Abstract
Infection with gastrointestinal parasitic nematodes is a major cause of chronic morbidity and economic burden around the world, particularly in low-resource settings. Some parasitic nematode species, including the human-parasitic threadworm Strongyloides stercoralis and human-parasitic hookworms in the genera Ancylostoma and Necator, feature a soil-dwelling infective larval stage that seeks out hosts for infection using a variety of host-emitted sensory cues. Here, we review our current understanding of the behavioral responses of soil-dwelling infective larvae to host-emitted sensory cues, and the molecular and cellular mechanisms that mediate these responses. We also discuss the development of methods for transgenesis and CRISPR/Cas9-mediated targeted mutagenesis in Strongyloides stercoralis and the closely related rat parasite Strongyloides ratti. These methods have established S. stercoralis and S. ratti as genetic model systems for gastrointestinal parasitic nematodes and are enabling more detailed investigations into the neural mechanisms that underlie the sensory-driven behaviors of this medically and economically important class of parasites.
Keywords: Parasitic helminth, Parasitic nematode, Host seeking, Chemosensation, Thermosensation, Sensory behavior, Strongyloides
Graphical abstract
Highlights
-
•
We review the behavioral responses of parasitic nematodes to host-emitted sensory cues.
-
•
Chemosensation and thermosensation are critical for sensory-driven host seeking.
-
•
The neural and molecular basis of host seeking remains poorly understood.
-
•
CRISPR/Cas9-mediated mutagenesis has recently become feasible in parasitic worms.
-
•
Sensory cascades found in free-living nematodes also drive parasite host seeking.
1. Introduction
Soil-transmitted gastrointestinal parasitic nematodes such as the human threadworm Strongyloides stercoralis and hookworms in the genera Ancylostoma and Necator infect over 600 million people and are a major source of neglected tropical disease. Infections are predominantly reported in resource-limited countries located in the tropical and subtropical regions of the world (Bethony et al., 2006; Bisoffi et al., 2013; Buonfrate et al., 2013; Schar et al., 2013; Nutman, 2017). However, nematode infections persist even within resource-rich countries such as the United States, primarily in areas of extreme poverty (McKenna et al., 2017). Although infections are often asymptomatic, symptoms of chronic, heavy infection can include gastrointestinal distress, stunted growth and cognitive impairment in children, anemia in the case of hookworms, and death in the case of untreated S. stercoralis infections (Bethony et al., 2006; Bisoffi et al., 2013; Buonfrate et al., 2013; Forrer et al., 2017; McKenna et al., 2017; Nutman, 2017). In humans, chronic nematode infections are estimated to cause an annual disease burden of over 5 million disability adjusted life years (DALYs) (Boatin et al., 2012; Pullan et al., 2014). Furthermore, parasitic nematodes that infect grazing livestock are a major economic threat in both developed and underdeveloped countries (Kumar et al., 2013; Roeber et al., 2013; Emery et al., 2016).
Current treatments for gastrointestinal parasitic nematodes rely on anthelminthic drugs to reduce the worm burden of ongoing infections. This strategy does not prevent reinfection, a serious issue in regions where parasitic nematodes are endemic. In addition, drug resistance resulting from repeated treatments in humans is expected to emerge in the near future (Keiser and Utzinger, 2008; Diawara et al., 2013). Indeed, for livestock-parasitic nematodes, resistance to anthelminthic drugs stemming from mass drug administration is a worldwide phenomenon with significant consequences for treatment (Kumar et al., 2013; Roeber et al., 2013; Emery et al., 2016; Learmount et al., 2016). Thus, the identification of new strategies for treating or preventing parasitic nematode infections has clear relevance to global health and economics.
Many gastrointestinal parasitic nematodes, including hookworms and Strongyloides species, invade hosts as environmentally motile infective third-stage larvae (iL3s). The iL3 stage of parasitic nematodes is similar to the dauer larval stage of C. elegans and other free-living nematodes (Hotez et al., 1993; Viney et al., 2005; Crook, 2014). For parasitic nematodes with an iL3 stage, parasitic adults reside in the mucosal lining of the host intestine, where they reproduce. Eggs or young larvae pass into the environment with feces and then mature on feces, passing through multiple larval stages until they developmentally arrest as iL3s. In the case of some Strongyloides species, the nematodes can develop through a limited number of free-living generations before arresting as iL3s (Roberts et al., 2005). For example, S. stercoralis can develop through a single free-living generation (Roberts et al., 2005). S. stercoralis can also cycle through multiple generations in the same host (Roberts et al., 2005). iL3s must infect a host to continue their life cycle. Different species use different strategies for infecting hosts. Some iL3s actively invade by skin penetration, while other iL3s invade passively when they are ingested by a host. Skin-penetrating nematodes include hookworms and Strongyloides species (Roberts et al., 2005; Nutman, 2017; Velikkakam et al., 2017). Passively ingested nematodes include human-infective nodular worms in the genus Oesophagostomum (Storey et al., 2000; Ghai et al., 2014; Cibot et al., 2015), as well as the ruminant-parasitic nematode Haemonchus contortus (Parkins and Holmes, 1989; Terrill et al., 2012). In addition, hookworms in the genus Ancylostoma can infect by both skin penetration and passive ingestion (Yokogawa and Oiso, 1926; Landmann and Prociv, 2003; Haas et al., 2005a; Traub, 2013). Once inside the host, skin-penetrating iL3s migrate through the body, and finally colonize the small intestine (Roberts et al., 2005; Schafer and Skopic, 2006). Passively ingested species travel to the small intestine after being swallowed (Roberts et al., 2005; O'Connor et al., 2006).
Gastrointestinal parasitic nematodes generally have narrow host ranges (Haley, 1961; Bezubik, 1965; Nolan et al., 2007; Viney and Lok, 2007; Viney and Kikuchi, 2017). For example, S. stercoralis naturally infects humans, non-human primates, and dogs; other species in the Strongyloides genus naturally infect other limited subsets of mammals, birds, reptiles, or amphibians (Viney and Lok, 2007). The process by which iL3s locate and infect hosts is likely comprised of several sensory-driven behaviors, including long-range navigation toward a potential host, short-range host identification, and host invasion (Haas, 2003). Together, long-range navigation and short-range identification are collectively referred to as “host seeking,” and involve the recognition of multiple host-emitted sensory cues (Gang and Hallem, 2016). Although it was long thought that only skin-penetrating iL3s participated in sensory-driven host seeking, several studies have suggested that at least some passively ingested iL3s also use host-emitted sensory cues to position themselves near potential hosts (Hernandez and Sukhdeo, 1995; Castelletto et al., 2014; Ruiz et al., 2017; Bryant et al., 2018).
2. Chemosensory responses of skin-penetrating nematodes
Species-specific volatile and soluble chemicals are produced by the skin, sweat, breath, serum, skin microbiota, urine, and feces of all mammals (Sharaf et al., 1977; Cork and Park, 1996; Arnould et al., 1998; Bernier et al., 1999, 2000, 2002; Safer et al., 2007; Gallagher et al., 2008; Psychogios et al., 2011; Verhulst et al., 2011; Starkenmann, 2017; Moulvi et al., 2018). Skin extracts appear to be attractive to the iL3s of some skin-penetrating species but not others (Table 1). For example, both the dog hookworm Ancylostoma caninum and S. stercoralis, which naturally infects dogs as well as humans, are attracted to dog skin extract (Granzer and Haas, 1991; Safer et al., 2007). Skin extracts are also known to stimulate skin penetration in A. caninum and some Strongyloides species (Granzer and Haas, 1991; Sakura and Uga, 2010). For these species, iL3s penetrate host skin at a higher frequency than non-host skin, an effect thought to result from host-specific penetration-stimulating chemical cues (Granzer and Haas, 1991; Sakura and Uga, 2010). In contrast, human skin extract does not appear to attract the human hookworms Ancylostoma duodenale and Necator americanus, although it does trigger increased crawling and skin penetration (Haas et al., 2005a, 2005b). Host serum and serum components such as sodium chloride are attractive for skin-penetrating iL3s in the genera Ancylostoma, Necator, and Strongyloides (Table 1) (Zietse et al., 1981; Wauters et al., 1982; Vetter et al., 1985; Ma, 1987; Granzer and Haas, 1991; Tada et al., 1997; Koga and Tada, 2000; Tobata-Kudo et al., 2000a; Forbes et al., 2003, 2004; Koga et al., 2004, 2005; Haas et al., 2005a).
Table 1.
The known responses of selected environmentally motile parasitic nematode infective larvae to host-emitted chemical attractants and thermosensory cues. The behaviors listed are those that have been described for iL3s of the indicated species; behaviors not listed either have not been tested or have been tested but not observed. The chemical attractants listed include only known chemical attractants for iL3s prior to host infection.
| Nematode Species | Hosts | Infection Mode | Chemical Attractants | Temperature-Driven Behaviors | References |
|---|---|---|---|---|---|
|
Necator americanus |
humans |
skin penetration |
NaCl |
arousal positive thermotaxis skin penetration |
Haas et al., 2005a,b Koga et al., 2004 |
|
Ancylostoma duodenale |
humans |
skin penetration oral infection |
arousal positive thermotaxis skin penetration nictation |
Haas et al., 2005a,b |
|
|
Ancylostoma ceylanicum |
humans dogs cats other carnivores |
skin penetration oral infection |
positive thermotaxis negative thermotaxis migration speed altered path curvature |
Bryant et al., 2018 |
|
|
Ancylostoma caninum |
dogs |
skin penetration oral infection |
serum skin extract |
arousal positive thermotaxis skin penetration nictation |
Bhopale et al., 2001 Granzer and Haas, 1991 Vetter et al., 1985 Wauters et al., 1982 Zietse et al., 1981 |
|
Strongyloides stercoralis |
humans primates dogs |
skin penetration |
host odorants skin extract serum NaCl sweat |
arousal positive thermotaxis negative thermotaxis migration speed altered path curvature |
Bryant et al., 2018 Castelletto et al., 2014 Forbes et al., 2003, 2004 Koga et al., 2005 Lopez et al., 2000 Safer et al., 2007 |
|
Strongyloides ratti |
rats |
skin penetration |
host odorants serum NaCl |
arousal positive thermotaxis negative thermotaxis skin penetration altered path curvature |
Barrett, 1968 Bryant et al., 2018 Castelletto et al., 2014 Koga and Tada, 2000 Lee et al., 2016 Sakura and Uga, 2010 Tada et al., 1997 Tobata-Kudo et al., 2000a,b |
|
Nippostrongylus brasiliensis |
rats |
skin penetration |
host odorants |
arousal positive thermotaxis negative thermotaxis altered path curvature |
Bryant et al., 2018 Castelletto et al., 2014 Parker and Haley, 1960 |
|
Heligmosomoides polygyrus |
mice |
oral infection |
host odorants fecal odor urine skin extract CO2 |
positive thermotaxis negative thermotaxis |
Bryant et al., 2018 Hernandez and Sukhdeo, 1995 Ruiz et al., 2017 |
| Haemonchus contortus | ruminants | oral infection | host odorants CO2 |
thermotaxis toward previous cultivation temperature |
Castelletto et al., 2014 Li et al., 2000a, 2000b Ruiz et al., 2017 |
A number of olfactory cues have also been identified as attractants for skin-penetrating nematodes (Table 1) (Gang and Hallem, 2016). For example, S. stercoralis iL3s are attracted to urocanic acid, a component of mammalian skin extract (Safer et al., 2007). S. stercoralis iL3s are also attracted to other odorants found in human skin and sweat, including many that are known to attract mosquitoes (Castelletto et al., 2014). Comparisons of the olfactory behaviors of diverse parasitic nematode species have revealed that olfactory preferences are species-specific (Castelletto et al., 2014; Ruiz et al., 2017). Furthermore, olfactory preferences reflect host range rather than genetic relatedness. For example, the chemosensory responses of the rat parasite Strongyloides ratti are more similar to those of the distantly related rat parasite Nippostrongylus brasiliensis than to those of a close relative, S. stercoralis (Castelletto et al., 2014). These results suggest that species-specific olfactory cues play a role in the ability of parasitic nematodes to locate and identify hosts. In addition, the olfactory preferences of at least some skin-penetrating iL3s can be modulated over the course of multiple days by the cultivation temperature recently experienced by the iL3s (Lee et al., 2016), perhaps enabling the iL3s to adapt to seasonal changes in environmental or host-emitted chemosensory cues (Ferkin et al., 1995; Heth et al., 1996; Zhang et al., 2005).
While the iL3s of Strongyloides species are attracted to skin and sweat odorants, they are not attracted to fecal odor (Castelletto et al., 2014). In contrast, the non-infective free-living larval and adult stages of Strongyloides species are attracted to fecal odor (Castelletto et al., 2014). The free-living life stages of Strongyloides species inhabit feces and feed off fecal bacteria, whereas the iL3s migrate off feces and into the soil to host seek (Gang and Hallem, 2016). Thus, loss of attraction to fecal odor specifically at the infective stage may enable iL3s to disperse from feces to host seek (Castelletto et al., 2014). The mechanisms that generate this life-stage-specific change in the valence of olfactory responses have not yet been investigated. Furthermore, the responses of hookworm species to fecal odor have not yet been examined.
An important host-emitted cue for many parasitic animals is the respiratory byproduct carbon dioxide (CO2) (Gang and Hallem, 2016; Takken and Verhulst, 2017). While the CO2 concentration in ambient air is ∼0.038% (Scott, 2011), the CO2 concentration in mammalian breath is ∼5% (Byrnes et al., 1997; Buszewski et al., 2007). The responses of skin-penetrating iL3s to CO2 vary across species. Early studies of iL3 responses to CO2 found that both A. caninum and S. stercoralis iL3s showed increased crawling in response to CO2 (Sciacca et al., 2002). A. caninum also showed CO2-induced increases in nictation, a host-seeking behavior thought to facilitate attachment to host skin in which the worm stands on its tail and waves its head (Granzer and Haas, 1991). In contrast, N. americanus and A. duodenale showed increased crawling only in response to CO2 in combination with warmth (35°C) or moisture (Haas et al., 2005a). More recently, the iL3s of multiple skin-penetrating species, including S. stercoralis, A. ceylanicum, S. ratti, and N. brasiliensis, were found to be repelled by CO2 in a chemotaxis assay (Castelletto et al., 2014; Ruiz et al., 2017). A lack of CO2 attraction by skin-penetrating iL3s is consistent with their infection route, since only low levels of CO2 are emitted from mammalian skin (Alkalay et al., 1971). Furthermore, fecal deposits emit high concentrations of CO2 (Jensen and Jorgensen, 1994; de Lacy Costello et al., 2014; Rotbart et al., 2017), and CO2 repulsion may facilitate host seeking by driving dispersal from feces into the soil environment.
