Abstract
Background
Although high BP is one of the most important factors affecting renal function, whether longitudinal BP trajectories in early life course are associated with renal function damage in later life is unclear.
Methods
To investigate the correlation between BP trajectories from childhood to adulthood and renal function in middle age, we used group-based trajectory models to identify BP trajectories in 2430 individuals (aged 6–15 years old at baseline) participating in the ongoing Hanzhong Adolescent Hypertension Cohort. We tested the association between these trajectories and subclinical renal damage in middle age, adjusting for several covariates.
Results
We identified four distinct systolic BP trajectories among 2430 subjects: low stable, moderate stable, high stable, and moderate increasing on the basis of systolic BP levels at baseline and during the 30-year follow-up period. The urinary albumin-to-creatinine ratio (uACR) was higher in moderate stable, high stable, and moderate increasing groups compared with the low stable group. A total of 228 individuals had subclinical renal disease by 2017. Compared with the low stable trajectory group, the other groups had increasingly greater odds of experiencing subclinical renal disease in middle age. These associations were not altered after adjustment for other covariates, except for in the moderate stable group. Analyzed results were similar for the mean arterial pressure and diastolic BP trajectory groups.
Conclusions
Higher BP trajectories were correlated with higher of uACR levels and risk of subclinical renal disease in middle age. Identifying long-term BP trajectories from early age may assist in predicting individuals’ renal function in later life.
Keywords: blood pressure trajectory, eGFR, subclinical renal damage, prospective cohort study, uACR
CKD is now one of the most significant global health problems. The population prevalence of CKD is >10%, and over 50% of the population are at high risk for this disease.1 Other studies reported that the prevalence of CKD among adults was 10.8% in China2 and 13.0% in the United States.3 The 2016 European Guidelines on cardiovascular disease prevention reported that high-risk and very high–risk cardiovascular disease categories correspond to subjects with moderate CKD (30≤eGFR<59 ml/min per 1.73 m2) and severe CKD (eGFR<30 ml/min per 1.73 m2), respectively.4 In addition, subjects with the highest risk benefit most from preventive efforts. Therefore, early renal damage detection or screening and intervention are of utmost importance.
Hypertension is one of the most important CKD risk factors.5,6 Elevated BP may lead to peripheral arterial disease, which in turn, contributes to the development of CKD. The relationship between hypertension and progression of CKD is direct and progressive.7 In addition, antihypertensive therapy has an additional protective effect on renal function.8
Population-based cohorts support the view that high BP in later life originates in childhood.9–11 However, available evidence on the association between BP and CKD mainly concerns adult or elderly subjects with or without hypertension.12 Few take into account the potential effect of BP levels experienced earlier in life or changes in BP levels over time. Long-term BP trajectory patterns from childhood to middle age and their effect on CKD risk remain unknown. Consequently, the aims of this study are to identify subgroups of population with similar trajectories in BP development from childhood to middle age and reveal the relationship of BP trajectories with urinary albumin-to-creatinine ratio (uACR) and the presence of subclinical renal damage (SRD).
Methods
Study Population
This study was conducted within the Hanzhong Adolescent Hypertension Cohort, an ongoing prospective study focusing on evaluating the development of cardiovascular risk factors originating from children and young adults. The study began in 1987 when 4623 schoolchildren were enrolled from 26 rural sites of three towns (Qili, Laojun, and Shayan) in Hanzhong, Shaanxi, China.13 During baseline evaluation, the inclusion criteria were as follows: aged 6–15 years old in 1987, no chronic disease in the child’s medical history, and can communicate normally in Mandarin. Later information collection was conducted in 1989, 1992, 1995, 2005, 2013, and 2017, resulting in a maximum follow-up time of 30 years. Among those follow-up activities, we randomly selected several subjects to visit in 2005 and obtained BP and other data from 436 individuals. Except for the visit in 2005, other follow-ups were large in scale and aimed to visit each individual who was enrolled in 1987. The response rate was 77.7% (n=3592) in 1989, 84.8% (n=3918) in 1992, 82.1% (n=3794) in 1995, 65.3% (n=3018) in 2013, and 60.1% (n=2780) in 2017. Reasons for loss of follow-up mainly included death, mental illness, military service, and migration.
The study was approved by the Academic Committee of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2015LSL-047) and clinically registered (NCT02734472). All participants in this study signed informed consent for each visit, and for those <18 years of age at baseline, consent of a parent/guardian was obtained. These analyses included individuals with BP values available at three or more examinations and excluded subjects with three or more BP measurements only from the initial four visits (1987, 1989, 1992, and 1995) or the last three follow-ups (2005, 2013, and 2017).
Anthropometric Measurements
Personal information, including demographic characteristics, personal/family medical history (hypertension, diabetes, hyperlipidemia, and stroke), cigarette smoking, and drinking history, was obtained through a questionnaire. Height, body weight, hip/waist circumference, and bust size were measured with underwear through appropriate scales by trained staff. Replicate measurements of these indices were made, and the mean values were used for analysis. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height (kilograms per meter squared); waist-to-hip ratio (WHR) was calculated as waist circumference divided by the hip circumference, both in centimeters.
BP Measurements
Participants were required to avoid coffee/tea, alcohol, cigarette smoking, and strenuous exercise for at least 30 minutes before BP measurement. BP was measured three times in a seated position on the right upper arm after a 5-minute rest, with a 2-minute interval between measurements. The average of three BP values was used in the analyses. A Hawksley random zero sphygmomanometer was used14 for the first six visits, and an Omron M6 (Omron, Kyoto, Japan) device was used15 in 2017 for BP measurements by trained and certified staff. An appropriate cuff size was used, and the procedure was performed in a quiet and comfortable environment. Mean arterial pressure (MAP) was calculated by the formula: 1/3 systolic BP (SBP) +2/3 diastolic BP (DBP; millimeters of Hg).
Biochemical Parameter Measurements
Fasting venous blood samples were obtained by experienced nurses in the morning after fasting for 8–10 hours. Blood samples were immediately centrifuged at 3000×g for 10 minutes and stored at −80°C until analysis. Urine samples were collected for the first time in the morning. The samples were kept frozen at −40°C until analysis. Certain biomarkers, including total bilirubin, total cholesterol (TC), triglyceride (TG), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), serum creatinine, fasting plasma blood glucose (GLU), urinary uric acid (UA), creatinine, and albumin levels, were evaluated with an automatic biochemical analyzer (model 7600; Hitachi Ltd., Tokyo, Japan). Serum creatinine was used to evaluate eGFR, and urine microalbumin and creatinine were used to evaluate uACR.
