Abstract
Dioscorea L., the largest genus of the family Dioscoreaceae with over 600 species, is not only an important food but also a medicinal plant. The identification and classification of Dioscorea L. is a rather difficult task. In this study, we sequenced five Dioscorea chloroplast genomes, and analyzed with four other chloroplast genomes of Dioscorea species from GenBank. The Dioscorea chloroplast genomes displayed the typical quadripartite structure of angiosperms, which consisted of a pair of inverted repeats separated by a large single-copy region, and a small single-copy region. The location and distribution of repeat sequences and microsatellites were determined, and the rapidly evolving chloroplast genome regions (trnK-trnQ, trnS-trnG, trnC-petN, trnE-trnT, petG-trnW-trnP, ndhF, trnL-rpl32, and ycf1) were detected. Phylogenetic relationships of Dioscorea inferred from chloroplast genomes obtained high support even in shortest internodes. Thus, chloroplast genome sequences provide potential molecular markers and genomic resources for phylogeny and species identification.
Keywords: Chloroplast genome, Dioscorea, Phylogeny, Single sequence repeats, Variable marker
Introdution
Dioscorea L. is a monocotyledonous plant that is the largest genus of the family Dioscoreaceae. It comprises more than 600 plant species, almost all of which are distributed in Southeast Asia, Africa, Central America, South America, and other tropical or subtropical regions of the world, while a few occur in Europe and North America (Caddick et al., 2002; Hsu et al., 2013). Some Dioscorea species are an economically important supply of starch in the staple diet such as D. alata, D. esculenta, D. cayenensis, D. dumetorum, and D. rotundata. The genus is also a favored source of medicinal plants, such as D. nipponica, D. opposita, and D. zingiberensis. (Zhai et al., 2009).
The identification of Dioscorea L. has presented a challenge to systematists because of its great morphological variations, especially the aerial parts, such as leaves (Wilkin et al., 2005). Furthermore, its hypanthium is relatively small and dioecious, which makes the classification of Dioscorea L. into a rather difficult task (Hsu et al., 2013; Wilkin et al., 2005). For phylogenetic studies of Dioscorea, some chloroplast molecular markers (such as rbcL, matK, trnH-psbA, trnL-F), have been analyzed (Gao et al., 2008; Hsu et al., 2013; Wilkin et al., 2005). Although molecular markers provide some information for the taxonomy of Dioscorea, phylogenetic analyses are low resolution due to these limited data. Further studies to seek high resolution molecular markers in the species level to the success of identification and phylogeny in Dioscorea is necessary. Four complete Dioscorea chloroplast genomes (D. elephantipes, D. rotundata, D. villosa, and D. zingiberensis) have been released in GenBank (Hansen et al., 2007; Mariac et al., 2014), which provides opportunities to develop more genetic resources for discriminating between species and populations. The chloroplast genome in angiosperms has a typical quadripartite structure, with two copies of inverted repeats (IRs) separating the large single-copy (LSC) and small single-copy regions (SSC), and the genome size ranging from 120 to 170 kb in length. Because of maternal inheritance, low rates of nucleotide substitutions and very low recombination, chloroplast DNA sequences have often been used for phylogenetic studies of higher plants in order to resolve complex evolutionary relationships (Burke et al., 2016; Dong et al., 2017). Comparisons of chloroplast genomes provide additional effective resources for the development of variable markers, which are used for phylogeny or species identification (Dong et al., 2012). Next Generation Sequencing (NGS) technique generates the large numbers of DNA sequences at relatively low cost and promptly extended gene-based phylogenetics to phylogenomics.
Here, we investigated the complete chloroplast genomes of five Dioscorea species through NGS and compared them with four previously sequenced species. The comparative analysis of nine complete Dioscorea chloroplast genomes was conducted to demonstrate the features and structural differentiation of the sequences, also to provide valuable chloroplast molecular markers for further phylogenetic and species identification. Furthermore, we tested the feasibility of phylogeny reconstruction using chloroplast genome data.
Materials and Methods
Sample materials and DNA extraction
Young leaves of five Dioscorea species were harvested from Lijiang, Yunan, China (D. aspersa), Lushan, Sichuan, China (D. alata), Lin’an, Zhejiang, China (D. bulbifera), and Minhou, Fujian, China (D. futschauensis and D. polystachya). Voucher specimens were deposited in the herbaria of CMMI (Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences). Total genomic DNA was extracted from silica-dried leaves following the method of Li et al. protocol (2013). Then, DNA was purified by the Wizard DNA CleanUp System (Promega, Madison, WI, USA). Final DNA quality was assessed on spectrophotometry, and their integrity was evaluated using a 1% (w/v) agarose gel.
Illumina sequencing, assembly, and annotation
DNA was sheared to fragments of 400–600 bp with an ultrasonic disruptor. An Illumina paired-end library was constructed with the NEBNext® Ultra™ DNA Library Prep Kit according to the manufacturer’s protocol. Paired-end sequencing was conducted on an Illumina HiSeq X 10 platform. For each species, approximately 10.0 Gb of raw data were generated with pair-end 150 bp read length. A four-step approach was employed to assemble the chloroplast genome. First, raw sequence reads were filtered for primer/adaptor sequences and low-quality reads with the NGS QC Tool Kit (Patel & Jain, 2012). By using the assembly program SPAdes 3.6.1 (Bankevich et al., 2012), with parameters, kmer = 95, contigs were generated from high quality paired-end reads. Second, chloroplast genome sequence contigs were selected from the initial assembly by performing a BLAST search using the D. elephantipes chloroplast genome sequence as a reference (GenBank accession number: EF380353). A high copy number of extranuclear DNA was present in the total DNA, usually around 5–10% of chloroplast DNA and around 1–2% of mitochondrial DNA. After the de novo assembling, the coverages of the contigs are significantly different among three genomes. Coverage of chloroplast contigs are much higher than those in nuclear and mitochondrial genome. In this study, we also used this method to select chloroplast genome contigs. The selected contigs from chloroplast genomes were further assembled using Sequencher 5.4.5. Third, ambiguous nucleotides or gaps and the four junctional regions between the IRs and SSC/LSC in the chloroplast genome sequences were further confirmed by PCR amplification and Sanger sequencing with specific primers (Dong et al., 2013). Finally, clean reads were remapped to the draft genome sequences and yielded the sequences.
