Abstract
Elevated major ion concentrations in streams are commonly observed as a consequence of resource extraction, de-icing and other anthropogenic activities. Ecologists report biodiversity losses associated with increasing salinity, with mayflies typically being highly responsive to increases of different major ions. In this study, we evaluated the performance of the mayfly Neocloeon triangulifer reared for its entire larval phase in a gradient of sulfate concentrations. Two natural waters were amended with SO4 as a blend of CaSO4 and MgSO4 and exposures ranged from 5 to 1500 mg l–1 SO4. Survival (per cent successful emergence to the subimago stage) was significantly reduced at the highest SO4 concentration in both waters, while development was significantly delayed at 667 mg l−1 SO4. Final sub-adult body weights were consistent across treatments, except at the highest treatment concentration. Despite evidence for sulfate uptake rates increasing with exposure concentrations and not being saturated at even extremely high SO4 concentrations, total body sulfur changed little in subimagos. Together, these results suggest that elevated SO4 imposes an energetic demand associated with maintaining homeostasis that is manifested primarily as reduced growth rates and associated developmental delays. We identified two genes related to sulfate transport in N. triangulifer.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: salinity stress, ion transport, mayfly, development, freshwater ecosystem
1. Introduction
Freshwater salinization has emerged as a major issue in freshwater ecosystems throughout the world [1–6]. Anthropogenic causes of salinization include road salting [7,8], irrigation of arid lands [9,10], urbanization [11,12], natural gas extraction and associated brine spills [13,14] and mountaintop removal/valley fill mining [6]. Climatic related changes in freshwater salinities include altered precipitation patterns and drought [15] and seawater intrusion from rising sea levels. These diverse causes of salinization occur in different geologies and result in a wide variety of salinity signatures. It is important that we significantly improve our understanding of how different salinity regimes affect aquatic life and how they might be mitigated.
Surface coal mining in many parts of the world can result in rather unique stream chemistries marked by dramatic increases in sulfate, bicarbonate, calcium and magnesium [6]. These streams are often low in the concentrations of other ions (e.g. Na and Cl) commonly observed to be elevated in other anthropogenic causes of increased salinity. Ecological responses to sulfate-dominated increases in salinity include the loss of mayflies and other taxa [6], which may be locally adapted to dilute habitats.
Because mayflies are important players in freshwater ecosystems and are responsive to environmental changes, several laboratories have begun working with Neocloeon triangulifer (formerly Cloeon triangulifer and Centroptilum triangulifer) as a mayfly model to explore responses to elevated salinity. Neocloeon triangulifer is one of a very few mayfly species that has been cultured successfully in the laboratory. Sweeney and co-workers [16,17] were the first to culture this species and use it in a bioassay context. Decades later, studies by Xie and co-workers [18,19], Conley et al. [20–23] and Kim et al. [24] demonstrated the use of this species to study trace metal accumulation and trophic exposures through periphytic biofilms. Other laboratories soon followed suit [25–27], and the United States (US) Environmental Protection Agency (EPA) began work to standardize rearing conditions so that the species could be used in different laboratories with established rearing protocols [28,29].
Scheibener et al. [30] was the first to study sulfate transport kinetics in N. triangulifer and other stream insects, and demonstrated that increasing sodium (Na) concentrations resulted in reduced sulfate uptake from solution, which corresponded with reduced sulfate toxicity on an acute basis. This finding was important, because the use of sodium sulfate to asses sulfate toxicity is the most standard practice in major ion toxicity studies, e.g. [27,31]. However, Scheibener's observation suggests that the use of sodium sulfate to assess sulfate toxicity may not appropriately reflect sulfate toxicity in the low Na environments common to coal mining impacted streams.
In this study, we assessed the toxicity of sulfate in two dilute natural waters characterized by low Na (≤2.5 mg l−1). A gradient of sulfate concentrations was prepared by spiking these natural waters with a blend of CaSO4 and MgSO4, maintaining a Ca : Mg mass ratio of 2.4 : 1, which was the ratio observed at site A, while site B had a ratio of 2.8 : 1. Newly hatched larvae were reared to the subimago stage across these sulfate gradients, and the percentage of larvae to successfully emergence, time to emergence and subimago weights were recorded. Total body sulfur (S) was measured in subimagos across treatments. Companion studies assessed the effect of sulfate and other major ion concentrations on mRNA expression levels using real time-quantitative polymerase chain reaction (RT-qPCR). We provide primer sequences for two genes associated with ion transport in N. triangulifer for the growing N. triangulifer research community.
2. Material and methods
(a). Test organisms and water preparation
The parthenogenetic mayfly N. triangulifer (clone WCC-2) was originally obtained from Stroud Water Research Center (Avondale, PA). Larvae were hatched in a modified artificial soft water (ASW) (mM: CaSO4 · 2H2O = 0.10, Na2HCO3 = 0.69, MgSO4 · 7H2O = 0.14, CaCl2 = 0.20 and K2HCO3 = 0.03). This recipe was obtained from the US EPA (D. Mount 2017, personal communication) based on a meta-analysis of surface waters throughout the conterminous USA.
