Summary
In the developing brain, heightened plasticity during the critical period enables the proper formation of neural circuits. Here we identify the “navigator” neurons, a group of perinatally born olfactory sensory neurons, as playing an essential role in establishing the olfactory map during the critical period. The navigator axons project circuitously in the olfactory bulb and traverse multiple glomeruli before terminating in perspective glomeruli. These neurons undergo a phase of exuberant axon growth and exhibit a shortened lifespan. Single cell transcriptome analyses reveal distinct molecular signatures for the navigators. Extending their lifespan prolongs the period of exuberant growth and perturbs axon convergence. Conversely, genetic ablation experiment indicates that, despite postnatal neurogenesis, only the navigators are endowed with the ability to establish a convergent map. The presence and the proper removal of the navigator neurons are both required to establish tight axon convergence into the glomeruli.
eTOC blurb:
Wu and colleagues identify a transient population of olfactory sensory neurons existing around the critical period. These neurons are morphologically and molecularly distinct from the adult olfactory sensory neurons and are critical for the formation of the olfactory map.
Introduction
The critical period represents a unique time window during which the developing nervous system is highly susceptible to environmental influence (Hubel and Wiesel, 1970). It has been described in various sensory systems, in different brain regions and across different species (Barkat et al., 2011; Berardi et al., 2000; Crair et al., 1998; Crowley and Katz, 2002; Erzurumlu, 2010; Katz and Crowley, 2002; Knudsen and Knudsen, 1990; Shatz and Stryker, 1978; Zhang et al., 2002). During the critical period, the nervous system exhibits heightened plasticity that allows the formation and reorganization of neuronal connections (Hensch, 2004; Levelt and Hubener, 2012). After the critical period, the architecture of neural circuits is maintained, and further remodeling is limited.
Recently, we and others have discovered a critical period in the formation of the olfactory map during postnatal development (Ma et al., 2014; Tsai and Barnea, 2014). Each olfactory sensory neuron (OSN) in the olfactory epithelium expresses a single type of odorant receptor (OR) gene. Axons expressing the same receptor converge into the same glomeruli in the olfactory bulb, forming a spatial map of discrete representation of odorant information (Mombaerts et al., 1996; Ressler et al., 1994; Vassar et al., 1994). This highly orchestrated axon path finding process is not limited to early development, as OSNs are continuously generated through the adult life of the animal and the convergent projection map remains constant (Costanzo, 1991; Graziadei and Graziadei, 1979). Despite the continuous neurogenesis, the ability of OSNs to restore disrupted projection is restricted to the first postnatal week (Ma et al., 2014). Beyond this time window, a disrupted map is maintained and cannot be restored.
The discovery of a critical period raises the questions as to what function it serves in the establishment of the olfactory map, and what cellular mechanisms govern the plasticity. Olfactory axons appear to converge to target glomeruli at birth (Mombaerts et al., 1996; Treloar et al., 1999), but it is not clear how individual axons navigate the developing olfactory bulb to reach their targets. Moreover, ectopic axon projections are observed during early postnatal stages (Chan et al., 2011; Royal and Key, 1999; Zou et al., 2004). These ectopic axons could merely be developmental errors, or they might serve a special function. In classic examples of neural development, including neuromuscular junction, retinocollicular and thalamocortical projections, the establishment of neuronal projections undergoes a postnatal refinement process (Allendoerfer and Shatz, 1994; Ghosh et al., 1990; Kanold et al., 2003; McLaughlin et al., 2003). The initial broad topographic projection is refined to a high precision map by pruning ectopic axons. In most systems, neurogenesis is largely completed before precise axonal connections are established. In contrast, a massive number of neurons are generated in the olfactory system postnatally, yet the ability to restore perturbed map is restricted to an early period. The influx of new axons creates a paradoxical situation in which continuous neurogenesis does not appear to be responsible for reorganization of axon projection. The mechanisms that enable early, but not late born neurons to restore convergence patterns is unknown.
In this study, we employ genetic, viral labeling of OSNs and tissue clearing technology to examine axon projection in intact olfactory bulb. By tracking individual axon trajectory at different developmental time points, we find unique morphology and axon dynamics in perinatal OSNs during the critical period. Single cell transcriptome analyses reveal that the perinatal OSN population is molecularly distinct from adult born neurons. Genetic labeling shows this population is quickly eliminated from the circuitry. Experiments that alter the timing of their elimination perturb the precision of axon targeting. Based on the specific properties of these neurons, we propose that a group of navigator cells establishes and refines OSN projection patterns during the critical period.
Results
Visualizing intact OSN axons in cleared olfactory bulb
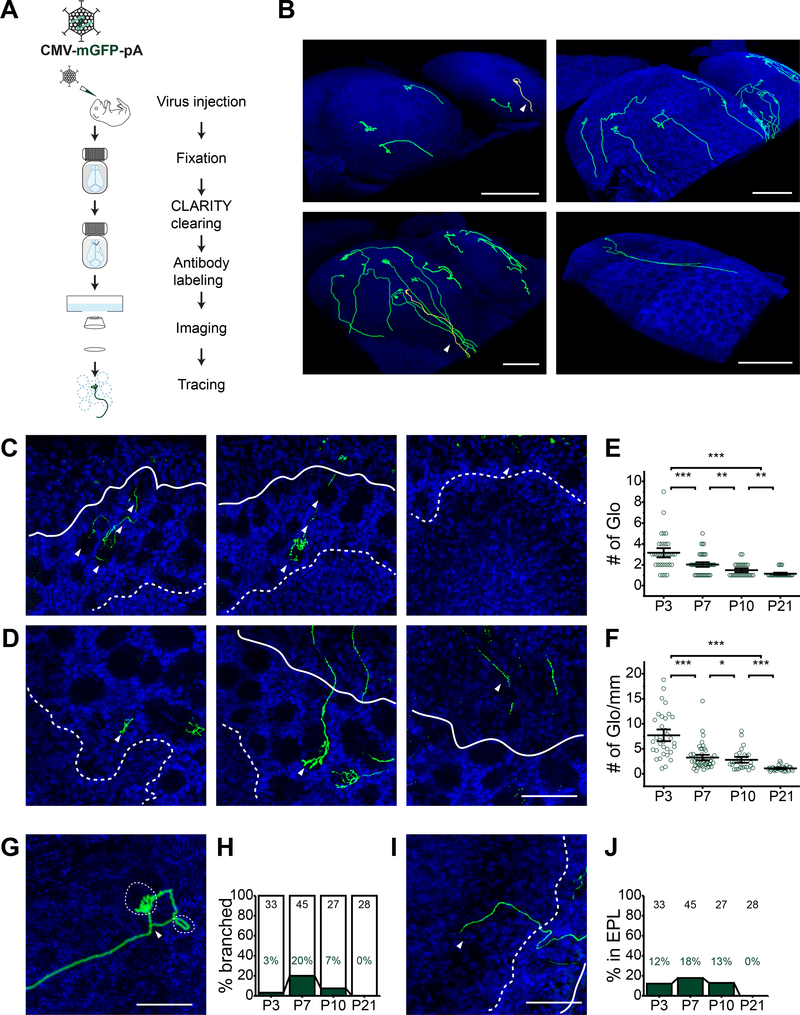
We first sought to determine whether OSNs targeted their glomeruli differently during and after the critical period. The advent of tissue clearing technology allowed us to investigate the OSN axon paths in intact olfactory bulb. We adopted the adenovirus system to stochastically label individual OSNs (Zhao et al., 1998; Zhao et al., 1996). We constructed an adenovirus (AV222) carrying membrane anchored GFP (mGFP) to enhance the labeling of axons (Muzumdar et al., 2007). After applying CLARITY method (Chung et al., 2013; Tomer et al., 2014), we stained the labeled axons with anti-GFP antibodies for confocal imaging (Figure 1A). This method allowed us to image and trace OSN axon paths at high resolution over ~2mm distance covering the entire olfactory bulb (Figure 1B, S1A, and Movie S1).
Figure 1. Visualization of individual OSN axons in intact olfactory bulb.
A. Schematic illustration of single axon tracing method in cleared olfactory bulb. Recombinant adenovirus carrying CMV-membrane GFP (AV222) is used to label individual OSNs. 48 hours after nasal infection, olfactory bulb is subject to tissue clearing treatment with CLARITY method combined with antibody staining of GFP. Cleared bulb is embedded and imaged using confocal microscope. B. Representative 3D rendering of OSN axons in P3, P7, P10 and P21 animals. Arrow heads indicate highlighted axons shown in C and D. Scale bar, 500 μm. C and D. Optical sections at different imaging depths of highlighted axons shown in B. Arrow heads indicate OSN axon terminals in glomeruli. Scale bar, 100 μm. E. Quantification of the OSN path in P3, P7, P10 and P21 animals. The total number of glomeruli that an axon traverses is summarized for each age group. F. Data in E are normalized to the length of the axon imaged from the entire bulb. Each circle in E and F represents one axon. Horizontal bars represent mean ± SEM with pvalues calculated from one tail Student’s t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant. G. 3D rendering of a bifurcate OSN axon in a P7 animal (also shown in B, P7). Arrow head indicates the branching point. Dotted circles delineate glomerular boundary. Scale bar, 100 μm. H. Stacked column plot showing the percentage of OSNs with branching axons in P3, P7, P10 and P21 animals. I. A representative image of an OSN axon invading the EPL. Arrow head points to the portion of axon growing into the EPL. Scale bar, 100 μm. J. Stacked column plot showing the percentage of OSNs extending axons to the EPL. The total number of axons is indicated for each age group at the top of the bars in H and J. ONL, olfactory nerve layer. Glo, glomeruli layer. EPL, external plexiform layer.
From these individually labeled axons, we made four observations. First, in animals younger than P10, the OSN axons followed circuitous paths and entered several glomeruli before arborizing in their destination glomeruli (Figure 1B-1F). In contrast, axons from P21 animals usually went directly to their targets without invading other glomeruli (Figure 1B, P21). The number of glomeruli that a single axon invaded before reaching its target decreased with age (Figure 1E and 1F). Second, in animals younger than P10, some axons branched out before reaching their target glomeruli (Figure 1B and 1G). The percentage of branching axons started at about 3% in P3 animals, reached its peak at P7 to about 20%, and then declined to ~7% at P10 (Figure 1H). No branching axons were observed in P21 animals (Figure 1H). Third, some axons in animals younger than P10 penetrated into the external plexiform layer (EPL) (Figure 1I). These axons did not arborize in the EPL; instead, they returned to the glomerular layer (Figure 1B and 1I). The proportion of the overshooting axons peaked at P7, reaching 18% of all axons (Figure 1J). No OSN axon was observed to invade the EPL in P21 animals (Figure 1J). Fourth, we observed OSNs that did not display the typical terminal arborization within a glomerulus (Figure S1B and S1C). Prior to CLARITY, the morphology of a single OSN was normally assessed in sections of ~50 – 100 μm thickness. It was difficult to assess whether the observed morphology reflected a severed en passant axon or the terminus of an intact axon. In cleared tissue, sampling of the entire axon trajectory in intact tissue led us to identify these axons. The lack of terminal branching likely represented a transient state of axons penetrating through the glomeruli.
Taken together, glomerular boundary crossing, axon branching, and exuberant growth into the EPL were observed only in young animals during the critical period. OSNs in young animals exhibited distinct dynamics.
