-repair systems, reductase systems, and chaperones)77. An intramitochondrial proteolytic system intervenes to clear irreversibly damaged mitochondrial proteins for their subsequent replacement. Sustained insults that overwhelm molecular MQC trigger pathways acting at the whole organelle level. Indeed, damaged mitochondria can fuse with neighbouring, intact organelles to dilute focal dysfunction, whereas severely damaged mitochondria are segregated from the network through fission and eventually degraded by a specialized form of autophagy78,79 (FIG. 2). Derangements at any level of the MQC axis can result in amplification of mitochondrial dysfunction, energy shortage, and ultimately loss of cell viability (FIG. 2). The following sections describe mitochondrial proteostasis, biogenesis, dynamics, and autophagy as core MQC mechanisms.
Proteostasis.
Cardiac homeostasis and activity are dependent on the tight regulation of protein synthesis and degradation, given that cardiomyocytes are enriched in the myofibrillar proteins myosin and actin that form, in association with other protein components, the contractile subunit known as the sarcomere80. In the context of cardiac workload, the demands of high contractile activity result in increased protein synthesis, and this enhanced rate of synthesis can lead to physiological or pathological cardiac hypertrophy. Reduced cardiac mass has been shown to result from either a substantial decrease in protein synthesis (by as much as 50% in <2 weeks)81 or an elevated activity of the ubiquitin-proteosome system (UPS)82. These responses suggest the existence of mechanosensors in cardiomyocytes ensuring a mechanoproteostasis link between the intensity of contraction and the molecular pathways regulating protein synthesis and degradation82.
Regulation of protein turnover, from synthesis to degradation, is also relevant to tissue architecture. Whereas mature myofibrils are confined to the perinuclear region, the Z-disc is located in a subcellular region where defined sarcomeric structures are assembled80. Through a highly dynamic process that depends on proper protein conformations and interactions, the addition of new sarcomeres to the structure occurs on a short timescale. In response to external mechanical stressors (pressure or volume overload), sarcomeric addition occurs either in parallel (concentric hypertrophy) or in series (eccentric hypertrophy). The production of functional proteins requires the completion of protein folding and 3D organization, which involves transient associations with molecular chaperones. An increased oxidative environment in cardiomyocytes might contribute to primary sequence modifications and protein misfolding.
Protein synthesis operates only through the ribosomal machinery, but three distinct and complementary systems have been identified for degrading and recycling cellular components and organelles: the calpain-calpastatin system, the UPS, and macroautophagy83–85. These three processes are intimately interconnected and orchestrate cardiac sarcomere degradation83–85. Cardiac proteostasis is also under circadian control. This observation is particularly relevant during periods of low activity when heart rate and blood pressure are low, creating optimal conditions for sarcomere repair and regeneration80.
Along with the UPS, mitoproteases act as a first line of defence against mild mitochondrial damage86. In the mitochondrial matrix, protein turnover is controlled by three AAA proteases: the soluble mitochondrial Lon protease homologue (LONP1) and mitochondrial ATP-dependent Clp protease (CLPP), and the mitochondrial inner membrane-bound m-AAA protease87. In the intermembrane space, mitochondrial protein quality is ensured by the membrane-bound ATP-dependent zinc metalloproteinase YME1L1, the soluble mitochondrial serine protease HTRA2, the mitochondrial metalloendopeptidase OMA1, and the mitochondrial presenilins-associated rhomboid-like protein (PARL)86. The level and activity of these mitoproteases change during ageing. For instance, the expression and function of LONP1 decrease with age88. The relevance of mitoprotease activity is epitomized by the deletion of genes encoding AFG3-like protein 2, CLPP, and PARL, which causes severe defects in mice (such as axonal degeneration, multisystem disorder, and cachexia) and ultimately shortens murine lifespan through mitochondrial dysfunction89–91.
A role for ER stress in mitochondrial proteostasis has also been acknowledged. cAMP-dependent transcription factor ATF4, which is involved in the unfolded protein response, has been shown to induce the expression of E3 ubiquitin-protein ligase parkin, which regulates mitochondrial fission, bioenergetics, and mitophagy92 by favouring transient Ca2+ transfer from the ER to mitochondria93. Another mediator of the unfolded protein response, eukaryotic translation initiation factor 2α-kinase 3, which is enriched at the mitochondria-associated ER membranes, favours the propagation of ROS from the ER to the mitochondria94.
Cardiac dysfunction of different aetiologies (including ischaemia, pressure or volume overload, and arrhythmia) has been associated with redox imbalance that affects several processes, including ROS-mediated regulatory pathways, the expression and/or function of proteins involved in Ca2+ homeostasis, and structural alterations of myofibrillar proteins95,96. In the context of an oxidative environment, post-translational modifications (such as disulfide bonds and carbonylation) alter the conformation of myofibrillar proteins and are likely to induce functional changes of the sarcomeric contractile apparatus. Furthermore, specific subunits of the 19S proteasome become oxidized, which results in a significant reduction in the 26S proteasome activity97.
As a countermeasure to the accumulation of damaged macromolecules within the cell, elevated levels of ROS have been reported to trigger autophagy by mechanisms that are not fully understood98,99. A deeper understanding of the molecular pathways (both deleterious and protective) that are activated by redox imbalance and that regulate cardiac proteostasis is highly important to develop therapeutic approaches aimed at reducing their harmful consequences and to prevent cardiac dysfunction.
Mitochondrial bioenergetics and biogenesis.
