Abstract
Context:
Thiazolidinediones have proven efficacy in preventing diabetes in high-risk individuals. However, the effect of thiazolidinediones on glucose tolerance after cessation of therapy is unclear.
Objective:
To examine the effect of pioglitazone (PIO) on incidence of diabetes after discontinuing therapy in ACT NOW.
Design, Settings and Patients:
Two-hundred ninety-three subjects (placebo [PLAC], n = 138; PIO, n = 152) completed a median followup of 11.7 mo after study medication was stopped.
Results:
Diabetes developed in 138 (12.3%) of PLAC vs 17 of 152 PIO patients (11.2%; P = not significant, PIO vs PLAC). However, the cumulative incidence of diabetes from start of study medication to end of washout period remained significantly lower in PIO vs PLAC (10.7 vs 22.3%; P < .005). After therapy was discontinued, 23.0% (35/152) of PIO-treated patients remained normal-glucose tolerant (NGT) vs 13.8% (19/138) of PLAC-treated patients (P = .04). Insulin secretion/insulin resistance index (I0–120/G0–120 × Matsuda index) was markedly lower in subjects with impaired glucose tolerance (IGT) who converted to diabetes during followup vs those who remained IGT or NGT. The decline in-cell function (insulin secretion/insulin resistance index) was similar in subjects with IGT who developed diabetes, irrespective of whether they were treated with PIO or PLAC.
Conclusions:
1) The protective effect of PIO on incidence of diabetes attenuates after discontinuation of therapy, 2) cumulative incidence of diabetes in individuals exposed to PIO remained significantly (56%) lower than PLAC and a greater number of PIO-treated individuals maintained NGT after median followup of 11.4 mo, and 3) low insulin secretion/insulin resistance index is a strong predictor of future diabetes following PIO discontinuation.
Approximately 30% of adults in the United States have impaired glucose tolerance (IGT) (1, 2). The conversion rate of IGT to type 2 diabetes mellitus (T2DM) varies from 3–11% per year, and the lifetime risk of T2DM is approximately 50% (3, 4). Hyperglycemia is the major risk factor for microvascular complications (UK Prospective Diabetes Study, Diabetes Control and Complications Trial), which account for a significant portion of the morbidity and mortality in T2DM. Early detection and treatment would be expected to prevent or delay the onset of these complications. Both lifestyle and pharmacologic interventions, including metformin, thiazolidinediones, and α-glucosidase inhibitors, have been shown to prevent or delay the progression of IGT to T2DM (5–9). However, it is not clear whether the protective effect of these agents persists after discontinuation of therapy.
Following completion of the Diabetes Prevention Program (DPP) study, subjects were invited to participate in a lifestyle modification (DPP Outcomes Study) and were followed for 10 years (6, 10). Most subjects in the lifestyle intervention arm regained the lost weight, and the difference in incidence of new diabetes between the lifestyle intervention, metformin, and PLAC groups was not significant during the follow-up period (11). However, the cumulative incidence of diabetes remained significantly lower in the group initially treated with lifestyle modification. In DPP, a group of subjects with IGT also received treatment with troglitazone, which was discontinued early because of liver toxicity (12). In troglitazone-treated subjects, a highly significant diabetes preventive effect was observed 1 year after troglitazone was discontinued (13). Similarly, in TRIPOD women with history of gestational diabetes and who were treated with troglitazone had a lower cumulative incidence of diabetes compared with those treated with PLAC 1 year after discontinuation of treatment (14). In DPP, subjects who reverted to normal glucose tolerance (NGT) any time during the trial, regardless of the treatment arm, had a lower incidence of diabetes (15). In ADOPT, rosiglitazone had the most durable effect on glycemic control in recently diagnosed T2DM individuals (16). In DREAM, 1.6 years after withdrawal of rosiglitazone the cumulative incidence of diabetes was 39% lower than those treated with PLAC (17). These results with thiazolidinediones (TZDs) (12–17) and lifestyle intervention (11, 17) demonstrate a true slowing of the disease (ie, reduced conversion of prediabetes to diabetes), and not a masking of the disease, given that there was not a higher rate of new cases of diabetes in the TZD or lifestyle group compared with the PLAC group after the therapy was stopped (18).