Three methods were used to check the assembling accuracy of chloroplast genome sequence. First, half of the amount of raw data was used to assemble the chloroplast genome; Second, original reads were assessed through strict quality control and then only high quality reads were filtered to assemble contigs. The third method was a four-step approach in this method. Three methods gave completely identical result of assemblage chloroplast genome sequence. Furthermore, the Geneious 11.1.2 was used to map all reads to the assembled chloroplast genome sequence. The consensus sequences were produced using mapped reads in Geneious.
Dual Organellar GenoMe Annotator (DOGMA) using the default parameters was used to annotate chloroplast genome sequences (Wyman, Jansen & Boore, 2004). BLASTX and BLASTN searches were utilized to accurately annotate the genes encoding proteins and the locations of the transfer RNAs (tRNAs). The Genome Vx software was used to draw a chloroplast genome map (Conant & Wolfe, 2008).
Analysis of tandem repeats and single sequence repeats
Three types of repeat sequences were identified in the Dioscorea chloroplast genome. We used REPuter to identify dispersed and palindromic repeats (Kurtz et al., 2001). The minimum similarity percentage of two repeat copies was limited to 90%, the minimum repeat size was 30 bp, and the hamming distance was 3. Tandem repeats were detected by Tandem Repeats Finder (https://tandem.bu.edu/trf/trf.html), with two, five, and seven set for the alignment parameters match, mismatch, and indel, respectively.
Single sequence repeats (SSRs) were detected by MISA (MIcroSAtellite; http://pgrc.ipk-gatersleben.de/misa) with the search parameters set at >10 repeat units for mononucleotide, >5 repeat units for dinucleotide, >4 repeat units for trinucleotide, and >3 repeat units for tetranucleotide, pentanucleotide, and hexanucleotide SSRs.
Comparison whole chloroplast genomes and divergent hotspot identification
The mVISTA program (http://genome.lbl.gov/vista/mvista/submit.shtml) with Shuffle-LAGAN mode (Frazer et al., 2004) was used to compare the Dioscorea chloroplast genomes. D. elephantipes chloroplast genome was used as reference. All Dioscorea sequenced chloroplast genomes and the other four Dioscorea species (D. elephantipes, GenBank accession: EF380353.1; D. villosa, GenBank accession: KY085893.1; D. zingiberensis, GenBank accession: KP899622.1; D. rotundata, GenBank accession: KJ490011.1) chloroplast genomes from GenBank were aligned using MAFFT v7 (Katoh & Standley, 2013) with default settings, assuming collinear genomes for the full alignment, and then we checked the small inversions through subsequent adjustment manually using Se-Al 2.0 (Rambaut, 1996). The nucleotide diversity of the chloroplast genome was conducted based on a sliding window analysis with the DnaSP v5.10 software (Librado & Rozas, 2009). The window length was set to 800 bp, with a 200 bp step size. Any large structural events, such as gene order rearrangements and IR expansions/contractions, among the nine species were ascertained.
Phylogenetic reconstruction
Phylogenetic relationships were reconstructed using nine Dioscorea species chloroplast genomes and Tacca chantrieri was used as an outgroup. The entire chloroplast genome, LSC, SSC, and IR regions were used to construct phylogenetic trees based on the differentiation of molecular evolutionary rates in chloroplast genome regions.
The program ModelFinder was used to find the optimal substitution mode (Kalyaanamoorthy et al., 2017). We performed Maximum Likelihood (ML) analyses using RAxML v.8.1.24. The general time reversible + G model was chosen in all analyses with 1,000 rapid bootstrap replicates.
Bayesian inference (BI) of the phylogenies was implemented with MrBayes v.3.2.2 (Ronquist et al., 2012). A Markov Chain Monte Carlo Analysis was run for 10,000,000 generations with trees sampled every 1,000 generations, with the first 25% discarded as burn-in. The remaining trees were used to construct a 50% majority-rule consensus tree.
Results
Genome sequencing and assembly
Five Dioscorea species were sequenced to produce 53,889,722-81,562,406 raw reads (150 bp for average read length). The complete chloroplast genomes of Dioscorea are 428,514–3,050,140 with 838× to 5,944× coverage (Table 1). The accuracy of inverted repeat junction regions in assembled sequences were further confirmed by PCR amplification and Sanger sequencing with specific primers. The five Dioscorea cp genome sequences were then submitted to GenBank (accession numbers MG267378, MG267381–MG267384).
Table 1. Sampling and assembly information for the five Dioscorea species.
| Species | ID | Raw data no. | Mapped read no. | Precent of chloroplast genome reads (%) | Chloroplast gemome coverage (X) | Accession number |
|---|---|---|---|---|---|---|
| D. aspersa | LJW01 | 69,648,118 | 428,514 | 0.62% | 838 | MG267381 |
| D. alata | LSC09 | 77,185,326 | 2,467,928 | 3.20% | 4,834 | MG267382 |
| D. bulbifera | LAW08 | 53,889,722 | 1,140,614 | 2.12% | 2,235 | MG267383 |
| D. futschauensis | MHW01 | 81,562,406 | 3,050,140 | 3.74% | 5,944 | MG267384 |
| D. polystachya | MHW08 | 62,610,816 | 1,119,774 | 1.79% | 2,192 | MG267378 |
Notes:
W, wild.
C, cultivated.
