Abstract
Deconstructive functionalizations involving scission of carbon-carbon double bonds are well established. In contrast, unstrained C(sp3)–C(sp3) bond cleavage and functionalization have less precedent. Here we report the use of deconstructive fluorination to access mono- and difluorinated amine derivatives by C(sp3)–C(sp3) bond cleavage in saturated nitrogen heterocycles such as piperidines and pyrrolidines. Silver-mediated ring-opening fluorination using Selectfluor highlights a strategy for cyclic amine functionalization and late-stage skeletal diversification, establishing cyclic amines as synthons for amino alkyl radicals and providing synthetic routes to valuable building blocks.
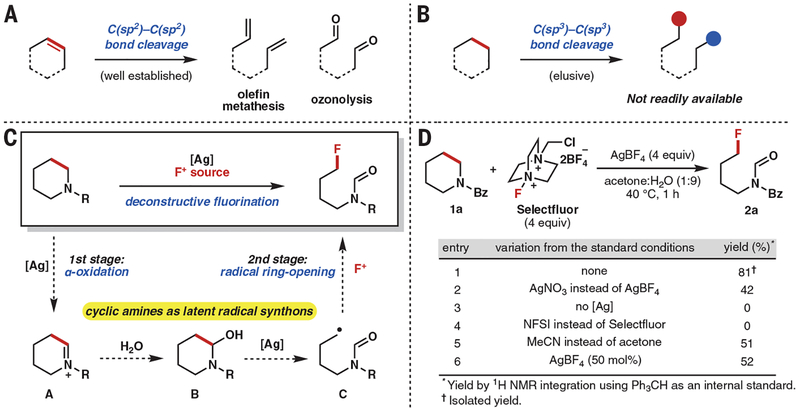
The ubiquity of carbon-carbon (C–C) bonds in organic compounds places a premium on methods that construct such bonds. In general, these bond constructions lead to an increase in structural complexity. However, in certain cases, the cleavage of C–C bonds may lead to more synthetically complex products that cannot be prepared efficiently in any other way. Historically, the full benefit of C–C bond cleavage (deconstructive strategies) has often been realized by coupling this process with value-added bond constructions such as C–C bond formation (e.g., in olefin metathesis processes) (1, 2) or C–O bond formation (e.g., in ozonolysis) (3) (Fig. 1A). Whereas the benefits of these C(sp2)=C(sp2) double-bond cleavage and functionalization processes are well established, the value of deconstructive processes becomes even more apparent when C(sp3)–C(sp3) single-bond cleavage and functionalization are considered, especially in the context of late-stage skeletal diversification to access unexplored chemical space (4) (Fig. 1B). The development of deconstructive functionalizations of cyclic amines would be particularly useful, given their ubiquity in pharmaceuticals and agrochemicals (5, 6). However, methods for the ring-opening of cyclic amines remain extremely limited and are dominated by C–O bond formation through α-oxidation followed by heterolytic C–N bond cleavage via the well-established equilibrium with the hemiaminal that forms (7–12). Although variants of the von Braun reaction of nucleophilic tertiary N-alkyl–substituted cyclic amines lead to C–Cl bond formation via heterolytic ring-opening, this strategy is limited to cyclic amines with small ring sizes owing to competing N-dealkylation (13). With these limitations in mind, we sought a mechanistically distinct strategy that would provide a general entry to the deconstructive functionalization of cyclic amines.
Fig. 1. Development of a deconstructive fluorination of cyclic amines.
(A) Well-established deconstructive functionalization. (B) An elusive deconstructive functionalization. (C) A blueprint for deconstructive fluorination of cyclic amines. (D) Optimization of silver-mediated deconstructive fluorination of N-Bz piperidine 1a. R, any functional group; Me, methyl; Ph, phenyl; equiv, equivalents; h, hour.
