Abstract
The frequent use of antibiotics contributes to antibiotic resistance in bacteria, resulting in an increase in infections that are difficult to treat. Livestock are commonly administered antibiotics in their feed, but there is current interest in raising animals that are only administered antibiotics during active infections. Staphylococcus aureus (SA) is a common pathogen of both humans and livestock raised for human consumption. SA has achieved high levels of antibiotic resistance, but the origins and locations of resistance selection are poorly understood. We determined the prevalence of SA and MRSA in conventional and antibiotic-free (AF) meat products, and also measured rates of antibiotic resistance in these isolates. We isolated SA from raw conventional turkey, chicken, beef, and pork samples and also from AF chicken and turkey samples. We found that SA contamination was common, with an overall prevalence of 22.6% (range of 2.8–30.8%) in conventional meats and 13.0% (range of 12.5–13.2%) in AF poultry meats. MRSA was isolated from 15.7% of conventional raw meats (range of 2.8–20.4%) but not from AF-free meats. The degree of antibiotic resistance in conventional poultry products was significantly higher vs AF poultry products for a number of different antibiotics, and while multi-drug resistant strains were relatively common in conventional meats none were detected in AF meats. The use of antibiotics in livestock contributes to high levels of antibiotic resistance in SA found in meat products. Our results support the use of AF conditions for livestock in order to prevent antibiotic resistance development in SA.
Introduction
The discovery of antibiotics has saved countless lives as they have been used to treat bacterial infections. However, bacteria can quickly develop resistance to antibiotics through mutation and by horizontal gene transfer [1]. Many bacterial species have acquired resistance to a number of antibiotics and the rate of development of new antibiotics is not keeping pace with the development of resistance. High rates of antibiotic use by humans, by livestock animals, and also the release of antibiotics into the environment continue to select for resistant hosts [2]. As a result, many bacterial infections are difficult to treat and future prospects are not promising that the trend will reverse.
Livestock animals are commonly raised in high density environments; thus infectious agents rapidly move through animals resulting in significant morbidity/mortality. These animals are commonly administered antibiotics prophylactically to prevent bacterial infections. Prophylactic use of antibiotics results in better animal survival, and also in higher meat yields. This practice is widespread around the world, and current estimates suggest that 80% of all antibiotics are administered to livestock [3]. This high rate of antibiotic use can result in the development of antibiotic resistance in livestock-associated bacterial species. Since many bacteria that infect livestock also infect humans (e.g., E. coli, S. aureus, Salmonella), the areas where livestock are raised are thought to be a breeding ground for antibiotic resistance [4].
Staphylococcus aureus (SA) is an opportunistic bacterial pathogen carried asymptomatically by healthy individuals; it is found consistently in 20% and intermittently in 60% of the human population [5]. SA can carry a number of virulence genes, including hemolysins, enterotoxins, and immune-modulatory factors [6, 7]. SA can cause a variety of human diseases including skin infections, sepsis, and pneumonia [7–9]. It is also likely the most common cause of food poisoning in the United States [10].
Methicillin-resistant Staphylococcus aureus (MRSA) is a group of SA strains that has become resistant to many common antibiotics (methicillin-susceptible strains referred to as MSSA). SA and MRSA have become an increasing problem in healthcare in the United States, where they cause an estimated 80–100,000 invasive infections and 11–19,000 deaths per year [11, 12]. In the U.S., the Centers for Disease Control and Prevention concluded that during the year 2012, the number of MRSA-infected patients admitted into the hospital was estimated to be just above 75,000 [13]. The most common mode of SA/MRSA transmission is person-to person contact, and transmission usually takes place either in hospitals or the community [14–20]. However, some individuals become infected with SA/MRSA through live animal contact, [16–20] and also potentially through contact with raw livestock meat. These bacteria can experience horizontal gene transfer by mobile genetic elements that confer antibiotic resistance, which is thought to be the cause of the emergence of resistant bacteria found in farms and farm workers [21]. There is a link between voluntary removal of antibiotics from large-scale farms and a significant reduction in rates of antibiotic resistance among Enterococcus isolates from those farms [22]. These data suggest that antibiotic resistance in livestock can be reversible.
The aim of this study was to determine the prevalence of SA and MRSA in raw beef, chicken, pork, and turkey meats from conventionally-raised animals, and also from chicken and turkey meats from antibiotic-free (AF) raised animals, and to characterize individual antibiotic resistance profiles of the isolates. Data obtained were then analyzed to determine correlations of meat types and levels of antibiotic resistance to see if there were differences in rates of antibiotic resistance in SA isolated from meats of a particular species. We also determined if there were differences in rates of antibiotic resistance in meats from animals raised with or without antibiotic use.
Materials and methods
Isolation and identification of SA/MRSA in meat samples
Conventional meat samples were collected from at least 11 different grocery stores/wholesale stores/ethnic markets in various cities in Utah County, Utah (see Table 1). Samples were obtained as packaged meats at grocery and wholesale stores and unpackaged meats from ethnic markets. Conventional meat products were collected over a period of about a year. AF meat samples were collected from 5 different stores, representing 6 different brands (see Table 2), all as packaged meats. All AF samples were clearly labeled with “antibiotic-free” on the packaging. All AF meat samples were collected on one of two separate days (several months apart), and also from grocery stores located in Utah County, Utah. In order to prevent over-representation of a a few isolates, a single swab or liquid sample was taken from meat within a given package.
