Abstract
Background
Exchange transfusion is a mainstay in the treatment of sickle cell anemia. Sickle cell recipients can be transfused over 10 units per therapy, an intervention that replaces circulating sickle red blood cells (RBCs) with donor RBCs. Storage of RBCs makes the intervention logistically feasible. The average storage duration for units transfused at the Duke University Medical Center is ~two weeks, a time window that should anticipate the accumulation of irreversible storage lesion to the erythrocyte. However, no metabolomics study has been performed to date to investigate the impact of exchange transfusion on recipients’ plasma and red blood cell phenotypes.
Study design and Methods
Plasma and red blood cells were collected from sickle cell patients before transfusion and within 5h from exchange transfusion with up to 11 units, prior to metabolomics analyses.
Results
Exchange transfusion significantly decreased plasma levels of markers of systemic hypoxemia like lactate, succinate, sphingosine 1-phosphate and 2-hydroxyglutarate. These metabolites accumulated in transfused RBCs, suggesting that RBCs may act as scavenger/reservoirs. Transfused RBCs displayed higher glycolysis, total adenylate pools and DPG, consistent with increased capacity to deliver oxygen. Plasma levels of acyl-carnitines and amino acids decreased, while fatty acids and potentially harmful phthalates increased upon exchange transfusion.
Conclusion
Metabolic phenotypes confirm the benefits of the transfusion therapy in sickle cell recipients and the reversibility of some of the metabolic storage lesion upon transfusion in vivo in two-week-old RBCs. However, results also suggest that potentially harmful plasticizers are transfused.
Keywords: Mass spectrometry, metabolomics, blood storage, transfusion medicine
Introduction
Sickle cell disease (SCD) is a monogenic hemoglobinopathy characterized by a point mutation in the β-globin gene. This mutation that results from a single nucleotide substitution (GAG to GTG) in the codon coding for amino acid 6 (glutamate → valine). Hemoglobin tetramers containing mutant β-globin chains tend to polymerize at the membrane, forming the so-called sickle hemoglobin (HbS).
While morbidity and mortality are significant in this population, patients demonstrate substantial phenotypic heterogeneity even when in presence of identical mutant alleles of the β-globin gene,1 which could be in part explained by underlying genotypic confounders, such as for example glucose 6-phosphate dehydrogenase deficiency.2 SCD is characterized by persistent episodes of hemolytic anemia and the occurrence of acute vaso-occlusive crises when the sickling red blood cells (RBCs) clog the fine capillary beds.3 The cumulative impact of recurring vaso-occlusive crises progressively damages many organs, including the kidneys, lungs and brain. Importantly, children and adults with SCD have a high prevalence (4%) of cerebrovascular accidents4 and an alarmingly high rate (~50%) of recurrence within 2 years without therapeutic intervention5. The use of RBC transfusions or performing exchange transfusions can significantly reduce the risk of overt stroke and recurrent stroke,6 but can be associated with both acute and chronic complications, including iron overload, iron-mediated hypercoagulability or development of alloimmunization over time.7,8 Indeed, transfusion of >10 units of RBCs (with an approximate total volume of >4.5L) is logistically constrained by the availability of fresh units to be transfused, which implies that such units ought to be stored under blood bank conditions. However, as RBCs are stored in the blood bank, they accumulate a series of biochemical and morphological alterations, collectively referred to as the storage lesion.9 The stored RBC is challenged by low storage temperatures,10 incubation with non-biological media (i.e. storage additives11–13) and exposure to (mM levels of) plasticizers,14 all factors impacting the energy15–18 and redox balance19–22 of the stored erythrocyte and, ultimately, its morphology,23 stiffness24 and hemolytic propensity in vitro and, upon transfusion, in vivo.25 However, reassuring evidence from randomized clinical trials mitigated most of the concerns related to the age of blood (reviewed in Ref.26). On the other hand, recent studies are highlighting the impact on RBC storability of biological confounders such as donor/recipient gender, age and ethnicity.27–29 Still, as RBCs approach the end of their shelf-life (42 days in the USA), they are characterized by an average ~17% loss of transfusion potency at outdate,30 based on 51Cr-labeled post-transfusion recovery studies in healthy human volunteers,31 an assay that provides information on the RBC’s ability to circulate, but not necessarily its ability to deliver oxygen. Storage-induced loss of potency may negatively impact massively transfused recipients, such as trauma patients.30 Similar considerations can be made for massively and chronically transfused SCD recipients undergoing lifelong transfusion therapy, where the opportunity to space out in time the frequency of transfusion therapies administered per year holds evident beneficial implications for the quality of life of the recipient as well as for the economic burden to the healthcare system. Therefore, transfusion to pediatric SCD patients often privileges fresher units (e.g. the average age of units transfused to sickle cell recipients at Duke is 15 days32). This time window anticipates the threshold for irreversible storage lesion(s) to key proteins and enzymes relevant to RBC function, energy and redox homeostasis, such as hemoglobin/band 3, glyceraldehyde 3-phosphate dehydrogenase and peroxiredoxin-2, respectively.33–36
Over the past few years, several studies have leveraged the power of omics technologies to investigate the impact of SCD on RBC biology – especially with respect to SCD specific adaptations at the protein37 and metabolic level.38–41 However, to the best of the authors’ knowledge no metabolomics study has hitherto characterized the metabolic changes in plasma and RBCs of SCD patients undergoing exchange transfusion. Moreover, while classic studies have anticipated that some of the metabolic lesions (i.e. depletion of high energy phosphate compounds adenosine triphosphate – ATP and 2,3-diphosphoglycerate – DPG) are restored within 72h from transfusion in vivo, no comprehensive metabolic assessment of the metabolic phenotypes of transfused RBCs (and recipients’ plasma) in comparison to pre-transfusion phenotypes has been described in the literature. In this study, we investigated the metabolic phenotypes of plasma and RBCs in SCD patients undergoing exchange transfusion.