In this context, reactions that form C(sp3)–F bonds are among the most valued bond constructions because of the influence of fluorine substitution on the properties of pharmaceuticals, agrochemicals, and organic materials (14–17). For example, installation of fluorine may lead to increased metabolic stability, altered physicochemical properties such as increased lipophilicity, reduced basicity of nearby nitrogen atoms, and conformational tuning. As a consequence, substantial progress has been made on site-selective reactions that form C(sp3)–F bonds (18, 19). Nevertheless, the development of methods for C(sp3)–F bond formation that facilitate the preparation of a variety of fluorine-containing building blocks from easily available starting materials remains a prominent goal. Here we report a deconstructive strategy to transform cyclic amine derivatives into versatile fluorine-containing acyclic amine derivatives, using commercially available reagents, through C(sp3)–C(sp3) single-bond cleavage followed by C(sp3)–F bond formation (Fig. 1C).
Our strategy for deconstructive fluorination of cyclic amine derivatives is based on two discrete stages, each mediated by a silver salt (Fig. 1C). In the first stage, we envisioned that under an appropriate set of oxidative conditions, a saturated cyclic amine would be oxidized to the corresponding iminium ion A, which would be trapped by H2O to form hemiaminal B. In the second stage, the resulting hemiaminal B could undergo homolytic ring-opening upon engaging the silver salt to yield primary radical C (20). A subsequent fluorine atom transfer would deliver the desired fluorinated product. On this basis, cyclic amines could be viewed as synthons for amino alkyl radicals, which have conventionally only been shown to arise from the corresponding halide, alcohol, or carboxylic acid derivatives (21). Although this strategy is conceptually simple, several challenges were inherent in putting it into practice. First, only a few methods exist for oxidation of amines to the corresponding hemiaminals owing to the competing over-oxidation to amides (22). Furthermore, no reports exist of α-oxidation of cyclic amine derivatives using silver salts. However, given the oxidation potential of N-protected cyclic amines such as 1a [anodic peak potential = +1.13 V versus saturated calomel electrode (SCE)] (fig. S1), we theorized that Ag(II) salts could be sufficiently oxidizing [standard reduction potential (Ag2+/Ag+) = +1.98 V versus SCE] (23) to effect single-electron transfer. Second, most of the reports of successful ring-opening fluorination are limited to strained tertiary cycloalkanols such as cyclobutanols (24–27); only limited examples that feature relatively unstrained cycloalkanols such as cyclopentanols and cyclohexanols are known, and these cases resulted in low yields (25). The challenge in achieving our envisioned transformation rested on identifying a silver salt and fluorinating reagent combination that would act in synergy to selectively cleave and functionalize the desired C–C bond.
We began our investigation by establishing the conditions for the overall transformation using N-Bz piperidine 1a as the substrate (Bz, benzoyl) (Fig. 1D). After extensive screening of various conditions, we identified the optimized conditions shown in entry 1, which use cheap and commercially available AgBF4 in a 9:1 (v/v) mixture of H2O/acetone at 40°C. Other silver sources led to lower yields, with AgNO3 providing the highest yield among them (entry 2). A control experiment established that a silver salt is essential to obtaining the desired fluorinated product (entry 3). Other fluorinating reagents such as N-fluorobenzenesulfonimide (NFSI) led to no reaction (entry 4). A 9:1 (v/v) mixture of H2O/MeCN gave a diminished yield (51%) of 2a (entry 5), pointing to the superiority of acetone as the cosolvent. The overall transformation can be conducted with substoichiometric amounts of AgBF4 to provide 2a, albeit in modest yield (entry 6).