Chronogenetic tracking reveals tight regulation of axon dynamics
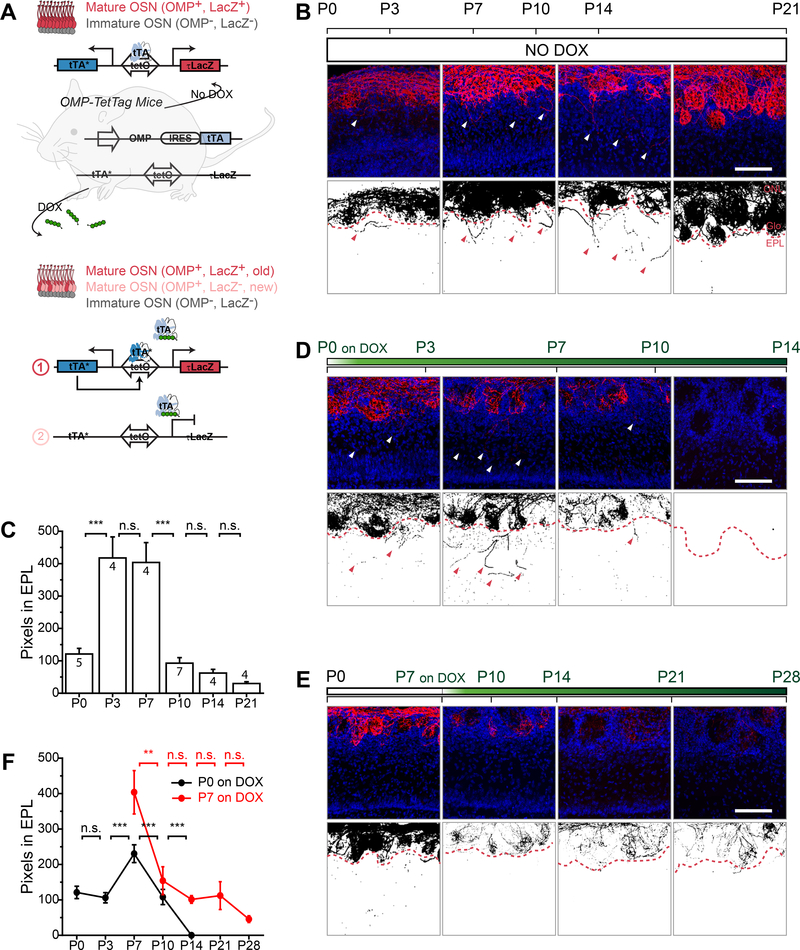
How do the navigating OSN axons contribute to the establishment of a precise olfactory map? We wondered whether we could track the perinatal OSN axons and examine their developmental trajectory over time. Although lineage tracing is available for several sensory systems (Lang and Fekete, 2001; Leung et al., 2007; Price et al., 1987; Rodriguez-Gil et al., 2015), most methods do not distinguish neurons of different birth orders. We developed a chronogenetic tracking system using the TetTag mouse model to label mature OSNs (mOSNs) using doxycycline (DOX) administration (Figure 2A). The TetTag mouse generated by Mayford’s group contained a bi-directional tetO promoter that could be activated by tetracycline trans-activator (tTA) to express LacZ and tTA*, a tetracycline insensitive variant (H100Y) of tTA (Reijmers et al., 2007). We generated Omp-TetTag mice by crossing the tetO-tTA*/LacZ line with Omp-IRES-tTA mouse, which expressed tTA from the olfactory marker protein (OMP) allele (Yu et al., 2004). Activation of the TetTag allele in mOSNs prior to DOX administration labeled these neurons and allowed the sustained expression of tTA* by a self-perpetuating loop in the presence of DOX (Figure 2A).
Figure 2. Chronogenetic labeling of OSNs reveals exuberant axon projection during critical period.
A. Schematic illustration of labeling OSNs with Omp-TetTag method. In the absence of DOX, OMP+ mOSNs are labeled with LacZ (red). When mice are fed with DOX diet, mOSNs that are OMP+ (red) before DOX treatment will maintain LacZ expression via persistent activity of tTA*. New mOSNs (pink) that become OMP+ after DOX treatment will not express LacZ. B, D and E. Representative images of olfactory bulb sections of Omp-TetTag mice fed with regular diet (B), DOX diet at P0 (D), and DOX diet at P7 (E). Upper panels, sections are stained with anti-βgal antibodies (red) and DAPI (blue). Lower panels, binary images of signals from anti-βgal staining. Arrow heads indicate exuberant axons invading the EPL. Red dashed line indicates the boundary between the glomerular layer and the EPL. C. Quantification of exuberant OSN axons in the EPL in mice fed with regular diet. The bar plot indicates the number of red pixel (LacZ staining) in the EPL. The number of bulbs analyzed are indicated for each bar. The bars represent mean ± SEM with p-values calculated from one tail Student’s t-test. ***, p < 0.001; n.s., not significant. F. Quantification of exuberant OSNs axons in the EPL in mice fed with DOX diet at P0 (black) and P7 (red). The scatter plot indicates the number of red pixel (LacZ staining) in the EPL. Each dot represents mean ± SEM. **, p < 0.01, ***, p < 0.001; n.s., not significant, one tail Student’s t-test. Scale bar, 100 μm. ONL, olfactory nerve layer. Glo, glomeruli layer. EPL, external plexiform layer.
To test whether this labeling approach could effectively mark the OSNs, we first examined mice between P0 to P21. In the absence of DOX treatment, tTA expressed from the Omp allele allowed the expression of LacZ in mOSNs (Figure 2B). We observed that ~80% of the mOSNs were labeled by LacZ, evident by the co-staining of OMP and LacZ in the neuroepithelium and the bulb (Figure S2A-C). The labeling efficiency remained relatively consistent across different ages of the animals (Figure S2B).
Consistent with the observation in adenovirus labeling, we found substantial amount of OSN axons invaded the EPL in young mice (Figure 2B). The most exuberant axon outgrowth was observed between P3 and P7, followed by a decline at P10 and a more gradual decrease afterwards (Figure 2C). The axon fibers in the EPL were less prominent at P0 and were absent by P21 (Figure 2B and 2C). We also observed some regional differences in the density of exuberant axons in the olfactory bulb (Figure S2D-F). For example, more exuberant axons were found in the medial and ventral parts of the olfactory bulb, with fewer in the dorsal region (Figure S2E). Along the anterior-posterior axis, more exuberant axons were found in the posterior part of the olfactory bulb at P3 than P7 (Figure S2F). In contrast, there were more exuberant axons at P7 in the anterior and middle regions (Figure S2F). These differences might reflect the temporal sequence of olfactory map development (Takeuchi et al., 2010).
We next treated the Omp-TetTag animal with DOX diet and traced the axons over a period of four weeks. In bulb sections, we found that axons labeled up to P0 (referred to as P0labeled) showed a significant increase in exuberant growth into the EPL at P7, followed by a rapid decline at P10 (Figure 2D). By P14, not only were exuberant axons completely absent in the EPL, few axons were found in any glomeruli (Figure 2D). This observation indicated that the P0-labeled OSNs underwent a dynamic phase of exuberant growth and were quickly removed from the bulbar circuitry. The elimination of these early axons was uniform; few labeled axons remained in any of the glomeruli examined at later time points.
In mice fed with DOX diet at P7, we did not observe an increase of exuberant axons in the EPL after labeling. Approximately 60% of the axons in the EPL disappeared by P10, with the remaining ones gradually declined over time (Figure 2E-2F). Consistent with the observation in the EPL, labeled axons in the glomeruli exhibited a rapid decline at P14, followed by a slower decline afterwards. The rapid decline suggested a fast turnover rate of axons during this time of development. Moreover, when we fed DOX at P3, and co-stained the OB sections with antibodies against OMP and LacZ, we did not observe axons that were only OMP+ in the EPL (Figure S3A). This result suggested that the late born OSNs (depicted as pink in Figure 2A) did not project exuberant axons. In the same line of experiments, we also specifically examined axons that projected to the M71 glomerulus. Some axons expressing M71 were found to cross glomerular boundaries towards neighboring glomeruli at P3 (Figure S3B).
In previous studies, we and others have shown that the convergent projection pattern of the OSNs required spontaneous neural activity (Nakashima et al., 2013; Yu et al., 2004). Expression of Kir2.1 channel suppressed spontaneous firing of action potentials and disrupted axon convergence. To address whether spontaneous activity was required for exuberant axon growth, we constructed an adenovirus that expressed Kir2.1(AV39) and RFP to sparsely label OSNs at early postnatal stages. AV39 labeled axons did not exhibit the characteristic exuberant growth found in control perinatal OSNs (Figure S3C). In addition, we examined Omp-IREStTA:tetO-Kir2.1-IRES-tauLacZ mice and observed significantly less exuberant axons in the EPL compared to the OMP-TetTag control animals (Figure S3D). Thus, neural activity was required for exuberant axon growth.
Finally, we tested whether the pioneering cells projected exuberant axons. Coined by Gong and Shipley, the pioneering neurons referred to a population of immature OSNs (iOSNs) generated around E13 whose axons were thought to induce the development of the primordial olfactory bulb (Gong and Shipley, 1995). We crossed Gγ8-tTA line (Nguyen et al., 2007) to TetTag mice to label iOSNs and examined their axon projection patterns (Figure S3E-F). Costaining of LacZ and GAP43 at E13.5 clearly showed co-localization of the signals, indicating that the Gγ8-tTA:TetTag approach could label GAP43-expressing pioneering neurons at this stage (Figure S3E). We then treated the animals with DOX from E13.5 to P7. We found that the LacZ signals were no longer detected in the OE (Figure S3E). In contrast, other cell types labeled in the olfactory bulb of the same animal continued to express LacZ at P7 (Figure S3F). This evidence suggested that the E13.5-labeled iOSNs were lost by P7. The exuberant axons were unlikely from the pioneering axons.
Perinatal OSNs are required for map formation
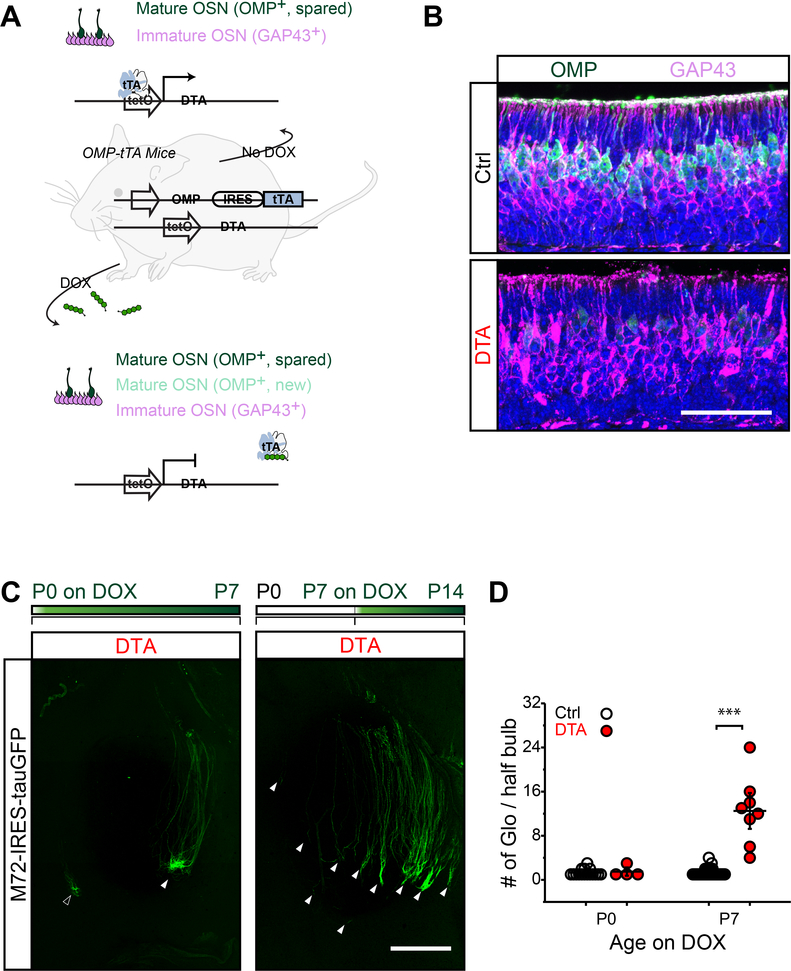
What was the role of the perinatal OSNs in olfactory map formation? To address this question, we ablated these OSNs using Omp-IRES-tTA:tetO-DTA line (DTA mouse in short) (Figure 3A) (Ma et al., 2014). DTA effectively eliminated mOSNs but left iOSNs intact (Figure 3B). We examined the convergence of M72 axons in the DTA mice after DOX treatment. When DOX was given at P0, newly generated M72 axons exhibited a convergent projection pattern similar to that in wildtype animals (Figure 3C and 3D). In contrast, when DTA mice were treated with DOX diet at P7, newly generated M72 axons projected diffusely in the olfactory bulb (Figure 3C and 3D). The same observations were made for MOR28 and M71 expressing OSNs (Figure S4A and S4B)
Figure 3. Perinatal OSNs are critical for axon targeting.
