Abstract
Cancer treatment with local administration of armed oncolytic viruses could potentially induce systemic antitumor effects, or the abscopal effect, as they self-amplify in tumors, induce danger signaling, and promote tumor-associated antigen presentation. In this study, oncolytic adenovirus coding for human tumor necrosis factor alpha (TNF-α) and interleukin-2 (IL-2) Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 (also known as [a.k.a.] TILT-123) provoked antitumor efficacy in tumors that were injected with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 and those that were left non-injected in the same animal. Importantly, the virus was able to travel to distant tumors. To dissect the effects of oncolysis and cytokines, we studied replication-incompetent viruses in mice. Systemic antitumor effects were similar in both models, highlighting the importance of the arming device. The cytokines induced positive changes in immune cell infiltrates and induced the expression of several immune-reaction-related genes in tumors. In addition, Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 was able to increase homing of adoptively transferred tumor-infiltrating lymphocytes into both injected and non-injected tumors, possibly mediated through chemokine expression. In summary, local treatment with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 resulted in systemic antitumor efficacy by inducing immune cell infiltration and trafficking into both treated and untreated tumors. Moreover, the oncolytic adenovirus platform had superior systemic effects over replication-deficient vector through spreading into distant tumors.
Keywords: immunotherapy, adenovirus, abscopal effect
Introduction
Eight million cancer deaths occur globally each year, and almost all of them result from metastatic cancer.1 New therapeutic approaches are thus urgently needed. Because the patients in need of novel treatments typically have metastatic disease, systemic therapeutic efficacy is required. After a century of developing immunotherapies, the first products have recently entered routine use. Many of them, including monoclonal checkpoint blocking antibodies and recombinant cytokines, are used systemically, which can cause severe adverse events and even mortality by affecting normal tissues.2, 3 In contrast, the typical embodiment of oncolytic immunotherapy is a local injection into tumors. Several types of oncolytic viruses are being investigated in preclinical and clinical studies, and one product, talimogene laherparepvec (also known as [a.k.a.] T-Vec or Imlygic), has already been approved.4, 5, 6 Even though T-Vec is not capable of spreading to distant tumors, local injection causes immunological reactions in distant metastases, a phenomenon known as abscopal effect.6, 7
The abscopal effect has been proposed as potentially relevant in patients being treated with systemic immunotherapy, such as checkpoint blocking antibodies, and local radiation.8, 9 The biological rationale is that radiation can cause immunogenic cell death, allowing the induction of new T cells against the tumor, while concurrent checkpoint inhibitors prevent immunosuppression from occurring. This approach is now being tested in dozens of trials.10 Oncolytic viruses, such as T-Vec, are able to induce an abscopal effect without the need for radiation.6, 7 The biological rationale is the same: oncolytic replication can induce immunogenic cell death and immunological danger signaling, both of which can induce de novo immunity against the tumor.
With regard to oncolytic viruses under development, but not yet approved (except oncolytic adenovirus Oncorine in China), oncolytic adenoviruses are well tolerated in humans and excellent devices for transgene delivery.5, 11, 12 For example, toxic systemic delivery of IL-2, regularly used in adoptive cell therapy protocols, is replaceable with virus-vectored IL-2 gene therapy in the context of T cell transfer.13 In addition to immune stimulation by the transgene, adenoviral oncolysis induces immunogenic cell death and the release of danger signals and tumor-associated antigens, which increase tumor immunogenicity.14, 15, 16 Importantly, adenovirally delivered cytokines provide enhanced antitumor efficacy with minimal or nonexistent toxicity.13, 17
To decrease the toxicity and increase the efficacy of T cell-related immunotherapies, such as adoptive cell therapy and checkpoint inhibitors, we have developed an oncolytic adenovirus coding for human tumor necrosis factor alpha (TNF-α) and interleukin-2 (IL-2) (Ad5/3-E2F-d24-hTNF-α-internal ribosome entry site [IRES]-hIL-2, a.k.a. TILT-123).17, 18, 19 We hypothesized that oncolytic adenovirus replication accompanied by IL-2 and TNF-α production from tumor cells induces immunological effects that are powerful not only locally but also system-wide. Because we have seen Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 inducing positive changes locally in the tumor-infiltrating immune cell milieu, as well as on a systemic level,17 we studied whether a local treatment would be able to generate an abscopal effect on distant tumors and the mechanisms behind it.
Results
Cytokine-Armed Oncolytic Adenoviruses Induce Systemic Antitumor Responses
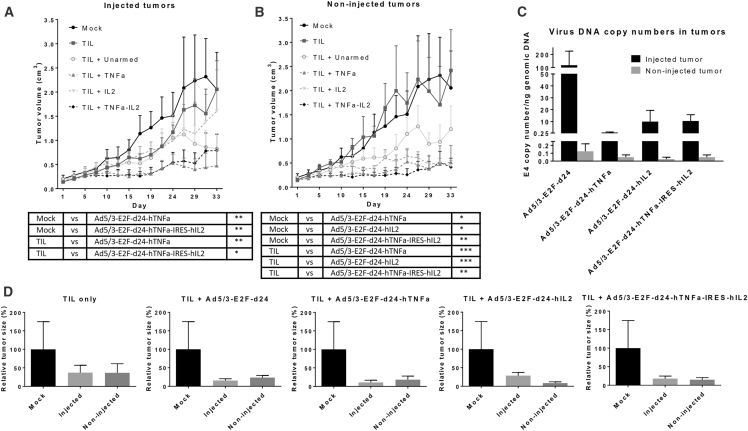
The systemic effects of a local treatment with oncolytic Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 were studied in Syrian hamsters that are semi-permissive for human adenovirus replication.20 In addition, certain human cytokines, including TNF-α and IL-2, are bioactive in hamsters.17, 20 Because this virus was developed to enable T cell therapies, the experimental settings included a treatment with tumor-infiltrating lymphocyte (TIL) graft. We observed tumor growth reduction in both injected and non-injected tumors without differences in tumor sizes between these tumors (Figures 1A, 1B, and 1D).