The base waters for the life cycle bioassay were collected from two different waterbodies at a metallurgical coal mine site in British Columbia (BC), Canada, where there was an interest in establishing a suitable limit for sulfate concentration in order to protect aquatic life. Concentrations of major ions in the waters, prior to amending with sulfate, are shown in table 1. The waters were amended with nominal concentration of 296, 444, 667, 1000 and 1500 mg l−1 sulfate using CaSO4 · 2H2O and MgSO4 · 7H2O (at a 2.4 : 1 mass ratio, or 1.45 molar ratio of Ca : Mg), which is consistent with the site A ratio. Concentrations of sulfate in the test solutions at the start of the test were measured by ALS Environmental (Burnaby, BC, Canada) by ion chromatography (table 2) with a subset of samples verified by inductively coupled plasma-mass spectrometry (ICP-MS) at North Carolina State University (NCSU). At the termination of the experiment, the SO4 concentrations were measured by ICP-MS in a single sample each treatment group and all were within 9% of the initial (table 2) values.
Table 1.
Major ion composition of the two site waters used as the base water for SO4 exposures.
| ion | site A (mg l−1) | site B (mg l−1) |
|---|---|---|
| Ca | 23.0 | 13.9 |
| Mg | 9.3 | 5.0 |
| Na | 2.5 | 0.4 |
| K | 0.7 | 0.4 |
| HCO3 | 74.5 | 59.8 |
| SO4 | 30.1 | 5.6 |
| Cl | <0.5 | <0.5 |
| TDS | 119 | 70 |
Table 2.
Measured sulfate concentrations and total dissolved solids as sum of major ions.
| nominal SO4 addition (mg l−1) | measured SO4 (mg l−1) |
TDS (mg l−1) |
conductivity (µS cm−1) |
|||
|---|---|---|---|---|---|---|
| site A | site B | site A | site B | site A | site B | |
| +296 | 330 | 312 | 525 | 487 | 729 | 673 |
| +444 | 475 | 456 | 724 | 681 | 967 | 905 |
| +667 | 706 | 678 | 1044 | 987 | 1307 | 1246 |
| +1000 | 1070 | 1050 | 1540 | 1491 | 1779 | 1698 |
| +1500 | 1640 | 1580 | 2301 | 2201 | 2386 | 2292 |
(b). Life cycle bioassay
Treatment solutions were aliquoted into replicate 1.8 l Ball™ glass jars with 1.75 l of water in each jar (three replicates per treatment). In addition to stream base water controls, a modified ASW control (see above for chemistry) (n = 4) was also included. Newly hatched larvae were provided a diet of natural periphyton to ensure survivorship for the first 24 h post-hatch until the start of the experiment. Upon the start of the experiment, mayflies (1–2 days old) were transferred to rearing jars provisioned with acrylic plates (6.5 × 23 × 0.15 cm) colonized by an assemblage of natural periphyton (a mixture of algae, diatoms, microbiota and detritus). Periphyton plates were produced at Stroud Water Research Center by allowing stream water (White Clay Creek, PA) to flow continuously over plates contained in a greenhouse as described previously [20,21]. Plates were shipped overnight to NCSU and upon reception; one plate was placed into each replicate jar, 1 day prior to the introduction of mayfly larvae. A second plate was placed 15 days after the start of the experiment to ensure adequate food supply throughout life cycle. Water was not changed during the experiment.
Each replicate was aerated and Parafilm™ was used to reduce evaporative loss, and deionized water was added to marked ‘fill’ lines on each vessel as needed. Conductivity, pH and dissolved O2 were measured approximately 3 days per week in a minimum of two replicates per treatment. Conductivities were relatively stable for the duration of the experiment (electronic supplementary material, figure S1). pH measures (n = 390) averaged 8.13 ± 0.25, with the 7.57 and 8.86 being the extreme low and high measures recorded, respectively. Dissolved oxygen measures (n = 390) averaged 7.7 ± 0.7 mg l−1 through the duration of the experiment, with 5.6 mg l−1 being the lowest value recorded. A Hobo™ temperature probe monitored temperature every hour for the entirety of the experiment and varied between 23.3 and 24.8°C. After approximately 2.5 weeks, mesh-lined collection lids were placed on top of each jar. Subimagos emerged in late afternoon/evening and were collected in 1.5 ml centrifuge tubes and placed in a −20°C freezer overnight. The mayflies were removed from the freezer, dried overnight at 60°C and weighed dry to the nearest 0.001 mg using a Sartorius CPA2P microbalance.
Dried subimagos were taken for whole body S concentrations across treatments from each water type. We also obtained dried subimagos from sulfate exposures conducted at the Stroud Water Research Center to assess variability in whole body S. Three individuals were composited to form a replicate and were microwave-digested (CEM MARSXpress) in 1 ml Omnitrace Ultra High Purity Nitric Acid (EMD Chemicals, Darmstadt, Germany). Filtered water samples (14 ml) were taken for each treatment pre- and post-test. Samples were filtered through 0.45 µm nylon syringe filters. Each sample was then acidified with 1 ml of Omnitrace Ultra High Purity Nitric Acid (EMD Chemicals, Darmstadt, Germany). Subimago digests and water samples were processed by the Environmental and Agricultural Testing Services Laboratory (Department of Soil Science, North Carolina State University, Raleigh, NC, USA) for quantification of major ions (Na, Ca, Mg, K, Cl and S) via ICP-OES. Quality control blanks were below detection limits. Standard reference material (NIST 2976 mussel tissue (freeze-dried)) was within 10% of expected concentrations.