A. Schematic illustration of DTA mice. In the absence of DOX, most of OMP+ OSNs are killed by DTA activity with a small number of mOSNs spared (dark green). When mice are fed with DOX diet, DTA expression is turned off. New mOSNs (green) that become OMP+ after DOX treatment replenish the neuroepithelium. GAP43+ iOSNs (magenta) are not affected by DTA. B. Representative images of olfactory epithelia stained with antibodies against GAP43 (magenta) and OMP (green). DAPI, blue. Epithelia from control and DTA mice at P3 are shown. Scale bar, 50 μm. C. Whole mount images of M72 glomeruli in DTA/M72-IRES-tauGFP mice treated with DOX for 7 days, from P0 to P7, and P7 to P14, reespctively. Scale bar, 500 μm. D. The number of M72 glomeruli per half bulb in control and DTA animals treated with DOX for 7 days. DOX treatment period is indicated. Each dot represents a single data point and the horizontal bars represent mean ± SEM with p-value calculated from one tail Student’s t-test. ***, p < 0.001.
There was a significant number of axons from the iOSNs arriving at the bulb at birth (Gong et al., 2009). In DTA mice treated with DOX at P0, these iOSNs could undergo maturation, allowing their axons to converge into the proper glomeruli. We therefore treated the DTA mice with DOX at P3 to examine whether eliminating newly matured OSNs till this time point would disrupt the proper axon projections. Indeed, M72 axons in these mice projected diffusely at P9 (Figure S4C). Moreover, in DTA mice treated with DOX at P3, we observed much fewer exuberant axons in the EPL layer at P7 (Figure S4D and S4E). Thus, elimination of the perinatal OSNs eliminated exuberant axons and severely affected the pattern of axon convergence in the olfactory bulb.
Lifespan of postnatal OSNs
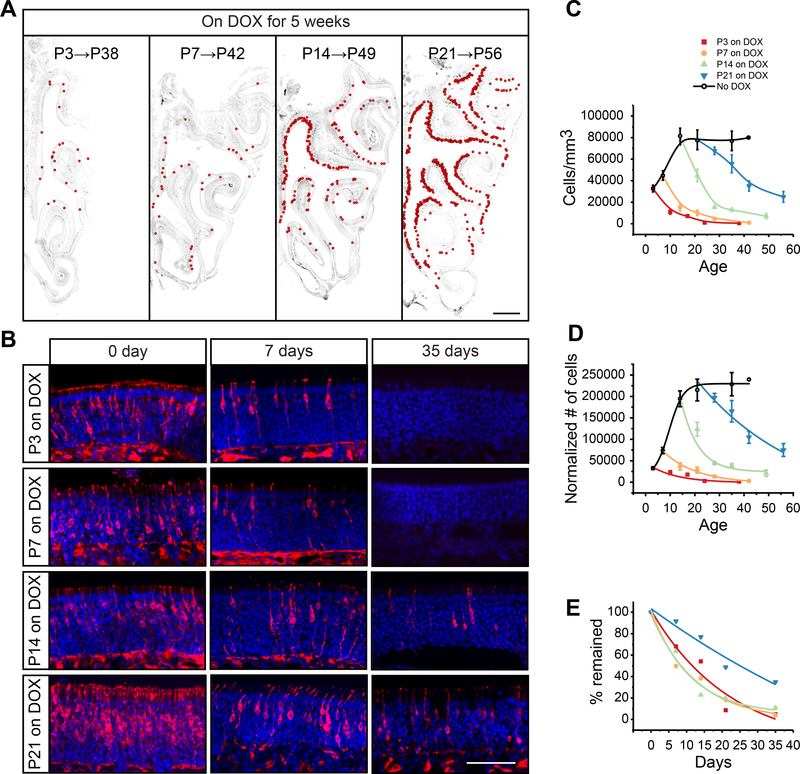
The rapid decline of perinatally labeled axons in the olfactory bulb during the critical period suggested a loss of OSNs in neuroepithelium during this time. We thus tested how long these neurons were able to survive (Figure 4). We used the OMP-TetTag mice and treated them with DOX diet at different postnatal time points (Figure 4A). After five weeks, sparsely labeled OSNs were observed in the epithelia of animals treated at P3 and P7 (Figure 4A). P3-labeled cells were largely gone by P21. In contrast, nearly half of the P21-labeled OSNs remained 5 weeks later, whereas moderate numbers of labeled cells were observed from P14-labeled animals (Figure 4A). To obtain a quantitative measurement of OSN lifespan, we systematically counted OSN numbers in Omp-TetTag mice with or without DOX (Figure 4B-4E). In the absence of DOX, cell density and total number of OSNs continued to increase from birth to P21 (Figure 4C and 4D, black curve, No DOX). This was consistent with previous studies using BrdU labeling (Kondo et al., 2010). When animals were treated at different time points, we observed various levels of reduction of labeled OSNs in different age groups (Figure 4C and 4D). When we normalized the cell numbers to that of the initially labeled OSNs, we were able to compare the decline rate of different age groups (Figure 4E). We found that OSNs in mice younger than P14 had faster decline rate and shorter lifespan than those in older animals (P21; Figure 4E). The half-lives were similar among neurons labeled before P14 (~10–14 days). In conclusion, a cell elimination program was in place to prune back all axons in the olfactory bulb up to P14 indiscriminately.
Figure 4. Tracking OSN lifespan with Omp-TetTag labeling.
A. Coronal sections of the nose through the turbinate. Omp-TetTag mice are fed with DOX diet during indicated time periods. Sections are stained with antibodies against βgal (gray). Each red circle indicates a LacZ+ OSN with clearly identifiable cell body and a dendrite. Scale bar, 500 μm. B. Representative images of neuroepithelia from DOX fed animals. Sections are stained with antibodies against βgal (red) and DAPI (blue). Omp-TetTag mice are fed with DOX diet starting at indicated ages. The number of days on DOX treatment is shown at the top. Scale bar, 50 μm. C and D. Quantification of labeled OSN in Omp-TetTag mice. The number of LacZ+ OSNs per mm3 (C) and the number of LacZ+ OSNs normalized to P3 (D) are plotted as a function of the age of the animals. Sigmoidal (black) and exponential decay (other colors) fittings are performed. E. Percentage of LacZ+ cells plotted as the function of the duration of DOX treatment. The initial value of cell number is set to 100% for each group. C-E. Mice are treated with either no DOX (black open circle), DOX at P3 (red square), P7 (orange filled circle), P14 (green triangle), or P21 (blue inverted triangle).
Single cell transcriptome analysis reveals distinct OSN populations
Our tracing experiments revealed a chronological change in axon dynamics. Experiments using the Omp-TetTag and DTA mice further suggested that a specific population of OSNs at birth played a critical role in establishing axon convergence. What could be the molecular mechanisms that govern the axon dynamics and the elimination of young OSNs? As neurogenesis occurred at a rapid pace postnatally, neurons born at different times intermingled in the olfactory epithelium. Analysis at single cell level could distinguish different cell populations at the molecular level (Fletcher et al., 2017; Hanchate et al., 2015; Tan et al., 2015). We, therefore, performed single cell transcriptome analysis of the olfactory epithelia during postnatal development (Figure 5).
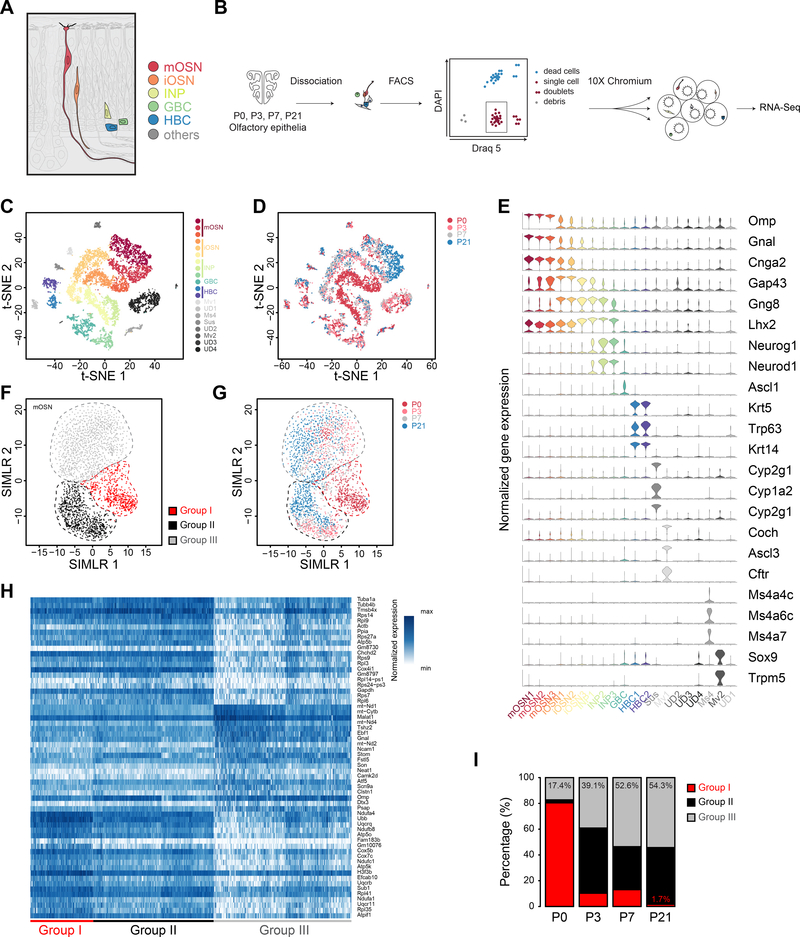
Figure 5. Single cell transcriptome analysis of olfactory epithelium during postnatal development.