3. Chemosensory responses of passively ingested nematodes
Although passively ingested nematodes do not actively invade hosts, a number of studies have revealed that they nevertheless respond robustly to host-emitted chemosensory cues (Table 1). For example, the passively ingested murine parasite Heligmosomoides polygyrus is attracted to mouse urine and epidermal lipids (Hernandez and Sukhdeo, 1995), mammalian skin and sweat odorants (Ruiz et al., 2017), and host fecal odor (Ruiz et al., 2017). Attraction to mammalian-derived odorants may drive increased contact with host skin and fur, which can lead to ingestion during grooming (Hernandez and Sukhdeo, 1995). Similarly, attraction to fecal odor may facilitate ingestion during coprophagy. Despite their attraction to fecal odor, H. polygyrus iL3s nevertheless eventually disperse from feces to engage in environmental navigation (Ruiz et al., 2017). The sensory cues that drive dispersal from feces are not yet clear. However, the ability of H. polygyrus iL3s to disperse from feces and migrate toward host-emitted odors may indicate that if a new host does not ingest their initial fecal source, passively ingested iL3s will seek out either a new fecal source or a host (Ruiz et al., 2017). As with H. polygyrus, the passively ingested ruminant parasite Haemonchus contortus is attracted to some mammalian odorants (Castelletto et al., 2014; Ruiz et al., 2017). H. contortus iL3s are also attracted to the smell of cut grass, which may enable them to migrate off feces and into grass, where they are more likely to encounter grazing ruminants (Castelletto et al., 2014). Together, these results suggest that orally ingested infective larvae use sensory-driven navigation to facilitate host engagement (Hernandez and Sukhdeo, 1995; Castelletto et al., 2014; Ruiz et al., 2017).
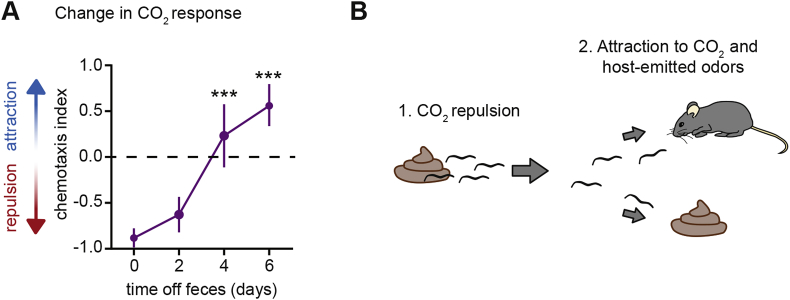
Interestingly, passively ingested iL3s show experience-dependent responses to CO2 (Ruiz et al., 2017). In the case of H. polygyrus, iL3s cultivated on feces are repelled by CO2, whereas iL3s that have been removed from feces for multiple days are attracted to CO2 (Fig. 1A). Cultivation under high CO2 conditions (2.5% ambient CO2) mimics the on-feces condition, such that iL3s removed from feces and then cultivated at high CO2 remain repelled by CO2. The response of H. polygyrus to the odorant benzaldehyde also changes from repulsive to attractive following removal from feces (Ruiz et al., 2017). Thus, H. polygyrus shows experience-dependent changes in the valence of multiple chemosensory responses. In the case of H. contortus, iL3s that are cultivated on feces are neutral to CO2; iL3s cultivated off feces for multiple days are attracted to CO2 (Castelletto et al., 2014; Ruiz et al., 2017). Whether the responses of H. contortus to other odorants also change following removal from feces has not yet been investigated. Both CO2 and benzaldehyde are emitted from mammalian feces (Jensen and Jorgensen, 1994; Garner et al., 2007; De Preter et al., 2009; Martin et al., 2010; Apps et al., 2012; de Lacy Costello et al., 2014; Rotbart et al., 2017; Uetake et al., 2017). The experience-dependent plasticity exhibited by passively ingested iL3s in response to fecal-associated chemosensory cues, in combination with their attraction to mammalian skin odorants, suggests that chemosensation contributes to a highly flexible infection strategy wherein iL3s that are not rapidly ingested by a host can abandon their original fecal sources, disperse into the environment, and then navigate toward more favorable ingestion conditions (Fig. 1B) (Ruiz et al., 2017).
Fig. 1.
The chemosensory preferences of passively ingested H. polygyrus iL3s are highly flexible. A. H. polygyrus iL3s display an experience-dependent shift in CO2 preference; the response of iL3s to 10% CO2 switches from repulsive to attractive over the course of 6 days following removal from feces. The chemotaxis index is a measure of chemosensory preference that ranges from +1 to −1, with +1 indicating maximum attraction and −1 indicating maximum repulsion. ***p < 0.001 relative to day 0, Kruskal-Wallis test with Dunn's post-test. Figure reproduced from Ruiz et al. (2017). B. Diagram representing a model wherein experience-dependent responses to CO2 and other fecal odorants contribute to a flexible infection strategy in which passively ingested iL3s abandon older feces that are not rapidly ingested by a host, in favor of seeking out either new fecal sources or host animals. iL3s are not drawn to scale.
4. Thermosensory responses of skin-penetrating nematodes
Unlike chemosensory cues, thermosensory cues are not thought to contribute to the selectivity of environmentally motile mammalian-parasitic iL3s for their host species. However, directional navigation in response to thermal gradients is likely a major feature of the ability of these nematodes to migrate rapidly toward potential hosts. Consistent with a critical role for thermotaxis in host seeking, iL3s display robust temperature-driven behaviors. In the case of many skin-penetrating iL3s, sudden exposure to temperatures approximating host body temperature results in behavioral arousal, as defined by a non-directional increase in locomotion. For example, a number of species in the genera Strongyloides and Ancylostoma exhibit an increase in crawling behavior and an increase in crawling speed in response to heat (Table 1) (Croll and Smith, 1972; Granzer and Haas, 1991; Haas et al., 2005a; Castelletto et al., 2014; Bryant et al., 2018).
Early studies testing temperature-driven navigation used small agar surfaces to observe relatively straight short-range migration toward heat by the iL3s of multiple skin-penetrating parasitic nematode species, including those that infect humans (Table 1) (Reesal, 1951; Parker and Haley, 1960; Gupta, 1963; Barrett, 1968; Croll and Smith, 1972; Granzer and Haas, 1991; Lopez et al., 2000; Tobata-Kudo et al., 2000b; Haas et al., 2005b). The parasitic adults of several species were also reported to swim up thermal gradients (McCue and Thorson, 1964). However, these experiments are difficult to interpret given the possible contribution of convection currents (Croll and Smith, 1972). Thus, the responses of non-infective life stages to thermal gradients remain unclear.
4.1. Skin-penetrating iL3s exhibit positive and negative thermotaxis
More recently, the ability of thermosensation to act as a long-distance host cue was assayed using a large-format thermal arena based on those designed for C. elegans (Goodman et al., 2014) but modified for use with iL3s. For a phylogenetically diverse set of skin-penetrating nematodes, iL3s were found to display both positive and negative thermotaxis, with the thermal switch point between positive and negative thermotaxis determined by the recently experienced environmental temperature (Table 1) (Tobata-Kudo et al., 2000b; Bryant et al., 2018). iL3s exposed to temperatures above the thermal switch point engage in positive thermotaxis toward temperatures above mammalian skin surface temperature (∼31–34°C) (Benedict et al., 1919; Burton, 1935; Haas et al., 2005a, 2005b; Bryant et al., 2018). Having a preferred temperature above skin surface temperature may drive migration that does not attenuate as the iL3s approach a heat source (Bryant et al., 2018). Negative thermotaxis is characterized by robust movement toward colder temperatures and has been observed with multiple species of skin-penetrating iL3s (Table 1) (Tobata-Kudo et al., 2000b; Bryant et al., 2018). For parasitic iL3s, negative thermotaxis may act as a dispersal mechanism, promoting movement away from temperatures that do not clearly signal the presence of a mammalian host. By moving iL3s toward cooler temperatures, negative thermotaxis may enhance subsequent detection of host-emitted heat.
Could skin-penetrating iL3s sense and respond to thermal cues emitted from host animals? At foot to hip level, the thermal microclimate surrounding the human body is approximately 8 cm thick (Gao and Niu, 2005; Voelker et al., 2014). Thus, when skin-penetrating iL3s encounter heat emitted from a standing or sitting human, they will be at most ∼8 cm away. In an artificial thermal gradient, iL3s were observed to crawl distances of over 15 cm in order to reach mammalian skin temperature (Bryant et al., 2018). The ability of S. stercoralis iL3s to engage in positive thermotaxis toward distantly located host temperature suggests that they are likely capable of migrating toward thermal stimuli emitted naturally from a human host.
C. elegans adults also engage in positive and negative thermotaxis to navigate in relation to a “remembered” cultivation temperature (TC), such that at temperatures above TC they display negative thermotaxis and at temperatures below TC they display positive thermotaxis (Hedgecock and Russell, 1975; Mori and Ohshima, 1995; Ryu and Samuel, 2002; Ito et al., 2006; Clark et al., 2007b; Ramot et al., 2008b; Garrity et al., 2010; Jurado et al., 2010). However, the temperature range over which C. elegans navigates is very different from that over which skin-penetrating iL3s navigate. C. elegans adults engage in thermotaxis within their physiological temperature range of ∼15°C–25°C and show noxious avoidance of temperatures above 25°C (Hedgecock and Russell, 1975; Mori and Ohshima, 1995; Wittenburg and Baumeister, 1999; Ryu and Samuel, 2002; Ito et al., 2006; Clark et al., 2007b; Ramot et al., 2008b; Garrity et al., 2010; Jurado et al., 2010; Glauser et al., 2011; Glauser, 2013; Schild and Glauser, 2013; Glauser and Goodman, 2016; Bryant et al., 2018). The responses of C. elegans dauers to thermal stimuli remain poorly understood, but they appear to be insensitive to thermal stimuli that are noxious for adults (Wittenburg and Baumeister, 1999; Bryant et al., 2018).
4.2. Recently experienced environmental temperatures regulate thermosensory behaviors
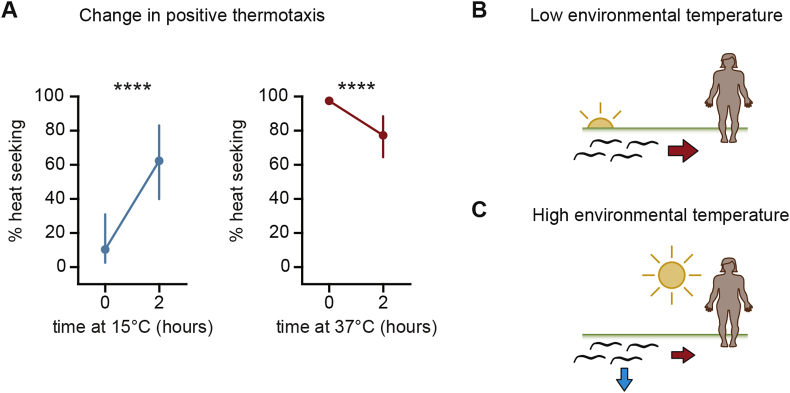
For C. elegans adults, exposure to a new ambient temperature resets TC within hours and alters thermotaxis behavior (Hedgecock and Russell, 1975; Mohri et al., 2005; Kodama et al., 2006; Kuhara and Mori, 2006; Clark et al., 2007b; Garrity et al., 2010). Similarly, changes to the ambient temperature modulate the threshold for triggering noxious heat avoidance (Schild et al., 2014). As in C. elegans, the thermal switch point between positive and negative thermotaxis in skin-penetrating iL3s is regulated by the recently experienced environmental temperature (Tobata-Kudo et al., 2000b; Bryant et al., 2018), and changing the ambient temperature shifts the thermal switch point toward the new ambient temperature within hours (Bryant et al., 2018). For example, iL3s cultivated at 15°C are more likely to engage in positive thermotaxis when placed at temperatures between 21°C and 23°C than iL3s cultivated at 23°C (Fig. 2A) (Bryant et al., 2018). In addition, prolonged cultivation of A. caninum iL3s at 7°C was found to significantly reduce both the preferred temperature of the larvae and the temperature that triggers skin-penetration behavior (Granzer and Haas, 1991). The rapid increase in positive thermotaxis following cultivation at cooler temperatures suggests that iL3s can modulate their behavior in response to diurnal temperature fluctuations (Bryant et al., 2018). Specifically, it suggests that iL3s may be more likely to engage in temperature-driven host seeking in the late evening or early morning, when environmental temperatures are low (Bennett et al., 1988; Robinson, 1994) and hosts are active (Fig. 2B). However, when iL3s are cultivated near host body temperature, thermal plasticity is reduced, such that iL3s still display positive thermotaxis and skin-penetration behaviors (Fig. 2A) (Granzer and Haas, 1991; Bryant et al., 2018). Thus, iL3s will likely engage in temperature-driven host seeking even when environmental temperatures are high (Fig. 2C).
Fig. 2.
Recently experienced environmental temperatures alter the thermosensory preferences of environmentally motile S. stercoralis iL3s. A. Left: S. stercoralis iL3s initially cultured at 23°C and then shifted to 15°C for 2 h show a dramatic increase in positive thermotaxis when placed at ∼22°C in a 20–33°C thermal gradient. Right: S. stercoralis iL3s initially cultured at 23°C and then shifted to 37°C for 2 h show a slight decrease in positive thermotaxis when placed at ∼30°C in a 21–33°C thermal gradient. ****p < 0.0001, two-way ANOVA with Tukey's post-test. Graphs depict medians and interquartile ranges. In some cases, error bars are too small to be visible. Figure modified from Bryant et al. (2018) with permission (Bryant et al., 2018). B. Model showing the possible temperature-driven movements of iL3s that have recently experienced cooler environmental temperatures. iL3s primarily display positive thermotaxis, which may direct them toward potential hosts. Ethologically, this scenario may arise in the late evening or early morning, when environmental temperatures are low and hosts are active. iL3s are not drawn to scale. C. Model showing the possible temperature-driven movements of iL3s that have recently experienced warmer environmental temperatures. iL3s engage in both positive and negative thermotaxis, which may direct some iL3s toward hosts and some deeper in the soil, where temperatures are cooler. Ethologically, this scenario may arise in the daytime, when environmental temperatures are highest. iL3s are not drawn to scale.