Table 1. Origin of Staphylococcus aureus isolates (conventional meats) by store location.
| Store | Beef MRSA | Chicken MSSA | Chicken MRSA | Pork MSSA | Pork MRSA | Turkey MSSA | Turkey MRSA | Total |
|---|---|---|---|---|---|---|---|---|
| Grocery store A | 1 | 1 | ||||||
| Grocery store B | 1 | 1 | ||||||
| Grocery store C | 1 | 1 | 2 | |||||
| Grocery store D | 1 | 1 | ||||||
| Grocery store E | 2 | 1 | 2 | 1 | 6 | |||
| Grocery Store F | 1 | 1 | ||||||
| Wholesale store A | 1 | 1 | 2 | |||||
| Wholesale store B | 2 | 1 | 3 | |||||
| Small ethnic market A | 1 | 1 | ||||||
| Small ethnic market B | 1 | 1 | ||||||
| Small ethnic market C | 1 | 1 | ||||||
| Unknown origin* | 1 | 2 | 3 | 1 | 4 | 5 | 16 | |
| Total | 1 | 4 | 10 | 5 | 7 | 2 | 7 | 36 |
Store locations and specific isolates per store are listed to show that SA isolates were collected from diverse locations.
*Some meat samples were provided without any information on the store of origin.
Table 2. Origin of staphylococcus aureus isolates (antibiotic-free meats) by store location.
| Store | Chicken MSSA | Chicken MRSA | Turkey MSSA | Turkey MRSA | Total |
|---|---|---|---|---|---|
| Grocery store A, Brand 1 | 2 | 0 | 2 | 0 | 4 |
| Grocery store B, Brand 1 | 5 | 0 | 1 | 0 | 6 |
| Grocery store C, Brand 1 | 0 | 0 | 0 | 0 | 0 |
| Grocery store D, Brand 1 | 0 | 0 | 0 | 0 | 0 |
| Grocery store E, Brand 1 | 0 | 0 | 0 | 0 | 0 |
| Grocery store E, Brand 2 | 0 | 0 | 0 | 0 | 0 |
| Total | 7 | 0 | 3 | 0 | 0 |
Store locations and specific isolates per store are listed to show that SA isolates were collected from diverse locations.
Samples were tested for SA by swabbing a 2.5x2.5cm2 section of meat with a sterile swab or pipetting 10μl of meat juice directly onto Mannitol Salt Agar (MSA) plates. If meat juice was apparent in the meat packaging, then juice was preferentially used over swabbing under the premise that contamination would be less likely if pipetting juice. MSA plates that showed no growth were scored as negative for SA. Growth on MSA plates, accompanied by fermentation, initially indicated SA detection. Gram stains were performed to confirm the presence of gram-positive cocci, and all isolates were also catalase and coagulase positive. Genotyping was performed by PCR to detect the presence of Staphylococcus-specific 16S rDNA sequences, and nucA detection was used to confirm S. aureus [23]. MRSA was detected by the same procedure in the presence of 2 μg/mL oxacillin. To confirm MRSA detection, PCR was used to detect mecA [23]; products were separated on a 1.5% agarose gel.
Disk diffusion test
The disk diffusion test was used to classify the resistance of each isolate to antibiotics. We used a standard protocol [24] and used ATCC S. aureus reference strain 25923 as a control; if that strain failed to show established resistance values then the test was repeated. Mueller-Hinton agar plates were used for growth, supplemented with 2% NaCl. McFarland standards were used to verify that bacterial density was in the appropriate range. Amounts of antibiotic per disk were as follows: clindamycin 2 μg, cefotaxime 30 μg, gentamicin 10 μg, erythromycin 15 μg, tetracycline 30 μg, ciprofloxacin 5 μg, chloramphenicol 30 μg, and rifampin 5 μg (Sigma Aldrich). Susceptibility of isolates was identified by zone diameters as determined by CLSI standards. Plates were incubated at 37°C for 24–48 hours.
Minimum inhibitory concentration (MIC)
MIC tests were performed by CLSI standards for vancomycin at concentrations of 6 μg/mL (intermediate resistance) and 16 μg/mL (complete resistance). Mueller-Hinton agar plates were prepared with vancomycin, then inoculated with isolates and analyzed for growth after 24–48 hours at 37°C. MRSA was detected by the same procedure in the presence of 4 μg/mL oxacillin. Plates were observed to see if growth occurred; if only a few colonies were detected, then samples were re-tested. Presence of just a few colonies was not scored as resistant.
Statistical analysis
To analyze the prevalence of SA, MSSA, or MRSA in different types of raw meat samples, a chi-squared test was performed. A two sample t-test with unequal variance was used to determine if the sum of all meat isolates was more or less resistant/susceptible to a certain antibiotic. For all antibiotics tested with zone diameter measurements, t-tests were performed, while a 2-sample z-test of proportions was performed on oxacillin and vancomycin and also for differences in the prevalence of multi-drug resistant (MDR) strains. Multi-drug comparison was performed by an asymptotic chi-square test. A Fishers Exact test was used to examine differences in rates of antibiotic resistance to oxacillin and vancomycin.