Methods
Patient population
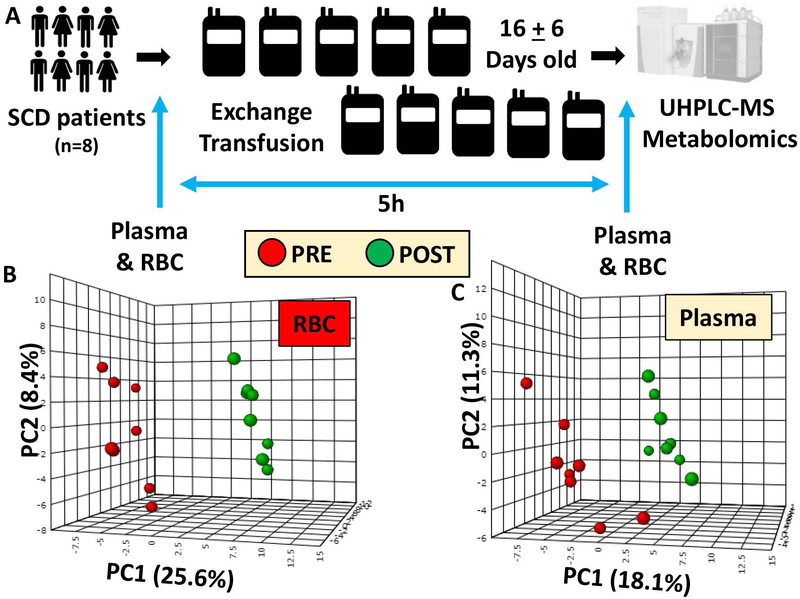
Blood samples were collected from SCD recipients prior to or 5h after exchange transfusion (8 exchange transfusion treatments; min = 3:37; max = 6:01 – Figure 1.A), upon receiving written informed consent and in conformity with the Declarations of Helsinki under protocol approved by the Duke University Medical Center (no. NCT02731157). All patients were primarily sickle cell type SS, in their baseline state of health and receiving chronic exchange transfusions for stroke prophylaxis. None of the patients had acute illness during the 2 weeks prior or during the exchange transfusion. Patients with stable chronic exchange protocols (as defined with consistent intervals between exchange) were enrolled. Only adult patients were enrolled to further minimize age-related differences. Although hydroxyurea is standard of care for patients to prevent complications, this therapy is not standard of practice for patients undergoing exchange transfusions. Therefore, none of the patients enrolled in this study were receiving hydroxyurea therapy. All patients were either at risk for stroke (based on elevated transcranial doppler velocities) or had a history of stroke, therefore being high-risk individuals for additional complications. Commercial reagents were purchased from Sigma-Aldrich (Saint Louis, MO) unless otherwise noted. Plasma and RBCs were separated by centrifugation at 2500 x g for 10 min at 4°C.
Figure 1 – An overview of the experimental design.
(A) and partial least square-discriminant analysis (PLS-DA) of red blood cell (RBC – B) and plasma (C) metabolic phenotypes in sickle cell diseases (SCD) patients prior to (red - left) or after (green - right) exchange transfusion.
Sample processing and metabolite extraction
A volume of 50 µl of RBCs and 20 µl of plasma were extracted in 450 and 980 µl, respectively of lysis buffer (methanol:acetonitrile:water 5:3:2), before ice cold extraction by vortexing for 30 minutes at 4ºC.42,43 Insoluble proteins were pelleted by centrifugation (10 minutes at 4ºC and 10,000 x g) and supernatants were collected and stored at −80°C until analysis.