Figure 1.
Treatment with Oncolytic Virus Controls the Growth of Both Injected and Non-injected Tumors
Hamsters were treated on days 1, 8, 15, 22, and 29 with 1 × 108 VPs intratumorally (i.t.) and with 5 × 107 TILs on day 1 intraperitoneally (i.p.). The growth of injected (A) and non-injected (B) hamster tumors (n = 5–6) was measured every 2–3 days until day 33. During the follow-up period, two animals were sacrificed from the mock group (day 24), two animals from the group receiving TILs only (day 22), and one animal from groups treated with Ad5/3-E2F-d24-hTNF-α (day 29) and Ad5/3-E2F-d24-hIL-2 (day 29). Small amounts of viral DNA were detectable also in non-injected tumors on day 16 (C). There were no differences between the injected and non-injected tumor sizes on day 33 (D). The graphs show mean plus SEM. Statistical differences were evaluated with mixed model analysis; ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05.
The arming devices resulted in a benefit in tumor control over the respective unarmed virus. With regard to injected tumors, the best groups were Ad5/3-E2F-d24-hTNF-α and Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 (p = 0.002 and p = 0.0034 compared with TILs alone, respectively; p = 0.002 and p = 0.01 compared with mock). Regarding non-injected tumors, all armed viruses had enhanced antitumor efficacy at the non-injected site, unlike the unarmed virus, when compared with mock and TILs alone (TIL versus TIL + TNF-α: p = 0.001; TIL versus TIL + IL-2: p = 0.000427; TIL versus TIL + TNF-α-IL-2: p = 0.00007; mock versus TIL + TNF-α: p = 0.011; mock versus TIL + IL-2: p = 0.022; mock versus TIL + TNF-α-IL-2: p = 0.006).
The viruses were present in injected tumors at high levels on day 16, 8 days after the last intratumoral injection. The highest values were observed in the group treated with the unarmed virus (Figure 1C). Viral DNA levels were low in non-injected tumors and normal tissues (Figures 1C and S1A). The highest individual values were detected in spleen, liver, and lung, but there were no differences in biodistribution between viruses or organs.
After treatments, animals were monitored and sacrificed according to animal regulations (tumor size reaching 20 mm). The group treated with the double-armed virus had the best survival (p = 0.03 and p = 0.0159 compared with mock and TILs alone, respectively), whereas TILs alone had a minimal effect on survival (Figure S1B).
Arming with TNF-α and IL-2 Results in an Abscopal Effect Even without Oncolysis
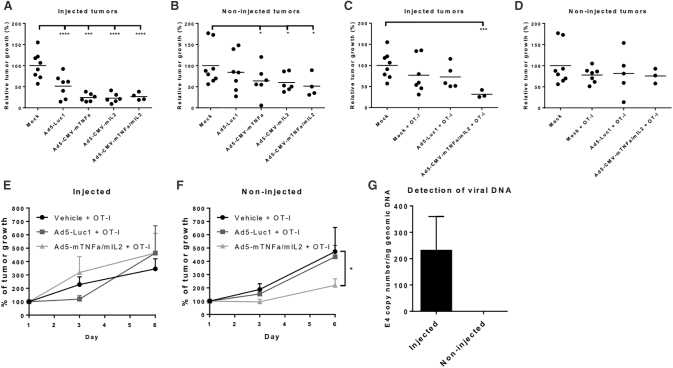
Next, we sought to dissect the effect of the transgenes from the effects of oncolysis. This could be achieved by using replication-incompetent adenoviruses with murine cytokines in immunocompetent mice bearing B16 melanoma tumors expressing chicken ovalbumin (OVA). By day eight, an early time point, a difference in tumor size could be seen in both injected and non-injected tumors (Figures 2A and 2B). The tumors injected with IL-2- and TNF-α-armed viruses were about 70% smaller than the PBS-injected vehicle control tumors (p < 0.0001). The control virus Ad5-Luc1, which lacks an immunologically active transgene, had a minor yet statistically significant effect (p = 0.0004).
Figure 2.
Replication-Incompetent Virus Induces Growth Control Also in the Non-injected Tumor at an Early Time Point
The sizes of injected (A and C) and non-injected (B and D) B16-OVA tumors in mice on day 8 (n = 3–8) without T cell transfer (A and B) or with OT-I cells (C and D) were compared with average mock tumor size on the same time point. Similar results were obtained for injected (E) and non-injected (F) tumors when the experiment was repeated. Viral DNA was detected only in the injected tumors (G). The bars show mean plus SEM. Statistical significance was evaluated with 2-way ANOVA: ****p < 0.0001; ***p < 0.001; *p < 0.05.