Genome size and features
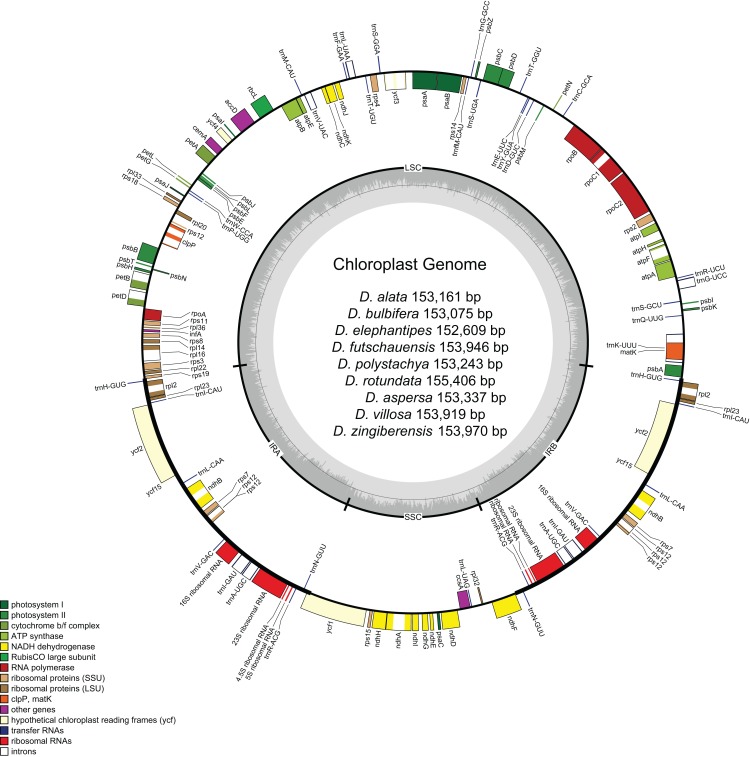
The total chloroplast genome sizes of Dioscorea are 152,609 in D. elephantipes to 155,418 in D. rotundata (Table 2) with a pair of IR regions (25,464–25,576 bp) separated by an LSC region (82,777–85,600 bp), and an SSC region (18,806–19,038 bp). The overall GC content was 37–37.2%, indicating nearly identical levels among the Dioscorea chloroplast genome. The IR regions have a higher GC content (43.0%) than the LSC regions (34.9%) and the SSC regions (31.0%) (Table 2). The high GC content of the IR regions is possibly due to the high GC content of the four rRNA genes in these regions.
Table 2. Characteristics of the chloroplast genomes of nine Dioscorea species.
| Genme features | D. aspersa | D. alata | D. bulbifera | D. futschauensis | D. polystachya | D. elephantipes | D. villosa | D. zingiberensis | D. rotundata |
|---|---|---|---|---|---|---|---|---|---|
| Size (bp) | 153,337 | 153,161 | 153,075 | 153,946 | 153,243 | 152,609 | 153,919 | 153,970 | 155,418 |
| LSC length (bp) | 83,517 | 83,414 | 83,226 | 83,979 | 83,431 | 82,777 | 83,865 | 83,950 | 85,600 |
| IR length (bp) | 25,478 | 25,464 | 25,499 | 25,529 | 25,489 | 25,513 | 25,576 | 25,491 | 25,484 |
| SSC length (bp) | 18,864 | 18,819 | 18,851 | 18,909 | 18,834 | 18,806 | 18,902 | 19,038 | 18,850 |
| Total genes | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 |
| Protein coding genes | 78 | 78 | 78 | 78 | 78 | 78 | 78 | 78 | 78 |
| tRNA genes | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| rRNA genes | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Overall GC content (%) | 37.0 | 37.0 | 37.0 | 37.2 | 37 | 37.2 | 37.2 | 37.2 | 37.2 |
| GC content in LSC (%) | 34.8 | 34.8 | 34.8 | 35.0 | 34.8 | 34.9 | 35.0 | 35.1 | 35.2 |
| GC content in SSC (%) | 31.0 | 31.0 | 30.8 | 31.2 | 30.9 | 31.2 | 31.2 | 31.2 | 30.9 |
| GC content in IR (%) | 43.0 | 43.0 | 43.0 | 43.0 | 42.9 | 43.0 | 43.0 | 43.0 | 42.9 |
The Dioscorea chloroplast genomes encoded 112 unique genes, with 79 protein-coding genes, 29 tRNA genes, and 4 ribosomal RNA genes (Fig. 1; Table S1). The LSC region comprised 62 protein-coding and 22 tRNA genes, and the SSC region was composed of 12 protein-coding genes and one tRNA gene. Six protein-coding (ndhB, rpl23, rps7, rps12, ycf2, rpl2), eight tRNAs (trnA-UGC, trnH-GUG, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC) and four rRNA genes (rrn4.5, rrn5, rrn16, rrn23) were found be duplicated in IRA and IRB. There are 17 intron-containing genes, of which 12 were protein coding genes and five were tRNA genes. clpP and ycf3 had two introns, whereas the rest contained single introns. The rps12 is a trans-splicing gene, having the first exon in the LSC region and the second and third exons in the IR regions.
Figure 1. Gene maps of chloroplast genomes of Dioscorea.
Genes on the inside of the large circle are transcribed clockwise and those on the outside are transcribed counter clockwise. The genes are color-coded based on their functions. The dashed area represents the GC composition of the chloroplast genome.
Repeat analysis and single sequence repeats
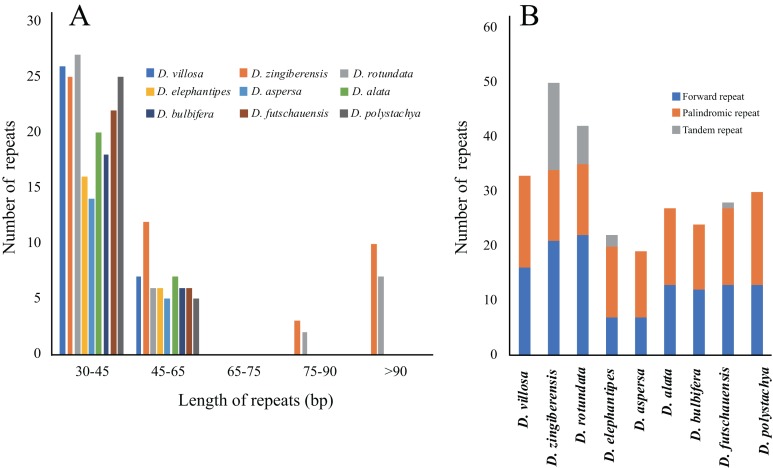
We used REPUTER and Tandem Repeats Finder for the identification of the repeats, which are at least 30 bp. In total, 275 repeats were detected in the nine Dioscorea chloroplast genomes (Fig. 2). Each Dioscorea chloroplast genome contained 19–50 repeat sequences, including 7–72 forward repeats and 12–17 palindromic repeats. There were many fewer tandem repeats in Dioscorea. The tandem repeats analysis detected one in D. futschauensis, two in D. elephantipes, and 16 in D. zingiberensis. Among these repeats, most repeats (70.18%) were 30–45 bp in length, while those with more than 75 bp were few (8%).
