Abstract
Background
Gastroenteritis caused by rotavirus accounts for considerable morbidity in young children. We aimed to assess the vaccine effectiveness (VE) of the oral rotavirus vaccine Rotarix, as measured by laboratory-confirmed rotavirus infection after referral to hospital and/or emergency departments in children aged <5 years with gastroenteritis.
Methods
We performed a systematic search for peer-reviewed studies conducted in real-life settings published between 2006 and 2016 and a meta-analysis to calculate the overall Rotarix VE, which was further discriminated through stratified analyses.
Results
The overall VE estimate was 69% (95% confidence interval [CI], 62% to 75%); stratified analyses revealed a non-negligible impact of factors such as study design and socioeconomic status. Depending on the control group, VE ranged from 63% (95% CI, 52% to 72%) to 81% (95% CI, 69% to 88%) for unmatched and matched rotavirus test–negative controls. VE varied with socioeconomic status: 81% (95% CI, 74% to 86%) in high-income countries, 54% (95% CI, 39% to 65%) in upper-middle-income countries, and 63% (95% CI, 50% to 72%) in lower-middle-income countries. Age, rotavirus strain, and disease severity were also shown to impact VE, but to a lesser extent.
Conclusions
This meta-analysis of real-world studies showed that Rotarix is effective in helping to prevent hospitalizations and/or emergency department visits due to rotavirus infection.
Keywords: effectiveness, meta-analysis, Rotarix, rotavirus, systematic literature review
Rotavirus (RV) is the major cause of severe gastroenteritis (GE) diseases, which amount to a considerable burden of disease in children younger than age 5 years [1]. Although a global decline in mortality was observed in the last decades, RV diseases still accounted for an estimated 215 000 deaths in this age group in 2013 [2].
Vaccination is the best preventive approach against RV diseases [1]. The oral live-attenuated human RV vaccine Rotarix (GSK, Belgium) was introduced in routine immunization programs as of 2006. In 2009, the World Health Organization recommended the global implementation of RV vaccination in infants [3]. By June 2017, 85 countries had introduced RV vaccination in their national immunization program (NIP), with an additional 7 countries including it in subnational programs and other countries making the vaccines available for private market use [4, 5].
Rotarix is a 2-dose-shedule oral live-attenuated human RV vaccine, recommended for active immunization against GE due to RV infection (RVGE) in infants aged 6–24 weeks [6]. The 2 doses of Rotarix should be administered at least 4 weeks apart, and the vaccination course must be completed by 24 weeks of age [6]. Rotarix was shown to have a favorable benefit/risk profile in infants and was efficacious against severe GE- or RV-associated hospitalization in several large clinical trials conducted worldwide [7–14], with a vaccine efficacy of 85%–96% demonstrated against these end points [13, 14]. Favorable data from clinical trials were further supported by postlicensure studies conducted over a period of more than 10 years since the introduction of the vaccine in routine immunization programs [5]. Rotarix was shown to provide broad protection against severe RVGE caused by nonvaccine RV strains; that is, efficacy or effectiveness has been demonstrated against 9 different strains [6].
This study evaluated the vaccine effectiveness (VE) of Rotarix, as measured by laboratory-confirmed RV infection after referral to hospitals and/or the emergency department (ED), in children with GE diseases in real-world settings. We conducted a systematic literature review and a meta-analysis of the VE of 2- or 1-dose Rotarix vaccination data published between January 1, 2006, and July 7, 2016.
Figure 1 represents a “Focus on the Patient Section,” which elaborates on the clinical relevance and impact of the study, to be shared with patients by health care professionals.
Figure 1.
Focus on the patient section.
METHODS
Systematic Literature Review
We performed a systematic search of the PubMed and Cochrane databases for peer-reviewed articles published from January 1, 2006, to July 7, 2016, using prespecified terms related to RV vaccines, as detailed in the Supplementary Data. We included papers in any language reporting data from postlicensure or original studies assessing the VE of Rotarix (full inclusion and exclusion criteria are presented in the Supplementary Data).
Relevant references were selected by a 3-step selection procedure. First, titles and abstracts identified through the search were screened based on their relevance to the objectives, with a random sample of 30% of titles and abstracts being screened in duplicate. Second, a full-text review of articles selected during the first step was performed, with the first 10% of the articles being appraised by 2 reviewers. Third, further scrutiny of the articles during the data extraction phase was applied. For example, when 2 included articles described results of the same study, we only included 1 of the articles in the meta-analysis to avoid double inclusion of data (ie, the article published most recently or with the most relevant data). In addition, the reference list of meta-analyses or systematic reviews was checked for relevant articles that could have been missed. The quality of the selected articles was assessed using the Coordination of Cancer Clinical Practice Guidelines (CoCanCPG) [15].
We extracted and summarized the following data as a minimum: study design, setting and period, study objectives, study country and its socioeconomic status (SES; according to the World Bank list of economies classification [16]), type of control group used (matched/unmatched hospital, test-negative, community/neighborhood controls), clinical setting (hospitalizations or ED visits), RV strain type (homotypic, fully/partly heterotypic), disease severity (mild, moderate, severe, and very severe, according to the Vesikari score list [17]), reports on vaccine introduction in the NIP, and vaccination coverage, when available.
Meta-analyses
A meta-analysis was performed to assess the overall VE of Rotarix, as measured by laboratory-confirmed RV infection after referral to hospitals and/or EDs in children with GE under 5 years of age, as reported by observational studies identified by the systematic literature search (see the Supplementary Data for a full list of inclusion criteria).
We estimated the overall VE in children receiving 2 doses of Rotarix (main analysis). The secondary objectives of the meta-analysis were to assess the VE according to the number of Rotarix doses provided (1 or 2), type of controls used in the studies, the SES of the country, RV strain type, age (<1 or ≥1 year), and disease severity. Stratification analyses were carried out on 1 level (age, RV strain, SES, and disease severity) for 1 Rotarix dose and the complete schedule, whereas 2-level stratified analyses were conducted only for 2 doses of Rotarix (by age and SES and by strain and SES). A meta-analysis was performed only when at least 4 VE estimates could be included. To investigate the effect of specific study parameters on the VE, sensitivity analyses were conducted by excluding studies reporting on ED referrals only or primary health care centers, high-income countries and unmatched control groups, or unadjusted data.