4.3. Thermal drive regulates multiple aspects of iL3 movement
The movement patterns of skin-penetrating iL3s are regulated by thermal cues, such that thermal conditions drive transitions between long-range navigation, local search, and skin-penetration behavior. Across species, skin-penetrating iL3s that are engaged in positive thermotaxis at temperatures well below host body temperature travel in relatively straight trajectories (Croll and Smith, 1972; Tobata-Kudo et al., 2000b; Bryant et al., 2018). Their tracks become more curved as the iL3s approach their preferred temperature, suggesting that iL3s transition from long-range navigation to local search as they gain proximity to a potential host (Haas, 2003; Castelletto et al., 2014; Bryant et al., 2018). The unstimulated movements of iL3s are also highly curved (Croll and Smith, 1972; Bryant et al., 2018). Together, this suggests that local search is a basal behavior that can be suppressed by strong thermal drive. Once skin-penetrating iL3s have neared host body temperature, they re-engage in local search and initiate other behaviors associated with host invasion, such as nictation and skin penetration (Granzer and Haas, 1991; Haas et al., 2005a; Sakura and Uga, 2010; Castelletto et al., 2014; Bryant et al., 2018).
4.4. Thermal drive can overcome chemosensory responses
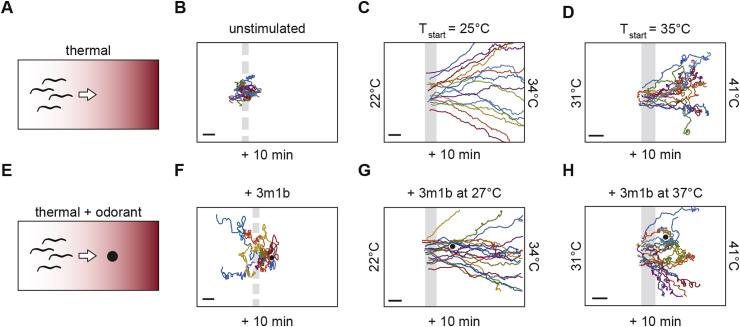
The potent ability of heat and odorants to drive host-seeking behaviors in skin-penetrating iL3s raises the question of whether these two sensory modalities interact to regulate behavior. The anthropophilic mosquito Aedes aegypti integrates multiple sensory modalities during host seeking, such that CO2 stimuli enable behavioral responses to heat and host-emitted odorants (McMeniman et al., 2014). Similarly, for A. duodenale and N. americanus iL3s, a stream of CO2 only stimulates movement in the presence of warmth or moisture, an effect thought to reflect a response to human breath (Haas et al., 2005a). During directed navigation, the presence of strong thermal drive can overcome the attraction of S. stercoralis iL3s to host-emitted odorants, suggesting that thermal cues can be dominant to olfactory cues (Fig. 3) (Bryant et al., 2018). Specifically, when an attractive host odorant is placed below mammalian body temperature in a thermal gradient, iL3s bypass the odorant to travel up the thermal gradient. However, when the odorant is placed at mammalian body temperature, iL3s are less likely to exceed the odorant's temperature as compared to their migration in pure thermal gradients (Fig. 3) (Bryant et al., 2018). Additional experiments are needed to determine whether thermal drive can overcome repulsive odorants, gate responses to neutral odorants, or enhance the responses of iL3s to other sensory modalities.
Fig. 3.
Sensory-driven migration of S. stercoralis iL3s. A. Diagram of an experiment testing the migration of iL3s in a linear thermal gradient. The temperature increases from left to right across the gradient. iL3s are not drawn to scale. B. Tracks of individual S. stercoralis iL3s displaying unstimulated movement patterns while migrating for 10 min on an isothermal room temperature agar plate. Dashed bar indicates approximate starting location of iL3s. C. Tracks of individual S. stercoralis iL3s displaying positive thermotaxis at temperatures below host body temperature. The gradient ranges from 20 to 34°C; the starting temperature of the iL3s (Tstart) = ∼25°C. Only a portion of the plate is shown. D. Tracks of individual S. stercoralis iL3s displaying positive thermotaxis at temperatures near host body temperature. The gradient ranges from 29 to 41°C; Tstart = ∼35°C. Only a portion of the plate is shown. iL3s migrate up the gradient, although with increased tortuosity relative to their migration at lower temperatures. E. Diagram of an experiment testing the interaction between a thermal gradient and an odorant. Black dot indicates a point source of the odorant. F. Tracks of S. stercoralis iL3s displaying chemosensory-driven movement toward the attractive host odorant 3-methyl-1-butanol (3m1b, 5 μL undiluted) under isothermal room temperature conditions. Dashed bar indicates approximate starting location of iL3s. G.S. stercoralis iL3s bypass the host odorant to travel up the thermal gradient when exposed to 3m1b in thermal gradients below host body temperature. The gradient ranges from 20 to 34°C; Tstart = ∼25°C and the temperature at which the odorant is placed (Todorant) = 27°C. Only a portion of the plate is shown. H. The temperature-driven migration of S. stercoralis iL3s near host body temperatures is attenuated by the presence of 3m1b. The gradient ranges from 29 to 41°C; Tstart = ∼35°C and Todorant = 37°C. For B-D and F--H, scale bar indicates 2 cm. Assays in B-D and F-H ran for 10 min. B-D and F are reproduced from Bryant et al. (2018) with permission (Bryant et al., 2018); G-H are modified from Bryant et al. (2018) with permission (Bryant et al., 2018).
5. Thermosensory responses of passively ingested nematodes
Attraction to mammalian body temperature is not limited to skin-penetrating iL3s. iL3s of the passively ingested murine parasite H. polygyrus also display robust positive thermotaxis to temperatures near host body temperature (Table 1) (Bryant et al., 2018). The attraction of H. polygyrus iL3s to heat is consistent with their attraction to mammalian odorants (Hernandez and Sukhdeo, 1995; Ruiz et al., 2017), and suggests that H. polygyrus iL3s navigate toward hosts to position themselves where they are more likely to be ingested. Moreover, like skin-penetrating iL3s, H. polygyrus iL3s can display either positive or negative thermotaxis, depending on the precise thermal gradient and their prior cultivation temperature (Bryant et al., 2018). In contrast to H. polygyrus, iL3s of the passively ingested ruminant parasite H. contortus display an experience-dependent preference for their previous cultivation temperature, similar to C. elegans (Li et al., 2000b). The responses of other passively ingested nematodes to thermal stimuli have not yet been investigated.
6. Mechanosensory responses of parasitic nematodes
The mechanosensory responses of C. elegans have been widely studied and consist of a diverse group of behaviors (Goodman, 2006; Chalfie et al., 2014). Mechanosensory stimuli that are known to generate behavioral responses in C. elegans adults include but are not limited to gentle touch (Sulston et al., 1975; Chalfie and Sulston, 1981; Wicks and Rankin, 1995; Wicks et al., 1996; Goodman, 2006), harsh touch (Way and Chalfie, 1989; Chalfie and Wolinsky, 1990), nose touch (Kaplan and Horvitz, 1993), and vibration (Goodman, 2006; Holbrook and Mortimer, 2018). The specific behavior produced is dependent on the precise nature of the mechanical stimulus (Goodman, 2006; Chalfie et al., 2014). For example, a gentle touch stimulus delivered selectively to the nose can elicit one of two distinct behavioral responses, depending on the orientation of the stimulus: gentle touch to the front of the nose triggers backward locomotion, while gentle touch to the side of the nose triggers a head-withdrawal reflex (Kaplan and Horvitz, 1993; Driscoll and Kaplan, 1997; Chalfie et al., 2014). Mechanosensory responses can habituate and sensitize following repeated stimulation (Rankin et al., 1990; Hobert, 2003; Chen and Chalfie, 2014), and can be modulated by internal and external conditions such as the presence of bacterial food and starvation state (Way and Chalfie, 1989; Chao et al., 2004; Goodman, 2006; Chalfie et al., 2014). In addition, C. elegans dauers engage in nictation, a behavior associated with mechanosensory circuits (Lee et al., 2012). Nictation is thought to act as a dispersal mechanism for dauers by facilitating phoretic associations with insects and other invertebrates (Lee et al., 2012).
Unlike C. elegans, the mechanosensory responses of soil-transmitted parasitic nematodes have not been closely examined. Nevertheless, several observations indicate that for soil-transmitted iL3s, tactile responses are likely to be involved in multiple behaviors, including host detection, host contact, and host invasion. Substrate vibration has been found to trigger arousal in multiple mammalian-parasitic species (Granzer and Haas, 1991; Haas et al., 2005a); this observation suggests a strategy wherein iL3s transition from quiescence to host seeking when they detect the seismic signals generated by the movement of a potential host (Haas, 2003). Further experiments are needed to characterize the effect of substrate vibration on iL3 behavior and to determine how responses to vibration differ across species with different hosts and infection routes.
Mechanosensory stimuli are also likely to contribute to short-range host attachment and host invasion. Touch stimulates arousal and increases crawling speed (Haas, 2003; Castelletto et al., 2014), which may increase the likelihood of host contact. In addition, the iL3s of many parasitic nematode species engage in nictation, which facilitates host attachment (Granzer and Haas, 1991). Mechanosensation is also thought to play a role in skin penetration (Fine et al., 1997). Interestingly, skin-penetrating iL3s have been shown to enter host tissue nose-first (Zaman et al., 1980; Sakura and Uga, 2010), suggesting their response to nose touch is likely very different from that of C. elegans. However, the precise responses of parasitic nematodes to nose touch and other touch stimuli remain to be investigated.
7. Other sensory modalities that contribute to host seeking
Additional sensory responses may contribute to the host-seeking behaviors of iL3s, including responses to moisture and light. Responses to moisture are poorly understood, although changes in humidity have been shown to drive behavioral arousal in skin-penetrating iL3s and to enhance nictation and skin-penetration behavior (Haas, 2003; Haas et al., 2005a). These effects may be due in part to moist environments promoting greater motility (Granzer and Haas, 1991; Haas, 2003). In addition, passively ingested H. contortus iL3s display greater vertical migration in moist environments (Rees, 1950). However, whether iL3s display directional migration toward moisture remains unclear. Some parasitic species may also display rheotaxis – swimming against current flow – although others do not (Fulleborn, 1924; Bone, 1985). Phototactic responses have been reported for some iL3s but not others (Rees, 1950; Reesal, 1951; Parker and Haley, 1960; Croll and Smith, 1972; Granzer and Haas, 1991; Haas et al., 2005a, 2005b). However, positive responses to light sources are difficult to interpret, since the infrared component of bright light may activate thermotaxis behaviors (Parker and Haley, 1960).
8. Neural circuits underlying host seeking
The neural basis of sensory-driven host seeking by parasitic nematodes is not well understood. In contrast, sensory behaviors have been studied extensively in C. elegans, as have the underlying genetic and neural mechanisms. Sensory neuroanatomy and function are at least partly conserved across many nematode species, including free-living and parasitic species (Ashton et al., 1995, 1998, 1999, 2007; Ashton and Schad, 1996; Fine et al., 1997; Li et al., 2000a, 2000b, 2001; Bhopale et al., 2001; Forbes et al., 2004; Ketschek et al., 2004; Zhu et al., 2011; Gang and Hallem, 2016). Thus, C. elegans serves as a powerful starting point for elucidating the molecular and cellular mechanisms that drive host seeking, since many of the neurons that mediate sensory behaviors in C. elegans are also likely to be critical for sensory behaviors in parasitic nematodes. However, a complete mechanistic understanding of host seeking will require identification of the specific molecular and cellular adaptations that enable parasitic but not free-living nematodes to host seek. In addition, since parasitism has arisen multiple times within the phylum Nematoda (Blaxter et al., 1998; Meldal et al., 2007; van Megan et al., 2009; Blaxter and Koutsovoulos, 2014), some of the parasite-specific adaptations that drive host seeking may differ across phylogenetically distant species such as those in the genus Strongyloides and those in the genera Ancylostoma and Necator.
8.1. Sensory neuron function in C. elegans
In C. elegans, the primary sensory organs are the bilateral amphid sensilla in the head; each amphid contains the ciliated dendrites of 12 sensory neurons that are named based on the unique morphology of their sensory endings (Perkins et al., 1986; Bargmann, 2006). In each amphid, 11 sensory neurons detect chemosensory cues such as odorants, tastants, and pheromones (Bargmann, 2006; Rengarajan and Hallem, 2016). For example, olfactory attraction is mediated primarily by the AWA and AWC neurons, while olfactory repulsion is mediated primarily by the AWB, ASH, and ADL neurons (Bargmann et al., 1990, 1993; Troemel et al., 1997; Bargmann, 2006; Rengarajan and Hallem, 2016). An additional amphidial sensory neuron pair, AFD, acts as the primary thermosensory neuron pair (Mori and Ohshima, 1995; Bargmann, 2006; Chung et al., 2006; Ramot et al., 2008a; Garrity et al., 2010; Wasserman et al., 2011; Luo et al., 2014; Goodman and Sengupta, 2018; Hawk et al., 2018). The AWC olfactory neurons also respond to thermal stimuli, including noxious heat (Biron et al., 2008; Kuhara et al., 2008; Garrity et al., 2010; Kotera et al., 2016). Several non-amphidial sensory neurons contribute to the detection of noxious thermal cues (Glauser et al., 2011; Liu et al., 2012). In addition, a number of amphidial and non-amphidial neurons mediate the detection of oxygen (O2) and CO2 (Cheung et al., 2004; Gray et al., 2004; Zimmer et al., 2009; Couto et al., 2013; Kodama-Namba et al., 2013; Carrillo and Hallem, 2015; Fenk and de Bono, 2015; Rengarajan and Hallem, 2016), including the BAG neurons in the head, which detect molecular CO2 (Hallem and Sternberg, 2008; Bretscher et al., 2011; Guillermin et al., 2011, 2017; Hallem et al., 2011b; Carrillo et al., 2013; Kodama-Namba et al., 2013; Smith et al., 2013).
For C. elegans hermaphrodites, 30 mechanoreceptor neurons contribute to the detection of tactile cues, including some that innervate the body wall and others that innervate the tip of the nose (Goodman, 2006). Several of these neurons detect mechanosensory cues delivered to the head, including the multimodal amphidial ASH neurons (Kaplan and Horvitz, 1993), which contribute to nose-touch-triggered reversals. In addition, some of the neurons that innervate the cephalic sensilla contribute to the detection of texture, and some of the neurons that innervate the inner and outer labial sensilla participate in the head-withdrawal reflex (Altun and Hall, 2010).