Regarding non-injected tumors, the armed viruses were the only ones able to induce tumor growth control (vehicle versus Ad5-CMV-mTNF-α: p = 0.0162; vehicle versus Ad5-CMV-mIL-2: p = 0.0475; vehicle versus Ad5-CMV-mTNF-α/mIL-2: p = 0.0212). The best tumor control was induced by the combination of the cytokines, mean tumor size being half of the size of the vehicle tumors. The addition of OVA-specific OT-I T cells resulted in similar outcomes but did not enhance tumor growth control at this early time point (Figures 2C and 2D).
The experiment was repeated with the most relevant treatments: OT-I T cells with vehicle control, control virus Ad5-Luc1, and Ad5-CMV-mTNF-α/mIL-2. Tumor growth was followed for 6 days. The treatment did not influence the growth of injected tumors, but the animals treated with cytokine-armed viruses clearly showed a delay in non-injected tumor growth as compared with vehicle treatment (p = 0.04; Figures 2E and 2F). Of note, the effect was not due to virus spread because viral genomes at the non-injected tumors were undetectable (Figure 2G). Thus, the viral transduction of distant tumors seems to require oncolysis and subsequent shedding of the virus from tumors into blood.
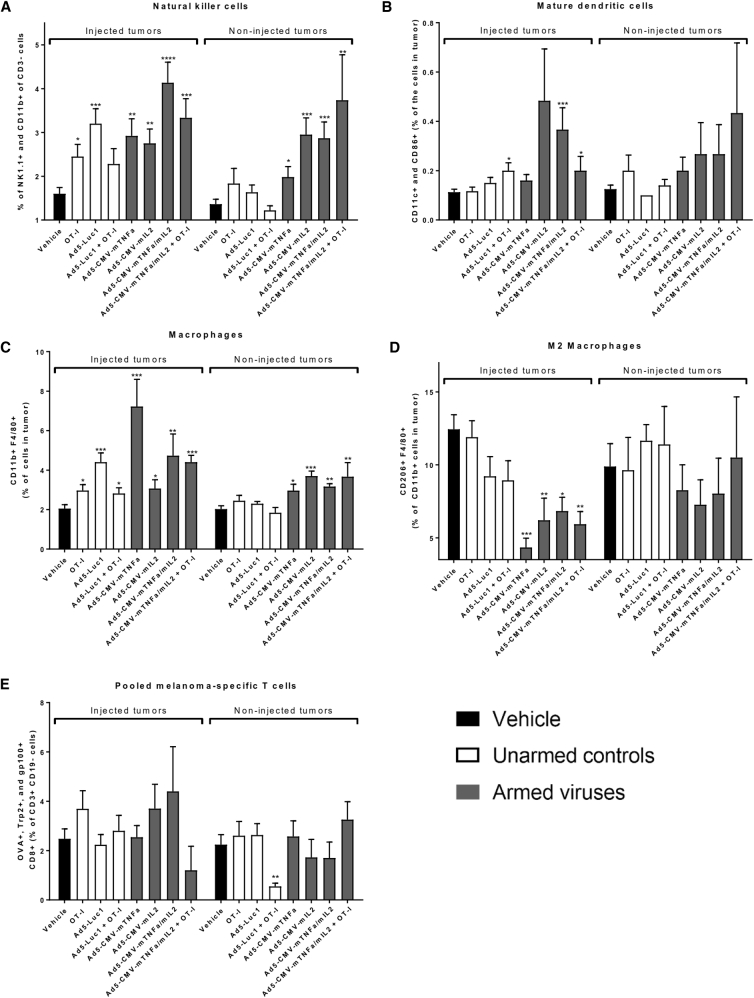
Day eight tumors were collected and analyzed for intratumoral immune cell populations. The percentage of natural killer (NK) cells (NK1.1. and CD11b double-positive cells in the CD3-negative cell population) was elevated in both injected and non-injected tumors following treatment with cytokine-coding viruses (Figure 3A). In addition, the cytokine combination was able to increase the levels of CD11c- and CD86-positive dendritic cells in treated tumors, and the trend was similar in non-injected tumors (Figure 3B). Moreover, there was a positive correlation between the presence of NK cells and dendritic cells (Figures S2A and S2B).
Figure 3.
Treatment with Armed Viruses Induces Positive Changes in Immune Cells in the Tumor Microenvironment
Tumor samples were collected on day 8, and the presence of natural killer cells (A), mature dendritic cells (B), macrophages (C), and M2-like macrophages (D) in tumors was detected with flow cytometry. Melanoma-specific T cells were detected with pentamers and the results with OVA+, Trp2+, and gp100+ cells pooled into one graph (E). Statistical differences were analyzed against corresponding mock tumor. The bars show mean plus SEM. Black bar indicates mock control, white bars unarmed virus or OT-I controls, and gray bars treatment groups with armed viruses. Unpaired t test was performed to analyze statistical differences; ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05.
An increase of F4/80 and CD11b-positive macrophages was observed in both tumors (Figure 3C). Interestingly, the portion of immunosuppressive M2-like macrophages (differentiated by CD206 expression) was decreased when tumors received cytokine-coding viruses (Figure 3D). Again, a similar, yet not significant, trend was seen in non-injected tumors. Out of other markers for immunosuppression, the treatments did not affect the expression of TGF-β or FoxP3 (Figures S2C and S2D). No major differences were observed between the groups regarding melanoma-specific (OVA, Trp2, and gp100) CD8-positive T cells (Figure 3E). The presence of any of the immune cell populations studied here did not correlate with tumor volumes (Figures S2E–S2J).
