Abstract
Retinoic acid, vitamin A metabolite, plays a role in oocyte development and maturation in different ways including gene expression alteration and/or prohibiting oxidative stress. The objective of this study was to examine the effect of 9-cis-retinoic acid (9-cisRA) on the quality and maturation rate of buffalo oocytes. Cumulus-oocyte complexes (COCs, n = 460) were collected from ovaries of slaughtered buffalos. Varying concentrations of 9-cisRA (0, 5, 50, and 200 nM) were added to the maturation medium, and the following parameters were analyzed: (i) maturation and cleavage rates, (ii) mitochondrial activity and reactive oxygen species (ROS) levels, (iii) expression level of antioxidant-related genes (PRDX1, SOD1, CAT, HOMX1, and GPX4) using RT-qPCR. Maturation rate was significantly improved in 5 nM 9-cisRA oocyte group (95.8%, P < .05) compared to control and other treatment groups (86.7% in control group). The same oocyte group exhibited significantly higher mitochondrial membrane potential activity and lower ROS accumulation level compared to other treatment groups. Antioxidant-related genes were up-regulated in oocytes matured with 5 or 50 nM 9-cisRA compared to control and 200 nM 9-cisRA groups. In contrast, 200 nM of 9-cisRA showed a clear down-regulation for antioxidant-related genes except for PRDX1. In conclusion, supplementation of 9-cisRA with a lower concentration (5 nM) to the buffalo oocytes maturation media promotes maturation rate through a protection mechanism that maintains adequate levels of antioxidant-related transcripts and improves mitochondrial activity. However, 9-cisRA has no significant effect on the cleavage rate of the treated oocytes.
Keywords: Antioxidant genes, Cleavage rate, Follicular fluid, Mitochondrial activity, Reactive oxygen species
1. Introduction
Buffalos (Bubalus bubalis) are a multi-purpose animal that has a significant contribution to agriculture economy. In comparison to cattle, buffaloes show more infertility related issues that reduce the productivity and profitability of this species. These problems include ovarian inactivity, long postpartum period, lower number of ovarian follicles and lower response for superovulation treatments [1]. Assisted reproductive technologies (ART) including in vitro production (IVP) of embryos have been successfully used in buffaloes to overcome some of these infertility problems, but still with low overall efficiency [2]. The first buffalo calf produced via assisted reproductive technology and in vitro fertilization has been reported in 1991 [3]. Since that date, several studies have been done to examine the influences of different culture conditions on oocyte and embryo development in buffaloes [4], [5]. In addition, several studies were focused on the low cleavage and developmental rates in in vitro produced buffalo embryos compared to cattle. These studies suggested that the reasons behind this lower embryo production efficiency could be the different nutritional requirements between both species, the varying time of nuclear and cytoplasmic maturation and/or the lower quality of frozen semen [6], [7]. On the ultrastructure level, buffalo immature oocytes showed high lipid contents which increase the sensitivity to oxidative stress under in vitro conditions [8]. Therefore, specific culture conditions should be optimized for buffalo embryos based on their special needs. Recently, conditioned media of stem cells supplemented with growth factors have been used to improve the developmental rates of buffalo embryos [9]. In addition, different molecules, including vitamins, hormones and growth factors, that known to play important roles in controlling oocyte maturation and embryo development have been tested with buffalo and other mammalian oocytes and showed positive results in relation with embryonic developmental rates and pregnancy outcomes [10], [11], [12].
Vitamin A is known to play an essential role in female reproduction including follicular growth, steroidogenesis and oocyte/embryo development. In ruminants, vitamin A is taken as β-carotene (BC) from forages, absorbed by intestinal mucosal cells and enzymatically cleavages to produce retinal which is subsequently reduced to retinol [13]. It has been reported that both BC and retinol are present in bovine follicular fluid [14] with follicular internal activity to convert BC into retinol that converts inside the target cells into 9-cis-retinoic acid (9-cisRA) and All-trans-retinoic acid (AtRA), the active forms of vitamin A [15]. These active forms can bind with retinoid X and RA receptors (RXR and RAR) and interact with the promoter region of some specific genes to control their expressions [16]. Transcripts of RAR are expressed in bovine oocytes, cumulus and granulosa cells indicating the existence of utilization mechanisms of vitamin A in these cells [17]. Retinoic acid has been used in vitro as a supplement to oocyte maturation media to improve maturation and embryo developmental rates in several species including cows [18], goats [19], mice [20] and, human [21] but has not been tested with buffalo oocytes or embryos. Moreover, the exact mechanism of action of 9-cisRA during the maturation process is differed between studies. Some studies reported that adding of RA during in vitro maturation (IVM) of oocytes increases the developmental capacity through the reduction of apoptosis rates of cumulus or embryonic cells by regulating expressions of signaling pathways and some related genes, like TNF-α [19] or cell cycle-related genes [22]. Other studies demonstrated the role of RA as an antioxidant agent protecting oocytes and embryonic cells from the excessive levels of ROS by scavenging the peroxyl radical and thus reducing ROS level [23]. Furthermore, the concentration of 9-cisRA in maturation media is an important factor determining the quality and developmental rate of produced embryos. High concentration of 9-cisRA (500 nM) found to be toxic and impairing nuclear maturation in bovine, porcine and canine oocytes, however, 5 nM was stimulatory [24], [25]. To the best of our knowledge, no studies determined the optimum concentration of RA during IVM and its effects on oocyte development and quality in the buffalo model. Therefore, the objective of this study was to examine the effect of adding 9-cisRA with different concentrations to the maturation media on development and quality of buffalo oocytes.