A. Illustration of the major cell types in the olfactory epithelium. B. Schematic illustration of the single cell RNA-Seq procedures. Olfactory epithelia are dissected from P0, P3, P7 and P21 animals and dissociated. Single cells are size-selected and stained with DAPI and Draq5. Single cells are purified using FACS. Libraries constructed using 10X Genomics platform are used for RNA-Seq. C. t-SNE plot showing 20 clusters assigned by the expression of marker genes. D. Cells isolated from different ages are mapped to the t-SNE plot. E. Violin plots showing the expression levels of the known cell-type markers. Cell clusters are identical to those in C. F. Two-dimension plot showing distinct mOSN sub-groups identified through SIMLR. G. Cells from different ages of the animals are mapped to the SIMLR plot. H. Heatmap showing differentially expressed genes among the three mOSN groups. I. Stacked column plot showing the proportion of cells belonging to each group at different ages. Abbreviations: mOSN, mature olfactory sensory neurons; iOSN, immature olfactory sensory neurons; INP, immediate neuronal progenitor; GBC, globose basal cell; HBC, horizontal basal cell; Mv, microvilli cell; Ms4, cells expressing Ms4a receptors; Sus, sustentacular cell; UD, undetermined cell type.
We dissociated single cells from P0, P3, P7 and P21 olfactory epithelia and performed single cell RNA-Seq (scRNA-Seq) on the 10X Genomics platform. Isolation of single, live, and nucleated cells was ensured by size selection using physical filtering and fluorescence-activated cell sorting (FACS) based on nuclear staining. We obtained 9112 cells that passed quality control filters (Figure 5C). The first 20 principal components from principal component analysis were used to generate tight and stable clusters. The clusters were visualized in two dimensions via tdistributed stochastic neighbor embedding (t-SNE; Figures 5C and 5D) (Maaten and Hinton, 2008). Based on the expression patterns of known marker genes, we made cluster assignment for horizontal basal cell (HBC), globose basal cell (GBC), immediate neuronal precursor (INP), iOSN, mOSN, microvillous cell (Mv), sustentacular cell (Sus), Ms4a-expressing chemosensory receptor cell (Ms4), and other cell types (Figures 5C, 5E and S5A). Most clusters contained cells from all four age groups. However, we observed sub-clusters predominantly contained P0 cells in the iOSN and mOSN clusters (Figure 5D).
We focused on the mOSNs and performed clustering on these cells using SIMLR, a method that used multi-kernel learning to maximize cluster distances (Wang et al., 2017). We identified three sub-clusters among the mOSNs (Figure 5F). The three sub-clusters contained cells from different ages, but Group I primarily contained P0 neurons (Figure 5G). Although these cells all expressed mOSN markers, they were distinguished by a set of differentiallyexpressed genes (Figure 5H; Tables S1-S3). We quantified the percentage of cells in each group during development (Figure 5I). The P0 sample contained 80.4% of Group I cells. The number decreased to 10.3% and 13.1% for P3 and P7 samples, respectively, and further decreased to 1.7% at P21. For Group II and III cells, the number generally increased over time. The progressive decrease of Group I cells during development was consistent with the decrease of cells exhibiting distinct axon growth phenotype.
Molecular characteristics of distinct mOSN populations
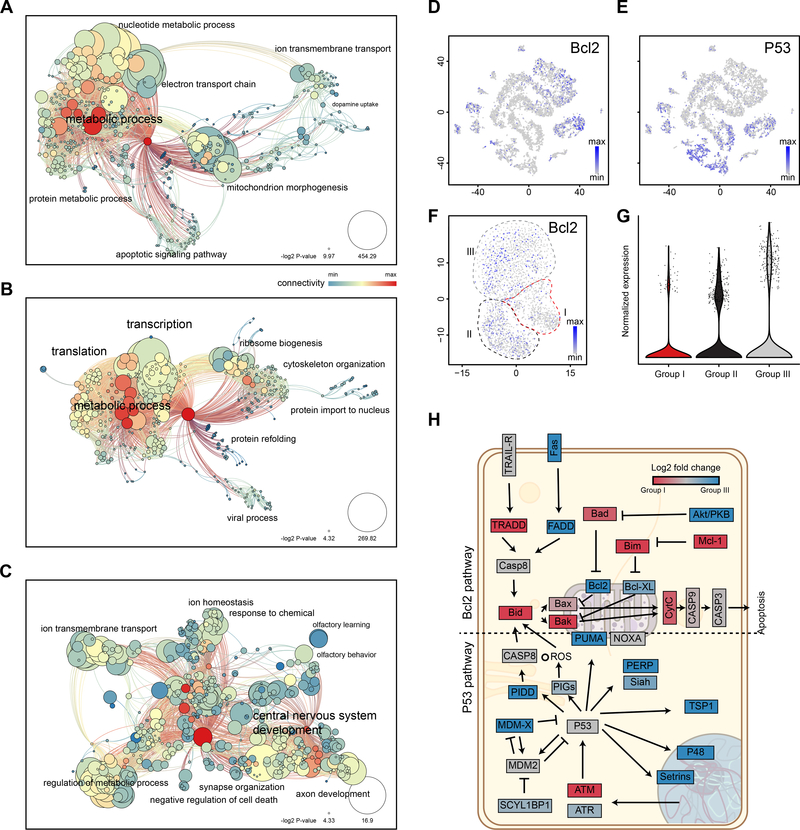
We performed gene ontology (GO) analysis to investigate whether the differentially expressed genes belonged to certain functional groups that were coordinately regulated (Figure 6A-C). GO terms enriched in Group I cells were predominantly associated with cell metabolism, especially with nucleotide, electron transport chain and mitochondrion morphogenesis. GO terms associated with Group II were enriched for transcription and translation. Those in Group III were related to nervous system development, ion transport, synaptic organization and transmission, and sensory perception. A closer look at the genes indicated that Group I cell had relatively lower expression of OMP and Gnal when compared with Group III (Figure 5H and Tables S1-S3). Genes associated with iOSNs (e.g. GAP43 and Gng8) were enriched in both Group I and II cells, suggesting that they likely had retained some characteristics of newly differentiated neurons. These cells were also enriched in genes associated with cytoskeleton organization, possibly enabling more dynamic regulation of neuronal processes. Although many GO terms and genes were shared between Groups I and II cells, the two groups appeared to be distinguished by two non-coding RNAs. Gm10076 was highly expressed in Group I, whereas Gm8730 was highly expressed by Group II cells. Group I cells were also marked by higher levels of mitochondrion-associated genes, indicating that these neurons were likely to be more metabolically active.
Figure 6. GO term analyses of mOSN populations.
A-C. GO term analyses of mOSN Groups I-III, respectively. GO term enrichment is represented as a hierarchical map built from differentially expressed genes in the group. The size of the circle represents -log2 p-value derived from Fisher’s test. Colors indicate the level of connectivity of a GO term. Only major GO terms are shown to simplify the graphs. D and E. Cells expressing Bcl2 (D) and P53 (E) are mapped to t-SNE plot. H. KEGG pathway analysis of Bcl2 and P53 apoptosis pathways. Color code indicates the mean log2 fold change of the genes between Groups I and III mOSNs. Values between −0.5 and 0.5 are color coded with a gradient. Values below or above −0.5 and 0.5 are coded as blue and red.
Group I cells were also enriched in GO terms associated with apoptosis (Figure 6A). We examined genes belonging to the two known apoptotic pathways (Bcl2 and P53 pathways). The anti-apoptotic gene Bcl2 was detected mainly in mOSNs (Figure 6D), whereas P53 was lower in OSNs (Figure 6E). Within the mOSNs, Bcl2 was mainly found in Group II and III cells, with fewer cells expressing it at a lower level in Group I (Figure 6F and 6G). KEGG pathway analysis indicated that pro-apoptotic genes were preferentially expressed by Group I mOSNs, whereas anti-apoptotic genes were preferentially expressed in Group II and III mOSNs (Figure 6H). This observation was further supported by our analysis of a published dataset that included scRNASeq of newborn and adult OSNs (Tan et al., 2015) (Figure S6A and S6B). Bcl2 was high in adult cells (Figure S6C). Casp3, the caspase involved in apoptotic cascade, was detected at a higher level in newborn OSNs (Figure S6D). With all things considered, the evidence indicated that Group I cells were likely to be the population with a shorter lifespan, whose elimination by DTA caused mistargeting. They were also more likely to have dynamically regulated axons. Group II cells likely represented a transitory state of mOSNs coming from iOSNs. They were present in all ages.
In contrast to Groups I and II, molecules involved in olfactory signal transduction and axon maintenance (e.g., Cnga4, Gng13, Kirrel2 and Epha5) were all expressed at higher levels in Group III OSNs. This evidence indicated that Group III cells consisted fully matured OSNs. Consistent with this finding, cells collected at P21, when neurogenesis reached a steady state, had a high percentage of Group III cells.
We also studied the OR expression pattern among the three groups. We detected 909 ORs from our dataset, with Olfr77, Olfr532, Olfr1176 being the most frequently expressed receptors (Figure S5B). A vast majority of OSNs expressed a single OR. We observed some mOSNs expressed 2–3 receptors, which was consistent with a previous report (Hanchate et al., 2015). The mOSNs that expressed two ORs were found as small portions in all the three groups. The mOSNs that expressed three receptors were rare and only seen in Group III cells. These observations indicated that the dynamic axon growth phenotype of perinatal neurons was not associated with neurons expressing multiple receptors.
Preserving perinatal neurons leads to ectopic exuberant axons and perturbs axon convergence
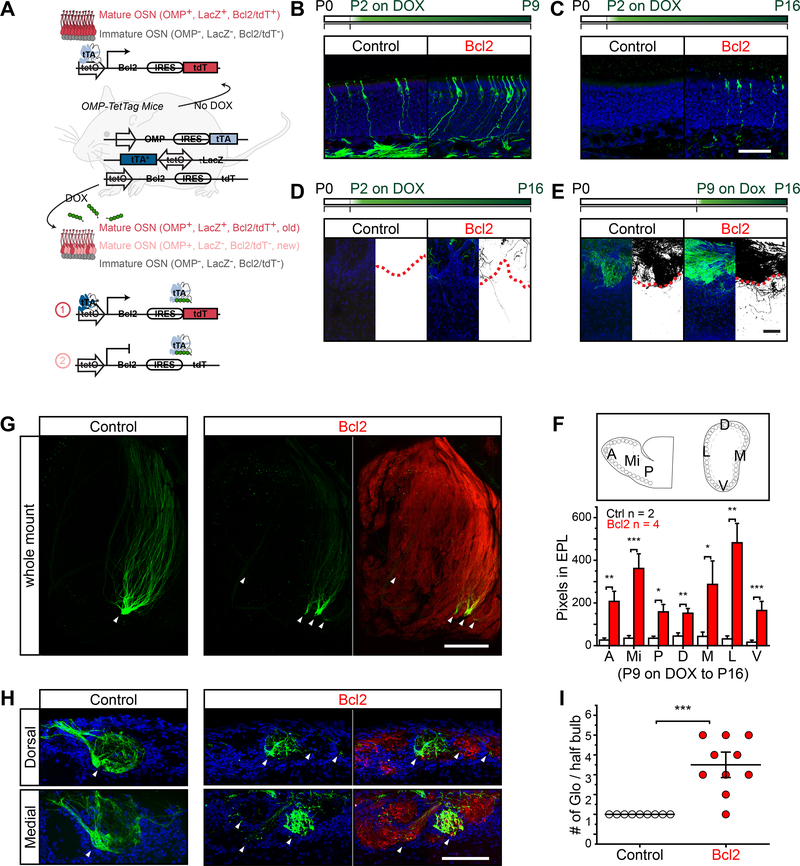
We sought to test whether perturbation of the apoptotic pathway could alter the lifespan of the perinatal OSNs. Moreover, this perturbation allowed us to test whether there was a functional consequence in preserving the perinatal neurons after the critical period. We generated mice carrying tetO-Bcl2-IRES-tdTomato transgene that expressed the long isoform of Bcl2 (Figure 7A) (McDonnell et al., 1989; Sentman et al., 1991; Strasser et al., 1991). In compound heterozygotic Omp-TetTag:tetO-Bcl2-IRES-tdTomato mice (Bcl2 mice for short), the expression of Bcl2 was sustained after DOX treatment. Indeed, for mice treated with DOX for 7 or 14 days at P2, more LacZ+ neurons were observed in the neuroepithelium than those in the control animals (Figure 7B and 7C). Accompanied by the extended survival of OSNs in the epithelium, we observed more exuberant axons in the EPL of Bcl2 mice than the controls (Figure 7D and 7E).