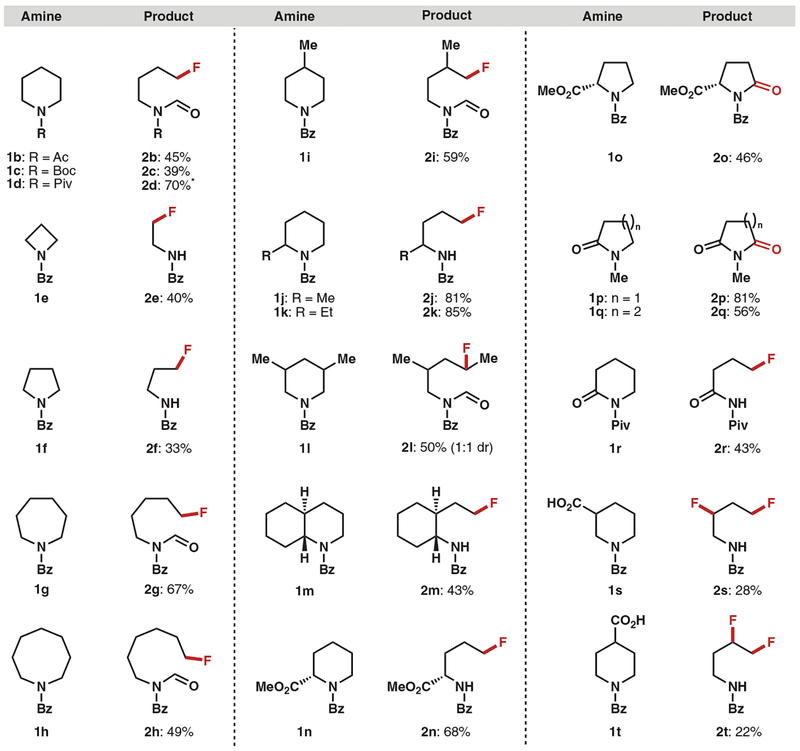
With the optimized conditions established, we proceeded to investigate the scope of the deconstructive fluorination process. As shown in Fig. 2, several structurally and electronically distinct N-substituted piperidine derivatives were fluorinated effectively, including those bearing acetyl (Ac, 1b), tert-butoxycarbonyl (Boc, 1c), and pivaloyl (Piv, 1d) groups. The deconstructive fluorination method is not limited to piperidine derivatives; a range of N-benzoylated saturated azacycles including azetidine 1e, pyrrolidine 1f, azepane 1g, and azocane 1h were all viable in the deconstructive fluorination reaction. Fluorinated products 2e and 2f were obtained in the deformylated form (vide infra). The variation of the cyclic amine substrate ring size led to fluoroamine derivatives bearing carbon chains of varying lengths. A variety of substitution patterns on the piperidine ring were also well tolerated, and the corresponding acyclic fluorinated amines were obtained in moderate to good yields (50 to 85%). Some of the fluorinated alkyl amine products have not been previously reported and would not be readily accessible by conventional deoxyfluorination strategies owing to the limited availability of the corresponding substituted linear amino alcohols. For example, 2-substituted piperidines 1j and 1k afforded the corresponding fluoroamines 2j and 2k, respectively, with complete positional selectivity. The observed selectivity for cleavage away from the substituents may be attributed to the steric hindrance imparted by these groups at the α-position of the cyclic amines. 3-Substituted piperidines were also good substrates, as evidenced by 1l undergoing ring-opening and fluorination to provide 2l in 50% yield, demonstrating that secondary alkyl fluorides can be accessed by this method. Fused piperidines such as 1m underwent deconstructive fluorination to provide 2m in 43% yield. This example demonstrates that polycyclic molecules can be functionalized as well, paving the way for late-stage skeletal diversification of complex molecules. Moreover, L-pipecolic acid derivative 1n gave 5-fluoro- L-norvaline derivative 2n in 68% yield (three steps from L-pipecolic acid), considerably shortening the synthesis of 5-fluoro-L-norvaline (previously prepared in seven steps from L-glutamic acid) (28). L-proline methyl ester derivative 1o was converted to pyrrolidinone derivative 2o, presumably by over-oxidation of an intermediate 5-hydroxyproline derivative. Similarly, N-methyl-2-piperidinone (1p) and N-methyl-2-pyrrolidinone (1q) were oxygenated under the reaction conditions to give N-methyl imides 2p and 2q, respectively. These oxygenation reactions are important in their own right because methods for the direct α-oxygenation of cyclic amides are dominated by strongly oxidizing RuO4 (22). N-Piv-2-pyrrolidinone 1r afforded fluorinated product 2r under the same conditions. Moreover, piperidines containing carboxylic acid groups underwent dual functionalization to provide difluorinated amines through decarboxylative (29) and deconstructive fluorination. For example, N-Bz piperidine 1s and 1t underwent dual fluorination to provide 3,5-difluorinated amine 2s (28%) and 4,5-difluorinated amine 2t (22%), along with N-Bz 3-fluoropiperidine (28%) and N-Bz 4-fluoropiperidine (20%), respectively.