To better understand performance differences of N. triangulifer in the different site waters, we used Visual MINTEQ, v. 3.0 (JP Gustafsson, Royal Institute of Technology, Department of Land and Water Resources Engineering, Stockholm, Sweden) to calculate the activity of all potential ionic species based on mean measured anion and cation concentrations. This is informative, because in more concentrated solutions, it may be necessary to account for the fact that ions behave as though their concentrations are lower than nominal or measured concentrations, i.e. an ion's ‘activity’ will probably be less than its concentration [32] (see [33]). For activity modelling, we input fixed pH values based on the average measured pH for each test.
(c). RNA isolation and quantitative polymerase chain reaction analysis for gene expression study
To compare the gene expression associated with sulfate stress, primers were designed based on a de novo assembly of compiled N. triangulifer cDNA sequence data (both 454 and Illumina platforms) resulting in approximately 23 000 contigs with associated bioinformatics. Based on these annotated contigs, we selected two genes of interest, a sulfate transporter and a Na-independent sulfate transporter, and developed probes for gene expression studies (table 3). Tubulin is served as a reference gene. Each gene of interest was inserted into a pCR2.1®-TOPO® TA vector (Life Technologies) expression vector and produced sequences that were independently confirmed. All primers were designed with IDTSciTools (http://www.idtdna.com/SciTools/SciTools.aspx) and were synthesized by Life Technologies, (Carlsbad, CA, USA).
Table 3.
List of primer sequences for qPCR gene expression studies.
| gene name | accession number | amplicon | primer sequences |
|---|---|---|---|
| tubulin | MF463012 | 219 | forward: 5′-ATGCCCTCTGACAAGACTGTTGGA-3′ |
| reverse: 5′-ATAGTGACCGCGAGCGTAGTTGTT-3′ | |||
| sulfate transporter | MH549240 | 204 | forward: 5′-TGATCATTACCGGCATCATCGGCT-3′ |
| reverse: 5′-TTTCCAGAATGGCGAGGATAGGCA-3′ | |||
| Na-independent sulfate transporter | MH549239 | 181 | forward: 5′-CTTCATTCCACGCACTACC-3′ |
| reverse: 5′-CACCACCATGCCGATTAG-3′ |
For gene expression studies, N. triangulifer mature larvae were exposed to ASW amended with Mg/CaSO4 and Na2SO4 (both containing 600 mg l−1 SO4) for 24 h. Total RNA was isolated (two larvae pooled for one biological replicate) following the SV Total RNA Isolation System protocol (Promega, Madison, WI). First-strand cDNA was synthesized from the same amount of each total RNA by MultiScribe™ MuLV reverse transcriptase using random primers (Applied Biosystems, Carlsbad, CA, USA) and all thermocycling was done using a Bio-Rad iCycler (Bio-Rad, Hercules, CA). RT-qPCR was performed on an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) using default parameters: 2 min at 94°C, followed by 40 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. Relative expression of each amplicon was calculated by the corrected delta–delta Ct method [34], with all expression normalized to Tubulin levels in initial control samples. Relative levels of Tubulin were confirmed to be approximately equal across all treatments. Results were plotted using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). All treatments were compared to ASW (control group) and a Student's t-test was performed to analyse the differences of gene expression between treatments and control. All biological replicates for treatment groups and control group had n = 6.
3. Results
Measured sulfate concentrations were in good agreement with targeted values (mean and s.d. of 101 ± 3% of target values, after taking into consideration the sulfate in the site waters) (table 2). Control survival (% of larvae to successfully emerge to the subimago stage) in ASW was 82 ± 2%, while survival in unmodified site water (site controls) was 76 ± 2% and 76 ± 6% in site A and B water controls, respectively. In site waters amended with SO4 between 296 and 1000 mg l−1 SO4, survival varied between 58 ± 12 and 80 ± 12%. This variation was idiosyncratic and not systematically associated with changes in exposure concentration. However, at 1500 mg l−1 SO4, survival decreased to 33 ± 4% and 29 ± 6% in site A and B waters, respectively (figure 1).
Figure 1.
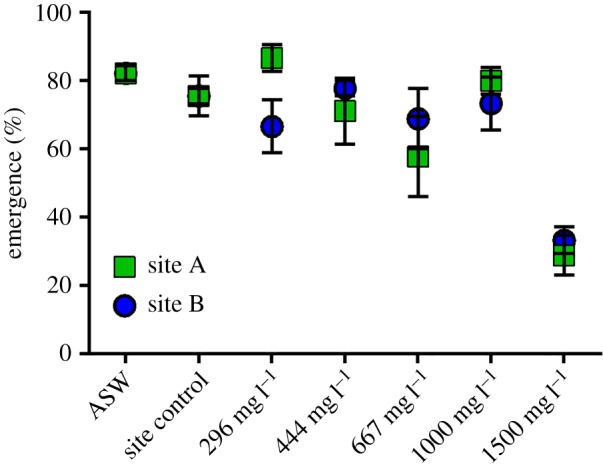
Emergence percentage of N. triangulifer subimagos reared in two site waters amended with SO4 as a blend of CaSO4 and MgSO4. Each point represents the mean of three replicate rearing chambers each seeded with 15 larvae 1 day post egg hatch. (Online version in colour.)