8.2. Sensory neuroanatomy of environmentally motile iL3s
Structural studies of amphid morphology in environmentally motile parasitic nematodes found that the amphids of these nematodes resemble those of C. elegans. However, the exact number of neurons innervating the amphids varies slightly by species, from 12 to 13 (Ashton et al., 1995, 1999; Ashton and Schad, 1996; Li et al., 2000a, 2001; Zhu et al., 2011). In addition, the structural details of dendritic processes can vary significantly between species and life stages, making it difficult to conclusively identify the parasite homologs of some C. elegans amphidial neurons based on structure alone. For example, hookworms and H. contortus have a pair of neurons with characteristic “finger-like” dendritic processes that are easily identifiable as homologs of the C. elegans AFD neurons (Ashton et al., 1999; Li et al., 2000a, 2001; Bhopale et al., 2001; Goodman and Sengupta, 2018), whereas the more phylogenetically distant species S. stercoralis lacks these projections (Ashton et al., 1995, 1999; Ashton and Schad, 1996). Instead, S. stercoralis has a “lamellar cell” pair called ALD, yet whether ALD is more similar to C. elegans AFD or AWC remains unclear (Ashton et al., 1995, 1999; Ashton and Schad, 1996; Lopez et al., 2000).
In S. stercoralis and A. duodenale iL3s, the cephalic and labial sensilla are generally organized as in C. elegans dauers; these sensilla are thought to be mechanosensory based on their position and structural characteristics (Ashton et al., 1995; Ashton and Schad, 1996; Fine et al., 1997). As in the amphids, the exact number of neurons per sensillum varies between parasitic and free-living species, as does the structure of the dendritic endings (Fine et al., 1997). The functional roles of the labial and cephalic neurons have not yet been tested in parasitic nematodes, although they are thought to play a role in skin penetration. Similarly, the effect of specific structural characteristics of the cephalic and labial sensilla on mechanosensory responses in iL3s is unknown.
8.3. Chemosensory neuron function in environmentally motile iL3s
The functions of sensory neurons in parasitic iL3s have so far been assessed by combining laser ablation with behavioral analysis. In parasitic nematodes, laser ablation studies have identified several amphidial neurons that functionally contribute to chemosensory behaviors. S. stercoralis ASE is involved in iL3 attraction to skin extracts and sodium chloride (Ashton et al., 1999; Forbes et al., 2004), similar to the role of C. elegans ASE in mediating attraction to sodium chloride and other gustatory cues (Bargmann and Horvitz, 1991). In S. stercoralis, the ALD neurons also contribute to attraction to skin extracts (Ashton et al., 1999). The ASH neurons of S. stercoralis and A. caninum mediate repulsion from chemical stimuli (Forbes et al., 2004; Ketschek et al., 2004), as in C. elegans (Bargmann and Horvitz, 1991; Bargmann, 2006). Whether the mechanosensory function of C. elegans ASH neurons is also conserved in the ASH neurons of parasitic nematodes remains to be determined. The ADL neurons of A. caninum have been shown to contribute to chemical avoidance (Ketschek et al., 2004), consistent with their role in mediating chemical avoidance in C. elegans (Sambongi et al., 1999; Chao et al., 2004; Bargmann, 2006). The sensory neurons that mediate responses to olfactory cues and gases have not yet been identified in mammalian-parasitic iL3s. However, the BAG sensory neurons were found to mediate CO2 response in two phylogenetically distant species of entomopathogenic nematodes in addition to C. elegans (Hallem and Sternberg, 2008; Hallem et al., 2011a; Rengarajan and Hallem, 2016), suggesting BAG neurons may also mediate CO2 detection in mammalian-parasitic iL3s.
8.4. Thermosensory neuron function in environmentally motile iL3s
The C. elegans AFD and AWC neurons possess complex dendritic structures: AFD is characterized by an elaborate “finger-like” ciliary structure and AWC by a large and highly developed “wing-like” ciliary structure (Perkins et al., 1986; Doroquez et al., 2014; Goodman and Sengupta, 2018). Neurons identified as parasite homologs of AFD based on the position of their cell bodies and a ciliary morphology resembling that of C. elegans AFD were found to be required for thermotaxis in both the skin-penetrating hookworm A. caninum and the passively ingested nematode H. contortus (Li et al., 2000b; Bhopale et al., 2001). In H. contortus, AWC is not required for thermotaxis (Li et al., 2000b), although an accessory role in thermosensation similar to that of C. elegans AWC cannot be excluded. In S. stercoralis, the ALD neuron is required for positive thermotaxis by iL3s (Ashton et al., 1999; Lopez et al., 2000).
8.5. Neural mechanisms underlying parasite-specific behaviors
The mechanisms that underlie parasite-specific host-seeking behaviors are not known but may arise from differences at the level of cellular or circuit function. Differences in the structure of amphid neuron processes across nematode species raise the question of whether these differences reflect functional adaptations. In C. elegans, many sensory neurons possess intricate receptive endings that are required for normal sensory function (Perkins et al., 1986; Satterlee et al., 2001; Bargmann, 2006; Inglis et al., 2006; Doroquez et al., 2014; Singhvi et al., 2016; Goodman and Sengupta, 2018). In the case of the AFD neurons, the complexity of the “finger-like” processes may enhance neuronal sensitivity by increasing the membrane surface-area-to-volume ratio, thereby enabling increased localized expression of membrane-bound thermosensory molecules (Goodman and Sengupta, 2018). Whether structural differences in parasitic sensory neurons, such as the unique morphology of the S. stercoralis ALD neurons (Ashton et al., 1995, 1999; Ashton and Schad, 1996; Lopez et al., 2000), result in functional specializations for responding to host-emitted sensory cues is unclear. In C. elegans, a fully assembled wiring diagram provides a detailed map of neuronal structure and connectivity (White et al., 1986; Varshney et al., 2011; Emmons, 2016). The construction of similar wiring diagrams for parasitic nematodes may enable the identification of changes in connectivity that give rise to parasite-specific behaviors.
In addition, future experiments will need to test whether parasite-specific host-seeking behaviors are generated by specialized functional properties of parasite sensory neural circuits. These efforts will likely require the use of genetically encoded calcium sensors to monitor neural activity in parasitic nematodes, as has been done in C. elegans. For example, in the case of thermosensation, neural imaging studies in C. elegans have identified multiple mechanisms that regulate thermosensory coding, including sensory adaptation in primary sensory neurons and synaptic plasticity between sensory neurons and downstream interneurons (Biron et al., 2006; Clark et al., 2006, 2007a; Yu et al., 2014; Hawk et al., 2018). In the case of CO2 response in C. elegans, neural imaging revealed that CO2-response valence is regulated by experience-dependent modulation of sensory-neuron-to-interneuron synapses (Guillermin et al., 2017). Whether the experience-dependent and life-stage-specific changes in the sensory-driven behaviors of parasitic nematodes are mediated by similar mechanisms, or by parasite-specific adaptations to underlying neural circuits, has not yet been tested.
9. A molecular toolkit for parasitic nematodes
Until recently, the lack of tools for genetic manipulation of parasitic nematodes severely limited our knowledge of the cellular and molecular mechanisms underlying parasitic behaviors. Several technical advances are fostering a new era for genetic analysis and manipulation in parasitic nematodes. The fully-sequenced genomes of many parasitic nematodes are publicly available (Howe et al., 2017), as is life-stage-specific RNA-Seq data from multiple species, including S. stercoralis (Stoltzfus et al., 2012b; Hunt et al., 2016; Howe et al., 2017). These high-quality reference genomes are facilitating the identification of homologous genes in free-living and parasitic nematodes. Furthermore, life-stage-specific datasets support efforts to identify genes critical for parasitism (Hunt et al., 2016).
Several techniques have been adapted for the study of gene function in parasitic nematodes with an environmentally motile infective stage. In particular, efforts to achieve transgenesis have been highly successful in Strongyloides species (Lok and Massey, 2002; Junio et al., 2008; Li et al., 2011; Lok, 2012, 2013; Shao et al., 2012; Lok et al., 2017). This success is enabled by the free-living adult life stage of Strongyloides species (Lok et al., 2017). The free-living Strongyloides adults are morphologically similar to C. elegans adults; thus, foreign DNA can be introduced by intragonadal microinjection using modifications of techniques originally developed for use in C. elegans (Evans, 2006). Injection of plasmid constructs can result in expression of exogenous genes through the formation of extrachromosomal arrays, as in C. elegans (Li et al., 2006, 2011; Junio et al., 2008; Lok et al., 2017). Furthermore, several studies have identified S. stercoralis promoters that drive expression in specific neurons or subsets of neurons (Junio et al., 2008; Stoltzfus et al., 2012a; Bryant et al., 2018). Thus, it should be possible to express genetically encoded calcium sensors such as GCaMP or cameleon (Nagai et al., 2004; Chen et al., 2013) and tools for genetic cell ablation or silencing (Schiavo et al., 1992; Pokala et al., 2014) in specific subsets of S. stercoralis neurons. However, whereas stable expression from extrachromosomal arrays occurs across generations in C. elegans (Evans, 2006), expression from extrachromosomal arrays is limited to the F1 generation in Strongyloides (Junio et al., 2008). Expression across multiple generations in Strongyloides requires genomic integration, which can be achieved by either transposon-mediated random integration or CRISPR/Cas9-mediated targeted integration (Shao et al., 2012; Lok, 2013; Gang et al., 2017).
The CRISPR/Cas9 system was recently adapted for use in S. stercoralis and S. ratti (Gang et al., 2017; Lok et al., 2017). This system can be used to obtain homozygous gene disruptions in the F1 generation, thereby enabling the study of genes with recessive mutant phenotypes, including those that impair or eliminate infectivity and would therefore preclude host passage (Gang et al., 2017; Bryant et al., 2018). To date, the efficacy of CRISPR/Cas9-mediated mutagenesis has been relatively consistent across genes and CRISPR target sites (Gang et al., 2017; Bryant et al., 2018). Gene disruptions have been generated by both deletions at the target region and homology-directed repair (HDR)-mediated integration of a repair template (Gang et al., 2017; Lok et al., 2017; Bryant et al., 2018). Deletions at the target region can, in at least some cases, be large deletions of >500 base pairs (Gang et al., 2017). Large deletions generated by CRISPR/Cas9 have also been observed in a number of contexts, including mammalian cell lines (Kosicki et al., 2018). Whether large deletions occur at all CRISPR target sites in Strongyloides or only a subset remains to be determined.
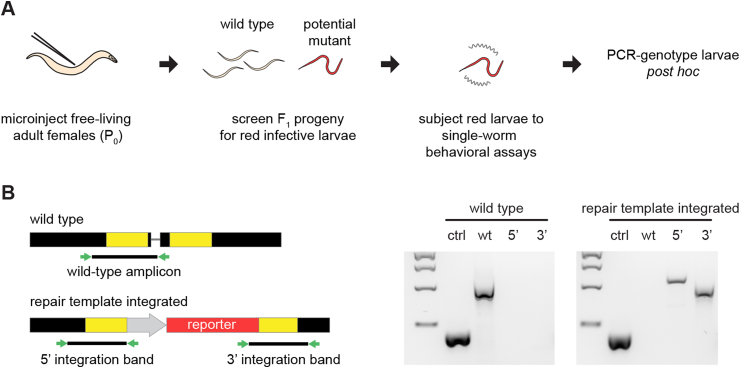
A pipeline for CRISPR/Cas9-mediated targeted mutagenesis in Strongyloides has now been established (Fig. 4) (Gang et al., 2017; Bryant et al., 2018). DNA plasmids containing the CRISPR components, including a repair template for HDR containing a fluorescent reporter, are introduced into free-living adult females by gonadal microinjection. F1 iL3s are then screened by epifluorescence microscopy for the presence of the reporter. Since the repair template can form an extrachromosomal array, some animals will show reporter expression that is independent of gene disruption. Thus, iL3s expressing the reporter are subjected to single-worm behavioral or functional assays, and then genotyped post hoc for homozygous disruption of the gene of interest and integration of the repair template (Fig. 4). A subset of the animals with homozygous gene disruptions will not show integration of the repair template (Gang et al., 2017; Bryant et al., 2018); whether these animals are excluded from subsequent analyses depends on the likelihood that a large deletion at the target region will disrupt neighboring genes. In addition to generating gene knockouts, CRISPR/Cas9-mediated HDR can be used to knock in exogenous genes at specific genetic loci (Gang et al., 2017; Bryant et al., 2018), an attractive alternative to the random integration produced by transposon-mediated techniques.
Fig. 4.
A pipeline for CRISPR/Cas9-mediated targeted mutagenesis in S. stercoralis. A. DNA plasmids containing the CRISPR components (the Cas9 gene, a single guide RNA, and a repair template containing a red fluorescent marker for homology-directed repair) are introduced into free-living adult females (P0) by gonadal microinjection. The F1 iL3 progeny are then screened for the presence of the fluorescent marker. Individual F1 iL3s expressing the fluorescent marker are subjected to single-worm behavioral or physiological assays, and then PCR-genotyped post hoc. B. Individual F1 iL3s are PCR-genotyped for homozygous disruption of the gene of interest and integration of the repair template (Gang et al., 2017; Bryant et al., 2018). Left, diagram depicting the expected PCR products from single-worm genotyping. Approximate locations of primer binding sites are shown as green arrows. In the diagram of the wild-type chromosomal locus (top), the thin grey line represents the location of the CRISPR target site and the yellow regions indicate the DNA sequences that match the homology arms of the repair template. In the diagram of the chromosomal locus after the repair template has integrated, the grey arrow indicates the promoter used to drive expression of the fluorescent reporter gene and the red region indicates the coding sequence of the reporter gene. The wild-type amplicon will yield a PCR product only if the unedited wild-type locus is present. The 5′ integration band tests for integration of the 5′ end of the repair template; the 3′ integration band tests for integration of the 3′ end of the repair template. For both PCR reactions testing for integration of the repair template, one primer matches a sequence in the repair template and the other matches a sequence in the flanking genomic DNA. Thus, these reactions will only yield a PCR product following successful homology-directed repair. Right, examples from DNA gels showing the PCR products obtained from a wild-type iL3 and a repair-template-integrated iL3. ctrl = a positive control that amplifies a wild-type region of the S. stercoralis actin-2 gene; wt = wild-type amplicon; 5’ = 5′ integration band; 3’ = 3′ integration band. DNA ladder shows 2 kb, 1.5 kb, 1 kb, and 500 bp bands from top to bottom. Figure adapted from Gang et al. (2017). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In our hands, testing the function of a single gene in S. stercoralis using CRISPR/Cas9-mediated targeted mutagenesis can take ∼3–6 months, from the initial design of the CRISPR plasmids to the accumulation of enough single-worm behavioral assays with post-hoc-genotyped F1 iL3s to ensure sufficiently powered statistical testing (Bryant et al., 2018). A major bottleneck is the screening of the F1 generation for transgenic animals expressing the fluorescent reporter (Fig. 4), because these animals generally represent only a small percentage of the total F1 population. Furthermore, although generating stable knockout lines with S. stercoralis is feasible (Gang et al., 2017), it is labor-intensive because hundreds of mutant iL3s must be obtained to achieve a patent infection in gerbils, the laboratory host for S. stercoralis (Lok, 2007). In addition, iL3s with mutations in genes that are required for infectivity or development inside the host cannot be propagated through gerbils as homozygotes but must instead be propagated as heterozygotes and then homozygosed in the free-living generation prior to behavioral testing. Thus, testing F1 iL3s directly without generating a stable line is generally a more efficient approach. Given the labor-intensive nature of the CRISPR pipeline even when F1 iL3s are tested directly, scaling up CRISPR/Cas9-mediated mutagenesis to more than a few genes at a time is not yet practical. Several technical advances that increase HDR efficacy have recently been developed in C. elegans, including the use of modified double-stranded DNA repair templates (Dokshin et al., 2018; Ghanta et al., 2018). These techniques may be applicable to parasitic nematodes; dramatic increases in efficacy could enable the use of CRISPR/Cas9-mediated mutagenesis for small-scale functional screens.