Figure 2. Analysis of repeated sequences in nine Dioscorea species.
(A) Number of repeated sequences by length; (B) Number of types repeated three times in the nine chloroplast genomes.
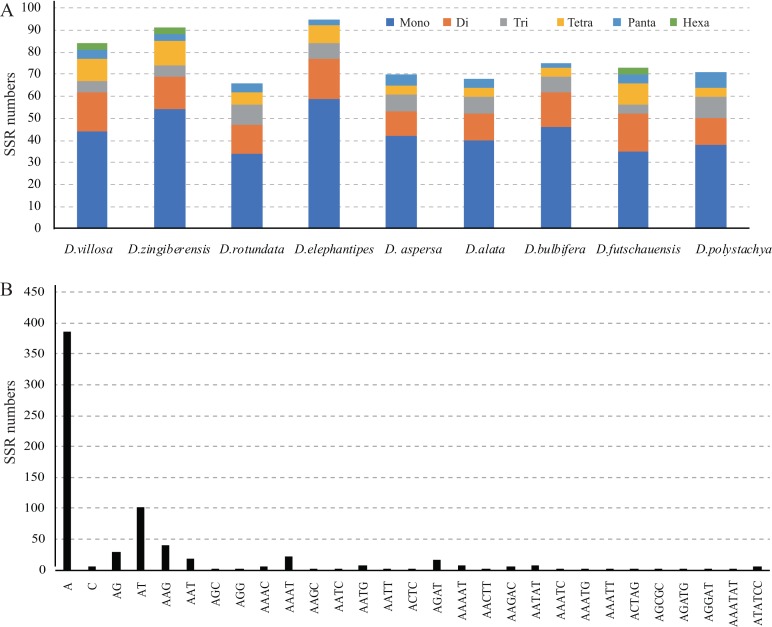
Moreover, SSRs of the nine chloroplast genomes were analyzed (Fig. 3). Among them, D. elephantipes (95) had the most SSRs, and D. rotundata (66) had the least. The majority of the SSRs in these chloroplast genomes consist of mono- and dinucleotide repeat motifs, varying from 34 in D. rotundata to 59 in D. elephantipes for mononucleotide repeats, while dinucleotide repeats varied from 11 in D. aspersa to 18 in D. villosa, and D. elephantipes (Fig. 3A). Trinucleotide and tetranucleotide SSRs were the second most common, ranging from four in D. futschauensis to 10 in D. polystachya for trinucleotide repeats, while tetraucleotide repeats varied from 4 to 11. Furthermore, three hexanucleotide repeats were found in D. villosa, D. zingiberensis, and D. futschauensi. SSRs were particularly rich in AT in the Dioscorea chloroplast genomes. The majority of SSRs in all species were A/T mononucleotides (Fig. 3B).
Figure 3. Analysis of simple sequence repeats (SSR) in the chloroplast genomes of nine Dioscorea species.
(A) Number of different SSR types detected in the nine genomes; (B) Number of identified SSR motifs in different repeat class types.
Phylogenetic analysis
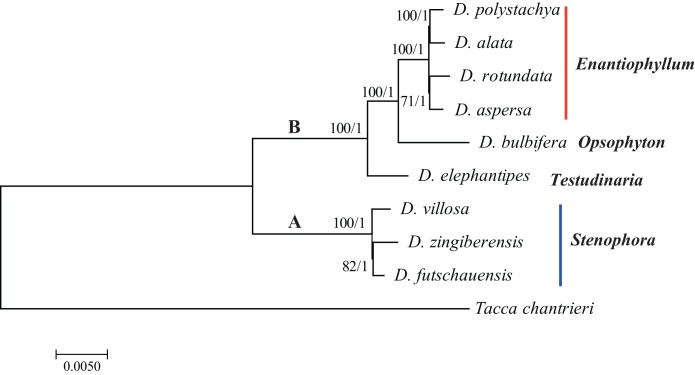
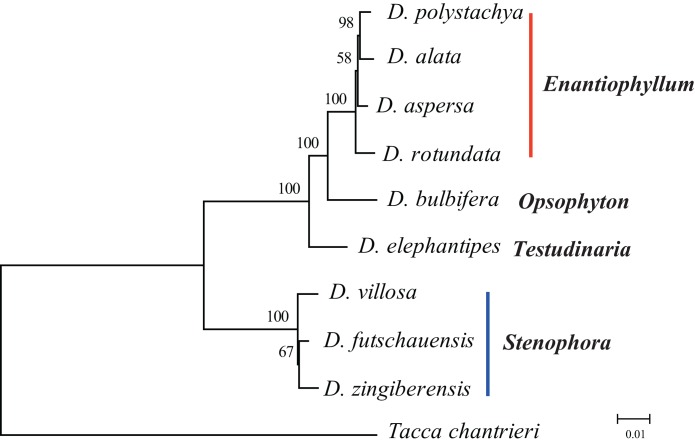
Phylogenomic analysis within Dioscorea were reconstructed using ML and BI methods. The topologies based on analyses using the two methods were highly concordant in each dataset, as well as phylogenetic trees with moderate-to-high support (Fig. 4; Fig. S1). For the nine Dioscorea species, they were grouped into two branches: A clade (including D. villosa, D. zingiberensis, and D. futschauensis) and B clade (including D. elephantipesn, D. bulbifera, D. aspersa, D. rotundata, D. alata, and D. polystachya). In B clade, D. elephantipes was the sister of D. bulbifera and sect. Enantiophyllum. The short branch lengths in the sect. Enantiophyllum and Stenophora suggest rapid radiation evolutionary history in these clades. The phylogenetic positions of these groups are in agreement with recent studies (Hsu et al., 2013; Wilkin et al., 2005).
Figure 4. Phylogenetic tree reconstruction using maximum likelihood, and Bayesian inference methods based on the complete chloroplast genome sequences.
ML topology shown with ML bootstrap support values/Bayesian posterior probability listed at each node.
Genome divergence and divergence hotspot
Nine complete Dioscorea chloroplast genomes were used for comparative analyses. The genome size of D. rotundata (155,418) is the largest of these, and this difference was mostly attributed to variation in the length of the LSC region (Table 2). Dioscorea had the highest chloroplast genome homologies, while there were more common linear relationships among the other plants (Jiang et al., 2017; Xu et al., 2017).
