Abstract
An important step toward successful pregnancy involves invasion of the trophoblast cells into the decidua for placentation. Herein, we show that in the human and baboon decidua HOXA10 expression is downregulated after implantation and that this reduction is most prominent in the decidual cells juxtaposed to the invading placental villi. The supernatants derived from HOXA10-depleted human decidual cells increase the invasiveness of the trophoblast cell lines ACH-3P and JEG3 in vitro; this increase is due to higher expression and activity of matrix metalloproteases (MMPs) and reduced expression of tissue inhibitors of MMPs in both the cell lines. The proinvasive ability of HOXA10-depleted decidual cells is due to increased levels and secretion of leukemia inhibitor factor (LIF) and interleukin (IL)-6. Both these cytokines individually promote invasion of ACH-3P and JEG3 cell by increasing the activities of MMPs and decreasing mRNA levels of TIMPs. Finally, we demonstrate that the supernatants derived from HOXA10-depleted decidual cell–phosphorylated STAT3 (Tyr 705) and knocking down STAT3 in ACH-3P and JEG3 cells restrained the invasion mediated by supernatants derived from HOXA10-depleted decidual cells. These results imply that STAT3 activity is essential and sufficient to promote invasion in response to downregulation of HOXA10 in decidual cells. We propose that downregulation of HOXA10 in the decidual cells promotes the expression of LIF and IL-6, which, in a paracrine manner, activates STAT3 in the trophoblast cells, leading to an increase in MMPs to facilitate invasion.
In this study, we demonstrate that HOXA10 is downregulated in the decidua after implantation, which promotes trophoblast invasion.
Successful pregnancy requires implantation of the blastocyst in the maternal endometrium and subsequent placentation. In this multistep process, the trophectodermal cells of the blastocyst first adhere and then invade into the endometrium; the endometrial stromal cells at the same time transform into the decidua, where the trophectodermal cells differentiate to become extravillous and villous trophoblasts (1). The extravillous trophoblasts (EVTs) eventually invade the decidua for placentation, leading to spiral artery remodeling (2). The invasion of EVTs in the decidua has to be tightly regulated both spatially and temporally, and any defects in this process can cause shallow placentation, which is associated with pregnancy disorders like preeclampsia and fetal growth restriction, which are major causes of feto-maternal morbidity and maternal deaths (2–4).
At the time of implantation, the endometrial stromal cells transform into specialized secretory decidual cells. This transformation promotes influx and differentiation of natural killer cells and macrophages (5). It is believed that the decidual natural killer cells are critical regulators of trophoblast invasion (6, 7), and this invasion-promoting activity of the decidual natural killer cells is believed to be due to their ability to secrete proinvasive factors (mainly cytokines) (8). However, mice that lack natural killer cells are generally not infertile and do not have major defects in placentation, suggesting that other mechanisms might govern trophoblast invasion (9). Recent evidence suggests that decidualized endometrial stromal cells may be regulators of trophoblast invasion (10). Incubation of trophoblast cells with conditioned medium from decidual cells increases their invasive behavior and changes the expression of proteases and integrins resembling a molecular phenotype of invasive EVTs (11–13). Interestingly, the decidual cells also secrete cytokines [e.g., interleukin (IL)-11 and IL-15] that are direct regulators of trophoblast invasion (10, 14, 15), suggesting that the decidualized endometrial stromal cells control trophoblast invasion. Indeed, defective decidualization is observed in women with pregnancy complications such as early spontaneous abortions and preeclampsia, which are associated with defective placentation (3–5). These observations imply that decidual cells are regulators of trophoblast invasion and that any alterations in their functions can lead to defective placentation. Although the role of decidual cells in governing trophoblast invasion is becoming clear, the molecular mechanisms by which it does so are yet not known.
Among the various genes that control decidualization, the homeobox transcription factor HOXA10 is indispensable for the decidual transformation of endometrial stromal cells. HOXA10 belongs to the class of Abdominal B–like homeobox genes and is essential for specification of anterior uterus during embryogenesis (16). In the adult endometrium, HOXA10 is expressed by both epithelial and stromal cells in a menstrual cycle–dependent manner and is regulated by progesterone (17–20). The expression of HOXA10 peaks in the endometrium at the time of embryo implantation, and this peak is required for decidualization of stromal cells (21–24). In an attempt to understand the mechanisms by which HOXA10 would govern decidualization, we performed microarray of human in vitro decidual cells lacking HOXA10 and found increased expression of a large number of genes that had known roles in trophoblast invasion (Supplemental Table 1). These observations prompted us to speculate that decidual HOXA10 might have some roles in regulation of trophoblast invasion. However, to the best of our knowledge, the involvement of decidual HOXA10 in the regulation of trophoblast invasion has not been investigated.
Thus, in the current study we aimed to determine the role of decidual HOXA10 in trophoblast invasion. We demonstrate in vivo that downregulation of HOXA10 in decidual cells after implantation enhances the levels of gp130 cytokines, which in a paracrine manner activate STAT3 in trophoblast cells to stimulate invasion.
Materials and Methods
Ethics statement
Human samples were collected after written informed consent, and the protocol was approved by the Institutional Ethics Committee (NIRRH, Mumbai, India) and Ethics Committee for Research on Human Subjects, King Edward Memorial Hospital, Mumbai, India. Collection of tissues from baboons was approved by the Institutional Animal Care and Use Committees of the University of Illinois at Chicago and Michigan State University.
Collection of human and baboon tissues
Proliferative-phase human endometrium was obtained from five subjects undergoing gynecological surgery. Luteal-phase endometrial biopsies were obtained from healthy normally cycling women. The phase of the cycle was estimated by last menstrual period and verified histologically by a pathologist. Decidual tissues were archived samples used previously (25) obtained from women undergoing medical termination of pregnancy in the first trimester (10 to 12 weeks of gestation). Mature cycling female baboons (Papio anubis) were mated with fertile male baboons during the periovulatory period, as determined by sex skin tumescence. The stage of pregnancy was confirmed by ultrasound, circulating levels of chorionic gonadotropin, estradiol, and progesterone as described previously (26, 27). Tissues were obtained on days 18 to 25 (n = 3) and on days 40 to 60 (n = 5) of the last menstrual bleed corresponding to implantation and postimplantation stages, respectively. All the tissues were processed for RNA extraction and paraffin embedding and sectioning.
Immunohistochemistry and immunofluorescence
Immunohistochemistry for HOXA10 was performed as detailed previously (23). Briefly, sections were deparaffinized and probed overnight with the primary antibody against HOXA10. On the next day, the slides were washed and incubated in biotinylated secondary antibody followed by incubation with streptavidin-labeled horseradish peroxidase. Detection was done using the ABC Staining System (Santa Cruz Biotech, Dallas, TX). All the sections were counterstained with hematoxylin and scored for the intensity of the nuclear and cytoplasmic staining by H score method (Supplemental Table 2).
Double immunofluorescence was performed, where the slides incubated simultaneously with antibodies against HOXA10 and cytokeratin (to detect trophoblasts). After extensive washings, the sections were incubated with Alexa 568–conjugated anti-goat secondary antibody to detect HOXA10 and FITC-labeled anti-rabbit secondary antibody to detect cytokeratin. The sections were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich Inc., St. Louis, MO) and mounted in antifade medium (Vector Laboratories, Burlingame, CA). The negative control sections were incubated without the primary antibody. Images were taken under a confocal microscope (Leica SP8, Mannheim, Germany). The details of the antibodies used and their dilutions are given in the Appendix.
Primary cultures of endometrial stromal cells and in vitro decidualization
Stromal cells from proliferative-phase human endometrial tissue were isolated and cultured as described previously (14). Stromal cells in the fourth passage (purity >98% as judged by vimentin immunostaining) were decidualized by treatment with 17-β estradiol (10−8 M) and progesterone (10−6 M) (Sigma-Aldrich) for 21 days as described earlier (14). To check for decidualization, the levels of prolactin and IGFBP-1 were measured in the culture supernatants using commercially available ELISA kits (R&D Systems, Minneapolis, MN, for IGFBP-1; Calbiotech, Spring Valley, CA, for prolactin).
HOXA10 knockdown in decidual cells and collection of conditioned medium
The endogenous expression of HOXA10 in the in vitro decidualized endometrial stromal cells was knocked down by small interfering RNA (siRNA) as described previously (14). Briefly, the decidualized cells (day 21 of steroid treatment) were transfected with scrambled or HOXA10-specific siRNA (sequences in Supplemental Table 3) using HiPerFect transfection reagent (Qiagen, Germantown, MD). Previous studies have shown that HOXA10 in the stromal cells is maximally downregulated by 3 days after transfection (14); thus, the amounts of HOXA10 mRNA and protein were assessed at 72 hours of transfection by real-time polymerase chain reaction (PCR) and Western blotting as described later.
To obtain the conditioned medium, the cells were fed with fresh medium, and after 24 hours the supernatants were collected and centrifuged to remove cellular debris. The medium was immediately frozen in aliquots at −80°C until use. Each vial of supernatant was thawed and used immediately for experiments, and the leftover medium was discarded.