In ACT NOW, pioglitazone (PIO) reduced the prevalence of T2DM by 72% (5). Herein, we describe the incidence of diabetes and glucose tolerance after cessation of PIO in ACT NOW.
Materials and Methods
Patients and study design
The details of recruitment, inclusion and exclusion criteria, study design, and patient characteristics of ACT NOW participants have been published (5). At baseline, 602 subjects with IGT received 2-hour oral glucose tolerance test (OGTT), and plasma samples were obtained every 15 minutes for determination of glucose and insulin concentrations. Participants were then randomly assigned to PIO (30 mg/d) or PLAC. One month after randomization, PIO was increased to 45 mg/d. Baseline measurements were repeated at study end (2.4 y after recruitment of last subject), at time of dropout or loss to followup (last observation carried forward), or at time of conversion to T2DM. After the closeout visit, PIO and PLAC were discontinued and subjects were asked to return for follow-up visits at 6-month intervals. Of 443 subjects who had a closeout visit, 290 (PIO, n = 152 and PLAC, n = 138) returned for at least one 6-month follow-up visit, had repeat OGTT, and were included in the present analysis. Appropriate informed consent was obtained from all study participants and appropriate treatment of research subjects was carried out.
Measurements
Plasma glucose was measured by glucose oxidase reaction, plasma insulin by RIA (Diagnostic Products) (interassay and intra-assay coefficient of variation, 7.1 and 5.1%, respectively), and glycated hemoglobin A1c with DCA 2000 Analyzer (Bayer). Total plasma cholesterol, triglycerides, and high-density lipoprotein cholesterol were measured using enzymatic assay (Stanbio Laboratory). Low-density lipoprotein cholesterol was calculated using Friedewald equation.
Calculations
Incremental area under the curve (AUC) for plasma glucose and insulin during OGTT was calculated according to trapezoidal rule. The primary stimulus for insulin secretion is the increment in plasma glucose concentration, and insulin section was calculated as the increment in plasma insulin concentration (ΔI) (AUC) divided by the increment in plasma glucose concentration (ΔG) (AUC) from 0 to 120 minutes (ΔI/ΔG). Insulin sensitivity during OGTT was calculated from the Matsuda index (MI), and β-cell function was calculated as the insulin secretion (IS)/insulin resistance (IR) index (ΔI0–120/ΔG0–120 × (MI) during OGTT. We have previously shown that IS/IR index calculated with ΔI0–120/ΔG0–120 × MI yields values similar to those calculated with ΔCpep0–120/ΔG0–120 (5, 19).
Statistical analysis
Statistical analyses were performed using SPSS, version 21 (IBM). Differences between values before and after treatment (within PLAC and PIO groups) were analyzed using paired Student t test. Comparisons between PLAC and PIO groups were made by independent-samples t test (or appropriate nonparametric method) or χ2, as appropriate. Risk of developing diabetes was analyzed by Cox proportional hazards regression. Comparison between different stages of glucose tolerance was performed using ANOVA with Bonferroni post-hoc testing when appropriate. The original sample size calculation (n = 600) was provided in an earlier publication (5). The present study represents a post-hoc analysis of subjects who participated in at least one 6-month followup visit. Data are presented as mean ± SEM.