Assessment of Renal Function
Renal function was assessed with eGFR and uACR. eGFR was calculated using the formula adapted from the Modification of Diet in Renal Disease equation on the basis of data from Chinese subjects with CKD.16 The specific formula is as follows: eGFR=175× serum creatinine−1.234 × age−0.179 (×0.79 for girls/women), where serum creatinine concentration is in milligrams per deciliter and age is in years.2 uACR was calculated as urine albumin in milligrams divided by the urine creatinine in millimole (milligrams per millimole). Presence of SRD was defined as eGFR between 30 and 60 ml/min per 1.73 m217 or elevated uACR of at least 2.5 mg/mmol in men and 3.5 mg/mmol in women as previously described.18
Definitions
Participants who reported continuous or cumulative smoking for 6 months or more during their lifetime were defined as cigarette smokers.13 Alcohol consumption was defined as subjects who claimed that they consumed alcohol (liquor, beer, or wine) every day and that consumption lasted for >6 months.19 The bust size is defined as the chest size of the annular double-nipple level.20 Hypertension was defined as an average measured SBP of at least 140 mm Hg and/or DBP at least 90 mm Hg or currently receiving antihypertension treatment. Diabetes was defined as GLU≥7.0 mmol/L or a physician diagnosis of diabetes that was self-reported by the individuals.21 Hyperlipidemia was defined as the occurrence of any one of the following four situations: hypertriglyceridemia (TG≥2.26 mmol/L), hypercholesterolemia (TC≥6.22 mmol/L), high levels of LDL-C (≥4.14 mmol/L), or low levels of HDL-C (<1.04 mmol/L).22
Statistical Analyses
During the 30-year follow-up period, SBP, DBP, and MAP trajectories were identified by using latent mixture modeling within the SAS Proc Traj.23,24 BP trajectories were modeled among 2430 subjects with three or more BP examinations, excluding those with only the initial four visits data (1987, 1989, 1992, and 1995) or only BP measurements for the last three visits (2005, 2013, and 2017). The “latent mixture modeling” estimated the model parameters through the maximum likelihood method and assigned each individual to a corresponding group with the greatest posteriori probability. We first constructed trajectory shape with a cubic specification and then, quadratic or linear if necessary.25 The best-fitting trajectory models were evaluated using the Bayesian information criterion, and the appropriate average posterior probability (>0.7) with a minimum sample size in each group accounted for >5.0%.
Continuous data were reported as means±SDs if normally distributed; otherwise, they were shown as medians (25th, 75th percentile ranges). Categorical data were presented as frequency and percentage. Differences between continuous variables were analyzed by t test for two-group comparisons and one-way ANOVA for three or more groups when the distribution and variance met the conditions; otherwise, Mann–Whitney U test and Kruskal–Wallis test were used. Categorical variables were analyzed by chi-squared tests. Correlation analysis was determined with the Pearson correlation coefficient or the Spearman correlation coefficient when appropriate.
Logistic regression analysis was used to determine the association between SRD in 2017 and different BP trajectory groups. Strengths of associations were determined by estimating the odds ratios (ORs) and their 95% confidence intervals (95% CIs). To detect changes in associations between outcome and main exposures, several models were fitted on unadjusted analysis as follows: model 1 = sex, race, BMI, heart rate (HR), and WHR in 2017; model 2 = model 1 + hypertension, diabetes, hyperlipidemia, smoking, and drinking in 2017; and model 3 = model 2 + GLU (millimoles per liter), serum UA (micromoles per liter), TC (millimoles per liter), TG (millimoles per liter), LDL-C (millimoles per liter), and HDL-C (millimoles per liter) in 2017. Furthermore, because of the fact that use of several medications may affect urinary protein and renal function, a sensitivity analysis was conducted by excluding individuals who had diabetes or received antihypertensives therapy (n=101).
All statistical tests were two sided, and statistical significance was considered at P<0.05. Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL).
Results
The Hanzhong Adolescent Hypertension Cohort enrolled 4623 schoolchildren in 1987. To fit BP trajectories, we included subjects with three or more BP measurements, except for those with data only derived from the initial four visits or the last three visits during the follow-up period. As a result, a total of 2430 individuals were included in the BP trajectory analysis. In this population, 1732 individuals with eGFR values and 1647 participants with uACR data available in 2017 were included in verifying the association between BP trajectories and eGFR, uACR, or SRD.
Characteristics of the Trajectory Groups
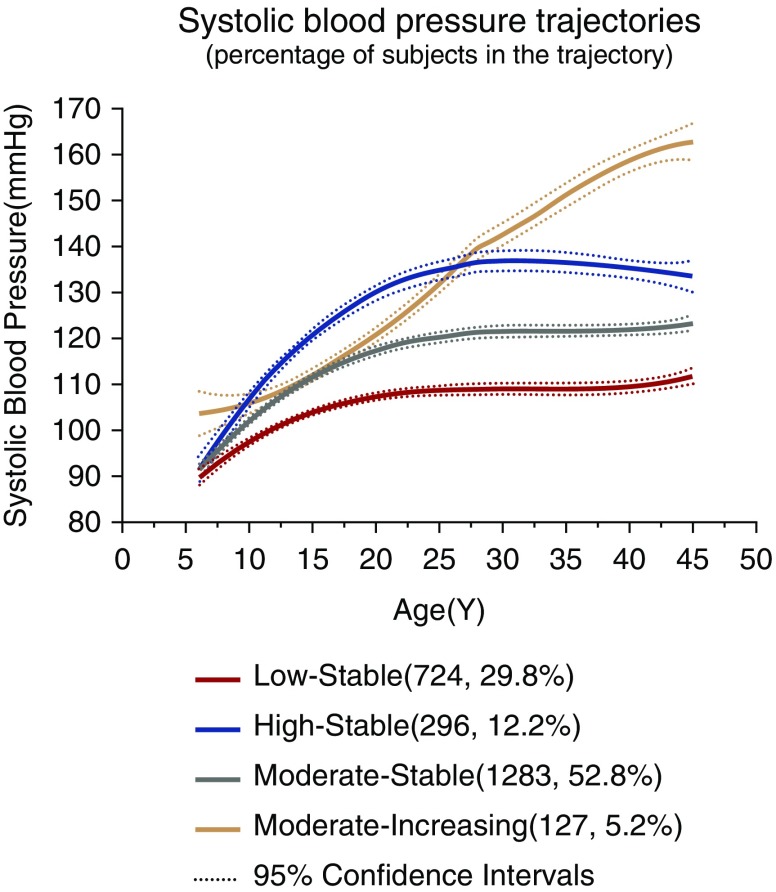
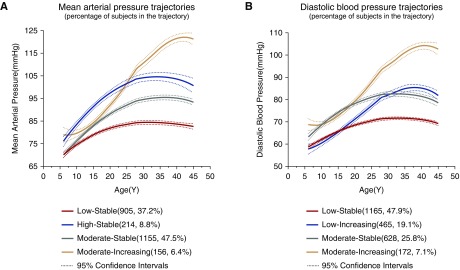
Four separate trajectories in SBP from childhood to middle age were identified (Figure 1). We refer to these trajectories as “low stable,” “moderate stable,” “high stable,” and “moderate increasing” groups. The low stable group (n=724; 29.8%) was characterized by maintaining relatively lower SBP levels. The moderate stable group (n=1283; 52.8%) was characterized by sharing moderate SBP levels. The high stable group (n=296; 12.2%) was characterized by enduring relatively higher SBP levels. The moderate increasing group (n=127; 5.2%) was characterized by experiencing a rapid increase in SBP, which started at moderate level and overtook the high stable group at about 30 years old (Figure 1, Table 1). Similar to SBP trajectories, four isolated trajectory groups were identified in MAP (Figure 2A). The numbers and proportions of participants were 905 (37.2%), 1155 (47.5%), 214 (8.8%), and 156 (6.4%) in “low stable,” “moderate stable,” “high stable,” and “moderate increasing” groups of MAP trajectories, respectively (Figure 2A, Supplemental Table 1).