Mean subimago body masses were remarkably consistent across controls and SO4 treatments with the exception of the 1500 mg l−1 SO4 treatment group (figure 2). Relative to their respective site controls, the 1500 mg l−1 treated subimago body weights were reduced 16% and 22% in site A and B waters, respectively. In general, larvae seemed to grow better in the site A (control and amended) water than in site B counterpart treatments, but these differences were only significantly different (p < 0.05) for site controls, 296 mg l−1 and 1000 mg l−1 SO4 treatments (Tukey's multiple comparison test).
Figure 2.
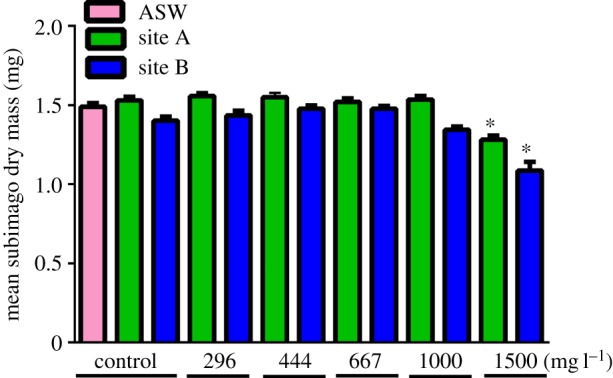
Mean of individual subimago weights (dry mass) emerging from different sulfate exposures.
Development time was significantly affected by sulfate exposures to 667 mg l−1 (site A), 1000 mg l−1 (both waters) and 1500 mg l−1 (both waters) (figure 3). Larvae that survived exposure to 1500 mg l−1 SO4 took 33% (site A water) and 40% (site B water) longer to reach the subimago stage than did their respective controls. Exposure to 1000 mg l−1 prolonged development by 9% and 13% in site A and B waters, respectively. Exposure to 667 mg l−1 (site A) prolonged development by 8%, whereas in site B water, this exposure did not significantly delay development.
Figure 3.
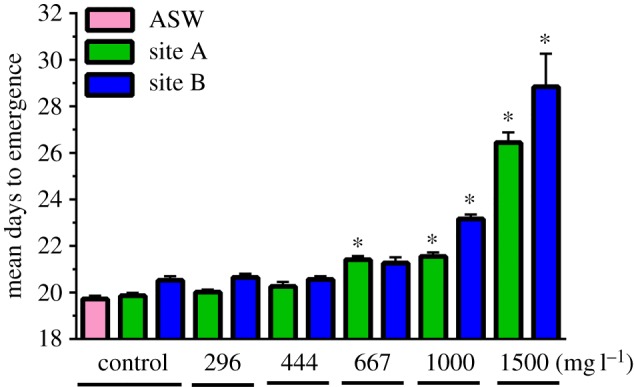
Average time (days) required for completion of larval development in natural waters amended with SO4 as a blend of CaSO4 and MgSO4.
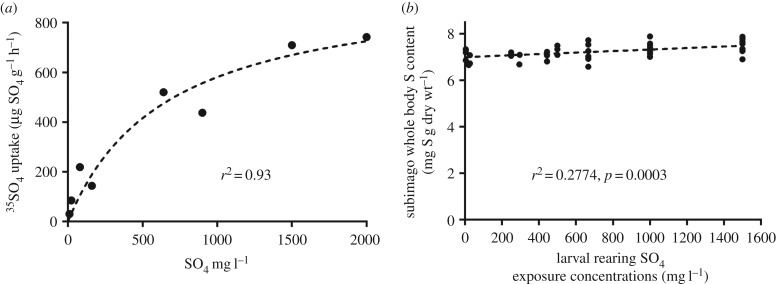
Previous work examining the kinetics of 35SO4 uptake as a function of concentration [30] showed expected Michaelis–Menten-type kinetics, but surprisingly did not fully saturate even at the highest SO4 concentrations tested (figure 4a). However, this rapid SO4 uptake in larvae did not result in appreciable changes in whole body S content in subimagos (figure 4b). Though statistically significant (R2 = 0.28, p = 0.0003), there was little appreciable increase in whole body S across four orders of magnitude of SO4 exposure concentrations. Across all treatments, S content varied between 6.6 and 7.8 mg g−1 on a dry weight basis, (mean = 7.2 ± 0.05), suggesting that N. triangulifer strongly regulates S content.
Figure 4.
(a) 35SO4 uptake rates in larvae of the mayfly N. triangulifer as a function of SO4 concentrations (re-drawn from Scheibener et al. [30]). (b) Total body S concentrations in subimagos reared for their entire larval stage at different SO4 concentrations.