Statistical Methods
In performing the analyses, several considerations were predefined for each included study. If VE estimates were presented for multiple control groups, only 1 estimate was selected as follows: hospital controls were preferred above community controls; in case of multiple hospital controls, matched controls were used; and when nonmatched control groups were studied, test-negative controls were preferred. When both crude and covariate-adjusted VE were provided in studies, adjusted estimates were used for the meta-analysis.
VE was defined as the percent reduction in the odds of referral to hospitals and/or EDs due to RVGE disease among vaccinated children compared with unvaccinated children. Meta-analyses were performed on odds ratios (ORs) and 95% confidence intervals (CIs); log(OR) and standard error of log(OR) were computed. If the ORs and 95% CIs were not available in the included studies, these were calculated from the estimates included in the articles using the formula OR = 1 – (VE/100) [18].
The random-effect model (using the DerSimonian-Laird approach) [19] was used for the main model, but the fixed-effect model (using the inverse variance method) [20] was also employed to calculate pooled ORs. The level of study heterogeneity was assessed by computing the Higgins I2 test, along with visual assessment of the funnel plots [21]. I2 values of <25%, 25%–50%, 50%–75%, and >75% were considered very low, low, medium, and high heterogeneity, respectively [22].
Publication bias was investigated for the overall 1- and 2-dose VE analyses, by visual assessment of funnel plots, and by Egger’s weighted regression test, with a 2-sided P value of <.10 considered significant [23].
All analyses were performed without any adjustment for multiplicity using STATA v13.1.
RESULTS
Systematic Literature Review
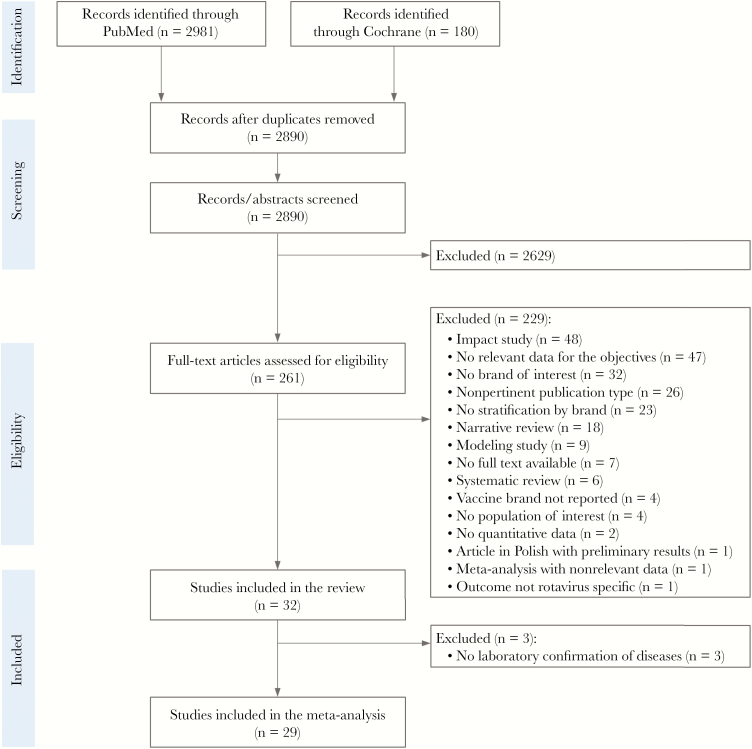
After removal of duplicates, the search strategy yielded 2890 unique records. Following the screening of titles and abstracts, we retained 261 articles for full-text review, from which 32 studies [24–55] were identified as relevant for the assessed outcomes. Figure 2 gives a schematic overview of the selection procedure used. Study characteristics are summarized in Table 1.
Figure 2.
PRISMA flowchart.
Table 1.
Characteristics of Studies Identified Through the Systematic Search
| Country/Region (SES) | NIP Introductiona VC, % |
Study Setting | Outcome | Study Design | Study Period |
Age at Study Enrollment | Case confirmation | |
|---|---|---|---|---|---|---|---|---|
| Studies included in the meta-analysis | ||||||||
| Armah et al. 2016 [24] | Ghana (LMIC) | NIP since 2012 VC: >90% |
Hospital and ED | AGE RV | Case-control (test-negative) | Jan 2013–Dec 2014 | 6wk–5y | EIA |
| Bar-Zeev et al. 2015 [26] | Malawi (LIC) | NIP since Oct 2012 VC: 74.6% (2013), 92.4% (2014), 95.1% (2015) |
ED | RV AGE | Matched case-control (matched community control, unmatched for test-negative control) | Oct 2012–Jun 2014 | ≥6wk–<5y | ELISA |
| Bar-Zeev et al. 2016 [25] | Malawi (LIC) | NIP since Oct 2012 VC: 74.6% (2013), 92.4% (2014), 95.1% (2015) |
Hospital and ED | Acute RV diarrhea | Case-control (test-negative) | Oct 2012–Jun 2015 | <5y | EIA + PCR |
| Beres et al. 2016 [27] | Zambia (LMIC) | NIP since 2013 VC: 58%–70% |
Hospitalb | Moderate to severe RV diarrhea | Case-control (test-negative) | Jul 2012–Oct 2013 | 0–59mo | EIA |
| Braeckman et al. 2012 [41] | Belgium (HIC) | NIP since Oct 2006 VC: 90% |
Hospitalb | RV GE | Matched case-control (hospital control) | Feb 2008–Jun 2010 | 14wk–39mo | Rapid test |
| Castilla et al. 2012 [42] | Spain/Navarre (HIC) | NIP since Aug 2006 but suspended in Mar 2010 VC: NR |
Health care databasec | RV GE | Nested case-control (test-negative) | Jan 2008–Jun 2011 | 3–59mo | ICA |
| Chang et al. 2014 [47] | Taiwan (HIC) | Private market since Aug 2006 VC: 24%–28% in controls |
Hospitalb | RV AGE | Matched case-control (test-negative and hospital control) | May 2009–Apr 2011 | 8–35mo | EIA |
| Correia et al. 2010 [32] | Brazil (UMIC) | NIP since Mar 2006 VC: >50% |
Hospital and ED | Severe RV diarrhea | Unmatched case-control (test-negative and hospital control) | Mar 2006–Sep 2008 | 6mo–5y | ELISA |