Results
Baseline clinical characteristics
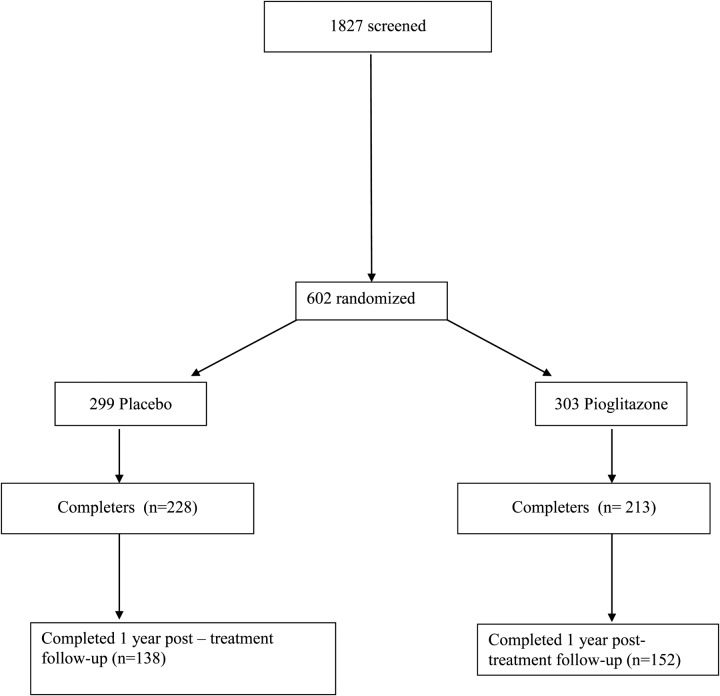
Of the initial cohort of 602 subjects with IGT, 441 individuals received a closeout visit with OGTT, and 290 came for at least the 6-month follow-up visit (Figure 1). Table 1 shows clinical characteristics of the post-treatment follow-up cohort that are very similar to the total cohort of 602 individuals (Supplemental Table 1). There were slightly more females in the PLAC group. Other clinical, anthropometric, and laboratory parameters were similar in PIO and PLAC groups. As expected, at the closeout visit, PIO-treated subjects had lower fasting and 2-hour plasma glucose concentrations and there were more individuals with NGT in PIO vs PLAC group.
Figure 1.
Flow diagram representing number of subjects at time of randomization, at end of active treatment period, and at 11.4 mo following cessation of therapy.
Table 1.
Clinical, Anthropometric, and Laboratory Data at the Time of Entry Into the Post-treatment Follow-up Period
| Characteristic | PIO | PLAC |
|---|---|---|
| n | 152 | 138 |
| NGT/IGT | 81/71 | 49/89 |
| Age, y | 54.2 ± 0.7 | 52 ± 0.7 |
| Weight, kg | 97.1 ± 1.6 | 95.3 ± 1.7 |
| BMI, kg/m2 | 33.4 ± 0.4 | 34.1 ± 0.4 |
| Male/female, n | 74/78 | 59/79 |
| HbA1c, % | 5.5 ± 0.3 | 5.5 ± 0.3 |
| FPG, mg/dL | 91.8 ± 0.7a | 94.4 ± 0.7 |
| 2-h PG, mg/dL | 134 ± 2.5a | 149 ± 2.6 |
| FPI, mU/L | 10.1 ± 0.6 | 10.2 ± 0.5 |
| Total Chol, mg/dL | 170 ± 2 | 171 ± 7 |
| LDL Chol, mg/dL | 105 ± 2 | 106 ± 2 |
| Triglyceride, mg/dL | 121 ± 4 | 120 ± 2 |
| HDL Chol, mg/dL | 40.5 ± 7 | 40.9 ± 2 |
| SBP, mm Hg | 127 ± 1.1 | 127 ± 0.2 |
| DBP, mm Hg | 74 ± 0.6 | 73 ± 0.1 |
Abbreviations: BMI, body mass index; Chol, cholesterol; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FPI, fasting plasma insulin; LDL, low-density lipoprotein; PG, plasma glucose; SBP, systolic blood pressure.
P < .05, PIO vs PLAC.
Incidence of diabetes
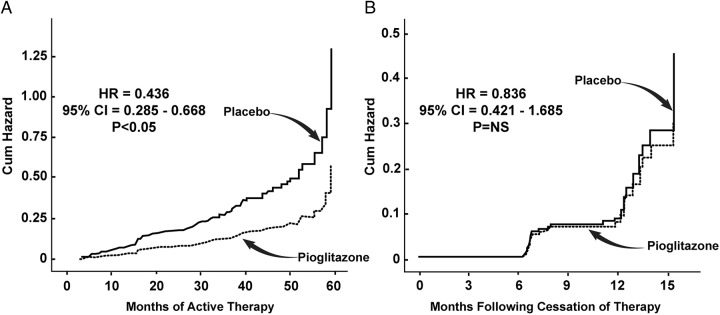
In ACT NOW, median followup was 2.4 years and 50 PLAC subjects and 15 PIO subjects developed diabetes (hazard ratio [HR], 0.28; P < .005). Following cessation of therapy, median follow-up period was 11.4 months in both PIO- and PLAC-treated groups. Diabetes developed in 17/138 (12.3%) in PLAC vs 17/152 PIO (11.2%) (HR, 0.836; 95% confidence interval [CI], 0.421–1.658; P = not significant) (Figure 2B). However, the cumulative incidence of diabetes from time of initial randomization to end of washout period (11.4 mo following cessation of therapy) remained significantly lower in PIO vs PLAC (HR, 0.436; 95% CI, 0.285–0.668; P < .005) (Figure 2A).