Modelled sulfate activities in test treatments ranged from approximately 0.2 (controls) to approximately 5 mM in the highest test treatments in both waters (see the electronic supplementary material, table S1). In both waters, activities decreased as a per cent of concentration with increasing Ca and Mg in dilution water. For example, in the controls for both waters, free sulfate activity was approximately 80% of the measured concentration, whereas in the 1500 mg l−1 treatments, free sulfate activity decreased to approximately 48% of the measured concentration. The slightly better performance of N. triangulifer in site A waters relative to site B was not directly attributable to differences in SO4 activity, but both differences in activities and performance were relatively subtle.
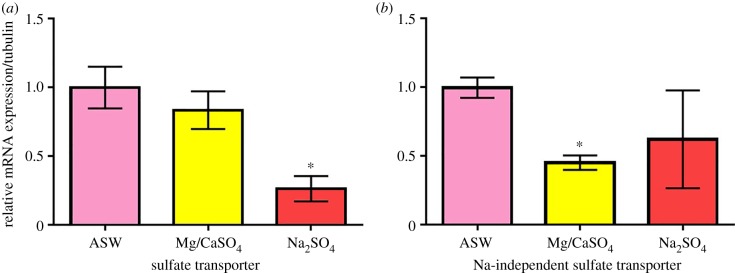
To further our understanding of the osmoregulatory physiology of N. triangulifer, we examined the expression patterns of two genes that encode a sulfate transporter protein. Gene expression results showed that the expression level of sulfate transporter only reduced slightly under MgSO4 and CaSO4 but had a significant 74% reduction under Na2SO4 treatment (p = 0.002) (figure 5a). The expression level of the sodium-independent sulfate transporter gene, on the other hand, had a significant 55% reduction (p = 0.0001) under MgSO4 and CaSO4 treatment but no statistically significant differences under Na2SO4 treatment (figure 5b).
Figure 5.
Relative mRNA expression of N. triangulifer larvae subjected to 24 h sulfate stress treatment. All data are normalized to control (ASW). Error bars represent standard deviations from the mean (n = 6). A Student's t-test was performed and an asterisk indicates significant difference compared to ASW control. (Online version in colour.)
4. Discussion
The emergence of freshwater salinization as an ecological problem [1–3,5,11] requires that we rapidly fill gaps in our understanding of how major ions affect aquatic life. It is important to acknowledge that mechanisms of major ion toxicity may differ among faunal groups, particularly in relation to evolutionary history. Insects dominate the ecology of running waters, and this alone should compel us to better understand how major ions shape their distributions and responses to changes in salinity regimes being reported around the globe. Moreover, the physiology of aquatic insects may be unique resulting from their history as secondarily aquatic species [35,36], and this should compel us to better understand fundamental physiological processes in relation to salinity.
Previous work [37] showed that major ion toxicity in the mayfly N. triangulifer could not be attributed to conductivity alone. Somehow, the ionic composition of the water mattered as waters high in sulfate, but low in sodium, were toxic to the mayfly, but when the ratios of SO4 to Na were considerably lower, toxicity was not observed despite the high conductivity. Subsequent work identified Na as a modifier of acute SO4 toxicity, with increasing Na associated with decreased SO4 uptake and toxicity [30]. This finding is important, because in streams affected by surface coal mining, it is common to find elevated SO4 against a low Na background.
In this paper, we evaluated the toxicity of SO4 in two natural waters with relatively low Na. Sulfate exposures were created by amending these waters with a blend of Ca and Mg sulfate to maintain this low Na background. We anticipated that low Na waters might produce toxicity at lower SO4 concentrations than those reported by Soucek & Dickinson [27] who used Na2SO4 and reported an EC20 for per cent emergence at 145 mg l−1 (95% CI 69–305). In our study, per cent emergence was not affected at 1000 mg l−1 SO4, but was markedly reduced at 1500 mg l−1 SO4.
There are a few possible explanations for the discrepancy between these results and those of the Soucek & Dickinson study [27]. First, our experimental temperatures varied between 23.3 and 24.8°C, while the Soucek & Dickinson study was conducted at 25°C, which is quite close to the edge of this species' thermal acclimation zone (see [38] and [39] for a discussion of the importance of temperature to mayfly growth and development). The Soucek & Dickinson study reports pre-egg laying fresh body weights of 2.5–2.6 mg per individual (wet weight), where our dry weights were typically 1.5 mg (approx. 6.8 mg wet weight based on a typical body water content of N. triangulifer of approx. 78%). Thus, there were substantial differences in larval growth in these studies that can be partially attributable to rearing temperature. Another strong possibility is that there are nutritional differences between the natural periphyton plate diet provided in the present study and the cultured diatom diet provided in the Soucek & Dickinson study. A third and potentially important factor is that the use of concentration unit (mg l−1) rather than ionic activities as a descriptor of toxicity may confound comparisons among studies. The 1500 mg l−1 treatments in both waters of the present study had sulfate activities (5.06 and 4.86 mM for sites A and B, respectively) that approached the average 96 h median lethal sulfate activity (average of approx. 5.5 mM) in experiments with Na2SO4, MgSO4 and K2SO4 in a study by Soucek et al. [40]. Additionally, the 1000 mg l−1 treatment consistently caused delayed development in both waters in the present study had SO4 activities that were only slightly higher than activity determined by Soucek & Dickinson [27] to cause developmental delay in their study. This difference in performance might be attributable to nutritional and thermal differences between the studies as described above. Nonetheless, it is apparent that using ionic activity as a descriptor of toxicity makes the results of the present study and the Soucek & Dickinson study more comparable than if concentration is used as a descriptor of toxicity.