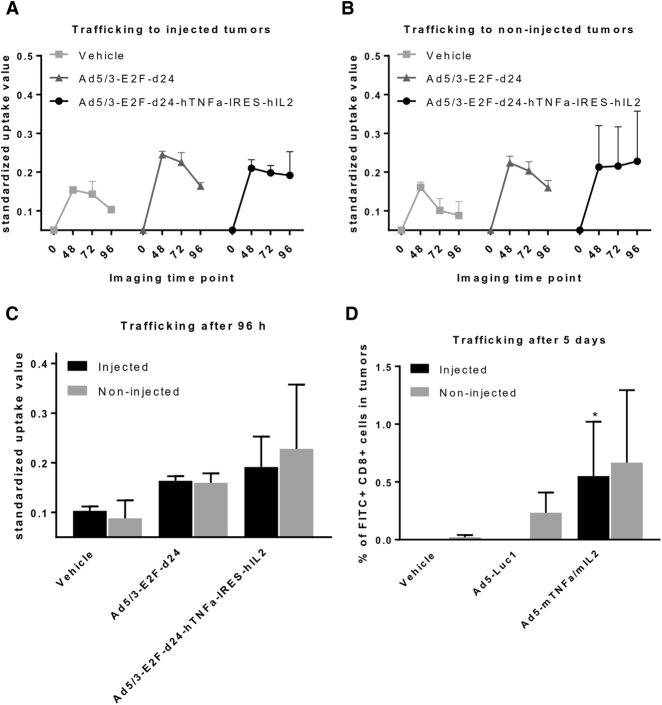
Armed Adenovirus Induces the T Cell Graft Trafficking into Both Injected and Non-injected Tumors
Because the oncolytic virus was able to travel to the non-injected tumors, we wanted to study whether the virus induces TIL graft trafficking into both injected and non-injected tumors. Distribution and tumor accumulation of the radiolabeled cells (111In-oxine) were determined by single-photon emission computed tomography/computed tomography (SPECT/CT) imaging at 48, 72, and 96 hr after administration (Figures 4A and 4B). A trend of increased trafficking into both injected and non-injected tumors was observed in animals treated with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 compared with the unarmed virus and the vehicle control (Figure 4C). In addition, the cells seemed to be more persistent in the tumors when the animals were treated with the cytokine-armed virus: there was no decrease over time in the injected or non-injected tumors, whereas in the vehicle and unarmed virus control groups the signal decreased over time.
Figure 4.
Armed Virus Induces TIL and TCR Graft Trafficking to and Persistence in Both Tumors
TILs were labeled with radioactive indium, and the trafficking into (A) injected and (B) non-injected tumors was followed with SPECT/CT imaging over time. On day 0, the hamsters received the labeled TILs intraperitoneally and viruses or PBS control intratumorally into one of the two tumors (n = 2/group). The difference between the groups was most prominent 96 hr after the administration of the cells (C). TCR-modified OT-I cells were labeled with fluorescent-labeled nanoparticles, and their presence in injected and non-injected tumors was investigated after 5 days (n = 6) (D). Tumors injected with viruses coding for TNF-α and IL-2 had significantly higher numbers of infused OT-I cells compared with vehicle-treated tumors (Kruskal-Wallis test, *p < 0.05).
To estimate the effect of the cytokines on T cell graft trafficking, fluorescently labeled OT-I T cells were administered to animals receiving vehicle, Ad5-Luc1 control virus, or Ad5-CMV-mTNF-α/IL-2 into one of the two tumors. Five days later, the tumors were collected and the presence of transferred T cells detected with flow cytometry. Even in the absence of oncolysis, treatment of one tumor was able to induce OT-I cell trafficking into both injected and non-injected tumors (Figure 4D). The transferred T cells did not find their way into the tumors when the animals were treated with vehicle control, and only a low signal was detected in non-injected tumors in animals receiving the control virus. There were no statistically significant differences between the injected and non-injected tumors.
Oncolytic Adenovirus Induces the Expression of Immunologically Relevant Genes in Injected and Non-injected Tumors
In order to uncover the mechanisms of action underlying the systemic effects and the trafficking of adoptively transferred cell graft, we analyzed the gene expression profiles of injected and non-injected tumors in hamsters receiving TIL therapy. In the vehicle group, PBS injection induced immune reactions by the upregulation of the genes related to humoral immune responses, chemokine and cytokine production, inflammatory responses, and complement activation, among others (Table S1). In addition, the PBS injection downregulated genes related to cytoskeleton organization and other filament-related processes. To allow for the effects of the needle puncture and vehicle injection, we analyzed treatment group expression levels against corresponding vehicle group tumors.
Over 2-fold upregulation was observed for 445 genes in injected tumors and for 165 genes in non-injected tumors (Figures S3 and 5). The number of downregulated genes was 45 and 70 in injected and non-injected tumors, respectively (Figures S3 and 6). TIL treatment without viruses induced the expression of chemokines (Ccl4, Ccl7, Ccl11), but also immune checkpoint molecules Pdl1 (or Cd274) in the PBS-injected tumors and Lag3 in the non-injected tumors. In addition, we saw upregulation of a variety of lymphocyte-related genes (Sash3, Fgl2, Txk, Gzmk, Bst1, Pik3ap1, Pik3cd, Prkcb, Nkg7, Spn), T cell activators (Prkcq, Tagap) and inhibitors (Ptprc, Ptpn22), dendritic cell marker Itgax (or Cd11c), genes promoting cytokine production (Themis2, Fgr, Trem1), and Rnf144b, whose product functions in major histocompatibility complex (MHC) class I antigen processing and presentation (Figures 5 and S5).