| Cortese et al. 2013 [48] | US (HIC) | NIP since 2008 VC: NR |
Hospital and ED | RV AGE | Matched case-control (matched test-negative IIS restricted control; unmatched for test-negative control) | Jan 2010–Jun 2011 | ≥56d–23mo | EIA |
| Cotes-Cantillo et al. 2014 [33] | Colombia (UMIC) | NIP since Jan 2009 VC: 94% |
ED | RV diarrhea | Case-control (unmatched test-negative) | Jan 2011–Jan 2013 | ≥8wk–4y | Rapid test + ELISA |
| de Palma et al. 2010 [34] | El Salvador (LMIC) | NIP since Oct 2006 VC estimated at 50% |
Hospitalb | RV diarrhea | Matched case-control (neighborhood control) | Jan 2007–Jun 2009 | <5y | EIA |
| Doll et al. 2015 [49] | Canada/Quebec (HIC) | NIP since Nov 2011 VC: 0% (2012), 90% (2014) |
Hospital and ED | Acute diarrhea RV | Matched case-control (test-negative optimal matching based upon symptom onset) | Feb 2012–May 2014 | 8wk–3y | EIA+PCR |
| Gastanaduy et al. 2016a [35] | Guatemala (LMIC) | NIP since July 2010 VC estimated at 50% |
Hospital and ED | RV diarrhea | Matched case-control (hospital control matched and unmatched test-negative control) | Jan 2012–Aug 2013 | 2–46mo | EIA |
| Gastanaduy et al. 2016b [29] | Botswana (UMIC) | NIP since July 2012 VC estimated at 70% |
Hospital and ED | RV diarrhea | Case-control (test-negative) | Jun 2013–Apr 2015 | ≥4mo–<5y | EIA |
| Gheorghita et al. 2016 [43] | Moldova (LMIC) | NIP since July 2012 VC: 20% (2012), 40% (2013) |
Hospital and ED | Acute RV diarrhea | Case-control (test-negative) | Oct 2012–July 2013 | 6mo–5y | EIA |
| Groome et al. 2014 [28] | South Africa (UMIC) | NIP since Aug 2009 VC: 96% |
Hospitalb | Acute diarrhea | Case-control (not matched, test-negative, and hospital) | Apr 2010–Oct 2012 | 18wk–23mo | ELISA |
| Ichihara et al. 2014 [36] | Brazil (UMIC) | NIP since Mar 2006 VC estimated at 70% |
Hospitalb | Acute diarrhea | Matched case-control (hospital control) | July 2008–Aug 2011 | 4–24mo | EIA+PAGE |
| Immergluck et al. 2016 [50] | US, Georgia (HIC) | NIP since 2008 in the US, since 2009 in Georgia VC: NR |
Hospital and ED | RV diarrhea care | Case-control (test-negative) | Jan 2013–Jun 2013 | ≥56d–4y | EIA |
| Justino et al. 2011 [37] | Brazil, Belem (UMIC) | NIP since Mar 2006 VC: estimated 52%–69% |
Hospitalb | Severe RV GE | Matched case-control (hospital and neighborhood control) | May 2008–May 2009 | ≥12wk–5y | ELISA |
| Marlow et al. 2015 [44] | Portugal (HIC) | Private market since 2006 VC: 30% |
ED | RV AGE | Matched case-control (test-negative) | Jan 2007–Jun 2012 | 8wk–36mo | Rapid test |
| Matthijnssen et al. 2014 [45] | Belgium (HIC) | NIP since Oct 2006 VC: 90% |
Hospitalb | RV GE | Matched case-control (hospital control) | Feb 2008–Jun 2010 | 14wk–39mo | PCR |
| Patel et al. 2013 [38] | Bolivia (LMIC) | NIP since Aug 2008 VC: 80% |
Hospital and ED | RV diarrhea | Matched case-control (matched hospital control and unmatched for test-negative control) | Mar 2010–Jun 2011 | 8wk–36mo | ELISA |
| Payne et al. 2013 [51] | US (HIC) | NIP since 2008 VC: low |
Hospital and ED; separate estimates available | RV AGE | Matched case-control (test-negative) | Nov 2009–Jun 2011 | <5y | EIA |
| Payne et al. 2015 [52] | US (HIC) | NIP since 2008 VC: low |
Hospital and ED; separate estimates available | RV AGE | Case-control (test-negative) | Dec 2011–Nov 2013 | ≥8mo–8yd | EIA+PCR |
| Pringle et al. 2016 [39] | Bolivia (LMIC) | NIP since Aug 2008 VC: NR |
Hospital and ED | RV AGE | Case-control (test-negative) | Apr 2013–Mar 2014 | 2–59mo | EIA |
| Sahakyan et al. 2016 [46] | Armenia (LMIC) | NIP since Nov 2012 VC: 16% (Jan 2013), 57% (Jan 2013), 77% (Jan 2015) |
Hospitalb | RV AGE | Case-control (test-negative) | Nov 2012–Jun 2015 | 0–59mo | EIA |
| Snelling et al. 2009 [30] | Central Australia (HIC) | NIP since late 2006 VC: partial/low |
Hospitalb | AGE | Matched case-control (community control) | Mar 2007–Jul 2007 | 10wk–5y | Immunoassay |
| Snelling et al. 2011 [31] | Central Australia (HIC) | NIP since late 2006 VC: partial/low |
Hospitalb | GE | Nested and matched case-control (matched risk-cohort control; unmatched hospital control) | Sep 2008–Jun 2009 | 6wk–36mo | ICA |