Trophoblast invasion assay
JEG3 cells (DZMO, Braunschweig, Germany) and ACH-3P cells (kind gift from Dr. Gernot Desoye, Medical University Graz, Austria) were maintained as detailed earlier (28, 29). JEG3 is a choriocarcinoma cell line, and ACH-3P are hybrid cells from the fusion of primary human first-trimester trophoblasts (week 12 of gestation) with a human choriocarcinoma cell line (AC1-1). Both the cell lines have a molecular repertoire identical to the human first-trimester invasive trophoblasts (30, 31).
Matrigel matrix–based invasion assay was performed in triplicate as described previously (12, 29). Briefly, the trophoblast cells were challenged with neat or 25% diluted decidual cell conditioned medium and loaded onto the growth factor–reduced Matrigel matrix (BD Biosciences, Bedford, MA) in the upper chamber of the transwell inserts. To study the effects of leukemia inhibitor factor (LIF) and IL-6 on trophoblast invasion, ACH-3P and JEG3 cells were treated individually with varying concentrations of recombinant LIF (Sigma-Aldrich) or IL-6 (Peprotech, Rocky Hill, NJ) and loaded on to Matrigel inserts. The control cells were treated with plain medium. After 24 hours, the cells on the lower side of the membrane were stained with 0.2 μM Hoechst 33342 nuclear dye (Biotium Inc., Hayward, CA) and counted under a fluorescent microscope (Eclipse 80i; Nikon, Chiyoda Ku, Japan).
To study the effect of STAT3 silencing on trophoblast invasion, ACH-3P and JEG3 cells were transfected with an siRNA against STAT3; scrambled siRNA was used as control (sequences in Supplemental Table 3). The extent of knockdown was determined by Western blot 48 hours after transfection, and invasion assay was carried out as described previously using 25% dilution of the conditioned medium from decidual cells.
Gelatin zymography
The supernatants of JEG3 and ACH-3P cells were used to measure the activity of matrix metalloproteinase (MMP)-2 and MMP-9 by gelatin zymography as described previously (12). Briefly, ACH-3P and JEG3 cells were treated with conditioned medium derived from the decidual cells or recombinant LIF or IL-6 for 24 h. The medium was collected and resolved on nondenaturing 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis containing gelatin (Sigma-Aldrich). The gels were washed in Triton X-100 and incubated overnight in the reaction buffer [50 mM Tris-HCl (pH 7.6), 10 mM CaCl2, and 0.02% sodium azide], stained with Coomassie Brilliant Blue (Sigma-Aldrich), and washed until clear bands appeared on the dark background. The intensity of bands was measured by gel documentation system.
Western blot
Western blotting was used to determine the level of HOXA10 in decidual cells and STAT3 and phospho-STAT3 (Tyr 705) in trophoblast cells. β-Actin was used as loading control as described previously (29, 32). Briefly, proteins were extracted using RIPA buffer (Cell Signaling Technology, Danvers, MA), separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. The blots were probed with an optimized concentration of the primary antibody followed by an appropriate horseradish peroxidase–labeled secondary antibody, and detection was done using the chemiluminescence detection kit (Pierce, Thermo Scientific, Rockford, IL). As negative control, blots were incubated without the primary antibody. Densitometric analysis of the bands was done using a gel documentation system. Band intensities were normalized to the housekeeping (β-actin or total STAT3) proteins.
Real-time reverse transcription-PCR
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA), treated with DNAse I, and reverse transcribed using random hexamers (BD Biosciences). Real-time PCR was performed in triplicate using SYBR green super mix (Biorad, Hercules, CA) as described earlier (23). The sequences of the primers used and their optimized annealing temperatures are detailed in Supplemental Table 3. The mean cycle threshold value for the gene of interest was normalized to the corresponding 18S rRNA value for each sample, and the relative expression was calculated.
Bead arrays
The levels of LIF, IL-6, and IL-15 in the culture supernatants were measured using the Bio-Plex system (Bio-Rad Laboratories, Singapore). Briefly, 50 μL of neat medium and appropriately diluted standards were incubated in 96-well plates with the primary antibody–coated beads. After incubation, the beads were probed with a biotinylated secondary antibody, and detection was done using phycoerythrin-conjugated streptavidin. The fluorescence intensities on the beads were read using the Bio-plex reader, and data were analyzed using the Bio-Plex Manager software (Bio-Rad).
Results
HOXA10 is downregulated in human and baboon decidua during pregnancy
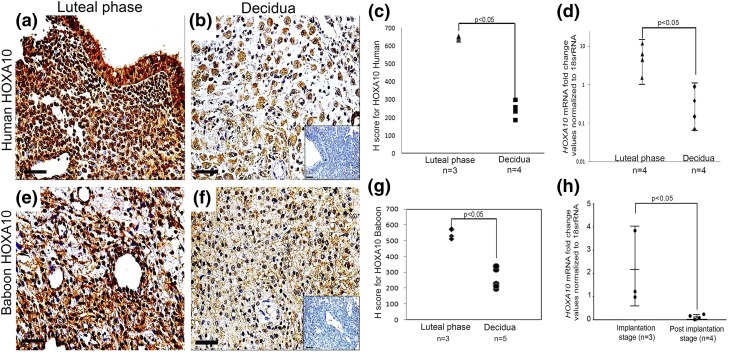
As compared with the luteal-phase human endometrium, the expression of HOXA10 was found to be weaker in the first-trimester human decidual tissue [Fig. 1(a) and 1(b)]. No staining was detected in negative controls [Fig. 1(b), inset]. Semiquantitative analysis by H scoring revealed a significant reduction in the expression of HOXA10 in decidual tissue as compared with luteal-phase endometrium [Fig. 1(c)]. This reduced expression in the decidua was not due to any generalized changes in the tissues during processing because subsequent sections from the same tissues intensely stained for IGFBP-1 (Supplemental Fig. 1). Real-time PCR was carried out to determine if the reduction in HOXA10 protein was due to reduction in its mRNA. In the human decidual tissue, levels of HOXA10 mRNA were significantly lower as compared with those observed in the luteal-phase endometrium [Fig. 1(d)].
Figure 1.
Expression of HOXA10 in the human and baboon luteal-phase endometrium and in the decidua. Immunohistochemistry for HOXA10 was done on (a) luteal-phase human endometrium, (b) first-trimester human decidua, (e) day 18 baboon endometrium, and (f) day 40 baboon decidua. The respective negative controls without the primary antibody are shown in insets in (b) and (f). The sections are counterstained with hematoxylin. Scale bar, 50 µm. HOXA10 immunostaining was evaluated semiquantitatively (Supplemental Table 2), and the H scores for the biological replicates (n) are represented in (c) and (g) for human and baboon, respectively, where each dot represents one sample. Levels of HOXA10 mRNA were measured by real-time reverse transcription PCR in the (d) luteal-phase human endometrium, first-trimester decidua (10 to 12 weeks of pregnancy), and (h) implantation-stage baboon endometrium (days 20 to 25) and decidua (days 40 to 60). Values on the y-axis are fold change in expression of HOXA10. The number of samples (n) analyzed in each group are given in each graph along with the respective P values.
In pregnant baboons, HOXA10 was detected in the nucleus and cytoplasm of stromal cells of the implantation-stage endometrium (days 18 to 25 from the last menstrual bleed); after implantation (days 40 to 60), when the endometrial stromal cells decidualized, the expression of HOXA10 was lower and mainly cytoplasmic [Fig. 1(e) and 1(f)]. No staining was detected in the negative controls [Fig. 1(f), inset]. Semiquantitative analysis by H scoring revealed a significant reduction in expression of HOXA10 in the postimplantation-stage decidua as compared with implantation-stage endometrium [Fig. 1(g)]. The mRNA levels of HOXA10 [Fig. 1(h)] were also significantly lower in the decidua of pregnant baboons (days 40 to 60) as compared with those observed in the implantation-stage endometrium (days 18 to 25).