Results
Detection and isolation of SA in conventional raw meat samples
159 different conventional meat samples (beef, chicken, pork and turkey) were tested for SA, as described in Materials and Methods. 1 of 36 beef samples was positive (2.8%), 14 of 49 chicken samples were positive (28.6%), 12 of 39 pork samples were positive (30.8%), and 9 of 35 turkey samples were positive (25.7%). SA contamination in beef was significantly lower than all other meat types (p<0.001; chi-squared test), but there were no other significant differences in prevalence. The overall frequency of SA isolation from the 159 samples was 36 (22.6%; see Table 3). The meat samples were collected from at least 11 different stores, as summarized in Table 1. Information on the store of origin was not available for all 36 isolates, but the origin is reported for 20 of the 36 isolates (55.6%).
Table 3. Prevalence of Staphylococcus aureus in raw meat samples.
| Meat type | Number of samples tested | Number of SA isolated | % with SA | Number of MSSA isolated | % with MSSA | Number of MRSA isolated | % with MRSA |
|---|---|---|---|---|---|---|---|
| C Beef | 36 | 1 | 2.8% | 0 | 0.0% | 1 | 2.8% |
| C Chicken | 49 | 14 | 28.6% | 4 | 8.2% | 10 | 20.4% |
| C Pork | 39 | 12 | 30.8% | 5 | 12.8% | 7 | 18.0% |
| C Turkey | 35 | 9 | 25.7% | 2 | 5.7% | 7 | 20.0% |
| C Total | 159 | 36 | 22.6% | 11 | 6.9% | 25 | 15.7% |
| AF Chicken | 53 | 7 | 13.2% | 7 | 13.2% | 0 | 0% |
| AF Turkey | 24 | 3 | 12.5% | 3 | 12.5% | 0 | 0% |
| AF Total | 77 | 10 | 13.0% | 10 | 13.0% | 0 | 0% |
Prevalence of Staphylococcus aureus (SA) in raw meat samples, which is further divided into Methicillin-Susceptible Staphylococcus aureus (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA). C = Conventional meat sample (raised with antibiotics) and AF = Antibiotic-free meat sample.
Screening of SA isolates from conventional meats for MRSA
SA isolates were re-plated on MSA plates in the presence of 2 μg/ml oxacillin to determine resistance to oxacillin. Any isolates found to be resistant to oxacillin were initially classified as MRSA, and all others were determined to be MSSA. To confirm MRSA, PCR genotyping was performed to detect the mecA gene; all isolates reported as MRSA produced a band of ~533bp (see Methods). MRSA was detected only rarely in beef (1 of 36 meat samples positive; 2.8%), but was common in the other three meat types: 10 of 49 meat samples positive in chicken (20.4%), 7 of 39 meat samples positive in pork (18.0%), and 7 of 35 meat samples positive in turkey (20.0%). The overall frequency of MRSA isolation from the 159 samples was 15.7% (Table 3), consistent with reports by others in different locations [25]. Of samples where SA was detected, 100% were MRSA in beef (but n = 1), 77.8% were MRSA in turkey, 71.4% were MRSA in chicken, and 58.3% were MRSA in pork. Overall, the majority of SA isolates were MRSA (69.4%). Beef had significantly fewer MSSA (p≤0.05) and MRSA (p≤0.02) contamination as compared to other meat types, but there were no other significant differences by meat type.
Antibiotic resistance in conventional raw meat SA isolates
We next measured antibiotic resistance in all MSSA and MRSA isolates. Resistance levels were determined by disk diffusion for eight common antibiotics. S1 Table shows disk diffusion distances for each isolate, with zone diameters (in millimeters) for all antibiotics except for oxacillin and vancomycin. Relative antibiotic resistance (complete resistance, intermediate resistance, and complete susceptibility) is also indicated for each isolate. We detected two isolates with intermediate resistance to vancomycin, one of which was also a MRSA strain. Mean disk diffusion distances are shown in Fig 1 to better illustrate relative differences by meat type and by antibiotic. Of note, the frequency of isolates that were multi-drug resistant (MDR; complete resistance to three or more antibiotics) per meat group were found to be: 100% (beef; but n = 1), 66.7% (chicken), 55.5% (turkey), and 54.5% (pork); the overall frequency of multi-drug resistance was 20/33 or 60.6%. No significant differences in multi-drug resistance were found by meat type; beef was excluded due to the small sample size. Complete susceptibility to all antibiotics tested was not detected in any isolate.
Fig 1. Mean antibiotic resistance in conventional raw meat SA isolates for eight common antibiotics.