2. Materials and methods
2.1. Oocyte collection, in vitro maturation and fertilization
Ovaries were collected from a local abattoir in warm saline (0.9% NaCl) including 100 IU/mL penicillin and 100 µg/mL streptomycin sulfate at 37°C and transported within 2 h to the lab. The ovaries were then washed with freshly prepared phosphate buffer saline (PBS). Follicles with 2–8 mm size were puncture using an 18-gauge needle and COCs were collected in HEPES buffered solution of medium-199 (22340; Gibco, UK) including antibiotics (100 IU/mL penicillin, 100 µg/mL streptomycin sulfate and 50 µg/mL gentamicin). COCs surrounded by at least three layers of compact cumulus cells were used for IVM. For each treatment, a total of 115 oocytes (3 biological replicates) were incubated in maturation media (400 µL) after supplementation with 0, 5, 50 and 200 nM of 9-cisRA (Sigma-Aldrich St. Louis, MO, USA) for 24 h at 38.5°C under 5% CO2 atmosphere. TCM199 medium (Sigma-Aldrich, Munich, Germany) supplemented with 10% (v/v) Fetal Bovine Serum (FBS), 4.4 mM HEPES, 55 mg/mL gentamicin, 2.9 mM calcium lactate, 33.9 mM NaCHO3 and 2 mM pyruvate were used for IVM. Following IVM, matured oocytes were washed with TCM-199 medium containing 25 mM HEPES and 0.3% bovine serum albumin (BSA). In a separate experiment and based on the maturation rates, oocytes matured with 0, 5 and 50 nM of 9-cisRA were washed twice with a pre-fertilization medium containing TCM-199 supplemented with 0.3% bovine serum albumin (BSA, Sigma A-9647) and 25 mM HEPES. In vitro fertilization (IVF) was then performed in Fert-TALP medium supplemented with 10 mM hypotaurine, 20 mM penicillinamine, 50 mg/mL gentamicin 2 mM nor-adrenaline, 1 mg/mL heparin and 6 mg/mL BSA. The spermatozoa were processed for IVF as described previously [26]. A total of 25–30 oocytes/well were placed into the sperm suspension (2 × 106 sperms/mL) then kept at 38.5°C in a Multi-gas incubator (5% CO2 and 5% O2) for 18 h. The presumptive zygotes obtained after IVF were denuded by gentle repetitive pipetting, washed three times with modified synthetic oviductal fluid (mSOFaa) medium supplemented with 5 mg/mL BSA + 5 ng/mL insulin and 50 μg/mL gentamycin and subsequently cultured in the same medium at 38.5°C in a humidified Multigas CO2 incubator. The cleavage rate was checked on day 2 post insemination.
2.2. Mitochondrial membrane potential activity
Mitochondrial membrane potential, as an indicator of mitochondrial activity and number, was assessed in control and 9-cisRA treated buffalo oocytes using MitoTracker1-Red CMXRos (M7512; Invitrogen) as previously reported [27]. For each treatment, ten oocytes were incubated with 600 µL of MitoTracker1-Red dye in a concentration of 200 nM for 45 min in dark followed by three times washing in PBS for 10 min. Samples were fixed in formaldehyde (4%) at 4°C overnight. In the next day, oocytes were mounted on a glass slide with Vectashield (H-1200) mounting media. The mitochondrial membrane potential activity was then visualized under a fluorescence microscope (Leica DMI 3000 B, Leica, Germany) at the appropriate excitation wavelength (579–599 nm).
2.3. ROS accumulation level
Intracellular ROS level in control and 9-cisRA treated buffalo oocytes was detected using fluorescent dye-based H2DCFDA (6-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate, di (acetoxymethyl ester), C-6827; Invitrogen) according to the manufacturer’s protocol [28]. A group of fifteen oocytes from each treatment was stained with 400 µL of 20 µM H2DCFDA at 37°C for 20 min with no light then washed for three times in PBS. ROS levels were visualized using an appropriate green-fluorescence filter under a fluorescence microscope (Leica DMI 3000B, Leica, Germany) at Ex/Em: approx 492-495/517-527 nm and images were immediately captured.
2.4. Fluorescence level quantification
To quantify fluorescence levels for both mitochondrial activity and ROS accumulation in oocytes, ImageJ software (v1.50i, NIH, USA, http://imagej.nih.gov/ij) was used. Mean fluorescence and background readings were measured for each oocyte. Fluorescence signals were quantified from at least 8 individual oocytes per each group. The total corrected fluorescence (TCF) was calculated according to the previous method [29].
2.5. Expression analysis of antioxidant genes using quantitative real-time PCR (RT-qPCR)
2.5.1. RNA isolation and cDNA synthesis
Three biological replicates were collected, each containing 30 matured oocytes, from control and each 9-cisRA treatment group for RNA isolation and cDNA synthesis. Total RNA was isolated using PicoPure isolation kit (Arcturus, Munich, Germany). Total RNA was then purified using on-column DNase digestion sets containing DNase enzyme (QiagenGmbH, Hilden, Germany). Finally, RNA was collected in a total volume of 11 µL using elution buffer after washing twice using two different washing buffers. RNA quantity and quality were determined using a Nanodrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA).
cDNA was synthesized from mRNA of each sample using random primers (Promega Madison WI, USA), oligo (dT)25 and SuperScript™ II reverse transcriptase (Invitrogen, Karlsruhe, Germany). Random primers (1 µL), oligo (dT)25 (1 µL) were incubated with 10 μL RNA sample for 3 min at 70°C. The RNA-primers mixture was then chilled on ice. After that, cDNA synthesis master mix containing 1 µL dNTP (10 pmol/μL), 0.3 µL RNase inhibitor, 2 µL of 0.1 M dithiothreitol (DTT, Promega, Madison, WI,USA), 4 µL of 5X first strand buffer (15 mM MgCl2, 375 mM KCl, 250 mM Tris–HCl, pH 8.3) and 0.7 µL of invitrogen SuperScript™ II reverse transcriptase (200 unit/µL) was added to the RNA-primers mix and incubated for 90 min at 42°C followed by denaturation at 70°C for 15 min. cDNA was stored at −20°C until use.
2.5.2. Gene expression analysis
Expression analysis of 5 antioxidant-related genes namely superoxide dismutase-1 (SOD1), heme oxygenase decycling-1 (HMOX1), peroxiredoxin-1 (PRDX1), catalase (CAT) and Glutathione peroxidase 4 (GPX4) have been analyzed in buffalo oocytes matured in vitro under different concentrations (0, 5, 50 and 200 nM) of 9-cisRA using sequence-specific primers (Table 1). A primer pair, forward and reverse, for each tested gene was designed using Primer Express version 2.0 software (Applied Biosystems, Foster City, CA).
Table 1.