Other techniques have also been adapted for genetic manipulation in parasitic nematodes. For example, chemical mutagenesis screens have been used to generate non-targeted dominant mutations in S. ratti iL3s, although mapping the genes responsible for these mutations has not yet been feasible (Viney et al., 2002; Guo et al., 2015). RNA interference (RNAi) has been used for targeted repression of gene expression in several parasitic nematode species, although its efficacy can be variable (Geldhof et al., 2006; Kotze and Bagnall, 2006; Visser et al., 2006; Kang and Hong, 2008; Lendner et al., 2008; Viney and Thompson, 2008; Samarasinghe et al., 2011; Britton et al., 2012; Zawadzki et al., 2012; Tzelos et al., 2015). Furthermore, in many species, including Strongyloides species, successful application of RNAi has not yet been reported (Geldhof et al., 2007). In the human-parasitic nematode Brugia malayi, the rate of effective gene knockdown following application of heterogenous short interfering RNA (hsiRNA) mixtures appears to be higher than the rates of gene knockdown using long double-stranded RNA (dsRNA) or synthetic short interfering RNAs (siRNAs) (Landmann et al., 2012). In contrast, preliminary efforts to adapt hsiRNA methodologies for soil-transmitted iL3s have not been promising: hsiRNA is less effective than dsRNA in the ovine-parasitic nematode Teladorsagia circumcincta (Tzelos, 2014), and does not induce RNA silencing in N. brasiliensis (Roberts, 2017). Thus, the development of a method for achieving RNA interference in soil-transmitted iL3s will likely require a substantial technical investment.
10. Molecular mechanisms of host seeking
A multitude of tools available for use in C. elegans has resulted in a deep understanding of the molecular basis of C. elegans sensory transmission for multiple sensory modalities (Bargmann, 2006; Garrity et al., 2010; Hart and Chao, 2010; Aoki and Mori, 2015; Carrillo and Hallem, 2015; Glauser and Goodman, 2016; Rengarajan and Hallem, 2016). Knowledge of C. elegans molecular mechanisms can now be combined with comparative genomic analyses and CRISPR/Cas9-mediated mutagenesis in Strongyloides species to test the role of specific genes in sensory-driven host-seeking behaviors (Bryant et al., 2018).
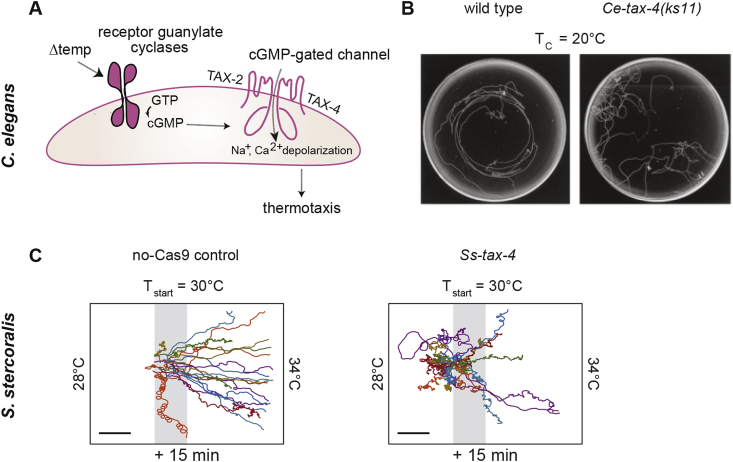
The tax-4 gene encodes a cyclic nucleotide-gated channel subunit that is expressed in multiple head sensory neurons in C. elegans and is required for many sensory-driven behaviors, including thermotaxis navigation (Fig. 5A and B), attraction to aqueous and volatile chemoattractants, and avoidance of volatile chemorepellants (Bargmann et al., 1993; Mori and Ohshima, 1995; Coburn and Bargmann, 1996; Komatsu et al., 1996, 1999; Satterlee et al., 2004; Bargmann, 2006; Ito et al., 2006; Kuhara et al., 2008; Jurado et al., 2010). The S. stercoralis genome contains a tax-4 homolog; consistent with its expression pattern in C. elegans, Ss-tax-4 is expressed in multiple head neurons (Gang et al., 2017; Bryant et al., 2018). Homozygous disruption of Ss-tax-4 (Gang et al., 2017; Bryant et al., 2018) resulted in multiple deficits in the temperature-driven behaviors of S. stercoralis iL3s, including the loss of positive thermotaxis toward mammalian body temperatures (Fig. 5C) (Bryant et al., 2018). Whether Ss-tax-4 is also required for chemosensory behaviors in S. stercoralis iL3s has not yet been tested. Nevertheless, the finding that Ss-tax-4 is required for positive thermotaxis in S. stercoralis iL3s demonstrates that tax-4 plays a conserved role in mediating at least some aspects of sensory transduction across free-living and parasitic nematode species (Bryant et al., 2018).
Fig. 5.
The tax-4 gene is required for both C. elegans and S. stercoralis thermotaxis behavior. A. Diagram of the C. elegans TAX-4-dependent thermosensory signal transduction pathway. Changes in temperature activate receptor guanylate cyclases, which in turn activate TAX-2/TAX-4 cation channels via cGMP signaling. This pathway is required for thermotaxis navigation in C. elegans. Figure adapted from Bargmann (2006). B. Left: Tracks of wild-type C. elegans adults migrating isothermally in a radial thermal gradient. Right: A null mutation in the C. elegans tax-4 gene abolishes the temperature-driven behavior of C. elegans adults. Cultivation temperature (TC) = 20°C. Figure reproduced from Komatsu et al., 1996 with permission (Komatsu et al., 1996). C. Left: Tracks of no-Cas9-control S. stercoralis iL3s displaying positive thermotaxis. Right: CRISPR/Cas9 targeting of the Ss-tax-4 gene results in reduced positive thermotaxis toward host body temperatures by Ss-tax-4 iL3s. The gradient ranges from ∼22 to 34°C; Tstart = ∼30°C. Only a portion of the full gradient is shown. Assays ran for 15 min. Scale bar indicates 2 cm. No-Cas9-control iL3s were generated following the same procedure used to generate the Ss-tax-4 iL3s, except that the plasmid encoding Cas9 was omitted from the microinjection mix. Figure reproduced from Bryant et al., 2018 with permission (Bryant et al., 2018).
Our understanding of the molecular mechanisms underlying sensory responses in C. elegans provides multiple gene targets for future investigations of S. stercoralis sensory transduction. For example, in C. elegans, a trio of receptor guanylate cyclases (GCY-8, GCY-18, GCY-23) act upstream of TAX-4 to enable thermosensory responses in AFD (Inada et al., 2006; Kuhara et al., 2008, 2011; Ramot et al., 2008a; Wasserman et al., 2011; Liu et al., 2012; Takeishi et al., 2016). In addition, the C. elegans odr-3 gene encodes a G protein alpha subunit that mediates chemosensory responses in multiple chemosensory neurons (Roayaie et al., 1998; Lans et al., 2004; Bargmann, 2006; Yoshida et al., 2012; Harris et al., 2014), and the C. elegans osm-9 gene encodes a transient receptor potential V (TRPV) channel involved in some chemosensory responses, osmotic avoidance, and detection of noxious temperatures (Colbert et al., 1997; Tobin et al., 2002; Glauser et al., 2011; Liu et al., 2012; Schild and Glauser, 2013; Venkatachalam et al., 2014). Whether homologs of these genes, other genes involved in C. elegans sensory transduction, or parasite-specific genes also play a role in sensory-driven host seeking by parasitic nematodes requires investigation, ideally via CRISPR/Cas9-mediated gene disruption.
11. Conclusions
Environmentally motile parasitic nematode infective larvae find and infect hosts by sampling various aspects of their sensory environment. Multiple studies have demonstrated the importance of thermal and chemical cues in driving host-seeking behaviors. Future research will characterize quantitatively the interaction between thermosensation and chemosensation in parasitic nematode infective larvae and will elucidate the role of additional understudied sensory modalities in driving host seeking. Furthermore, recent methodological advances will enable a better understanding of the molecular, cellular, and circuit mechanisms that underlie sensory behaviors in parasitic nematodes. This knowledge will provide insights into how the sensory systems of parasitic nematodes are adapted to mediate host seeking and may enable the development of new strategies for nematode control. Parasitic nematode infections are one of the most neglected sources of chronic debilitating disease and economic burden, and parasitic nematode sensory circuits are a promising yet largely unexplored target for intervention.
Declarations of interest
None.
Acknowledgements
We give special thanks to Taylor Brown, who gave invaluable feedback on this manuscript and other manuscripts on this topic. We also thank Michelle Castelletto, Felicitas Ruiz, and Spencer Gang for insightful comments and interactions. We thank Samata Katta (Miriam Goodman lab) for her thoughts on mechanosensation. This work was supported by an A.P. Giannini Postdoctoral Fellowship and NIH Ruth L. Kirschstein National Research Service Award T32NS058280 (A.S.B.); and NIH New Innovator Award 1DP2DC014596, a Burroughs-Wellcome Fund Investigators in the Pathogenesis of Disease Award, and a Howard Hughes Medical Institute Faculty Scholar Award (E.A.H.).