| Yen et al. 2011 [40] | Mexico (UMIC) | NIP since 2006–2007 VC: 70% (age <5 y) |
Hospitalb | Severe GE | Matched case-control (community control) | Mar 2010–May 2010 | 15d–<2y | RNA extraction, electrophoresis |
| Studies excluded from the meta-analysis | ||||||||
| Gosselin et al. 2016 [53] | Canada/Quebec (HIC) | NIP since 2011 VC: 13.6% (Jan 2012), 85.9% (2014) |
Health care databasec | RV AGE and RVGE | Retrospective cohort (3 cohorts: vaccinated, unvaccinated, historical vaccinated [Aug 2008–Dec 2010]) | Aug 2011–Dec 2013 | <3y | ICD-10 diagnosis |
| Muhsen et al. 2010 [54] | Macabi, Israel (HIC) | Private market since 2007 VC: 55% |
Health care databasec | RV AGE | Incidence rate ratio (incidence rate in vaccinated individuals/incidence rate in nonvaccinated individuals) | Sep 2008–Jan 2009 | <12mo | ICD-9 diagnosis |
| Perez-Vilar et al. 2015 [55] | Spain, Navarre region (HIC) | NIP since Aug 2006, suspended in Mar 2010 | Health care databasec | RV disease | Retrospective cohort | Jan 2007–Jun 2012 | <3y | ICD-10 diagnosis |
Abbreviations: AGE, acute gastroenteritis; ED, emergency department; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; GE, gastroenteritis; HIC, high-income country; ICA, immuno-chromatographic assay; ICD-9, International Classification of Diseases, 9th Revision; ICD-10, ICD, 10th Revision; IIS, immunization information system; LIC, low-income country; LMIC, lower-middle-income country; NIP, national immunization program; NR, not reported; PAGE, polyacrylamide gel electrophoresis; PCR, polymerase chain reaction; RNA, ribonucleic acid; RV, rotavirus; SES, socioeconomic status; UMIC, upper middle-income country; VC, vaccination coverage.
aFor countries where Rotarix was not included in the NIP, the date of its introduction on the private market was provided.
bNot clear if emergency departments were included.
cIncludes hospital, ED setting, and primary health care centers.
dEstimates of vaccine effectiveness for Rotarix were obtained in children ≤62 months of age.
Meta-analysis
The meta-analysis included 29 studies (Figure 2). For the 3 studies excluded [53–55], RV disease was only confirmed based on International Classification of Diseases codes and/or electronic medical records and was not based on laboratory results (Table 1).
Characteristics of Selected Studies
Among the 29 studies included in the meta-analysis, 6 were conducted in African countries [24–29], 2 in Central Australia [30, 31], 9 in countries or regions from Latin America [32–40], 6 in Europe [41–46], 1 in Asia [47], and 5 in North America [48–52]. Most of them (27) were retrospective case-control studies conducted in hospital settings, and 2 were prospective case-control studies using electronic medical records from health care facilities [29, 41]. In all 29 studies, RV diseases requiring hospitalization or ED visits were assessed using robust laboratory testing to confirm the RV disease status. Based on the World Bank classification of economies, 2 studies were conducted in low-income countries [25, 26], 15 in lower and upper-middle-income countries, and 12 in high-income countries. Ten of the 29 studies presented results for more than 1 virus strain (Table 1).
All 29 studies included in the meta-analysis were case-control studies and fulfilled the quality criteria of the CoCanCPG checklist.
Meta-analysis of the Effectiveness of Rotarix Vaccine
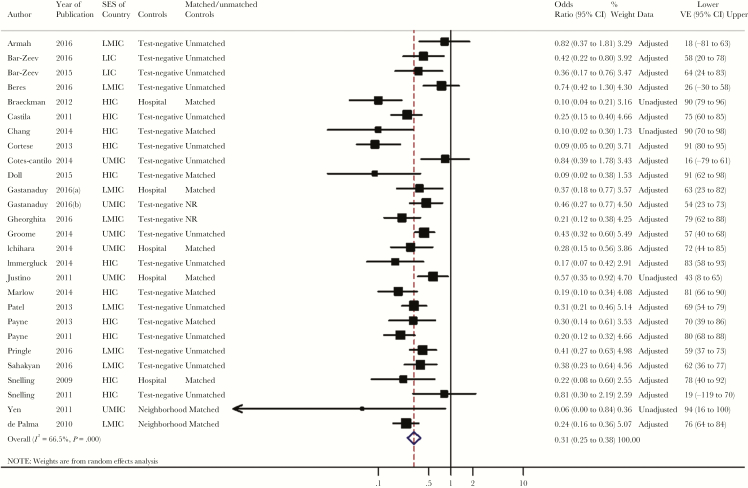
The main analysis included 27 studies evaluating the overall VE of 2 doses of Rotarix. Two [32, 45] of the 29 studies identified by the systematic search were excluded from the main analysis, as only stratified data were presented without reporting overall results. The reported or calculated ORs per study ranged from 0.06 to 0.84. The overall VE estimate was 69% (95% CI, 62% to 75%), with medium heterogeneity (I2 = 67%; 95% CI, 50% to 78%) observed between studies (Table 2, Figure 3). Among the 12 studies included in the secondary analysis for 1 dose of Rotarix, the pooled VE was 46% (95% CI, 34% to 57%) with an I2 of 34% (95% CI, 0% to 67%), showing low between-study heterogeneity (Supplementary Figure 1).
Table 2.
