Abstract
Background
Epidemiological, laboratory and clinical studies have established an association between elevated urate and high blood pressure (BP). However, the inference of causality remains controversial. A naturally occurring antioxidant, urate may also be neuroprotective, and urate-elevating treatment with its precursor inosine is currently under clinical development as a potential disease-modifying strategy for Parkinson's disease (PD).
Methods
Our study takes advantage of a recently completed phase II trial evaluating oral inosine in de novo non-disabling early PD with no major cardiovascular and nephrological conditions, and of three lines of genetically engineered mice: urate oxidase (UOx) global knockout (gKO), conditional KO (cKO), and transgenic (Tg) mice with markedly elevated, mildly elevated, and substantially reduced serum urate, respectively, to systematically investigate effects of urate-modifying manipulation on BP.
Findings
Among clinical trial participants, change in serum urate but not changes in systolic, diastolic and orthostatic BP differed by treatment group. There was no positive correlation between urate elevations and changes in systolic, diastolic and orthostatic BP ((p = .05 (in inverse direction), 0.30 and 0.63, respectively)). Between UOx gKO, cKO, or Tg mice and their respective wildtype littermates there were no significant differences in systolic or diastolic BP or in their responses to BP-regulating interventions.
Interpretation
Our complementary preclinical and human studies of urate modulation in animal models and in generally healthy early PD do not support a hypertensive effect of urate elevation or an association between urate and BP.
Fund
U.S. Department of Defense, RJG Foundation, Michael J. Fox Foundation LEAPS program, National Institutes of Health, American Federation for Aging Research, Parkinson's Disease Foundation Advancing Parkinson's Therapies initiative.
Keywords: Urate, Hyperuricemia, Urate oxidase, Blood pressure, Hypertension
Abbreviations: BP, blood pressure; PD, Parkinson's disease; UOx, urate oxidase; gKO, global knockout mice; cKO, conditional KO mice; Tg, transgenic; SURE-PD, The Safety of Urate Elevation in PD; PSG, Parkinson's Disease Study Group; SBP, systolic blood pressure; DBP, diastolic blood pressure; OBP, orthostatic blood pressure; HR, heart rate; BMI, body mass index; VPR, volume pressure recording; HPLC, high performance liquid chromatography; WT, wildtype; OSBP, OBP for SBP; MAP, mean arterial pressure; NONOate, diazeniumdiolate; NO, nitrate oxide; L-NAME, NG-nitro-L-arginine methyl ester
Research in context.
Evidence before this study
Epidemiological links between higher blood urate levels and BP support a theory that urate elevates BP. However, the inference of causality remains controversial. In addition, there are also emerging lines of evidence supporting disassociation between urate elevation and higher BP. Systematic searches were performed on MEDLINE, PubMed, Informit, ClinicalTrials.gov and CINAHL databases regarding the effect of urate on BP. Different Medical Subject Headings (MeSH) were combined with all of its synonymies using “OR” to identify all possible relevant articles. Using the Boolean search mode, key words “urate* OR uric acid*” were combined with “blood pressure* OR hypertension* OR diastolic pressure OR systolic pressure” using “and”. Then results were combined with those from MeSH to find primary and review articles before August 1, 2018. Literatures were either excluded or included based on the relevance to the current study.
Added value of this study
Our preclinical experiments in three lines of genetically engineered mice with markedly elevated, mildly elevated, and substantially reduced urate, did not show an association between urate levels and BP. Further, we did not detect a positive association between urate elevation and changes in BP in de novo non-disabling early PD with no major cardiovascular and nephrological conditions from the inosine PD trial. Our results do not support an association between urate levels and BP.
Implications of all the available evidence
The general view of hypertensive effect of urate elevation is not supported by our data. Our findings highlight the need for a more careful evaluation of urate-lowering treatments for hypertension and related conditions with well-designed clinical trials.
Alt-text: Unlabelled Box
1. Introduction
Urate, the anionic form of uric acid, is the end product of enzymatic purine metabolism in hominoids. The discovery of its antioxidant properties strengthened the hypothesis that beneficial effects of increased urate concentrations in our primate ancestors led to the evolutionary selection of disrupted urate oxidase (UOx), the enzyme that catalyzes the oxidative degradation of urate to allantoin in most mammals including rodents [[1], [2], [3]]. Urate may confer advantages in multiple neurological disorders, most notably Parkinson's disease (PD), one of the most common neurodegenerative diseases [4]. Higher urate levels are associated with a lower risk of developing PD, and among those who already diagnosed with PD, urate is associated with presence and progression of both motor and non-motor symptoms in PD. [[5], [6], [7], [8]] In cellular and animal models of PD, urate protects against dopaminergic neurodegeneration [9,10]. The Safety of Urate Elevation in PD (SURE-PD) study assessed the safety, tolerability and urate-elevating ability of oral inosine, a urate precursor, in generally healthy people with de novo non-disabling early PD. [11] Following the success of this phase II study, a phase III trial has been initiated to evaluate inosine as a potential urate-elevating strategy for disease modification in PD. [12]
By contrast, outside the central nervous system urate is generally viewed as a marker if not a mediator of systemic diseases. Elevated urate is known to cause the crystallopathic disorders of gout and uric acid urolithiasis [13]. Urate is also positively correlated with other conditions such as cardiovascular disease and metabolic syndrome [[14], [15], [16]]. Various studies have shown an association between hyperuricemia and increased risk of hypertension especially in the adolescents [[17], [18], [19], [20]], and the association appears to be dose-responsive [18]. Clinical trials revealed reduced blood pressure (BP) in obese adolescents by allopurinol, a urate-lowering xanthine oxidase inhibitor [[21], [22], [23]]. Probenecid, a uricosuric, urate-lowering agent had a similar BP-lowering response in obese adolescents [22]. In another recent, single-centered, retrospective Japanese cohort study, hyperuricemia in lean/normal individuals without metabolic syndrome carried an increased risk for hypertension [24]. In a rat model of hyperuricemia induced by the UOx inhibitor oxonic acid, BP was elevated, and both allopurinol and benziodarone, another uricosuric agent, prevented hypertension development [25]. Collectively, these studies suggested that urate levels may modulate BP. However, it remains to be determined whether higher urate is a cause or consequence of hypertension. None of the pharmacological agents evaluated in the aforementioned studies selectively modulate urate. Allopurinol, for example, increases hypoxanthine and xanthine in addition to reducing urate, and it can also inhibit other enzymes in purine and pyrimidine metabolic pathways [26]. In addition, these studies encompassed relatively short time periods of urate alteration [[21], [22], [23]]. Furthermore, emerging evidence argues against a causal effect of urate on BP. In a genetic model of mild hyperuricemia by disruption of Glut9, a urate transporter that is a key regulator of urate homeostasis, long-term inosine administration markedly further increased urate without elevating BP [27]. In humans, elevated urate induced by inosine administration in patients with multiple sclerosis was not associated with changes in BP in a clinical trial [28]. Across larger human cohorts, mendelian randomization and genetic risk studies have generally found no evidence that genetic determinants of higher urate can cause higher BP [13,[29], [30], [31]].
The present clinical study analyses whether urate elevation by inosine is associated with changes in BP in early PD patients from the SURE-PD trial. Parallel preclinical experiments entail BP measurements on three complementary lines of genetically engineered mice: UOx conditional knockout (cKO), global KO (gKO), and transgenic (Tg) mice with mildly elevated, markedly elevated, and substantially reduced serum urate, respectively.