Figure 2.
A, HR for the development of diabetes in subjects with IGT who participated in the post-treatment follow-up period (median, 11.4 mo). B, Cumulative HR for the development of diabetes in subjects with IGT from the time of randomization until the end of post-treatment follow-up period.
Presence of NGT
After therapy was discontinued (11.4 mo), 23.0% (35/152) of PIO-treated subjects remained at NGT vs 13.8% (19/138) of PLAC-treated individuals (P = .04). Cumulative incidence of NGT (at least once during the entire followup) was higher in PIO vs PLAC (101/213 vs 61/228; P < .05).
Incidence of diabetes in relation to glucose tolerance at time of therapy cessation
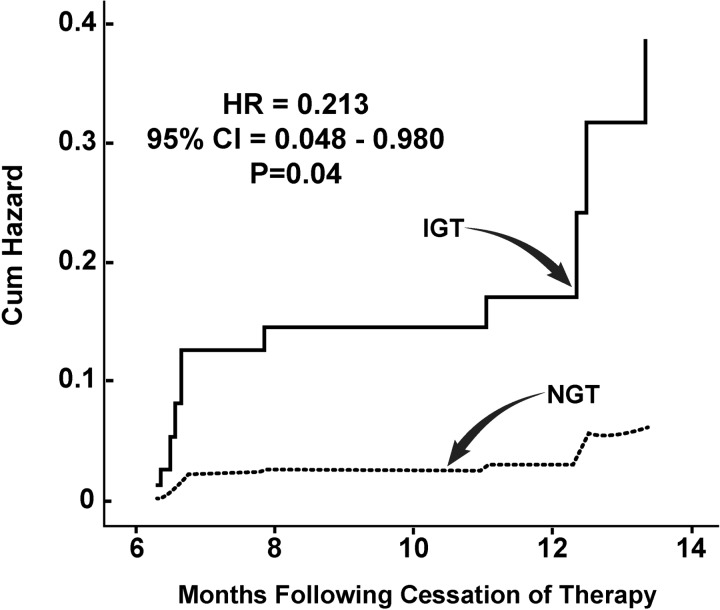
In the PIO group at the end of active treatment (median followup, 2.4 y), 81 subjects had NGT and 71 had IGT. Of subjects with NGT only three (4%) converted to T2DM after 11.4 months vs 13 (24%) who had IGT (HR, 0.176; 95% CI, 0.05–0.618; P = .007) (Figure 3).
Figure 3.
HR for the development of diabetes in relation to glucose tolerance status (NGT, IGT) at entry into the post-treatment follow-up period in PIO-treated subjects.
In the PLAC group at the end of active treatment, 49 had NGT and 89 had IGT (both P < .01 vs PIO). Similarly, the rate of conversion to T2DM was significantly lower in subjects with NGT (2/49; 4%) vs subjects with IGT (15/89; 17%) (HR, 0.213; 95% CI, 0.048–0.96; P = .04) after 11.4 months.
Changes in insulin sensitivity and insulin secretion
After 2.4 years of PIO treatment, both MI (4.11 ± 0.2 to 8.20 ± 0.4; P < .005) and insulin secretion/insulin resistance (IS/IR) index (3.30 ± 0.15 to 5.88 ± 0.4; P < .005) increased markedly. After 2.4 years of PLAC treatment, there was slight improvement in MI (4.12 ± 0.2 to 5.51 ± 0.4; P < .05; P < .01 vs PIO) and IS/IR index (3.32 ± 0.3 to 4.31 ± 0.2; P < .05; P < .01 vs PIO).
Following discontinuation of therapy (11.4 mo), there was no difference in MI (5.15 ± 0.36 vs 5.23 ± 0.45) or IS/IR index (4.03 ± 0.30 vs 3.83 ± 0.27) between PIO and PLAC groups. However, β-cell function (IS/IR index) in the PIO group was significantly improved compared with baseline (ie, time of randomization).