Depletion of HOXA10 in the in vitro decidualized endometrial stroma cells increases the expression of IL-15
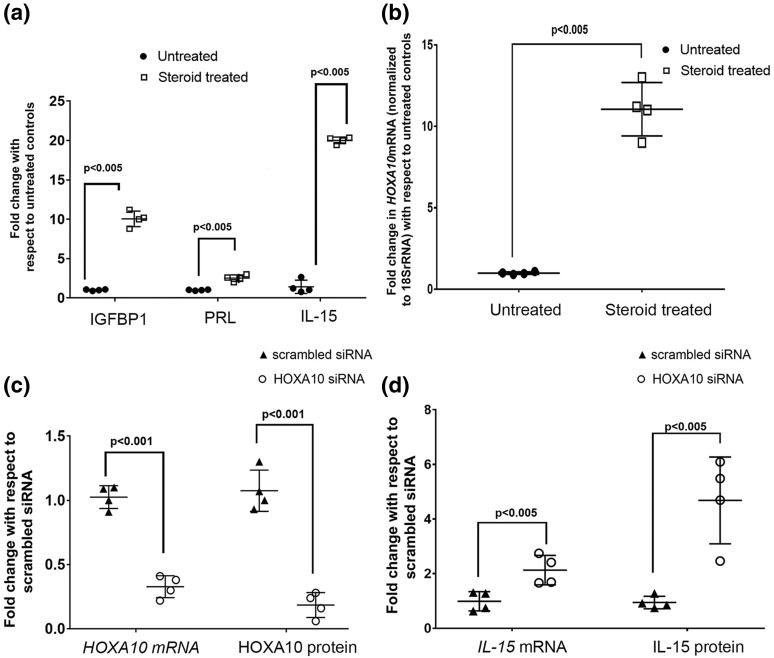
As compared with untreated controls, there was significant increase in levels of IGFBP-1, prolactin, and IL-15 in supernatants of stromal cells treated with steroids [Fig. 2(a), which is indicative of successful decidualization. In the decidualized endometrial stromal cells, the mRNA levels of HOXA10 were almost 8- to 10-fold higher as compared with nondecidualized controls [Fig. 2(b)]; the difference was statistically significant. Further, expression of HOXA10 was knocked down by siRNA in the in vitro decidualized cells, and the levels of HOXA10 mRNA and protein were measured. By real-time PCR, the mRNA levels of HOXA10 were found to be 80% lower in the HOXA10 siRNA–transfected cells as compared with scrambled siRNA–transfected controls [Fig. 2(c)]. By Western blotting, the intensity of the band corresponding to HOXA10 was found to be lower in the HOXA10 siRNA–transfected cells as compared with the cells transfected with scrambled siRNA. The bands corresponding to β-actin did not differ between the two groups (Supplemental Fig. 2). There was an almost 70% reduction in levels of HOXA10 protein in the decidual cells after 72 hours of siRNA transfection. This difference was statistically significant.
Figure 2.
Effects of HOXA10 knockdown on expression of IL-15 in decidualized human endometrial stromal cells. Human endometrial stromal cells were decidualized by treatment with estradiol and progesterone (steroid treated) for 21 days. (a) Expression of IGFBP-1, prolactin, and IL-15 in the culture supernatants and (b) the level of HOXA10 mRNA are compared in stromal and decidualized cells. In (a) and (b), the values on the y-axis are expressed as fold change with respect to untreated controls. HOXA10 was knocked down by siRNA transfection in decidualized cells, and levels of HOXA10 mRNA and protein after 72 hours of transfection are shown in (c). (d) IL-15 mRNA and protein expression in the HOXA10-silenced decidualized cells. In (c) and (d), the values on the y-axis are expressed as fold change with respect to scrambled transfected control. In all the graphs, each dot represents one sample, and the data are derived out of four biological replicates. Values that are statistically significant are denoted by respective bars along with the respective P values.
The levels of IL-15 mRNA were measured in decidual cells by real-time PCR. As compared with the scrambled transfected controls, the levels of IL-15 mRNA were significantly higher in the HOXA10-silenced cells [Fig. 2(d)], and the protein was found to be significantly higher in the supernatants of the same cells [Fig. 2(d)].
HOXA10 is differentially expressed in the decidua at the sites of trophoblast invasion compared with noninvading sites
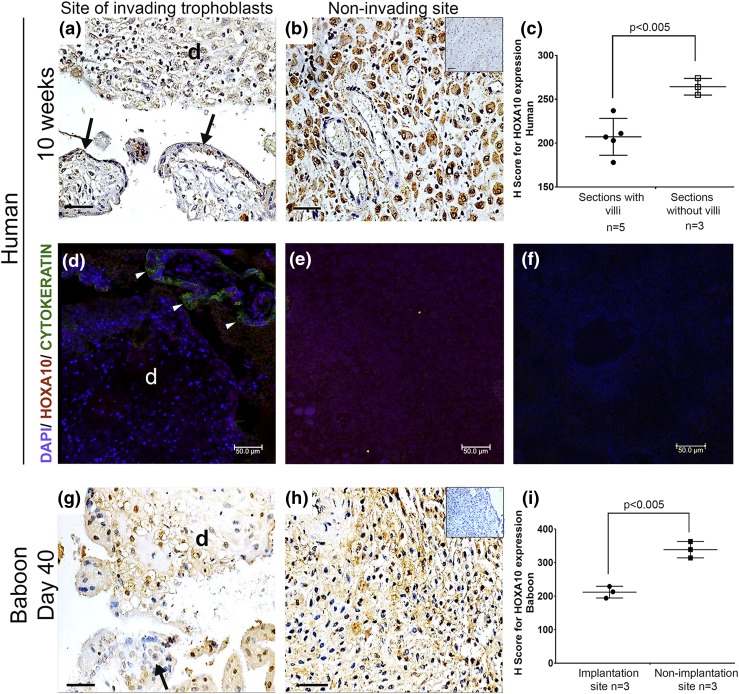
Immunohistochemically, in the human decidual sections where placental villi were detected [Fig. 3(a)], the expression of HOXA10 was weaker as compared with that observed in decidual tissue that did not contain villous tissues [Fig. 3(b)]. The H score for HOXA10 was significantly lower in the implantation site decidua as compared with decidua from the nonimplantation site [Fig. 3(c)]. To verify this, immunofluorescence for HOXA10 and cytokeratin (trophoblast marker) was performed and demonstrated weak staining for HOXA10 in decidual tissue juxtaposed to the placental villi [Fig. 3(d)] that were stained green for cytokeratin as compared with those sections where the villi were not detected [Fig. 3(e)]. The negative control [Fig. 3(f)] did not show any staining.
Figure 3.
Differential expression of HOXA10 at implantation and nonimplantation sites in the human and baboon decidua. Immunohistochemistry for HOXA10 in the decidual sections was performed. (a and g) The section of the decidua (marked as “d”) in human and baboon, respectively, at implantation site as judged by the presence of placental villi (marked with arrows). (b and h) The section of the decidua from the nonimplantation site as judged by the absence of placental villi in human and baboon, respectively. Negative control section incubated without primary antibody is shown in the inset of (b). Bar, 50 µm. (c and i) Graphs representing the H-scores for HOXA10 in sections with and without placental villi in humans and implantation and nonimplantation sites in baboons, respectively. (d) Double immunofluorescence for HOXA10 and cytokeratin in human decidua was performed; the nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Arrowheads indicate the section where the cytokeratin-positive trophoblast cells in the villi were detected. The decidual tissue is marked as “d.” (e) Decidual section without placental villi. (f) Negative control without primary antibody. In all the graphs, each dot represents one sample, and the mean values that are statistically significant are denoted by bars along with the respective P values. The number of biological replicates analyzed in case is represented as (n).
In the tissue sections from the implantation site of pregnant baboon (40 to 60 days from last menstrual bleed), invading trophoblast cells and placental villi were seen. The expression of HOXA10 was weaker in the decidual cells as compared with the decidua from the nonimplantation sites [Fig. 3(g) and 3(h)]. No staining was detected in the negative sections. The H score for HOXA10 in the decidual tissue from the implantation sites was significantly lower (P < 0.005) as compared with that for the decidua from the nonimplantation site [Fig. 3(i)]. The levels of HOXA10 mRNA were also found to be lower in implantation site as compared with nonimplantation sites (Supplemental Fig 2).