Body weights were unaffected by SO4 concentrations up to 1000 mg l−1, but the development time required to achieve those weights was increased at 667 mg l−1 SO4. This observation is consistent with previous studies showing significant developmental delays associated with exposure to ion-rich waters [27,41]. These observations are suggestive of increased energetic demand associated with development in ion-rich waters in mayflies.
Scheibener et al. [30] previously characterized Michaelis–Menten-type uptake kinetics for SO4 in N. triangulifer and showed that even at a very high SO4 concentration, uptake of the ion was not saturated. To determine whether this situation could lead to ‘self-poisoning’ through excessive SO4 accumulation in tissues, we analysed the total body S content of the subimagos that emerged during the study. We found that total body S was relatively stable across treatments, which suggests that N. triangulifer must be strongly regulating its SO4 content by increasing SO4 excretion rates commensurate with uptake rates. This finding is similar to previous observations of Na regulation in Hydropsyche sparna and Maccaffertium sp. [42], and related observation of the maintenance of haemolymph osmolality in the mayfly Austrophlebioides pusillus even at lethal salinities [43].
Together, these observations suggest that the energetic cost of regulation, rather than the loss of regulation, might drive the effects of elevated major ions on aquatic insects. What is intriguing about this proposition is that sensitivity to elevated major ions might be predictable based on how species regulate the uptake of a given ion when it is present at high concentrations. Species that maintain relatively low uptake rates might be expected to be less responsive to salinity increases. However, our knowledge of the specific ion transporters and how the expression and activities of these transporters are regulated remains virtually unknown.
Our identification of two distinct genes that respond differently to SO4 exposures is a small first step towards understanding these processes. Both our sulfate transporter and Na-independent sulfate transporter genes are annotated using bioinformatics gene prediction methods. Both genes are suggested to encode proteins that belong to the sulfate permease (SulP) family, which mostly consists of inorganic anion uptake transporters or anion:anion exchange transporters [44]. While the sulfate transporter gene was also suggested as a Na-independent sulfate transporter gene in orthologues, experimental results in this study suggest the sulfate transporter gene expression is different than the Na-dependent sulfate transporter in N. triangulifer. Therefore, it is likely that the two genes encode different functional proteins. However, we posit that expression levels of the two genes may also vary in different tissues, and thus it is inconclusive for our data whether the two gene-encoded proteins have different functions or not. Na dependent or not, our data support the likely existence of sulfate transporters in N. triangulifer, and high sulfate treatments affect its gene expression levels. It remains unknown which tissues have more sulfate transporters and the differences between expression levels.
Finally, if salinity differentially challenges aquatic species via the energetic costs of regulation, then modifying factors such as temperature and nutritional availability may play huge roles in ultimately determining how communities and biodiversity change in the face of increasing salinization. If, for example, increasing temperature increases the rates of ion uptake commensurate with basic metabolic rates, the potential for salinity–temperature interactions are great. We might hypothesize that sensitivity to major ions might increase with increasing temperature. Similarly, individuals that are developing with access to sub-optimal nutrition (more oligotrophic conditions) might be considerably more sensitive to changes in salinity regimes than individuals developing with closer-to-optimal dietary resources.
Supplementary Material
Supplementary Material
Acknowledgements
We thank undergraduate research assistants Sophie Westrud (UNC—Chapel Hill) and Maya Houn (NCSU) for their help in executing experiments.
Data accessibility
Data available as part of the electronic supplementary material and on request from dbbuchwa@ncsu.edu.
Authors' contributions
J.E. measured concentrations of sulfate in the test solutions and provided test waters to NCSU. S.S. and D.B. conducted the life cycle bioassay and data analyses. H.C. conducted the gene expression studies and analyses. D.B. and J.E. conceived of the research. D.B. and H.C. wrote the paper.
Competing interests
The authors declare no competing financial interests in this work.
Funding
This work was supported by Nautilus Environmental, Burnaby, BC, Canada.