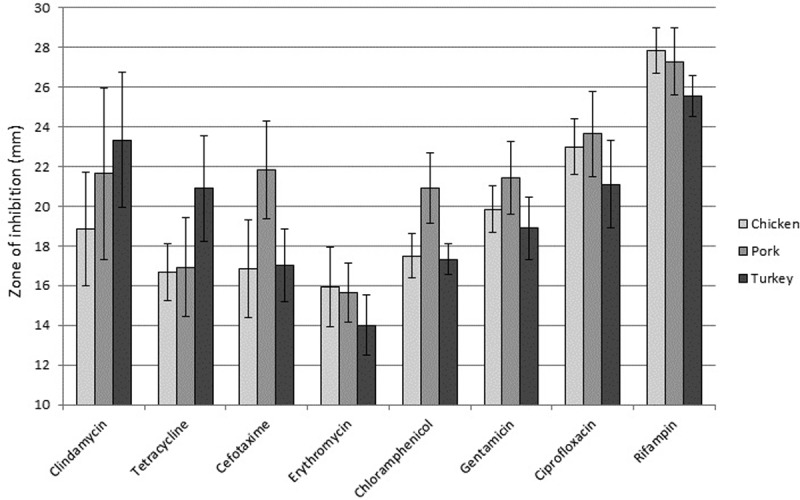
Disk diffusion tests were performed to determine the amount of antibiotic required to prevent growth of the various SA raw meat isolates. There was only a single SA isolate from beef, and thus no results are reported here for that meat type. Means were calculated and standard error is indicated. Antibiotic concentrations used and significance of zone diameters are detailed in Methods. A two sample t-test with unequal variance test was used to determine if there were significant differences in rates of antibiotic resistance or susceptibility amongst the various meat types.
We then determined if there were any significant differences in rates of antibiotic resistance when comparing meat types. The only significant result was that pork SA isolates were significantly more susceptible to cefotaxime as compared to other SA isolates (p = 0.03 for susceptibility, or p = 0.97 for resistance). We compared rates of antibiotic resistance amongst all SA samples to determine if SA from raw meat samples showed significant differences in antibiotic susceptibility across the 10 antibiotics tested (Fig 1). Clindamycin resistance was significantly higher that rifampin (p = 0.005); tetracycline resistance was significantly higher than rifampin (p<0.001) and ciprofloxacin (p = 0.003); cefotaxime resistance was significantly higher than rifampin (p<0.001) and ciprofloxacin (p = 0.011); erythromycin resistance was significantly higher than all other antibiotics (p≤0.05); chloramphenicol resistance was significantly higher than rifampin (p<0.001) and ciprofloxacin (p = 0.002); gentamicin resistance was significantly higher than rifampin (p<0.001) and ciprofloxacin (p = 0.035); and ciprofloxacin resistance was significantly higher than rifampin (p = 0.001).
Detection and isolation of SA and MRSA in AF raw poultry samples
Raw meat samples were tested for the presence of SA using the same methods outlined above, but using raw meat samples marked as “antibiotic-free”. In total, 77 different raw poultry meat samples (chicken and turkey) were tested. AF meat sources are shown in Table 2. 7 of 53 chicken samples were positive for SA (13.2%) and 3 of 24 turkey samples were positive (12.5%) for SA. SA was found at significantly higher levels in conventional meats as compared to AF meats for chicken (p = 0.03), but not for turkey (p = 0.11). Isolates were genotyped as above to confirm SA and MRSA. None of the 10 isolates from AF poultry meats were positive for the mecA gene, indicating a lack of MRSA amongst all AF poultry isolates. These results were further confirmed by a lack of growth on MSA plates with 2 μg/ml oxacillin. MRSA was found at significantly higher levels in conventional meats as compared to AF meats for both chicken (p = 0.0004) and turkey (p = 0.0002).
Antibiotic resistance in AF poultry SA isolates
We next measured antibiotic resistance in all SA isolates from AF meats, as above for conventional meat SA isolates. S1 Table shows disk diffusion distances for each isolate, with zone diameters (in millimeters) for all antibiotics except for oxacillin and vancomycin; relative rates of antibiotic resistance are also indicated for each isolate. Mean disk diffusion distances are shown in Fig 2. We did not detect any MDR isolates in the AF meat samples. Complete susceptibility to all antibiotics tested was detected in one chicken SA isolate. All AF SA isolates were susceptible to chloramphenicol, oxacillin, and vancomycin.
Fig 2. Mean antibiotic resistance in AF raw poultry SA isolates for eight common antibiotics.
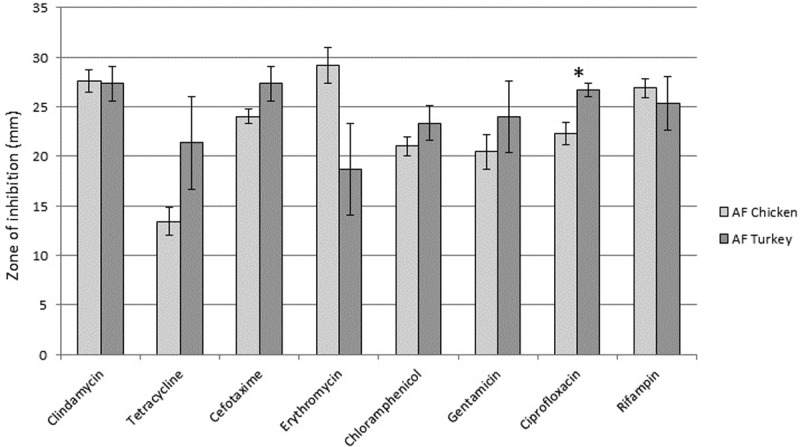
Disk diffusion tests were performed to determine the amount of antibiotic required to prevent growth of the various SA raw meat isolates. A two sample t-test with unequal variance test was used to determine if there were significant differences in rates of antibiotic resistance or susceptibility amongst the various meat types. * indicates p≤0.05.