Sequence identity comparisons among the nine chloroplast genomes were plotted using the program mVISTA with the annotated D. elephantipes sequence as a reference (Fig. S2). The whole aligned sequences showed high similarities with only a few regions below 90%, suggesting that Dioscorea chloroplast genomes were rather conserved. In addition, the IRs regions were more conserved than the single-copy regions, and noncoding regions exhibited a higher level of divergence than coding regions in the complete chloroplast genomes.
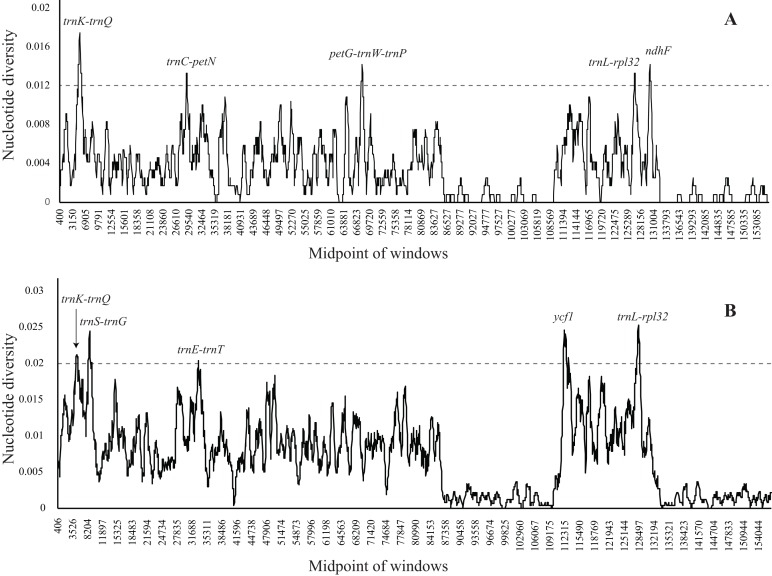
To identify the divergence hotspot regions, nucleotide diversity values within 800 bp in the nine Dioscorea chloroplast genomes were calculated with the DnaSP v5.10 software (Fig. 5). According to the phylogeny results, nucleotide diversity was calculated in both A clade and B clade. In the Dioscorea chloroplast genomes, nucleotide diversity values within 800 bp varied from 0 to 0.0175 in A clade and 0 to 0.02533 in B clade, respectively. The average value of nucleotide diversity was 0.00334 in A clade and 0.00926 in B clade. We identified eight divergence hotspot regions that could be utilized as potential makers to reconstruct the phylogeny and plant identification in this genus. Two are in the coding regions ndhF and ycf1, and six are in the intergenic regions (trnK-trnQ, trnS-trnG, trnC-petN, trnE-trnT, petG-trnW-trnP, and trnL-rpl32). Five of these regions lie in the LSC, and three are in the SSC. Phylogenetic tree reconstruction using ML methods based on eight divergence hotspot regions showed moderate-to-high support (Fig. 6), similar to the tree topology based on the complete chloroplast genomes. trnK-trnQ, trnS-trnG, and trnE-trnT had higher resolution among the eight hotspots (Fig. S3).
Figure 5. Sliding window analysis of the Dioscorea chloroplast genomes (window length: 800 bp; step size: 200 bp).
(A) Nucleotide diversity of A-clade dataset; (B) Nucleotide diversity of B-clade dataset. X-axis: position of the midpoint of a window; Y-axis: nucleotide diversity of each window.
Figure 6. Phylogeny of the nine Dioscorea species constructed using eight regions of highly variable sequences.
Numbers above nodes are support values with ML bootstrap values.
Discussion
Chloroplast genome sequence variation and evolution
In this study, five new chloroplast genome sequences of Dioscorea were sequenced using Illumina sequencing technology, and another four additional Dioscorea species chloroplast genomes from GenBank were simultaneously taken into consideration for comparative analyses. Gene and intron content are highly conserved among land plant plastomes (Dong et al., 2013), although losses have been identified in several angiosperm lineages. The chloroplast genomes of Dioscorea species are structurally conserved and no rearrangement events were detected in this study. Meanwhile, the genome divergence was low. mVISTA results revealed high similarities among chloroplast genomes, suggesting that the Dioscorea cpDNAs were rather conserved. Similar results have been reported previously in angiosperms chloroplast genomes, and the lower sequence divergence in the IR regions compared to the SSC and LSC regions is possibly due to copy corrections between IR sequences by gene conversion (Zhang et al., 2017). Furthermore, the divergent regions included trnK-trnQ, trnS-trnG, trnC-petN, trnE-trnT, petG-trnW-trnP, which were consistent with previous reports that these divergent regions were mostly present in the SSC and LSC regions and showed a trend toward more rapid evolution (Rogalski et al., 2015; Scarcelli et al., 2011; Shaw et al., 2007).
We identified 275 repeats in the nine Dioscorea chloroplast genomes, which include dispersed, palindromic, and tandem repeats. Previous studies have shown that repeat sequences may play roles in rearranging sequences and producing variation through slipped-strand mispairing and illegitimate recombination (Morrison, 2009; Ochoterena, 2009). Furthermore, the presence of these repeats indicates that the region is a crucial hotspot for genome reconfiguration. The majority were distributed in non-coding regions, which were the highly variable regions in the chloroplast genomes (Asaf et al., 2017). Additionally, these repeats are an informative source for phylogenetic studies (Le Flèche et al., 2001; Rokas & Holland, 2000).
Potential DNA barcodes for yam
Because of the more than 600 species, great morphological diversity, dioecism, and small flowers in Dioscorea, its DNA barcoding and taxonomy is still difficult to unravel after many years (Hsu et al., 2013; Wilkin et al., 2005). The chloroplast genome markers matK, rbcL, and psbA-trnH have been widely served as universal barcoding applications in plants (Borisenko, Sones & Hebert, 2009; Hollingsworth, Graham & Little, 2011); however, these markers had extremely low discriminatory power (Sun et al., 2012). matK only successfully identified 23.26%, compared with 9.30% for rbcL and 11.63% for psbA-trnH (Sun et al., 2012). Therefore, the development of reliable and effective DNA barcodes with high percentage of variable sites is very important for Dioscorea.