Details of primers used for quantitative real-time PCR.
| Gene | Accession number | Primer sequences | Product size (bp) |
|---|---|---|---|
| SOD1 | XM_006053564 | F: gagaggcatgttggagacct R: ctgcccaagtcatctggtt |
153 |
| GPX4 | XM_006050496 | F: agccagggagtaatgcagag R: cacacagccgttcttgtcaa |
203 |
| CAT | XM_006062382 | F: agatggacacaggcacatga R: attgaaaagatcgcggaggc |
184 |
| PRDX1 | XM_006052557 | F: aaacaaggaggactgggacc R: gcacacttccccatgtttgt |
243 |
| HMOX1 | XM_006074428 | F: atgccccaggatttgtcaga R: aggggagtatagacggggtt |
206 |
| GAPDH | XM_006065800 | F: agatggtgaaggtcggagtg R: tggaagatggtgatggcctt |
229 |
Step-One Plus real-time PCR system (Applied Biosystems) was used to analyze the expression level of transcripts using SYBR green fluorescent dye. RT-qPCR reactions (20 µL) were contained 2 µL cDNA, 10 µL iTaq SYBR Green master mix with ROX (Bio-Rad Laboratories, Munich, Germany), 7.4 µL H2O and 0.3 µL of each forward and reverse primers (20 µM). The cycling conditions were adjusted to 95°C for 3 min as an initial denaturation followed by 15 s at 95°C and 45 s at 60°C (annealing/extension) for 40 cycles. Comparative CT (2−ΔΔCT) method was used to analyze RT-qPCR data and determine the transcript abundance in the tested samples. The relative CT value of each gene was normalized using Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene to compare transcript abundance differences between the groups of buffalo oocytes matured under different levels of 9-cisRA.
2.6. Statistical analysis
Data were analyzed statistically using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Maturation and cleavage rates were log transformed and analyzed by one-way ANOVA followed by Tukey’s test (α = 0.05). Gene expression data were analyzed using SAS General Linear Model (GLM). Differences in mean values were tested amongst treatment groups using ANOVA followed by Student t-test. The significance level was considered at P < .05.
3. Results
3.1. Maturation and cleavage rates
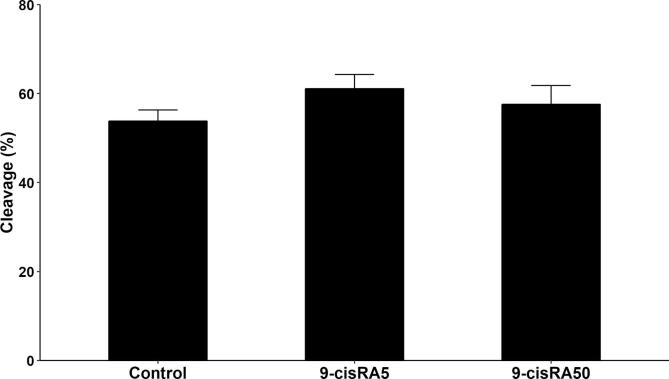
A total number of 460 oocytes have been aspirated and randomly distributed into 4 groups (115 oocytes each). Polar body extrusion and cumulus cells expansion rates were recorded and analyzed. The 9-cisRA treatment with a concentration of 5 nM showed higher maturation rate (P < .05) represented as expansion and polar body rates (95.8 and 45.5%, respectively) compared to other treatments (Table 2). In contrast, treatment with high concentrations of 9-cisRA (50 and 200 nM) during maturation showed the lowest maturation rates (P < .05, Table 2). The cleavage rates of oocytes matured with 0, 5 and 50 nM 9-cisRA have been recorded. The results showed a tendency of higher cleavage rate in 5 nM 9-cisRA treatment group (61.1%) compared to control and 50 nM groups (53.8 and 57.6%, respectively) but with no significant differences (Fig. 1).
Table 2.
Expansion and polar body appearance rates under different concentrations (0, 5, 50 and 200 nM) of 9-cisRA during in vitro maturation of buffalo oocytes.
| Groups | Total oocyte | Expanded oocytes (% ± S.D) | Polar body appearance (% ± S.D) |
|---|---|---|---|
| Control | 115 | 86.7 ± 5.4b | 29.7 ± 1b |
| 9-cisRA5 | 115 | 95.8 ± 5a | 45.5 ± 5.2a |
| 9-cisRA50 | 115 | 80.5 ± 1.8c | 26.4 ± 5c |
| 9-cisRA200 | 115 | 79.1 ± 3c | 23.6 ± 5c |
a, b, c values with different letters in the same column are significantly different (P < .05).
Fig. 1.
Cleavage rates per total fertilized oocytes after maturation in vitro with a supplementation of 0 (control), 5, and 50 nM of 9-cisRA.
3.2. Mitochondrial membrane potential activity
The oocytes mitochondrial membrane potential, as indicator of mitochondrial activity, was detected using MitoTracker1-Red. 5 nM 9-cisRA treatment group exhibited significantly higher mitochondrial activity (high fluorescence intensity) compared to other treatment groups (Fig. 2). In contrast, oocytes matured with 50 and 200 nM 9-cisRA exhibited lower mitochondrial activity compared to control and 5 nM 9-cisRA groups.
Fig. 2.
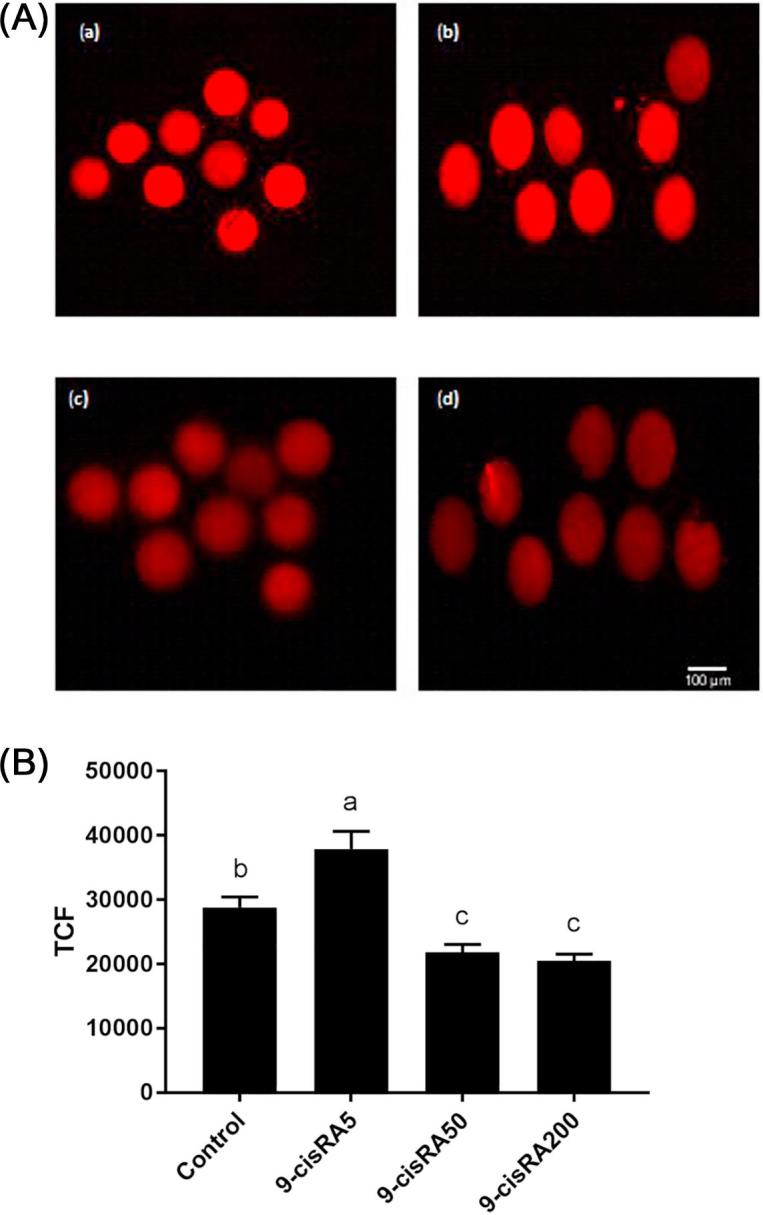
(A) Mitochondrial membrane potential activity (MitoTracker1-Red stain) of buffalo oocytes matured in vitro with a supplementation of (a) 0 (control), (b) 5, (c) 50 and (d) 200 nM of 9-cisRA. Scale bar, 100 μm. (B) Total corrected fluorescence (TCF) levels of oocytes from different groups. a, b, c values with different letters are significantly different (P < .05).