Several studies have reported a decline in mitochondrial respiration and decreases in mitochondrial membrane potential during ageing both in laboratory rodents and in humans34,41. However, the extent to which ETC complex I-IV-supported respiration is reduced and the tissue-specificity of these reductions are controversial34,100,101. Furthermore, whether ETC functional decline and increased ROS generation are upstream or secondary to age-related changes in mitochondrial energy transfer systems (for example, creatine kinase (CK) energy exchange) or organelle content variations is uncertain100.
A causal link between the age-dependent decline in heart muscle performance and the decrease in the CK transfer system is plausible considering both its role in supplying high-energy compounds to the cytosol and substrate to the ETC and the observation of decreased phosphocreatine:ATP ratios in aged human and rodent tissues100,102–105. However, additional explanations are available for the age-related decline in ETC function, including changes in diffusion barriers within the myocardium that limit mitochondrial substrate availability and variations in paracrine signalling103,106. In addition, the increased susceptibility to heart disease in male animals suggests a role for age-related changes in sex hormone levels differentially influencing ETC function in the two sexes107. Indeed, mitochondria from the heart of ovariectomized rats show aberrant mitochondrial morphology, overproduction of ROS, and impairment in basal and stress-induced mitochondrial fission, which are prevented by treatment with oestrogens and progesterone107. Oestrogens can also modulate ATP synthesis107. Moreover, results from muscle-specific oestrogen receptor knockout (Esrl−/−) mice suggest a link between nuclear receptor signalling and hormonal control of mitochondrial function and metabolism107. The role of sex hormones in the bioenergetic decline observed during ageing is an attractive area of investigation106,108.
Cardiac mitochondriogenesis is a complex nuclear-mitochondrial process that orchestrates both genome transcription and replication109 (FIG. 1). PGC1α is amaster regulator of mitochondrial biogenesis and energy metabolism110,111. In the heart, PGC1α is induced at birth to support the metabolic shift in fuel preference from glucose and lactate during the fetal period to fatty acids after birth112. The transcriptional activities of a set of factors, including peroxisome proliferator-activated receptor-α, steroid hormone receptor ERR1, and nuclear respiratory factor 1 (NRF1), are under the control of PGCtα111. Through this regulation, PGC1α modulates mitochondrial biogenesis and energy metabolism. Mitochondrial content is substantially reduced in the failing hearts of both rodents and humans113,114. Moreover, downregulation of PGC1α signalling has been observed in the setting of experimental heart failure115. As such, discovering the cardiac mechanisms regulating PGC1α signalling might lead to the development of therapies aimed at stimulating mitochondrial biogenesis and increasing energy production in the setting of higher contractile demand114. Hydrogen sulfide has been indicated as an important regulator of cardiac mitochondrial content and an inducer of mitochondrial biogenesis via a 5’-AMP-activated protein kinase (AMPK)-PGC1α signalling cascade116.
In addition to PGC1α, the mitochondrial transcription factor A (TFAM), through its binding to mtDNA, has been indicated as a relevant modulator of mitochondrial biogenesis117 (FIG. 1). This interaction is modulated by several mechanisms, including regulation of TFAM expression and turnover, post-translational modifications and differential affinity of TFAM to specific mtDNA regions, TFAM sliding on mtDNA filaments and cooperative binding among TFAM molecules, and modulation of protein-protein interactions117.
Apart from the specific mechanisms responsible for mitochondrial functional impairment, differential decline in ETC activity has been found between inter-fibrillar mitochondria and subsarcolemmal mitochondria44, suggesting that subclasses of mitochondria are differentially susceptible to dysfunction118. Indeed, studies by Hoppel and colleagues reported increased activity of mitochondrial citrate synthase and ETC complexes, state 3 respiration rates, and abundance of respiratory cytochromes in interfibrillar mitochondria isolated from the rat heart119,120. This possibility suggests that distinct therapeutic approaches are necessary to restore bioenergetics in the two mitochondrial subpopulations.
Mitochondrial dynamics.
Mitochondria exist in a reticular state in the myocardium, allowing electrochemical conductance of the proton motive force throughout the mitochondrial network tofacilitate network-wide ATP synthesis121–123 (FIG. 1). This structural organization, reminiscent of an electrical power grid, has been revealed from classic electron microscopy-based studies and more recent fluorescence and 3D focused ion beam scanning electron microscopy structural analyses122–124. Mitochondrial-mitochondrial contact sites — gap junction-like structures — are thought to facilitate energy distribution between organelles. However, the molecular structure of these junctions and how they change during ageing remain important unanswered aspects. Morphometric analysis of mitochondrial structure has demonstrated that cardiomyocyte mitochondrial networks deteriorate with age125,126. In particular, the area of the inner mitochondrial membrane substantially decreases with age, as shown by morphometric analysis of electron microscopy images of rodent heart muscle sections125. The inner mitochondrial membrane houses the ETC, the site of respiration and ATP production; ETC functional changes can, therefore, be expected to accompany changes in inner membrane morphology.
An important area of future research will be to determine the role of fission factors, such as dynamin 1-like protein (DNM1L) and mitochondrial fission factor (MFF)127, and fusion factors, such as mitochondrial dynamin-like 120 kDa protein (OPA1) and mitofusin 1 (MFN1) and MFN2, in regulating mitochondrial morphology, Ca2+, and energy conductance inside adult cardiomyocytes. Towards this understanding, murine knockout of Dnm1l results in dilated cardiomyopathy128. Similarly, heart-specific Mfn1 and Mfn2 double knockout results in cardiac dysfunction in mice128. However, heart-specific Dnm1l, Mfn1, and Mfn2 triple-knockout mice show a partial rescue of myocardial function, demonstrating that the balance between fission and fusion is an important requirement for maintaining heart health129.