In the chloroplast genome, indels and SNPs were not random but clustered as “hotspots” (Scarcelli et al., 2011). Those “hotspots” regions were defined as highly variable locies. Based on the nine compared Dioscorea cpDNAs, eight highly variable regions (trnK-trnQ, trnS-trnG, trnC-petN, trnE-trnT, petG-trnW-trnP, ndhF, trnL-rpl32, and ycf1) are identified (Fig. 5). ndhF, trnL-rpl32, and ycf1 have been the focus of DNA barcodes and hypervariable markers for phylogenetic reconstruction in previous studies (Dong et al., 2015; Shaw et al., 2007).
Recently, ycf1, a gene essential for plant viability and encodes Tic214, which was a vital component of the Arabidopsis TIC complex, was more focused on DNA barcoding and phylogeny (Dong et al., 2012, 2015; Xu et al., 2017). It was more variable than the matK and rbcL in most plant lineages (Dong et al., 2015; Neubig et al., 2009). Ycf1 exhibited high variability in B clade of Dioscorea (Fig. 5). NdhF was widely used in tree of life and was considered as a variable coding gene in chloroplast genome (Chen et al., 2016; Kim & Jansen, 1995; Prather, Ferguson & Jansen, 2000). It exhibited relatively high variability in A clade of Dioscorea (Fig. 5). Rpl32-trnL and trnS-G showed considerable length variation across taxa and a high level of positional variability (Shaw et al., 2007). The trnC-petN was part of trnC-trnD IGS which was divided into three IGS, trnC-petN, petN-psbM, and psbM-trnD. This region appeared to contain large indels, and the length of this IGS was variable (615–989 bp) across taxa. The nucleotide diversity of trnK-trnQ was 0.0175 in A clade of Dioscorea (Fig. 5), which were the highest markers. The trnE-trnT and petG-trnW-trnP were less used in plant phylogeny and DNA barcoding before. Therefore, further work on investigating whether these markers could recommend as effective, specific barcodes for Dioscorea species is necessary.
Phylogenetic analysis
Recently, plastome information has provided a large amount of data for improving phylogenetic resolution. Chloroplast genome sequences have been widely used for the reconstruction of phylogenetic relationships among plant lineages (Burke et al., 2016; Dong et al., 2017; Du et al., 2017; Sun et al., 2016). Phylogenetic analyses of plant species using a small number of loci might frequently be insufficient to resolve evolutionary relationships, particularly at low taxonomic levels (Hilu & Alice, 2001; Majure et al., 2012). Many previous phylogenetic work based on whole chloroplast genomes have been used to resolve difficult phylogenetic relationships among closely related species (Carbonell-Caballero et al., 2015; Dong et al., 2017) and to enhance our understanding of the evolutionary relationships among angiosperms (Goremykin et al., 2013; Luo et al., 2016). Phylogenetic relationships of Dioscorea were estimated using several chloroplast DNA markers (rbcL, matK, trnH-psbA). However, they are insufficient to resolve evolutionary relationships (Gao et al., 2008; Hsu et al., 2013; Wilkin et al., 2005). Our phylogenetic analysis based on the dataset of complete chloroplast genomes indicated very clear internal relationships of Dioscorea. The phylogenetic trees indicated that the nine species of Dioscorea clustered into two groups. Furthermore, the phylogenetic trees indicated clear internal relationships of sect. Enantiophyllum and Stenophora, which may result from ancient, rapid radiations. However, our study was just a glimpse of phylogenetic relationships within the genus Dioscorea, and we will sequence more Dioscorea chloroplast genomes to estimate solid phylogenetic relationships and enhance our understanding of the evolution and diversification of characteristics of Dioscorea in the future.
Conclusions
We assembled, annotated and analyzed five new complete chloroplast genome sequences of Dioscorea, and compared them with four chloroplast genomes from GenBank. The repeated sequences, microsatellites and eight highly variable regions (trnK-trnQ, trnS-trnG, trnC-petN, trnE-trnT, petG-trnW-trnP, ndhF, trnL-rpl32, and ycf1) were identified in Dioscorea chloroplast genome. Phylogenetic relationships of the Dioscorea species inferred from chloroplast genomes obtained high support even at the shortest internode. Furthermore, chloroplast genomic resources, in combination with other informative molecular markers from the mitochondrial and/or nuclear genomes, could be useful for phylogenetic analysis and species identification of the genus Dioscorea, as well as for population genetics.
Supplemental Information
ML topology shown with ML bootstrap support values/Bayesian posterior probability listed at each node.
VISTA based similarity graphical information portraying the sequence identity of Dioscorea with reference D. elephantipes chloroplast genome. Grey arrows above the alignment indicate the orientation of genes. Purple bars represent exons, blue ones represent introns, and pink bars represent non-coding sequences (CNS). A cut-off of 50% identity was used for the plots. The Y-scale axis represents the percent identity within 50%–100%.
The figures above the lines are the bootstrap values for the clades.
Funding Statement
This work was funded by the National Natural Science Foundation of China (NSFC: 81325023 and 31671092), the National Key Research and Development Program of China (2017YFC1703700: 2017YFC1703704) and Key Project at Central Government Level: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (2060302). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Qingjun Yuan, Email: yuanqingjun@icmm.ac.cn.
Luqi Huang, Email: huangluqi01@126.com.
Additional Information and Declarations
Competing Interests
Yi Yu is an employee of Infinitus (China) Company Ltd. The authors declare that they have no competing interests.
Author Contributions
Zhenyu Zhao performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xin Wang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Yi Yu performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Subo Yuan analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Dan Jiang analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Yujun Zhang performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Teng Zhang analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Wenhao Zhong analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Qingjun Yuan conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Luqi Huang conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data can be found at GenBank SRA: SRR7062185, SRR7062349, SRR7062357, SRR7062294, SRR7062296.