Figure 7. Extending the lifespan of navigator OSNs perturbs axon convergence.
A. Schematic illustration of Bcl2 mice. In the absence of DOX, OMP+ OSNs labeled with LacZ also express Bcl2/tdTomato (red). When mice are fed with DOX diet, OSNs that are OMP+ (red) before DOX treatment will maintain LacZ/Bcl2/tdTomato expression via persistent activity of tTA*. New OSNs (pink) that become OMP+ after DOX treatment will not express LacZ. B and C. Confocal images of neuroepithelium stained with antibodies against β-gal (green) and DAPI (blue) in control and Bcl2 mice. Mice were on DOX diet for 7 days (P2-P9; B) and 14 days (P2P16; C). Scale bar, 50 μm. D and E. Confocal images of olfactory bulb sections stained with antibodies against β-gal (green) and DAPI (blue) in control and Bcl2 mice. Mice were on DOX diet for 14 days (P2-P16; D) and 7 days (P9-P16; E). Binary images show the staining signals of βgal. Red dotted line indicates the glomerular boundary. Scale bar, 50 μm. F. Bar graph represents the quantification of exuberant axons in the EPL. The number of pixels in the EPL is quantified in anterior (A), middle (Mi), posterior (P), dorsal (D), medial (M), lateral (L), and ventral (V) regions of olfactory bulb of control (open bars) and Bcl2 (red bars) mice treated with DOX diet from P9 to P16. The bars represent mean ± SEM with p-value calculated from one tail Student’s t-test. The number of animals used in the analysis is indicated. *, p < 0.05, **, p < 0.01, ***, p < 0.001. G. Whole mount images of M72 glomeruli in M72-IRES-tauGFP (control) and Bcl2/M72-IRES-tauGFP mice. M72-GFP, green; tdTomato, red. Arrowheads indicate glomeruli containing M72-GFP axons. Scale bar, 500 μm. H. Images of olfactory bulb sections of M72-IRES-tauGFP (control) and Bcl2/M72-IRES-tauGFP mice. GFP, green; tdTomato, red; DAPI, blue. Both medial and dorsal M72 glomeruli are shown. Arrowheads indicate M72 axons. Scale bar, 100 μm. I. The number of M72 glomeruli in control (open circle) and Bcl2 (red circle) mice. Each dot represents a half bulb and horizontal bars represent mean ± SEM. n = 4–5 mice. ***, p < 0.001, one tail Student’s t-test. Glo, glomeruli.
We next examined the projection patterns of the OSNs. In adult Bcl2 mice, ectopic projections were observed in the vicinity of the M72 glomerulus (Figure 7G). Individual axon fibers were also detected in a wide area of the dorsal olfactory bulb (Figure 7G). In coronal sections, axons were observed entering different glomeruli in the vicinity of the main targets (Figure 7H). Compare to the control animals, there was a significantly increase in the number of glomeruli receiving input from the M72 axons in the Bcl2 mice (Figure 7I). These results demonstrated that a tight regulation of the lifespan of the perinatal cells was a crucial step in establishing the convergent projection patterns. It was likely that maintaining exuberant axons beyond the critical period prevented the formation of a precise olfactory map.
Discussion:
The “navigator” cells
Previous studies demonstrated that the olfactory map observed in adulthood is imprinted from that formed during postnatal development (Ma et al., 2014; Tsai and Barnea, 2014). OSNs generated in adult stage recapitulate the projection patterns formed during the critical period. In this study, we use genetic tracking and viral tracing experiments to obtain a detailed view of the axon dynamics in the first postnatal week of OSN development. The results indicate that the perinatal OSNs exhibit unique characteristics in their pathfinding dynamics during the critical period. These OSNs are a transient population that is eliminated after the critical period. Our single cell transcriptome analyses indicate that the Group I OSNs are distinct from the late-born cells. The change in the percentage of Group I cells over time is aligned with the changes in axon dynamics and the decline of perinatal cell population during postnatal development. The differentially expressed genes in Group I cells are found in pathways that are involved in the dynamic regulation of axon, and in controlling the lifespan of perinatal OSNs. We thus favor a model that the molecularly-defined Group I cells are a special population whose axons navigate the landscape in the olfactory bulb to establish and refine the olfactory map. We refer to them as the “navigator” OSNs.
The navigator cells likely are born before birth and exist in both mature and immature forms. At the perinatal stage, axons from the mature navigators follow a circuitous path, traverse multiple glomeruli, branch out, and overshoot into the EPL. These characteristics could enable them to sample the environment, find partners expressing the same OR and coalesce into protoglomeruli. As iOSNs become mOSNs, their axons continue to converge. The overall percentage of navigator cells among the total OSNs decreases over time, consistent with the various types of axon phenotypes observed with the tracing experiments.
Exuberant growth of navigator axons coincides with the critical period when projection mistakes can be corrected. Results from Omp-TetTag tracking show that the navigator neurons do not last in the olfactory epithelium and are eliminated from the circuitry after the critical period. Despite their transient existence, the navigator neurons are required to establish the OSN convergence pattern in the olfactory bulb. Thus, the functional roles of the navigator OSNs are likely to be twofold: to form a map de novo by establishing convergent axon projection patterns; and to correct aberrant projections. These characteristics define the navigator cells as a special population during an essential stage of olfactory system development. By following the projection patterns established during the critical period, later born OSNs maintain a more stable olfactory system with new OSNs integrating into the circuitry throughout the life of the animal.
Distinct OSN axon dynamics during critical period
Our results show that the regulation of olfactory axon dynamics is distinct from the classic model. In the development of neuromuscular junction (Sanes and Lichtman, 1999; Tapia et al., 2012), as well as in the central nervous systems including the retinal ganglion cell projection and climbing fiber – Purkinje cell synapses (Chen and Regehr, 2000; Kano and Hashimoto, 2009; Lohof et al., 1996; Luo and O’Leary, 2005), neurons in the early stage of development project exuberant axons to reach numerous targets. The extranumeric contacts are eliminated through competition at later stages.
The observations of ectopic axons in the olfactory bulb of young rats have led earlier studies to conclude that the development of olfactory system follows similar rules (Chan et al., 2011; Royal and Key, 1999; Tenne-Brown and Key, 1999). Our results demonstrate four new characteristics of the dynamics of olfactory axon projection. First, early axons arriving at the bulb follow circuitous routes and cross glomerular boundaries. At any given time, there does not appear to have high error rate of axon projection. Second, our evidence indicates that all neurons do not engage in exuberant axon growth in the postnatal period. Although a large number of neurons is added postnatally, only the navigator neurons extend exuberant axons. Third, exuberant axon growth is dynamically regulated. Exuberant growth is observed at P0 but is heightened between P3 and P7. Lastly, the navigators have short lifespan and are eliminated after the closure of the critical period. Moreover, premature ablation of these neurons eliminates exuberant axons. Conversely, extending the lifespan of these neurons leads to protracted exuberant axon growth. These observations suggest that the ectopic projections in the olfactory system are not merely developmental errors.
A model of olfactory map formation
The mammalian olfactory system is a continuously regenerating system. Yet exuberant growth is only enabled in the navigator cells. The timing coincides with the critical period, when OSNs have the capacity to re-establish single glomerular projection patterns. This observation raises the question as to how a small number of neurons with exuberant axons could help establish the single receptor type convergence and restore aberrant projections.
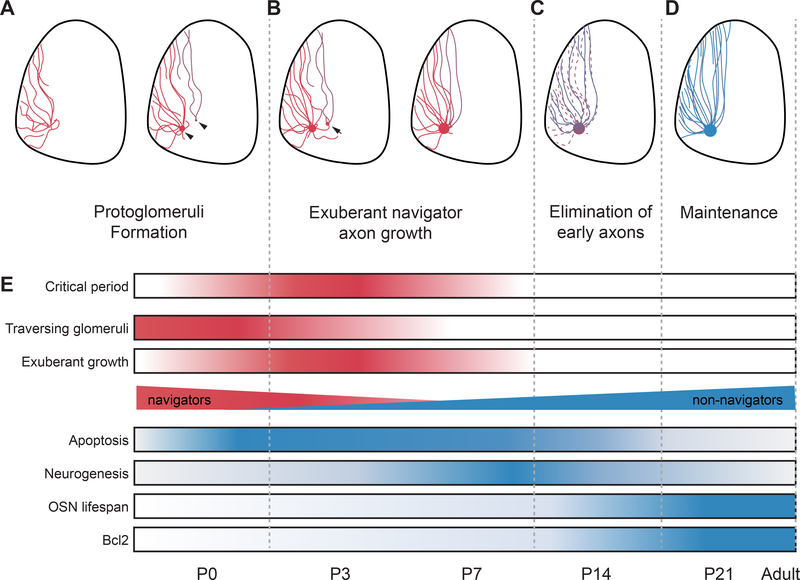
Our study, together with previous observations, point to a series of events during postnatal development of the olfactory system (Figure 8). During the early stage of map formation, axons project to stereotypical areas along the dorsal-ventral and anterior-posterior axes in the bulb. This process is likely mediated through gradients of guidance molecules expressed by the OSN axons (Cutforth et al., 2003; Imai et al., 2009; Takeuchi et al., 2010). Past studies have indicated that molecular recognition of discrete guidance cues plays an important role in allowing axons expressing the same OR to converge into the same glomeruli (Kaneko-Goto et al., 2008; Nakashima et al., 2013; Sakano, 2010; Serizawa et al., 2006; St John et al., 2002). The circuitous routes followed by navigator axons, or the “roaming” of the axons, could provide an opportunity for axons to encounter others bearing the same OR and form the initial coalescing of axons into protoglomeruli (Figure 8A). This roaming model predicts an active role of the navigator axons in establishing the protoglomeruli, but a causal relationship between the roaming of axons and their convergence is yet to be established. An alternative possibility is that the navigator cells express a distinct set, or different levels of guidance molecules that are needed to establish axon convergence. To identify these genes, an approach that provides better sensitivity than our current single cell sequencing may be required. In either case, our results indicate that the navigators are crucial for the proper formation of the protoglomeruli.
Figure 8. A model of olfactory map formation during the critical period.
A-D. A model for establishing olfactory circuitry by navigator OSNs. (A) Initial axon projection forms protoglomeruli (red) as axons expressing the same OR progressively coalesce with each other. The projection may contain errors or straying fibers (dark red). (B) During the critical period, exuberant growth of navigator axons allows these OSNs to explore large areas and make contacts with axons expressing the same odorant receptor. These contacts can help axons to converge into the proper glomeruli. (C) Following the critical period, axons from newly generated OSNs (dark blue) reach target glomeruli while navigator cells are eliminated from the olfactory epithelium and their axons from the olfactory bulb. (D) Maintenance of the map. (E) Color coded illustration of OSN developmental events. Color intensity indicates activity level.