Overview of Overall Odds Ratios and Vaccine Effectiveness Against Laboratory-Confirmed Rotavirus Infection After Hospital and/or Emergency Department Visits for Rotarix Vaccination Resulting From Meta-analysisa
| Analyses | No. | OR (95% CI) | I 2 (95% CI) |
P Value (Cochrane Q-Test) |
VE (95% CI)b | |
|---|---|---|---|---|---|---|
| RE Model | FE Model | |||||
| Primary analysis | ||||||
| 2 doses | 27 | 0.31 (0.25–0.38) | 0.33 (0.29–0.37) | 67 (50–78) | <.001 | 69 (62–75) |
| 1 dose | 12 | 0.54 (0.43–0.66) | 0.57 (0.49–0.66) | 34 (0–67) | .115 | 46 (34–57) |
| Secondary analyses, 2 doses | ||||||
| Hospital controls, 2 doses, matched | 9 | 0.24 (0.15–0.36) | 0.26 (0.22–0.35) | 62 (22–82) | .007 | 76 (64–85) |
| Test-negative matched controls | 4 | 0.19 (0.12–0.31) | 0.20 (0.13–0.30) | 10 (0–86) | .345 | 81 (69–88) |
| Other hospital matched controls | 5 | 0.28 (0.16–0.51) | 0.28 (0.22–0.36) | 71 (27–89) | .008 | 72 (49–84) |
| Hospital controls, 2 doses, unmatched | 14 | 0.37 (0.28–0.48) | 0.36 (0.31–0.42) | 72 (51–83) | <.001 | 63 (52–72) |
| Test-negative unmatched controls | 14 | 0.37 (0.28–0.48) | 0.36 (0.31–0.42) | 72 (51–83) | <.001 | 63 (52–72) |
| Neighborhood/community controls, 2 doses | 5 | 0.32 (0.20–0.49) | 0.29 (0.22–0.39) | 42 (0–79) | .140 | 68 (51–80) |
| Matched controls | 5 | 0.32 (0.20–0.49) | 0.29 (0.22–0.39) | 42 (0–79) | .140 | 68 (51–80) |
| Stratified analyses | ||||||
| 2 doses, LMIC | 8 | 0.37 (0.28–0.50) | 0.35 (0.30–0.42) | 62 (18–82) | .010 | 63 (50–72) |
| 2 doses, UMIC | 6 | 0.46 (0.35–0.61) | 0.46 (0.37–0.57) | 28 (0–70) | .225 | 54 (39–65) |
| 2 doses, HIC | 11 | 0.19 (0.14–0.26) | 0.19 (0.16–0.24) | 49 (0–75) | .033 | 81 (74–86) |
| 1 dose, LMIC | 5 | 0.63 (0.52–0.77) | 0.63 (0.52–0.77) | 0 (0–79) | .454 | 37 (23–48) |
| 1 dose, UMIC | 4 | 0.52 (0.40–0.67) | 0.52 (0.40–0.67) | 0 (0–85) | .506 | 48 (33–60) |
| 2 doses, high severity | 14 | 0.36 (0.26–0.50) | 0.35 (0.29–0.42) | 67 (41–81) | <.001 | 64 (50–74) |
| 2 doses, very high severity | 9 | 0.40 (0.26–0.62) | 0.37 (0.26–0.53) | 30 (0–68) | .177 | 60 (38–74) |
| 1 dose, high severity | 6 | 0.62 (0.46–0.84) | 0.62 (0.46–0.84) | 0 (0–75) | .577 | 38 (16–54) |
| 1 dose, very high severity | 4 | 0.70 (0.38–1.28) | 0.73 (0.43–1.23) | 20 (0–88) | .289 | 30 (-28–62) |
| 2 doses, homotypic strain | 5 | 0.11 (0.07–0.18) | 0.11 (0.07–0.18) | 0 (0–79) | .728 | 89 (82–93) |
| 2 doses, partly heterotypic strain | 12 | 0.28 (0.22–0.35) | 0.28 (0.22–0.35) | 0 (0–58) | .902 | 72 (65–78) |
| 2 doses, fully heterotypic strains | 15 | 0.35 (0.26–0.46) | 0.38 (0.23–0.45) | 53 (16–74) | .007 | 65 (54–74) |
| 2 doses, strains unspecified | 10 | 0.36 (0.28–0.45) | 0.36 (0.28–0.45) | 0 (0–69) | .682 | 64 (55–72) |
| 2 doses, age <1 y | 13 | 0.30 (0.23–0.40) | 0.33 (0.27–0.40) | 33 (0–65) | .118 | 70 (60–77) |
| 2 doses, age ≥1 y | 13 | 0.42 (0.29–0.61) | 0.44 (0.36–0.54) | 70 (46–83) | <.001 | 58 (39–71) |
| Sensitivity analyses | ||||||
| 2 doses, excluding referral to ED only or primary healthcare centers | 23 | 0.31 (0.24–0.39) | 0.33 (0.29–0.37) | 67 (50–79) | <0.001 | 69 (61–76) |
| 2 doses, excluding HIC countries | 16 | 0.40 (0.33–0.49) | 0.39 (0.35–0.45) | 48 (7–71) | .016 | 60 (51–67) |
| 1 dose, excluding HIC countries | 9 | 0.58 (0.50–0.68) | 0.58 (0.50–0.68) | 0 (0–65) | .488 | 42 (32–50) |
| 2 doses, excluding unmatched controls or unclear matching process | 11 | 0.24 (0.17–0.34) | 0.27 (0.21–0.33) | 55 (11–77) | .014 | 76 (66–83) |
| 1 dose, excluding unmatched controls or unclear matching process | 4 | 0.44 (0.33–0.60) | 0.44 (0.33–0.60) | 0 (0–85) | .868 | 56 (40–67) |
Abbreviations: CI, confidence interval; ED, emergency department; FE, fixed effect; HIC, high-income country; LMIC, lower-middle-income country; No., number of studies/subgroups included in the analyses; OR, odds ratio; RE, random effect; UMIC, upper-middle-income country; VE, vaccine effectiveness.
aPlanned analyses for which an insufficient number of articles were identified were not performed (secondary analyses: hospital controls, 2 doses, unmatched/other hospital controls; neighborhood/community controls, 2 doses, unmatched controls; 2 doses, database controls; stratified by SES: 2 doses, low-income countries; 1 dose, HIC; stratified by disease severity: 2 doses, mild severity; 1 dose, mild severity; 1 dose, moderate severity; stratified by strain: all analyses for 1-dose VE; stratified by age: all analyses for 1-dose VE).
bCalculated using the RE model.
Figure 3.