3.3. ROS level
ROS accumulation level was detected in fifteen oocytes from each group. Oocytes matured with 50 and 200 nM of 9-cisRA showed higher level of ROS accumulation. In contrast, control and 5 nM groups showed lower levels of ROS as a lower intensity of fluorescent dye (Fig. 3).
Fig. 3.
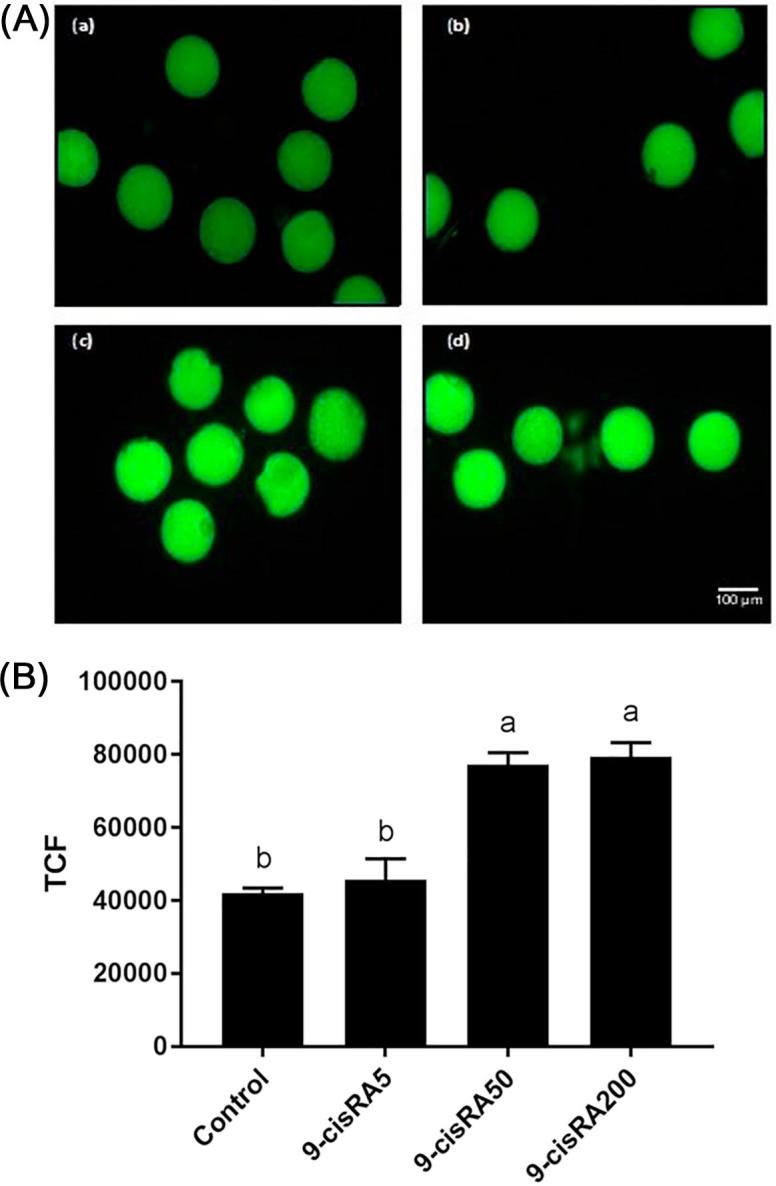
(A) ROS levels (H2DCFDA stain) in buffalo oocytes matured in vitro with supplementation of (a) 0 (control), (b) 5, (c) 50 and (d) 200 nM of 9-cisRA. Scale bar, 100 μm. (B) Total corrected fluorescence (TCF) levels of oocytes from different groups. a, b, c values with different letters are significantly different (P < .05).