Fig. 2. Deconstructive fluorination: cyclic amine scope.
Only isolated yields are shown. Reaction conditions: 1 (0.1 mmol), AgBF4 (4 equivalents), Selectfluor (4 equivalents), acetone:H2O (1:9), 40°C, 1 hour. *Deformylated product obtained. dr, diastereomeric ratio.
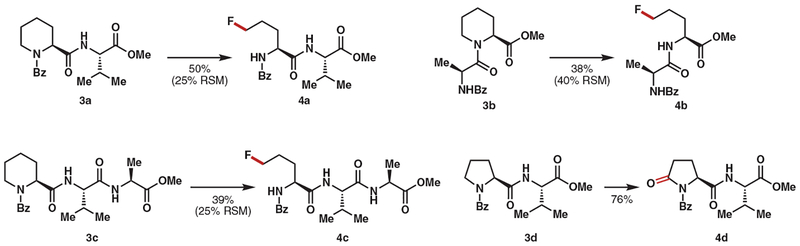
As a demonstration of the utility of this method, we considered functionalizing synthetic peptides, which continue to see widespread use in drug discovery (30–32). Deconstructive functionalization of peptides can provide orthogonal and complementary skeletal diversification and add profitably to the toolbox of available methods (33). When dipeptide 3a, which has a valine residue, was subjected to our reaction conditions, the fluorination proceeded readily to afford fluorinated dipeptide 4a in 50% yield along with 25% recovered starting material (Fig. 3). In this case, prolonged reaction times did not lead to an increase in the product yield, presumably owing to product inhibition. Internal peptides such as 3b also underwent deconstructive fluorination to provide 4b in 38% yield (accompanied by 40% of recovered starting material). Likewise, tripeptide 3c was converted to 4c in 39% yield, along with 29% of recovered starting material. This methodology could also be applied to the selective C5-oxygenation of L-proline–containing peptides. For example, peptide 3d was oxygenated in 76% yield to provide 4d. N-Benzoyl imide–containing peptide 4d served as a versatile intermediate for further functionalization. We did not observe racemization of the fluorinated and oxygenated peptides, which were obtained as single diastereomers.
Fig. 3. Diversification of pipecolic acid and proline residues in peptides.
Isolated yields are shown. Isolated yields of recovered starting material (RSM) are given in parentheses. Reaction conditions: 3 (0.1 mmol), AgBF4 (4 equivalents), Selectfluor (4 equivalents), acetone:H2O (1:9), room temperature, 15 hours.