Estimated pooled vaccine effectiveness for 2 doses of Rotarix against laboratory-confirmed rotavirus infection after hospital and/or emergency department visits. Abbreviations: CI, confidence interval; HIC, high-income country; LIC, low-income country; LMIC, lower-middle-income country; NR, not reported; SES, socioeconomic status; UMIC, upper-middle-income country; VE, vaccine effectiveness.
VE for 2 doses of Rotarix was 81% (95% CI, 74% to 86%), 54% (95% CI, 39% to 65%), and 63% (95% CI, 50% to 72%) in high-, upper-middle-, and lower-middle-income countries, respectively. One-dose VE also varied slightly with the SES of the countries, from 48% (95% CI, 33% to 60%) for upper-middle-income to 37% (95% CI, 23% to 48%) for lower-middle-income countries (Table 2). In a stratified analysis by type of control, the pooled 2-dose VE of Rotarix varied between 81% (95% CI, 69% to 88%) for matched and 63% (95% CI, 52% to 72%) for unmatched RV test–negative controls (Table 2).
When the analysis was performed by strain type, the estimated 2-dose VE of Rotarix was 89% (95% CI, 82% to 93%), 72% (95% CI, 65% to 78%), and 65% (95% CI, 54% to 74%) for the G1P [8] genotype, partially heterotypic strains, and fully heterotypic strains, respectively (Table 2). Stratified analysis by age groups showed higher 2-dose VE point estimates in children aged <1 year (70%; 95% CI, 60% to 77%) than in those ≥1 year of age (58%; 95% CI, 39% to 71%) (Table 2; Supplementary Figure 2). Two-dose VEs for high and very high disease severity were 64% (95% CI, 50% to 74%) and 60% (95% CI, 38% to 74%), respectively; 1-dose VEs for high and very high disease severity were 38% (95% CI, 16% to 54%) and 30% (95% CI, –28% to 62%), respectively.
Stratified analyses on 2 levels performed for the 2-dose VE confirmed a higher VE in children aged <1 year than in those ≥1 year of age, in both upper-middle- and lower-middle-income countries (Supplementary Table 1).
When excluding data from studies conducted only in ED settings or primary health care centers (not in hospital settings), sensitivity analyses yielded a 2-dose VE of 69% (95% CI, 61% to 76%), similar to that obtained from the main analysis. When omitting data from high-income countries, VE estimates were found to be in the same ranges as those obtained for the main analysis (ie, 60%; 95% CI, 51% to 67%; and 42%; 95% CI, 32% to 50%; for 2 doses and 1 dose, respectively). Higher VE estimates for both 2 doses and 1 dose of Rotarix were observed when excluding unmatched controls or studies where the matching process was not clear (Table 2).
Funnel plots assessing publication bias are presented in Supplementary Figure 3. Egger’s regression test showed no significant funnel plot asymmetry for the 2-dose VE analysis (calculated coefficient of –1.23; 95% CI, –3.08 to 0.63; P = .19), whereas significant asymmetry was shown by the coefficient for the 1-dose VE (–1.46; 95% CI, –3.73 to –0.19; P = .03).
DISCUSSION
This meta-analysis of the VE of the Rotarix vaccine includes peer-reviewed data publicly available on its use in real-world settings over a period of 10 years. Although several meta-analyses assessing Rotarix effectiveness against various end points have already been published [5, 56–58], we included worldwide data and performed subgroup analyses, thus providing an exhaustive view on the VE of Rotarix against RVGE-related hospitalizations or ED visits. Our systematic review and meta-analysis showed that programmatic use of Rotarix prevents hospital admission or ED visits due to RVGE in children under 5 years of age.
We analyzed data from 29 studies assessing postlicensure VE, conducted in various geographical regions, and covering the entire range of SES. Most of the studies included in the review were undertaken in countries where Rotarix is implemented in the NIP. Studies from Taiwan and Portugal, where the vaccine is only available on the private market, were also included.
The meta-analysis showed that a 2-dose schedule of Rotarix provided considerable prevention of RV disease–related hospitalizations or referrals to ED, with a pooled VE estimate of 69%, but increasing to up to 81% in high-income countries. The estimated VE for 1 dose was 46%, indicating that partial reduction of hospitalization is also provided by 1 dose of Rotarix. However, our results highlight the importance of a full vaccination schedule.
Overall VE values obtained from stratified analyses were the highest in high-income countries. This is consistent with results from clinical trials, showing lower vaccine efficacy in African countries [11] than in industrialized countries [13]. Lower VE in low-income countries was also noted for other live oral vaccines and has been attributed to differences between countries of different SES in breastfeeding practices, micronutrient malnutrition, gut flora, RV epidemiology, and underlying medical conditions [59].
Because case-control study design is a widely used method to assess VE, only this type of study was included in our meta-analysis. In addition, to ensure high specificity of the outcome and avoid any potential misclassification, only studies with laboratory-confirmed RVGE diagnosis were considered when conducting the meta-analysis. The type of controls used was previously shown to impact VE to some extent, as observed in a review performed to assess VE of RV vaccines in Latin America [56]. In our study, we considered all types of controls, in line with other meta-analyses performed for RV vaccines [5, 56, 57] and selected the control groups according to a prespecified selection method. Only peer-reviewed studies that were checked for quality before inclusion in the meta-analysis were used; therefore, an adequate homogeneity between cases and controls in terms of exposure to the disease was assumed. Of the selected studies, when available, we preferentially included in the meta-analysis those reporting estimates based on hospital controls over community controls and studies using matched controls to limit bias due to any potential confounders. To assess the choice of controls on the overall VE estimate, we also performed subgroup analyses by type of control. Similar to previous meta-analyses [56], we evidenced a relationship between VE and the type of controls used, as studies using hospital matched controls yielded higher VE of the 2-dose schedule of Rotarix than those with unmatched or neighborhood/community controls, with values varying from 81% to 63%. Furthermore, RV test–negative control types are highly specific for non-RV diseases and thereby increase the robustness of the estimate. The overall VE of 2 Rotarix doses obtained using matched RV test–negative controls was 81%, but only 4 studies could be included in the analysis.