UHPLC-MS metabolomics
Analyses were performed using a Vanquish UHPLC system coupled online to a Q Exactive mass spectrometer (Thermo Fisher, Bremen, Germany). Samples were resolved over a Kinetex C18 column (2.1 × 150 mm, 1.7 µm; Phenomenex, Torrance, CA, USA) at 25ºC using a three minute isocratic condition of 5% acetonitrile, 95% water, and 0.1% formic acid flowing at 250 µl/min,44 or using a 9 min gradient at 400 µl/min from 5–95% B (A: water/0.1% formic acid; B: acetonitrile/0.1% formic acid).43 MS analysis and data elaboration was performed as described.43 Metabolite assignments were performed using MAVEN (Princeton, NJ, USA), as described.44
Graphs and statistical analyses (either t-test) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA) and principal component analyses (PCA) were performed through the software Metaboanalyst 3.0.45
Results
Exchange transfusion significantly impacts the metabolic phenotypes of red blood cells and plasma in SCD patients
Metabolomics analyses were performed via UHPLC-MS in RBCs and plasma from 8 SCD patients prior to or 5h after the beginning of exchange transfusion therapies (Figure 1.A). Each patient received up to 11 units of blood. The average storage duration of the transfused units was 16 ± 6 days. Information on pre and post-exchange transfusion hematological parameters, including hemoglobin concentration, hematocrit, mean cell volume, mean cell hemoglobin, mean corpuscular hemoglobin concentrations and RBC counts are provided in Supplementary Table 1. A detailed report for 160 metabolites is provided in Supplementary Table 1, including compound names, KEGG IDs, metabolite mass to charge ratios (m/z), chromatographic retention times (RT) and the polarity in which each metabolite was detected. Results indicate significant impacts on RBC and plasma metabolic phenotypes following exchange transfusion, as gleaned by partially supervised statistical analysis (PLS-DA in Figure 1.B and C for RBCs and plasma, respectively). The impact of exchange transfusion on metabolic phenotypes informed the clustering across principal component 1, which explained 25.6 and 18.1% of the total metabolic variance for RBCs and plasma, respectively – three times or twice as much the impact of biological variability across the subjects enrolled in this study (principal component 2 – Figure 1.B and C). Hierarchical clustering analyses are reported in Supplementary Figures 1 and 2 for RBCs and plasma, respectively.
Transfused RBCs are more metabolically active and less energetically challenged than recipients’ pre-transfusion RBCs
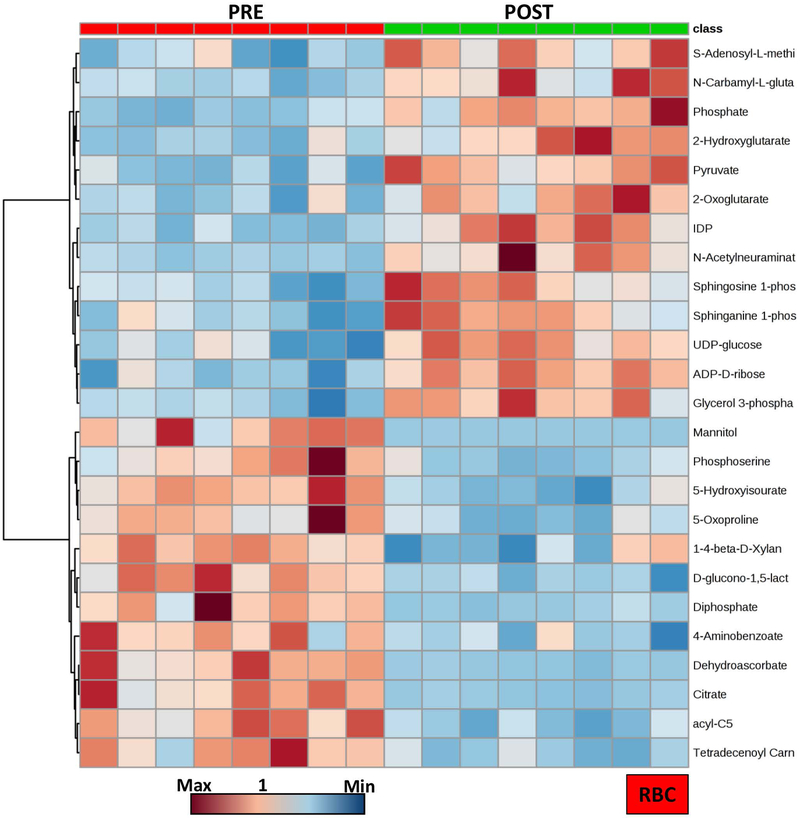
The heat map in Figure 2 shows a detail of the top 25 most significant features (paired T-test) in RBCs prior to and 5h after exchange transfusion. Results show a clear phenotype, in part explained by the fact that – upon exchange transfusion – the majority of the circulating RBCs come from the transfused units.
Figure 2 –
Hierarchical clustering analysis of the top 25 significant metabolites in RBCs from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).