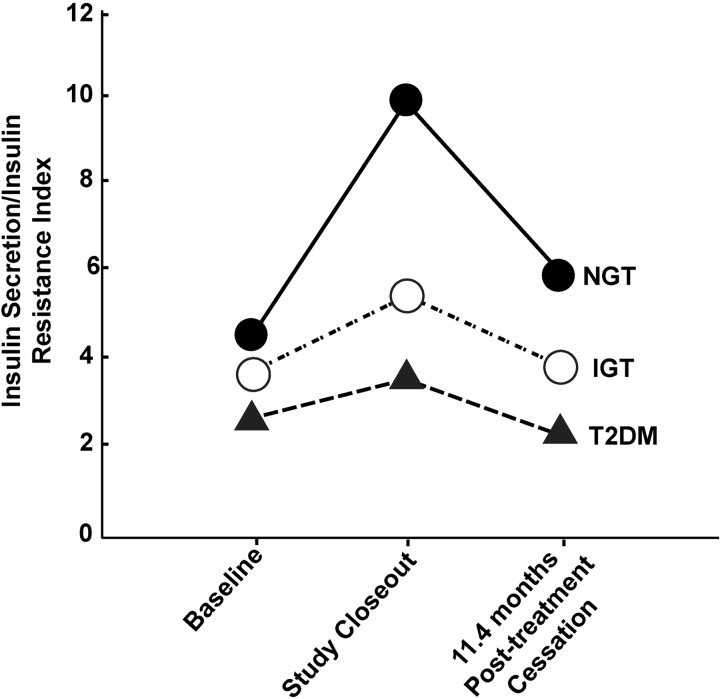
During the 11.4-month follow-up period following therapy cessation, the IS/IR index (ΔI0–120/ΔG0–120 × MI) was markedly lower in subjects with IGT who converted to diabetes compared with subjects who remained IGT (1.30 ± 0.1 vs 3.52 ± 0.2; P < .001) or NGT (1.31 ± 0.1 vs 5.70 ± 0.4; P < .001) (Figure 4). The decline in β-cell function (IS/IR index) was similar in subjects with IGT who developed diabetes, irrespective of whether they were treated with PIO or PLAC, whereas IS/IR index improved in subjects who reverted from IGT to NGT irrespective of treatment with PIO or PLAC.
Figure 4.
IS/IR index in relation to glucose tolerance status at study end.
Change in weight following cessation of therapy
Subjects receiving PIO gained 3.9 kg during active treatment period. Following PIO cessation, body weight decreased by 1.2 kg. Weight gain during the active treatment period and weight loss following PIO cessation was similar in all age and sex groups and in all glucose-tolerance categories. In subjects with IGT treated with PLAC, no significant changes in body weight were observed during active intervention period and following cessation of therapy.
Discussion
The present results are consistent with previously published results (9, 19, 20) and demonstrate that the effect of PIO on T2DM prevention in high-risk subjects with IGT wanes after discontinuation of study medication and that the rate of IGT conversion to T2DM 11.4 months after cessation of therapy is similar in PIO- and PLAC-treated groups. However, the cumulative incidence of diabetes from time of initial randomization to end of followup at 11.4 months (median) remained lower in the PIO vs PLAC group (Figure 2). In addition, a greater number of individuals treated with PIO reverted to NGT compared with PLAC. These findings demonstrate that following discontinuation of PIO disease progression (18) in high-risk individuals with IGT can be slowed although ultimately (ie, 11.4 mo in the present study) the rate of development of diabetes in the PIO-treated group becomes similar to that in the PLAC-treated group.
At the end of the active treatment period in ACT NOW (2.4 y), 58% of PIO-treated subjects reverted to NGT vs 35% of PLAC-treated subjects (5). Ten-year followup of DPP study showed that reversion to NGT at any time during the study was associated with a lower risk of progression to T2DM. In the current study we show that the rate of progression to diabetes following cessation of therapy was only 4% in subjects with NGT vs 24% in individuals with IGT. Although the rate of reversion to NGT in both PIO and PLAC groups was similar during the 11.4 months following cessation of therapy, a greater number of subjects achieved normoglycemia with PIO.