3.4. Gene expression results
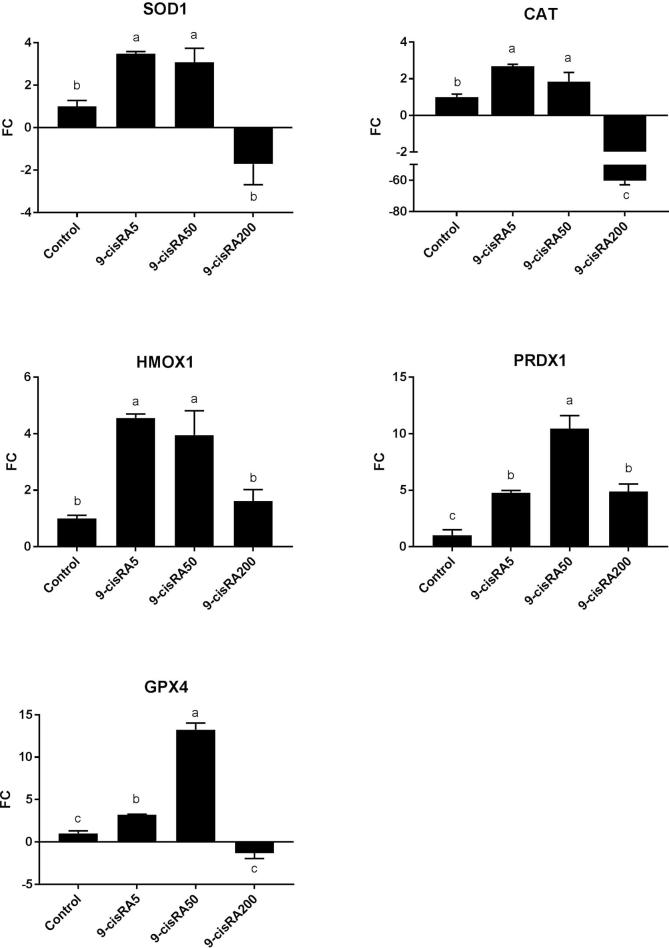
Gene expression analysis revealed that 5 and 50 nM 9-cisRA treatments significantly up-regulated all antioxidant-related genes compared to control and 200 nM 9-cisRA groups (Fig. 4). In contrast, 200 nM 9-cisRA treatment dramatically decreased the expression of CAT gene compared to all other groups, in addition to a down-regulation for other antioxidant-related genes except for PRDX1 (Fig. 4).
Fig. 4.
Relative expression of antioxidant-related genes (SOD1, CAT, HOMX1, PRDX1, and GPX4) represented as fold change (FC) in buffalo oocytes matured in vitro with supplementation of 0 (control), 5, 50 and 200 nM of 9-cisRA. a,b,c values with different letters are significantly different (P < .05).
4. Discussion
In this study we examined the effect of 9-cisRA with different concentrations on buffalo oocytes maturation rate and quality in regards to mitochondrial activity, ROS levels and gene expression during IVM. Our results showed that 9-cisRA with a concentration of 5 nM during IVM significantly improves the nuclear and cytoplasmic maturation in buffalo oocytes. However, higher concentrations (50 and 200 nM) reduce the maturation rate compared to control group. Meanwhile, we recorded the cleavage rates of the fertilized oocytes from control, 5 nM and 50 nM 9-cisRA groups. In the 5 nM 9-cisRA group there was a tendency for an improvement in cleavage rate compared to the other groups but with no significant differences (P > .05). Several studies discussed the beneficial and detrimental effects of RA during IVM and development of embryos in different species. RA concentration in maturation media is an essential factor affecting oocytes and embryos developmental competency. In agreement with our findings, 5 nM of 9-cisRA during IVM was supportive to the maturation of bovine [30] porcine [24] and canine [25] oocytes. The susceptibility of oocytes to different retinoid concentrations seems to vary between species and in vitro culture conditions. For instance, higher concentrations of RA (500 nM) were cytotoxic for oocytes during IVM in bovine [30] and porcine [31]. In contrast, basic maturation media supplemented with FSH and 500 nM RA improved blastocyst formation of bovine embryos cultured in potassium simplex optimized medium (KSOM) [32]. In another study, the same concentration of RA improved the developmental capacity in goat embryos [33] or even with higher concentration (2 μM), it showed beneficial effects on mouse oocyte IVM [34]. One explanation of these contrary results may be the different used media with different compositions in which interaction between RA and hormones supplemented to the maturation media has been proved to affect the action mechanisms inside cells and oocytes [30]. On the other hand, species-specific nutritional requirements and time required for in vitro nuclear maturation may account for these variations [35].
Oocyte developmental competence and subsequent preimplantation embryonic development are highly correlated with the potentiality and distribution of mitochondria [36]. Production of ATP by mitochondria using oxidizable energy substrates is essential for all metabolic, transcription and translation processes required for normal nuclear and cytoplasmic maturation [37]. Although the high efficiency of energy production in mitochondria via oxidative phosphorylation pathway, it is considered as a main source of ROS. High levels of ROS lead to cell apoptosis through impairment of the mitochondrial activity in oocytes and embryos [38]. Internally produced or externally added antioxidants play important roles to maintain the oocyte’s oxidant/antioxidant balance during IVM. Several antioxidants, including enzymatic and non-enzymatic, were tested with in vitro cultured oocytes and embryos in different mammalian species and found to be efficient protectants against ROS [39]. We reported here an increase in mitochondrial membrane potential activity in oocyte group matured with 5 nM 9-cisRA compared with control and other treatments. The same oocyte group showed a reduction of ROS level compared to the higher concentrations of 9-cisRA. These results indicated that an appropriate concentration of 9-cisRA can maintain the energy production process via mitochondria, meanwhile keeping adequate levels of ROS during oocytes IVM. In mouse liver and a model of human liver, the isomer for 9-cisRA (AtRA) was found to increase fatty acid oxidation and mitochondrial function [40]. In contrast, higher concentrations of AtRA induce electron leakage from mitochondrial membranes and subsequently reduce mitochondrial activity, increase ROS generation and lead to cell apoptosis [41]. These findings confirmed the contrary effects of RA in its dose-specific manner and could explain the reported lower mitochondrial activity and higher ROS levels in 50 and 200 nM 9-cisRA groups compared to the lower concentration and control groups.