Mechanistically, impairment of mitochondrial dynamics enhances mitophagy128,130,131. Excess mitophagy might lead to loss of healthy mitochondria and energy decline128,130. However, because active mitochondrial dynamics and mitophagy have not been observed in vivo in the heart, the role of mitochondrial dynamics factors in the heart remains unclear. In addition, because heart-specific knockout of essential genes is expected to result in pathological phenotypes, how more subtle alterations in mitochondrial dynamics factors contribute to cardiovascular disease susceptibility and cardiac ageing remains to be shown. When a molecular understanding of the role of mitochondrial dynamics in the heart is obtained, therapeutic manipulation of mitochondrial fission and fusion might become a powerful tool to combat cardiovascular disease and promote ‘healthy’ cardiac ageing.
Mitochondrial autophagy.
Autophagy is an evolutionarily conserved catabolic pathway that selectively or nonselectively eliminates long-lived, unnecessary, or damaged proteins and organelles to ensure cell homeostasis132. Of the three known types of autophagy (including macroautophagy, chaperone-mediated autophagy, and microautophagy), macroautophagy is the best-understood pathway. When autophagy is stimulated by starvation and other stresses, a double-membrane phagophore proximal to the cellular cargos expands to generate the autophagosome, which ultimately fuses with the late endosome or lysosome to hydrolyse the engulfed constituents132.
Mitophagy is a form of selective autophagy that not only prevents the accumulation of abnormal or damaged mitochondria but also promotes the maintenance of a stable number of healthy mitochondria within cells (FIG. 1). A study in mito-Keima-expressing mice demonstrated that the heart was one of the most robust mitophagic organs, substantiating the importance of mitophagy for normal cardiac function133. The onset of mitophagy can be triggered by at least three different mechanisms in the cell134. Type I mitophagy largely resembles canonical autophagy and utilizes the classical autophagy machinery, involving phosphatidylinositol 3-kinase class III (PI3K-III) and phagophore assembly.
Type II mitophagy is instigated by recruitment and activation of the mitochondrial serine/threonine-protein kinase PINK1 and the E3 ubiquitin-protein ligase parkin to the outer mitochondrial membrane135 (FIG. 1). In healthy polarized mitochondria, PINK1 is imported into the inner mitochondrial membrane and matrix via the translocase of the inner membrane (TIM)-translocase of the outer membrane (TOM) complex, where it is degraded by PARL. Upon depolarization, PINK1 is stabilized within the TOM complex in the outer mitochondrial membrane. Outer membrane stabilization prevents matrix-localized proteases from cleaving PINK1, resulting in its accumulation on the outer membrane of mitochondria with compromised membrane potential136,137. Under these conditions, PINK1 can phosphorylate ubiquitin138,139, which in turn recruits and activates parkin. PINK1-induced phosphorylation of parkin at its Ser65 residue140 further drives the activation of parkin activity, resulting in a feedforward phosphor-ubiquitylation cascade, which drives mitophagy to completion. Ubiquitylated targets then interact with mitophagy receptors, including sequestosome 1 (p62)141, optineurin, tax1-binding protein 1, calcium-binding and coiled-coil domain-containing protein 2 (REF142), next to BRCA1 gene 1 protein, and histone deacetylase 6 (REF142). In the heart, PINK1 might have a role in phosphorylating MFN2 at Thr11 and Ser442 (REF138), and phosphorylated MFN2 is a well-characterized substrate of parkin137. However, mitophagy can occur independently of parkin and PINK1. BCL-2-like protein 13, BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3, and FUN14 domain-containing 1 are known to induce mitophagy in a parkin-independent manner143,146.
Type III m itophagy refers to unconventional mitophagy in which damaged regions of an individual mitochondrion selectively bud off as a form of mitochondrion-derived vesicles that subsequently transit to lysosomes147. Although the onset of this non-canonical mitophagy does not require mitochondrial depolarization, a subset of mitochondrion-derived vesicles needs parkin and PINK1 for their form ation148. Membrane potential-independent PINK1-parkin-mediated piecemeal mitophagy has also been demonstrated in cell lines expressing a mitochondrial matrix-localized misfolded protein130,149. Loss of mitochondrial fission in Dnm1l−/− cells results in the loss of the specificity of mitophagy130 and enhanced mitophagic flux in cell lines and Dnm1l−/− mouse hearts128,131.
Autophagy is a central cellular process involved in ageing and lifespan in many organisms and progressively declines in the heart during ageing, leading to increased susceptibility to stress149. Mechanisms underlying this age-mediated decrease in autophagy include downregulation of important autophagy-related proteins and autophagy regulators, as well as modification of autophagy-related proteins and altered autophagy signalling. Aged mice have a substantial loss of beclin 1 and microtubule-associated proteins 1A/1B light chain 3A and 3B as compared with younger littermates, suggesting impaired formation of autophagosomes with advancing age150,151. In addition, important regulators of autophagy such as forkhead box protein O1 (FOXO1), transcription factor EB, PI3K-III, and glycogen synthase kinase 3α are reduced in the aged heart151,152. In further support of an integral role of autophagy in cardiac ageing, heart-specific overexpression of foxo improves cardiac function in aged Drosophila153.
Post-translational modifications markedly influence the stability and activity of proteins. NAD-dependent protein deacetylase sirtuin 1 (SIRT1), located in both the nucleus and the cytosol, regulates autophagy, mitochondrial biogenesis, and antioxidant defence154. Many non-histone targets are deacetylated by SIRT1, including PGC1α, CREB-regulated transcription co-activator 2, FOXO1 and FOXO3, fibroblast growth factor 21, microtubule-associated proteins 1A/1B light chain 3A, autophagy protein 5, autophagy-related protein 7, and signal transducer and activator of transcription 3, which are all closely linked to energy homeostasis and autophagy154,155. In a rodent model, ageing substantially reduced SIRT1 activity in the heart, accompanied by increased cardiac oxidative injury156,157. Cytoprotective effects of SIRT1 are further substantiated by a study showing that cardiac-specific deletion of Sirt1 markedly impairs myocardium contractility together with increased ER stress and apoptosis158. Therefore, reduced expression or activity of SIRT1 with advancing age might alter the status of acetylation or deacetylation of target substrates, ultimately resulting in autophagy defects in aged hearts.