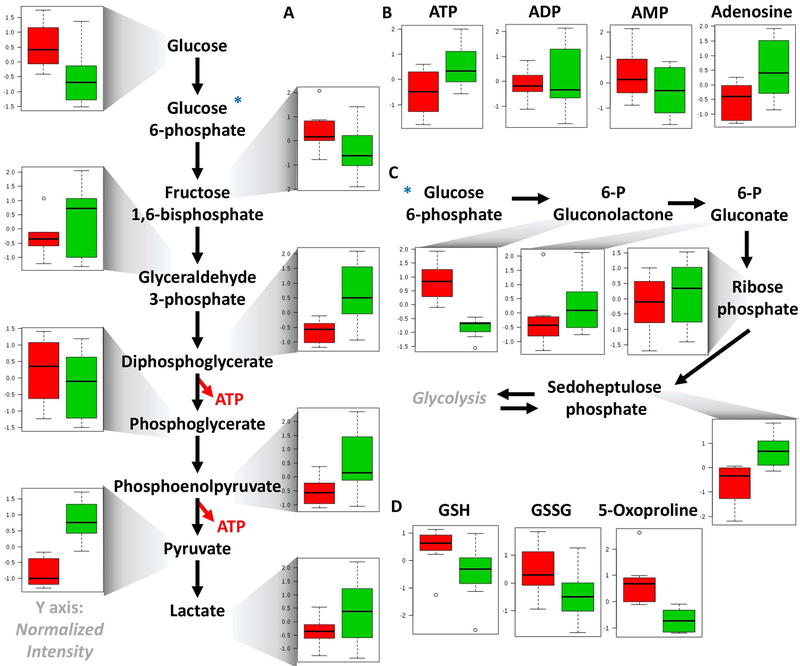
Pathway analyses revealed that transfused RBCs were characterized by higher glycolytic activity, which resulted in significantly lower levels of RBC glucose and higher levels of all glycolytic intermediates, including fructose bisphosphate, glyceraldehyde 3-phosphate, diphosphoglycerate, phosphoenolpyruvate, pyruvate and lactate (Figure 3.A). Increased glycolytic intermediates in RBCs upon exchange transfusion were also accompanied by significantly higher levels of high energy phosphate compounds – adenosine tri- and di-phosphate (ATP and ADP), lower levels of low energy adenosine monophosphate (AMP) and increases in RBC adenosine, an observation with overall improved preservation of the total adenylate pool in transfused RBCs in comparison to pre-transfusion SCD RBCs (Figure 3.B). Increased RBC levels of high energy S-Adenosyl-methionine, mono phosphate and phosphocreatine upon exchange transfusion are consistent with this observation (Supplementary Figure 3).
Figure 3 –
Glycolysis (A), energy metabolism (adenylate pool - B), pentose phosphate pathway (C) and glutathione homeostasis (D) in RBCs from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).
Transfused RBCs were also characterized by lower levels of early pentose phosphate pathway (PPP) oxidative phase intermediates (6-phosphogluconolactone) and increased levels of PPP byproducts, ribose phosphate (and isobaric isomers) and sedoheptulose phosphate (Figure 3.C). In the absence of in vivo tracing data to reveal metabolic fluxes, steady state observations could be suggestive of increased PPP activation to cope with exacerbated oxidative stress upon transfusion. Decreased reduced and oxidized glutathione (GSH and GSSG, respectively) and 5-oxoproline (a byproduct of the gamma-glutamyl cycle – Figure 3.D) are suggestive of an overall decrease in the total glutathione pool and potentially indicate that transfused RBCs appear to be characterized by higher energy metabolism in exchange for a decreased antioxidant metabolic capacity. However, these results are in part explained by decreased levels of dehydroascorbate (oxidized vitamin C - Supplementary Figure 3), which is converted to its reduced form through consumption of reduced glutathione.