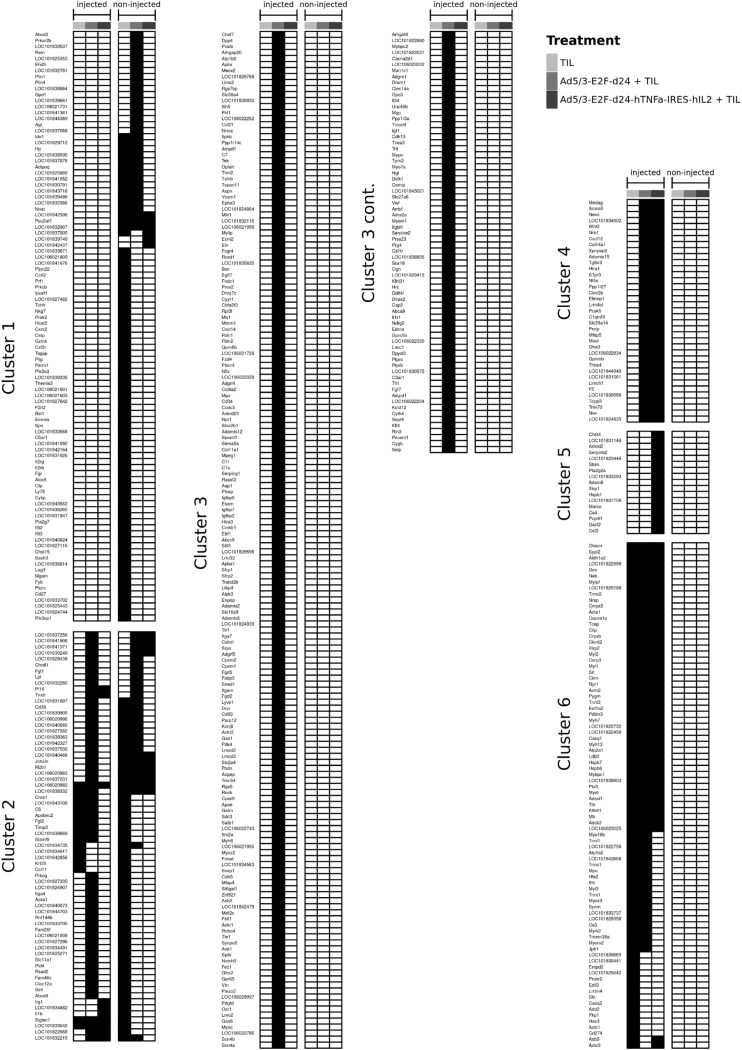
Figure 5.
List of Genes Upregulated over 2-Fold Compared with Corresponding Mock Group
Genes in cluster 1 are upregulated only in non-injected tumors, genes in cluster 2 are upregulated in both tumors, genes in cluster 3 are upregulated with Ad5/3-E2F-d24 injection, genes in cluster 4 are upregulated with either virus, genes in cluster 5 are upregulated with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 only, and genes in cluster 6 with any injection. Black box indicates over 2-fold upregulation.
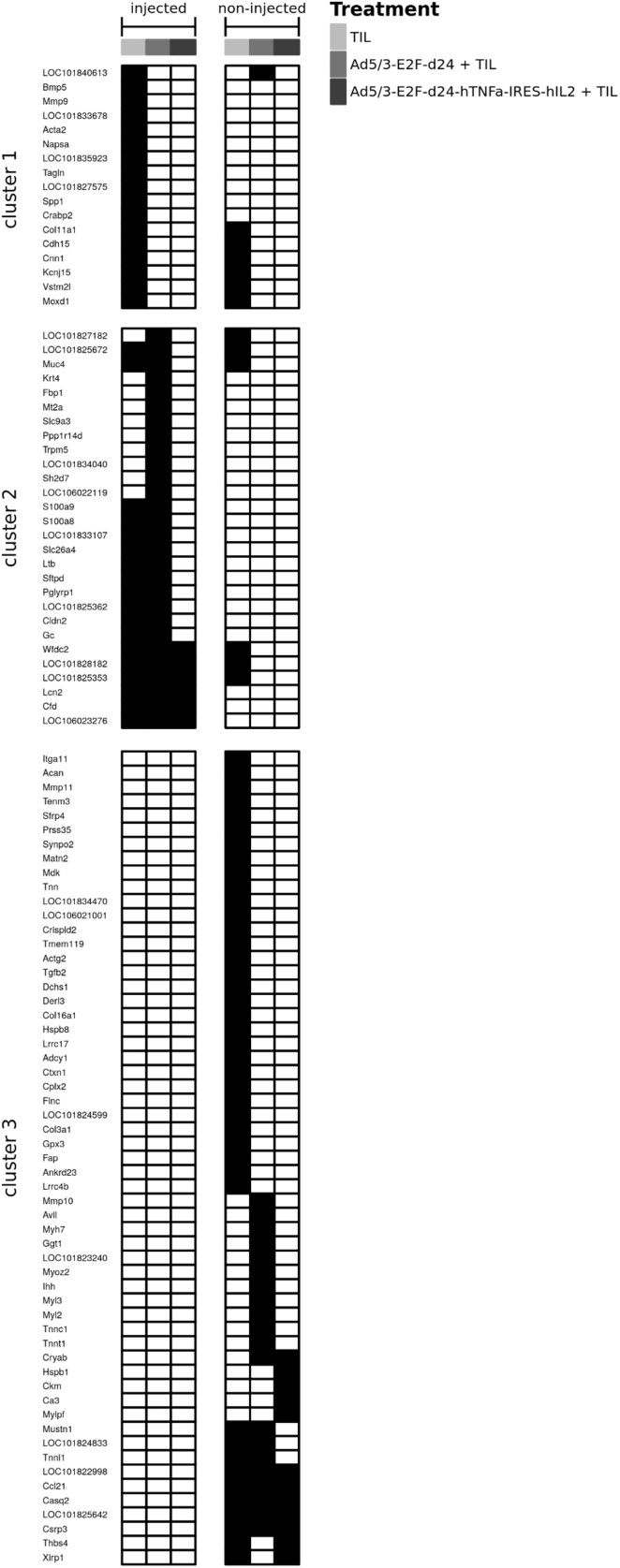
Figure 6.