Downregulation of HOXA10 in decidual cells increased the invasion of trophoblast cells
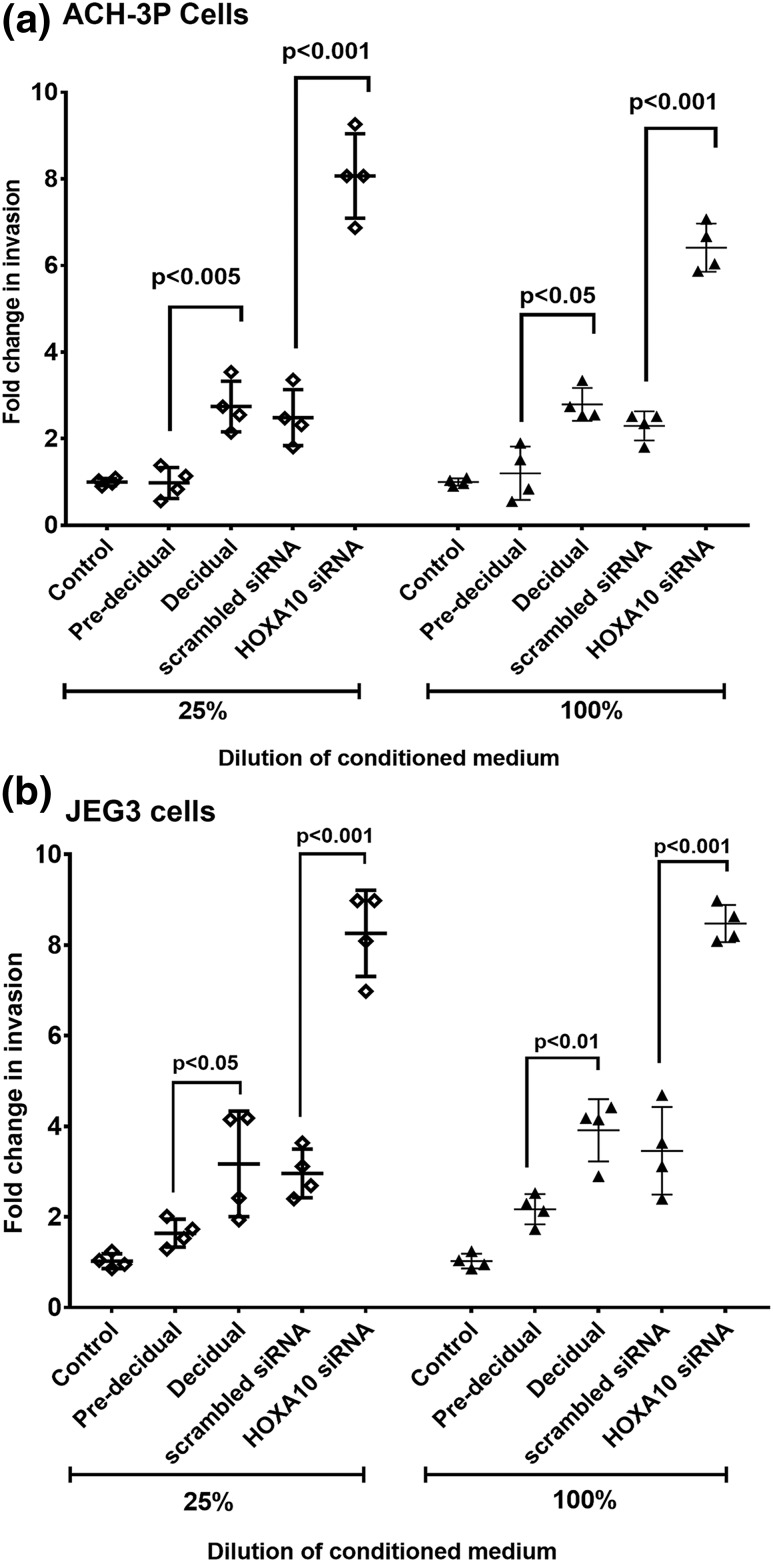
To examine if the loss of HOXA10 in decidual cells affects trophoblast invasion, culture supernatant derived from HOXA10 knockdown decidual cells was used to test the invasiveness of ACH-3P and JEG3 cells (Fig. 4). As compared with controls (culture supernatant obtained from predecidualized stromal cells), the medium derived from decidualized stromal cells significantly increased the invasion of ACH-3P and JEG3 cells. However, when HOXA10 was knocked down in the decidual cells, the medium increased the number of invading ACH-3P and JEG3 cells further by six- to eightfold over predecidualized controls; this increase was statistically significant. There was no significant difference in invasiveness of ACH-3P or JEG3 cells with neat or 25% diluted medium (Fig. 4).
Figure 4.
Effect of HOXA10 knockdown in decidual cells on invasion of trophoblast cells. Trophoblast cell lines (a) ACH-3P and (b) JEG3 were treated with diluted (25%) and neat (100%) conditioned medium and loaded onto the Matrigel. After 24 hours of incubation, cells present in the lower chamber were counted. The different types of conditioned media used are shown on the x-axis. Predecidual refers to conditioned medium from endometrial stromal cells treated with steroids for 8 days; decidual refers to conditioned medium from endometrial stromal cells treated with steroids for 21 days. Scrambled siRNA and HOXA10 siRNA samples are the conditioned medium obtained from decidual cells (day 21 of steroid treatment) after 72 hours of transfection with respective siRNAs. Control represents nontransfected control cells. Values on the y-axis represent the fold change in the number of invading cells with respect to control medium (taken as 1). Each dot represents value obtained from one experiment. Mean values that are statistically significant (n = 4) are denoted by bars, along with the respective P values.
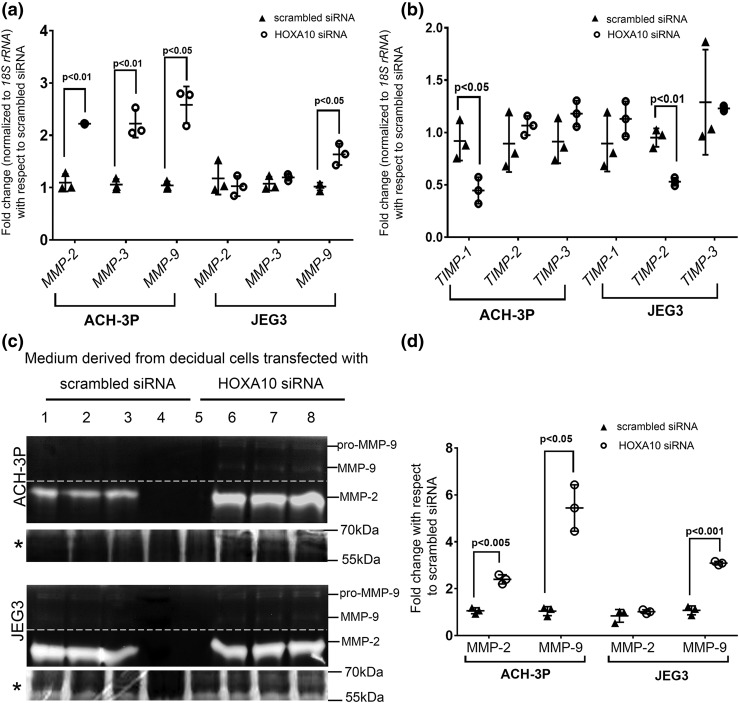
Downregulation of HOXA10 in decidual cells increased the expression of MMPs and repressed tissue inhibitors of matrix metalloprotease in the trophoblast cells
As compared with the medium derived from scrambled siRNA–transfected controls, treatment of ACH-3P cells with culture medium derived from HOXA10-depleted decidualized cells significantly increased the levels of MMP-2, -3, and -9 mRNA. In ACH3P cells, the expression of MMP-2 and MMP-3 mRNA increased by approximately twofold, and the MMP-9 mRNA expression increased by approximately threefold [Fig. 5(a)]. In case of JEG3 cells, as compared with controls, medium derived from HOXA10-depleted decidual cells increased the expression of MMP-9 mRNA by approximately twofold. However, there were no significant differences in levels of MMP-2 and MMP-3 mRNA between the two groups [Fig. 5(a)].
Figure 5.
Effects of HOXA10 knockdown in decidual cells on expression of the MMPs and tissue inhibitors of TIMPs in trophoblast cells. Human endometrial stromal cells were decidualized in vitro, and the cells were transfected with siRNA against HOXA10. Cells transfected with scrambled siRNAs were kept as transfection control. The conditioned medium from these cells was collected after 72 hours. ACH-3P and JEG3 cells were challenged with the conditioned medium for 24 hours. Medium was collected to perform zymography, and cells were harvested to extract the RNA. The mRNA levels of (a) MMP-2, -3, and -9 and (b) TIMP-1, -2, and -3 were measured by real-time PCR. Gene expression data are represented as fold change where the control value was taken as 1. (c) Representative image of the gelatin zymogram. Lanes 1, 2, and 3 are supernatants of JEG3/ACH-3P cells challenged with the medium from scrambled siRNA-transfected decidual cells. Lanes 4 and 5 are loaded with heat-inactivated medium. Lanes 6, 7, and 8 are supernatants of JEG3/ACH-3P cells challenged with supernatant obtained from HOXA10 siRNA-transfected decidual cells (each representing three different experiments). The bands for pro- and active MMP-9 and MMP-2 are marked. The dotted line indicates that the contrast was adjusted separately for MMP-9 and MMP-2 bands for visual representation, and the gels were merged. The Coomassie-stained gel image is shown below each zymogram and is denoted by an asterisk. The intensities of the bands were densitometrically analyzed. (d) Activity of active MMP-9 and total MMP-2 as fold change. The value of the control (medium from scrambled siRNA–transfected decidual cells) has been taken as 1. In all the graphs, each dot represents one value. The statistically significant mean values derived from three independent experiments (n = 3) are denoted by bars along with the respective P values.
In the case of tissue inhibitors of matrix metalloprotease (TIMPs), as compared with medium from scrambled siRNA–transfected controls, the medium from HOXA10-depleted decidual cells significantly reduced the expression of TIMP-1 mRNA in ACH-3P cells and TIMP-2 mRNA in JEG3 cells. The levels of other TIMPs were not significantly different in both the groups [Fig. 5(b)]. Gelatin zymography was performed to analyze the observed changes in the expression of MMPs. Bands corresponding to MMP-9 and MMP-2 were observed in supernatants obtained from both ACH-3P and JEG3 cells. No bands were detected in the lanes loaded with heat-inactivated culture supernatant, indicating the specificity of the assay. All the lanes had equal loading as judged by Coomassie blue staining of cognate gels [Fig. 5(c)]. In the case of ACH-3P cells challenged with culture medium derived from HOXA10-depleted decidual cells, bands corresponding to both MMP-9 and MMP-2 were more intense as compared with those observed for ACH-3P cells incubated with culture medium derived from scrambled siRNA–transfected decidual cells. Quantitatively, the activity of MMP-2 and -9 increased significantly by ∼1.5- and 5-fold, respectively [Fig. 5(d)]. In the case of JEG3 cells challenged with the culture supernatant derived from HOXA10-silenced decidual cells, a significant increase (approximately threefold) in activity of MMP-9 was observed [Fig. 5(d)]. However, the activity of MMP-2 remained unchanged between the two groups.