2. Materials and methods
2.1. Study design
The objective of the study was to determine whether urate-modifying interventions alter BP in a clinical cohort of early PD patients and in complementary genetically engineered mouse models. The clinical data set was from a phase II trial rigorously designed and successfully conducted by the authors testing urate-elevating ability and safety of oral inosine [11]. Data analyses including group comparisons and correlations between changes in urate and changes in BP were led by coauthor Dr. Eric Macklin, the trial statistician. Preclinical study entailed BP measurements in constitutively and conditionally UOx KO mice, and UOx Tg mice with markedly elevated and mildly elevated, and substantially reduced urate, respectively. Sample sizes were determined by power calculation to provide 80% power to detect 20–30% changes in primary outcome measure (systolic BP (SBP)). For all the animal experiments, only littermates were used as controls. Invasive and non-invasive BP assessments and urate analyses were conducted by investigators blind to genotypes. Dr. Macklin also provided professional assistance in experimental design and statistical analyses of the preclinical study.
2.2. Clinical study
The SURE-PD study was a randomized, double blind, placebo-controlled trial of the urate-elevating drug, inosine; ClinicalTrials.gov registration # NCT00833690 [32]. Participants enrolled at 16 US Parkinson Study Group (PSG) sites had a mean age of 62 years and mild, early PD patients with baseline serum urate <6 mg/dL. They were randomized to three treatment arms: placebo or inosine titrated to produce mild or moderate serum urate elevation to 6·1–7·0 or 7·1–8·0 mg/dL for two years, respectively, with a primary focus on safety and tolerability of urate elevation in PD. The detailed study methods and primary results have been published [11]. The study protocol of PD patients was approved by the institutional review boards of the Administrative Coordination Center at Massachusetts General Hospital (MGH), the Clinical Coordination Center at University of Rochester and all clinical sites, and participants provided written informed consent. Serum urate levels were measured at screening, baseline and subsequent follow-up visits on study drug for up to 24 months (18 months on average). Vital signs (BP and heart rate (HR)) measurements were performed after patients had been sitting for one to three minutes with the back supported and feet on the floor in supine positions, and then BP in standing positions was taken immediately after the supine BP was recorded. BP measurements were made on each patient by using the same right bare forearm and the same manometer with an appropriate cuff size. BP readings were obtained by palpating the brachial artery, positioning the cuff's bladder over the brachial artery, and then applying the cuff above the antecubital fossa. Orthostatic BP (OBP) was calculated for each of the measurements. Changes of SBP, diastolic BP (DBP), OBP, supine HR, and changes of urate were calculated for each of the participants as the difference between final visit on the treatment vs. baseline visit. Association between urate elevation and BP were estimated from mixed models for comparisons of treatment groups and by Spearman rank correlations between changes in serum urate and BP, adjusting for age, gender, and body mass index (BMI).
2.3. Animal study
All experimental animal procedures were conducted at MGH and were approved prior to implementation by the Institutional Animal Care and Use Committee. Mice were housed in a controlled environment for humidity and temperature with 12-hour light/dark cycles and ad libitum food and water. UOx gKO and UOx Tg mouse strains have been described in detail previously [10]. The gKO strain, originally established by Wu and colleagues [33], was obtained through Jackson Laboratory. The Tg strain was obtained from Kenneth L. Rock (Department of Immunology, University of Massachusetts, Worcester, MA) [34]. Both strains have been backcrossed to C57/BL 6 J background for >10 generations in our laboratory. UOx cKO: To generate the cKO mouse line, we first created (with ingenious Targeting Laboratory, Inc., NY) a floxed UOx line (on C57BL/6 genetic background) with loxP sequences flanking critical exons (3&4) of UOx. The floxed UOx line was then mated with a transgenic inducible (tamoxifen-responsive) cre line (UBC-cre-ERT, Stock 008085, Jackson Laboratory, ME) (on congenic C57BL/6 background). Adult mice homozygous for the floxed UOx gene and hemizygous for the transgene (cre-ERT) have no discernable phenotype as adults until they are systemically exposed to the estrogen receptor ligand tamoxifen by i.p. injection (once daily at a concentration of 75 mg/kg for five consecutive days), triggering ubiquitous cre expression and consequently recombination and disruption of the floxed UOx gene. As a result, these mice demonstrated abolished hepatic UOx (Fig. S2) detected by Western Blot and increased serum urate levels in cheek blood taken two weeks after completion of the tamoxifen regimen [10].
2.3.1. BP measurements
Invasive[35,36]: Mice were anesthetized with intraperitoneal administration of ketamine 120 mg/kg and xylazine 5 mg/kg. This mixture induces deep anesthesia in mice with minimum effects on hemodynamics. The mice were placed on a heated table. ECG was monitored. The electrodes were placed on one upper limb and the two lower limbs. After endotracheal intubation (20G Angiocath), volume-controlled ventilation was initiated (respiratory rate of 110–120 breaths per minute, fraction of inspired oxygen = 1. Rodent Ventilator, model 687; Harvard Apparatus). An incision was made in the chest, and a catheter was placed in the carotid artery to administer saline and drugs and to monitor SBP and DBP and HR before and after vehicle (saline) and NOC-9 (NO donor) administration (doses = 3·3 and 10 μg/kg). During surgery, additional ketamine and xylazine was administered every 15 min or if there is evidence of discomfort (e.g. withdrawal to pain, tachycardia, etc.). At the conclusion of the invasive hemodynamic measurements, the mice received 125 mg/kg pentobarbital intravenously for euthanasia. For each genetic line, male mice and littermate controls were used.
Noninvasive: noninvasive BP was measured using CODA™ noninvasive BP system (Kent Scientific Cooperation, Torrington, CT). To increase the accuracy of BP measurements, room temperature was set at 26 °C and mice were placed in the holder 15 min before beginning of BP measurements. An infrared warming blanket was used to maintain body core temperature and adequate blood flow in the tail. A cuff was placed around the animal's tail to occlude the blood flow. A volume pressure recording (VPR) sensor was placed distal to the occlusion cuff to monitor BP. VPR uses a specially designed differential pressure transducer to measure the blood volume in the tail non-invasively and measures four parameters simultaneously: SBP, DBP, mean BP, and HR. Female mice were used from the UOx cKO line and males from the UOx Tg line.
All mice were pre-trained for five consecutive days. Baseline BP was measured one week after cheek blood withdrawal for serum urate measurement. Mice were then provided with a high salt diet (4% NaCl, TestDiet, Richmond, IN) for three weeks followed by a low salt (0·125% NaCl, TestDiet) diet for three weeks. BP was measured once every week during the diet changes by the same researcher around the same time of the day. After three-week washout with regular 0·26% NaCl diet, L-NAME (NG-nitro-L-arginine methyl ester; an NOS inhibitor) was introduced (100 mg/kg i.p.), and BP was measured 15 min and three days after L-NAME injection. Mice were then sacrificed, and cheek blood was saved for urate analysis. No data were excluded.