References
- Alkalay I., Suetsugu S., Constantine H., Stein M. Carbon dioxide elimination across human skin. Am. J. Physiol. 1971;220:1434–1436. doi: 10.1152/ajplegacy.1971.220.5.1434. [DOI] [PubMed] [Google Scholar]
- Altun Z.F., Hall D.H. WormAtlas. 2010. Nervous system, neuronal support cells.www.wormatlas.org [Google Scholar]
- Aoki I., Mori I. Molecular biology of thermosensory transduction in C. elegans. Curr. Opin. Neurobiol. 2015;34:117–124. doi: 10.1016/j.conb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Apps P., Mmualefe L., McNutt J.W. Identification of volatiles from the secretions and excretions of African wild dogs (Lycaon pictus) J. Chem. Ecol. 2012;38:1450–1461. doi: 10.1007/s10886-012-0206-7. [DOI] [PubMed] [Google Scholar]
- Arnould C., Malosse C., Signoret J.-P., Descoins C. Which chemical constituents from dog feces are involved in its food repellent effect in sheep? J. Chem. Ecol. 1998;24:559–576. [Google Scholar]
- Ashton F.T., Bhopale V.M., Fine A.E., Schad G.A. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J. Comp. Neurol. 1995;357:281–295. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- Ashton F.T., Bhopale V.M., Holt D., Smith G., Schad G.A. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J. Parasitol. 1998;84:691–695. [PubMed] [Google Scholar]
- Ashton F.T., Li J., Schad G.A. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Vet. Parasitol. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- Ashton F.T., Schad G.A. Amphids in Strongyloides stercoralis and other parasitic nematodes. Parasitol. Today. 1996;12:187–194. doi: 10.1016/0169-4758(96)10012-0. [DOI] [PubMed] [Google Scholar]
- Ashton F.T., Zhu X., Boston R., Lok J.B., Schad G.A. Strongyloides stercoralis: amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp. Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C.I. WormBook. 2006. Chemosensation in C. elegans; pp. 1–29.www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C.I., Hartwieg E., Horvitz H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bargmann C.I., Horvitz H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Bargmann C.I., Thomas J.H., Horvitz H.R. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harbor Symp. Quant. Biol. 1990;55:529–538. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- Barrett J. The effect of temperature on the development and survival of the infective larvae of Strongyloides ratti Sandground, 1925. Parasitology. 1968;58:641–651. doi: 10.1017/s0031182000028936. [DOI] [PubMed] [Google Scholar]
- Benedict F.G., Miles W.R., Johnson A. The temperature of the human skin. Proc. Natl. Acad. Sci. U.S.A. 1919;5:218–222. doi: 10.1073/pnas.5.6.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N.C., Jarvis J.U.M., Davies K.C. Daily and seasonal temperatures in the burrows of African rodent moles. S. Afr. J. Zool. 1988;23:189–195. [Google Scholar]
- Bernier U.R., Booth M.M., Yost R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal. Chem. 1999;71:1–7. doi: 10.1021/ac980990v. [DOI] [PubMed] [Google Scholar]
- Bernier U.R., Kline D.L., Barnard D.R., Schreck C.E., Yost R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti) Anal. Chem. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- Bernier U.R., Kline D.L., Schreck C.E., Yost R.A., Barnard D.R. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae) J. Am. Mosq. Contr. Assoc. 2002;18:186–195. [PubMed] [Google Scholar]
- Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D., Hotez P.J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Bezubik B. Failure to establish infection in rats and Guinea pigs exposed to the larvae of Strongyloides papillosus. Acta Parasitol. 1965;13:349–354. [Google Scholar]
- Bhopale V.M., Kupprion E.K., Ashton F.T., Boston R., Schad G.A. Ancylostoma caninum: the finger cell neurons mediate thermotactic behavior by infective larvae of the dog hookworm. Exp. Parasitol. 2001;97:70–76. doi: 10.1006/expr.2000.4575. [DOI] [PubMed] [Google Scholar]
- Biron D., Shibuya M., Gabel C., Wasserman S.M., Clark D.A., Brown A., Sengupta P., Samuel A.D.T. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat. Neurosci. 2006;9:1499–1505. doi: 10.1038/nn1796. [DOI] [PubMed] [Google Scholar]
- Biron D., Wasserman S., Thomas J.H., Samuel A.D., Sengupta P. An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11002–11007. doi: 10.1073/pnas.0805004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi Z., Buonfrate D., Montresor A., Requena-Mendez A., Munoz J., Krolewiecki A.J., Gotuzzo E., Mena M.A., Chiodini P.L., Anselmi M. Strongyloides stercoralis: a plea for action. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M., Koutsovoulos G. The evolution of parasitism in Nematoda. Parasitology. 2014;142(Suppl. 1):S26–S39. doi: 10.1017/S0031182014000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M.L., De Ley P., Garey J.R., Liu L.X., Scheldeman P., Vierstraete A., Vanfleteren J.R., Mackey L.Y., Dorris M., Frisse L.M. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Boatin B.A., Basanez M.G., Prichard R.K., Awadzi K., Barakat R.M., Garcia H.H., Gazzinelli A., Grant W.N., McCarthy J.S., N'Goran E.K. A research agenda for helminth diseases of humans: towards control and elimination. PLoS Negl. Trop. Dis. 2012;6:e1547. doi: 10.1371/journal.pntd.0001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone L.W. Response of adult Nippostrongylus brasiliensis (Nematoda) to fluid velocity. Proc. Helminthol. Soc. Wash. 1985;52:244–246. [Google Scholar]
- Bretscher A.J., Kodama-Namba E., Busch K.E., Murphy R.J., Soltesz Z., Laurent P., de Bono M. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69:1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton C., Samarasinghe B., Knox D.P. Ups and downs of RNA interference in parasitic nematodes. Exp. Parasitol. 2012;132:56–61. doi: 10.1016/j.exppara.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Bryant A.S., Ruiz F., Gang S.S., Castelletto M.L., Lopez J.B., Hallem E.A. A critical role for thermosensation in host seeking by skin-penetrating nematodes. Curr. Biol. 2018;28:2338–2347. doi: 10.1016/j.cub.2018.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfrate D., Requena-Mendez A., Angheben A., Munoz J., Gobbi F., Van Den Ende J., Bisoffi Z. Severe strongyloidiasis: a systematic review of case reports. BMC Infect. Dis. 2013;13:78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A.C. Human calorimetry: II. The average temperature of the tissues of the body: three figures. J. Nutr. 1935;9:261–280. [Google Scholar]
- Buszewski B., Kesy M., Ligor T., Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed. Chromatogr. 2007;21:553–566. doi: 10.1002/bmc.835. [DOI] [PubMed] [Google Scholar]
- Byrnes C.A., Dinarevic S., Shinebourne E.A., Barnes P.J., Bush A. Exhaled nitric oxide measurements in normal and asthmatic children. Pediatr. Pulmonol. 1997;24:312–318. doi: 10.1002/(sici)1099-0496(199711)24:5<312::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Carrillo M.A., Guillermin M.L., Rengarajan S., Okubo R., Hallem E.A. O2-sensing neurons control CO2 response in C. elegans. J. Neurosci. 2013;33:9675–9683. doi: 10.1523/JNEUROSCI.4541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo M.A., Hallem E.A. Gas sensing in nematodes. Mol. Neurobiol. 2015;51:919–931. doi: 10.1007/s12035-014-8748-z. [DOI] [PubMed] [Google Scholar]
- Castelletto M.L., Gang S.S., Okubo R.P., Tselikova A.A., Nolan T.J., Platzer E.G., Lok J.B., Hallem E.A. Diverse host-seeking behaviors of skin-penetrating nematodes. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Hart A.C., Rankin C.H., Goodman M.B. WormBook. 2014. Assaying mechanosensation; pp. 1–13.www.wormbook.org [Google Scholar]
- Chalfie M., Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature. 1990;345:410–416. doi: 10.1038/345410a0. [DOI] [PubMed] [Google Scholar]
- Chao M.Y., Komatsu H., Fukuto H.S., Dionne H.M., Hart A.C. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chalfie M. Modulation of C. elegans touch sensitivity is integrated at multiple levels. J. Neurosci. 2014;34:6522–6536. doi: 10.1523/JNEUROSCI.0022-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B.H., Arellano-Carbajal F., Rybicki I., de Bono M. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr. Biol. 2004;14:1105–1111. doi: 10.1016/j.cub.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Chung S.H., Clark D.A., Gabel C.V., Mazur E., Samuel A.D.T. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibot M., Guillot J., Lafosse S., Bon C., Seguya A., Krief S. Nodular worm infections in wild non-human primates and humans living in the Sebitoli area (Kibale National Park, Uganda): do high spatial proximity favor zoonotic transmission? PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Biron D., Sengupta P., Samuel A.D. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Gabel C.V., Gabel H., Samuel A.D.T. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J. Neurosci. 2007;27:6083–6090. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Gabel C.V., Lee T.M., Samuel A.D. Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J. Neurophysiol. 2007;97:1903–1910. doi: 10.1152/jn.00892.2006. [DOI] [PubMed] [Google Scholar]
- Coburn C.M., Bargmann C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- Colbert H.A., Smith T.L., Bargmann C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork A., Park K.C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Couto A., Oda S., Nikolaev V.O., Soltesz Z., de Bono M. In vivo genetic dissection of O2-evoked cGMP dynamics in a Caenorhabditis elegans gas sensor. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E3301–E3310. doi: 10.1073/pnas.1217428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll N.A., Smith J.M. Mechanism of thermopositive behavior in larval hookworms. J. Parasitol. 1972;58:891–896. [PubMed] [Google Scholar]
- Crook M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014;44:1–8. doi: 10.1016/j.ijpara.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., Ratcliffe N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- De Preter V., Van Staeyen G., Esser D., Rutgeerts P., Verbeke K. Development of a screening method to determine the pattern of fermentation metabolites in faecal samples using on-line purge-and-trap gas chromatographic-mass spectrometric analysis. J. Chromatogr. A. 2009;1216:1476–1483. doi: 10.1016/j.chroma.2008.12.095. [DOI] [PubMed] [Google Scholar]
- Diawara A., Schwenkenbecher J.M., Kaplan R.M., Prichard R.K. Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2013;88:1052–1061. doi: 10.4269/ajtmh.12-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokshin G.A., Ghanta K.S., Piscopo K.M., Mello C.C. Robust genome editing with short single-stranded and long, partially single-stranded DNA donors in Caenorhabditis elegans. Genetics. 2018 doi: 10.1534/genetics.118.301532. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroquez D.B., Berciu C., Anderson J.R., Sengupta P., Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife. 2014;3 doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., Kaplan J. Mechanotransduction. In: Riddle D.L., Blumenthal T., Meyer B.J., Priess J.R., editors. C elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 1997. [Google Scholar]
- Emery D.L., Hunt P.W., Le Jambre L.F. Haemonchus contortus: the then and now, and where to from here? Int. J. Parasitol. 2016;46:755–769. doi: 10.1016/j.ijpara.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Emmons S.W. Connectomics, the final frontier. Curr. Top. Dev. Biol. 2016;116:315–330. doi: 10.1016/bs.ctdb.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.C. WormBook. 2006. Transformation and microinjection; pp. 1–15.www.wormbook.org [Google Scholar]
- Fenk L.A., de Bono M. Environmental CO2 inhibits Caenorhabditis elegans egg-laying by modulating olfactory neurons and evokes widespread changes in neural activity. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E3525–E3534. doi: 10.1073/pnas.1423808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin M.H., Sorokin E.S., Johnston R.E. Seasonal changes in scents and responses to them in meadow voles: evidence for the co-evolution of signals and response mechanisms. Ethology. 1995;100:89–98. [Google Scholar]
- Fine A.E., Ashton F.T., Bhopale V.M., Schad G.A. Sensory neuroanatomy of a skin-penetrating nematode parasite Strongyloides stercoralis. II. Labial and cephalic neurons. J. Comp. Neurol. 1997;389:212–223. [PubMed] [Google Scholar]
- Forbes W.M., Ashton F.T., Boston R., Schad G.A. Chemotactic behaviour of Strongyloides stercoralis infective larvae on a sodium chloride gradient. Parasitology. 2003;127:189–197. doi: 10.1017/s0031182003003433. [DOI] [PubMed] [Google Scholar]
- Forbes W.M., Ashton F.T., Boston R., Zhu X., Schad G.A. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet. Parasitol. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Forrer A., Khieu V., Schar F., Hattendorf J., Marti H., Neumayr A., Char M.C., Hatz C., Muth S., Odermatt P. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulleborn F. Tropisms of Strongyloides & Ancylostome larvae. Arch. f Schiffs u Tropen. Hyg. 1924;28:144–165. [Google Scholar]
- Gallagher M., Wysocki C.J., Leyden J.J., Spielman A.I., Sun X., Preti G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008;159:780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang S.S., Castelletto M.L., Bryant A.S., Yang E., Mancuso N., Lopez J.B., Pellegrini M., Hallem E.A. Targeted mutagenesis in a human-parasitic nematode. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang S.S., Hallem E.A. Mechanisms of host seeking by parasitic nematodes. Mol. Biochem. Parasitol. 2016;208:23–32. doi: 10.1016/j.molbiopara.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N.P., Niu J.L. CFD study of the thermal environment around a human body: a review. Indoor Built Environ. 2005;14:5–16. [Google Scholar]
- Garner C.E., Smith S., de Lacy Costello B., White P., Spencer R., Probert C.S., Ratcliffe N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. Faseb. J. 2007;21:1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- Garrity P.A., Goodman M.B., Samuel A.D., Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldhof P., Murray L., Couthier A., Gilleard J.S., McLauchlan G., Knox D.P., Britton C. Testing the efficacy of RNA interference in Haemonchus contortus. Int. J. Parasitol. 2006;36:801–810. doi: 10.1016/j.ijpara.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Geldhof P., Visser A., Clark D., Saunders G., Britton C., Gilleard J., Berriman M., Knox D. RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects. Parasitology. 2007;134:609–619. doi: 10.1017/S0031182006002071. [DOI] [PubMed] [Google Scholar]
- Ghai R.R., Chapman C.A., Omeja P.A., Davies T.J., Goldberg T.L. Nodule worm infection in humans and wild primates in Uganda: cryptic species in a newly identified region of human transmission. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta K.S., Dokshin G.A., Mir A., Krishnamurthy P.M., Gneid H., Edraki A., Watts J.K., Sontheimer E.J., Mello C.C. 5' Modifications improve potency and efficacy of DNA donors for precision genome editing. BioRxiv. 2018 doi: 10.1101/354480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser D.A. How and why Caenorhabditis elegans uses distinct escape and avoidance regimes to minimize exposure to noxious heat. Worm. 2013;2 doi: 10.4161/worm.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser D.A., Chen W.C., Agin R., Macinnis B.L., Hellman A.B., Garrity P.A., Tan M.W., Goodman M.B. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser D.A., Goodman M.B. Molecules empowering animals to sense and respond to temperature in changing environments. Curr. Opin. Neurobiol. 2016;41:92–98. doi: 10.1016/j.conb.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Goodman M.B. WormBook. 2006. Mechanosensation; pp. 1–14.www.wormbook.org [Google Scholar]
- Goodman M.B., Klein M., Lasse S., Luo L., Mori I., Samuel A., Sengupta P., Wang D. WormBook. 2014. Thermotaxis navigation behavior; pp. 1–10.www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M.B., Sengupta P. The extraordinary AFD thermosensor of C. elegans. Pflugers Arch. - Eur. J. Physiol. 2018;470:839–849. doi: 10.1007/s00424-017-2089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzer M., Haas W. Host-finding and host recognition of infective Ancylostoma caninum larvae. Int. J. Parasitol. 1991;21:429–440. doi: 10.1016/0020-7519(91)90100-l. [DOI] [PubMed] [Google Scholar]
- Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C.I. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Guillermin M.L., Carrillo M.A., Hallem E.A. A single set of interneurons drives opposite behaviors in C. elegans. Curr. Biol. 2017;27:2630–2639. doi: 10.1016/j.cub.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermin M.L., Castelletto M.L., Hallem E.A. Differentiation of carbon dioxide-sensing neurons in Caenorhabditis elegans requires the ETS-5 transcription factor. Genetics. 2011;189:1327–1339. doi: 10.1534/genetics.111.133835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Chang Z., Dieterich C., Streit A. A protocol for chemical mutagenesis in Strongyloides ratti. Exp. Parasitol. 2015;158:2–7. doi: 10.1016/j.exppara.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Gupta S.P. Mode of infection and biology of infective larvae of Molineus barbatus Chandler, 1942. Exp. Parasitol. 1963;13:252–255. doi: 10.1016/0014-4894(63)90077-8. [DOI] [PubMed] [Google Scholar]
- Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology. 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- Haas W., Haberl B., Idris S.I., Kallert D., Kersten S., Stiegeler P. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol. Res. 2005;95:30–39. doi: 10.1007/s00436-004-1257-7. [DOI] [PubMed] [Google Scholar]
- Haas W., Haberl B., Idris S.I., Kersten S. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol. Res. 2005;95:25–29. doi: 10.1007/s00436-004-1256-8. [DOI] [PubMed] [Google Scholar]
- Haley A.J. Biology of the rat nematode Nippostrongylus brasiliensis (Travassos, 1914). I. Systematics, hosts and geographic distribution. J. Parasitol. 1961;47:727–732. [PubMed] [Google Scholar]
- Hallem E.A., Dillman A.R., Hong A.V., Zhang Y., Yano J.M., DeMarco S.F., Sternberg P.W. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Spencer W.C., McWhirter R.D., Zeller G., Henz S.R., Ratsch G., Miller D.M., Horvitz H.R., Sternberg P.W., Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2011;108:254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Sternberg P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., Shen Y., Ha H., Donato A., Wallis S., Zhang X., Zhang Y. Dissecting the signaling mechanisms underlying recognition and preference of food odors. J. Neurosci. 2014;34:9389–9403. doi: 10.1523/JNEUROSCI.0012-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A.C., Chao M.Y. From odors to behaviors in Caenorhabditis elegans. In: Menini A., editor. The Neurobiology of Olfaction. CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- Hawk J.D., Calvo A.C., Liu P., Almoril-Porras A., Aljobeh A., Torruella-Suarez M.L., Ren I., Cook N., Greenwood J., Luo L. Integration of plasticity mechanisms within a single sensory neuron of C. elegans actuates a memory. Neuron. 2018;97:356–367. doi: 10.1016/j.neuron.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E.M., Russell R.L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A.D., Sukhdeo M.V.K. Host grooming and the transmission strategy of Heligmosomoides polygyrus. J. Parasitol. 1995;81:865–869. [PubMed] [Google Scholar]
- Heth G., Nevo E., Todrank J. Seasonal changes in urinary odors and in responses to them by blind subterranean mole rats. Physiol. Behav. 1996;60:963–968. doi: 10.1016/0031-9384(96)00077-7. [DOI] [PubMed] [Google Scholar]
- Hobert O. Behavioral plasticity in C. elegans: paradigms, circuits, genes. J. Neurobiol. 2003;54:203–223. doi: 10.1002/neu.10168. [DOI] [PubMed] [Google Scholar]
- Holbrook R.I., Mortimer B. Vibration sensitivity found in Caenorhabditis elegans. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.178947. [DOI] [PubMed] [Google Scholar]
- Hotez P., Hawdon J., Schad G.A. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol. Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Howe K.L., Bolt B.J., Shafie M., Kersey P., Berriman M. WormBase ParaSite − a comprehensive resource for helminth genomics. Mol. Biochem. Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt V.L., Tsai I.J., Coghlan A., Reid A.J., Holroyd N., Foth B.J., Tracey A., Cotton J.A., Stanley E.J., Beasley H. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat. Genet. 2016;48:299–307. doi: 10.1038/ng.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H., Ito H., Satterlee J., Sengupta P., Matsumoto K., Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis P.N., Ou G., Leroux M.R., Scholey J.M. WormBook. 2006. The sensory cilia of Caenorhabditis elegans; pp. 1–22.www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Inada H., Mori I. Quantitative analysis of thermotaxis in the nematode Caenorhabditis elegans. J. Neurosci. Methods. 2006;154:45–52. doi: 10.1016/j.jneumeth.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Jensen B.B., Jorgensen H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol. 1994;60:1897–1904. doi: 10.1128/aem.60.6.1897-1904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junio A.B., Li X., Massey H.C., Jr., Nolan T.J., Todd Lamitina S., Sundaram M.V., Lok J.B. Strongyloides stercoralis: cell- and tissue-specific transgene expression and co-transformation with vector constructs incorporating a common multifunctional 3' UTR. Exp. Parasitol. 2008;118:253–265. doi: 10.1016/j.exppara.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado P., Kodama E., Tanizawa Y., Mori I. Distinct thermal migration behaviors in response to different thermal gradients in Caenorhabditis elegans. Gene Brain Behav. 2010;9:120–127. doi: 10.1111/j.1601-183X.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- Kang S., Hong Y.S. RNA interference in infectious tropical diseases. Kor. J. Parasitol. 2008;46:1–15. doi: 10.3347/kjp.2008.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.M., Horvitz H.R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. J. Am. Med. Assoc. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Ketschek A.R., Joseph R., Boston R., Ashton F.T., Schad G.A. Amphidial neurons ADL and ASH initiate sodium dodecyl sulphate avoidance responses in the infective larva of the dog hookworm Anclyostoma caninum. Int. J. Parasitol. 2004;34:1333–1336. doi: 10.1016/j.ijpara.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Kodama-Namba E., Fenk L.A., Bretscher A.J., Gross E., Busch K.E., de Bono M. Cross-modulation of homeostatic responses to temperature, oxygen and carbon dioxide in C. elegans. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama E., Kuhara A., Mohri-Shiomi A., Kimura K.D., Okumura M., Tomioka M., Iino Y., Mori I. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20:2955–2960. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M., Nuamtanong S., Dekumyoy P., Yoonuan T., Maipanich W., Rojekittikhun W., Waikagul J. Host-finding behavior of Strongyloides stercoralis infective larvae to sodium cation, human serum, and sweat. Southeast Asian J. Trop. Med. Publ. Health. 2005;36:93–98. [PubMed] [Google Scholar]
- Koga M., Sa-Nguankiat S., Muennoo C., Pubampen S., Nuamtanong S., Maipanich W., Rojekittikhun W., Dekumyoy P., Waikagul J. Chemotactic attraction of Necator hookworm filariform larvae to sodium chloride. Southeast Asian J. Trop. Med. Public Health. 2004;35:112–115. [Google Scholar]
- Koga M., Tada I. Strongyloides ratti: chemotactic responses of third-stage larvae to selected serum proteins and albumins. J. Helminthol. 2000;74:247–252. [PubMed] [Google Scholar]
- Komatsu H., Jin Y.H., L'Etoile N., Mori I., Bargmann C.I., Akaike N., Ohshima Y. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 1999;821:160–168. doi: 10.1016/s0006-8993(99)01111-7. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Mori I., Rhee J.S., Akaike N., Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Kosicki M., Tomberg K., Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera I., Tran N.A., Fu D., Kim J.H.J., Byrne Rodgers J., Ryu W.S. Pan-neuronal screening in Caenorhabditis elegans reveals asymmetric dynamics of AWC neurons is critical for thermal avoidance behavior. Elife. 2016;5 doi: 10.7554/eLife.19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze A.C., Bagnall N.H. RNA interference in Haemonchus contortus: suppression of β-tubulin gene expression in L3, L4 and adult worms in vitro. Mol. Biochem. Parasitol. 2006;145:101–110. doi: 10.1016/j.molbiopara.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Kuhara A., Mori I. Molecular physiology of the neural circuit for calcineurin-dependent associative learning in Caenorhabditis elegans. J. Neurosci. 2006;26:9355–9364. doi: 10.1523/JNEUROSCI.0517-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A., Ohnishi N., Shimowada T., Mori I. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat. Commun. 2011;2:355. doi: 10.1038/ncomms1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A., Okumura M., Kimata T., Tanizawa Y., Takano R., Kimura K.D., Inada H., Matsumoto K., Mori I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science. 2008;320:803–807. doi: 10.1126/science.1148922. [DOI] [PubMed] [Google Scholar]
- Kumar N., Rao T.K., Varghese A., Rathor V.S. Internal parasite management in grazing livestock. J. Parasit. Dis. 2013;37:151–157. doi: 10.1007/s12639-012-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F., Foster J.M., Slatko B.E., Sullivan W. Efficient in vitro RNA interference and immunofluorescence-based phenotype analysis in a human parasitic nematode, Brugia malayi. Parasites Vectors. 2012;5:16. doi: 10.1186/1756-3305-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann J.K., Prociv P. Experimental human infection with the dog hookworm, Ancylostoma caninum. Med. J. Aust. 2003;178:69–71. doi: 10.5694/j.1326-5377.2003.tb05222.x. [DOI] [PubMed] [Google Scholar]
- Lans H., Rademakers S., Jansen G. A network of stimulatory and inhibitory Gα-subunits regulates olfaction in Caenorhabditis elegans. Genetics. 2004;167:1677–1687. doi: 10.1534/genetics.103.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmount J., Stephens N., Boughtflower V., Barrecheguren A., Rickell K. The development of anthelmintic resistance with best practice control of nematodes on commercial sheep farms in the UK. Vet. Parasitol. 2016;229:9–14. doi: 10.1016/j.vetpar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Lee H., Choi M.K., Lee D., Kim H.S., Hwang H., Kim H., Park S., Paik Y.K., Lee J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat. Neurosci. 2012;15:107–112. doi: 10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- Lee J., Dillman A.R., Hallem E.A. Temperature-dependent changes in the host-seeking behaviors of parasitic nematodes. BMC Biol. 2016;14:36. doi: 10.1186/s12915-016-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendner M., Doligalska M., Lucius R., Hartmann S. Attempts to establish RNA interference in the parasitic nematode Heligmosomoides polygyrus. Mol. Biochem. Parasitol. 2008;161:21–31. doi: 10.1016/j.molbiopara.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Li J., Ashton F.T., Gamble H.R., Schad G.A. Sensory neuroanatomy of a passively ingested nematode parasite, Haemonchus contortus: amphidial neurons of the first stage larva. J. Comp. Neurol. 2000;417:299–314. [PubMed] [Google Scholar]
- Li J., Zhu X., Ashton F.T., Gamble H.R., Schad G.A. Sensory neuroanatomy of a passively ingested nematode parasite, Haemonchus contortus: amphidial neurons of the third-stage larva. J. Parasitol. 2001;87:65–72. doi: 10.1645/0022-3395(2001)087[0065:SNOAPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Li J., Zhu X., Boston R., Ashton F.T., Gamble H.R., Schad G.A. Thermotaxis and thermosensory neurons in infective larvae of Haemonchus contortus, a passively ingested nematode parasite. J. Comp. Neurol. 2000;424:58–73. doi: 10.1002/1096-9861(20000814)424:1<58::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Li X., Massey H.C., Jr., Nolan T.J., Schad G.A., Kraus K., Sundaram M., Lok J.B. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. Int. J. Parasitol. 2006;36:671–679. doi: 10.1016/j.ijpara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Li X., Shao H., Junio A., Nolan T.J., Massey H.C., Jr., Pearce E.J., Viney M.E., Lok J.B. Transgenesis in the parasitic nematode Strongyloides ratti. Mol. Biochem. Parasitol. 2011;179:114–119. doi: 10.1016/j.molbiopara.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Schulze E., Baumeister R. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J. piggyBac: a vehicle for integrative DNA transformation of parasitic nematodes. Mobile Genet. Elem. 2013;3 doi: 10.4161/mge.24417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J.B. WormBook. 2007. Strongyloides stercoralis: a model for translational research on parasitic nematode biology; pp. 1–18.www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J.B. Nucleic acid transfection and transgenesis in parasitic nematodes. Parasitology. 2012;139:574–588. doi: 10.1017/S0031182011001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J.B., Massey H.C., Jr. Transgene expression in Strongyloides stercoralis following gonadal microinjection of DNA constructs. Mol. Biochem. Parasitol. 2002;119:279–284. doi: 10.1016/s0166-6851(01)00414-5. [DOI] [PubMed] [Google Scholar]
- Lok J.B., Shao H., Massey H.C., Li X. Transgenesis in Strongyloides and related parasitic nematodes: historical perspectives, current functional genomic applications and progress towards gene disruption and editing. Parasitology. 2017;144:327–342. doi: 10.1017/S0031182016000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P.M., Boston R., Ashton F.T., Schad G.A. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int. J. Parasitol. 2000;30:1115–1121. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Luo L., Cook N., Venkatachalam V., Martinez-Velazquez L.A., Zhang X., Calvo A.C., Hawk J., MacInnis B.L., Frank M., Ng J.H. Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2776–2781. doi: 10.1073/pnas.1315205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R. Chemoattraction of infective larvae of Ancylostoma braziliense to rodent plasmas and to salts. Acta Biol. Hung. 1987;38:235–245. [PubMed] [Google Scholar]
- Martin J., Barja I., Lopez P. Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus) Biochem. Syst. Ecol. 2010;38:1096–1102. [Google Scholar]
- McCue J.F., Thorson R.E. Behavior of parasitic stages of helminths in a thermal gradient. J. Parasitol. 1964;50:67–71. [PubMed] [Google Scholar]
- McKenna M.L., McAtee S., Bryan P.E., Jeun R., Ward T., Kraus J., Bottazzi M.E., Hotez P.J., Flowers C.C., Mejia R. Human intestinal parasite burden and poor sanitation in rural Alabama. Am. J. Trop. Med. Hyg. 2017;97:1623–1628. doi: 10.4269/ajtmh.17-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman C.J., Corfas R.A., Matthews B.J., Ritchie S.A., Vosshall L.B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldal B.H., Debenham N.J., De Ley P., De Ley I.T., Vanfleteren J.R., Vierstraete A.R., Bert W., Borgonie G., Moens T., Tyler P.A. An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol. Phylogenet. Evol. 2007;42:622–636. doi: 10.1016/j.ympev.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Mohri A., Kodama E., Kimura K.D., Koike M., Mizuno T., Mori I. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169:1437–1450. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Moulvi A., Minz P., Rath S., Ashma R. Characterization of chemical constituents of human sweat: a study based on Indian population. Am. J. Forensic Med. Pathol. 2018;39:141–147. doi: 10.1097/PAF.0000000000000388. [DOI] [PubMed] [Google Scholar]
- Nagai T., Yamada S., Tominaga T., Ichikawa M., Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T.J., Zhu X., Ketschek A., Cole J., Grant W., Lok J.B., Schad G.A. The sugar glider (Petaurus breviceps): a laboratory host for the nematode Parastrongyloides trichosuri. J. Parasitol. 2007;93:1084–1089. doi: 10.1645/GE-1234R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman T.B. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:263–273. doi: 10.1017/S0031182016000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L.J., Walkden-Brown S.W., Kahn L.P. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet. Parasitol. 2006;142:1–15. doi: 10.1016/j.vetpar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Parker J.C., Haley A.J. Phototactic and thermotactic responses of the filariform larvae of the rat nematode Nippostrongylus muris. Exp. Parasitol. 1960;9:92–97. doi: 10.1016/0014-4894(60)90015-1. [DOI] [PubMed] [Google Scholar]