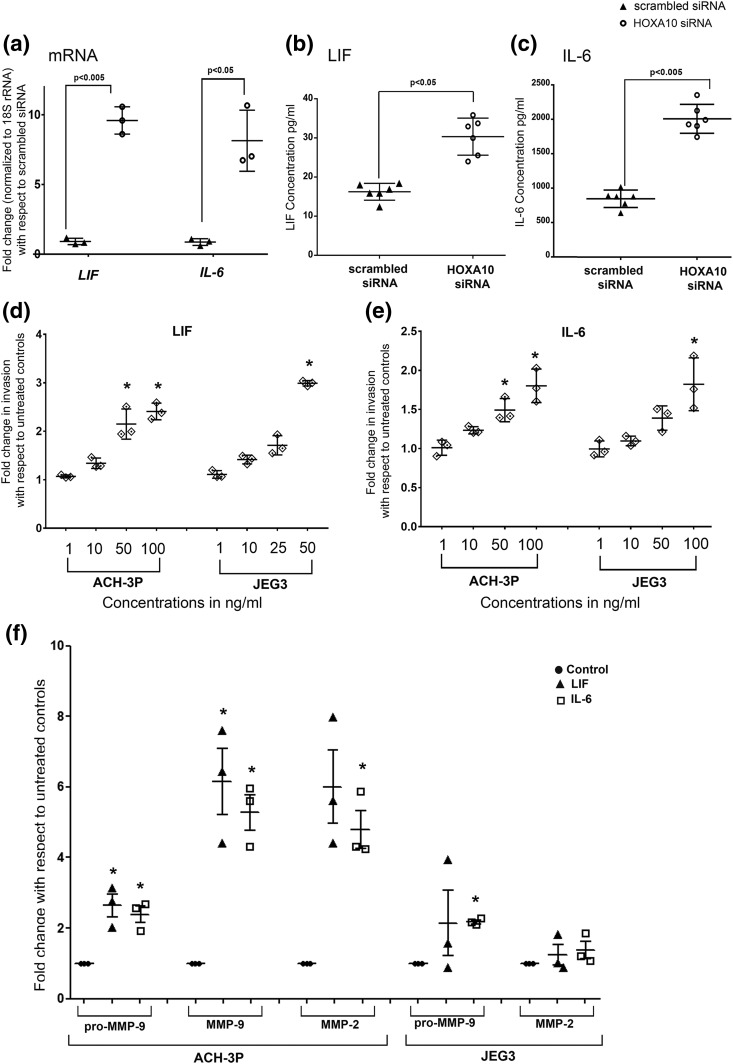
Downregulation of HOXA10 increased the expression and secretion of LIF and IL-6 in the decidual cells
In the decidual cells depleted for HOXA10, the mRNA levels of LIF and IL-6 were significantly higher (∼10- and 9-fold, respectively) as compared with scrambled siRNA–transfected controls [Fig. 6(a)]. Similar increases in levels of LIF and IL-6 were observed in the culture supernatant [Fig. 6(b) and 6(c)].
Figure 6.
Effects of HOXA10 knockdown in the in vitro decidualized human endometrial stromal cells on expression of LIF and IL-6 and the effect of recombinant LIF and IL-6 on trophoblast invasion and activity of MMPs. In vitro decidualized endometrial cells were transfected with scrambled and HOXA10 siRNA. After 72 hours, the RNA was isolated and processed for the analysis of the expression of LIF and IL-6 by real-time PCR. Supernatants were used to measure the levels of secreted LIF and IL-6 proteins through bead arrays. (a) Expression of LIF and IL-6 as fold change with respect to scrambled siRNA. (b and c) LIF and IL-6 expression, respectively, in the medium after HOXA10 silencing in the decidual cells. Values on the y-axis represent the mean of three independent experiments performed in duplicate. (d and e) The effect of increasing concentration of recombinant LIF and IL-6 on invasion of trophoblast cells. (f) The effect of LIF and IL-6 treatment on activity of MMP-2 and MMP-9 (as assessed by gelatin zymography) in ACH-3P and JEG3 cells. In all the graphs, values observed in untreated controls were taken as one, and the data are represented as fold change over untreated controls. Each dot represents value from one experiment. Statistically significant mean values (as compared with controls) derived from independent experiments (n = 3 or 4) are denoted by an asterisk.
LIF and IL-6 promote invasion, increase the activity of MMPs, and inhibit TIMP expression in trophoblast cells
Treatment of both ACH-3P and JEG3 cells with LIF or IL-6 increased their invasiveness. As compared with controls, 50 ng of LIF significantly increased invasion of both ACH-3P and JEG3 cells [Fig. 6(d)]. Concentrations of 50 and 100 ng/mL of IL-6 also significantly increased the invasion of ACH-3P cells. A significant increase in the invasion of JEG3 cells was observed only at 100 ng/mL of IL-6 [Fig. 6(e)]. Both recombinant LIF and IL-6 independently increased the pro-MMP-9, MMP-9, and MMP-2 activity in ACH-3P cells; in JEG3 cells, only IL-6 caused a significant increase in the activity of MMP-9 [Fig. 6(f); Supplemental Fig. 3].
We also measured the mRNA levels of TIMP-1, -2, and -3 in ACH-3P and JEG3 cells challenged with LIF and IL-6 (Supplemental Fig. 3). In ACH-3P cells, LIF significantly reduced the mRNA levels of all the three TIMPs. In case of JEG3 cells, the levels of TIMP-1 mRNA were unaffected, whereas TIMP-2 and TIMP-3 levels were reduced significantly by LIF. IL-6 significantly reduced the mRNA levels of TIMP-2 and -3 in both ACH-3P and JEG-3 cells, but the level of TIMP-1 mRNA was unaffected in both the cell lines.
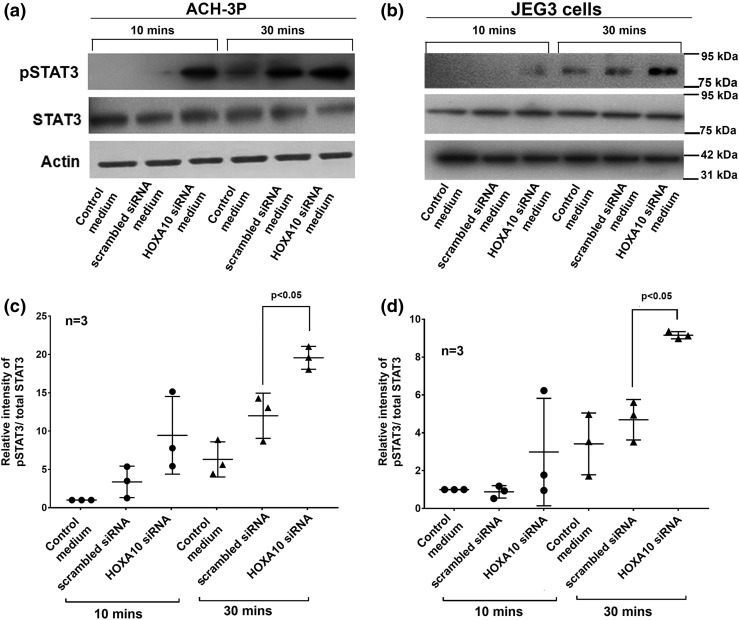
Silencing of HOXA10 in decidual cells increased STAT3 phosphorylation in trophoblast cells
As compared with controls (medium from scrambled siRNA–transfected cells), treatment of ACH-3P and JEG3 cells with supernatants from HOXA10 siRNA-transfected cells modestly increased the phosphorylation of STAT3 (Tyr 705) at 10 minutes, which was not statistically significant (Fig. 7). However, at 30 minutes of incubation phosphorylation of STAT3 (Tyr 705) increased significantly in both ACH-3P and JEG3 cells as compared with controls [Fig. 7(c) and 7(d)]. The levels of total STAT3 and β-actin did not differ between the groups [Fig. 7(a) and 7(b)].
Figure 7.
Effects of conditioned medium from HOXA10 knockdown decidual cells on phosphorylation of STAT3 in trophoblast cells. (a and b) Representative Western blots for phosphorylated STAT3 (pSTAT3 -Tyr705), total STAT3, and β-actin in ACH-3P and JEG3 cells challenged with conditioned medium from scrambled siRNA–transfected or HOXA10 siRNA–transfected decidual cells for 10 and 30 minutes. Supernatant derived from nontransfected controls were used as negative control. The position of the nearest molecular weight markers is shown next to each image. (c and d) Densitometric analysis of the band intensities from three independent experiments. Band intensities were densitometrically calculated, and values of pSTAT3 were normalized with total STAT3. Values on the y-axis represent the fold change in pSTAT3 in cells treated with culture supernatants derived from scrambled and HOXA10 siRNA–treated decidual cells with respect to control culture medium (taken as one). In all the graphs, each dot represents value from one experiment, and the statistically significant mean values (as compared with controls) derived from independent experiments (n = 3 or 4) are denoted by bars along with the respective P values.