Exchange transfusion impacts plasma metabolic phenotypes by increasing circulating levels of fatty acids and other metabolites that accumulate over storage in the RBCs transfused
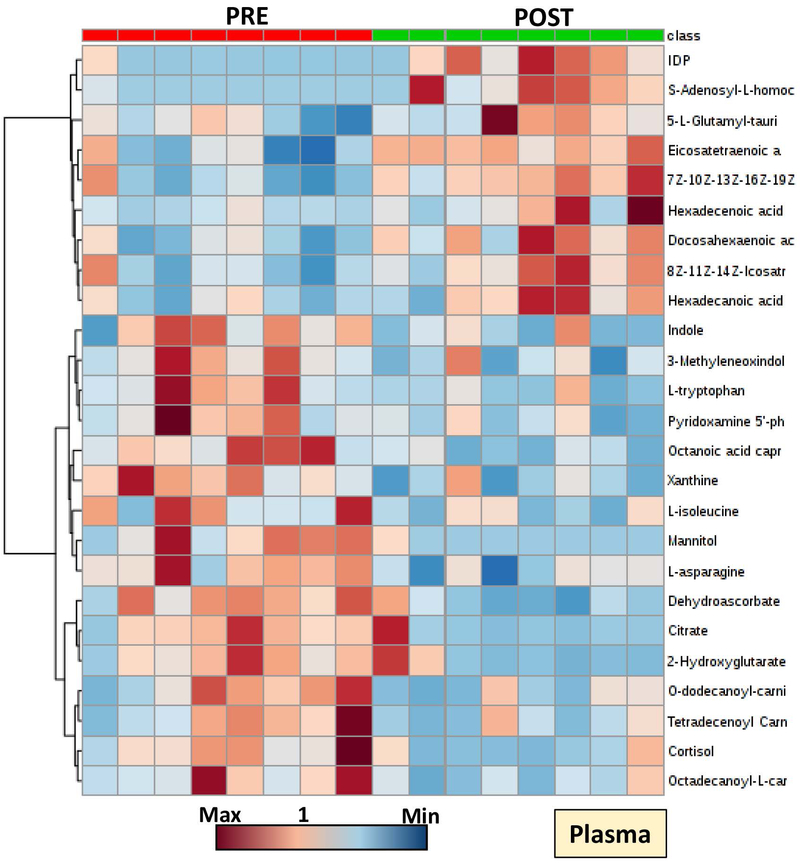
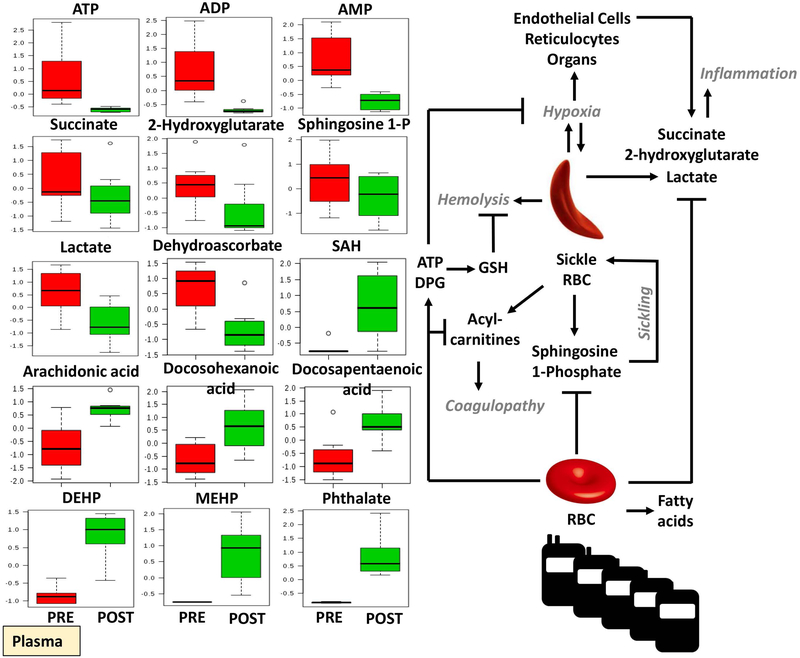
Significant alterations were observed in the plasma metabolic phenotypes of transfused SCD patients (Figure 4; Supplementary Figure 2). Since exchange transfusion therapies are administered to counteract systemic hypoxemia, the observed decreases in systemic markers of hypoxia such as lactate, sphingosine 1-phosphate, succinate and 2-hydroxyglutarate were expected (Figure 4). Decreases in oxidative stress (dehydroascorbate, hypoxanthine) and hemolysis markers (bilirubin, biliverdin; plasma ATP, ADP and AMP) were noted upon exchange transfusion (Figure 5; Supplementary Figure 2). Unexpectedly, exchange transfusion significantly decreased the circulating levels of fatty acyl-conjugated carnitines (including hexanoyl-, octanoyl-, decanoyl-, decenoyl-, dodecanoyl-, dodecenoyl-, tetradecenoyl- and palmitoyl-carnitines) and amino acids (including valine, leucine, isoleucine, proline, tyrosine, glycine, serine, glutamine, arginine, asparagine and methionine - Figure 4; Supplementary Figure 2). On the other hand, post-exchange plasma was characterized by increases in free fatty acids, including arachidonic acid, docosahexaenoic acid and docosapentaenoic acid (Figure 5), all metabolites that increase in stored RBC supernatants during storage in the blood bank.9 Finally, phthalate plasticizers (phthalate, monoethylhexyl-phthalate – MEHP and di-ethylhexylphthalate – DEHP) – which leak from the bag during storage in the blood bank up to mM levels – are significantly increased in post-transfusion plasma of SCD recipients (Figure 5).
Figure 4 –
Hierarchical clustering analysis of the top 25 significant metabolites in plasma from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).
Figure 5 –
Significant metabolic changes in plasma from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).