As postnatal development ensues, an active phase of exuberant axon growth from the navigator cells occurs prior to P7, just before the closure of the critical period (Figure 8B). As the number of navigator cell does not increase, an increase in the number of exuberant axons may reflect extrusion from those already innervated a glomerulus. It is possible that these exuberant axons, anchored in a glomerulus to expand their reaches to broader areas, can scour stray axons into the glomeruli expressing the same OR. This hypothesis remains to be tested.
The navigator axons are eliminated after the critical period. This appears to be an important step to remove traces of circuitous routes and lingering exuberant axons (Figure 8C). Although the mechanisms are currently unknown, the closure of the critical period likely will restrict the plasticity of axon projection. Exuberant axons that linger after the closure of the critical period, as in the case of the Bcl2 mice, may not be able to remodel their projections the way they do during the critical period. Our experiments show that, even though we only extend the lifespan of a small number of OSNs, the stray axons could direct late arrival axons to project to ectopic sites, resulting in a perturbed map.
Late born neurons do not project exuberantly. They can track existing axon bundles to reach target glomeruli (Ma et al., 2014; Tsai and Barnea, 2014) (Figure 8D). These “follower” neurons, therefore, allow the early formed maps to be imprinted throughout the life of the animal. We suggest that exuberant axon growth of the navigators provides the capacity to solve the problem of axon mistargeting and allow the establishment of a precise map. Notably, the exuberant axon growth also coincides with the refinement of the primary dendrite of the mitral cell (Lin et al., 2000). This coincidence potentially offers a period of synaptic matching, when pre-synaptic and post-synaptic neurons form synapses and undergo reorganization. Although pruning of ectopic glomeruli continue to occur after the closure of the critical period (Zou et al., 2004), different mechanisms are likely being utilized.
The events described here distinguish the navigator neurons from the pioneering neurons (Gong and Shipley, 1995; Yu and Wu, 2016). The pioneering neurons are the first group of iOSNs (GAP43+) that arrives at the developing telencephalon around E13. They are hypothesized to induce the evagination of the primordial olfactory bulb. In contrast, the navigator neurons are the mature neurons (OMP+) critical for the formation of the olfactory map. Although it is possible that the pioneering neurons remain till early postnatal days and mature into navigator cells, our experiment labeling iOSNs at E13.5 showed that these cells are absent during critical period.
Molecular signature of the navigator cells
Our single cell transcriptome analyses have revealed differentially expressed genes, but few of them uniquely mark the navigator cells. This may in part be attributed to the sequencing depth of the scRNA-Seq platform, which detects on average 2000 highly expressed genes per cell in our experiment. It is possible that differentially expressed genes at moderate levels may distinguish the populations uniquely. Nevertheless, our data suggest pathways such as those in the mitochondria function are associated with the navigator cells. Moreover, a non-coding RNA with unknown function also appears to mark the navigator cells. These genes and pathways provide a handle with which we can use to specifically manipulate the navigator cells and further test their functions.
Our transcriptome analyses do not provide a clear answer as to how the timing of exuberant growth is controlled at the molecular level. It is possible that an intrinsic cell signaling program determines the timed transcription of genes associated with dynamic axon growth. A timed expression of a ligand, either by the OSNs or by the cells in the olfactory bulb, may drive these genes to induce the exuberant growth. Alternatively, the transient expression of receptors by the navigator cells may allow them to respond to an existing ligand and permit transient axon sprouting. In either scenario, further experiments are required to identify specific transcription factors or cell signaling molecules during the critical period.
Lifespan of the olfactory sensory neurons.
There has been considerable debate over the lifespan of OSNs (Graziadei and Graziadei, 1979; Hinds et al., 1984; Mackay-Sim and Kittel, 1991; Moulton, 1974; Schwob et al., 1992). Different studies showed varied results. The lifespan of OSN estimated by these studies varies from 30 days to years. The inconsistency in the lifespan studies comes from at least three major variables: technical caveat from the chemical tracers, the environment and the age of the animal. Since most estimates were made using BrdU or radioactive thymidine, which label dividing progenitor cells, the labels are diluted as these cells divide and differentiate (Graziadei and Graziadei, 1979; Hinds et al., 1984; Kondo et al., 2010; Mackay-Sim and Kittel, 1991; Moulton, 1974; Weiler and Farbman, 1997). The Omp-TetTag animal provides a genetic method to permanently label a neuronal population and trace its fate. We find the lifespan varies among the neurons generated at different developmental stages. Neurons generated during the critical period are short-lived. They are eliminated from the system after the closure of critical period. Neurons generated in adults are much more stable compared to those generated during postnatal period. The differential expression of pro-apoptotic and anti-apoptotic genes provide molecular explanation of the differences in lifespan. Furthermore, our study suggests an intricate connection among lifespan, exuberance growth and axon guidance in establishing and maintaining the olfactory map.
STAR method
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, C. Ron Yu (cry@stowers.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines and cell culture
HEK293 and Neuro2A cell lines were obtained from ATCC. Cultures were maintained in DMEM with 10% FBS and GlutaMAX (Thermo Fisher Scientific) at 5% CO2 in humidified incubators.
Animals
The TetTag (tetO-tTA*-tauLacZ allele), tetO-DTA, M72-IRES-tauGFP, M71-IRES-tauGFP, Omp-IRES-tTA, mT/mG, and tetO-Kir2.1-IRES-tauLacZ (Jackson laboratory, stock number
008344, 008468, 006678, 006676, 017754, 007576 and 009136 respectively), MOR28-IRESGFP, and Gγ8-tTA mice were described previously (Feinstein and Mombaerts, 2004; Lee et al., 1998; Muzumdar et al., 2007; Nguyen et al., 2007; Reijmers et al., 2007; Yu et al., 2004). TetTag and Omp-IRES-tTA mice are mated to create Omp-TetTag mice (Omp-IRES-tTA:tetO-tTA*tauLacZ). C57BL/6J and CD1 mice were obtained from Lab Animal Services Facility of Stowers Institute (Stowers LASF). Mice containing individual alleles were maintained by breeding with C57BL/6J strain until mating with mice carrying other alleles for experiment. All animals were maintained in Stowers LASF with a 14:10 light cycle and provided with food and water ad libitum. DOX treatment was performed by either weaning the pups onto DOX diet or by fostering the pups to CD1 moms fed with DOX diet at least 48 hours earlier. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Stowers Institute and in compliance with the NIH Guide for Care and Use of Animals. Both male and female mice were used in the experiment without discrimination.
Generation of tetO-Bcl2-IRES-tdTomato transgenic mouse
A DNA fragment encoding the Bcl2 gene was PCR-amplified from olfactory epithelium cDNA with forward primer (ACGCGTGCCACCATGGCGCAAGCCGGGAGAACAG) and reverse primer (TTAATTAATCACTTGTGGCCCAGGTATGCA). A DNA construct bearing the tetOBcl2-IRES-tdTomato transgene was generated by Gibson Assembly (NEB). Transgenesis was conducted by standard pronuclei injection method at the LASF of Stowers Institute.
METHOD DETAILS
Adenovirus production
All virus work was performed at BSL2 facilities at Stowers Institute. DNA fragment containing mGFP was amplified from mTmG mice genomic DNA (Muzumdar et al., 2007). Kir2.1 cDNA was amplified from tetO-Kir2.1-IRES-tauLacZ plasmid (Addgene 32642) (Yu et al., 2004). Shuttle vectors containing mGFP and Kir2.1 were constructed from a vector (Addgene plasmid #50957, a gift from James Bamburg) using Gibson Assembly (NEB). Production of recombinant adenoviruses AV222 and AV39 was conducted using the pADeasy-1 vector (Addgene #16400) modified from published protocol (He et al., 1998; Luo et al., 2007). Electrocompetent ADeasier cells were prepared by electroporation of pADeasy-1 vector into BJ5183 cells (Agilent). The clones carrying pADeasy-1 were confirmed by restriction digestion mapping and sequencing. pADeasy-1 positive cells were made electrocompetent by washing the cells with 10% glycerol at 4ºC. Electrocompetent ADeasier cells were transformed with linearized AV222. Positive clones were selected by kanamycin resistance and the digestion patterns of restriction enzymes PacI and NcoI. Correctly recombined plasmid was confirmed by DNA sequencing and further amplified in One Shot TOP10 Electrocomp E. coli (Thermo Fisher Scientific). The plasmid was digested with PacI and transfected into HEK293 cells using Lipofectamine 3000 (Thermo Fisher Scientific). After fluorescent plaques formed, adenovirus was released from the infected HEK293 cells by 3 freeze and thaw cycles, using ethanol dry ice bath and 37ºC water bath, respectively. Released viruses were further purified by polyethylglycol-8000 (PEG 8000; Sigma) precipitation. The titer of the virus was determined by infecting Neuro2A cells.
Adenovirus labeling of single OSN
All virus infection experiments were conducted at BSL2 facilities at Stowers LASF. CD1 animals were infected with the adenovirus for 2 days before sacrifice. P0–7 CD1 pups were anesthetized on ice in latex gloves. Pups older than P7 were anesthetized using isoflurane. Each animal was infected with 10 uL viral solution at 107-1013 infectious particles/mL. Viruses were delivered by quick injection into the nasal cavity via pulled pipette tips attached to a syringe. Animals with sparse labeling were used for the downstream analysis.
CLARITY
Passive CLARITY was conducted to visualize the OSN axons. Animals were anesthetized on ice covered with latex gloves (P0–7), or with urethane (2 mg/g body weight; > P7). Animals were then perfused with 10 mL ice cold PBS, 10 mL ice cold CLARITY monomer. The skull was not removed until after the polymerization to protect the integrity of the tissue. Samples were then post-fixed in CLARITY monomer solution overnight at 4ºC. The solution was then equilibrated with nitrogen for 5 minutes, polymerized directly at 37ºC without vacuum for 3 hours. The olfactory bulb and part of the prefrontal cortex were used in the clearing process. The samples were passively cleared in the clearing solution for overnight (P0–7) or 2 days (> P7), then washed with PBST (0.1 M PBS with 0.1% Triton X-100) overnight. Immunofluorescent staining was performed by incubating the cleared sample with primary antibodies overnight, washing with PBST for 3 hours, incubating with secondary antibodies and DAPI overnight, and washing with PBST for 3 hours. Clearing and staining steps were done at 37ºC with gentle rotation. Samples were then incubated with CUBIC reagent 2 (Susaki et al., 2014) for 3 hours before being mounted onto an imaging dish with NO. 1.5 glass bottom (Cellvis) using Y-mount. Y-mount is a mounting medium that matches the refractive index of the cleared sample. The recipe for Ymount is: 215 g H2O, 35 g porcine gelatin, 250 g sucrose, 2.5 g n-propyl-gallate. Y-mount is a thermo-reversible gel that melts at 50ºC and solidifies at room temperature. Imaging of the CLARITY processed samples was conducted with a 10X (NA 0.45) lens on LSM 700 confocal microscope (Zeiss).
Single axon tracing
Axons were traced manually using Simple neurite tracer (Longair et al., 2011) in Fiji ImageJ. Imaris was used to create 3D rendering of the axon traces.