2.3.2. Serum urate analysis
Serum urate levels were assessed by high performance liquid chromatography (HPLC) [37]. Sample Preparation: Whole blood samples were collected from the submandibular vein in mice and centrifuged at 13,000 rpm for 20 min at 4 °C. 50 μL supernatant was removed, and 30 μL of a solution consisting of 0·4 M perchloric acid and 100 μM methyldopa were added to denature proteins. Methyldopa served as an internal standard for HPLC analysis. After 10-min incubation, samples were again centrifuged at 4 °C and 13,000 rpm for 15 min. The resultant 50 μL supernatant was then combined with 0·2 M potassium phosphate and centrifuged through a Corning Costar Spin-X 0·22 μm filter tube. The filtrate was then directly loaded into the autosampler. HPLC: The isocratic HPLC system used was comprised of an ESA model 584 pump, a Dionex Ultimate 3000 autosampler, a Varian microsorb-MV reversed-phase C18 column (150 × 4·46 mm i.d. 5 μm), two Dionex model 5011A coulometric cells, and an ESA CoulArray 5600A. mobile phase consisted of 200 mM potassium phosphate monobasic and 10% sodium 1-pentanesulfonate monohydrate, brought to pH 3·5 with 85% phosphoric acid. All chemicals were purchased through Sigma-Aldrich. Run time was 10 min. The electrodes were set at −150 mV and + 150 mV.
2.4. Statistical analysis
All graphs and error bars are expressed as mean ± standard error of the mean. For human study, change in serum urate levels and changes in BP outcome measures from baseline to last measured on study drug at average of 18 months were calculated for individual participants. Mean differences in change in serum urate levels and changes in BP outcome measures between groups were calculated using Student's t-test. Tests of difference from slope of zero were performed for correlation between changes in BP measurements and change in serum urate from baseline from baseline to last measured on study drug. Association between urate elevation and BP were estimated from mixed models for comparisons of treatment groups and by Spearman rank correlations between changes in serum urate and BP, adjusting for age, gender, and BMI.
For animal study, mean differences in serum urate levels and BP outcome measures between groups were assessed using Student's t-test. Tests of difference from slope of zero were performed for correlation between BP measurements and serum urate. Two-way ANOVA was performed to compare effect of genotype and effect of given manipulations on invasive BP measurements, adjusting for multiple comparisons using the Šidák correction. Two-way ANOVA was performed to compare the effect between the genetically modified mice and their wildtype (WT) littermate controls across noninvasive BP measurement time points and to compare the time effect of given manipulations within groups, adjusting for multiple comparisons using the Šidák correction.
3. Results
3.1. Urate elevation is not associated with changes in BP in SURE-PD trial participants
The SURE-PD trial enrolled subjects with early PD whose screening serum urate concentrations were at or below the population median of ~5·7 mg/dL (with a mean baseline value of 4·5 mg/dL among the 41 women and 34 men randomized). Oral inosine dosed to produce mild (~2 mg/dL to 6·1–7·0 mg/dL) and moderate (~3 mg/dL to 7·1–8·0 mg/dL) urate elevation significantly and dose-dependently increased serum urate compared to placebo (Fig. 1a) and did so chronically for up to 24 months (mean treatment duration of 18 months) [11]. SBP and DBP were measured in the supine and then standing positions at baseline and at follow-up visits on study drug.
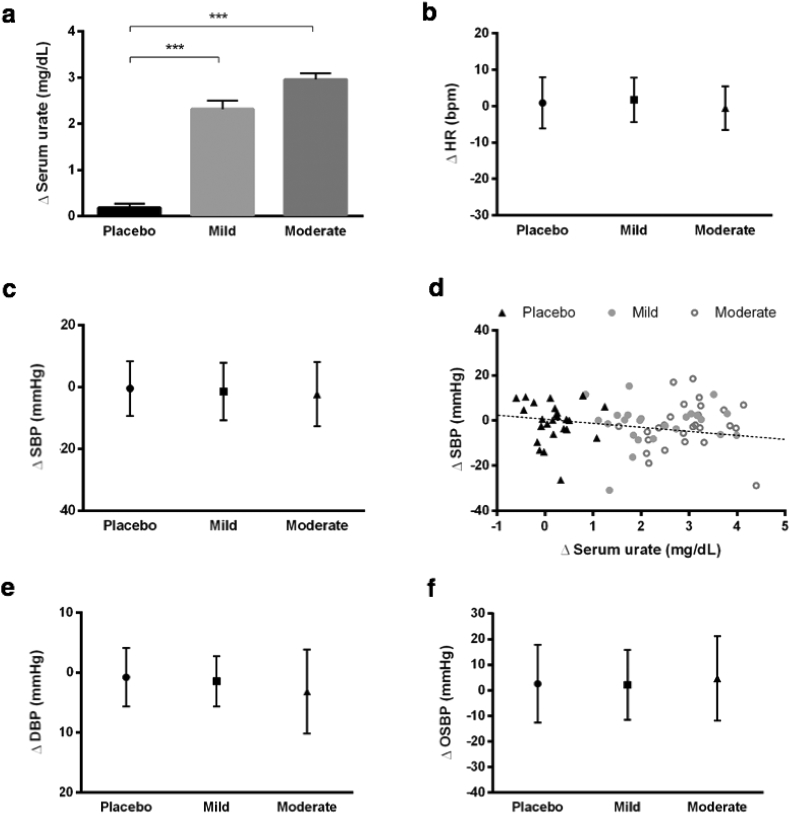
Fig. 1.
Hemodynamic effect of urate-elevating inosine treatment in patients of the SURE-PD trial. (a) Change in serum urate levels from baseline to last measured at average of 18 months on study drug in subjects randomized to placebo or inosine titrated to produce a mild or moderate serum urate elevation (from a mean pre-treatment value of 4·5 ± 0·9 mg/dL to target ranges of 6·1-7·0 or 7·1-8·0 mg/dL, respectively). (b) Change in supine HR from baseline to last measured on study drug in mild and moderate inosine groups and placebo group. (c) Change in supine SBP from baseline to last measured on study drug. (d) Change in supine SBP versus change in serum urate of all participants from baseline to last measured on study drug (r2=-0·23, p=0·05). (e) Change in supine DBP from baseline to last measured on study drug. (f) Change in OSBP from baseline to last measured on study drug. n=25, 24, and 26, placebo, mild, and moderate inosine treatment group, respectively. ***p<0·001, adjusted for age, gender, and BMI.
There was no significant difference in change in supine HR (Fig. 1b) or change in supine SBP (Fig. 1c) from baseline to the last visit on study drug comparing the placebo group and the groups with mild and moderate urate elevation, and none of the treatments resulted in significant differences between the last visit on the treatment and the baseline from the baseline within groups. Supine SBP at the last visit in subjects on placebo or inosine producing mild or moderate urate elevation was 126 ± 14·2, 129 ± 18, and 128 ± 17·1 mmHg, respectively and the baseline was 127 ± 14·8, 131 ± 13·5, and 131 ± 15·4 mmHg, respectively. Correlation analysis revealed a marginally significant inverse relationship (r2 = −0·23, p = 0·05) between change in supine SBP and change in urate from baseline over average of 18 months among the three treatment groups (Fig. 1d). Stratifying our data by gender (an important covariate of BP as well as urate), this inverse correlation appeared stronger in female subjects (r2 = −0·32, p = 0·04). However, the association in men (r2 = −0·02, p = 0·92), and in all subjects for standing SBP, were not statistically significant (Table S1).