Vaccine efficacy as assessed in clinical trials was previously shown to increase with the severity of the disease [13], a finding partially confirmed in real-world settings [57]. In our analysis, Rotarix VE was similar against disease of high and very high severity regardless of the number of doses but could not be estimated against mild and moderately severe disease due to the lack of data.
VE against RVGE-related hospitalizations or ED visits varied between 64% and 89% with the type of RV strain, in line with efficacy results observed in clinical trials that demonstrated broad protection against severe RVGE by different RV types [10, 14]. The highest VE estimates were found for homotypic strains and confirm the high protective effect of Rotarix against fully homotypic strains. A higher point estimate for VE was observed in children aged <1 year compared with children aged ≥1 year. Nevertheless, lower odds of RV-related hospitalizations or referrals to ED were still observed in our study in vaccinated vs unvaccinated children ≥1 year of age.
A rigorous quality control procedure has been applied for both the systematic review and the meta-analysis. Additional analyses investigating the publication bias have been performed. The study’s main strength was the inclusion of robust case-control studies using laboratory confirmation of RV status. To minimize the risk of bias, adjusted results were preferentially included in the analysis. Nevertheless, although the case-control design allowed for stratified meta-analyses performed by type of control, this type of study also presents a certain risk of bias, as it relies on retrospectively assessed chart-based data and thorough documentation of vaccination history that might not have been correctly reported.
The study also has several limitations. Although covering various geographical settings, the data used in the meta-analysis originated from only 21 countries, with a small amount of data from low-income countries. Similarly, a relatively small number of studies reported strain-specific data. For certain subgroups, only a limited number of studies could be included in the analyses, or no meta-analyses could be performed due to insufficient data. The funnel plots suggest publication bias for some small studies with high OR (or low VE). However, as these studies had very low weights, their impact on the overall estimates is low.
Importantly, as previously discussed in relation with postlicensure studies [60], values derived from case-control studies can only provide information on the direct effectiveness of a vaccine and do not fully account for indirect protection afforded by herd effect, following the implementation of a vaccination program [60]. Given that a disease reduction of up to 75% was estimated in age groups that were not vaccine-eligible in countries with national RV immunization programs [61–63], VE values from our study are likely to underestimate the true global impact of Rotarix vaccination.
CONCLUSIONS
This meta-analysis provides strong evidence that vaccination with 2 doses of Rotarix has a substantial preventive effect against hospitalizations and ED visits due to RVGE, further confirming the effectiveness of Rotarix in children younger than age 5 years across various geographic and economic settings.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors are indebted to Leentje Moerman and Bernd Benninghoff for providing valuable contributions to the design and/or the interpretation of the results.
The authors would like to thank Petronela M. Petrar and Urszula Miecielica (XPE Pharma and Science c/o GSK) for medical writing assistance and Emmanuelle Ghys (XPE Pharma and Science c/o GSK) for editorial support and publication coordination on behalf of GSK.
Author contributions. C.W. and D.R. were involved in the study conception. C.W., N.P., and A.O.S. performed the systematic literature search. C.W., K.K., R.v.H., and M.V.N.S. contributed to the data extraction, and R.V.H. and M.V.N.S. conducted the meta-analysis. All authors contributed to interpretation of the data and critical review of the paper for important intellectual content.
Financial support. This work was supported by GlaxoSmithKline Biologicals S.A. GlaxoSmithKline Biologicals S.A. also supported all costs associated with the development and publication of this manuscript.
Trademark statement. Rotarix is a trademark owned by the GSK group of companies. RotaTeq is a registered trademark of Merck&Co., Inc.
Potential conflicts of interest. C.W., N.P., and D.R. are employed by the GSK group of companies. N.P. and D.R. have unrestricted GSK shares. E.G. worked as a consultant for the GSK group of companies at the time the study was conducted and is now an employee of the GSK group of companies. A.O.S., M.V.N.S., K.K., and R.v.H. are employees of Pallas, a commercial entity that has received grants from the GSK group of companies and that carried out the submitted work as a supplier to GSK; Pallas also received grants from Sanofi Pasteur and Daiichy Sankyo outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tate JE, Patel MM, Steele AD, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9:395–407. [DOI] [PubMed] [Google Scholar]
- 2. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62(Suppl 2):S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49–64. [PubMed] [Google Scholar]
- 4. International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub global vaccine introduction and implementation. 2017 https://www.jhsph.edu/research/centers-and-institutes/ivac/resources/IVAC_VIEW-hub_Report%202017Jun.pdf. Accessed 10 November 2017.
- 5. Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global post-licensure data, 2006–2016. Clin Infect Dis 2017; 65:840–50. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency. Summary of product characteristics. Rotarix 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000639/WC500054789.pdf. Accesssed 22 October 2017.
- 7. Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30(Suppl 1):A36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawamura N, Tokoeda Y, Oshima M, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335–41. [DOI] [PubMed] [Google Scholar]
- 9. Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother 2014; 10:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linhares AC, Velázquez FR, Pérez-Schael I, et al. ; Human Rotavirus Vaccine Study Group Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181–9. [DOI] [PubMed] [Google Scholar]
- 11. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 12. Phua KB, Lim FS, Lau YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine 2012; 30:4552–7. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. ; Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 14. Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757–63. [DOI] [PubMed] [Google Scholar]
- 15. Fervers B, Remy-Stockinger M, Mazeau-Woynar V, et al. CoCanCPG. Coordination of cancer clinical practice in Europe. Tumori 2008; 94:154–9. [DOI] [PubMed] [Google Scholar]
- 16. World Bank list of economies. 2016. http://www.google.be/url?url=http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS&rct=j&frm=1&q=&esrc=s&sa=U&ved=0ahUKEwjDiZ6_yI7PAhVCtBQKHXPVBmQQFggUMAA&sig2=BlToa6MCoZ-3AR4C8eDqQQ&usg=AFQjCNHDdewhv68_lyv7Lp_cP3BoRwRnqw. Accessed 15 June 2017.
- 17. Lewis K. Vesikary Clinical Severity Scoring System Manual. Version 1.3. 2011 https://www.path.org/publications/files/VAD_vesikari_scoring_manual.pdf. Accessed 10 July 2017.