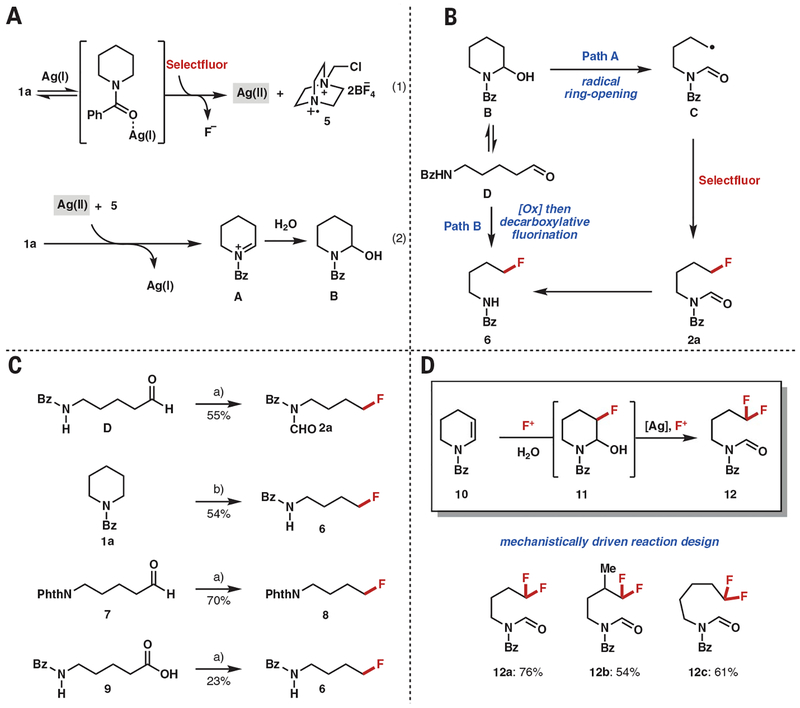
A number of additional experiments were performed to elucidate the reaction mechanism. We began by using nuclear magnetic resonance (NMR) spectroscopy to investigate the interaction of Ag(I) and Selectfluor. Unexpectedly, a 19F NMR spectrum of an equimolar mixture of Selectfluor and AgBF4 in a 1:9 (v/v) mixture of acetone-d6/D2O, acquired after stirring at 40°C for 1 hour (fig. S2), displayed no consumption of Selectfluor (34, 35). However, in the presence of an equivalent of 1a, consumption of Selectfluor was observed, suggesting that the N-protected cyclic amine substrates play an important role in initiating the ring-opening and fluorination process. In addition, line broadening in the 1H NMR spectrum was observed, suggesting the formation of a paramagnetic Ag(II) complex. Furthermore, downfield shifts of NMR resonances of 1a were observed in the 1H NMR spectra upon addition of AgBF4 (fig. S3), suggesting the binding of Ag(I) to the amide moiety of 1a (36, 37). On the basis of these NMR experiments, we propose a mechanism that involves initial coordination of Ag(I) to 1a, followed by single-electron oxidation by Selectfluor to form Ag(II) and radical dication 5 (Eq. 1 in Fig. 4A) (35). The resulting Ag(II) species would undergo single-electron transfer from 1a. Subsequent hydrogen-atom abstraction by 5 (38) would deliver iminium ion A, and this would be followed by trapping by H2O to give hemiaminal B (Eq. 2 in Fig. 4A). An alternative mechanistic pathway wherein radical dication 5 undergoes α-amino C−H abstraction of 1a followed by single-electron transfer by Ag(II) to generate the same iminium ion A cannot be ruled out. From hemiaminal B, an alkoxy Ag(II) intermediate may form (not shown). Opening of this intermediate to primary radical C would achieve the desired C(sp3)–C(sp3) bond cleavage, and attendant fluorination of the radical by Selectfluor would yield 2a (path A in Fig. 4B). However, we recognize that another pathway could be operable based on the fact that deformylated products were obtained in some cases. In this alternate pathway, opening of the hemiaminal to linear aldehyde D and subsequent oxidation to the corresponding carboxylic acid would then set the stage for a decarboxylative fluorination—in line with the precedent of (29) (path B in Fig. 4B). In a series of experiments to support or refute either mechanism (Fig. 4C), aldehyde D, which likely exists in equilibrium with hemiaminal B, was subjected to our reaction conditions and gave fluoroamine 2a in 55% yield, which can only be accessed through path A. In addition, when the reaction was conducted over a prolonged period, the benzoyl amide was obtained as the major product, indicating that the conversion of 2a to 6 likely occurs through a deformylation process. However, the successful fluorination of N-phthaloyl aldehyde 7 demonstrates that fluorination can proceed from the aldehyde, which cannot form the hemiaminal. That 7 gave a higher yield than 1a and D is a result of the relative stability of the product 8 under the oxidative reaction