References
- 1.Canedo-Arguelles M, Kefford BJ, Piscart C, Prat N, Schafer RB, Schulz CJ. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 2.Canedo-Arguelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 3.Kaushal SS, Likens GE, Pace ML, Utz RM, Haq S, Gorman J, Grese M.. 2018. Freshwater salinization syndrome on a continental scale. Proc. Natl Acad. Sci. USA 115, E574–E583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kefford BJ, Hickey GL, Gasith A, Ben-David E, Dunlop JE, Palmer CG, Allan K, Choy SC, Piscart C. 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: eastern Australia, France, Israel and South Africa. PLoS ONE 7, e35224 ( 10.1371/journal.pone.0035224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kefford BJ, Buchwalter D, Canedo-Arguelles M, Davis J, Duncan RP, Hoffmann A, Thompson R. 2016. Salinized rivers: degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 12, 20151072 ( 10.1098/rsbl.2015.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pond GJ, Passmore ME, Borsuk FA, Reynolds L, Rose CA. 2008. Downstream effects of mountaintop coal mining: comparing biological conditions using family and genus-level macroinvertebrate bioasessment tools. J. North Am. Benthol. Soc. 127, 717–737. ( 10.1899/08-015.1) [DOI] [Google Scholar]
- 7.Karraker NE, Gibbs JP, Vonesh JR. 2008. Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecol. Appl. 18, 724–734. ( 10.1890/07-1644.1) [DOI] [PubMed] [Google Scholar]
- 8.Clements WH, Kotalik C. 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 35, 126–138. ( 10.1086/685085) [DOI] [Google Scholar]
- 9.Williams WD. 2001. Salinization: unplumbed salt in a parched landscape. Water Sci. Technol. 43, 85–91. ( 10.2166/wst.2001.0186) [DOI] [PubMed] [Google Scholar]
- 10.Williams WD. 2001. Anthropogenic salinization of inland waters. Hydrobiologia 466, 329–337. ( 10.1023/A:1014598509028) [DOI] [Google Scholar]
- 11.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. Proc. Natl Acad. Sci. USA 102, 13 517–13 520. ( 10.1073/pnas.0506414102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushall SS, et al. 2017. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 83, 121–135. ( 10.1016/j.apgeochem.2017.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entrekin S, Evans-White M, Hagenbuch E. 2011. Rapid expansion of natural gas development poses a threat to surface waters. Front. Ecol. Environ. 9, 503–511. ( 10.1890/110053) [DOI] [Google Scholar]
- 14.Lauer NE, Harkness JS, Vengosh A. 2016. Brine spills associated with unconventional oil development in North Dakota. Environ. Sci. Technol. 50, 5389–5397. ( 10.1021/acs.est.5b06349) [DOI] [PubMed] [Google Scholar]
- 15.Mosley LM. 2017. Drought impacts on the water quality of freshwater systems; review and integration. Earth Sci. Rev. 140, 203–214. ( 10.1016/j.earscirev.2014.11.010) [DOI] [Google Scholar]
- 16.Sweeney BW, Vannote RL. 1984. Influence of food quality and temperature on life-history characteristics of the parthenogenetic mayfly, Cloeon triangulifer. Freshw. Biol. 14, 621–630. ( 10.1111/j.1365-2427.1984.tb00181.x) [DOI] [Google Scholar]
- 17.Sweeney BW, Funk DH, Standley LJ. 1993. Use of the stream mayfly Cloeon triangulifer as a bioassay organism—life-history response and body burden following exposure to technical Chlordane. Environ. Toxicol. Chem. 12, 115–125. ( 10.1002/etc.5620120113) [DOI] [Google Scholar]
- 18.Xie L, Funk DH, Buchwalter DB. 2010. Trophic transfer of Cd from natural periphyton biofilms to the grazing mayfly Centroptilum triangulifer in a life cycle test. Environ. Pollut 158, 272–277. ( 10.1016/j.envpol.2009.07.010) [DOI] [PubMed] [Google Scholar]
- 19.Xie L, Buchwalter DB. 2011. Cadmium exposure route affects antioxidant responses in the mayfly Centroptilum triangulifer. Aquat. Toxicol. 105, 199–205. ( 10.1016/j.aquatox.2011.06.009) [DOI] [PubMed] [Google Scholar]
- 20.Conley JM, Funk DH, Buchwalter DB. 2009. Selenium bioaccumulation and maternal transfer in the mayfly Centroptilum triangulifer in a life-cycle, periphyton-biofilm trophic assay. Environ. Sci. Technol. 43, 7952–7957. ( 10.1021/es9016377) [DOI] [PubMed] [Google Scholar]
- 21.Conley JM, Funk DH, Cariello NJ, Buchwalter DB. 2011. Food rationing affects dietary selenium bioaccumulation and life cycle performance in the mayfly Centroptilum triangulifer. Ecotoxicology 20, 1840–1851. ( 10.1007/s10646-011-0722-1) [DOI] [PubMed] [Google Scholar]
- 22.Conley JM, Funk DH, Hesterberg DH, Hsu LC, Kan J, Liu YT, Buchwalter DB. 2013. Bioconcentration and biotransformation of selenite versus selenate exposed periphyton and subsequent toxicity to the mayfly Centroptilum triangulifer. Environ. Sci. Technol. 47, 7965–7973. ( 10.1021/es400643x) [DOI] [PubMed] [Google Scholar]