Figure 1.
Trajectory modeling identified four distinct systolic BP trajectory groups from childhood to middle age in the Hanzhong Adolescent Hypertension Cohort.
Table 1.
Average systolic and diastolic BP (millimeters of Hg) by age periods in systolic BP trajectory groups
| Trajectory Groups | SBP, mm Hg | DBP, mm Hg | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Stable | Moderate Stable | High Stable | Moderate Increasing | Low Stable | Moderate Stable | High Stable | Moderate Increasing | |
| N (%) | 724 (29.8) | 1283 (52.8) | 296 (12.2) | 127 (5.2) | 724 (29.8) | 1283 (52.8) | 296 (12.2) | 127 (5.2) |
| 6–10, yr | 96.5±8.0 | 101.1±8.3 | 105.4±8.5 | 107.7±10.6 | 61.2±7.8 | 63.9±8.4 | 66.9±8.0 | 70.6±8.2 |
| 11–15, yr | 98.6±8.7 | 104.9±9.5 | 112.2±10.2 | 106.9±9.0 | 62.4±8.1 | 65.6±8.4 | 69.7±8.9 | 67.8±9.0 |
| 16–20, yr | 107.5±9.9 | 117.1±10.2 | 127.1±11.8 | 117.9±11.2 | 67.1±7.8 | 71.7±8.9 | 76.3±9.2 | 73.8±8.6 |
| 21–25, yr | 110.2±10.9 | 119.7±9.8 | 132.3±11.8 | 124.6±12.7 | 70.5±8.3 | 74.2±8.2 | 79.4±10.0 | 77.4±7.8 |
| 26–30, yr | 109.4±7.2 | 118.8±8.2 | 133.3±7.7 | 135.5±10.6 | 72.0±6.7 | 74.6±8.8 | 82.3±10.0 | 87.5±7.7 |
| 31–35, yr | 105.8±9.5 | 121.0±9.6 | 133.1±11.1 | 147.2±10.0 | 71.2±8.1 | 80.3±9.1 | 88.6±11.4 | 100.8±10.3 |
| 36–40, yr | 109.0±9.8 | 121.5±10.3 | 135.6±11.6 | 157.3±19.1 | 71.4±8.2 | 79.0±9.6 | 88.7±10.1 | 103.2±12.9 |
| 41–45, yr | 111.1±10.4 | 123.4±11.5 | 135.7±11.2 | 157.2±19.6 | 70.6±7.9 | 78.4±9.7 | 87.6±10.2 | 100.6±12.5 |
SBP, systolic BP; DBP, diastolic BP.
Figure 2.
Trajectory modeling identified four discrete (A) mean arterial pressure and (B) diastolic BP trajectory groups from childhood to middle age in the Hanzhong Adolescent Hypertension Cohort.
In addition, four discrete trajectory groups were identified in DBP (Figure 2B). The “low stable” group (1165; 47.9%) holds a low DBP level during the 30-year follow-up period. The “moderate stable” group (628; 25.8%) kept moderate DBP levels. The “low increasing” group (465; 19.1%) started at the lowest DBP levels and shared a rapid increase in DBP, which surpassed low stable and moderate stable groups subsequently. The “moderate increasing” group (172; 7.1%) originated in moderate DBP levels and experienced a rapid increase in DBP at about 20 years old (Supplemental Table 2).
The BP trajectory curve was not smooth, and it fluctuated over time. Generally, the SBP, MAP, and DBP trajectories showed larger BP increase during the children and adolescents period (6–19 years old) than after adulthood. Specially, the BP of the low increasing and moderate increasing groups increased with age, even after adulthood (Figures 1 and 2). Each individual was not always assigned to the same level group identified by SBP, DBP, or MAP trajectories. For example, individuals assigned to the low stable group in DBP or MAP trajectory had partial overlap with the low stable group in SBP trajectory; however, they were not identical, because individuals belonging to the low stable group in the SBP trajectory could also be assigned to low increasing (n=53) or moderate stable (n=34) groups in DBP trajectories (Supplemental Figures 1 and 2). Rarely, 14 subjects in the high stable group by SBP trajectories were assigned to the low stable group by DBP trajectories (Supplemental Figure 1).
Cardiovascular Risk Factors by BP Trajectory Groups
We analyzed partial anthropometry (age and bust in 1987, WHR in 2017, and HR and BMI in both 1987 and 2017) and biochemical indicator tests in 2017 in each group. The median age of the 2430 analyzed subjects was 12 (9, 14) years old at baseline. Among them, 1378 participants (56.7%) were boys, and 1052 (43.3%) were girls. Differences in the proportion of boys, highest level of education, smoking, drinking, and anthropometric measurements (including age, bust at baseline, WHR and HR in 2017, and BMI in both 1987 and 2017) were statistically significant (P<0.05) (Table 2). Biochemical parameters (GLU, TC, TG, LDL-C, HDL-C, serum creatinine, serum UA, urine UA, and urine albumin) tested in 2017 were also significantly different (P<0.05) (Table 2) among the four SBP trajectory groups. In addition, the incidence of hypertension and hyperlipidemia in 2017 increased with the trajectories of SBP (P<0.05) (Table 2).
Table 2.