Hoshino and colleagues reported that p53, a transcription factor known to promote DNA repair and apoptosis, prevents the onset of mitophagy by binding to the RING0 domain of parkin and consequently inhibiting parkin translocation to mitochondria159. Importantly, the age-dependent decline in mitochondrial bioenergetics was substantially alleviated by Tp53 knockdown. Furthermore, cardiac function was well maintained in old Tp53 heterozygous mice, suggesting that p53 and parkin have a pivotal role in cardiac ageing through their regulation of mitophagy. Indeed, gene-expression profiling studies of mouse muscles revealed a substantial increase in Tp53 mRNA levels in the old mice160.
In the context of cardiac ageing, energy stress modulation of autophagy involves AMPK and insulin signalling pathways (FIG. 3). Although not fully elucidated, this regulation seems to be mediated by the nutrient-sensing mechanistic target of rapamycin complex 1 (mTORC1) that integrates the metabolic signals from both AMPK and insulin to induce autophagy161. Indeed, the activation of mTORC1 inhibits both serine/threonine-protein kinase ULK1 (a key instigator of autophagosome formation)162 and transcription factor EB (a lysosomal biogenesis modulator)163. In particular, changes in amino acid availability influence mTORC1 and regulate macrophagic protein breakdown161.
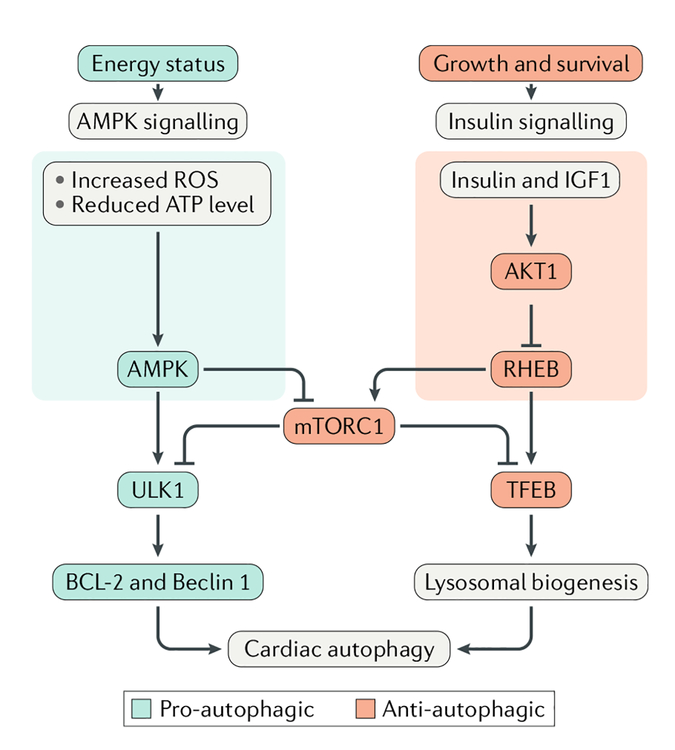
Fig. 3 |. regulation of cardiac autophagy.
Cardiomyocyte energy status regulates autophagy via metabolic signalling. Under substrate deficit conditions or oxidative stress, decreased ATP levels stimulate 5′-AMP-activated protein kinase (AMPK) activity and, therefore, autophagy via activation of serine/threonine-protein kinase ULK1 and downstream apoptosis regulator BCL-2 and beclin 1 through inhibition of autophagy suppressors (such as mechanistic target of rapamycin complex 1 (mTORC1)). At the same time, growth or cell survival stimuli activate insulin or insulin-like growth factor I (IGF1) signalling in cardiomyocytes, leading to the induction of the insulin–RACα serine/threonine-protein kinase (AKT1) pathway. Activation of GTP-binding protein RHEB results in inhibition of autophagy by autophagy suppressors (primarily mTORC1) and transcription factors related to lysosomal biogenesis (such as transcription factor EB (TFEB)). ROS, reactive oxygen species.
Beclin 1 is another relevant regulator of autophagy in the heart164. In particular, beclin 2 and BCL-2-like protein 1 are negative regulators of autophagy through binding to beclin 1. The modulation of this binding occurs via phosphorylation and can inhibit or activate cardiomyocyte autophagy depending on the amino acid residues being phosphorylated165–167.
Although induction of autophagy is essential to ensure heart function during fasting168, fasted mice that are deficient in IGF1 show cardiac atrophy following overactivation of autophagy169. The insulin signalling pathway operates through activation of PI3K, RACα serine/threonine-protein kinase (AKT1), GTP-binding protein RHEB, and mechanistic target of rapamycin (mTOR), and results in inhibition of autophagic activity (FIG. 3). Emerging evidence suggests that activation of autophagy elicits a prosurvival response in cardiomyocytes, but has also been linked to cardiomyocyte death when overactivated. Indeed, glucose deprivation in cardiomyocytes overexpressing Rheb induces activation of autophagy via downregulation of the RHEB-mTOR signalling pathway and is essential for cardiomyocyte survival. Similarly, in response to cardiac ischaemia in vivo, Rheb overexpression prevents ischaemia-induced autophagy and increases infarct size170.