It has been reported that retinoids can quench oxygen molecules and subsequently regulates redox status and pathways [42]. IVM medium supplemented with AtRA showed a significant reduction in ROS level and improved blastocyst rate in bovine [23]. The antioxidant activity of AtRA has been elucidated through its ability to scavenge a spectrum of ROS molecules in in vitro cultured cells [43]. This may suggest a scavenging ability of 9-cisRA, as a more potent isomer for AtRA [44], against ROS as a mechanism of reducing ROS level, however, this mechanism needs more research to be confirmed. Previously, It has been reported that retinoids can maintain adequate endogenous levels of antioxidants which can be used to protect oocyte and embryo during maturation and development against ROS accumulation [45]. These antioxidants regulate the internal protection mechanisms of oocytes against ROS [46]. It is already known that RA regulates the transcription of several genes through the nuclear receptors RAR and RXR that hetero- or homo-dimerize after binding with RA and then interact with RARE in the promoter regions of target genes [47]. Anti-apoptotic and oocyte quality related genes were found to be up-regulated in IVM oocyte cultured with RA [30]. In addition, it has been reported that RA improves the polyadenylation process and thus increases mRNA quality [22]. In the current study, we observed a significant up-regulation of all tested antioxidant-related genes in 5 and 50 nM 9-cisRA groups compared to control and 200 nM 9-cisRA groups. It is accepted that during the transition from meiosis I (MI) to meiosis II (MII), oocyte progressively decreases its transcriptional activities and becomes highly dependent on the stored maternal RNA and proteins. Therefore, protecting the existed transcripts against degradation is an essential process for further development [48]. RA proved to protect and prevent decreases in transcript and protein levels of SOD in primary cultured rat cells [49]. This protection strengthened the endogenous antioxidant defense system and subsequently reduced ROS levels. Moreover, it has been reported that 9-cisRA with a concentration of 5 nM during IVM can stabilize the transcription of prostaglandin-endoperoxide synthase2 (PTGS2), which has an important role in the process of cumulus cell expansion, and subsequently improves maturation and embryo development in porcine [24]. This could explain the higher levels of antioxidant-related genes in 5 and 50 nM 9-cisRA groups as a protective role of RA against transcripts degradation. Our results regarding SOD1 and GPX4 are in agreement with the previous studies which explained that the addition of antioxidant upregulated these genes as an antioxidant behavior [50], [51]. Moreover, SOD1 has been reported as one of the important genes which supports buffalo oocytes against oxidative stress [26] as it is involved in the breakdown of ROS during stress conditions. In addition, several antioxidant enzymes including CAT and GPX play an important role in protecting oocytes and embryos against pro-oxidative damage [52]. SOD1 is responsible for the first enzymatic step that protects cells against toxic oxygen radicals and produces hydrogen peroxide (H2O2) as a by-product which is then eliminated either by catalase or GPX [45]. Peroxiredoxin1 (Prdx1) is an antioxidant enzyme that regulates the cellular ROS levels through catalyzation of the reduction of H2O2 and alkyl hydroperoxide. The result of the current study supports the antioxidant action of Prdx1 as previously stated [53] and its function as a downstream mediator of the retinoic acid signaling pathway during embryogenesis [54]. The inducible isoform heme-oxygenase 1 (HMOX1) plays an important physiological role as an antioxidant enzyme. It is involved in oxidative degradation of heme into equimolar amounts of carbon monoxide, ferrous iron, and biliverdin, which, per se and particularly after conversion into bilirubin, is known to have potent antioxidant properties [55]. The expression of HMOX1 gene is regulated by the Keap1-Nrf2 pathway after binding of Nrf2 to the consensus binding sequence and activates a cascade of events which, in the end, provides robust protection against oxidative challenge [56]. The importance of HMOX1 during oocyte maturation has been illustrated when female Hmox1−/− mice showed lower levels of oocyte maturation and overall lower fertility compared to the wild-type mice [57]. Although 5 and 50 nM 9-cisRA groups showed the same expression pattern of antioxidant genes, only 5 nM 9-cisRA group showed a significant reduction in ROS level with increased mitochondrial activity and maturation rate. Although the 50 nM of 9-cisRA can maintain the higher levels of antioxidant genes, it could be a slightly toxic concentration for buffalo oocytes through its negative effects on mitochondrial activity. In addition, it could negatively affect the antioxidant capacity on the level of enzyme activities as it has been reported previously that RA caused an increase in ROS and decrease in peroxidase activity [58]. However, highly toxic concentrations, as 200 nM in this study, negatively affected the antioxidant defense system on the expression level and reduced mitochondrial activity that leading to a high ROS level.
5. Conclusions
Supplementation of 9-cisRA with lower concentration (5 nM) to the maturation media is beneficial for buffalo oocytes. It promotes maturation rate through a protection mechanism that maintains adequate levels of antioxidant-related transcripts and improves mitochondrial activity. However, 9-cisRA has no significant effect on the cleavage rate of the treated oocytes.
Acknowledgments
Acknowledgements
This work was supported by a research grant financed by Cairo University (project no. 57/16). The authors thank the student group: Ms. Huda Hassan, Ms. Alyaa Mohamed, Mr. Ahmed Abed and Mr. Hesham Fawzy for their contribution and technical assistance.
Competing interests
There is no competing interest to declare.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Van L., Nguyen B.X., Son H.N., Driancourt M.A. Superovulation and ovarian follicular population of juvenile buffaloes and calves. Anim Reprod Sci. 1994;35:191–199. [Google Scholar]
- 2.Hufana-Duran D., Pedro P.B., Venturina H.V., Duran P.G., Cruz L.C. Full-term delivery of river buffalo calves (2n=50) from in vitro-derived vitrified embryos by swamp buffalo recipients (2n=48) Livest Sci. 2007;107:213–219. [Google Scholar]
- 3.Madan M., Singla S., Jailkhari S., Ambrose J. Proc. third world buffalo Congr. 1991. In vitro fertilization in buffalo and birth of first ever IVF buffalo calf; pp. 11–17. [Google Scholar]