Demographic characteristics and cardiovascular risk factors by the systolic BP trajectory group
| Parameter | Total, N | Low Stable | Moderate Stable | High Stable | Moderate Increasing | P Value |
|---|---|---|---|---|---|---|
| Boys (%) | 1378 | 283 (39.09) | 785 (61.18) | 227 (76.69) | 83 (65.35) | <0.001 |
| Age in 1987, yr | 2430 | 12 (9, 14) | 12 (9, 14) | 12 (10, 14) | 12 (10, 14) | 0.03 |
| Bust in 1987, cm | 2421 | 60.0 (55.5, 66.0) | 61.0 (57.0, 67.0) | 63.0 (59.0, 70.0) | 64.0 (60.0, 70.0) | <0.001 |
| HR in 1987, times per min | 2425 | 78 (72, 84) | 78 (72, 84) | 80 (72, 84) | 78 (72, 84) | 0.09 |
| HR in 2017, times per min | 1907 | 73 (66, 80) | 73 (67, 79) | 75 (67, 82) | 77 (68, 85) | <0.01 |
| BMI in 1987, kg/m2 | 2419 | 15.6 (14.4, 17.2) | 15.9 (14.7, 17.4) | 16.1 (15.0, 17.8) | 16.3 (15.1, 18.1) | <0.001 |
| BMI in 2017, kg/m2 | 1904 | 22.9 (21.1, 24.6) | 23.8 (22.0, 26.0) | 25.1 (23.2, 26.9) | 26.6 (23.9, 29.2) | <0.001 |
| WHR in 2017, m | 1901 | |||||
| Men | 1097 | 0.925±0.057 | 0.940±0.063 | 0.958±0.053 | 0.987±0.061 | <0.001 |
| Women | 804 | 0.879±0.060 | 0.886±0.066 | 0.889±0.051 | 0.924±0.062 | 0.003 |
| Occupation (%) | 2170 | 0.20 | ||||
| Farmer | 871 | 256 (39.81) | 457 (39.91) | 109 (41.60) | 49 (40.83) | |
| Worker | 424 | 105 (16.33) | 227 (19.83) | 61 (23.28) | 31 (25.83) | |
| Businessman | 173 | 56 (8.71) | 91 (7.95) | 19 (7.25) | 7 (5.83) | |
| Governor | 71 | 19 (2.95) | 37 (3.23) | 10 (3.82) | 5 (4.17) | |
| Other | 631 | 207 (32.19) | 333 (29.08) | 63 (24.05) | 28 (23.33) | |
| Education (%) | 2206 | 0.01 | ||||
| Primary school or less | 179 | 51 (7.74) | 96 (8.25) | 27 (10.07) | 5 (4.31) | |
| Middle school | 1328 | 375 (56.90) | 719 (61.82) | 150 (55.97) | 84 (72.41) | |
| High school | 504 | 159 (24.13) | 251 (21.58) | 71 (26.49) | 23 (19.83) | |
| College or more | 195 | 74 (11.23) | 97 (8.34) | 20 (7.46) | 4 (3.45) | |
| Marital status (%) | 2255 | 0.20 | ||||
| Married | 2162 | 648 (96.57) | 1131 (95.12) | 267 (97.45) | 116 (95.87) | |
| Divorced | 46 | 15 (2.24) | 26 (2.19) | 4 (1.46) | 1 (0.83) | |
| Unmarried or other | 47 | 8 (1.19) | 32 (2.69) | 3 (1.09) | 4 (3.31) | |
| Smoking (%) | 1908 | 185 (32.23) | 470 (47.33) | 148 (60.66) | 57 (58.16) | <0.001 |
| Drinking (%) | 1908 | 118 (20.59) | 326 (32.83) | 97 (39.75) | 34 (34.69) | <0.001 |
| Hypertension (%) | 1908 | 9 (1.57) | 63 (6.34) | 78 (31.97) | 64 (65.31) | <0.001 |
| Diabetes (%) | 1908 | 9 (1.57) | 34 (3.42) | 4 (1.64) | 7 (7.22) | <0.01 |
| Hyperlipidemia (%) | 1908 | 26 (4.54) | 95 (9.57) | 29 (11.89) | 19 (19.39) | <0.001 |
| GLU, mmol/L | 1730 | 4.52 (4.25, 4.80) | 4.55 (4.27, 4.93) | 4.69 (4.32, 5.02) | 4.74 (4.39, 5.19) | <0.001 |
| TC, mmol/L | 1732 | 4.43 (3.98, 4.90) | 4.49 (4.04, 5.01) | 4.69 (4.14, 5.10) | 4.52 (4.08, 5.17) | 0.02 |
| Triglycerides, mmol/L | 1732 | 1.22 (0.89, 1.77) | 1.35 (0.97, 1.94) | 1.50 (1.01, 2.04) | 1.69 (1.17, 2.52) | <0.001 |
| LDL-C, mmol/L | 1732 | 2.44 (2.07, 2.79) | 2.51 (2.15, 2.91) | 2.60 (2.18, 3.05) | 2.55 (2.18, 3.00) | <0.001 |
| HDL-C, mmol/L | 1732 | 1.19 (1.03, 1.38) | 1.14 (0.97, 1.32) | 1.11 (0.98, 1.27) | 1.04 (0.90, 1.19) | <0.001 |
| Serum uric acid, μmol/L | 1732 | 254.6 (211.5, 312.5) | 283.1 (229.6, 334.4) | 310.7 (263.6, 358.2) | 329.6 (262.4, 379.8) | <0.001 |
| Urine uric acid, μmol/L | 1671 | 1251 (876, 1855) | 1315 (959, 2083) | 1403 (991, 2243) | 1522 (948, 2206) | <0.01 |
| Serum creatinine, μmol/L | 1669 | 71.60 (63.60, 83.08) | 76.70 (68.00, 86.80) | 78.85 (71.53, 89.73) | 77.45 (68.70, 88.10) | <0.001 |
| Urine albumin, mg/L | 1671 | 6.20 (3.20, 11.20) | 7.70 (4.20, 13.73) | 10.35 (5.40, 21.58) | 14.40 (7.00, 45.50) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 1732 | 98.47 (88.39, 112.26) | 97.09 (87.80, 110.33) | 96.21 (85.19, 109.20) | 95.35 (85.62, 111.56) | 0.34 |
| uACR, mg/mmol | 1669 | 0.878 (0.593, 1.439) | 0.932 (0.619, 1.631) | 1.182 (0.738, 2.367) | 2.574 (1.020, 6.392) | <0.001 |
Continuous variables were shown as mean±SD if normally distributed or median (quartile 1, quartile 3) if non-normally distributed. Categorical variables were expressed as numbers and percentages of subjects. Statistical ANOVA was performed by one-way ANOVA when normally distributed; otherwise, the Kruskal–Wallis test was used. Differences between groups of categorical variables were compared with chi-squared tests. HR, heart rate; BMI, body mass index; WHR, waist-to-hip ratio; GLU, fasting plasma blood glucose; TC, total cholesterol; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; uACR, urinary albumin-to-creatinine ratio.
Individuals in the low stable group were more likely to be girls; have lower BMI, WHR, GLU, TC, TG, LDL-C, serum UA, and serum creatinine; and have higher HDL-C than those in other groups. Boys with high BMI or WHR were more likely to suffer from high SBP throughout or rapid increase in SBP during the follow-up. In addition, high-risk SBP trajectory groups were associated with high rates of smoking and drinking (Table 2). Findings were similar for the isolated DBP (Supplemental Table 3) and MAP trajectories (Supplemental Table 4). Interestingly, values of BMI and WHR in 2017, the percentage of hypertension, GLU, TC, serum UA, and urine albumin increased with the trajectories of SBP, DBP, and MAP, whereas eGFR values decreased (Supplemental Tables 3 and 4, Table 2). However, no significant differences existed in the percentages of employment type and marital status among SBP, DBP, and MAP trajectories (Supplemental Tables 3 and 4, Table 2).