Taken together, these results highlight the debate on whether upregulation of autophagy is cardioprotective or harmful. Extrapolation of findings in other tissues might be helpful given that large knowledge gaps exist in the field of cardiac autophagy. The vital roles of general and selective autophagy in the setting of cardiac ageing and cardiovascular disease make this cellular process highly attractive for developing targeted therapeutic approaches (BOX 2).
Box 2 |. Calorie restriction, autophagy, and cardiac ageing.
Exploiting the capacity of cells to clear irreversibly damaged molecules and organelles is an appealing approach to delay cardiac ageing and to prevent or treat cardiovascular disease. Particularly intriguing is the prospect of preserving cardiac health through fine-tuning cardiomyocyte autophagy. Calorie restriction (CR), defined as a reduction in food intake without malnutrition, is among the most powerful inducers of autophagy171. Indeed, the modulation of autophagy is a primary mechanism underlying the lifespan-extending and health-promoting properties of CR172. Studies in old rodents have shown that CR delays cardiac ageing, possibly via improved mitochondrial quality control processes, including autophagy. For instance, lifelong 40% CR increased the protein expression of autophagy-related protein 7, autophagy-related protein 9, and lipidated microtubule-associated proteins 1A/1B light chain 3B in the hearts of old rats, which was associated with a higher occurrence of autophagic vacuoles173. A similar degree of CR increased the autophagic flux in the heart of old rats via suppression of mechanistic target of rapamycin signalling174. This suppression was accompanied by reduced myocardial lipofuscin accumulation, decreased cardiomyocyte apoptosis, and preservation of left ventricular diastolic function. Induction of cardiac autophagy and amelioration of cardiomyocyte contractile performance were also observed in old mice with lifelong 40% CR175. Challenges and concerns associated with long-term CR implementation in humans have instigated a great deal of research aimed at identifying compounds to recapitulate the autophagy-inducing properties of CR7. Several agents, such as 2-deoxy-d-glucose, metformin, rapamycin, resveratrol, salicylates, spermidine, TEMPOL, and verapamil, have shown promising results in experimental animal models, but whether these agents can exert cardioprotection in humans through modulation of autophagy remains to be established176.
Conclusions
Although we are still far from fully understanding the events that trigger cardiomyocyte senescence and underpin cardiac ageing, wide consensus exists on the central role of mitochondrial dysfunction. MQC mechanisms operate through an integrated hierarchical network of pathways, and derangements at any level of the MQC machinery can affect the whole system. During ageing and in the setting of cardiovascular disease, failing MQC might allow primary mitochondrial defects to expand until a critical threshold is breached and mitochondrial dysfunction becomes phenotypically evident.
The recently identified SASP has been recognized to establish direct or indirect contacts between cellular components (including ER, peroxisomes, lysosomes, and vacuoles) and the extracellular environment through m itochondrion-derived vesicle release. However, the functional consequences of these interactions in the context of cardiac ageing are not fully understood, and several research questions remain unanswered (BOX 3). Dissecting the interaction between MQC pathways and the pattern of circulating mediators associated with cardiac senescence might be exploited to unveil new pathways for the prevention and treatment of age-related heart dysfunction.
Box 3 |. Unanswered research questions.
How much of cardiomyocyte age-related dysfunction is attributable to failing mitochondrial quality control?
In the context of mitochondrial quality control, are there pathways that are primarily involved in heart senescence and that might be better targets to achieve cardioprotection?
What is the optimal window of mitochondrial quality control functioning to achieve cardioprotection without disrupting cardiomyocyte homeostasis?
To what extent can data obtained from pharmacological or behavioural modulation of mitochondrial quality control in model organisms be translated to humans?
When should an intervention that modulates cardiac mitochondrial quality control be started and for how long should it be administered?
How can mitochondrial quality control adaptations elicited by experimental drugs be monitored noninvasively in humans?
Key points.
Older adults are especially vulnerable to developing cardiovascular disease owing to long-term exposure to risk factors and intrinsic cardiovascular alterations occurring during ageing.
Mitochondrial quality control (MQC) operates through the coordination of various processes (proteostasis, biogenesis, dynamics, and mitophagy) to ensure cell homeostasis.
Mitochondrial dysfunction, amplified by failing quality control processes, is believed to be a major mechanism underlying cardiac ageing and cardiovascular disease.
Preclinical evidence suggests that modulation of MQC can be harnessed for therapeutic benefit against cardiac ageing and cardiovascular disease.
Current unknowns include the optimal window of MQC functioning to achieve cardioprotection, the timing and intensity of interventions, and noninvasively accessible biomarkers of MQC in the heart.