Similarly, change in supine DBP from baseline to end of treatment follow-up did not significantly differ between the three groups (Fig. 1e) and was not correlated with change in urate levels (Table. S1). OBP was closely examined given its hypothesized role in our evolutionary loss of functional UOx as well as its impact on disability in PD. [38,39] The change in OBP for SBP (OSBP) from baseline to end of treatment follow-up was also not significantly different between the three groups (Fig. 1f), although in this relatively healthy early PD cohort there was no appreciable orthostatic hypotension at baseline (placebo = 0·12 ± 2·20; mild = 0·83 ± 2·29; moderate = −1·62 ± 2·42 for OSBP and placebo = 2·08 ± 1·09; mild = 1·83 ± 1·36; moderate = −0·42 ± 1·34 for ODBP) or end of treatment follow-up (placebo = 1·24 ± 3·07, mild = −3·33 ± 1·77, moderate = −2·42 ± 1·50 for OSBP and placebo = 2·38 ± 1·36, mild = −0·42 ± 2·13, moderate = 2·30 ± 1·27 for ODBP). Similarly, change in OBP for SBP and DBP did not significantly correlate with change in serum urate (Table. S1).
3.2. Markedly elevated urate in UOx gKO mice is not associated with BP changes
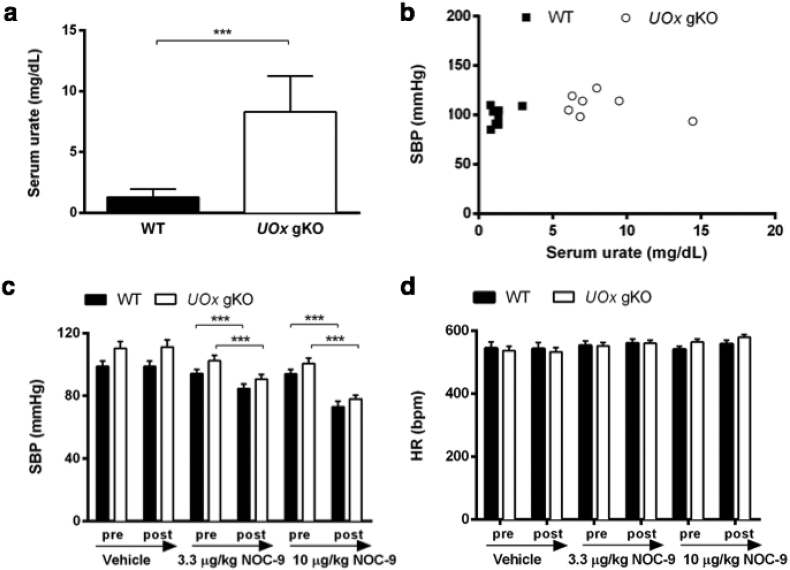
Silencing the UOx gene in mice recapitulates its evolutionary silencing in human ancestors resulting in hyperuricemia and serves as a model of human purine catabolism to investigate the relationship between urate elevation and BP change [33]. While serum urate levels are typically lower in mice than humans, urate levels in UOx gKO mice were markedly higher than WT littermate controls and reached levels of those in humans (Fig. 2a) [10]. BP was initially measured in anesthetized male, nine-month old UOx gKO mice and littermate WT controls through invasive carotid artery catheterization. There was no significant difference in their body weights (29 ± 0·4 vs. 33 ± 1·8 g, UOx gKO vs. WT). Despite substantially elevated blood urate level and a trend towards higher baseline SBP in UOx gKO compared to WT (p = 0·07), baseline SBP was uncorrelated with urate (Fig. 2b), as was DBP and mean arterial pressure (MAP) (Fig. S1 a and b). We then investigated whether serum urate levels impacted the response to nitric oxide (NO), a known vasodilator. SBP of both WT and UOx gKO mice decreased dose-dependently after administration of NOC-9, a diazeniumdiolate (NONOate) small molecule NO donor (Fig. 2c). SBP decreased 11% and 22% in UOx gKO mice and 10% and 22% in WT mice following administration of 3·3 and 10 μg/kg NOC-9, respectively. Similarly, the NOC-9-induced decreases in MAP were indistinguishable between UOx gKO and WT mice (11% and 24%, and 9 and 23% after 3·3 μg/kg and 10 μg/kg NOC-9 administration, respectively). HR did not differ between UOx gKO and WT mice across all treatments (Fig. 2d). BP was similar between UOx gKO and WT littermates before or after all the treatments. These results demonstrated that BP and the response to a vasodilator were not affected in UOx gKO despite markedly elevated serum urate levels.
Fig. 2.
Markedly elevated urate in UOx gKO mice is not associated with BP changes. (a) HPLC analysis of serum urate levels in UOx gKO and WT mice. (b) Invasive SBP versus serum urate of UOx gKO and WT mice at baseline (WT: r2=0·007, p=0·85; UOx gKO: r2=0·012, p=0·38; pooled: r2=0·10, p=0·24). Invasive SBP (c) and HR (D) measurement before (baseline) and after vehicle (saline), 3·3 μg/kg, and 10 μg/kg NOC-9 administration. n=8 and 7, male nine-month old UOx gKO and littermate WT mice. ***p<0·001.
3.3. Mildly elevated urate in UOx cKO mice is not associated with BP changes
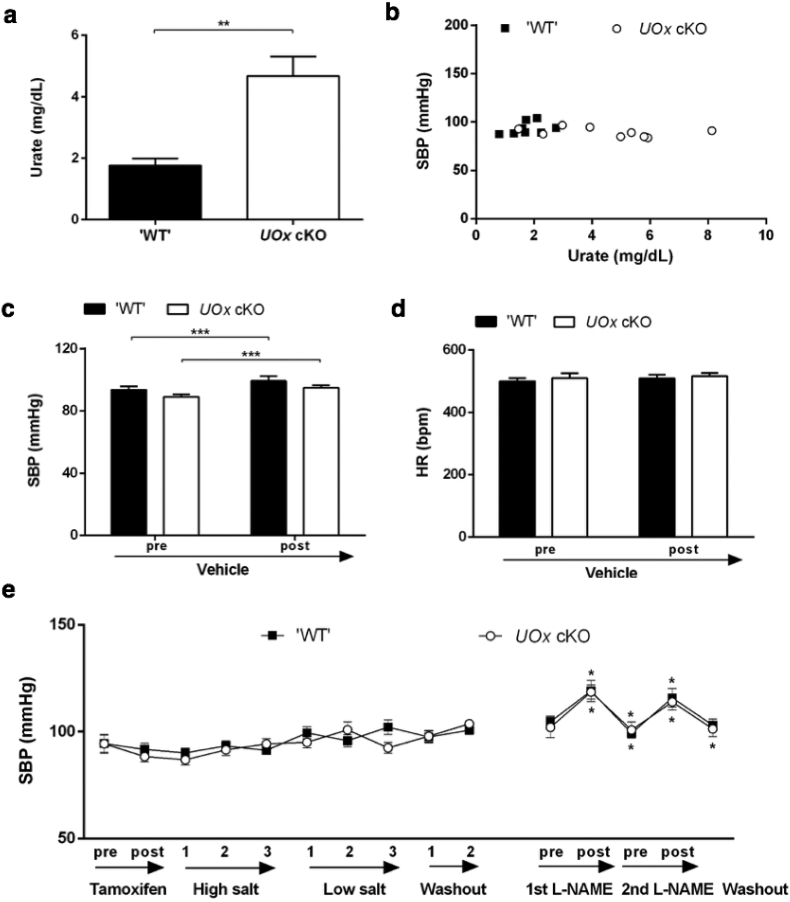
Although UOx gKO mice display high urate levels comparable to those observed in adult humans, their germline disruption of UOx produces embryonic or post-natal developmental effects such as nephrotoxicity [11,32]. As these may confound the interpretation of urate elevation in adult mice, we generated UOx cKO with a conditional and inducible disruption of UOx to obviate the concern that a developmental phenotype might obscure urate effects in mature animals. BP in these mice was monitored using both invasive (male, average four-month old) and noninvasive methods (female, average three-month old). Two weeks after completion of the tamoxifen injection regimen in UOx cKO mice, resulting in disruption of UOx and deletion of UOx (Fig. S2a), serum urate was moderately and significantly higher than ‘WT’ littermates (UOx floxed with no cre transgene, and the mice have WT phenotype) (Fig. 3a). Baseline SBP assessed invasively was similar between UOx cKO and littermate controls, and urate levels do not correlate with SBP (Fig. 3b and c; DBP and MAP in Fig. S2 b and c). Both UOx cKO and their ‘WT’ littermates had mild but significantly increased SBP following saline administration (WT: +6·1%, p < 0·001; cKO: +6·6%, p < 0·001) (Fig. 3c). The slightly differing responses of control mice from UOx cKO and from gKO lines may be due to their age difference. Also HR was similar in ‘WT’ and UOx cKO mice (Fig. 3d). Body weights of UOx cKO (24 ± 0·6 g) and ‘WT’ littermates (24 ± 0·8 g) were similar.