We further classified BMI, SBP, and DBP according to age periods and analyzed the relationship between BMI and BP. BMI had group differences in prepuberty, puberty, and middle-aged adulthood by SBP trajectories (Supplemental Table 5) (P<0.05). SBP and DBP showed significant group differences from prepuberty to middle-aged adulthood (Supplemental Table 5) (P<0.05). Results were similar in DBP and MAP trajectories (Supplemental Tables 6 and 7). Additional analyses showed that the SBP was positively correlated with BMI in prepuberty (r=0.239; P<0.01), puberty (r=0.350; P<0.01), young adulthood (r=0.226; P<0.01) and middle-aged adulthood (r=0.336; P<0.01). In addition, DBP and MAP were also significantly positively correlated with BMI (Supplemental Table 8).
Association between BP Trajectories and eGFR or uACR
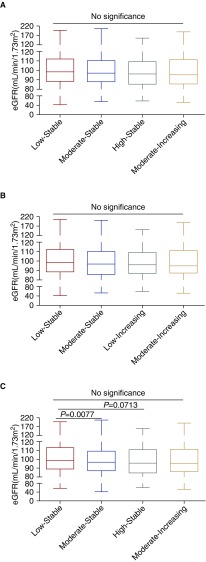
As shown in Supplemental Table 9, eGFR≥90 ml/min per 1.73 m2 accounts for the majority of the proportions (>60%) in each SBP trajectory followed by 60≤eGFR<90 ml/min per 1.73 m2 (>25%). Because subjects included in this study were relatively young (the oldest subjects were 45 years old in 2017), individuals with eGFR<60 ml/min per 1.73 m2 were limited. The median of eGFR was higher in the low stable group in comparison with other trajectories of SBP, DBP, and MAP (Supplemental Tables 3 and 4, Table 2).We used eGFR values as continuous variables to analyze the association between discrete BP trajectories and eGFR level. Compared with the low stable group, eGFR values were significantly lower in the moderate stable group of MAP trajectories (P<0.01) (Figure 3). However, there were no differences between eGFR and other SBP, DBP, or MAP trajectories (Figure 3).
Figure 3.
No differences in eGFR among groups in (A) systolic BP, (B) diastolic BP, and (C) mean arterial pressure trajectories except for the moderate stable group of MAP trajectories in comparison with the low stable group (C).
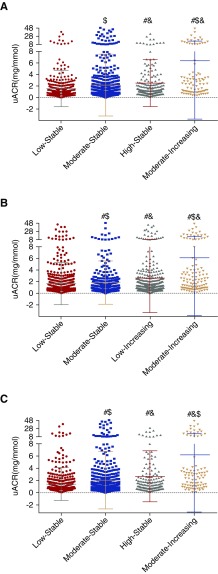
Then, we analyzed the relationship between uACR levels and isolated BP trajectories. Interestingly, the uACR levels were significantly higher in high stable (1.182 [0.738, 2.367] mg/mmol) and moderate increasing (2.574 [1.020, 6.392] mg/mmol) groups compared with the low stable group (0.878 [0.593, 1.439] mg/mmol) in SBP trajectories (P<0.05) (Figure 4A). In the DBP trajectory groups, the uACR levels were significantly higher in moderate stable (0.990 [0.621, 1.653] mg/mmol), low increasing (1.134 [0.696, 2.134] mg/mmol), and moderate increasing (2.233 [0.930, 4.802] mg/mmol) groups in comparison with the low stable group (0.872 [0.583, 1.463] mg/mmol; P<0.05) (Figure 4B). In the MAP trajectories, compared with the low stable group (0.868 [0.583, 1.398] mg/mmol), the uACR levels were significantly higher in moderate stable (0.990 [0.635, 1.670] mg/mmol), high stable (1.195 [0.716, 2.572] mg/mmol), and moderate increasing (2.323 [0.988, 4.197] mg/mmol) groups (P<0.05) (Figure 4C). Moreover, the uACR levels between moderate increasing, high stable (low increasing), and moderate stable groups in SBP, MAP, and DBP trajectories were also significantly different, respectively (P<0.05) (Figure 4).
Figure 4.
The urinary albumin-to-creatinine ratio (uACR) values shows significant differences among groups in (A) systolic BP, (B) diastolic BP, and (C) mean arterial pressure trajectories. #P<0.05 compared with the low stable group; &P<0.05 compared with the moderate stable group; $P<0.05 compared with the high stable group.
Risk of Subclinical Renal Damage for the Discrete Trajectories
We evaluated renal function by eGFR and uACR jointly. Among the 2430 subjects who were included to fit the BP trajectories, 1732 individuals had eGFR values and 1647 had uACR data in 2017. As a result, a total of 1738 participates obtained either eGFR or uACR data. Among them, 228 individuals developed SRD by the most recent follow-up in April 2017 (Supplemental Table 10). The prevalence of SRD varied from 7.85% in the low stable group up to 43.62% in the moderate increasing group in the SBP trajectories (Table 3). To examine the association between BP trajectory groups and SRD, the groups were included as independent variables in a logistic regression model estimating the predictors of the presence of SRD in the most recent visit, and the low stable group was considered as the control group. Compared with the low stable group, other trajectory groups had increasingly greater odds of existing SRD in 2017. In unadjusted analyses, ORs (95% CIs) were 1.45 (95% CI, 0.99 to 2.12) for moderate stable, 3.25 (95% CI, 2.07 to 5.10) for high stable, and 9.08 (95% CI, 5.42 to 15.20) for moderate increasing in comparison with low stable. Adjustment for subjects’ demographic characteristics (sex and race) and anthropometric parameters in 2017 (BMI, HR, and WHR) significantly attenuated the ORs (Table 3, model 1). Additional adjustment for cardiovascular risk factors in 2017 (hypertension, diabetes, hyperlipidemia, smoking, and drinking) led to slightly attenuated ORs for the high-risk groups (Table 3, model 2). In the final model, additional adjustment was performed with individuals’ biochemical indicators in 2017 (GLU [millimoles per liter], serum UA [micromoles per liter], TC [millimoles per liter], TG [millimoles per liter], LDL-C [millimoles per liter], and HDL-C [millimoles per liter]) (Table 4, model 3), resulting in additional attenuations of the ORs for the high-risk groups.
Table 3.