List of Genes Downregulated over 2-Fold Compared with Corresponding Mock Group
Genes in cluster 1 are downregulated in TIL group, genes in cluster 2 are downregulated with a virus injection, and genes in cluster 3 are downregulated only in non-injected tumors. Black box indicates over 2-fold downregulation.
Virus Injection Induces the Upregulation of Genes Related to Both Innate and Adaptive Immunity
Out of immunologically relevant genes, injection with either virus induced the expression of macrophage marker Scara5, chemokine gene Cxcl12, lymphocyte differentiation marker Nt5e, and two genes, Retnla and Retnlb, related to T cell and dendritic cell recruitment, respectively. In addition, we saw upregulation of T cell inhibitor Ido1 in non-injected tumors in the group treated with the unarmed virus and TILs. In addition, as seen in mice, virus injection was able to upregulate NK cell activation markers and dendritic cell differentiation (Figure S5).
The unarmed virus induced the upregulation of the highest number of genes. Among the genes upregulated in the unarmed group, there were several T cell markers (Itm2a, Prkcq, Dpp4, Aplnr), B cell-related genes (Jchain, Ebf1, Fgd2, Mzb1), chemokines and their receptors (Ccl21, Ackr1, Ccr5, Cmklr1, Cxcl14), complement system-related genes (C1s, C1r, C3ar1 C4, C6, C7), and antigen presentation-related genes (B2m, Tap1, Tap2, Tapbp; Figure S4). Interestingly, Ad5/3-E2F-d24 also upregulated genes that are required for lymphocyte migration and invasion, such as Vcam1, Pecam1, and Esam, in addition to CD34 and Sell, coding for the binding partner of CD34 on T cells, L-selectin. Another interesting detail unique to the unarmed virus was the upregulation of genes related to viral processes, for example, antiviral Rsad2 (Figure S5). In addition, we saw downregulation of inflammation-related genes such as S100a9, S100a8, Orm1, Ltb (or Tnfc), and Gc (Figures 6 and S6).
Armed Adenovirus Induces Chemokine Expression in Both Injected and Non-injected Tumors
Tumors injected with armed virus showed unique upregulation of cytokine genes Csf3 and Il1b. In addition, we saw an upregulation of macrophage-related gene Marco, and Ackr3 coding for an atypical chemokine receptor. In the same group, the non-injected tumors showed upregulation of immunoglobulin lambda- and kappa-like genes. When expanding the observations to all significantly differentially expressed genes present both in injected and non-injected tumors uniquely in this group, we observed upregulation of Cxcl5 coding for TNF-α-inducible chemokine and Rnase2 that attracts dendritic cells (Table S2). Furthermore, we saw upregulation of the following genes related to immune reactions: Ier3 that has functions in TNF-α-stimulated apoptosis and T cell apoptosis inhibition; Lrmp, whose protein product delivers peptides to MHC class I molecules; B cell regulator Rgs13; and Sult1e1 that is involved in inflammatory-response regulation. By contrast, immune-reaction-related genes that were downregulated in this group in both tumors were Jak3 (mediates IL-2R signaling in T cells and NK cells) and three genes coding for parts of MHC class II complex (Table S2).
Because the double-armed virus had more prominent antitumor abscopal effects on the non-injected tumors in comparison to the same virus without arming devices, it was of interest to study those genes that changed only in non-injected tumors, and only when the double-armed virus was used for injection of the other tumor. In theory, this could allow dissection of the abscopal effects of oncolysis from the effects of the transgenes. However, only a few such genes were identified (upregulation of LOC101839749 and LOC101842437, annotated as immunoglobulin lambda-1 light chain-like and immunoglobulin kappa variable 4-1-like, respectively, and downregulation of Ca3, Mylpf, Ckm, and Hspb1), but they are currently of unknown significance in the context of immunotherapy. Further cell biology research could eventually help understand the role of these transcripts in our findings.