The effect of PIO to prevent diabetes 11.4 months after discontinuation of study medication in the present study is similar (56 vs 39%) to that observed in DREAM with rosiglitazone (17). The results of the present study are somewhat different from those of the TRIPOD study in which women with a history of gestational diabetes treated with troglitazone had a lower conversion rate to diabetes compared with PLAC-treated women 8 months after discontinuation of troglitazone. It is possible that the different study populations (ie, history of gestational diabetes in TRIPOD vs IGT in ACT NOW) or a stronger effect of troglitazone vs PIO on IS could explain this difference between the two studies.
PIO therapy was associated with weight gain of 3.9 kg during active treatment period, and there was a modest weight loss of 1.2 kg when medication was stopped. Body weight of individuals treated with PLAC was stable during active treatment period, as well as during post-treatment followup. Despite weight gain, PIO was associated with better glycemic control and improved insulin sensitivity (MI) and β-cell function. Therefore, weight gain was not associated with any adverse metabolic effects on glucose tolerance, insulin sensitivity, or β-cell function The beneficial effects of PIO, despite weight gain, are mediated by an increase in plasma adiponectin concentration (7, 21), direct effect on insulin sensitivity, improved β-cell function mediated by PPARγ activation (22, 23) and reversal of lipotoxicity (22, 23).
It can be debated whether PIO prevents or just delays or slows development of diabetes. Regardless, there is a lower cumulative exposure to hyperglycemia in subjects with IGT treated with PIO and it is well established that diabetic microvascular complications are related to both the severity and duration of hyperglycemia in type 1 and type 2 diabetes (24, 25). Thus, lower exposure to hyperglycemia is likely to have some protective effect against long-term microvascular complications.
The physiologic mechanisms responsible for IGT conversion to T2DM seem to be similar irrespective of prior treatment. Thus, a decline in β-cell function (IS/IR index) was associated with worsening glycemic control in both PIO and PLAC groups. We previously demonstrated that poor β-cell function at baseline and improved β-cell function in response to therapy were strong predictors of final glucose tolerance status at the end of the active treatment period (19, 26). During active treatment with PIO MI also improved significantly (19), whereas following cessation of PIO therapy insulin sensitivity declined. Thus, PIO not only reduced glycemia, it also improved both core defects present in T2DM (27), whereas cessation of PIO therapy was associated with deterioration of both β-cell function and insulin sensitivity.
In conclusion, our results support previous observations (12–18) that the effect of TZDs on diabetes prevention is attenuated after discontinuation of study drug, but the cumulative incidence of diabetes remains lower in TZD-treated subjects. The novel observation of the present study is that deterioration of glucose tolerance status following discontinuation of PIO therapy is associated with declines in β-cell function and insulin sensitivity, just as improved β-cell function and insulin sensitivity were strong predictors of IGT reversion to NGT and protection against development of diabetes. The potential success, or failure, of a specific medication is related to how effectively the therapy corrects the underlying pathophysiologic defects, ie, β-cell dysfunction and insulin resistance.
Supplementary Material
Acknowledgments
We appreciate the enormous and expert help of our nurses and other technical staff without whom this study would not have been possible. We also are indebted to the 602 patients with impaired glucose tolerance who participated in this study. Lorrie Albarado and Amy Richardson of the University of Texas Health Science Center at San Antonio provided expert secretarial assistance in preparation of the manuscript.
Author Contributions: D.T., T.A.B., P.D.R., and R.A.D. all participated in writing and viewing the first draft of the manuscript, which then was reviewed by all contributing authors (D.C.S., M.A.B., G.A.B., S.C.C., R.R.H., A.E.K., S.M., R.E.R., F.B.S., N.M.) prior to submission. R.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was registered in ClinicalTrials.gov as trial number NCT00220961.
This work was supported in part by GCRC Grant MO1-RR00221 at the University of Tennessee Health Science Center, Clinical and Translational Science Award Grant UL1TR000130 to the University of Southern California, and the South Texas Veterans Health Care System–Audie Murphy Division. The study was supported by an investigator-initiated and unrestricted research grant from Takeda Pharmaceuticals North America. Takeda Pharmaceuticals played no role in the study design, data collection/analysis, or manuscript preparation/review. The salaries of D.T. and R.A.D. are in part supported by the South Texas Veterans Health Care System.