Adjusted odds ratios and 95% confidence intervals of the association of BP trajectory groups with subclinical kidney damage
| Trajectory Groups | No. of Subjects with SRD in 2017 (%) | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|
| SBP trajectory group | |||||
| Low stable | 42 (7.85) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 98 (10.99) | 1.45 (0.99 to 2.12) | 1.19 (0.79 to 1.78) | 1.16 (0.77 to 1.74) | 1.10 (0.73 to 1.67) |
| High stable | 47 (21.66) | 3.25 (2.07 to 5.10) | 2.12 (1.29 to 3.49) | 1.76 (1.03 to 2.99) | 1.63 (0.95 to 2.81) |
| Moderate increasing | 41 (43.62) | 9.08 (5.42 to 15.20) | 5.03 (2.86 to 8.87) | 3.21 (1.70 to 6.08) | 3.04 (1.58 to 5.85) |
| DBP trajectory group | |||||
| Low stable | 67 (7.94) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 52 (11.71) | 1.54 (1.05 to 2.25) | 1.30 (0.86 to 1.96) | 1.23 (0.81 to 1.87) | 1.16 (0.76 to 1.78) |
| High stable | 58 (17.63) | 2.48 (1.70 to 3.62) | 1.82 (1.19 to 2.78) | 1.74 (1.13 to 2.68) | 1.63 (1.04 to 2.53) |
| Moderate increasing | 51 (42.15) | 8.45 (5.45 to 13.10) | 5.27 (3.22 to 8.63) | 3.43 (1.93 to 6.12) | 3.38 (1.87 to 6.12) |
| MAP trajectory group | |||||
| Low stable | 47 (6.93) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 98 (12.41) | 1.90 (1.32 to 2.74) | 1.47 (0.98 to 2.19) | 1.44 (0.96 to 2.16) | 1.33 (0.88 to 2.01) |
| High stable | 37 (23.42) | 4.11 (2.56 to 6.59) | 2.73 (1.62 to 4.60) | 2.37 (1.36 to 4.11) | 2.29 (1.30 to 4.03) |
| Moderate increasing | 46 (41.07) | 9.36 (5.80 to 15.11) | 5.42 (3.17 to 9.28) | 3.67 (1.96 to 6.87) | 3.37 (1.76 to 6.43) |
Model 1 = sex, race, body mass index, heart rate, and waist-to-hip ratio in 2017. Model 2 = model 1 + hypertension, diabetes, hyperlipidemia, smoking, and drinking in 2017. Model 3 = model 2 + fasting blood glucose (millimoles per liter), serum uric acid (micromoles per liter), total cholesterol (millimoles per liter), triglycerides (millimoles per liter), LDL (millimoles per liter), and HDL (millimoles per liter) in 2017. SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure.
Table 4.
Adjusted odds ratios and 95% confidence intervals of the association of BP trajectory groups with subclinical kidney damage (sensitivity analysis)
| Trajectory Groups | No. of Subjects with SRD in 2017 (%) | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|
| SBP trajectory group | |||||
| Low stable | 47 (8.23) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 89 (10.48) | 1.31 (0.90 to 1.89) | 1.12 (0.75 to 1.66) | 1.09 (0.73 to 1.63) | 1.06 (0.70 to 1.58) |
| High stable | 28 (19.44) | 2.69 (1.62 to 4.48) | 1.81 (1.03 to 3.18) | 1.48 (0.82 to 2.68) | 1.51 (0.83 to 2.77) |
| Moderate increasing | 34 (41.98) | 8.07 (4.73 to 13.74) | 4.93 (2.73 to 8.91) | 3.44 (1.80 to 6.55) | 3.24 (1.67 to 6.28) |
| DBP trajectory group | |||||
| Low stable | 60 (7.58) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 47 (10.54) | 1.44 (0.96 to 2.15) | 1.20 (0.78 to 1.84) | 1.17 (0.76 to 1.80) | 1.12 (0.73 to 1.74) |
| High stable | 53 (17.15) | 2.53 (1.70 to 3.75) | 1.88 (1.21 to 2.94) | 1.80 (1.14 to 2.82) | 1.74 (1.09 to 2.76) |
| Moderate increasing | 38 (38.78) | 7.73 (4.76 to 12.54) | 5.11 (2.97 to 8.79) | 3.59 (1.94 to 6.64) | 3.63 (1.94 to 6.78) |
| MAP trajectory group | |||||
| Low stable | 43 (6.60) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate stable | 91 (11.86) | 1.91 (1.31 to 2.78) | 1.54 (1.02 to 2.32) | 1.51 (0.99 to 2.28) | 1.43 (0.94 to 2.18) |
| High stable | 24 (18.32) | 3.18 (1.85 to 5.45) | 2.30 (1.28 to 4.12) | 2.07 (1.13 to 3.79) | 2.05 (1.11 to 3.80) |
| Moderate increasing | 40 (42.11) | 10.30 (6.18 to 17.18) | 6.15 (3.46 to 10.95) | 4.48 (2.33 to 8.62) | 4.28 (2.19 to 8.37) |
Model 1 = sex, race, body mass index, heart rate, and waist-to-hip ratio in 2017. Model 2 = Model 1 + hypertension, diabetes, hyperlipidemia, smoking, and drinking in 2017. Model 3 = model 2 + fasting blood glucose (millimoles per liter), serum uric acid (micromoles per liter), total cholesterol (millimoles per liter), triglycerides (millimoles per liter), LDL (millimoles per liter), and HDL (millimoles per liter) in 2017. SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure.
Similarly, relative risk was evaluated in discrete DBP and MAP trajectories. ORs (95% CIs) were 1.54 (95% CI, 1.05 to 2.25) for moderate stable, 2.48 (95% CI, 1.70 to 3.62) for low increasing, and 8.45 (95% CI, 5.45 to 13.10) for moderate increasing in the DBP trajectories and 1.90 (95% CI, 1.32 to 2.74) for moderate stable, 4.11 (95% CI, 2.56 to 6.59) for high stable, and 9.36 (95% CI, 5.80 to 15.11) for moderate increasing in the MAP trajectories. The relative risk still existed, although ORs were attenuated after adjusting for confounding factors, except for the moderate stable group in both DBP and MAP trajectories (Table 3).
Sensitivity Analyses
We performed additional analyses after excluding individuals who had diabetes or received antihypertensive drugs (n=101) to eliminate the effect of these factors on the results. Trajectories in BP were identified among the rest of the 2329 participants. There was no significant change found in the characteristics of BP trajectories (Supplemental Figure 3). Among the 2329 subjects, there were 1639 eGFR values and 1560 uACR data available in 2017, leading to a total of 1645 individuals who had either a calculated eGFR or an uACR value. We analyzed the risk of SRD for each trajectory, and the results remained the same (Table 4). Taken together, the results of our study indicated that the trajectories of BP during early life were important for the development of CKD in later life.
Discussion
Four trajectory groups in SBP from childhood to middle age were identified. We illustrated that the SBP trajectories were significantly associated with uACR levels and the incidence of SRD in middle age (that is, the higher the level of SBP in early life course, the higher uACR and risk of SRD in middle age). In addition, rapid increase in SBP throughout the follow-up period predicted the highest uACR and risk of SRD in middle age. The analyzed results were similar for MAP trajectories. In DBP trajectory groups, subjects with DBP starting at either low or moderate levels with rapid increase during the follow-up period had higher uACR and risk of SRD in middle age.