Discussion
Cytokines, such as TNF-α and IL-2, are potent inducers of antitumor immunity. TNF-α has both direct and indirect effects on cancer cells by inducing necrosis and apoptosis, but also inducing immunologic reactions via acute inflammation.21 Recombinant TNF-α is routinely used in isolated limb perfusion of, for example, sarcoma and melanoma, but it is too toxic to be used systemically.22, 23 With adenoviral delivery, high local concentration of TNF-α and IL-2 is achievable without significant systemic exposure.17, 18 IL-2 functions as a T cell propagator and activator, and it is used as a treatment for melanoma and renal cell carcinoma. IL-2 is also included in many adoptive cell therapy trials, especially in TIL therapy and in many solid tumor trials with chimeric antigen receptor T cells (CART) or with receptor-modified T cells (TCR).24, 25, 26 Again, vectored delivery can achieve the beneficial effects of IL-2 without systemic toxicity.13 Here, we inserted TNF-α and IL-2 into an oncolytic adenovirus and studied the systemic antitumor effects of Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 with adoptive T cell transfer.
Oncolytic adenoviruses whose capsid is a chimera between serotype 5 and serotype 3 appear useful with regard to systemic delivery. In line with the results obtained in this study, we have previously shown in both laboratory animals and humans that virus administered intratumorally or intravenously can transduce distant metastases.11, 27 It has even been possible to grow out the treatment virus from non-injected brain metastases of a cancer patient.11 Injected and non-injected tumors were transduced to the same degree in patients. Thus, the 5/3 chimeric platform is appealing for achieving systemic effects also through viral transduction, in addition to immunological effects.
Consistent with previous results, the oncolytic virus studied here could be detected in both injected and non-injected tumors. The virus spread was linked to replication, because the replication-incompetent viruses were not found in non-injected tumors. Oncolytic virus levels were higher in injected tumors than in non-injected tumors, but the presence in non-injected tumors in immune-competent hosts constitutes an important proof-of-concept. Moreover, the level of unarmed virus in injected tumors was higher than that of armed viruses, suggesting more extensive immunogenicity of the armed viruses. Because the viruses were found in non-injected tumors, it was not surprising to find them also in normal tissues. The selectivity of the virus, however, is not at the level of entry, but on the level of replication.28 Because the virus does not replicate in normal cells, these cells are not damaged and no adverse events are seen.17 In fact, higher DNA copy number can sometimes be seen in normal human tissues than in tumors.11
In contrast with oncolytic adenovirus, the first oncolytic virus approved by authorities in the United States and European Union (EU), T-Vec, has not been detected from non-injected tumors in humans or in animals.29 Nevertheless, T-Vec induces immunological responses in distant tumors, even though at a lower level.6, 7 In the phase 3 OPTIM trial, T-Vec resulted in a 26% response rate in injected tumors and 15% in non-injected visceral metastases.6 Because our approach induced similar efficacy in injected and in non-injected tumors, one can speculate that overall clinical benefits could be more pronounced if the virus is capable of transducing non-injected tumors. Thus, there is a major difference between oncolytic herpes type 1 and oncolytic 5/3 chimeric adenovirus, and this difference is potentially very important clinically. The former can produce systemic effects through the immune system, whereas the latter can achieve body-wide effects though two mechanisms: (1) viral dissemination through the vasculature, transduction of distant tumors followed by transgene expression, and oncolysis; and (2) systemic immunological effects. Considering there are several oncolytic viruses in development, it is clear that they are not all alike with regard to the mechanisms of action and systemic efficacy.
As a result of virus spread into non-injected tumors, there were no significant differences between the sizes of injected and non-injected tumors in this study. Moreover, cytokine-armed viruses influenced circulating T cell graft seen as increased trafficking into distant tumors, both with oncolytic and replication-incompetent viruses. The experiment with replication-incompetent viruses without T cell therapy highlighted the importance of the transgenes in inducing the systemic antitumor effects. Even in the absence of oncolysis, the replication-incompetent viruses were able to inhibit the growth of the non-injected tumors when armed with TNF-α and IL-2. Moreover, with oncolytic viruses, the unarmed virus was not able to induce as strong antitumor effects as the armed viruses in the non-injected tumors despite higher copy number. The results are in line with our previous results with the same constructs: TNF-α and IL-2 are necessary for enabling curative treatment with TIL therapy and inducing immunological memory against tumor rechallenge.17 Here, the two different animal models, hamsters and mice, both suggest that the transgenes have an importance in inducing systemic antitumor effects.
Due to limited availability of hamster-specific or cross-reactive reagents, a mouse model provided us a means to study immune cell compartments in tumors, having the focus on innate immunity. Interestingly, treating just one tumor induced immune cell infiltration also into the non-injected tumor. Moreover, the presence of immune cells in tumors did not correlate with tumor sizes, suggesting that the treatment influences tumor microenvironment regardless of the volume. The clearest difference was seen with NK cells that are known to attack cells with low MHC class I expression, such as B16-OVA.30, 31 The presence of any virus induced NK levels in the injected tumors, but an arming device was required for NK cell induction in the non-injected tumor. Interestingly, also Balasa et al.32 reported the importance of the combination of IL-2 and TNF-α with regard to NK activation.