Discussion
Here we provided the first metabolic characterization of plasma and RBCs from SCD patients prior to or after exchange transfusion. The results are novel in that they confirm that – despite the well-established storage-induced depletion of DPG within the first two weeks of storage46–48 – RBCs stored for ~2 weeks are still capable of regenerating high energy phosphate compounds ATP and DPG within 5h from transfusion to levels above those measured in patients’ own sickle RBCs before transfusion. This observation is consistent with historical observations on the in vivo reversibility of some of the metabolic lesions for RBCs stored,49 though the rate at which this phenomenon occurs may be influenced by the storage-dependent percentage of reversible/irreversible lesions to key glycolytic enzymes such as glyceraldehyde 3-phosphate dehydrogenase. However, in the present study the absence of longitudinal samples post-transfusion limits any sort of speculation with respect to kinetics for the observed phenomenon. Transfused RBCs in this study were more metabolically active, as inferred from lower steady state levels of glucose and higher levels of all glycolytic intermediates and byproducts in comparison to pre-transfusion sickle RBCs – which are already characterized by supra-physiological glycolytic fluxes, as reported in other studies where stable isotope-labeled tracers were used.41
RBC ATP, DPG and sphingosine 1-phosphate were higher in transfused RBCs than in pre-transfusion sickle RBCs. These metabolites play a key physiological role in stabilizing the tense deoxygenated state of hemoglobin and promoting oxygen off-loading,50 which would in turn counteract systemic hypoxemia. Consistently, exchange transfusion resulted in decreases in circulating levels of systemic markers of hypoxia such as lactate, sphingosine 1-phosphate, hypoxanthine51 2-hydroxyglutarate52 and succinate, all decreasing in post-exchange transfusion plasma and increasing in post-transfusion RBCs that could function as a reservoir for these compounds. Therefore, our data clearly indicate that exchange transfusion was effective in restoring systemic tissue oxygenation in SCD recipients. In addition, these observations are interesting in the light of the recently appreciated role of succinate53 and sphingosine 1-phosphate in mediating inflammatory sequelae and oxygen off-loading50 in response to pathological systemic hypoxemia, such as in the case of trauma/hemorrhagic shock54 and ischemia.55
Plasma levels of succinate (and other carboxylic acids such as 2-hydroxyglutarate) have long been associated with hypoxic responses, in that they prevent the degradation of the hypoxia inducible factor 1alpha (HIF1a) by inhibiting its hydroxylation by prolyl hydroxylases.56 By regulating the activities of transcriptional activators TET enzymes (alpha-ketoglutarate-dependent dioxygenase that hydroxylate methylcytosines in DNA), succinate (and fumarate) regulate the expression of hypoxia-inducible genes.57 Circulating levels of carboxylic acids like succinate derive from decreased oxygen availability, which limits electron flow through the electron transport chain (ETC) in mitochondria and results in ETC uncoupling and accumulation of dicarboxylates.58,59 In SCD patients, this may occur in in mitochondria-endowed cells, such as endothelial cells or reticulocytes (since SCD patients have high reticulocyte counts) or abnormal mature RBCs, which have been reported to contain residual mitochondria in SCD patients.60 Plasma levels of succinate in SCD patients may thus trigger inflammatory responses through cell-autonomous mechanisms, though activation of the succinate receptor GPR91 has also been associated with late termination of inflammatory processes via non-cell autonomous mechanisms.61 Decreases of plasma dicarboxylates in SCD recipients upon exchange transfusion may thus contribute to the beneficial impact of such therapy in mitigating not just systemic hypoxemia, but also inflammatory complications in SCD recipients.
While sphingosine 1-phosphate is generated by RBCs sphingosine kinase 1 in response to hypoxia under physiological conditions (e.g. high-altitude hypoxia),50 the same metabolite holds relevant physiological implications in SCD patients, where it induces sickling by promoting the translocation to the membrane of sickle hemoglobin and its polymerization.41,62 Therefore, by decreasing circulating levels of sphingosine 1-phosphate, exchange transfusion contributes to mitigating sickling of the recipient’s own RBCs.
Of note, the overall antioxidant capacity of transfused RBCs in comparison to pre-transfusion SCD is decreased, suggestive of (i) compensatory mechanisms in the sickle RBC to mitigate oxidative injury and/or (ii) a long lasting impact of storage-induced decreased in the total glutathione pool11,63,64 that persists within 5h from the start of exchange transfusion. However, post-transfusion RBCs were characterized by higher steady state levels of non-oxidative phase PPP metabolites (ribose phosphate and sedoheptulose phosphate), suggestive of an improved capacity to activate NADPH-generating redox pathways in the transfused cells.
Moreover, a beneficial impact of exchange transfusion was immediately evident with respect of decreases levels of circulating markers of hemolysis and oxidative stress, including extracellular ATP, ADP and AMP, biliverdin/bilirubin. Exchange transfusion also results in significant dilution of circulating levels of amino acids, which are included in current storage additive formulations. Though future studies will have to investigate the functional implications of this observation, it is interesting to speculate that amino acids such as glutamine, glutamate and arginine play a key role in redox and nitric oxide homeostasis. Therefore, post-transfusion plasma may not adequately sustain the metabolic demands of circulating cells/organs within the first few hours from transfusion unless novel amino acid-rich additives are introduced, or amino acids are supplemented in transfused recipients, either orally, intravenously or, in trauma patients, enterally (though reviews of clinical data highlight some controversies65).
Plasma levels of circulating fatty acids – which are known to accumulate in stored RBC units as a function of storage age47,63,66 – increased upon exchange transfusion in comparison to pre-transfusion levels, while plasma levels of acyl-carnitines decreased. Normalization of circulating levels of acyl-carnitines by exchange transfusion may hold significant implications with respect to bleeding phenotypes of transfusion recipients, in the light of the anticoagulant effect of this class of compounds.67,68
Despite the many beneficial effects of exchange transfusion highlighted above, plasticizers leaking from the storage bags were found to significantly accumulate in the plasma of transfused SCD recipients. It is interesting to note that chronic exposure to plasticizers has been previously associated with prostate cancer and fertility issues in males69 and reproductive outcomes in females in laboratory studies.70,71 Since phthalate plasticizers progressively accumulate in storage units as a function of storage time up to mM levels,14 it is interesting to note that exchange transfusion would theoretically result in hundred uM to low mM levels of phthalate plasticizers in plasma and, in a population exposed chronically to transfusion such as SCD patients – could promote untoward consequences beyond factors such as mortality – as tested in recent randomized clinical trials on the age of blood. It will be relevant to understand whether the age of blood, while not significantly associated with increased mortality in transfusion recipients as the recent randomized clinical trials suggest, may indeed be negatively associated with other morbidities in chronically exposed populations such as SCD recipients. Whether clinical evidence of the aforementioned risks will be produced, the introduction of phthalate-free storage bags or alternative plasticizers with reduced toxicity may represent a readily available solution.