The risk of renal damage is reported to increase with the increase in BP.26–28 A meta-analysis among 4.2 million adults revealed that elevated SBP is closely related to high risk of peripheral arterial disease, which in turn, contributes to a nearly 30% increased risk of CKD.12 A randomized, multicenter study determined that SBP is an independent predictor of renal function insufficiency among older subjects with isolated systolic hypertension.29 These studies focusing on the relationship between BP and renal function status were mainly on the basis of BP data from single measurements, multiple measurements in a short time, or 24-hour ambulatory BP measurements. However, BP values were easily influenced by mental status, physical activity, and other factors, which may not adequately reflect individuals’ accurate BP level.
Group-based trajectory modeling takes into account variations in time to distinguish changes in BP over time and heterogeneity within multiple BP measurements. It assigns individuals sharing similar BP development trends to the same subgroup on the basis of multiple measurement data over a long time period. It provides an effective approach to reflect the life course association of certain predictors with its potential effect on target organ. Available evidence showed that continuous and high BP trajectories for years are closely correlated with subclinical atherosclerosis,30 intima media thickness, and left ventricular mass index.31
Studies indicated that high BP in later life originates in childhood,9 and severe BP abnormality in childhood is responsible for increased risk of target organ damage secondary to hypertension.32 BP trajectories are identifiable in childhood and associated with adverse childhood experiences11 and other modifiable risk factors over time.33 Similarly, our study identified individuals’ BP (SBP, DBP, and MAP) trajectories throughout childhood, puberty, early adulthood, and middle-aged adulthood.
Interestingly, several studies have reported that group-based trajectory may predict individuals’ renal function status. Data from a large population-based health examination have indicated that continuous high SBP was related to kidney damage in nonhypertensive subjects.34 Unfortunately, the study did not fit DBP or MAP trajectories. In our study, we found that continuous high SBP trajectory (high stable group) was closely associated with increased uACR levels and SRD, so did MAP trajectories. Furthermore, subjects with rapid increase in BP were assigned to moderate increasing or low increasing groups, which had stronger predictive ability for SRD in middle age. Evidence from the Mater-University of Queensland Study of Pregnancy (MUSP) birth cohort showed that continuous high and middle pulse pressures rather than MAP trajectories in early life can predict the risk of lower eGFR by 30 years of age.35 However, our study found that the predictive ability of MAP trajectories was similar to that of SBP trajectories. The difference may be caused by various characteristics of the analyzed population, because the population of the MUSP birth cohort study was relatively young (average age was 30 years old at the last follow-up) with a lower incidence of CKD, which was defined by eGFR using serum creatinine values alone. In our study, subjects were followed up for 30 years, and the renal function was assessed by eGFR and uACR jointly. In addition, we reported that uACR was higher in the moderate stable, high stable, and moderate increasing groups compared with the low stable group, and MAP trajectories were a strong predictor for the risk of SRD in middle age.
In addition to the effect of hypertension on SRD, notable differences in sex were observed in the different BP trajectory groups. Women were more likely to be included in the low stable group, whereas men were more likely to be included in the high stable (low increasing) and moderate increasing groups. This result is consistent with previous reports.9,35 Cigarette smoking and alcohol consumption are not only risk factors for hypertension but also, independently associated with CKD.36,37 In our study, the high stable (low increasing) and moderate increasing groups were associated with higher rates of smoking and drinking in SBP and DBP trajectories. Furthermore, higher-level trajectory groups had markedly abnormal metabolic risk factors, because the moderate stable, high stable (low increasing), and moderate increasing groups were related to higher incidence of diabetes; hyperlipidemia; higher BMI, GLU, TC, TG, and LDL-C; and lower HDL-C. BMI in early life played an important role in the development of hypertension38 and CKD.39 Locatelli et al.40 reported that patients with metabolic disorder were at higher risk for microalbuminuria or CKD, and the number of metabolic disorders determined the level of risk. Another study showed that GLU levels of 110 mg/dl, reduced HDL-C, and high TG levels were independently associated with the greatest risk for microalbuminuria and low eGFR.41 Although mechanisms of the inter-relationship between metabolic disorders and renal damage were not fully elucidated, several pathophysiologic factors were involved, including oxidative stress, inflammation, and adipocytokines.42 Serum UA proved to be a strong predictor for increased urine albumin excretion and the development of CKD43: the concentration of serum UA increased with the increase of BP trajectories in our study.
Although our study used a large population, had high response rate, was prospective in nature, and used a community-based cohort followed for 30 years, it had several limitations, which should be acknowledged. The participants were recruited from multiple rural areas in northern China, and most of them were Han nationality (98.2%). Therefore, our findings should be verified in other cohorts to determine generalizability to other ethnicities and populations with different backgrounds. In addition, not all subjects had BP data available during visits, especially follow-up in 2005; we only randomly selected about 500 individuals to visit, resulting in a large number of individuals’ data being unavailable between 1995 and 2013. However, the analyzed population had three or more BP measurements, including one or more measurements from visits in 1987, 1989, 1992, and 1995 and at least one measurement in 2005, 2013, and 2017. Indeed, the majority of the 2430 subjects (n=2054; 84.53%) had five or more BP measurements. Generally, variability in BP was more significant before than after adulthood; thus, we visited the population more frequently from 1987 to 1995 (average age was 11.6–19.6 years old), resulting in four visits within 8 years. We visited the population three times in their adulthood (2005, 2013, and 2017). These measures ensured the accuracy of the trajectory construction and preciseness of the conclusion. Finally, participants with mean age of 42 years old in the last follow-up experienced a relatively low prevalence of SRD; in particular, the number of individuals who met the eGFR range was rather small. To our knowledge, this is the first study that has taken into account the variability of SBP, DBP, and MAP in early life course and revealed the association between BP variation trends and SRD among healthy young adults in a community-based cohort. The prospective design of our research provided us an opportunity to achieve further follow-up in determining the future risk of clinical CKD development.
BP trajectories throughout early life are independently associated with uACR level and SRD risk. Higher BP trajectories were correlated with higher level of uACR and risk of SRD in middle age, indicating that identifying long-term trajectories of BP from early age may assist in predicting individuals’ renal function status in later life.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University of China grant XJTU1AF-CRF-2015-006; National Natural Science Foundation of China grants 81370357, 81570381 (to J.M.), 81700368 (to C.C.), and 81600327 (to Y.W.); Major Chronic Non-Communicable Disease Prevention and Control Research Key Project of the Ministry of Science and Technology of the People’s Republic of China grant 2017YFC1307604; and Key Research Project of Shaanxi Province grant 2017ZDXM-SF-107.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018030263/-/DCSupplemental.
Supplemental Material
Supplemental Table 1. Average systolic and diastolic BP (millimeters of Hg) by age periods in MAP trajectory groups.
Supplemental Table 2. Average systolic and diastolic BP (millimeters of Hg) by age periods in DBP trajectory groups.