Fig. 3.
Mildly elevated urate in UOx cKO mice is not associated with BP changes. (a) HPLC analysis of serum urate levels in UOx cKO mice and ‘WT’ (floxed UOx with no cre). (b) Invasive SBP versus serum urate of UOx cKO mice and ‘WT’ at baseline (‘WT’: r2=0·138, p=0·36; cKO: r2=0·193, p=0·20; pooled: r2=0·163, p=0·09). Invasive SBP (c) and HR (d) measurement before (baseline) and after vehicle administration. n=9 and 8, male four-month old UOx cKO and littermate ‘WT’ mice. (e) Noninvasive SBP measurement before and two weeks after completion of tamoxifen injection, during three weeks of high salt diet, low salt diet and two weeks of washout, and pre-, post (15 min) each L-NAME administration. The second L-NAME administration was seven days after first L-NAME administration. Asterisks for significant change comparing preceding time point from different treatment are above the plot for WT and below the plot for UOx cKO. n=16 and 14, UOx cKO and littermate WT mice, all female, average three-month old at the baseline. *p<0·05, **p<0·01, ***p<0·001.
Serial, noninvasive tail-cuff measurement of BP in awake female mice also revealed no difference in SBP between UOx cKO and their ‘WT’ littermates at baseline. Modulating dietary sodium levels did not alter BP in UOx cKO or littermate control mice. BP increased similarly in both UOx cKO and ‘WT’ mice immediately after administration of L-NAME, a NO synthase inhibitor known to cause vasoconstriction and hypertension. BP returned to baseline within two days (Fig. 3e). Serum urate measured in cheek blood was 1·9 ± 0·24-, 1·9 ± 0·33-, and 2·2 ± 0·29-fold higher in UOx cKO mice than control littermates at two weeks after tamoxifen injection, washout week two, and the end point of the experiment. Body weights were 20 ± 0·6 and 21 ± 0·3; 23 ± 0·7 and 23 ± 0·5, and 22 ± 1·1 and 24 ± 0·7 g for UOx cKO mice and control littermates, respectively, at these same time points, with no significant difference between the two groups throughout the experimental course. Unlike UOx gKO mice characterized by decreased kidney size [11], kidney weights were comparable in UOx cKO mice and control littermates (combined weight of both kidneys: 388 ± 15 vs. 373 ± 11 mg). These results demonstrate that serum urate levels were not associated with BP in a mouse model with moderately elevated urate and no developmental nor any apparent systemic toxicity of hyperuricemia.
3.4. Reduced urate in UOx Tg mice is not associated with BP changes
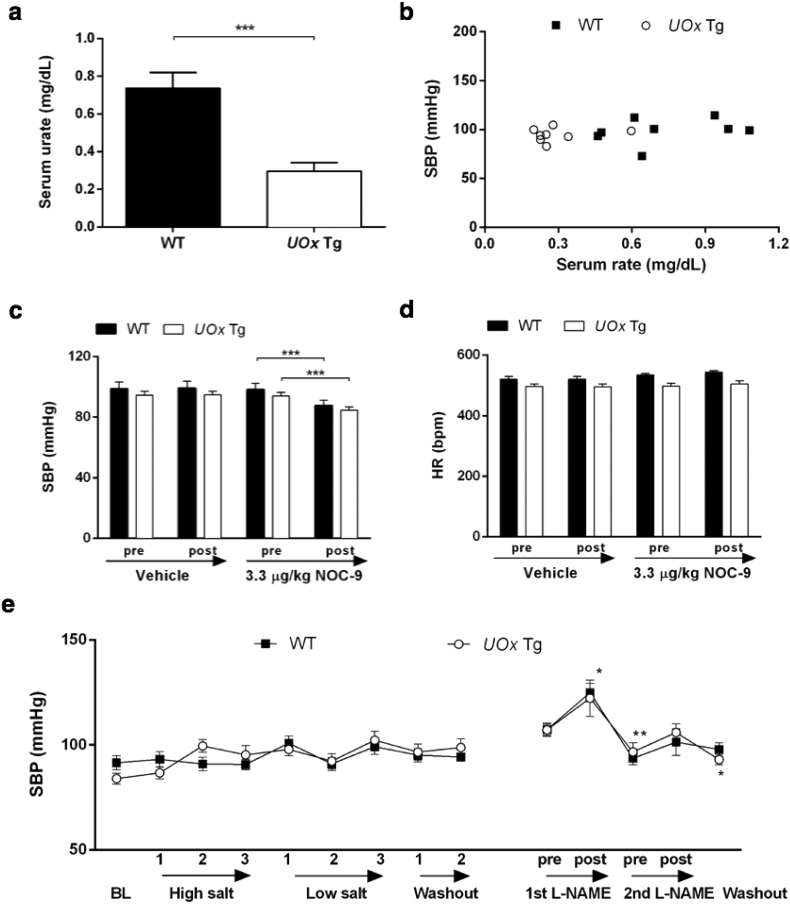
Male, anesthetized UOx Tg mice and WT littermates (three-month old) were used to measure BP invasively. As expected, serum urate levels were lower in UOx Tg mice than WT littermates (Fig. 4a) [11]. UOx Tg mice tended to be smaller, however, there was no statistical significance in body weight between UOx Tg (29 ± 0·8 g) and WT animals (32 ± 1·3 g). Baseline SBP, DBP and MAP were similar in UOx Tg and littermate WT mice and did not correlate with serum urate levels (Fig. 4a and Fig. S3 a and b). Administration of 0·3 μg/kg NOC-9 similarly decreased SBP of UOx Tg mice and WT littermates by 11% and 10%, respectively (Fig. 4c). HR responses to the treatments were similar in Tg and WT mice (Fig. 4d). Also in awake ten-month old male UOx Tg mice, SBP, assessed noninvasively, was similar to their WT littermates (Fig. 4e). Neither high nor low salt diet affected BP in either UOx Tg or WT mice. SBP increased indistinguishably following L-NAME administration in UOx Tg mice and WT littermates. BP returned to baseline two days after L-NAME injection in both UOx Tg mice and WT controls (Fig. 4e). Unlike younger mice, the weight difference between ten-month old UOx Tg mice and their WT littermates was statistically significant throughout the experimental course (average 30 ± 0·8 g for UOx Tg mice and 33 ± 0·7 g for WT, p = 0·008). However, we did not detect a correlation between body weight and SBP. In this experiment UOx Tg mice had a significant reduction (77 ± 3·0% in average) in serum urate compared to the controls (p < 0·001).