Statistical analysis was then performed to determine if there were any significant differences in antibiotic resistance when comparing AF poultry meat types. Most antibiotic resistances were not significantly different when comparing SA from chicken or turkey, with the exception being that SA from chicken was significantly more resistant to ciprofloxacin (p = 0.005).
Statistical analysis was also performed to determine if there were any significant differences in antibiotic resistance when comparing conventional to AF meat sources (Fig 3). We found significantly lower rates of resistance (p≤0.05) to the following antibiotics in AF chicken products: clindamycin, cefotaxime, erythromycin, chloramphenicol, and oxacillin (Fig 3A). In addition, there was a highly significant difference (p = 0.0003) in MDR strains, with none detected in AF chicken meat products. We found significantly lower rates of resistance (p≤0.05) to the following antibiotics in AF turkey products: cefotaxime, chloramphenicol, ciprofloxacin, and oxacillin (Fig 3B). There was a significant difference (p = 0.0067) in MDR strains, with none detected in AF turkey meat products.
Fig 3. Antibiotic resistance levels in AF meat products as compared to conventional meat products.
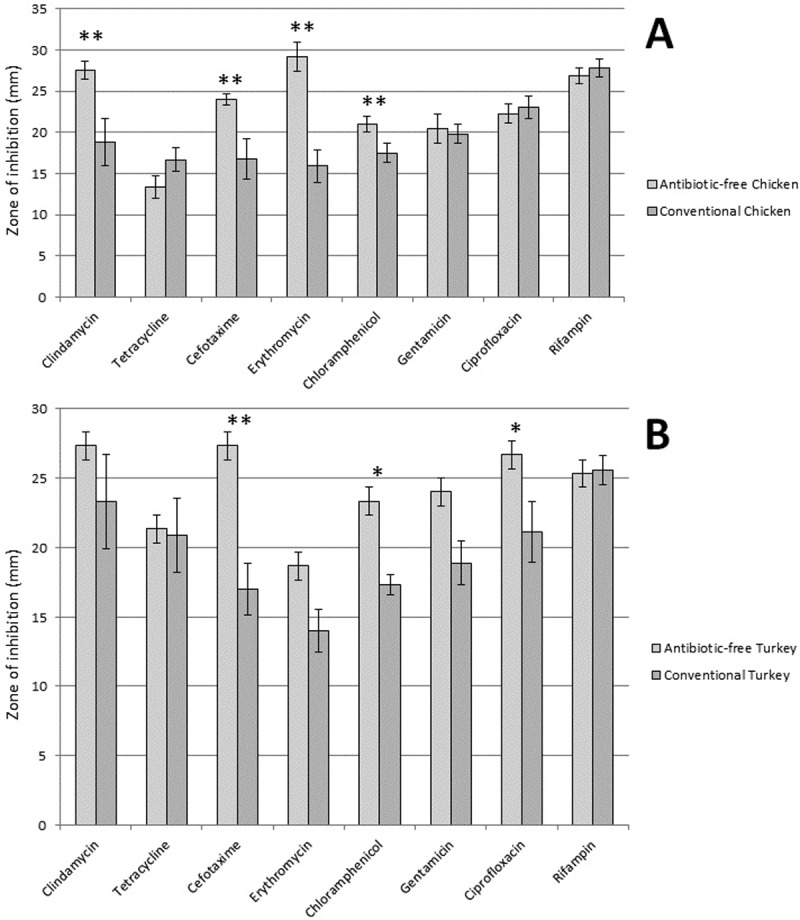
Panel A shows differences in antibiotic resistance in SA isolated from AF chicken products (n = 7) as compared to conventional chicken products (n = 12) and panel B shows differences in rates of antibiotic resistance in SA isolated from AF turkey products (n = 3) as compared to conventional turkey products (n = 9). A 2-sample z-test on proportions was used to determine if there were significant differences in rates of antibiotic resistance amongst the various meat types for all antibiotics tested by disk diffusion. A Fishers Exact test was used to examine significant differences in oxacillin and vancomycin resistance. * indicates p≤0.05; ** indicates p≤0.01.
Discussion
We isolated 36 Staphylococcus aureus (SA) strains from conventional raw meat products and 10 SA strains from AF meat products. SA was common in conventional raw meat products, with a combined prevalence of 22.6% amongst the four meat types. Beef contamination was significantly lower than other meat types, but no significant differences were seen between non-beef frequencies. MRSA isolates were also common in conventional meat products, with an overall prevalence of 15.7%, but there were no significant differences in either MRSA or MSSA detection with the exception that beef had significantly lower contamination with both types. SA contamination of AF poultry meats was significantly lower than in conventional meats (13.0% vs 22.6%; p = 0.02), and no MRSA was detected in the 77 AF poultry samples (0%; p<0.001). We also determined the antibiotic resistance profiles of each isolate for ten common antibiotics. Antibiotic resistance in SA was very common amongst conventional meat isolates, but less common in AF meat isolates. 20 conventional meat isolates showed resistance to at least three different antibiotics (60.6% of the isolates); while no isolates were multi-drug resistant in the AF group.