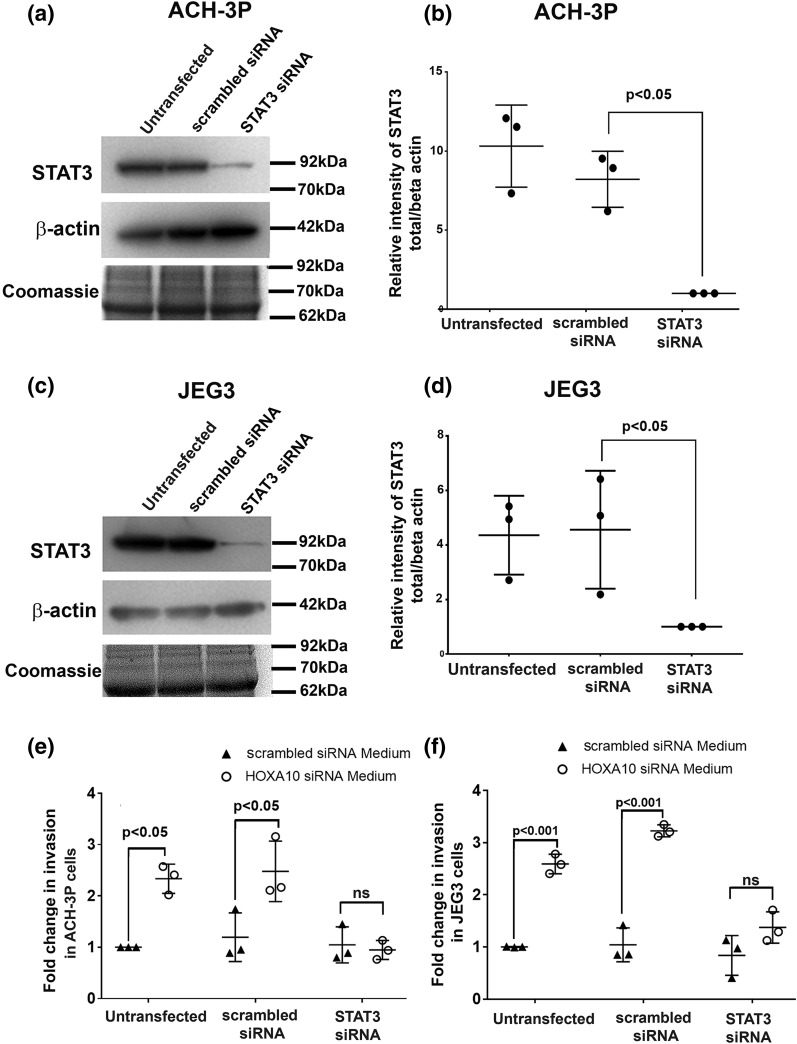
STAT3 in trophoblast cells is necessary and sufficient to increase invasion mediated by loss of HOXA10 in decidual cells
As compared with scrambled siRNA controls, there was a significant (∼90%) reduction in STAT3 protein in ACH-3P and JEG3 cells transfected with STAT3-specific siRNA [Fig. 8(a–d)]. These cells were then used for Matrigel-based invasion assay to study the effect of the conditioned medium derived from HOXA10-depleted cells in this system. As expected [Fig. 8(e) and 8(f)], the invasion of untransfected and scrambled siRNA–transfected ACH-3P and JEG3 cells increased by four- to fivefold in response to medium derived from HOXA10 knockdown decidual cells when compared with medium derived from controls (scrambled siRNA–transfected decidual cells). However, this increase was not observed when STAT3 was silenced in the trophoblast cells. The invasiveness of the STAT3 knockdown ACH-3P and JEG3 cells was identical when challenged with medium derived from HOXA10-knocked down decidual cells and medium derived from scrambled transfected decidual cells [Fig. 8(e) and 8(f)].
Figure 8.
Effect of STAT3 knockdown on the invasiveness of trophoblast cells in response to conditioned medium from HOXA10 knockdown decidual cells. ACH-3P and JEG3 cells were transfected with scrambled or STAT3 siRNAs. After 48 hours, the amount of total STAT3 was measured by Western blotting. (a and c) Representative Western blots for STAT3 and β-actin along with the Coomassie-stained gel image for total protein. The position of the nearest molecular weight markers is shown next to each image. Band intensities were desitometrically estimated, and values of STAT3 were normalized with respect to β-actin. (b and d) The relative intensity of STAT3 upon normalization after STAT3 silencing in ACH-3P and JEG-3 cells from three independent experiments. Scrambled or STAT3 siRNA–transfected ACH-3P and JEG3 cells were challenged with supernatants derived from scrambled or HOXA10 siRNA–transfected decidual cells, and the invasion was measured after 24 hours of treatment. (d and e) Fold change in invasiveness of cells as compared with nontransfection controls (taken as 1). Data are derived from three independent experiments involving different pools of supernatants from HOXA10-transfected and scrambled siRNA–transfected decidual cells. Each dot represents the value from one experiment. Statistically significant mean values (as compared with controls) derived from independent experiments are denoted by bars along with the respective P values. ns, not significant.
Discussion
Previous studies have shown that levels of HOXA10 in the endometrium increase from the follicular to luteal phase and increase further in the decidualizing stromal cells at the time of implantation (17–23). Transcripts for HOXA10 have also been reported in first-trimester human decidua (17, 33). Interestingly, mice knockout for HOXA10 have implantation failure, and the stromal cells fail to proliferate just prior to decidualization (20, 24). These observations led to the notion that HOXA10 is indispensable for implantation and can be considered as a hallmark of successful decidual transformation (18, 19). However, herein, we observed that, as compared with luteal-phase human endometrium, the expression of HOXA10 mRNA and protein are downregulated in first-trimester human decidua. Corroborating the human data, we also observed that even in baboons the levels of HOXA10 mRNA and protein are reduced in the postimplantation-stage decidua as compared with implantation-stage uterus. Similar to HOXA10, in the mouse, NOTCH1 peaks in the endometrium at the time of embryo implantation, whose expression gets rapidly downregulated in the decidua after implantation (34). These observations suggest that the decidual transformation may be a biphasic process that occurs first at the time of embryo implantation and then after implantation. We have previously shown that there is an increase in expression of HOXA10 in human endometrial stromal cells during in vitro decidualization (14); silencing the expression of HOXA10 in decidualized cells increases the expression of decidual marker IGFBP-1, whereas overexpressing HOXA10 leads to its downregulation (35), suggesting that reduction in HOXA10 may be required for further differentiation of the stromal cells. To address this further, we studied the level of IL-15, which is known to be required for decidualization (36, 37). We observed that the levels of IL-15 are increases in the endometrial stromal cells upon in vitro decidualization; its levels are elevated further upon HOXA10 knockdown. These observations imply that at the time of embryo implantation, HOXA10 is essential for initial transformation of endometrial stromal cells. Loss of HOXA10 subsequently leads to its further differentiation in to decidual cells.
We next asked what could be the functional significance of loss of HOXA10 in the decidual cells. In this study, we observed that although the expression of HOXA10 was reduced in the decidua, the levels were relatively lower in decidual cells juxtaposed to the placental villi as compared with the decidual tissue where the placental villi were absent. We also observed that the levels of HOXA10 mRNA were lower in the baboon decidual cells at the site of implantation site as compared with nonimplantation sites. These observations led us to speculate that loss of HOXA10 in the decidual cells may have an association with trophoblast invasion. To address this question, we studied the effects of culture supernatants derived from primary cultures of human endometrial stromal cells, in vitro decidualized stromal cells, and HOXA10 knockdown decidual cells on invasion of trophoblast cell lines JEG3 and ACH-3P. This experiment allowed us to specifically investigate the functional significance of HOXA10 downregulation in the decidual cells. The results revealed that although initial transformation of endometrial stromal cells is essential for trophoblast invasion, when HOXA10 is specifically depleted in the decidual cells there is an increase in invasion of both ACH-3P and JEG3 cells. These observations imply that decidual transformation of endometrial stromal cells is essential to permit trophoblast invasion, and loss of HOXA10 in these cells further potentiates the invasion process.