Fig. 4.
Reduced urate in UOx Tg mice is not associated with BP changes. (a) HPLC analysis of serum urate levels in UOx Tg and WT mice. (b) Invasive SBP versus serum urate of UOx Tg and WT mice at baseline (WT: r2=0·091, p=0·46; Tg: r2=0·046, p=0·60, pooled: r2=0·113, p=0·20). (c) Invasive SBP and HR (d) measurement before (baseline) and after vehicle and 3·3 μg/kg NOC-9 administration. n=8 and 8, UOx Tg and littermate WT mice, all male, average four-month old. (e) Noninvasive SBP and DBP measurement at the baseline (BL), during three weeks of high salt diet, low salt diet, and two weeks of washout, and pre-, post (15 min) each L-NAME administration. n=9 and 13, UOx Tg and littermate WT mice, all male, average ten-month old at the baseline. Asterisks for significant change comparing preceding time point from different treatment are above the plot for WT and below the plot for Tg. *p<0·05, **p<0·01, ***p<0·001.
4. Discussion
We have demonstrated that in the SURE-PD clinical trial, oral inosine over 24 months dose-dependently elevates serum urate without raising BP. There is no positive correlation between changes in serum urate and changes in all BP measures among its 75 early PD participants. In animal models, neither germline nor conditional disruption of UOx, nor overexpression of UOx, resulting in elevation or reduction in serum urate, respectively, was associated with changes in BP, measured by noninvasive or invasive methods. Responses to pharmacological BP stimulation were also indistinguishable between the genetically engineered animals and their littermate controls. The findings reveal no correlation between long-term urate elevation and BP increase, and that they do so in both laboratory animals and humans strengthens their biological and clinical relevance.
Our preclinical results in mice lacking UOx contrast those previously reported with hyperuricemic hypertension in rats, in which oxonic acid-induced mild hyperuricemia produced systemic hypertension that was reversible by urate-lowering agents allopurinol or benziodarone administration [24]. The ~two-fold urate elevation of ~43 uM (~0·7 mg/dL) achieved in the rat model is comparable to that in UOx cKO mice, yet UOx cKO mice displayed no phenotype of altered BP. The seemingly discordant findings may be due to differences between the species or between the genetic vs. pharmacological approaches taken to modulate UOx. Selective and irreversible targeting of UOx through its constitutive or conditional gene disruption or its transgenic overexpression and consequent stable alterations in urate concentrations could lead to compensatory hemodynamic effects, which may not occur with reversible pharmacological inhibition of UOx by oxonic acid. Conversely, oxonic acid is less selective for UOx as it can inhibit other metabolic processes [[40], [41], [42]], potentially confounding its interpretation.
Our preclinical findings generally corroborate those of a recent study reporting unaltered BP in eight-week old male constitutive UOx KO mice which was generated using the transcription activator-like effector nuclease technique, resulting in ~three-fold higher serum urate that is comparable to our cKO mice [43]. The same study, however, found significantly higher BP in female mice of the same age [43]. The discrepancy could be due to differences in BP measurement methods, animal gender and age, knock-out methods, genetic background or renal function status. Unaltered BP was reported in another recent murine study taking an alternative genetic approach to elevating systemic urate levels. Instead of recapitulating our evolutionary loss of UOx and its catabolism as in the present study, Preitner et al. [27] disrupted the gene for the urate transporter Glut9 (SLC2A9), which is a major genetic determinant of urate levels in humans. They reported that specifically eliminating hepatic Glut9, which transports urate into liver for enzymatic degradation by UOx, could raise serum urate (to 2 mg/dL, and then up to 5 mg/dL over six months on a purine-rich diet) without altering BP. However, spontaneous hypertension has been reported in another study using mice lacking the intestinal urate transporter Glut9, and it can be reversed by allopurinol [44]. Given the relative small urate effect (~35%) in this gut SLC2A9 KO model, there is a possibility that hypertension developed in this model was not specifically related to urate, and “the allopurinol effects are derived specifically from blocking oxidant production via xanthine oxidase inhibition per se” as the authors discussed [44].
Similarly supportive of dissociation between urate and BP are our clinical results that early, untreated PD patients responded to inosine test with a dose-dependent increase in serum urate with no BP increase. These findings are consistent with a smaller trial of urate-elevating inosine in patients with multiple sclerosis [27]. Increased urate levels have been demonstrated without accompanying changes in SBP in a longitudinal cohort of relapsing-remitting multiple sclerosis patients after treatment with natalizumab, α4 integrin antagonist, for 12 months [45]. The marginally inverse relationship between change in spine SPB and change in urate among the three treatment groups in our study appears to be in agreement with a previous study demonstrating an association between higher urate genetic risk score and lower BP [31]. This inverse correlation is stronger in female subjects; male subjects only show a trend. In a recent study in treated hypertensive patients, 25% of which have hyperuricemia, rate of uncontrolled BP is higher in men with hyperucicermia versus normouricemia whereas it is similar in normouricemic and hyperuricemic women [46]. Gender difference has also been demonstrated in another recent study, in which urate is positively associated with pulse pressure (difference between SBP and DBP) only in women not in men [47].
In the current study we also serially and systematically acquired OBP measurements, which may be of particular relevance to the evolutionary silencing of UOx in hominoids [38]. It has been suggested that reduced dietary salt availability among upright human ancestors some 15–25 million years ago threatened their ability to maintain adequate BP for survival and that elevated urate resulting from UOx mutations may have conferred a selection advantage [4,14,38,48]. Although SURE-PD subjects had early mild PD and did not demonstrate hypotensive or tachycardic responses in response to orthostatic maneuvers as seen typically in advanced PD [39], the lack of modulation of standing versus supine hemodynamic parameters argues against a hypertensive evolutionary advantage of UOx disruption.