Disclosure Summary: R.A.D. reports receiving grants from Amylin, Takeda, Bristol Myers Squibb, Astra Zeneca, and Janssen; serves on the advisory board for Amylin, Takeda, Bristol Myers Squibb, Novo Nordisk, Janssen, Astra Zeneca, Lexicon, and Boehringer Ingelheim; and is on the Speakers Bureau for Novo Nordisk, BMS, Janssen, and Astra Zeneca. R.A.D.'s salary is supported in part by the South Texas Veterans Health Care System–Audie L. Murphy Division. D.T. reports receiving consultant fees from HDL Diagnostics. D.C.S. reports receiving funding of the Phoenix Data Coordinating Center by a Takeda Grant. M.A.B. reports receiving consulting fees from Sanofi Aventis, Merck, Roche, and Boehringer Ingelheim; grants from Takeda and Merck; and fees for participation in review activities from Novartis and BMS. T.A.B. reports receiving grant support from Allergan and Takeda; advisory panel from Takeda; speakers bureau from Takeda; and stock options from Tethys Bioscience. S.C.C. reports that he is a full-time employee of Merck and Co. R.R.H. reports receiving grant support from AstraZeneca, BMS, Eli Lilly, Sanofi-Aventis, and Medtronics; is a consultant to Boehringer Ingelheim, Gilead, Intarcia, Isis, Eli Lilly, Novo Nordisk, Roche, and Medtronics; is on the advisory board to Amgen, AstraZeneca, BMS, Gilead, Intarcia, Johnson & Johnson/Janssen, Eli Lilly, Merck, Novo Nordisk, Roche, Sanofi-Aventis, Daiichi Sankyo, and Elcelyx. S.M. reports being a speaker to Takeda. R.E.R. reports receiving research support from Takeda. F.B.S. reports no conflict of interest. P.D.R. reports receiving research grants from BMS and Novo Nordisk, speaker support through Amylin, and is a consultant of BMS. G.A.B., A.E.K., and N.M. have nothing to disclose.
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- DPP
Diabetes Prevention Program
- ΔG
increment in plasma glucose concentration
- HR
hazard ratio
- ΔI
increment in plasma insulin concentration
- IGT
impaired glucose tolerance
- IR
insulin resistance
- IS
insulin secretion
- MI
Matsuda index of insulin sensitivity
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- PIO
pioglitazone
- PLAC
placebo
- T2DM
type 2 diabetes mellitus.
References
- 1. Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care. 2013;36(8):2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: Findings from the National Health Interview Survey. Diabetes Care. 2012;35:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magliano DJ, Shaw JE, Shortreed SM, et al. Lifetime risk and projected population prevalence of diabetes. Diabetologia. 2008;51:2179–2186. [DOI] [PubMed] [Google Scholar]
- 4. Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319:1500–1506. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. [DOI] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 8. Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–1105. [DOI] [PubMed] [Google Scholar]
- 10. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916–917. [DOI] [PubMed] [Google Scholar]
- 13. Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone In the Prevention Of Diabetes): A randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials. 1998;19:217–231. [DOI] [PubMed] [Google Scholar]
- 15. Perreault L, Pan Q, Mather KJ, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. [DOI] [PubMed] [Google Scholar]
- 17. Gerstein HC, Mohan V, Avezum A, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia. 2011;54:487–495. [DOI] [PubMed] [Google Scholar]
- 18. Buchanan TA. (How) can we prevent type 2 diabetes? Diabetes. 2007;56:1502–1507. [DOI] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tripathy D, Schwenke DC, et al. Prevention of diabetes with pioglitazone in ACT NOW: Physiologic correlates. Diabetes. 2013;62:3920–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tripathy D, Clement SC, Schwenke DC, et al. Baseline adiponectin levels do not influence the response to pioglitazone in ACT NOW. Diabetes Care. 2014;37:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007;50:1723–1731. [DOI] [PubMed] [Google Scholar]
- 23. Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–4319. [DOI] [PubMed] [Google Scholar]
- 24. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 25. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853. [PubMed] [Google Scholar]
- 26. DeFronzo RA, Tripathy D, Schwenke DC, et al. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care. 2013;36:3607–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.