Trophoblast invasion in the decidua is an active process that requires degradation of the extracellular matrix proteins. The EVTs produce a plethora of MMPs, of which MMP-2 and -9 are indispensable for invasion (38, 39). The activities of the MMPs are negatively regulated by the TIMPs, and the relative abundance of MMPs and TIMPs produced by the EVTs regulates invasion (38, 39). Herein we observed that the supernatants derived from HOXA10-depleted decidual cells significantly elevated mRNA expression levels of the MMP-2, -3, and -9 in ACH-3P cells and MMP-9 in JEG3 cells; there was also a concomitant increase in enzymatic activity of MMP-2 and -9 in ACH-3P cells and MMP-9 in JEG3 cells. Intriguingly, along with the increase in MMPs, there was a decrease in the mRNA levels of TIMP-1 in ACH-3P and TIMP-2 in JEG3 cells. The precise reason for such differential responses of the two cell lines in terms of regulation of MMPs and TIMPs is unclear. However, similar differences are observed in various trophoblast cell lines in response to different stimuli (10, 28, 31), and these are thought to occur due to the differences in the signaling mechanisms used by the different cell lines (28, 40). In our case too, it is possible that the JEG3 and ACH-3P cells use different signaling pathways to respond to the culture supernatants leading to such disparate responses. Nevertheless, irrespective of these differences, our results indicate that loss of HOXA10 in the decidual cells in a paracrine manner in general increases the MMPs and downregulates the TIMPs in the trophoblast cells to aid trophoblast invasion.
We next aimed to understand the mechanism by which loss of HOXA10 in the decidual cells increases trophoblast invasion. Because secretions derived from decidualized cells with reduced HOXA10 promoted trophoblast invasion, we hypothesized that downregulation of HOXA10 must increase the expression of some proinvasive factors in the decidual secretions. Analysis of the microarray data of the uterine/decidual cells depleted of HOXA10 identified several differentially expressed genes that are known regulators of trophoblast invasion (Supplemental Table 1). Among these, the increase in levels of LIF, IL-6, and IL-11 in the HOXA10 knockdown decidual cells was interesting because they are known to be abundantly produced by first-trimester human decidua and to promote trophoblast invasion (36, 37, 40–44). We have previously reported an increase in expression of IL-11 by HOXA10 knockdown in decidual cells (14). In this study we further validated the microarray data by reverse transcription PCR and observed that the mRNA expression of IL-6 and LIF also increased by severalfold in the HOXA10-depleted decidualized cells. Furthermore, the levels of LIF and IL-6 proteins are also higher in the secretions of HOXA10-depleted cells, suggesting that the loss of HOXA10 in the decidual cells would increase the production of gp130 cytokines, which, in a paracrine manner, would promote invasion. Indeed, both recombinant LIF and IL-6 individually were found to increase the invasion of ACH-3P and JEG3 cells; akin to the cell supernatants, both the cytokines increased the activity of MMP-2 and -9 in ACH-3P cells and MMP-9 in JEG3 cells. LIF and IL-6 also downregulated TIMP-1 and TIMP-2 mRNA levels in both cell lines. Taken together, our observations imply that loss of HOXA10 in decidual cells would increase the expression and secretion of LIF and IL-6, which in a paracrine manner would alter expression of MMPs and TIMPs in the trophoblast cells to promote invasion.
In most cells, including trophoblasts, LIF and IL-6 activate the Janus kinase pathway to phosphorylate cytoplasmic STAT3, which translocates to the nucleus and activates the expression of its target genes (mainly MMPs) to drive invasion (40, 44, 45). This phosphorylation is essential because reduced pSTAT3 is observed in trophoblast cells from placenta of pregnancies complicated with shallow invasion (46). Thus, we next tested the effects of supernatants derived from HOXA10-depleted decidual cells on STAT3 phosphorylation in trophoblast cells. The results revealed that the culture supernatants derived from HOXA10-silenced decidual cells phosphorylated STAT3 (Tyr 705) in both the cell lines and activated the STAT pathway. We next asked if the invasion-promoting ability of the paracrine signals from the HOXA10-depleted decidual cells is STAT3 dependent. For the purpose, the expression of STAT3 in ACH-3P and JEG3 cells was knocked down by siRNA, and their invasive ability in response to supernatants from decidual cells with reduced HOXA10 was tested. The results revealed that the invasion-promoting ability of supernatants derived from HOXA10-depleted decidual cells was significantly compromised when STAT3 was silenced in JEG3 and ACH-3P cells. A similar attenuation in invasion of trophoblast cells in response to LIF and IL-6 in the absence of STAT3 has been previously reported (29, 32, 44, 45). These results imply that STAT3 activation is necessary and sufficient to increase trophoblast invasion mediated by loss of HOXA10 in decidual cells. Together our data indicate that loss of HOXA10 in decidual cells increases the expression and secretion of LIF and IL-6, which in a paracrine manner phosphorylates STAT3 in the trophoblast cells to increase expression of MMPs and promote invasion.
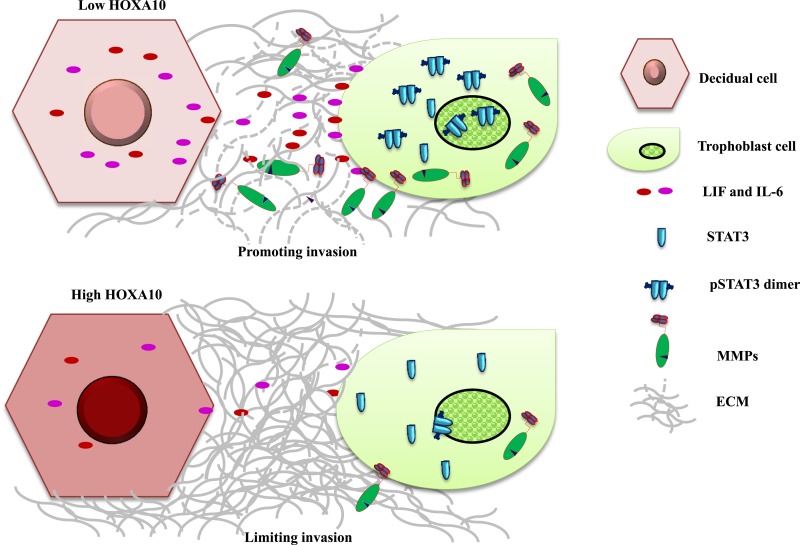
In summary, our results show that trophoblast invasion in the decidua appears to be a two-step process controlled by HOXA10 (Fig. 9). In the first step, at the time of implantation, high levels of HOXA10 in the stromal cells promote decidualization, making the tissue conducive for trophoblast invasion. In the second step, with the downregulation of HOXA10 in the decidual cells, there is a burst in production of LIF and IL-6, which in a paracrine manner activates STAT3 and stimulates MMP expression in the trophoblast cells to enhance their invasion. In decidual cells that retain HOXA10 (like at the nonimplantation sites), there is limited production of proinvasive molecules there by restraining invasion. Based on these data, it is tempting to postulate that, during the course of pregnancy, there could be zones of high and low amounts of HOXA10, creating localized gradients of proinvasive factors in the decidua, which would differentially regulate STAT3 activity and MMP production by the trophoblast cells, thereby permitting an ordered and judicious invasion (Fig. 9). Such an activity of HOXA10 would be highly desirous in vivo to avoid premature invasion and may aid in setting the limit of the placentation within the decidua. It will be of interest to study the expression of HOXA10 in the decidua of pregnancies complicated with defective trophoblast invasion and determine how defective HOXA10 signaling might contribute to placentopathies, which are the leading causes of obstetric complications.
Figure 9.
HOXA10-STAT3 crosstalk in governing trophoblast invasion. At the site of invasion where HOXA10 levels are low in the deicudal cells, there is increased secretion of gp130 cytokines. These cytokines in a paracrine manner phosphorylate STAT3 in trophoblast cells, thereby increasing the expression of MMPs to facilitate the degradation of extracellular matrix and increase their invasiveness. At sites where the decidual HOXA10 is high, the expression of gp130 cytokines is kept under check, thereby limiting the STAT3 activation, MMP production, and hence the trophoblast invasion (see text for further explanation).
Our findings have revealed important molecular steps in the process of decidualization and decidual control of placentation. These results will have critical relevance in basic understanding of trophoblast biology and may also explain the molecular basis of obstetric complications caused due to faulty trophoblast invasion.
Supplementary Material
Acknowledgments
We thank Dr. Stacy Colaco and Ms. Neha Singh (National Institute for Research in Reproductive Health) for technical help. The microarray dataset used in this study is available at http://www.ncbi.nlm.nih.gov/gds (accession no. GSE 35801).
Current Affiliation: G. Godbole’s current affiliation is the Department of Biological Sciences, Tata Institute for Fundamental Research, Mumbai 400005, India. P. Suman’s current affiliation is DBT-National Institute of Animal Biotechnology, Hyderabad, 500049, India.
This work (RA/284/08-2015) was supported by grants from the Department of Biotechnology, the Department of Science and Technology, and the Indian Council of Medical Research, Government of India (to D.M.); by Grant GIA/28/2014-DHR from the National Institute of Immunology, New Delhi, India, Department of Health Research (to S.K.G.); by a J. C. Bose Fellowship (SB/S2/JCB_040/2015) from the Science and Engineering Research Board, Department of Science and Technology, Government of India (to S.K.G.); by Junior and Senior Research Fellowships from the Lady Tata Memorial Trust, Mumbai, India, and Council of Scientific and Industrial Research (CSIR), Government of India (to G.G.); by an SRF grant by CSIR (to P.S.); and by a postdoctoral fellowship from Indian Council of Medical Research (to M.G.).