The prevalence of SA detected in our conventional meat samples was remarkably consistent amongst chicken, pork and turkey (range of 25.7–30.8%). We detected more MRSA than MSSA in raw meat samples (Table 3), and that trend held true across all meat types. Our MRSA detection was higher than reported in other areas of the USA, especially for MRSA in poultry, but were lower than those reported for pork in Canada (31/402 or 7.7%) [26]. For example, Pu et. al. reported that 45.6% pork samples and 20% of beef samples were contaminated with SA when sampling 120 retail meat samples taken from 30 different grocery stores in the Baton Rouge, Louisiana, USA region. Only 5% of their samples had MRSA [27]. Waters et. al. examined SA contamination in raw meat from 80 unique brands in 5 cities in the USA. They found the following for SA contamination in commercially available raw meats: 77% in turkey, 42% in pork, 41% in chicken, and 37% in beef. Only 4.7% of their samples contained MRSA, but 52% of their isolates were MDR with the MDR prevalence highest for turkey (79%), then pork (64%), then beef (35%), then chicken (26%) [28]. We found 55% of SA isolates from raw meat to be MDR, with the highest MDR prevalence in chicken (66.7%), then in turkey (55.6%), then in pork (54.5%), with beef omitted due to a small sample size. We detected 2 isolates with intermediate vancomycin resistance, while Waters et. al. found 1 such isolate [28].
It is possible that contamination of meat products at central processing locations could explain our results. Our isolates were obtained from at least 11 different stores, and the antibiotic resistance profiles shown provide some evidence that we did not re-isolate the same strains repeatedly because the drug resistance patterns vary widely amongst the various isolates (S1 and S2 Tables). Molecular typing of the isolates would further substantiate their origins. Regardless of their origins, our results nonetheless show that antibiotic resistance is rampant in SA isolates from raw meat, especially conventional meat products.
The United States Food and Drug Administration releases results of the total amounts of antibiotics sold for use in food-producing animals, and the 2014 report showed an increase of 22% in sales from 2009 to 2014. Tetracycline accounted for 70% of sales, followed by penicillin (9%), macrolides (7%), and sulfas (5%) with no other drugs over 3% [29]. We have shown that tetracycline resistance is significantly higher in SA isolates from conventional meats than that seen for rifampin or ciprofloxacin, but not for the other antibiotics tested (Fig 1), although there was no significant difference. High levels of antibiotic usage in livestock can also be seen on a worldwide scale; in 2012 it was estimated that 38.5 million kilograms of antibiotics were used exclusively in swine and poultry in China [30] and it is estimated that worldwide antibiotic usage will increase 67% from 2010 to 2030 due to growing demand for meat [31].
A handful of studies have analyzed the frequency of SA and MRSA in meats produced from animals raised under AF conditions. A study of AF vs conventional chicken products in Oklahoma, USA found a lower prevalence of SA contamination in AF meats vs. conventional meats (41% vs 53.8%) but the difference was not significant. MRSA contamination was very low in both types of chicken (0.9% in conventional chicken and 1.6% in AF chicken) [32]. SA was somewhat less common in AF pork (56.8%) as compared to conventional pork (67.3%), although the difference was not significant. MRSA frequency in raw pork was very similar in conventional pork (6.3%) and AF pork (7.4%) [33]. 27.8% of raw meat products contained SA in the state of Iowa, USA. A higher prevalence of both MSSA and MRSA was found in conventional meat as compared to antibiotic-free meat, and 23% of SA isolates were multi-drug resistant [34]. Our AF meats also had a significantly lower SA prevalence than for conventional meats. The reasons for this finding are not clear, but since SA has high rates of antibiotic resistance it is possible that this species can outcompete other species when antibiotics are present, but when they are not it is outcompeted by other bacteria due to higher fitness in other areas.
E. coli antibiotic resistance in organic vs conventionally-raised pigs in four European countries was found to be lower for a number of different antibiotics tested [35], and analysis of antimicrobial resistance genes across the microbiome of AF vs conventional chickens found that AF animals also had lower levels of antibiotic resistance genes in their associated bacteria [36]. These results indicate that the lower rates of antibiotic resistance in AF animals likely apply to other bacterial species and not just for SA. A poultry farm in the USA transitioned from common antibiotic use to organic practices, and two Enterococcus species were analyzed for antibiotic resistance before and after the transition. Interestingly, antibiotic resistance levels significantly decreased following the transition [22].
Conclusions
In conclusion, we have found that the use of antibiotics in livestock contributes to high levels of antibiotic resistance in SA isolates found in their resulting meat products. Our results suggest that the use of antibiotics in livestock promotes higher rates of antibiotic resistance in bacteria found in the meat products that consumers come into contact with and could be a source of transmission of antibiotic resistance bacteria to humans. AF conditions for livestock may prevent antibiotic resistance development in SA and in other microbes, and could relieve the continued development of antibiotic resistance.
Supporting information
Disk diffusion results are listed for all antibiotics except for oxacillin and vancomycin, for which standard inhibitory concentrations are listed. Dark Grey = resistant, light grey = intermediate, white = susceptible, black = MDR isolate. Those with complete resistance to at least three antibiotics were considered “multiple drug-resistant” (MDR). AFC = antibiotic-free chicken, AFT = antibiotic-free turkey, Cld = clindamycin, Tet = tetracycline, Cef = cefotaxime, Ery = erythromycin, Chl = chloramphenicol, Gen = gentamicin, Cip = ciprofloxacin, Rif = rifampin, Oxa = oxacillin, Van = vancomycin, SE = standard error, Susc = susceptible, Inter = intermediate resistance, Resis = complete resistance. All values for disk diffusion are in millimeters, which reflect the diameter of the growth ring around the antibiotic disk.