- 4.Nandi S., Raghu H.M., Ravindranatha B.M., Chauhan M.S. Production of buffalo (Bubalus bubalis) embryos in vitro: premises and promises. Reprod Domest Anim. 2002;37:65–74. doi: 10.1046/j.1439-0531.2002.00340.x. [DOI] [PubMed] [Google Scholar]
- 5.Sadeesh E.M., Selokar N.L., Balhara A.K., Yadav P.S. Differences in developmental competence and gene expression profiles between buffalo (Bubalus bubalis) preimplantation embryos cultured in three different embryo culture media. Cytotechnology. 2016;68:2035–2048. doi: 10.1007/s10616-016-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasparrini B. In vitro embryo production in buffalo species: state of the art. Theriogenology. 2002;57:237–256. doi: 10.1016/s0093-691x(01)00669-0. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D., Anand T. In vitro embryo production in buffalo: basic concepts. J Buffalo Sci. 2012;1:50–54. [Google Scholar]
- 8.Waiz S.A., Raies-ul-Haq M., Dhanda S., Kumar A., Goud T.S., Chauhan M.S. Heat stress and antioxidant enzyme activity in bubaline (Bubalus bubalis) oocytes during in vitro maturation. Int J Biometeorol. 2016;60:1357–1366. doi: 10.1007/s00484-015-1129-0. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj R., Ansari M.M., Parmar M.S., Chandra V., Sharma G.T. Stem cell conditioned media contains important growth factors and improves in vitro buffalo embryo production. Anim Biotechnol. 2016;27:118–125. doi: 10.1080/10495398.2015.1118383. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J., Moawad A.R., Wang C.-Y., Li H.-F., Ren J.-Y., Dai Y.-F. Advances in in vitro production of sheep embryos. Int J Vet Sci Med. 2018;6:S15–S26. doi: 10.1016/j.ijvsm.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raheem K.A. Cytokines, growth factors and macromolecules as mediators of implantation in mammalian species. Int J Vet Sci Med. 2018;6:S6–S14. doi: 10.1016/j.ijvsm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda B.S.K., Pandey S., Somal A., Parmar M.S., Bhat I.A., Baiju I. Leptin supplementation in vitro improved developmental competence of buffalo oocytes and embryos. Theriogenology. 2017;98:116–122. doi: 10.1016/j.theriogenology.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Weiss W.P. Requirements of fat-soluble vitamins for dairy cows: a review. J Dairy Sci. 1998;81:2493–2501. doi: 10.3168/jds.S0022-0302(98)70141-9. [DOI] [PubMed] [Google Scholar]
- 14.Haliloglu S., Baspinar N., Serpek B., Erdem H., Bulut Z. Vitamin A and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle. Reprod Domest Anim. 2002;37:96–99. doi: 10.1046/j.1439-0531.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Schweigert F.J., Zucker H. Concentrations of vitamin A, beta-carotene and vitamin E in individual bovine follicles of different quality. J Reprod Fertil. 1988;82:575–579. doi: 10.1530/jrf.0.0820575. [DOI] [PubMed] [Google Scholar]
- 16.di Masi A., Leboffe L., De Marinis E., Pagano F., Cicconi L., Rochette-Egly C. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Mohan M., Malayer J.R., Geisert R.D., Morgan G.L. Expression patterns of retinoid X receptors, retinaldehyde dehydrogenase, and peroxisome proliferator activated receptor gamma in bovine preattachment embryos. Biol Reprod. 2002;66:692–700. doi: 10.1095/biolreprod66.3.692. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo C.O., Díez C., Duque P., Facal N., Gómez E. Pregnancies and improved early embryonic development with bovine oocytes matured in vitro with 9-cis-retinoic acid. Reproduction. 2003;125:409–416. [PubMed] [Google Scholar]
- 19.Pu Y., Wang Z., Bian Y., Zhang F., Yang P., Li Y. All-trans retinoic acid improves goat oocyte nuclear maturation and reduces apoptotic cumulus cells during in vitro maturation. Anim Sci J. 2014;85:833–839. doi: 10.1111/asj.12216. [DOI] [PubMed] [Google Scholar]
- 20.Nasiri E., Mahmoudi R., Bahadori M.H., Amiri I. The effect of retinoic acid on in vitro maturation and fertilization rate of mouse germinal vesicle stage oocytes. Cell J. 2011;13:19–24. [PMC free article] [PubMed] [Google Scholar]
- 21.Best M.W., Wu J., Pauli S.A., Kane M.A., Pierzchalski K., Session D.R. A role for retinoids in human oocyte fertilization: regulation of connexin 43 by retinoic acid in cumulus granulosa cells. MHR Basic Sci Reprod Med. 2015;21:527–534. doi: 10.1093/molehr/gav017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez E., Rodríguez A., Goyache F., Dd́ez C., José Royo L., Moreira P.N. Retinoid-dependent mRNA expression and poly-(A) contents in bovine oocytes meiotically arrested and/or matured in vitro. Mol Reprod Dev. 2004;69:101–108. doi: 10.1002/mrd.20154. [DOI] [PubMed] [Google Scholar]
- 23.Lucas C.G., Remião M.H., Komninou E.R., Domingues W.B., Haas C., de Leon P.M.M. Tretinoin-loaded lipid-core nanocapsules decrease reactive oxygen species levels and improve bovine embryonic development during in vitro oocyte maturation. Reprod Toxicol. 2015;58:131–139. doi: 10.1016/j.reprotox.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Atikuzzaman M., Koo O.J., Kang J.T., Kwon D.K., Park S.J., Kim S.J. The 9-cis retinoic acid signaling pathway and its regulation of prostaglandin-endoperoxide synthase 2 during in vitro maturation of pig cumulus cell-oocyte complexes and effects on parthenogenetic embryo production. Biol Reprod. 2011;84:1272–1281. doi: 10.1095/biolreprod.110.086595. [DOI] [PubMed] [Google Scholar]
- 25.Liang S., Kang J., Jin H., Liu X., Li J., Li S. The influence of 9-cis-retinoic acid on nuclear and cytoplasmic maturation and gene expression in canine oocytes during in vitro maturation. Theriogenology. 2012;77:1198–1205. doi: 10.1016/j.theriogenology.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 26.El-Sayed A., Nagy R., El-Asheeri A.K., Eid L.N. Developmental and molecular responses of buffalo (Bubalus bubalis) cumulus–oocyte complex matured in vitro under heat shock conditions. Zygote. 2018;26:177–190. doi: 10.1017/S0967199418000072. [DOI] [PubMed] [Google Scholar]
- 27.Prastowo S., Amin A., Rings F., Held E., Wondim D.S., Gad A. Fateful triad of reactive oxygen species, mitochondrial dysfunction and lipid accumulation is associated with expression outline of the AMP-activated protein kinase pathway in bovine blastocysts. Reprod Fertil Dev. 2016;29:890. doi: 10.1071/RD15319. [DOI] [PubMed] [Google Scholar]
- 28.Amin A., Gad A., Salilew-Wondim D., Prastowo S., Held E., Hoelker M. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol Reprod Dev. 2014;81:497–513. doi: 10.1002/mrd.22316. [DOI] [PubMed] [Google Scholar]