In the present study exchange transfusion was performed by administering only relatively fresh units (~16 days old). In the light of reassuring evidence from randomized clinical trials, ethical concerns may be now mitigated and pave the way for future studies investigating whether similar phenotypes to those reported here would be observed in response to exchange transfusion with exclusively end of storage units. Finally, by impacting RBC hemolytic propensity, other factors such as donor gender, age, ethnicity or donation frequency72 may impact the transfusion efficacy and the metabolic phenotypes of transfused recipients, though the limited numbers of cases tested in the present study and the large number of units received from donors with different (and unknown) biology during the exchange transfusion therapy prevent us from drawing any reliable conclusion on these variables.
Supplementary Material
Supplementary Figure 1 – Hierarchical clustering analysis of RBCs metabolic phenotypes in SCD patients prior to and after exchange transfusion
Supplementary Figure 2 – Hierarchical clustering analysis of plasma metabolic phenotypes in SCD patients prior to and after exchange transfusion
Supplementary Figure 3 – Additional significant metabolic changes in RBCs from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).
Acknowledgments
Research reported in this publication was supported in part by funds from the Boettcher Webb-Waring Biomedical Research Award – Early Career grant (ADA). IW was funded by a pilot grant from the NC TraCS and Duke Translational Medicine Institutes, supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure of Conflict of interest ADA is a founder of Omix Technologies Inc. ADA is a consultant for New Health Sciences Inc. All the other authors disclose no conflict of interest relevant to this study.
References
- 1.Kutlar A. Sickle cell disease: a multigenic perspective of a single gene disorder. Hemoglobin. 2007;31(2):209–224. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E, Johnson C, Powars D, West C. Prevalence of Glucose-6-Phosphate Dehydrogenase Deficiency in Sickle-Cell Disease. N. Engl. J. Med 1974;290(15):826–828. [DOI] [PubMed] [Google Scholar]
- 3.Goodman SR, Pace BS, Hansen KC, et al. Minireview: Multiomic candidate biomarkers for clinical manifestations of sickle cell severity: Early steps to precision medicine. Exp. Biol. Med. Maywood NJ 2016;241(7):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 5.Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am. J. Med 1978;65(3):461–471. [DOI] [PubMed] [Google Scholar]
- 6.Kassim AA, Galadanci NA, Pruthi S, DeBaun MR. How I treat and manage strokes in sickle cell disease. Blood. 2015;125(22):3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wun T, Hassell K. Best practices for transfusion for patients with sickle cell disease. Hematol. Rev 2010;1(2):. [Google Scholar]
- 8.Shah N, Welsby IJ, Fielder MA, Jacobsen WK, Nielsen VG. Sickle cell disease is associated with iron mediated hypercoagulability. J. Thromb. Thrombolysis 2015;40(2):182–185. [DOI] [PubMed] [Google Scholar]
- 9.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55(1):205–219. [DOI] [PubMed] [Google Scholar]
- 10.Yurkovich JT, Zielinski DC, Yang L, et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J. Biol. Chem 2017;292(48):19556–19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessandro A, Nemkov T, Kelher M, et al. Routine Storage of Red Blood Cell Units in Additive Solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfsson Ó, Sigurjonsson ÓE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017;112(4):326–335. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016;14(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56(4):852–862. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of AS-1 RBC storage. Transfus. Med. Rev. 2014;28(2):41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertinhez TA, Casali E, Lindner L, et al. Biochemical assessment of red blood cells during storage by 1H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus. 2014;12(4):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56(2):421–426. [DOI] [PubMed] [Google Scholar]
- 20.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;blood-2016–05-714816. [DOI] [PubMed] [Google Scholar]
- 21.Harper VM, Oh JY, Stapley R, et al. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid. Redox Signal 2015;22(4):294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–1449. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Zheng Y, Wang X, et al. Stiffness increase of red blood cells during storage. Microsyst. Nanoeng 2018;4:17103. [Google Scholar]
- 25.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Invest 2017;127(1):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. Trasfus. Sangue. 2017;15(2):112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56(10):2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101(5):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mays JA, Hess JR. Modelling the effects of blood component storage lesions on the quality of haemostatic resuscitation in massive transfusion for trauma. Blood Transfus. Trasfus. Sangue 2017;15(2):153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. [DOI] [PubMed] [Google Scholar]
- 32.Gehrke S, Srinivasan AJ, Culp‐Hill R, et al. Metabolomics evaluation of early-storage red blood cell rejuvenation at 4°C and 37°C. Transfusion. 0(0): [DOI] [PubMed] [Google Scholar]
- 33.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32–42. [DOI] [PubMed] [Google Scholar]