References
- Asaf et al. (2017).Asaf S, Khan AL, Khan MA, Waqas M, Kang SM, Yun BW, Lee IJ. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Scientific Reports. 2017;7(1):7556. doi: 10.1038/s41598-017-07891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich et al. (2012).Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisenko, Sones & Hebert (2009).Borisenko AV, Sones JE, Hebert PD. The front-end logistics of DNA barcoding: challenges and prospects. Molecular Ecology Resources. 2009;9(Suppl s1):27–34. doi: 10.1111/j.1755-0998.2009.02629.x. [DOI] [PubMed] [Google Scholar]
- Burke et al. (2016).Burke SV, Lin CS, Wysocki WP, Clark LG, Duvall MR. Phylogenomics and plastome evolution of tropical forest grasses (Leptaspis, Streptochaeta: Poaceae) Frontiers in Plant Science. 2016;7:1993. doi: 10.3389/fpls.2016.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick et al. (2002).Caddick LR, Wilkin P, Rudall PJ, Hedderson TAJ, Chase MW. Yams reclassified: a recircumscription of Dioscoreaceae and Dioscoreales. Taxon. 2002;51(1):103–114. doi: 10.2307/1554967. [DOI] [Google Scholar]
- Carbonell-Caballero et al. (2015).Carbonell-Caballero J, Alonso R, Ibañez V, Terol J, Talon M, Dopazo J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Molecular Biology and Evolution. 2015;32(8):2015–2035. doi: 10.1093/molbev/msv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen ZD, Yang T, Lin L, Lu LM, Li HL, Sun M, Liu B, Chen M, Niu YT, Ye JF, Cao ZY, Liu HM, Wang XM, Wang W, Zhang JB, Meng Z, Cao W, Li JH, Wu SD, Zhao HL, Liu ZJ, Du ZY, Wang QF, Guo J, Tan XX, Su JX, Zhang LJ, Yang LL, Liao YY, Li MH, Zhang GQ, Chung SW, Zhang J, Xiang KL, Li RQ, Soltis DE, Soltis PS, Zhou SL, Ran JH, Wang XQ, Jin XH, Chen YS, Gao TG, Li JH, Zhang SZ, Lu AM, Consortium CP. Tree of life for the genera of Chinese vascular plants. Journal of Systematics and Evolution. 2016;54(4):277–306. doi: 10.1111/jse.12219. [DOI] [Google Scholar]
- Conant & Wolfe (2008).Conant GC, Wolfe KH. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 2008;24(6):861–862. doi: 10.1093/bioinformatics/btm598. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2012).Dong W, Liu J, Yu J, Wang L, Zhou S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLOS ONE. 2012;7(4):e35071. doi: 10.1371/journal.pone.0035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2013).Dong W, Xu C, Cheng T, Lin K, Zhou S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biology and Evolution. 2013;5(5):989–997. doi: 10.1093/gbe/evt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2015).Dong W, Xu C, Li C, Sun J, Zuo Y, Shi S, Cheng T, Guo J, Zhou S. ycf1, the most promising plastid DNA barcode of land plants. Scientific Reports. 2015;5(1):8348. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2017).Dong W, Xu C, Li W, Xie X, Lu Y, Liu Y, Jin X, Suo Z. Phylogenetic resolution in juglans based on complete chloroplast genomes and nuclear DNA Sequences. Frontiers in Plant Science. 2017;8:1148. doi: 10.3389/fpls.2017.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2017).Du YP, Bi Y, Yang FP, Zhang MF, Chen XQ, Xue J, Zhang XH. Complete chloroplast genome sequences of Lilium: insights into evolutionary dynamics and phylogenetic analyses. Scientific Reports. 2017;7(1):5751. doi: 10.1038/s41598-017-06210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer et al. (2004).Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Research. 2004;32(Web Server):W273–W279. doi: 10.1093/Nar/Gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2008).Gao X, Zhu YP, Wu BC, Zhao YM, Chen JQ, Hang YY. Phylogeny of Dioscorea sect. Stenophora based on chloroplast matK, rbcL and trnL-F sequences. Journal of Systematics and Evolution. 2008;46:315–321. doi: 10.3724/Sp.J.1002.2008.08007. [DOI] [Google Scholar]
- Goremykin et al. (2013).Goremykin VV, Nikiforova SV, Biggs PJ, Zhong B, Delange P, Martin W, Woetzel S, Atherton RA, McLenachan PA, Lockhart PJ. The evolutionary root of flowering plants. Systematic Biology. 2013;62(1):50–61. doi: 10.1093/sysbio/sys070. [DOI] [PubMed] [Google Scholar]
- Hansen et al. (2007).Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Jansen RK. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae) Molecular Phylogenetics and Evolution. 2007;45(2):547–563. doi: 10.1016/j.ympev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Hilu & Alice (2001).Hilu KW, Alice LA. A phylogeny of Chloridoideae (Poaceae) based on matK sequences. Systematic Botany. 2001;26(2):386–405. doi: 10.1043/0363-6445-26.2.386. [DOI] [Google Scholar]
- Hollingsworth, Graham & Little (2011).Hollingsworth PM, Graham SW, Little DP. Choosing and using a plant DNA barcode. PLOS ONE. 2011;6(5):e19254. doi: 10.1371/journal.pone.0019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu et al. (2013).Hsu KM, Tsai JL, Chen MY, Ku HM, Liu SC. Molecular phylogeny of Dioscorea (Dioscoreaceae) in East and Southeast Asia. Blumea-Biodiversity, Evolution and Biogeography of Plants. 2013;58(1):21–27. doi: 10.3767/000651913x669022. [DOI] [Google Scholar]
- Jiang et al. (2017).Jiang D, Zhao Z, Zhang T, Zhong W, Liu C, Yuan Q, Huang L. The Chloroplast Genome Sequence of Scutellaria baicalensis Provides Insight into Intraspecific and Interspecific Chloroplast Genome Diversity in Scutellaria. Genes. 2017;8(9):227. doi: 10.3390/genes8090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy et al. (2017).Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim & Jansen (1995).Kim KJ, Jansen RK. NdhF sequence evolution and the major clades in the sunflower Family. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(22):10379–10383. doi: 10.1073/pnas.92.22.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz et al. (2001).Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Research. 2001;29(22):4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Flèche et al. (2001).Le Flèche P, Hauck Y, Onteniente L, Prieur A, Denoeud F, Ramisse V, Sylvestre P, Benson G, Ramisse F, Vergnaud G. A tandem repeats database for bacterial genomes: application to the genotyping of Yersinia pestis and Bacillus anthracis. BMC Microbiology. 2001;1(1):2. doi: 10.1186/1471-2180-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2013).Li J, Wang S, Jing Y, Wang L, Zhou S. A modified CTAB protocol for plant DNA extraction. Chinese Bulletin of Botany. 2013;48(1):72–78. doi: 10.3724/sp.j.1259.2013.00072. [DOI] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2016).Luo Y, Ma P-F, Li H-T, Yang J-B, Wang H, Li D-Z. Plastid phylogenomic analyses resolve tofieldiaceae as the root of the early diverging monocot order alismatales. Genome Biology and Evolution. 2016;8(3):932–945. doi: 10.1093/gbe/evv260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majure et al. (2012).Majure LC, Puente R, Griffith MP, Judd WS, Soltis PS, Soltis DE. Phylogeny of Opuntia s.s. (Cactaceae): Clade delineation, geographic origins, and reticulate evolution. American Journal of Botany. 2012;99(5):847–864. doi: 10.3732/ajb.1100375. [DOI] [PubMed] [Google Scholar]