Conversely, urate-lowering medication trials have inconsistently demonstrated BP-lowering effects. The xanthine oxidase inhibitor febuxostat failed in a phase II trial in subjects with hyperuricemia and hypertension to show change in BP despite a substantial urate drop from 7·6 to 4·3 mg/dL [49]. A smaller study in a dialysis population recently reported a similar result [50]. Further, allopurinol decreased urate but did not show effects on BP in patients with mild to moderate renal disease [51]. A recent double-blinded randomized retrospective cohort study involving non-hypertensive overweight or obese adults also did not support the positive association between urate and BP [24]. The study found that urate-lowering therapies, allopurinol and probenecid, lowered serum urate after four and eight weeks but did not significantly alter the mean SBP [24]. The inconsistency between these findings and previously reported BP-lowering benefit of urate-lowering treatments in obese adolescents may be explained by differences of age between the study populations [21,22]. Indeed, the relationship between urate and BP in elderly populations is less significant and more controversial than in the adolescents [19,52]. All the aforementioned null results from interventional studies are in adult or senior patients. The average age of our SURE-PD participants was 62-year old at baseline [11]. Though the increasing incidence of hypertension due to other causes may contribute to the weakened urate-BP link with increasing age, the precise role of age is still unclear. Similarly, causal association between obesity, urate and BP remain to be elucidated given that the reported beneficial BP-lowering effects of urate-lowering agents are conducted in adolescent populations with obesity [21,22]. All our analyses for SURE-PD population are adjusted for age and BMI. Like these few pharmacological intervention trials, a growing number of ‘genetic trials’ via mendelian randomization, have also overall argued against a clinically significant hypertensive effect of urate elevation [31].
While the convergence of findings from complementary animal and clinical trial data is a particular strength of this study several limitations should be considered. The SURE-PD population comprises individuals with a specific neurodegenerative disease so their response to urate-elevating treatment may not be generalizable. The limitation is likely modest however as the trial enrolled only subjects with mild, early disease not yet warranting dopaminergic medication and without substantial cardiovascular or renal disease. Prior studies of urate-modifying drugs on BP have focused on populations with greater medical disability such as overweight or obese patients [21]. Another caveat is the relatively small trial population, which increases the possibility that BP confounders may have been unequally distributed among the treatment groups despite randomization. However, known BP covariates (age, sex, BMI, blood glucose, total cholesterol, high density lipoprotein, and triglycerides) were not different between the groups at baseline and after chronic urate-elevating treatment [11]. Another limitation on inferences of urate's role based on both the preclinical and clinical interventions is that in neither case was urate itself manipulated. Although the trial used the metabolic precursor inosine that is rapidly converted to urate it is possible that this conversion could have other effects (e.g., with xanthine oxidase-catalyzed conversion of xanthine to urate generating equimolar amounts of hydrogen peroxide) [53]. Similarly, the murine manipulations of UOx to increase or decrease urate would be expected to simultaneously decrease or increase, respectively, levels of UOx-catalyzed oxidation product allantoin. Lastly, an unavoidable limitation of even convergent evidence against an effect is that it's difficult to prove the absence of an effect.
Nevertheless, the mouse and human studies reported here yielded consistent null results that when combined with emerging lines of laboratory, human genetic and clinical evidence from other investigators provide a strengthening counter-argument to a causal link between hyperuricemia and hypertension in adults. As a growing number of effective urate-lowering medications have become available there has been an expansion of interest in the application of urate-lowering treatment to preventing or treating BP dysregulation in individuals with cardiovascular, renal and metabolic diseases [[54], [55], [56]]. While our findings reflect the need for more careful evaluation of urate-lowering treatments in pursuit of such indications and their potential cardiovascular risks [57], the conflicting evidence in the field highlights the importance of well-designed interventional studies in the future.
Acknowledgments
Acknowledgements
The authors thank K.L. Rock and J. Kono for providing UOx Tg mice and for advice on their use, and Y. Xu for animal breeding and genotyping.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding sources
This work is supported by the U.S. Department of Defense (grant W81XWH-11-1-0150 to M.A.S.), the RJG Foundation (2011D004473 to X.C.), Michael J. Fox Foundation LEAPS program (to M.A.S.), National Institutes of Health grant K24NS060991 (to M.A.S.), U01NS090259 (to M.A.S.), R21NS090246 (to X. C.), R01NS102735 (to X. C.), American Federation for Aging Research, and the Parkinson's Disease Foundation Advancing Parkinson's Therapies initiative.
Declaration of interests
Dr. Schwarzschild reports personal fees from Prevail Therapeutics, personal fees from Eli Lilly and Co., other from Acorda Therapeutics, outside the submitted work.
Author contributions
Study concept and design: X.C., E.S.B., A.A. and M.A.S.. Acquisition of data: R.E.T, M.M., F.Z., M.R. and R.L.. Statistical analysis: X.C., D.D.F. and E.A.M.. Analysis and interpretation of data: X.C., D.D.F, E.A.M., E.S.B. and M.A.S.. Drafting of the manuscript: X.C., D.D.F., C.C.U., X.W., E.A.M., E.S.B. and M.A.S contributed to critical revision of the manuscript for important intellectual content.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.10.039.
Appendix A. Supplementary data
Supplementary material
References
- 1.Proctor P. Similar functions of uric acid and ascorbate in man? Nature. 1970;228(5274):868. doi: 10.1038/228868a0. https://www.ncbi.nlm.nih.gov/pubmed/5477017 [DOI] [PubMed] [Google Scholar]
- 2.Ames B.N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. https://www.ncbi.nlm.nih.gov/pubmed/6947260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oda M., Satta Y., Takenaka O., Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 4.Chen X., Wu G., Schwarzschild M.A. Urate in Parkinson's disease: more than a biomarker? Curr Neurol Neurosci Rep. 2012;12(4):367–375. doi: 10.1007/s11910-012-0282-7. [DOI] [PubMed] [Google Scholar]
- 5.Weisskopf M.G., O'Reilly E., Chen H., Schwarzschild M.A., Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166(5):561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascherio A., Lewitt P.A., Xu K. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66(12):1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moccia M., Picillo M., Erro R. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson's disease. Eur J Neurol. 2015;22(1):93–98. doi: 10.1111/ene.12533. [DOI] [PubMed] [Google Scholar]
- 8.Huang X., Ng S.Y., Chia N.S. Serum uric acid level and its association with motor subtypes and non-motor symptoms in early Parkinson's disease: PALS study. Parkinsonism Relat Disord. 2018 doi: 10.1016/j.parkreldis.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Bakshi R., Zhang H., Logan R. Neuroprotective effects of urate are mediated by augmenting astrocytic glutathione synthesis and release. Neurobiol Dis. 2015;82:574–579. doi: 10.1016/j.nbd.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Burdett T.C., Desjardins C.A. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110(1):300–305. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkinson Study Group S-PDI, Schwarzschild M.A., Ascherio A. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzschild M. Study of Urate Elevation in Parkinson's Disease, Phase 3 (SURE-PD3). U.S National Library of Medicine Wed Site. https://clinicaltrials.gov/ct2/show/NCT02642393. Updated January 9, 2018. Accessed October 29, 2017.