conditions that were used. Subjecting carboxylic acid 9 to the optimized conditions resulted in 23% yield of fluorinated product 6. On the basis of these experiments, we cannot rule out the possibility that path B is operative for the small subset of substrates that gave exclusively deformylated products. Lastly, on the basis of our mechanistic proposal, we sought to explore the reactivity of enamides under our optimized reaction conditions. We envisioned enamide 10 undergoing electrophilic fluorination followed by trapping of the resulting carbocation with water to yield 11 (39). An alkoxy Ag(II) intermediate would follow, leading to C(sp3)–C(sp3) cleavage and fluorination to yield gem-difluorinated protected amine 12. As shown in Fig. 4D, in a reaction using catalytic amounts of silver salts, a variety of enamides related to 10 underwent the desired deconstructive difluorination to yield 12a to 12c in 54 to 76% yield. In support of path A (Fig. 4A), the formyl imide products were isolated as the major product under the optimized reaction conditions. These results are productive given the established importance of difluoromethyl groups (40, 41). For example, the difluoromethyl moiety serves as a lipophilic hydrogen bond donor that acts as a bioisostere for thiol and hydroxyl groups.
Fig. 4. Mechanistic studies.
(A) Proposed mechanism for 1a oxidation. (B) Possible mechanisms for fluorination of B. (C) Mechanistic studies. Reaction conditions: (a) starting material (0.1 mmol), AgBF4 (4 equivalents), Selectfluor (4 equivalents), acetone:H2O (1:9), 40°C, 1 hour; (b) 1a (0.5 mmol), AgBF4 (4 equivalents), Selectfluor (4 equivalents), acetone:H2O (1:9), room temperature, 16 hours. (D) Mechanistically driven gem-fluorination of enamide 10. Reaction conditions: 10 (0.1 mmol), AgBF4 (0.25 equivalents), Selectfluor (4 equivalents), acetone:H2O (1:1), room temperature, 15 hours. Phth, phthaloyl.
The simple protocol of the deconstructive fluorination, which proceeds in aqueous solvent mixtures as well as in water alone (42, 43), should lead to its widespread adoption for late-stage skeletal diversification in the pharmaceutical and agrochemical arena. From the retrosynthetic viewpoint, cyclic amines can now be regarded as synthons for amino alkyl radical intermediates, which can be engaged by a variety of coupling partners. Thus, we anticipate that this method will unlock fundamentally different disconnection strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Derrick for assistance with electrochemical measurements.
Funding: This work was supported by the National Institutes of Health (NIGMS RO1 086374). J.B.R. thanks the NIH (NIGMS RO1 086374) for a graduate diversity supplement fellowship. Y.K. thanks the Japan Society for the Promotion of Science (JSPS) for an Overseas Research Fellowship. L.T.G. thanks LMU PROSA and DAAD for financial support.
Footnotes
Competing interests: J.B.R., Y.K., L.T.G., and R.S. are listed as inventors on an initial patent application describing the Ag-mediated deconstructive functionalization of cyclic amines (052103-515P01US).