Supplemental Table 3. Demographic characteristics and cardiovascular risk factors by DBP trajectory group.
Supplemental Table 4. Demographic characteristics and cardiovascular risk factors by MAP trajectory group.
Supplemental Table 5. Characteristics in SBP, DBP, and BMI by SBP trajectories.
Supplemental Table 6. Characteristics in SBP, DBP, and BMI by DBP trajectories.
Supplemental Table 7. Characteristics in SBP, DBP, and BMI by MAP trajectories.
Supplemental Table 8. Pearson correlation coefficients between BMI and BP.
Supplemental Table 9. Comparison of eGFR (millimeters per minute per 1.73 m2) in the different groups.
Supplemental Table 10. Number and percentage of subjects with subclinical renal damage in BP trajectory groups.
Supplemental Figure 1. Percentage of SBP trajectories by DBP and MAP trajectory groups.
Supplemental Figure 2. (A) The percentage of DBP trajectories by SBP and MAP trajectory groups and (B) the percentage of MAP trajectories by SBP and DBP trajectory groups.
Supplemental Figure 3. Trajectories in (A) SBP, (B) DBP, and (C) MAP in the Hanzhong Adolescent Hypertension Cohort (sensitivity analysis).
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al.: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al.: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al.: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al.: ESC Scientific Document Group : 2016 European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J 37: 2315–2381, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al.: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al.: Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 387: 435–443, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al.: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Čabarkapa V, Ilinčić B, Đerić M, Radosavkić I, Šipovac M, Sudji J, et al.: Screening for chronic kidney disease in adult males in Vojvodina: A cross-sectional study. J Med Biochem 36: 153–162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, et al.: Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: The Bogalusa Heart Study. Hypertension 70: 66–74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oikonen M, Nuotio J, Magnussen CG, Viikari JS, Taittonen L, Laitinen T, et al.: Repeated blood pressure measurements in childhood in prediction of hypertension in adulthood. Hypertension 67: 41–47, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, et al.: Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: The Georgia stress and Heart study. Circulation 131: 1674–1681, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, et al.: Usual blood pressure, peripheral arterial disease, and vascular risk: Cohort study of 4.2 million adults. BMJ 351: h4865, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C, Dai Y, Mu J, Yang R, Wang M, Yang J, et al.: Associations of risk factors in childhood with arterial stiffness 26 years later: The Hanzhong adolescent hypertension cohort. J Hypertens 35[Suppl 1]: S10–S15, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Portegies ML, Mirza SS, Verlinden VJ, Hofman A, Koudstaal PJ, Swanson SA, et al.: Mid- to late-life trajectories of blood pressure and the risk of stroke: The Rotterdam Study. Hypertension 67: 1126–1132, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Kagura J, Adair LS, Munthali RJ, Pettifor JM, Norris SA: Association between early life growth and blood pressure trajectories in black South African children. Hypertension 68: 1123–1131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al.: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mulè G, Calcaterra I, Costanzo M, Geraci G, Guarino L, Foraci AC, et al.: Relationship between short-term blood pressure variability and subclinical renal damage in essential hypertensive patients. J Clin Hypertens (Greenwich) 17: 473–480, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leoncini G, Viazzi F, Conti N, Baratto E, Tomolillo C, Bezante GP, et al.: Renal and cardiac abnormalities in primary hypertension. J Hypertens 27(5): 1064–1073, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Li XX, Zhao Y, Huang LX, Xu HX, Liu XY, Yang JJ, et al.: Effects of smoking and alcohol consumption on lipid profile in male adults in northwest rural China. Public Health 157: 7–13, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Prantl L, Gründl M: Males prefer a larger bust size in women than females themselves: An experimental study on female bodily attractiveness with varying weight, bust size, waist width, hip width, and leg length independently. Aesthetic Plast Surg 35: 693–702, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Selvin E, Parrinello CM, Sacks DB, Coresh J: Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 160: 517–525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan J, Ren Z, Li W, Wei Z, Rao H, Ren H, et al.: Prevalence of hyperlipidemia in Shanxi Province, China and application of Bayesian networks to analyse its related factors. Sci Rep 8: 3750, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones BL, Nagin DS, Roeder K: A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 29: 374–393, 2001 [Google Scholar]
- 24.Nagin DS, Odgers CL: Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6: 109–138, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B: Latent class growth modelling: A tutorial. Tutor Quant Methods Psychol 5: 11–24, 2009 [Google Scholar]
- 26.Konno S, Hozawa A, Miura Y, Ito S, Munakata M: High-normal diastolic blood pressure is a risk for development of microalbuminuria in the general population: The Watari study. J Hypertens 31: 798–804, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, et al.: Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57: 846–851, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Wang JG, et al.: Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension 63: 790–796, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J: Blood pressure and decline in kidney function: Findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol 13: 2776–2782, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, et al.: Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 311: 490–497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S: Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: Results from the 23-year Longitudinal Georgia Stress and Heart Study. Hypertension 69: 435–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrindle BW: Assessment and management of hypertension in children and adolescents. Nat Rev Cardiol 7: 155–163, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, et al.: Childhood to early-midlife systolic blood pressure trajectories: Early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 66: 1108–1115, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang ZJ, Jia D, Tian J, Liu J, Li LJ, Huang YL, et al.: Correlation between the trajectory of systolic blood pressure and new renal damage in a nonhypertensive population. Blood Press Monit 22: 282–289, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Das SK, McIntyre HD, Mamun AA: An early life course association of pulse pressure with adulthood estimated glomerular filtration rate: Evidence from a large community-based birth cohort study. J Hypertens 35: 392–400, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Formanek P, Salisbury-Afshar E, Afshar M: Helping patients with ESRD and earlier stages of CKD to quit smoking. Am J Kidney Dis 72: 255–266, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosola C, Sabatino A, di Bari I, Fiaccadori E, Gesualdo L: Nutrients, nutraceuticals, and xenobiotics affecting renal health. Nutrients 10: E808, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, Zhang H, Li Y, Sun D, Li S, Fernandez C, et al.: Temporal relationship between childhood body mass index and insulin and its impact on adult hypertension: The Bogalusa Heart Study. Hypertension 68: 818–823, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverwood RJ, Pierce M, Thomas C, Hardy R, Ferro C, Sattar N, et al.: National Survey of Health and Development Scientific and Data Collection Teams : Association between younger age when first overweight and increased risk for CKD. J Am Soc Nephrol 24: 813–821, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locatelli F, Pozzoni P, Del Vecchio L: Renal manifestations in the metabolic syndrome. J Am Soc Nephrol 17[Suppl 2]: S81–S85, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al.: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Nashar K, Egan BM: Relationship between chronic kidney disease and metabolic syndrome: Current perspectives. Diabetes Metab Syndr Obes 7: 421–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharaf El Din UAA, Salem MM, Abdulazim DO: Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res 8: 537–548, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.