- 23.Conley JM, Watson AT, Xie L, Buchwalter DB. 2014. Dynamic selenium assimilation, distribution, efflux, and maternal transfer in Japanese medaka fed a diet of se-enriched mayflies. Environ. Sci. Technol. 48, 2971–2978. ( 10.1021/es404933t) [DOI] [PubMed] [Google Scholar]
- 24.Kim KS, Funk DH, Buchwalter DB. 2012. Dietary (periphyton) and aqueous Zn bioaccumulation dynamics in the mayfly Centroptilum triangulifer. Ecotoxicology 21, 2288–2296. ( 10.1007/s10646-012-0985-1) [DOI] [PubMed] [Google Scholar]
- 25.Wesner JS, Walters DM, Schmidt TS, Kraus JM, Stricker CA, Clements WH, Wolf RE. 2017. Metamorphosis affects metal concentrations and isotopic signatures in a mayfly (Baetis tricaudatus): implications for the aquatic-terrestrial transfer of metals. Environ. Sci. Technol. 51, 2438–2446. ( 10.1021/acs.est.6b05471) [DOI] [PubMed] [Google Scholar]
- 26.Wesner JS, Kraus JM, Schmidt TS, Walters DM, Clements WH. 2014. Metamorphosis enhances the effects of metal exposure on the mayfly, Centroptilum triangulifer. Environ. Sci. Technol. 48, 10 415–10 422. ( 10.1021/es501914y) [DOI] [PubMed] [Google Scholar]
- 27.Soucek DJ, Dickinson A. 2015. Full-life chronic toxicity of sodium salts to the mayfly Neocloeon triangulifer in tests with laboratory cultured food. Environ. Toxicol. Chem. 34, 2126–2137. ( 10.1002/etc.3038) [DOI] [PubMed] [Google Scholar]
- 28.Struewing KA, Lazorchak JM, Weaver PC, Johnson BR, Funk DH, Buchwalter DB. 2015. Part 2: sensitivity comparisons of the mayfly Centroptilum triangulifer to Ceriodaphnia dubia and Daphnia magna using standard reference toxicants; NaCl, KCl and CuSO4. Chemosphere 139, 597–603. ( 10.1016/j.chemosphere.2014.04.096) [DOI] [PubMed] [Google Scholar]
- 29.Weaver PC, Lazorchak JM, Struewing KA, DeCelles SJ, Funk DH, Buchwalter DB, Johnson BR. 2015. Part 1: laboratory culture of Centroptilum triangulifer (Ephemeroptera: Baetidae) using a defined diet of three diatoms. Chemosphere 139, 589–596. ( 10.1016/j.chemosphere.2014.04.092) [DOI] [PubMed] [Google Scholar]
- 30.Scheibener S, Conley JM, Buchwalter D. 2017. Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects. Aquat. Toxicol. 190, 62–69. ( 10.1016/j.aquatox.2017.06.027) [DOI] [PubMed] [Google Scholar]
- 31.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 16, 2009–2019. ( 10.1897/1551-5028(1997)016%3C2009:SMTPTT%3E2.3.CO;2) [DOI] [Google Scholar]
- 32.Snoeyink VL, Jenkins D. 1980. Water chemistry. New York, NY: John Wiley and Sons. [Google Scholar]
- 33.Erickson RJ, Mount DR, Highland TL, Hockett JR, Hoff DJ, Jenson CT, Norberg-King TJ, Peterson KN. 2018. The acute toxicity of major ion salts to Ceriodaphnia dubia. III. Modeling the toxicity of major ion mixtures. Environ. Toxicol. Chem. 37, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acid. Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 36.Kristensen NP. 1981. Phylogeny of insect orders. Annu. Rev. Entomol. 26, 135–157. ( 10.1146/annurev.en.26.010181.001031) [DOI] [Google Scholar]
- 37.Kunz JL, Conley JM, Buchwalter DB, Norberg-King TJ, Kemble NE, Wang N, Ingersoll CG. 2013. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 32, 2826–2835. ( 10.1002/etc.2391) [DOI] [PubMed] [Google Scholar]
- 38.Kim KS, Chou H, Funk DH, Jackson JK, Sweeney BW, Buchwalter DB. 2017. Physiological responses to short-term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits. J. Exp. Biol. 220, 2598–2605. ( 10.1242/jeb.156919) [DOI] [PubMed] [Google Scholar]
- 39.Sweeney BW, Funk DH, Jackson JK, Camp AA, Buchwalter D. 2018. Why a mayfly Cloeon dipterum (Ephemeroptera: Baetidae) gets smaller as temperatures warm. Freshw. Sci. 37, 64–81. ( 10.1086/696611) [DOI] [Google Scholar]
- 40.Soucek DJ, Mount DR, Dickinson A, Hockett JR. 2018. Influence of dilution water ionic composition on acute major ion toxicity to the mayfly Neocloeon triangulifer. Environ. Toxicol. Chem. 37, 1330–1339. ( 10.1002/etc.4072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson BR, Weaver PC, Nietch CT, Lazorchak JM, Struewing KA, Funk DH. 2015. Elevated major ion concentrations inhibit larval mayfly growth and development. Environ. Toxicol. Chem. 34, 167–172. ( 10.1002/etc.2777) [DOI] [PubMed] [Google Scholar]
- 42.Scheibener SA, Richardi VS, Buchwalter DB. 2016. Comparative sodium transport patterns provide clues for understanding salinity and metal responses in aquatic insects. Aquat. Toxicol. 171, 20–29. ( 10.1016/j.aquatox.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 43.Dowse R, Palmer CG, Hills K, Torpy F, Kefford BJ. 2017. The mayfly nymph Austrophlebioides pusillus Harker defies common osmoregulatory assumptions. R. Soc. open sci. 4, 160520 ( 10.1098/rsos.160520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piłsyk S, Paszewski A. 2009. Sulfate permeasesphylogenetic diversity of sulfate transport. Acta Biochim. Pol. 56, 375–384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available as part of the electronic supplementary material and on request from dbbuchwa@ncsu.edu.