- 13.Li X., Meng X., Timofeeva M. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357 doi: 10.1136/bmj.j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Lario B., Macarron-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010;49(11):2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 15.Kutzing M.K., Firestein B.L. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324(1) doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 16.Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayson P.C., Kim S.Y., Lavalley M., Choi H.K. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63(1):102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Qin T., Chen J. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.J., Ahn J., Hwang J. Relationship between uric acid and blood pressure in different age groups. Clin Hypertens. 2015;21 doi: 10.1186/s40885-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R.J. Why focus on uric acid? Curr Med Res Opin. 2015;31(Suppl. 2):3–7. doi: 10.1185/03007995.2015.1087979. [DOI] [PubMed] [Google Scholar]
- 21.Feig D.I., Soletsky B., Johnson R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soletsky B., Feig D.I. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5):1148–1156. doi: 10.1161/HYPERTENSIONAHA.112.196980. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal V., Hans N., Messerli F.H. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2013;15(6):435–442. doi: 10.1111/j.1751-7176.2012.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullan C.J., Borgi L., Fisher N., Curhan G., Forman J. Effect of Uric Acid Lowering on Renin-Angiotensin-System Activation and Ambulatory BP: a Randomized Controlled Trial. Clin J Am Soc Nephrol. 2017;12(5):807–816. doi: 10.2215/CJN.10771016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzali M., Hughes J., Kim Y.G. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. https://www.ncbi.nlm.nih.gov/pubmed/11711505 [DOI] [PubMed] [Google Scholar]
- 26.Girardet J.L., Miner J.N. Vol. 49. Academic press; 2014. Annual reports in Medicinal Chemistry; pp. 158–159. [Google Scholar]
- 27.Preitner F., Pimentel A., Metref S. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int. 2015;87(5):940–947. doi: 10.1038/ki.2014.385. [DOI] [PubMed] [Google Scholar]
- 28.Spitsin S., Markowitz C.E., Zimmerman V., Koprowski H., Hooper D.C. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens. 2010;24(5):359–362. doi: 10.1038/jhh.2009.83. [DOI] [PubMed] [Google Scholar]
- 29.Palmer T.M., Nordestgaard B.G., Benn M. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262. doi: 10.1136/bmj.f4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sedaghat S., Pazoki R., Uitterlinden A.G. Association of uric acid genetic risk score with blood pressure: the Rotterdam study. Hypertension. 2014;64(5):1061–1066. doi: 10.1161/HYPERTENSIONAHA.114.03757. [DOI] [PubMed] [Google Scholar]
- 31.Bardin T., Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. doi: 10.1186/s12916-017-0890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safety of Urate Elevation in Parkinson's Disease (SURE-PD) U.S. National Library of Medicine Web Site. https://clinicaltrials.gov/show/NCT00833690 Updated June 5, 2014. Accessed September 20, 2017.
- 33.Wu X., Wakamiya M., Vaishnav S. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91(2):742–746. doi: 10.1073/pnas.91.2.742. https://www.ncbi.nlm.nih.gov/pubmed/8290593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono H., Chen C.J., Ontiveros F., Rock K.L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120(6):1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thoonen R., Cauwels A., Decaluwe K. Cardiovascular and pharmacological implications of haem-deficient NO-unresponsive soluble guanylate cyclase knock-in mice. Nat Commun. 2015;6 doi: 10.1038/ncomms9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buys E.S., Raher M.J., Kirby A. Genetic modifiers of hypertension in soluble guanylate cyclase alpha1-deficient mice. J Clin Invest. 2012;122(6):2316–2325. doi: 10.1172/JCI60119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdett T.C., Desjardins C.A., Logan R., McFarland N.R., Chen X., Schwarzschild M.A. Efficient determination of purine metabolites in brain tissue and serum by high-performance liquid chromatography with electrochemical and UV detection. Biomed Chromatogr. 2013;27(1):122–129. doi: 10.1002/bmc.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe S., Kang D.H., Feng L. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. https://www.ncbi.nlm.nih.gov/pubmed/12215479 [DOI] [PubMed] [Google Scholar]
- 39.Espay A.J., Lewitt P.A., Hauser R.A., Merola A., Masellis M., Lang A.E. Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15(9):954–966. doi: 10.1016/S1474-4422(16)30079-5. [DOI] [PubMed] [Google Scholar]
- 40.Shirasaka T., Shimamoto Y., Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53(17):4004–4009. https://www.ncbi.nlm.nih.gov/pubmed/7689420 [PubMed] [Google Scholar]
- 41.Yamashita T., Ueda Y., Fuji N. Potassium oxonate, an enzyme inhibitor compounded in S-1, reduces the suppression of antitumor immunity induced by 5-fluorouracil. Cancer Chemother Pharmacol. 2006;58(2):183–188. doi: 10.1007/s00280-005-0150-0. [DOI] [PubMed] [Google Scholar]
- 42.Stavric B., Nera E.A. Use of the uricase-inhibited rat as an animal model in toxicology. Clin Toxicol. 1978;13(1):47–74. doi: 10.3109/15563657808988228. [DOI] [PubMed] [Google Scholar]
- 43.Lu J., Hou X., Yuan X. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018;93(1):69–80. doi: 10.1016/j.kint.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Debosch B.J., Kluth O., Fujiwara H., Schurmann A., Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5 doi: 10.1038/ncomms5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moccia M., Albero R., Lanzillo R. Cardiovascular profile improvement during Natalizumab treatment. Metab Brain Dis. 2018;33(3):981–986. doi: 10.1007/s11011-017-0169-z. [DOI] [PubMed] [Google Scholar]
- 46.Redon P., Maloberti A., Facchetti R. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood pressure control rate and CArdiovascular Risk profilE study. J Hypertens. 2018 doi: 10.1097/HJH.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 47.Park C.E., Sung H.H., Jung E.Y., Moon A.E., Kim H.S., Yoon H. Gender difference in the relationship between uric acid and pulse pressure among Korean adults. Clin Exp Hypertens. 2018:1–6. doi: 10.1080/10641963.2018.1510944. [DOI] [PubMed] [Google Scholar]
- 48.Johnson R.J., Gaucher E.A., Sautin Y.Y., Henderson G.N., Angerhofer A.J., Benner S.A. The planetary biology of ascorbate and uric acid and their relationship with the epidemic of obesity and cardiovascular disease. Med Hypotheses. 2008;71(1):22–31. doi: 10.1016/j.mehy.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda Effect of Febuxostat on Blood Press U.S National Library of Medicine Web Site. https://clinicaltrials.gov/show/NCT01496469 Updated August 31, 2015. Accessed September 10, 2017.
- 50.Tsuruta Y., Kikuchi K., Tsuruta Y. Febuxostat improves endothelial function in hemodialysis patients with hyperuricemia: a randomized controlled study. Hemodial Int. 2015;19(4):514–520. doi: 10.1111/hdi.12313. [DOI] [PubMed] [Google Scholar]
- 51.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Sundstrom J., Sullivan L., D'Agostino R.B., Levy D., Kannel W.B., Vasan R.S. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 53.Schwarzschild MA. Food-Drug interaction study of serum urate after oral inosine. U.S National Library of Medicine Web Site. https://clinicaltrials.gov/show/NCT02614469. Updated March 29, 2017. Accessed August 22, 2017.
- 54.Mazzali M., Kanbay M., Segal M.S. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep. 2010;12(2):108–117. doi: 10.1007/s11926-010-0094-1. [DOI] [PubMed] [Google Scholar]
- 55.Zoccali C., Mallamaci F. Uric acid, hypertension, and cardiovascular and renal complications. Curr Hypertens Rep. 2013;15(6):531–537. doi: 10.1007/s11906-013-0391-y. [DOI] [PubMed] [Google Scholar]
- 56.Kuwabara M. Hyperuricemia, Cardiovascular Disease, and Hypertension. Pulse (Basel) 2016;3(3–4):242–252. doi: 10.1159/000443769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White W.B., Saag K.G., Becker M.A. Cardiovascular Safety of Febuxostat or Allopurinol in patients with Gout. N Engl J Med. 2018;378(13):1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material