(DOCX)
Disk diffusion results are listed for all antibiotics except for oxacillin and vancomycin, for which standard inhibitory concentrations are listed. Dark Grey = resistant, light grey = intermediate, white = susceptible, black = MDR isolate. Isolates with complete resistance to at least three antibiotics were considered “multiple drug-resistant” (MDR). B = beef, C = chicken, P = pork, T = turkey, Cld = clindamycin, Tet = tetracycline, Cef = cefotaxime, Ery = erythromycin, Chl = chloramphenicol, Gen = gentamicin, Cip = ciprofloxacin, Rif = rifampin, Oxa = oxacillin, Van = vancomycin, SE = standard error, Susc = susceptible, Inter = intermediate resistance, Resis = complete resistance. All values for disk diffusion are in millimeters, which reflect the diameter of the growth ring around the antibiotic disk. Two chicken SA isolates and 1 turkey isolate were only tested for prevalence/genotyping and not by disk diffusion, which is why sample numbers differ for this table compared to other parts of the manuscript.
(DOCX)
Acknowledgments
We thank Kyle Jensen, Taalin Rasmussen, Kelsey Berges, and Chloe McCullough for help in collecting meat samples.
Abbreviations
- SA
Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- MRSA
Methicillin-resistant Staphylococcus aureus
- LA
livestock-associated
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by a Brigham Young University Mentoring Environment Grant and a Turkey Research Grant to BKB, and a Brigham Young University Office of Research and Creative Activities Grant to BBH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–33. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10 (Suppl):S122–S9. [DOI] [PubMed] [Google Scholar]
- 3.Ventola CL. The Antibiotic Resistance Crisis. Part 1: Causes and Threats. Pharmacy and Therapeutics. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 4.Barza M, Gorbach SL. The need to improve antimicrobial use in agriculture: ecological and human health consequences. Clin Infect Dis. 2002;34:S71–S144. 10.1086/34024111988874 [Google Scholar]
- 5.Von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. New England Journal of Medicine. 2001;344:11–6. 10.1056/NEJM200101043440102 [DOI] [PubMed] [Google Scholar]
- 6.Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. Expression of virulence factors by Staphylococcus aureus grown in serum. Applied and environmental microbiology. 2011;77:8097–105. 10.1128/AEM.05316-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bien J, Sokolova O, Bozko P. Characterization of virulence factors of Staphylococcus aureus: novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. Journal Pathog. 2011;2011:601905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clinical microbiology reviews. 2010;23:616–87. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? British Journal of Dermatology. 2008;158:442–55. 10.1111/j.1365-2133.2008.08437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluytmans JA. Methicillin-resistant Staphylococcus aureus in food products: cause for concern or case for complacency? Clin Microbiol Infect. 2010;16:11–5. 10.1111/j.1469-0691.2009.03110.x [DOI] [PubMed] [Google Scholar]
- 11.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S344–9. 10.1086/533590 [DOI] [PubMed] [Google Scholar]
- 12.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–6. 10.3201/eid1312.070629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevention CfDCa. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Methicillin—Resistant Staphylococcus aureus 2012 [updated 2012]. Available from: http://www.cdc.gov/abcs/reports-findings/survreports/mrsa12.pdf.
- 14.van Rijen MM, Bosch T, Heck ME, Kluytmans JA. Meticillin-resistant Staphylococcus aureus epidemiology and transmission in a Dutch hospital. J Hosp Infect. 2009;72:299–306. 10.1016/j.jhin.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Hughes C, Tunney M, Bradley MC. Infection control strategies for preventing the transmission of meticillin-resistant Staphylococcus aureus (MRSA) in nursing homes for older people. Cochrane Database Syst Rev. 2013;11:CD006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juhász-Kaszanyitzky É, Jánosi S, Somogyi P, Dán Á, vanderGraaf van Bloois L, Van Duijkeren E, et al. MRSA transmission between cows and humans. Emerg infect dis. 2007;13:630–2. 10.3201/eid1304.060833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, Van Duijkeren E, Heederik D. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE. 2010;5:e10990 10.1371/journal.pone.0010990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl Environ Microbiol. 2003;69:6489–94. 10.1128/AEM.69.11.6489-6494.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Duijkeren E, Ikawaty R, Broekhuizen-Stins MJ, Jansen MD, Spalburg EC, de Neeling AJ, et al. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet microbiol. 2008;126:383–9. 10.1016/j.vetmic.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 20.Armand-Lefevre L, Ruimy R, Andremont A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg Infect Dis. 2005;11(711–714). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay JA. Staphylococcus aureus genomics and the impact of horizontal gene transfer. Int J Med Microbiol. 2014;304(2):103–9. Epub 1 December 2013. 10.1016/j.ijmm.2013.11.010 . [DOI] [PubMed] [Google Scholar]