- 29.McCloy R.A., Rogers S., Caldon C.E., Lorca T., Castro A., Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–1412. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez E., Royo L.-J., Duque P., Carneiro G., Hidalgo C., Goyache F. 9-cis-retinoic acid during in vitro maturation improves development of the bovine oocyte and increases midkine but not IGF-I expression in cumulus-granulosa cells. Mol Reprod Dev. 2003;66:247–255. doi: 10.1002/mrd.10307. [DOI] [PubMed] [Google Scholar]
- 31.Alminana C., Gil M.A., Cuello C., Caballero I., Roca J., Vazquez J.M. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod Fertil Dev. 2008;20:483–489. doi: 10.1071/rd07175. [DOI] [PubMed] [Google Scholar]
- 32.Lima P., Oliveira M., Santos M., Reichenbach H., Weppert M., Paula-Lopes F. Effect of retinoids and growth factor on in vitro bovine embryos produced under chemically defined conditions. Anim Reprod Sci. 2006;95:184–192. doi: 10.1016/j.anireprosci.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Conceição J.C.Z., Moura M.T., Ferreira-Silva J.C., Cantanhêde L.F., Chaves R.M., Lima P.F. Incidence of apoptosis after retinoids and insulin-like growth factor-I (IGF-I) supplementation during goat in vitro embryo production. Zygote. 2016;24:808–813. doi: 10.1017/S0967199416000125. [DOI] [PubMed] [Google Scholar]
- 34.Abouzaripour M., Fathi F., Daneshi E., Mortezaee K., Rezaie M.J., Abdi M. Combined effect of retinoic acid and basic fibroblast growth factor on maturation of mouse oocyte and subsequent fertilization and development. Int J Fertil Steril. 2018;12:68–71. doi: 10.22074/ijfs.2018.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arhin S.K., Lu J., Xi H. Jin X. Energy requirements in mammalian oogenesis. Cell Mol Biol (Noisy-Le-Grand) 2018;64:12–19. [PubMed] [Google Scholar]
- 36.Zhuang X.-J., Huang Y., Duan Y.-P., Zhang M., Lu Y.-Q., Lu K.-H. Translocation of active mitochondria during buffalo (Bubalus bubalis) oocytes in vitro maturation, fertilization and preimplantation embryo development. Reprod Domest Anim. 2012;47:443–448. doi: 10.1111/j.1439-0531.2011.01900.x. [DOI] [PubMed] [Google Scholar]
- 37.Harris S.E., Picton H.M. Metabolism of follicles and oocytes during growth and maturation. In: Tan S., Chian R., Bucket W., editors. Vitr. Matur. Hum. Oocytes, informa UK Ltd. 2006. pp. 15–36. [Google Scholar]
- 38.Amoushahi M., Salehnia M., Ghorbanmehr N. The mitochondrial DNA copy number, cytochrome c oxidase activity and reactive oxygen species level in metaphase II oocytes obtained from in vitro culture of cryopreserved ovarian tissue in comparison with in vivo -obtained oocyte. J Obstet Gynaecol Res. 2018 doi: 10.1111/jog.13747. [DOI] [PubMed] [Google Scholar]
- 39.Khazaei M., Aghaz F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int J Fertil Steril. 2017;11:63–70. doi: 10.22074/ijfs.2017.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathy S., Chapman J.D., Han C.Y., Hogarth C.A., Arnold S.L.M., Onken J. All-trans-retinoic acid enhances mitochondrial function in models of human liver. Mol Pharmacol. 2016;89:560–574. doi: 10.1124/mol.116.103697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.de Oliveira M.R. Vitamin A and retinoids as mitochondrial toxicants. Oxid Med Cell Longev. 2015;2015:1–13. doi: 10.1155/2015/140267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imam A., Hoyos B., Swenson C., Levi E., Chua R., Viriya E. Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 2001;15:28–30. doi: 10.1096/fj.00-0329fje. [DOI] [PubMed] [Google Scholar]
- 43.Siddikuzzaman Grace V.M.B. Antioxidant potential of all- trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol Immunotoxicol. 2013;35:164–173. doi: 10.3109/08923973.2012.736520. [DOI] [PubMed] [Google Scholar]
- 44.Thaller C., Hofmann C., Eichele G. 9-cis-retinoic acid, a potent inducer of digit pattern duplications in the chick wing bud. Development. 1993;118:957–965. doi: 10.1242/dev.118.3.957. [DOI] [PubMed] [Google Scholar]
- 45.Guérin P., El Mouatassim S., Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 46.Khan I., Chowdhury M.M.R., Song S.-H., Mesalam A., Zhang S., Khan Khalil A.A. Lupeol supplementation improves the developmental competence of bovine embryos in vitro. Theriogenology. 2018;107:203–210. doi: 10.1016/j.theriogenology.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Amann P.M., Eichmuller S.B., Schmidt J., Bazhin A.V. Regulation of gene expression by retinoids. Curr Med Chem. 2011;18:1405–1412. doi: 10.2174/092986711795029618. [DOI] [PubMed] [Google Scholar]
- 48.Sirard M.-A. Factors affecting oocyte and embryo transcriptomes. Reprod Domest Anim. 2012;47:148–155. doi: 10.1111/j.1439-0531.2012.02069.x. [DOI] [PubMed] [Google Scholar]
- 49.Ahlemeyer B., Bauerbach E., Plath M., Steuber M., Heers C., Tegtmeier F. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med. 2001;30:1067–1077. doi: 10.1016/s0891-5849(01)00495-6. [DOI] [PubMed] [Google Scholar]
- 50.Mehaisen G.M.K., Saeed A.M., Gad A., Abass A.O., Arafa M., El-Sayed A. Antioxidant capacity of melatonin on preimplantation development of fresh and vitrified rabbit embryos: morphological and molecular aspects. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F., Tian X., Zhang L., Gao C., He C., Fu Y. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J Pineal Res. 2014;56:333–342. doi: 10.1111/jpi.12126. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Foote R.H., Simkin M. Development of rabbit zygotes cultured in protein-free medium with catalase, taurine, or superoxide dismutase1. Biol Reprod. 1993;49:33–37. doi: 10.1095/biolreprod49.1.33. [DOI] [PubMed] [Google Scholar]
- 53.Neumann C.A., Krause D.S., Carman C.V., Das S., Dubey D.P., Abraham J.L. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 54.Chae S., Lee H.-K., Kim Y.-K., Jung Sim H., Ji Y., Kim C. Peroxiredoxin1, a novel regulator of pronephros development, influences retinoic acid and Wnt signaling by controlling ROS levels. Sci Rep. 2017;7:8874. doi: 10.1038/s41598-017-09262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farombi E.O., Surh Y.J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 56.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zenclussen M.L., Jensen F., Rebelo S., El-Mousleh T., Casalis P.A., Zenclussen A.C. Heme oxygenase-1 expression in the ovary dictates a proper oocyte ovulation, fertilization, and corpora lutea maintenance. Am J Reprod Immunol. 2012;67:376–382. doi: 10.1111/j.1600-0897.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- 58.Schnabel D., Salas-Vidal E., Narváez V., del Rayo Sánchez-Carbente M, Hernández-García D., Cuervo R. Expression and regulation of antioxidant enzymes in the developing limb support a function of ROS in interdigital cell death. Dev Biol. 2006;291:291–299. doi: 10.1016/j.ydbio.2005.12.023. [DOI] [PubMed] [Google Scholar]