Data and materials availability: Experimental and characterization data are provided in the supplementary materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Hoveyda AH, Zhugralin AR, Nature 450, 243–251 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Vougioukalakis GC, Grubbs RH, Chem. Rev 110, 1746–1787 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Bailey PS, Chem. Rev 58, 925–1010 (1958). [Google Scholar]
- 4.Silverman SK, Hergenrother PJ, Curr. Opin. Chem. Biol 10, 185–187 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Vitaku E, Smith DT, Njardarson JT, J. Med. Chem 57, 10257–10274 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Lawrence SA, Amines: Synthesis, Properties and Applications (Cambridge Univ. Press, 2004). [Google Scholar]
- 7.Shawcross AP, Stanforth SP, J. Heterocycl. Chem 27, 367–369 (1990). [Google Scholar]
- 8.Han G, McIntosh MC, Weinreb SM, Tetrahedron Lett 35, 5813–5816 (1994). [Google Scholar]
- 9.Cocquet G, Ferroud C, Guy A, Tetrahedron 56, 2975–2984 (2000). [Google Scholar]
- 10.Ito R, Umezawa N, Higuchi T, J. Am. Chem. Soc 127, 834–835 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Kaname M, Yoshifuji S, Sashida H, Tetrahedron Lett 49, 2786–2788 (2008). [Google Scholar]
- 12.Osberger TJ, Rogness DC, Kohrt JT, Stepan AF,White MC, Nature 537, 214–219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C et al. , J. Org. Chem 82, 6615–6620 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ,Meanwell NA, J. Med. Chem 58, 8315–8359 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Wang J et al. , Chem. Rev 114, 2432–2506 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Purser S, Moore PR, Swallow S, Gouverneur V, Chem. Soc. Rev 37, 320–330 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Müller K, Faeh C, Diederich F, Science 317, 1881–1886 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Champagne PA, Desroches J, Hamel J-D, Vandamme M,Paquin J-F, Chem. Rev 115, 9073–9174 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Lantaño B, Postigo A, Org. Biomol. Chem 15, 9954–9973 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Murakami M, Ishida N, Chem. Lett 46, 1692–1700 (2017). [Google Scholar]
- 21.Yan M, Lo JC, Edwards JT, Baran PS, J. Am. Chem. Soc 138, 12692–12714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperry J, Synthesis 2011, 3569–3580 (2011). [Google Scholar]
- 23.Po HN, Coord. Chem. Rev 20, 171–195 (1976). [Google Scholar]
- 24.Zhao H, Fan X, Yu J, Zhu C, J. Am. Chem. Soc 137, 3490–3493 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Ren S, Feng C, Loh T-P, Org. Biomol. Chem 13, 5105–5109 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Tian Q, Chen B, Zhang G, Green Chem 18, 6236–6240 (2016). [Google Scholar]
- 27.Deng Y, Kauser NI, Islam SM, Mohr JT, Eur. J. Org. Chem 2017, 5872–5879 (2017). [Google Scholar]
- 28.Wang L et al. , Nucl. Med. Biol 39, 933–943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin F, Wang Z, Li Z, Li C, J. Am. Chem. Soc 134, 10401–10404 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Antosova Z, Mackova M, Kral V, Macek T, Trends Biotechnol 27, 628–635 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M, Drug Discov. Today 15, 40–56 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Kaspar AA, Reichert JM, Drug Discov. Today 18, 807–817 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Sengupta S, Mehta G, Tetrahedron Lett 58, 1357–1372 (2017). [Google Scholar]
- 34.Selectfluor is reported to react with AgNO3 in acetone-d6/D2O (35)
- 35.Patel NR, Flowers RA 2nd, J. Org. Chem 80, 5834–5841 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Romanov V et al. , J. Phys. Chem. A 112, 10912–10920 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Romanov V, Siu C-K, Verkerk UH, Hopkinson AC,Siu KWM, J. Phys. Chem. A 114, 6964–6971 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Pitts CR et al. , J. Am. Chem. Soc 136, 9780–9791 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Singh S et al. , Synlett 23, 2421–2425 (2012). [Google Scholar]
- 40.Meanwell NA, J. Med. Chem 54, 2529–2591 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Sessler CD et al. , J. Am. Chem. Soc 139, 9325–9332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li CJ, Chen L, Chem. Soc. Rev 35, 68–82 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Simon MO, Li CJ, Chem. Soc. Rev 41, 1415–1427 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.