Acknowledgements
The authors acknowledge support from Fondazione Roma (NCDs Call for Proposals 2013), Innovative Medicine Initiative-Joint Undertaking (IMI-JU 115621), intramural research grants from the Catholic University of the Sacred Heart (D3.2 2013 and D3.2 2015), the non-profit research foundation “Centro Studi Achille e Linda Lorenzon”, and the Claude D. Pepper Older Americans Independence Center at the University of Florida’s Institute on Aging (NIA 1P30AG028740).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Cardiology thanks E. Lesnefsky and the other anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Mozaffarian D et al. Heart disease and stroke statistics—2016 update. Circulation 133, e38–e360 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Chiao YA & Rabinovitch PS The aging heart. Cold Spring Harb. Perspect. Med. 5, a025148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med 71, 208–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baris OR et al. Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metab. 21, 667–677 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Lok NS & Lau CP Prevalence of palpitations, cardiac arrhythmias and their associated risk factors in ambulant elderly. Int. J. Cardiol 54, 231–236 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C & Marzetti E Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ. Res 110, 1125–1138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzetti E et al. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin. Geriatr. Med 25, 715–732 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Otín C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North BJ & Sinclair DA The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theurey P & Pizzo P The aging mitochondria. Genes (Basel) 9, 22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Segura A, Nehme J & Demaria M Hallmarks of cellular senescence. Trends Cell Biol 28, 436–453 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Garinis GA, van der Horst GTJ, Vijg J & Hoeijmakers JHJ DNA damage and ageing: new-age ideas for an age-old problem. Nat. Cell Biol 10, 1241–1247 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin E et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zierer J et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany, NY) 8, 77–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen W-L & Klionsky DJ How to live long and prosper: autophagy, mitochondria, and aging. Physiology 23, 248–262 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Marzetti E et al. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol 305, H459–H476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohlgemuth SE, Calvani R & Marzetti E The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J. Mol. Cell. Cardiol 71, 62–70 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Chung HY et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev 8, 18–30 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fougère B, Boulanger E, Nourhashémi F, Guyonnet S & Cesari M Chronic inflammation: accelerator of biological aging. J. Gerontol. A Biol. Sci. Med. Sci 72, 1218–1225 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Lin C-C et al. NADPH oxidase/ROS-dependent VCAM-1 induction on TNF-α-challenged human cardiac fibroblasts enhances monocyte adhesion. Front. Pharmacol 6, 310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallam N & Laher I Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev 2016, 7239639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett MR, Sinha S & Owens GK Vascular smooth muscle cells in atherosclerosis. Circ. Res 118, 692–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimbrone MA & García-Cardeña G Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res 118, 620–636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campisi J Aging, cellular senescence, and cancer. Annu. Rev. Physiol 75, 685–705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz-Espín D & Serrano M Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol 15, 482–496 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Senft D & Ronai ZA UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci 40, 141–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano R et al. Endoplasmic reticulum protein BI-1 regulates Ca2+-mediated bioenergetics to promote autophagy. Genes Dev 26, 1041–1054 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam-Vizi V & Starkov AA Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimers Dis 20 (Suppl. 2), S413–S426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson DC et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14, 196–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bánsághi S et al. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem 289, 8170–8181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee SG & Kil IS Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic. Biol. Med 100, 73–80 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Manella G & Asher G The circadian nature of mitochondrial biology. Front. Endocrinol. (Lausanne) 7, 162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harman D Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956). [DOI] [PubMed] [Google Scholar]
- 34.Duicu OM et al. Ageing-induced decrease in cardiac mitochondrial function in healthy rats. Can. J. Physiol. Pharmacol 91, 593–600 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Kuka S et al. Effect of aging on formation of reactive oxygen species by mitochondria of rat heart. Gen. Physiol. Biophys 32, 415–420 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Wong H-S, Dighe PA, Mezera V, Monternier P-A & Brand MD Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem 292, 16804–16809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy SR, Salk JJ, Schmitt MW & Loeb LA Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 9, e1003794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itsara LS et al. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet. 10, e1003974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trifunovic A et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Kujoth GC et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Dai D-F et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis KN, Andziak B, Yang T & Buffenstein R The naked mole-rat response to oxidative stress: just deal with it. Antioxid. Redox Signal 19, 1388–1399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Someya S et al. Effects of calorie restriction on the lifespan and healthspan of POLG mitochondrial mutator mice. PLoS ONE 12, e0171159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das KC & Muniyappa H Age-dependent mitochondrial energy dynamics in the mice heart: role of superoxide dismutase-2. Exp. Gerontol 48, 947–959 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logan A et al. In vivo levels of mitochondrial hydrogen peroxide increase with age in mtDNA mutator mice. Aging Cell 13, 765–768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberg T et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med 22, 1428–1438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng W et al. Increased age-related cardiac dysfunction in bradykinin B2 receptor-deficient mice. J. Gerontol. A Biol. Sci. Med. Sci 71, 178–187 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Marzetti E et al. Shorter telomeres in peripheral blood mononuclear cells from older persons with sarcopenia: results from an exploratory study. Front. Aging Neurosci 6, 233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tocchi A, Quarles EK, Basisty N, Gitari L & Rabinovitch PS Mitochondrial dysfunction in cardiac aging. Biochim. Biophys. Acta 1847, 1424–1433 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sena LA & Chandel NS Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojtovich AP, Nadtochiy SM, Brookes PS & Nehrke K Ischemic preconditioning: the role of mitochondria and aging. Exp. Gerontol 47, 1–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishra P & Chan DC Metabolic regulation of mitochondrial dynamics. J. Cell Biol 212, 379–387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H, Wei H, Sehgal SA, Liu L & Chen Q Mitophagy receptors sense stress signals and couple mitochondrial dynamic machinery for mitochondrial quality control. Free Radic. Biol. Med 100, 199–209 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Rossignol R et al. Mitochondrial threshold effects. Biochem. J 370, 751–762 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wanrooij S et al. In vivo mutagenesis reveals that OriL is essential for mitochondrial DNA replication. EMBO Rep 13, 1130–1137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elson JL, Samuels DC, Turnbull DM & Chinnery PF Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am. J. Hum. Genet 68, 802–806 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greaves LC et al. Comparison of mitochondrial mutation spectra in ageing human colonic epithelium and disease: absence of evidence for purifying selection in somatic mitochondrial DNA point mutations. PLoS Genet 8, e1003082 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kauppila TES, Kauppila JHK & Larsson N-G Mammalian mitochondria and aging: an update. Cell Metab 25, 57–71 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Müller-Höcker J, Droste M, Kadenbach B, Pongratz D & Hübner G Fatal mitochondrial myopathy with cytochrome-c-oxidase deficiency and subunit-restricted reduction of enzyme protein in two siblings: an autopsy-immunocytochemical study. Hum. Pathol 20, 666–672 (1989). [DOI] [PubMed] [Google Scholar]