- 34.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56(2):421–426. [DOI] [PubMed] [Google Scholar]
- 35.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–1449. [DOI] [PubMed] [Google Scholar]
- 36.Rinalducci S, Ferru E, Blasi B, Turrini F, Zolla L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. Trasfus. Sangue 2012;10 Suppl 2:s55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakhniashvili DG, Griko NB, Bulla LA, Goodman SR. The proteomics of sickle cell disease: profiling of erythrocyte membrane proteins by 2D-DIGE and tandem mass spectrometry. Exp. Biol. Med. Maywood NJ 2005;230(11):787–792. [DOI] [PubMed] [Google Scholar]
- 38.Darghouth D, Koehl B, Junot C, Roméo P-H. Metabolomic analysis of normal and sickle cell erythrocytes. Transfus. Clin. Biol. J. Soc. Francaise Transfus. Sang 2010;17(3):148–150. [DOI] [PubMed] [Google Scholar]
- 39.Darghouth D, Koehl B, Madalinski G, et al. Pathophysiology of sickle cell disease is mirrored by the red blood cell metabolome. Blood. 2011;117(6):e57–66. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Dai Y, Wen J, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat. Med. 2011;17(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun K, D’Alessandro A, Ahmed MH, et al. Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci. Rep 2017;7(1):15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56(4):980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 44.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. RCM 2017;31(8):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43(Web Server issue):W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J. Proteomics 2012;76 Spec No.:168–180. [DOI] [PubMed] [Google Scholar]
- 47.D’Alessandro A, Nemkov T, Hansen KC, Szczepiorkowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55(12):2955–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56(4):852–862. [DOI] [PubMed] [Google Scholar]
- 49.Heaton A, Keegan T, Holme S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. Br. J. Haematol 1989;71(1):131–136. [DOI] [PubMed] [Google Scholar]
- 50.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun 2016;7:12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103(2):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Intlekofer AM, Dematteo RG, Venneti S, et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metab. 2015;22(2):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DʼAlessandro A, Moore HB, Moore EE, et al. Plasma succinate is a predictor of mortality in critically injured patients. J. Trauma Acute Care Surg 2017;83(3):491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Alessandro A, Moore HB, Moore EE, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am. J. Physiol. Regul. Integr. Comp. Physiol 2015;308(12):R1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koivunen P, Hirsilä M, Remes AM, et al. Inhibition of Hypoxia-inducible Factor (HIF) Hydroxylases by Citric Acid Cycle Intermediates Possible links between cell metabolism and stabilization of hif. J. Biol. Chem 2007;282(7):4524–4532. [DOI] [PubMed] [Google Scholar]
- 57.Laukka T, Mariani CJ, Ihantola T, et al. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J. Biol. Chem 2016;291(8):4256–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, et al. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid. Redox Signal 2018;28(3):230–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 2016;1857(8):1086–1101. [DOI] [PubMed] [Google Scholar]
- 60.Jagadeeswaran R, Lenny H, Vazquez B, et al. The Abnormal Presence of Mitochondria in Circulating Red Blood Cells Cause an Increased Oxygen Consumption Rate, ROS Generation and Hemolysis in Patients with Sickle Cell Disease. Blood. 2017;130(Suppl 1):2237–2237.29170191 [Google Scholar]
- 61.Grimolizzi F, Arranz L. Multiple faces of succinate beyond metabolism in blood. Haematologica. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Berka V, Song A, et al. Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J. Clin. Invest 2014;124(6):2750–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 64.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51(7):1450–1459. [DOI] [PubMed] [Google Scholar]
- 65.van Zanten ARH, Dhaliwal R, Garrel D, Heyland DK. Enteral glutamine supplementation in critically ill patients: a systematic review and meta-analysis. Crit. Care Lond. Engl 2015;19:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56(10):2560–2570. [DOI] [PubMed] [Google Scholar]
- 67.Deguchi H, Banerjee Y, Trauger S, et al. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood. 2015;126(13):1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimring JC. Does AC stand for acylcarnitine, anticoagulant, or both? Blood. 2015;126(13):1524–1525. [DOI] [PubMed] [Google Scholar]
- 69.Kismali G, Yurdakok-Dikmen B, Kuzukiran O, Arslan P, Filazi A. Phthalate induced toxicity in prostate cancer cell lines and effects of alpha lipoic acid. Bratisl. Lek. Listy 2017;118(8):460–466. [DOI] [PubMed] [Google Scholar]
- 70.Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod. Toxicol 2015;53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol 2013;43(3):200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – Hierarchical clustering analysis of RBCs metabolic phenotypes in SCD patients prior to and after exchange transfusion
Supplementary Figure 2 – Hierarchical clustering analysis of plasma metabolic phenotypes in SCD patients prior to and after exchange transfusion
Supplementary Figure 3 – Additional significant metabolic changes in RBCs from SCD patients prior to and after exchange transfusion (red - left and green - right, respectively).