Author contributions: D.M. and G.G. conceived the study; G.G., A.M., P.S., and M.G. performed the experiments and analyzed the data; D.M., S.K.G., G.G., and P.S. interpreted the results; N.J. and A.F. provided the baboon data; and all authors contributed in writing the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- EVT
extravillous trophoblast
- IL
interleukin
- LIF
leukemia inhibitor factor
- MMP
matrix metalloprotease
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- TIMP
tissue inhibitors of matrix metalloprotease.
Appendix.
Antibodies Used in This Study
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Mono/Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| HOXA10 | Epitope mapping near the N-terminus | HOXA10 (N-20) | Santa Cruz, #sc-17158 | Goat; polyclonal | 1:100 | AB_2248649 |
| Phospho-STAT3 | Synthetic phosphopeptide of residues surrounding Tyr 705 of mouse STAT3 | Phospho-STAT3 (Tyr705) | Cell Signaling Technologies, #9131 | Rabbit; polyclonal | 1:1000 | AB_331586 |
| STAT3 | Synthetic peptide of residues surrounding Tyr705 of mouse STAT3 | STAT3 | Cell Signaling Technologies, #9132 | Rabbit; polyclonal | 1:1000 | AB_331588 |
| IGFBP-1 | E. coli–derived recombinant human IGFBP-1 Ala26-Asn259 | Human IGFBP-1 | R & D Systems, #AF871 | Goat; polyclonal | 1: 500 | AB_355674 |
| Cytokeratin 8 | Recombinant fragment within amino acids 227–483 of Human Cytokeratin 8 | Anticytokeratin 8 | Abcam, #ab51152 | Rabbit; polyclonal | 1:100 | AB_869900 |
| β-actin | Synthetic peptide of residues near the amino-terminus of human β-actin. | β-actin (13E5) | Cell Signaling Technologies, #4970 | Rabbit; monoclonal | 1:1000 | AB_2223172 |
Abbreviation: RRID, research resource identifier.
References
- 1. James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. Part I: what do we know about formative placental development following implantation? Placenta. 2012;33(5):327–334. [DOI] [PubMed] [Google Scholar]
- 2. Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):273–285. [DOI] [PubMed] [Google Scholar]
- 3. Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2007;14(Spec No 1):101–109. [DOI] [PubMed] [Google Scholar]
- 4. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 6. Hu Y, Eastabrook G, Tan R, MacCalman CD, Dutz JP, von Dadelszen P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta. 2010;31(3):213–221. [DOI] [PubMed] [Google Scholar]
- 7. Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18(4):458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. [DOI] [PubMed] [Google Scholar]
- 9. Croy BA, Gambel P, Rossant J, Wegmann TG. Characterization of murine decidual natural killer (NK) cells and their relevance to the success of pregnancy. Cell Immunol. 1985;93(2):315–326. [DOI] [PubMed] [Google Scholar]
- 10. Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75(3):341–350. [DOI] [PubMed] [Google Scholar]
- 11. Zhu XM, Han T, Sargent IL, Wang YL, Yao YQ. Conditioned medium from human decidual stromal cells has a concentration-dependent effect on trophoblast cell invasion. Placenta. 2009;30(1):74–78. [DOI] [PubMed] [Google Scholar]
- 12. Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95:1278–1283. [DOI] [PubMed] [Google Scholar]
- 13. Menkhorst EM, Lane N, Winship AL, Li P, Yap J, Meehan K, Rainczuk A, Stephens A, Dimitriadis E. Decidual-secreted factors alter invasive trophoblast membrane and secreted proteins implying a role for decidual cell regulation of placentation. PLoS One. 2012;7(2):e31418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godbole G, Modi D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol. 2010;85(2):130–139. [DOI] [PubMed] [Google Scholar]
- 15. Evans J, Salamonsen LA. Decidualized human endometrial stromal cells are sensors of hormone withdrawal in the menstrual inflammatory cascade. Biol Reprod. 2014;90(1):14. [DOI] [PubMed] [Google Scholar]
- 16. Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122(9):2687–2696. [DOI] [PubMed] [Google Scholar]
- 17. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27(4):331–355. [DOI] [PubMed] [Google Scholar]
- 19. Modi D, Godbole G. HOXA10 signals on the highway through pregnancy. J Reprod Immunol. 2009;83(1-2):72–78. [DOI] [PubMed] [Google Scholar]
- 20. Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13(6):1005–1017. [DOI] [PubMed] [Google Scholar]
- 21. Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290(1):105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Z, Hardt J, Kim JJ. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod. 2008;14(6):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Godbole GB, Modi DN, Puri CP. Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction. 2007;134(3):513–523. [DOI] [PubMed] [Google Scholar]
- 24. Yao MW, Lim H, Schust DJ, Choe SE, Farago A, Ding Y, Michaud S, Church GM, Maas RL. Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol Endocrinol. 2003;17(4):610–627. [DOI] [PubMed] [Google Scholar]
- 25. Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76(1):102–117. [DOI] [PubMed] [Google Scholar]
- 26. Tarantino S, Verhage HG, Fazleabas AT. Regulation of insulin-like growth factor-binding proteins in the baboon (Papio anubis) uterus during early pregnancy. Endocrinology. 1992;130(4):2354–2362. [DOI] [PubMed] [Google Scholar]
- 27. Hild-Petito S, Verhage HG, Fazleabas AT. Characterization, localization, and regulation of receptors for insulin-like growth factor I in the baboon uterus during the cycle and pregnancy. Biol Reprod. 1994;50(4):791–801. [DOI] [PubMed] [Google Scholar]
- 28. Suman P, Godbole G, Thakur R, Morales-Prieto DM, Modi DN, Markert UR, Gupta SK. AP-1 transcription factors, mucin-type molecules and MMPs regulate the IL-11 mediated invasiveness of JEG-3 and HTR-8/SVneo trophoblastic cells. PLoS One. 2012;7(1):e29745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suman P, Poehlmann TG, Prakash GJ, Markert UR, Gupta SK. Interleukin-11 increases invasiveness of JEG-3 choriocarcinoma cells by modulating STAT3 expression. J Reprod Immunol. 2009;82(1):1–11. [DOI] [PubMed] [Google Scholar]
- 30. Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, Schmitz U, Fast-Hirsch C, Hengstschläger M, Pötgens A, Rüben A, Knöfler M, Haslinger P, Huppertz B, Bilban M, Kaufmann P, Desoye G. The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations: TNF-alpha stimulates MMP15 expression. BMC Dev Biol. 2007;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82(2):235–245. [DOI] [PubMed] [Google Scholar]
- 32. Suman P, Gupta SK. STAT3 and ERK1/2 cross-talk in leukaemia inhibitory factor mediated trophoblastic JEG-3 cell invasion and expression of mucin 1 and Fos. Am J Reprod Immunol. 2014;72(1):65–74. [DOI] [PubMed] [Google Scholar]
- 33. Sarno J, Schatz F, Huang SJ, Lockwood C, Taylor HS. Thrombin and interleukin-1beta decrease HOX gene expression in human first trimester decidual cells: implications for pregnancy loss. Mol Hum Reprod. 2009;15(7):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26(1):282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13(5):323–332. [DOI] [PubMed] [Google Scholar]
- 36. Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Int J Dev Biol. 2010;54(2-3):313–322. [DOI] [PubMed] [Google Scholar]
- 37. Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11(6):613–630. [DOI] [PubMed] [Google Scholar]
- 38. Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27(8):783–793. [DOI] [PubMed] [Google Scholar]
- 39. Lala PK, Graham CH. Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer Metastasis Rev. 1990;9(4):369–379. [DOI] [PubMed] [Google Scholar]
- 40. Modi DN, Godbole G, Suman P, Gupta SK. Endometrial biology during trophoblast invasion. Front Biosci (Schol Ed). 2012;4:1151–1171. [DOI] [PubMed] [Google Scholar]
- 41. Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6(10):907–914. [DOI] [PubMed] [Google Scholar]
- 42. Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One. 2011;6(9):e25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suman P, Shembekar N, Gupta SK. Leukemia inhibitory factor increases the invasiveness of trophoblastic cells through integrated increase in the expression of adhesion molecules and pappalysin 1 with a concomitant decrease in the expression of tissue inhibitor of matrix metalloproteinases. Fertil Steril. 2013;99(2):533–542. [DOI] [PubMed] [Google Scholar]
- 44. Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, Markert UR. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005;26(Suppl A):S37–S41. [DOI] [PubMed] [Google Scholar]
- 45. Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update. 2008;14(4):335–344. [DOI] [PubMed] [Google Scholar]
- 46. Borg AJ, Yong HE, Lappas M, Degrelle SA, Keogh RJ, Da Silva-Costa F, Fournier T, Abumaree M, Keelan JA, Kalionis B, Murthi P. Decreased STAT3 in human idiopathic fetal growth restriction contributes to trophoblast dysfunction. Reproduction. 2015;149(5):523–532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.