- Mariac et al. (2014).Mariac C, Scarcelli N, Pouzadou J, Barnaud A, Billot C, Faye A, Kougbeadjo A, Maillol V, Martin G, Sabot F, Santoni S, Vigouroux Y, Couvreur TLP. Cost-effective enrichment hybridization capture of chloroplast genomes at deep multiplexing levels for population genetics and phylogeography studies. Molecular Ecology Resources. 2014;14(6):1103–1113. doi: 10.1111/1755-0998.12258. [DOI] [PubMed] [Google Scholar]
- Morrison (2009).Morrison DA. A framework for phylogenetic sequence alignment. Plant Systematics and Evolution. 2009;282(3–4):127–149. doi: 10.1007/s00606-008-0072-5. [DOI] [Google Scholar]
- Neubig et al. (2009).Neubig KM, Whitten WM, Carlsward BS, Blanco MA, Endara L, Williams NH, Moore M. Phylogenetic utility of ycf1 in orchids: a plastid gene more variable than matK. Plant Systematics and Evolution. 2009;277(1–2):75–84. doi: 10.1007/s00606-008-0105-0. [DOI] [Google Scholar]
- Ochoterena (2009).Ochoterena H. Homology in coding and non-coding DNA sequences: a parsimony perspective. Plant Systematics and Evolution. 2009;282(3–4):151–168. doi: 10.1007/s00606-008-0095-y. [DOI] [Google Scholar]
- Patel & Jain (2012).Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLOS ONE. 2012;7(2):e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, Ferguson & Jansen (2000).Prather LA, Ferguson CJ, Jansen RK. Polemoniaceae phylogeny and classification: Implications of sequence data from the chloroplast gene ndhF. American Journal of Botany. 2000;87(9):1300–1308. doi: 10.2307/2656723. [DOI] [PubMed] [Google Scholar]
- Rambaut (1996).Rambaut A. Se-Al: sequence alignment editor. Version 2.0http://tree.bio.ed.ac.uk/software/seal/ 1996
- Rogalski et al. (2015).Rogalski M, Do Nascimento Vieira L, Fraga HP, Guerra MP. Plastid genomics in horticultural species: importance and applications for plant population genetics, evolution, and biotechnology. Frontiers in Plant Science. 2015;6:586. doi: 10.3389/fpls.2015.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas & Holland (2000).Rokas A, Holland PWH. Rare genomic changes as a tool for phylogenetics. Trends in Ecology & Evolution. 2000;15(11):454–459. doi: 10.1016/S0169-5347(00)01967-4. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli et al. (2011).Scarcelli N, Barnaud A, Eiserhardt W, Treier UA, Seveno M, d’Anfray A, Vigouroux Y, Pintaud JC. A set of 100 chloroplast DNA primer pairs to study population genetics and phylogeny in monocotyledons. PLOS ONE. 2011;6(5):e19954. doi: 10.1371/journal.pone.0019954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw et al. (2007).Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. American Journal of Botany. 2007;94(3):275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2016).Sun L, Fang L, Zhang Z, Chang X, Penny D, Zhong B. Chloroplast phylogenomic inference of green algae relationships. Scientific Reports. 2016;6(1):20528. doi: 10.1038/srep20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2012).Sun XQ, Zhu YJ, Guo JL, Peng B, Bai MM, Hang YY. DNA barcoding the Dioscorea in China, a vital group in the evolution of Monocotyledon: use of matK gene for species discrimination. PLOS ONE. 2012;7(2):e32057. doi: 10.1371/journal.pone.0032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin et al. (2005).Wilkin P, Schols P, Chase MW, Chayamarit K, Furness CA, Huysmans S, Rakotonasolo F, Smets E, Thapyai C. A plastid gene phylogeny of the yam genus, Dioscorea: Roots, fruits and Madagascar. Systematic Botany. 2005;30(4):736–749. doi: 10.1600/036364405775097879. [DOI] [Google Scholar]
- Wyman, Jansen & Boore (2004).Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu C, Dong W, Li W, Lu Y, Xie X, Jin X, Shi J, He K, Suo Z. Comparative analysis of six Lagerstroemia complete chloroplast genomes. Frontiers in Plant Science. 2017;8:15. doi: 10.3389/fpls.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai et al. (2009).Zhai C, Lu Q, Chen X, Peng Y, Chen L, Du S. Molecularly imprinted layer-coated silica nanoparticles toward highly selective separation of active diosgenin from Dioscorea nipponica Makino. Journal of Chromatography A. 2009;1216(12):2254–2262. doi: 10.1016/j.chroma.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang Y, Iaffaldano BJ, Zhuang X, Cardina J, Cornish K. Chloroplast genome resources and molecular markers differentiate rubber dandelion species from weedy relatives. BMC Plant Biology. 2017;17(1):34. doi: 10.1186/s12870-016-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ML topology shown with ML bootstrap support values/Bayesian posterior probability listed at each node.
VISTA based similarity graphical information portraying the sequence identity of Dioscorea with reference D. elephantipes chloroplast genome. Grey arrows above the alignment indicate the orientation of genes. Purple bars represent exons, blue ones represent introns, and pink bars represent non-coding sequences (CNS). A cut-off of 50% identity was used for the plots. The Y-scale axis represents the percent identity within 50%–100%.
The figures above the lines are the bootstrap values for the clades.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data can be found at GenBank SRA: SRR7062185, SRR7062349, SRR7062357, SRR7062294, SRR7062296.