Tissue section histology
Animals were fully anesthetized with urethane (2 mg/g body weight) and perfused intracardiacally with 10 mL PBS and 10 mL 4% PFA. The olfactory epithelia and brain were dissected and post-fixed in 4% PFA overnight at 4ºC. Free floating coronal sections of the olfactory bulb were cut at 50 μm thickness using a Leica Vibratome system.
Cryosections were obtained from the olfactory epithelia. The olfactory epithelia were dissected, decalcified in PBS with 0.5 M EDTA and 30% sucrose at 4ºC overnight, and embedded in Tissue-Tek O.C.T. (Sakura) for sectioning. Coronal sections were cut with a cryostat (Leica microsystem) at 16 μm thickness.
Immunofluorescent staining was performed by incubating the sections with primary antibodies in PBST overnight at 4ºC, washed 3 times with PBST, incubated with secondary antibodies in PBST overnight at 4ºC, and mounted in VECTASHIELD (Vectorlabs). Primary antibodies used in the study were: chicken anti-βgal (Abcam, ab9361–250, 1:1000 dilution), goat anti-OMP (Waco, 544–10001, 1:500 dilution), rabbit anti-RFP (MBL, PM005, 1:1000 dilution), rabbit antiRFP (Rockland, 600–401-379, 1:1000 dilution), rabbit anti-GAP43 (Novus, B300–143; 1:1000 dilution) and chicken anti-GFP (Abcam, ab13970–100, 1:3000 dilution). Secondary antibodies used were: donkey anti-chicken Alexa Fluor 488, donkey anti-chicken Alexa Fluor 594 (Jackson ImmunoResearch Inc.), donkey anti-goat Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 488, and donkey anti-rabbit Alexa Fluor 594 (Thermo Fisher Scientific). DAPI (Thermo Fisher Scientific) and secondary antibodies were used at 1:1000 dilution from the stock solutions prepared according to the manufacture’s suggestion.
Quantification of axon exuberant growth
Confocal scanning images of the histological staining were projected into a single plane through maximal intensity projection in Fiji ImageJ. The image was then thresholded by the “Triangle” method. The layers of the olfactory bulb were identified based on DAPI staining. For each olfactory bulb, images were sampled randomly at dorsal, medial, lateral, ventral regions from sections from the anterior, middle, and posterior positions of the olfactory bulb (see QUANTIFICATION AND STATISTICAL ANALYSIS for numbers). These images were used for quantitative analysis. Pixels in the EPL were calculated as indicated in the figures.
Quantification of the lifespan of OSN
To quantify LacZ+ neurons in the olfactory epithelia, we made coronal sections at the middle position along the anterior-posterior axis of the olfactory epithelia and imaged the entire section. Images were stitched together in Fiji ImageJ using “Grid/Collection Stitching”. To quantify the number of labeled OSNs consistently, we only counted LacZ+ cells that included both the cell body and the dendrite, as well as with clear nuclear staining. The cell count was converted cell density, which was used to adjust for tissue expansion during development. During postnatal development, the nasal tissue undergoes expansion in all three dimensions. The changes in olfactory epithelia dimensions were measured using C57BL/6J strain pups by measuring the distance between the dorsal ridge to the ventral tip, and between the most anterior tip and the cribriform plate. An expansion factor for each age was calculated as the ratio of its volume over P3. Normalized density (in cells/mm3 equivalent to that of a P3 mouse) were calculated by multiplying the expansion factors with the actual density.
Half-life (T1/2) of the neurons was estimated by fitting the number of labeled neurons over time with an exponential decay curve using equation:
| (1) |
Preparation of single cell suspension for scRNA-Seq
CD1 pups at ages of postnatal day 0, 3, 7, and 21 were used for scRNA-Seq. Olfactory epithelia were dissected in oxygenized ACSF as described previously (Ma et al., 2011). Epithelial tissues were digested with papain solution containing DNase I. Digestion solution containing 117 mM NaCl, 5.3 mM KCl, 1.17 mM NaH2PO4, and 5.56 mM glucose was oxygenated by 95% O2/5% CO2 for 5 min before adding papain (20 mg/mL; Calbiochem), 10 u RNase free DNase I (NEB) and L-cysteine (3 mg/mL; Calbiochem). Epithelia were digested at 37oC for 10 min. Dissociated single cells were filtered by 70 μm, 30 μm and 10 μm filters (pluriSelect), washed twice with PBS-BSA (0.1 M PBS with 0.01% BSA), then stained with Draq5 (25 μΜ) and DAPI (0.5 μg/mL) on ice for 5 min. FACS was conducted using BD Influx cytometer (BD Bioscience) running Sortware v1.2 using a 100 μm nozzle tip, 20 psi system pressure and DPBS sheath fluid (Leinco S632). Cells were gated for high level of forward scattering, low level of side scattering, a single peak in electronic pulse, Draq5 staining positive, DAPI staining negative to purify the live nucleated single cell population.
Single cell RNA-Seq
Single cell RNA-Seq was performed using 10X Chromium single cell platform (10X Genomics). Around 6000 cells were used as input per time point. Libraries were prepared using Chromium Single Cell 3’ Library & Gel Bead Kit v2. All four libraries were sequenced on an Illumina HiSeq 2500 Rapid flowcell with two 50 bp paired-end kits using the following read length: 26 bp Read1, 8 bp I7 Index and 98 bp Read2. 20000–50000 reads were obtained per cell.
Single cell transcriptome analysis
Single cell RNA-Seq data were first analyzed by the Cell Ranger pipeline. The UMI counts from different samples were combined in Seurat for further analysis (Butler et al., 2018). Cells expressing mOSN markers were further sub-clustered using a multi-kernel learning method, SIMLR (Wang et al., 2017). For OR expression analysis, only ORs with normalized expression above 2.5 were used. For the GO term analysis, GO terms used in the analysis were from GO.db (Carlson M 2017). The enrichment was calculated by Goseq (Young MD et al. 2010). Enriched GO terms were connected by their hierarchies from GO.db and converted into gexf format using the rgexf function. The GO term topographic map was visualized in Gephi (gephi.org). KEGG pathway analysis was done using Pathview (Luo and Brouwer 2013).
Significant differentially expressed genes were shown in Tables S1-3. Differential expression was called using the Wilcoxen rank sum test for each of the three mOSN clusters versus the other two mOSN clusters using Seurat’s FindMarkers function. A Bonferroni correction was made based on the total number of genes in the dataset and was labeled as “p_val_adj” for the adjusted p-value. The proportion of cells in the primary cluster showing any expression for a gene was labeled as “pct.1” and the proportion in the two other clusters combined was labeled as “pct.2”. The average log fold change between the groupings was labeled “avg_logFC” with a positive value denoting a higher level of expression in the primary cluster and a negative value indicating a decreased expression level relative to the other two clusters. Only genes that pass an adjusted p-value cutoff of 0.05 were included in the list.
Data from Tan et al. 2016 were also used for the analysis. Differentially expressed genes were identified by two tailed Student’s t-test. Genes with log2 fold change higher than 1 and log2 pvalue larger than 5 were used to perform the GO term enrichment analysis.
Whole mount spectral imaging
Whole mount images of the olfactory bulb were acquired by spectral imaging. Animals were sacrificed by cervical dislocation. The skin and skull on the dorsal head were removed to expose the olfactory bulbs. Spectral imaging was conducted on LSM 780 (Zeiss). Samples were excited using 488 nm and 561 nm excitation laser. 32 channels of the detector were used, spanning 411965 nm. Linear unmixing was conducted in Zen (Zeiss).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses of the imaging experiments were performed in OriginPro 2016 (OriginLab Corporation). For comparisons between two groups, one-tail Student’s t-test was used unless specified in the legend. Sample numbers are shown in Figures and in the Figure Legends. The exact numbers are listed as follows. Figure 1: P3 data (1E, 1F, 1H, and 1J), n = 33 axons from 5 animals; P7 data (1E, 1F, 1H, and 1J), n = 45 axons from 3 animals; P10 data (1E, 1F, 1H, and 1J), n = 27 axons from 2 animals; P21 data (1E, 1F, 1H, and 1J), n = 28 axons from 5 animals. Figure 2C: P0 data, n = 44 images from 5 olfactory bulbs of 3 animals; P3, n = 38 images from 4 olfactory bulbs of 2 animals; P7, n = 26 images from 3 olfactory bulbs of 2 animals; P10, n = 62 images from 7 half bulbs of 4 animals; P14, n = 35 images from 4 olfactory bulbs of 2 animals; P21, n = 40 images from 4 olfactory bulbs of 2 animals. Figure 2F: P0 no DOX, n = 44 images from 5 olfactory bulbs of 3 animals; P0 on DOX for 3 days, n = 47 images from 6 olfactory bulbs of 3 animals; P0 on DOX for 7 days, n = 56 from 6 olfactory bulbs of 3 animals; P0 on DOX for 10 days, n = 35 images from 4 olfactory bulbs of 2 animals; P0 on DOX for 14 days, n = 2 images from 2 olfactory bulbs of 1 animal; P7 no DOX, n = 26 images from 3 olfactory bulbs of 2 animals; P7 on DOX for 3 days, n = 20 images from 2 olfactory bulbs of 1 animal; P7 on DOX for 7 days, n = 39 images from 4 olfactory bulbs of 2 animals; P7 on DOX for 14 days, n = 20 images from 2 olfactory bulbs of 1 animal; P7 on DOX for 21 days, n = 22 images from 3 olfactory bulbs of 2 animals. Figure 3D: control P0, n = 16 half bulbs from 8 animals; DTA P0, n = 4 half bulbs from 2 animals; control P7, n = 53 half bulbs from 28 animals; DTA P7 on DOX, n = 8 half bulbs from 4 animals. Figure 4, cell number in one section from one animal was considered as one sample; n = 3, 4, 4, 2, and 3 for 0, 7, 14, 21 and 35 days after P3 on DOX, respectively; n = 3, 3, 2, 3, and 2 for 0, 7, 14, 21 and 35 days after P7 on DOX, respectively; n = 4, 4, 2, 3 and 2 for 0, 7, 14, 21 and 35 days after P14 on DOX, respectively; n = 3, 3, 3, 3 and 3 for 0, 7, 14, 21 and 35 days after P21 on DOX, respectively; n = 4 for P35 no DOX; n = 1 for P42 no DOX. Figure 7F: n = 8, 8, 4, 4, 6, 4, and 6 images for anterior, middle, posterior, dorsal, medial, lateral, and ventral sections from 2 olfactory bulbs of 2 control animals, respectively; n = 9, 12, 8, 5, 9, 6, and 9 images for anterior, middle, posterior, dorsal, medial, lateral, and ventral sections from 4 olfactory bulbs of 3 Bcl2 animals, respectively. Figure 7I: n = 7 half bulbs from 4 animals for control mice; n = 10 half bulbs from 5 animals for Bcl2 mice.
DATA AND SOFTWARE AVAILABILITY
All original data and RNA-Seq analysis scripts are available through Stowers Institute for Medical Research Original Data Repository (https://www.stowers.org/research/publications/odr). Raw and normalized scRNA-Seq data from olfactory epithelia can be obtained at NCBI. The GEO accession super series ID number for the data reported in this paper is GEO: GSE120199.
Supplementary Material
Movie S1. Visualization of individual OSN axons in intact olfactory bulb. Related to Figure 1.
3-D rendering of an olfactory bulb from a P7 mouse with axons sparsely labeled with adenovirus AV222.
Table S1. Genes differentially expressed by Group I mOSNs. Related to Figure 5.
Table S2. Genes differentially expressed by Group II mOSNs. Related to Figure 5.
Table S3. Genes differentially expressed by Group III mOSNs. Related to Figure 5.