- 18. Centers for Disease Control and Prevention. Principles of epidemiology in public health practice. An introduction to applied epidemiology and biostatistics. Lesson 3: Measures of risk. 2012 https://www.cdc.gov/ophss/csels/dsepd/ss1978/ss1978.pdf. Accessed 10 July 2017.
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 20. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–48. [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?Psychol Methods 2006; 11:193–206. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis 2016; 62(Suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bar-Zeev N, Jere KC, Bennett A, et al. ; Vaccine Effectiveness and Disease Surveillance Programme, Malawi (VACSURV) Consortium Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis 2016; 62(Suppl 2):S213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bar-Zeev N, Kapanda L, Tate JE, et al. ; VacSurv Consortium Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015; 15:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis 2016; 62(Suppl 2):S175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014; 14:1096–104. [DOI] [PubMed] [Google Scholar]
- 29. Gastañaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis 2016; 62(Suppl 2):S161–7. [DOI] [PubMed] [Google Scholar]
- 30. Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 2009; 49:428–31. [DOI] [PubMed] [Google Scholar]
- 31. Snelling TL, Andrews RM, Kirkwood CD, et al. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis 2011; 52:191–9. [DOI] [PubMed] [Google Scholar]
- 32. Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010; 201:363–9. [DOI] [PubMed] [Google Scholar]
- 33. Cotes-Cantillo K, Paternina-Caicedo A, Coronell-Rodríguez W, et al. Effectiveness of the monovalent rotavirus vaccine in Colombia: a case-control study. Vaccine 2014; 32:3035–40. [DOI] [PubMed] [Google Scholar]
- 34. de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gastañaduy PA, Contreras-Roldán I, Bernart C, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Guatemala. Clin Infect Dis 2016; 62(Suppl 2):S121–6. [DOI] [PubMed] [Google Scholar]
- 36. Ichihara MY, Rodrigues LC, Teles Santos CA, et al. Effectiveness of rotavirus vaccine against hospitalized rotavirus diarrhea: a case-control study. Vaccine 2014; 32:2740–7. [DOI] [PubMed] [Google Scholar]
- 37. Justino MC, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J 2011; 30:396–401. [DOI] [PubMed] [Google Scholar]
- 38. Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pringle KD, Patzi M, Tate JE, et al. Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013–2014. Clin Infect Dis 2016; 62(Suppl 2):S115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yen C, Figueroa JR, Uribe ES, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis 2011; 204:783–6. [DOI] [PubMed] [Google Scholar]
- 41. Braeckman T, Van Herck K, Meyer N, et al. ; RotaBel Study Group Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 2012; 345:e4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castilla J, Beristain X, Martínez-Artola V, et al. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine 2012; 30:539–43. [DOI] [PubMed] [Google Scholar]
- 43. Gheorghita S, Birca L, Donos A, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the republic of Moldova. Clin Infect Dis 2016; 62(Suppl 2):S140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marlow R, Ferreira M, Cordeiro E, et al. Case control study of rotavirus vaccine effectiveness in Portugal during 6 years of private market use. Pediatr Infect Dis J 2015; 34:509–12. [DOI] [PubMed] [Google Scholar]
- 45. Matthijnssens J, Zeller M, Heylen E, et al. ; RotaBel study group Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect 2014; 20:O702–10. [DOI] [PubMed] [Google Scholar]
- 46. Sahakyan G, Grigoryan S, Wasley A, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis 2016; 62(Suppl 2):S147–54. [DOI] [PubMed] [Google Scholar]
- 47. Chang WC, Yen C, Wu FT, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J 2014; 33:e81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132:e25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doll MK, Buckeridge DL, Morrison KT, et al. Effectiveness of monovalent rotavirus vaccine in a high-income, predominant-use setting. Vaccine 2015; 33:7307–14. [DOI] [PubMed] [Google Scholar]
- 50. Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr 2016; 172:116–20.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis 2013; 57:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis 2015; 61:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gosselin V, Généreux M, Gagneur A, Petit G. Effectiveness of rotavirus vaccine in preventing severe gastroenteritis in young children according to socioeconomic status. Hum Vaccin Immunother 2016; 12:2572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muhsen K, Chodick G, Goren S, et al. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine 2010; 29:91–4. [DOI] [PubMed] [Google Scholar]
- 55. Pérez-Vilar S, Díez-Domingo J, López-Lacort M, et al. Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia region, Spain. BMC Infect Dis 2015; 15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Oliveira LH, Camacho LA, Coutinho ES, et al. Rotavirus vaccine effectiveness in Latin American and Caribbean countries: a systematic review and meta-analysis. Vaccine 2015; 33(Suppl 1):A248–54. [DOI] [PubMed] [Google Scholar]
- 57. Lamberti LM, Ashraf S, Walker CL, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatr Infect Dis J 2016; 35:992–8. [DOI] [PubMed] [Google Scholar]
- 58. Santos VS, Marques DP, Martins-Filho PR, et al. Effectiveness of rotavirus vaccines against rotavirus infection and hospitalization in Latin America: systematic review and meta-analysis. Infect Dis Poverty 2016; 5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patel M, Shane AL, Parashar UD, et al. Oral rotavirus vaccines: how well will they work where they are needed most?J Infect Dis 2009; 200(Suppl 1):S39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine 2013; 31:5634–42. [DOI] [PubMed] [Google Scholar]
- 61. Atchison C, Collins S, Brown D, et al. ; PHE Rotavirus Surveillance Network Reduction in rotavirus disease due to the infant immunisation programme in England; evidence from national surveillance. J Infect 2015; 71:128–31. [DOI] [PubMed] [Google Scholar]
- 62. Glass RI, Parashar U, Patel M, et al. Rotavirus vaccines: successes and challenges. J Infect 2014; 68(Suppl 1):S9–18. [DOI] [PubMed] [Google Scholar]
- 63. Inns T, Trindall A, Dunling-Hall S, Shankar AG. Introduction of a new rotavirus vaccine: initial results of uptake and impact on laboratory confirmed cases in Anglia and Essex, United Kingdom, July 2015. Hum Vaccin Immunother 2016; 12:1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.