- Parkins J.J., Holmes P.H. Effects of gastrointestinal helminth parasites on ruminant nutrition. Nutr. Res. Rev. 1989;2:227–246. doi: 10.1079/NRR19890016. [DOI] [PubMed] [Google Scholar]
- Perkins L.A., Hedgecock E.M., Thomson J.N., Culotti J.G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Pokala N., Liu Q., Gordus A., Bargmann C.I. Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2770–2775. doi: 10.1073/pnas.1400615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N., Hau D.D., Peng J., Guo A.C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B. The human serum metabolome. PloS One. 2011;6 doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B.L., Goodman M.B. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 2008;11:908–915. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B.L., Lee H.C., Goodman M.B. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J. Neurosci. 2008;28:12546–12557. doi: 10.1523/JNEUROSCI.2857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C.H., Beck C.D., Chiba C.M. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav. Brain Res. 1990;37:89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Rees G. Observations on the vertical migrations of the third-stage larva of Haemonchus contortus (Rud.) on experimental plots of Lolium perenne S24, in relation to meteorological and micrometeorological factors. Parasitology. 1950;40:127–143. doi: 10.1017/s0031182000017959. [DOI] [PubMed] [Google Scholar]
- Reesal M.R. Observations on the biology of the infective larvae of Strongyloides agoutii. Can. J. Zool. 1951;29:109–115. [Google Scholar]
- Rengarajan S., Hallem E.A. Olfactory circuits and behaviors of nematodes. Curr. Opin. Neurobiol. 2016;41:136–148. doi: 10.1016/j.conb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K., Crump J.G., Sagasti A., Bargmann C.I. The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Roberts L.B. Imperial College London; 2017. The Influence of Non-neuronal Cholinergic Signalling on the Type 2 Immune Response (PhD Dissertation) [Google Scholar]
- Roberts L.S., Janovy J., Schmidt P. seventh ed. McGraw-Hill; 2005. Foundations of Parasitology. [Google Scholar]
- Robinson A.F. Movement of five nematode species through sand subjected to natural temperature gradient fluctuations. J. Nematol. 1994;26:46–58. [PMC free article] [PubMed] [Google Scholar]
- Roeber F., Jex A.R., Gasser R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance - an Australian perspective. Parasites Vectors. 2013;6:153. doi: 10.1186/1756-3305-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart A., Yao C.K., Ha N., Chrispa M.D., Muir J.G., Gibson P.R., Kalantar-Zadeh K., Ou J.Z. Designing an in-vitro gas profiling system for human faecal samples. Sensor. Actuator. B Chem. 2017;238:754–764. [Google Scholar]
- Ruiz F., Castelletto M.L., Gang S.S., Hallem E.A. Experience-dependent olfactory behaviors of the parasitic nematode Heligmosomoides polygyrus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W.S., Samuel A.D. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined thermal stimuli. J. Neurosci. 2002;22:5727–5733. doi: 10.1523/JNEUROSCI.22-13-05727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D., Brenes M., Dunipace S., Schad G. Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1627–1630. doi: 10.1073/pnas.0610193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura T., Uga S. Assessment of skin penetration of third-stage larvae of Strongyloides ratti. Parasitol. Res. 2010;107:1307–1312. doi: 10.1007/s00436-010-1998-4. [DOI] [PubMed] [Google Scholar]
- Samarasinghe B., Knox D.P., Britton C. Factors affecting susceptibility to RNA interference in Haemonchus contortus and in vivo silencing of an H11 aminopeptidase gene. Int. J. Parasitol. 2011;41:51–59. doi: 10.1016/j.ijpara.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sambongi Y., Nagae T., Liu Y., Yoshimizu T., Takeda K., Wada Y., Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999;10:753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- Satterlee J.S., Ryu W.S., Sengupta P. The CMK-1 CaMKI and the TAX-4 cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr. Biol. 2004;14:62–68. doi: 10.1016/j.cub.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Satterlee J.S., Sasakura H., Kuhara A., Berkeley M., Mori I., Sengupta P. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Schafer T.W., Skopic A. Parasites of the small intestine. Curr. Gastroenterol. Rep. 2006;8:312–320. doi: 10.1007/s11894-006-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar F., Trostdorf U., Giardina F., Khieu V., Muth S., Marti H., Vounatsou P., Odermatt P. Strongyloides stercoralis: global distribution and risk factors. PLoS Neglected Trop. Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B.R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schild L.C., Glauser D.A. Dynamic switching between escape and avoidance regimes reduces Caenorhabditis elegans exposure to noxious heat. Nat. Commun. 2013;4:2198. doi: 10.1038/ncomms3198. [DOI] [PubMed] [Google Scholar]
- Schild L.C., Zbinden L., Bell H.W., Yu Y.X.V., Sengupta P., Goodman M.B., Glauser D.A. The balance between cytoplasmic and nuclear CaM Kinase-1 signaling controls the operating range of noxious heat avoidance. Neuron. 2014;84:983–996. doi: 10.1016/j.neuron.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca J., Forbes W.M., Ashton F.T., Lombardini E., Gamble H.R., Schad G.A. Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol. Int. 2002;51:53–62. doi: 10.1016/s1383-5769(01)00105-2. [DOI] [PubMed] [Google Scholar]
- Scott K. Out of thin air: sensory detection of oxygen and carbon dioxide. Neuron. 2011;69:194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Li X., Nolan T.J., Massey H.C., Jr., Pearce E.J., Lok J.B. Transposon-mediated chromosomal integration of transgenes in the parasitic nematode Strongyloides ratti and establishment of stable transgenic lines. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf D.M., Clark S.J., Downing D.T. Skin surface lipids of the dog. Lipids. 1977;12:786. doi: 10.1007/BF02533265. [DOI] [PubMed] [Google Scholar]
- Singhvi A., Liu B., Friedman C.J., Fong J., Lu Y., Huang X.Y., Shaham S. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell. 2016;165:936–948. doi: 10.1016/j.cell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.S., Martinez-Velazquez L., Ringstad N. A chemoreceptor that detects molecular carbon dioxide. J. Biol. Chem. 2013;288:37071–37081. doi: 10.1074/jbc.M113.517367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkenmann C. Springer Handbook of Odor. 2017. Analysis and chemistry of human odors; pp. 921–936. [Google Scholar]
- Stoltzfus J.D., Massey H.C., Jr., Nolan T.J., Griffith S.D., Lok J.B. Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PloS One. 2012;7 doi: 10.1371/journal.pone.0038587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus J.D., Minot S., Berriman M., Nolan T.J., Lok J.B. RNAseq analysis of the parasitic nematode Strongyloides stercoralis reveals divergent regulation of canonical dauer pathways. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey P.A., Faile G., Hewitt E., Yelifari L., Polderman A.M., Magnussen P. Clinical epidemiology and classification of human oesophagostomiasis. Trans. R. Soc. Trop. Med. Hyg. 2000;94:177–182. doi: 10.1016/s0035-9203(00)90267-0. [DOI] [PubMed] [Google Scholar]
- Sulston J., Dew M., Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975;163:215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- Tada I., Koga M., Hamano S., Higo H., Tanaka K. Strongyloides ratti: accumulating behavior of the third stage larvae to sodium ion. Jpn. J. Nematol. 1997;27:22–29. [Google Scholar]
- Takeishi A., Yu Y.V., Hapiak V.M., Bell H.W., O'Leary T., Sengupta P. Receptor-type guanylyl cyclases confer thermosensory responses in C. elegans. Neuron. 2016;90:235–244. doi: 10.1016/j.neuron.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W., Verhulst N.O. Chemical signaling in mosquito-host interactions: the role of human skin microbiota. Curr. Opin. Insect Sci. 2017;20:68–74. doi: 10.1016/j.cois.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Terrill T.H., Miller J.E., Burke J.M., Mosjidis J.A., Kaplan R.M. Experiences with integrated concepts for the control of Haemonchus contortus in sheep and goats in the United States. Vet. Parasitol. 2012;186:28–37. doi: 10.1016/j.vetpar.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Tobata-Kudo H., Higo H., Koga M., Tada I. Chemokinetic behavior of the infective third-stage larvae of Strongyloides ratti on a sodium chloride gradient. Parasitol. Int. 2000;49:183–188. doi: 10.1016/s1383-5769(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Tobata-Kudo H., Shimada M., Koga M., Tada I. Strongyloides ratti: thermokinetic behavior of third-stage larvae on a temperature gradient. Exp. Parasitol. 2000;95:196–201. doi: 10.1006/expr.2000.4526. [DOI] [PubMed] [Google Scholar]
- Tobin D., Madsen D., Kahn-Kirby A., Peckol E., Moulder G., Barstead R., Maricq A., Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Traub R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int. J. Parasitol. 2013;43:1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Troemel E.R., Kimmel B.E., Bargmann C.I. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Tzelos T. University of Edinburgh; 2014. RNA Interference in Parasitic Nematodes – from Genome to Control (PhD Dissertation) [Google Scholar]
- Tzelos T., Matthews J.B., Whitelaw B., Knox D.P. Marker genes for activation of the RNA interference (RNAi) pathway in the free-living nematode Caenorhabditis elegans and RNAi development in the ovine nematode Teladorsagia circumcincta. J. Helminthol. 2015;89:208–216. doi: 10.1017/S0022149X13000801. [DOI] [PubMed] [Google Scholar]
- Uetake K., Abumi T., Suzuki T., Hisamatsu S., Fukuda M. Volatile faecal components related to sex and age in domestic cats (Felis catus) J. Appl. Anim. Res. 2017;46:766–770. [Google Scholar]
- van Megan H., van den Elson S., Holterman M., Karssen G., Mooyman P., Bongers T., Holovachov O., Bakker J., Helder J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009;11:927–950. [Google Scholar]
- Varshney L.R., Chen B.L., Paniagua E., Hall D.H., Chklovskii D.B. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 2011;7 doi: 10.1371/journal.pcbi.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikkakam T., Fiuza J.A., Gaze S.T. Overview of hookworm infection in humans. In: Singh S.K., editor. Neglected Tropical Diseases - South Asia. Springer; Cham: 2017. [Google Scholar]
- Venkatachalam K., Luo J., Montell C. Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 2014;223:937–962. doi: 10.1007/978-3-319-05161-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst N.O., Mukabana W.R., Takken W., Smallegange R.C. Human skin microbiota and their volatiles as odour baits for the malaria mosquito Anopheles gambiae s.s. Entomol. Exp. Appl. 2011;139:170–179. [Google Scholar]
- Vetter J.C., Vingerhoed J., Schoeman E., Wauters H.W. Chemotactic attraction of infective hookworm larvae of Ancylostoma caninum by a dog serum factor. Z. Parasitenkd. 1985;71:539–543. doi: 10.1007/BF00928357. [DOI] [PubMed] [Google Scholar]
- Viney M., Kikuchi T. Strongyloides ratti and S. venezuelensis - rodent models of Strongyloides infection. Parasitology. 2017;144:285–294. doi: 10.1017/S0031182016000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney M.E., Green L.D., Brooks J.A., Grant W.N. Chemical mutagenesis of the parasitic nematode Strongyloides ratti to isolate ivermectin resistant mutants. Int. J. Parasitol. 2002;32:1677–1682. doi: 10.1016/s0020-7519(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Viney M.E., Lok J.B. WormBook. 2007. Strongyloides spp; pp. 1–15.www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney M.E., Thompson F.J. Two hypotheses to explain why RNA interference does not work in animal parasitic nematodes. Int. J. Parasitol. 2008;38:43–47. doi: 10.1016/j.ijpara.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Viney M.E., Thompson F.J., Crook M. TGF-β and the evolution of nematode parasitism. Int. J. Parasitol. 2005;35:1473–1475. doi: 10.1016/j.ijpara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Visser A., Geldhof P., de Maere V., Knox D.P., Vercruysse J., Claerebout E. Efficacy and specificity of RNA interference in larval life-stages of Ostertagia ostertagi. Parasitology. 2006;133:777–783. doi: 10.1017/S0031182006001004. [DOI] [PubMed] [Google Scholar]
- Voelker C., Maempel S., Kornadt O. Measuring the human body's microclimate using a thermal manikin. Indoor Air. 2014;24:567–579. doi: 10.1111/ina.12112. [DOI] [PubMed] [Google Scholar]
- Wasserman S.M., Beverly M., Bell H.W., Sengupta P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 2011;21:353–362. doi: 10.1016/j.cub.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters H.W., Klaver-Wesseling J.C., Vetter J.C. The effect of ultrafiltrated and dialysed dog serum on the chemotaxis of infective hookworm larvae of Ancylostoma caninum. Z. Parasitenkd. 1982;68:305–311. doi: 10.1007/BF00927408. [DOI] [PubMed] [Google Scholar]
- Way J.C., Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 1989;3:1823–1833. doi: 10.1101/gad.3.12a.1823. [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. Roy. Soc. Lond. B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wicks S.R., Rankin C.H. Integration of mechanosensory stimuli in Caenorhabditis elegans. J. Neurosci. 1995;15:2434–2444. doi: 10.1523/JNEUROSCI.15-03-02434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks S.R., Roehrig C.J., Rankin C.H. A dynamic network simulation of the nematode tap withdrawal circuit: predictions concerning synaptic function using behavioral criteria. J. Neurosci. 1996;16:4017–4031. doi: 10.1523/JNEUROSCI.16-12-04017.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg N., Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10477–10482. doi: 10.1073/pnas.96.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa S., Oiso T. Studies on oral infection with Ancylostoma. Am. J. Hyg. 1926;6:484–497. [Google Scholar]
- Yoshida K., Hirotsu T., Tagawa T., Oda S., Wakabayashi T., Iino Y., Ishihara T. Odour concentration-dependent olfactory preference change in C. elegans. Nat. Commun. 2012;3:739. doi: 10.1038/ncomms1750. [DOI] [PubMed] [Google Scholar]
- Yu Y.V., Bell H.W., Glauser D., Van Hooser S.D., Goodman M.B., Sengupta P. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron. 2014;84:919–926. doi: 10.1016/j.neuron.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman V., Dawkins H.J., Grove D.I. Scanning electron microscopy of the penetration of newborn mouse skin by Strongyloides ratti and Ancylostoma caninum larvae. Southeast Asian J. Trop. Med. Publ. Health. 1980;11:212–219. [PubMed] [Google Scholar]
- Zawadzki J.L., Kotze A.C., Fritz J.A., Johnson N.M., Hemsworth J.E., Hines B.M., Behm C.A. Silencing of essential genes by RNA interference in Haemonchus contortus. Parasitology. 2012;139:613–629. doi: 10.1017/S0031182012000121. [DOI] [PubMed] [Google Scholar]
- Zhang Z.M., Cai J.J., Ruan G.H., Li G.K. The study of fingerprint characteristics of the emanations from human arm skin using the original sampling system by SPME-GC/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;822:244–252. doi: 10.1016/j.jchromb.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Zhu H., Li J., Nolan T.J., Schad G.A., Lok J.B. Sensory neuroanatomy of Parastrongyloides trichosuri, a nematode parasite of mammals: amphidial neurons of the first-stage larva. J. Comp. Neurol. 2011;519:2493–2507. doi: 10.1002/cne.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietse M.A., Klaver-Wesseling J.C., Vetter J.C. The behaviour of infective Ancylostoma caninum larvae in serum gradients. J. Helminthol. 1981;55:203–207. [PubMed] [Google Scholar]
- Zimmer M., Gray J.M., Pokala N., Chang A.J., Karow D.S., Marletta M.A., Hudson M.L., Morton D.B., Chronis N., Bargmann C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]