Highlights:
Single cell analyses uncover a distinct population of olfactory neurons.
Chronogenetic labeling reveals distinct navigator cell axon morphology and lifespan.
Transgenic expression of Bcl2 extends the lifespan of navigator cells.
Alterations of navigator cell lifespan disrupt olfactory map formation.
Acknowledgement:
We thank Michael Durnin from Lab Animal Service Facility, Laura Holmes from Cytometry, Kate Hall from Molecular Biology, Shiyuan Chen from Computational Biology and Sean McKinney from Microscopy Center of Stowers Institute for technical assistance. This work fulfills, in part, requirements for Y.W. and K.D.’s Ph.D. theses with the Open University, United Kingdom. This work is supported by funding from Stowers Institute for Medical Research and the National Institutes of Health (R01DC016696).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- Allendoerfer KL, and Shatz CJ (1994). The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 17, 185–218. [DOI] [PubMed] [Google Scholar]
- Barkat TR, Polley DB, and Hensch TK (2011). A critical period for auditory thalamocortical connectivity. Nat Neurosci 14, 1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, and Maffei L (2000). Critical periods during sensory development. Curr Opin Neurobiol 10, 138–145. [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature biotechnology 36, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Ekberg JA, Lineburg KE, Scott SE, Chehrehasa F, Windus LC, Claxton C, Mackay-Sim A, Key B, and St John JA (2011). Correction of aberrant axon growth in the developing mouse olfactory bulb. Mol Cell Neurosci 46, 282–295. [DOI] [PubMed] [Google Scholar]
- Chen C, and Regehr WG (2000). Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, et al. (2013). Structural and molecular interrogation of intact biological systems. Nature 497, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo RM (1991). Regeneration of olfactory receptor cells. Ciba Found Symp 160, 233–242; discussion 243–238. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, and Stryker MP (1998). The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, and Katz LC (2002). Ocular dominance development revisited. Curr Opin Neurobiol 12, 104–109. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, Frisen J, and Axel R (2003). Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell 114, 311–322. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS (2010). Critical period for the whisker-barrel system. Exp Neurol 222, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein P, and Mombaerts P (2004). A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell 117, 817–831. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, Cole MB, Flores Q, Choi YG, Yosef N, et al. (2017). Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell 20, 817–830e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, and Shatz CJ (1990). Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347, 179–181. [DOI] [PubMed] [Google Scholar]
- Gong Q, Chen H, and Farbman AI (2009). Olfactory sensory axon growth and branching is influenced by sonic hedgehog. Dev Dyn 238, 1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, and Shipley MT (1995). Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron 14, 91–101. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, and Graziadei GA (1979). Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8, 1–18. [DOI] [PubMed] [Google Scholar]
- Hanchate NK, Kondoh K, Lu Z, Kuang D, Ye X, Qiu X, Pachter L, Trapnell C, and Buck LB (2015). Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science 350, 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, and Vogelstein B (1998). A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK (2004). Critical period regulation. Annu Rev Neurosci 27, 549–579. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL, and McNelly NA (1984). An autoradiographic study of the mouse olfactory epithelium: evidence for long-lived receptors. Anat Rec 210, 375–383. [DOI] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology 206, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, and Sakano H (2009). Pretarget axon sorting establishes the neural map topography. Science 325, 585–590. [DOI] [PubMed] [Google Scholar]
- Kaneko-Goto T, Yoshihara S, Miyazaki H, and Yoshihara Y (2008). BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron 57, 834–846. [DOI] [PubMed] [Google Scholar]
- Kano M, and Hashimoto K (2009). Synapse elimination in the central nervous system. Curr Opin Neurobiol 19, 154–161. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, and Shatz CJ (2003). Role of subplate neurons in functional maturation of visual cortical columns. Science 301, 521–525. [DOI] [PubMed] [Google Scholar]
- Katz LC, and Crowley JC (2002). Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci 3, 34–42. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, and Knudsen PF (1990). Sensitive and critical periods for visual calibration of sound localization by barn owls. J Neurosci 10, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M, Yamaguchi K, Nibu K, Kaga K, and Yamasoba T (2010). Age-related changes in cell dynamics of the postnatal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol 518, 1962–1975. [DOI] [PubMed] [Google Scholar]
- Lang H, and Fekete DM (2001). Lineage analysis in the chicken inner ear shows differences in clonal dispersion for epithelial, neuronal, and mesenchymal cells. Dev Biol 234, 120–137. [DOI] [PubMed] [Google Scholar]
- Lee P, Morley G, Huang Q, Fischer A, Seiler S, Horner JW, Factor S, Vaidya D, Jalife J, and Fishman GI (1998). Conditional lineage ablation to model human diseases. Proc Natl Acad Sci U S A 95, 11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, and Reed RR (2007). Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10, 720–726. [DOI] [PubMed] [Google Scholar]
- Levelt CN, and Hubener M (2012). Critical-period plasticity in the visual cortex. Annu Rev Neurosci 35, 309–330. [DOI] [PubMed] [Google Scholar]
- Lin DM, Wang F, Lowe G, Gold GH, Axel R, Ngai J, and Brunet L (2000). Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron 26, 69–80. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Delhaye-Bouchaud N, and Mariani J (1996). Synapse elimination in the central nervous system: functional significance and cellular mechanisms. Rev Neurosci 7, 85–101. [DOI] [PubMed] [Google Scholar]
- Longair MH, Baker DA, and Armstrong JD (2011). Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27, 2453–2454. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al. (2007). A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2, 1236–1247. [DOI] [PubMed] [Google Scholar]
- Luo L, and O’Leary DD (2005). Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28, 127–156. [DOI] [PubMed] [Google Scholar]
- Ma L, Haga-Yamanaka S, Yu QE, Qiu Q, Kim S, and Yu CR (2011). Imaging neuronal responses in slice preparations of vomeronasal organ expressing a genetically encoded calcium sensor. Journal of visualized experiments: JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wu Y, Qiu Q, Scheerer H, Moran A, and Yu CR (2014). A developmental switch of axon targeting in the continuously regenerating mouse olfactory system. Science 344, 194–197. [DOI] [PubMed] [Google Scholar]
- Maaten L.v.d., and Hinton G (2008). Visualizing data using t-SNE. Journal of machine learning research 9, 2579–2605. [Google Scholar]
- Mackay-Sim A, and Kittel P (1991). Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci 11, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, and Korsmeyer SJ (1989). bcl-2-Immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57, 79–88. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, and O’Leary DD (2003). Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol 13, 57–69. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, and Axel R (1996). Visualizing an olfactory sensory map. Cell 87, 675–686. [DOI] [PubMed] [Google Scholar]
- Moulton DG (1974). Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci 237, 52–61. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Takeuchi H, Imai T, Saito H, Kiyonari H, Abe T, Chen M, Weinstein LS, Yu CR, Storm DR, et al. (2013). Agonist-Independent GPCR Activity Regulates Anterior-Posterior Targeting of Olfactory Sensory Neurons. Cell 154, 1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Zhou Z, Marks CA, Ryba NJ, and Belluscio L (2007). Prominent roles for odorant receptor coding sequences in allelic exclusion. Cell 131, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Turner D, and Cepko C (1987). Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A 84, 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, and Mayford M (2007). Localization of a stable neural correlate of associative memory. Science 317, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, and Buck LB (1994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79, 1245–1255. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Bartel DL, Jaspers AW, Mobley AS, Imamura F, and Greer CA (2015). Odorant receptors regulate the final glomerular coalescence of olfactory sensory neuron axons. Proc Natl Acad Sci U S A 112, 5821–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal SJ, and Key B (1999). Development of P2 olfactory glomeruli in P2-internal ribosome entry sitetau-LacZ transgenic mice. J Neurosci 19, 9856–9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H (2010). Neural map formation in the mouse olfactory system. Neuron 67, 530–542. [DOI] [PubMed] [Google Scholar]
- Sanes JR, and Lichtman JW (1999). Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22, 389–442. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Mieleszko Szumowski KE, and Stasky AA (1992). Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci 12, 3896–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, and Korsmeyer SJ (1991). bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67, 879–888. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, and Sakano H (2006). A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127, 10571069. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, and Stryker MP (1978). Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol 281, 267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John JA, Clarris HJ, and Key B (2002). Multiple axon guidance cues establish the olfactory topographic map: how do these cues interact? Int J Dev Biol 46, 639–647. [PubMed] [Google Scholar]
- Strasser A, Harris AW, and Cory S (1991). bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67, 889–899. [DOI] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, et al. (2014). Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Inokuchi K, Aoki M, Suto F, Tsuboi A, Matsuda I, Suzuki M, Aiba A, Serizawa S, Yoshihara Y, et al. (2010). Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell 141, 1056–1067. [DOI] [PubMed] [Google Scholar]
- Tan L, Li Q, and Xie XS (2015). Olfactory sensory neurons transiently express multiple olfactory receptors during development. Mol Syst Biol 11, 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JC, Wylie JD, Kasthuri N, Hayworth KJ, Schalek R, Berger DR, Guatimosim C, Seung HS, and Lichtman JW (2012). Pervasive synaptic branch removal in the mammalian neuromuscular system at birth. Neuron 74, 816–829. [DOI] [PubMed] [Google Scholar]
- Tenne-Brown J, and Key B (1999). Errors in lamina growth of primary olfactory axons in the rat and mouse olfactory bulb. J Comp Neurol 410, 20–30. [DOI] [PubMed] [Google Scholar]
- Tomer R, Ye L, Hsueh B, and Deisseroth K (2014). Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc 9, 1682–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HB, Purcell AL, and Greer CA (1999). Glomerular formation in the developing rat olfactory bulb. J Comp Neurol 413, 289–304. [PubMed] [Google Scholar]
- Tsai L, and Barnea G (2014). A critical period defined by axon-targeting mechanisms in the murine olfactory bulb. Science 344, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, and Axel R (1994). Topographic organization of sensory projections to the olfactory bulb. Cell 79, 981–991. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhu J, Pierson E, Ramazzotti D, and Batzoglou S (2017). Visualization and analysis of single-cell RNA-seq data by kernel-based similarity learning. Nat Methods 14, 414–416. [DOI] [PubMed] [Google Scholar]
- Weiler E, and Farbman AI (1997). Proliferation in the rat olfactory epithelium: Age-dependent changes. J Neurosci 17, 3610–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, and Gogos JA (2004). Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron 42, 553–566. [DOI] [PubMed] [Google Scholar]
- Yu CR, and Wu Y (2016). Regeneration and rewiring of rodent olfactory sensory neurons. Exp Neurol. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, and Merzenich MM (2002). Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci U S A 99, 2309–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, and Firestein S (1998). Functional expression of a mammalian odorant receptor. Science 279, 237–242. [DOI] [PubMed] [Google Scholar]
- Zhao H, Otaki JM, and Firestein S (1996). Adenovirus-mediated gene transfer in olfactory neurons in vivo. J Neurobiol 30, 521–530. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, and Firestein S (2004). Postnatal refinement of peripheral olfactory projections. Science 304, 1976–1979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Visualization of individual OSN axons in intact olfactory bulb. Related to Figure 1.
3-D rendering of an olfactory bulb from a P7 mouse with axons sparsely labeled with adenovirus AV222.
Table S1. Genes differentially expressed by Group I mOSNs. Related to Figure 5.
Table S2. Genes differentially expressed by Group II mOSNs. Related to Figure 5.
Table S3. Genes differentially expressed by Group III mOSNs. Related to Figure 5.