- 60.Cottrell DA et al. Cytochrome c oxidase deficient cells accumulate in the hippocampus and choroid plexus with age. Neurobiol. Aging 22, 265–272 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Khrapko K, Kraytsberg Y, de Grey ADNJ, Vijg J & Schon EA Does premature aging of the mtDNA mutator mouse prove that mtDNA mutations are involved in natural aging? Aging Cell 5, 279–282 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Inoue K et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat. Genet 26, 176–181 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Nakada K et al. Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat. Med 7, 934–940 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Vermulst M et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet 39, 540–543 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Meissner C et al. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp. Gerontol 43, 645–652 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Kraytsberg Y et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet 38, 518–520 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Khrapko K & Vijg J Mitochondrial DNA mutations and aging: a case closed? Nat. Genet 39, 445–446 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Srivastava S The mitochondrial basis of aging and age-related disorders. Genes (Basel) 8, 398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor SD et al. Targeted enrichment and high-resolution digital profiling of mitochondrial DNA deletions in human brain. Aging Cell 13, 29–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor RW et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest 112, 1351–1360 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nag AC Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28, 41–61 (1980). [PubMed] [Google Scholar]
- 72.Vliegen HW, van der Laarse A, Cornelisse CJ & Eulderink F Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur. Heart J 12, 488–494 (1991). [DOI] [PubMed] [Google Scholar]
- 73.Bergmann O et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nunnari J & Suomalainen A Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bates MGD et al. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur. Heart J 33, 3023–3033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischer F, Hamann A & Osiewacz HD Mitochondrial quality control: an integrated network of pathways. Trends Biochem. Sci 37, 284–292 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Szklarczyk R, Nooteboom M & Osiewacz HD Control of mitochondrial integrity in ageing and disease. Phil. Trans. R. Soc. B Biol. Sci 369, 20130439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Twig G, Hyde B & Shirihai OS Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim. Biophys. Acta 1777, 1092–1097 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calvani R et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 394, 393–414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boateng SY & Goldspink PH Assembly and maintenance of the sarcomere night and day. Cardiovasc. Res. 77, 667–675 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Klein I, Samarel AM, Welikson R & Hong C Heterotopic cardiac transplantation decreases the capacity for rat myocardial protein synthesis. Circ. Res 68, 1100–1107 (1991). [DOI] [PubMed] [Google Scholar]
- 82.Razeghi P et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation 108, 2536–2541 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Patterson C, Portbury AL, Schisler JC & Willis MS Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ. Res 109, 453–462 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Portbury AL, Willis MS & Patterson C Tearin’ up my heart: proteolysis in the cardiac sarcomere. J. Biol. Chem 286, 9929–9934 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Powell SR The ubiquitin-proteasome system in cardiac physiology and pathology. Am. J. Physiol. Circ. Physiol 291, H1–H19 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Quirós PM, Langer T & López-Otín C New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol 16, 345–359 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Voos W Chaperone-protease networks in mitochondrial protein homeostasis. Biochim. Biophys. Acta 1833, 388–399 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Ngo JK & Davies KJA Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann. NY Acad. Sci 1119, 78–87 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Gispert S et al. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum. Mol. Genet 22, 4871–4887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cipolat S et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126, 163–175 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Maltecca F et al. The mitochondrial protease AFG3L2 is essential for axonal development. J. Neurosci 28, 2827–2836 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narendra D, Tanaka A, Suen D-F & Youle RJ Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol 183, 795–803 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calì T, Ottolini D, Negro A & Brini M Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim. Biophys. Acta 1832, 495–508 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Verfaillie T et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19, 1880–1891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos CXC, Anilkumar N, Zhang M, Brewer AC & Shah AM Redox signaling in cardiac myocytes. Free Radic. Biol. Med 50, 777–793 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sumandea MP & Steinberg SF Redox signaling and cardiac sarcomeres. J. Biol. Chem 286, 9921–9927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Divald A et al. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ. Res 106, 1829–1838 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Yuan H et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am. J. Physiol. Heart Circ. Physiol 296, H470–H479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haberland M, Montgomery RL & Olson EN The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet 10, 32–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tepp K et al. Changes in the mitochondrial function and in the efficiency of energy transfer pathways during cardiomyocyte aging. Mol. Cell. Biochem 432, 141–158 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Tatarková Z et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 60, 281–289 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Esterhammer R et al. Cardiac high-energy phosphate metabolism alters with age as studied in 196 healthy males with the help of 31-phosphorus 2-dimensional chemical shift imaging. PLoS ONE 9, e97368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yaniv Y, Juhaszova M & Sollott SJ Age-related changes of myocardial ATP supply and demand mechanisms. Trends Endocrinol. Metab 24, 495–505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nathania M et al. Impact of age on the association between cardiac high-energy phosphate metabolism and cardiac power in women. Heart 104, 111–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klepinin A et al. Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J. Bioenerg. Biomembr 48, 531–548 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Huss JM & Kelly DP Nuclear receptor signaling and cardiac energetics. Circ. Res 95, 568–578 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Rattanasopa C, Phungphong S, Wattanapermpool J & Bupha-Intr T Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol 147, 1–9 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Huss JM, Torra IP, Staels B, Giguère V & Kelly DP Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol 24, 9079–9091 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dorn GW, Vega RB & Kelly DP Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 29, 1981–1991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kubli DA & Gustafsson ÅB Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res 111, 1208–1221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez-Marcos PJ & Auwerx J Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr 93, 884S–890S (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leone TC & Kelly DP Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb. Symp. Quant. Biol 76, 175–182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karamanlidis G et al. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res 106, 1541–1548 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bayeva M, Gheorghiade M & Ardehali H Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol 61, 599–610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faerber G et al. Induction of heart failure by minimally invasive aortic constriction in mice: reduced peroxisome proliferator-activated receptor γ coactivator levels and mitochondrial dysfunction. J. Thorac. Cardiovasc. Surg 141, 492–500. e1 (2011). [DOI] [PubMed] [Google Scholar]