- 22.Sapkota AR, Hulet RM, Zhang G, McDermott P, Kinney EL, Schwab KJ, et al. Lower prevalence of antibiotic-resistant Enterococci on U.S. conventional poultry farms that transitioned to organic practices. Environ Health Perspect. 2011;119:1622–8. 10.1289/ehp.1003350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maes N, Magdalena J, Rottiers S, De Gheldre Y, Struelens MJ. Evaluation of a triplex PCR assay to discriminate Staphylococcus aureus from coagulase-negative Staphylococci and determine methicillin resistance from blood cultures. J Clin Microbiol. 2002;40:1514–7. 10.1128/JCM.40.4.1514-1517.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. CLSI document M11-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 25.Jackson CR, Davis JA, Barrett JB. Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J Clin Microbiol. 2013;51(4):1199–207. Epub 30 January 2013. 10.1128/JCM.03166-12 ; PubMed Central PMCID: PMCPMC3666775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weese JS, Reid-Smith R, Rousseau J, Avery B. Methicillin-resistant Staphylococcus aureus (MRSA) contamination of retail pork. Can Vet J. 2010;51:749–52. [PMC free article] [PubMed] [Google Scholar]
- 27.Pu S, Han F, Ge B. Isolation and characterization of methicillin-resistant Staphylococcus aureus strains from Louisiana retail meats. Appl Environ Microbiol. 2009;75:265–7. 10.1128/AEM.01110-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters AE, Contente-Cuomo T, Buchhagen J, Liu CM, Watson L, Pearce K, et al. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin Infect Dis. 2011;52:1227–30. 10.1093/cid/cir181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.2014 Summary Report on Antimicrobials Sold or Distrubuted for Use in Food-Producing Animals. In: Services HaH, editor. 2015.
- 30.Krishnasamy V, Otte J, Silbergeld E. Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob Resist Infect Control. 2015;4(17):17 Epub 28 April 2015. 10.1186/s13756-015-0050-y ; PubMed Central PMCID: PMCPMC4412119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. P Natl Acad Sci USA. 2015;112(18):5649–54. 10.1073/pnas.1503141112 PubMed PMID: WOS:000353953800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdalrahman LS, Stanley A, Wells H, Fakhr MK. Isolation, Virulence, and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus aureus (MRSA) and Methicillin Sensitive Staphylococcus aureus (MSSA) Strains from Oklahoma Retail Poultry Meats. Int J Environ Res Public Health. 2015;12:6148–61. 10.3390/ijerph120606148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien AM, Hanson BM, Farina SA, Wu JY, Simmering JE, Wardyn SE, et al. MRSA in conventional and alternative retail pork products. PLoS One. 2012;7:e30092 10.1371/journal.pone.0030092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thapaliya D, Forshey BM, Kadariya J, Quick MK, Farina S, O' Brien A, et al. Prevalence and molecular characterization of Staphylococcus aureus in commercially available meat over a one-year period in Iowa, USA. Food Microbiology. 2017;65:122–9. 10.1016/j.fm.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 35.Österberg J, Wingstrand A, Nygaard Jensen A, Kerouanton A, Cibin V, Barco L, et al. Antibiotic Resistance in Escherichia coli from Pigs in Organic and Conventional Farming in Four European Countries. PLoS One. 2016;11:e0157049 10.1371/journal.pone.0157049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegde NV, Kariyawasam S, DebRoy C. Comparison of antimicrobial resistant genes in chicken gut microbiome grown on organic and conventional diet. Veterinary and Animal Science. 2016;1:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disk diffusion results are listed for all antibiotics except for oxacillin and vancomycin, for which standard inhibitory concentrations are listed. Dark Grey = resistant, light grey = intermediate, white = susceptible, black = MDR isolate. Those with complete resistance to at least three antibiotics were considered “multiple drug-resistant” (MDR). AFC = antibiotic-free chicken, AFT = antibiotic-free turkey, Cld = clindamycin, Tet = tetracycline, Cef = cefotaxime, Ery = erythromycin, Chl = chloramphenicol, Gen = gentamicin, Cip = ciprofloxacin, Rif = rifampin, Oxa = oxacillin, Van = vancomycin, SE = standard error, Susc = susceptible, Inter = intermediate resistance, Resis = complete resistance. All values for disk diffusion are in millimeters, which reflect the diameter of the growth ring around the antibiotic disk.
(DOCX)
Disk diffusion results are listed for all antibiotics except for oxacillin and vancomycin, for which standard inhibitory concentrations are listed. Dark Grey = resistant, light grey = intermediate, white = susceptible, black = MDR isolate. Isolates with complete resistance to at least three antibiotics were considered “multiple drug-resistant” (MDR). B = beef, C = chicken, P = pork, T = turkey, Cld = clindamycin, Tet = tetracycline, Cef = cefotaxime, Ery = erythromycin, Chl = chloramphenicol, Gen = gentamicin, Cip = ciprofloxacin, Rif = rifampin, Oxa = oxacillin, Van = vancomycin, SE = standard error, Susc = susceptible, Inter = intermediate resistance, Resis = complete resistance. All values for disk diffusion are in millimeters, which reflect the diameter of the growth ring around the antibiotic disk. Two chicken SA isolates and 1 turkey isolate were only tested for prevalence/genotyping and not by disk diffusion, which is why sample numbers differ for this table compared to other parts of the manuscript.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