- 116.Shimizu Y et al. Hydrogen sulfide regulates cardiac mitochondrial biogenesis via the activation of AMPK. J. Mol. Cell. Cardiol 116, 29–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Picca A & Lezza AMS Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions. Useful insights from aging and calorie restriction studies. Mitochondrion 25, 67–75 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Anmann T et al. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim. Biophys. Acta 1837, 1350–1361 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Palmer JW, Tandler B & Hoppel CL Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem 252, 8731–8739 (1977). [PubMed] [Google Scholar]
- 120.Palmer JW, Tandler B & Hoppel CL Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch. Biochem. Biophys 236, 691–702 (1985). [DOI] [PubMed] [Google Scholar]
- 121.Ichas F, Jouaville LS & Mazat JP Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89, 1145–1153 (1997). [DOI] [PubMed] [Google Scholar]
- 122.Glancy B et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP & Zorov DB Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J. Cell Biol 107, 481–495 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glancy B et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep 19, 487–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.El’darov CM, Vays VB, Vangeli IM, Kolosova NG & Bakeeva LE Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochemistry (Mosc.) 80, 604–609 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Tate EL & Herbener GH A morphometric study of the density of mitochondrial cristae in heart and liver of aging mice. J. Gerontol 31, 129–134 (1976). [DOI] [PubMed] [Google Scholar]
- 127.Chen H et al. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J. Cell Biol 211, 795–805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song M, Mihara K, Chen Y, Scorrano L & Dorn GW Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21, 273–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song M, Franco A, Fleischer JA, Zhang L & Dorn GW Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26, 872–883. e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burman JL et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol 216, 3231–3247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parone PA et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 3, e3257 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mizushima N, Levine B, Cuervo AM & Klionsky DJ Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun N et al. Measuring in vivo mitophagy. Mol. Cell 60, 685–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemasters JJ Variants of mitochondrial autophagy: types 1 and 2 mitophagy and micromitophagy (type 3). Redox Biol. 2, 749–754 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Youle RJ & Narendra DP Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol 12, 9–14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin SM et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol 191, 933–942 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen Y & Dorn GW PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okatsu K et al. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol 209, 111–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koyano F et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Sarraf SA et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pankiv S et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem 282, 24131–24145 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Lazarou M et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murakawa T et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun 6, 7527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc. Natl Acad. Sci. USA 107, 9035–9042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanna RA et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem 287, 19094–19104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu L et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol 14, 177–185 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Soubannier V et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol 22, 135–141 (2012). [DOI] [PubMed] [Google Scholar]
- 148.McLelland G-L, Soubannier V, Chen CX, McBride HM & Fon EA Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33, 282–295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou J et al. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging (Albany, NY 9, 583–599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peng L et al. Changes in cell autophagy and apoptosis during age-related left ventricular remodeling in mice and their potential mechanisms. Biochem. Biophys. Res. Commun 430, 822–826 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y et al. Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochim. Biophys. Acta 1863, 1919–1932 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK & Sadoshima J Aging and autophagy in the heart. Circ. Res 118, 1563–1576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blice-Baum AC et al. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 16, 93–103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chun SK et al. Autophagy in ischemic livers: a critical role of sirtuin 1/mitofusin 2 axis in autophagy induction. Toxicol. Res 32, 35–46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang R et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Ferrara N et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11, 139–150 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Ren J et al. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell 16, 976–987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hsu Y-J et al. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int. J. Cardiol 228, 543–552 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Hoshino A et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun 4, 2308 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Edwards MG et al. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics 8, 80 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tan VP & Miyamoto S Nutrient-sensing mTORC1: Integration of metabolic and autophagic signals. J. Mol. Cell. Cardiol 95, 31–41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Egan D, Kim J, Shaw RJ & Guan K-L The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martina JA, Chen Y, Gucek M & Puertollano R MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maejima Y, Isobe M & Sadoshima J Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol 95, 19–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zalckvar E et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep 10, 285–292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gurkar AU et al. Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat. Commun 4, 2189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei Y, Pattingre S, Sinha S, Bassik M & Levine B JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ikeda Y et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res 116, 264–278 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Troncoso R et al. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc. Res 93, 320–329 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sciarretta S et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125, 1134–1146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cuervo AM Calorie restriction and aging: the ultimate “cleansing diet”. J. Gerontol. A Biol. Sci. Med. Sci 63, 547–549 (2008). [DOI] [PubMed] [Google Scholar]
- 172.Tóth ML et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4, 330–338 (2008). [DOI] [PubMed] [Google Scholar]
- 173.Wohlgemuth SE et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 10, 281–292 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Shinmura K et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J. Mol. Cell. Cardiol 50, 117–127 (2011). [DOI] [PubMed] [Google Scholar]
- 175.Han X et al. Influence of long-term caloric restriction on myocardial and cardiomyocyte contractile function and autophagy in mice. J. Nutr. Biochem 23, 1592–1599 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Delbridge LMD, Mellor KM, Taylor DJ & Gottlieb RA Myocardial stress and autophagy: mechanisms and potential therapies. Nat. Rev. Cardiol 14, 412–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]