In addition to NK cells, IL-2 stimulated the presence of dendritic cells expressing the maturation marker CD86. Moreover, the presence of mature dendritic cells positively correlated with higher numbers of NK cells in the tumor. Crosstalk between NK cells and dendritic cells has an interesting role in both innate and adaptive immunity. NK cells promote tumor antigen presentation by dendritic cells, but at the same time, CD11c-positive dendritic cells are required for NK cell priming.33, 34 Moreover, NK cells induce dendritic cell maturation both via cell-cell contacts and via secretion of TNF-α and interferon gamma.35 NK cells additionally secrete chemokines, such as MIP-1α, MIP-1β, and RANTES, which attract dendritic cells.36
In addition to inducing dendritic cell maturation, TNF-α is capable of suppressing the M2 macrophage phenotype.37 Immunosuppressive M2-like macrophages are known to associate with poor survival in cancer patients, and cancer cells appear to attempt to drive macrophage differentiation toward this phenotype.38, 39 We saw, however, a relative decrease in this subtype of macrophages when tumors were injected with armed viruses even though the overall macrophage percentage was increased in both tumors. The effect was most noticeable with TNF-α-coding virus. A similar trend was seen in gene expression profiles in hamster tumors where 12 out of 19 genes specific for M1 macrophages according to Kratochvill et al.37 were upregulated in tumors treated with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2. Likewise, the expression of 13 out of 20 genes that are linked to M2 phenotype was absent in the tumors.
When interpreting gene expression data, it is important to extract the effects derived from the delivery of the drug. We have previously observed that injection with saline causes immunological reactions in tumors.40 This phenomenon was confirmed in our observations with the upregulation of immune-reaction-related genes caused by injection of PBS. To control the effect of PBS injection, we compared the gene expression profiles of the virus-injected tumors with the saline-injected tumors and the non-injected tumors with the non-injected tumors in the vehicle group. Most changes in gene expression profile occurred in the group treated with the unarmed virus. Because this virus causes fewer immune responses toward tumor cells that allow replication, oncolysis is more prominent, and therefore the majority of the expression changes seen are due to this phenomenon. For example, the virus upregulated many of the complement system components, suggesting that the unarmed virus might have induced more antiviral immune responses than the armed virus.
The cytokines seemed to influence the trafficking and the persistence of the transferred cells in both injected and non-injected tumors, regardless of oncolysis. Previously, increased chemokine expression in the tumors treated with replication-incompetent adenoviruses armed with TNF-α and IL-2 was linked to increased T cell trafficking in mice.18 Here, we studied the effect of oncolytic viruses on chemotactic genes and genes promoting leukocyte proliferation and survival in both injected and non-injected tumors. Interestingly, many of these, such as Cxcl5, Rnase2, and Ier3, were uniquely upregulated in the group treated with Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2.
In addition to chemokines, we saw upregulation of a set of genes related to different immune cell compartments, invasion of T cells through the vessels, and antigen presentation. Recently, Patel et al.41 reported extensive analysis of genes important for responses to immunotherapy in cancer. Interestingly, the same genes related to MHC class I antigen processing and presentation (B2m, Tap1, Tap2, and Tapbp) were upregulated in tumors injected with Ad5/3-E2F-d24 and in animals treated with TILs only. Moreover, they discovered that loss-of-function mutations in Aplnr, coding for apelin receptor, reduce the effectiveness of T cell therapies, including anti-CTLA4 blockade. Of note, in our dataset, Aplnr was upregulated in all the injected tumors and in the non-injected tumors in the group that received Ad5/3-E2F-d24. Of course, the efficacy of an immunotherapeutic does not depend on the expression of a single gene, but is a matter of inducing the right set of genes. Nevertheless, the correlation of our data with Patel’s data is tantalizing, and because they used different methodology and made the observations from human data, the identified genes seem indeed relevant for cellular immunotherapy.
To conclude, our study demonstrates systemic effects induced by local injection of Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2. The virus was able to spread to non-injected tumors, while the experiments with replication-incompetent viruses pointed out the importance of the arming device. Together, the oncolytic replication and the arming devices induced upregulation of genes essential for successful immunotherapy. Thus, an abscopal effect was seen and it was caused by two synergistic phenomena: (1) viral dissemination through the bloodstream into distant tumors, followed by oncolysis, and (2) systemic immune response. Taken together with previous reports, the findings presented here underline the rationale for using Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 as an enabler of T cell therapies and checkpoint inhibitors. A clinical trial, where the virus is used in patients receiving adoptive TIL therapy, is in progress.
Materials and Methods
Cell Lines and Viruses
All cells lines were maintained under recommended conditions and tested to be pathogen-free. In mouse experiments, we used mouse melanoma cell line B16-OVA, a kind gift from Prof. Richard Vile (Mayo Clinic, Rochester, MN, USA), and replication-deficient adenoviruses Ad5-CMV-mIL-2, Ad5-CMV-mTNF-α, and Ad5-Luc1.18, 42 Oncolytic adenoviruses Ad5/3-E2F-d24, Ad5/3-E2F-d24-hTNF-α, Ad5/3-E2F-d24-hIL-2, and Ad5/3-E2F-d24-hTNF-α-IRES-IL-217 were studied with hamster pancreatic cancer cell line HapT1 (DSMZ, Braunschweig, Germany).
Hamster Experiments
Oncolytic adenoviruses armed with human TNF-α and IL-2 were studied in Syrian hamsters (Envigo). 4 × 106 HapT1 cells were implanted on both flanks of the animals and allowed to develop for 7 days. Tumors were treated with 1 × 108 viral particles (VPs) intratumorally once a week and once with 5 × 107 HapT1-derived TILs administered intraperitoneally. As a control, one of the two tumors in the TIL group was treated with PBS, whereas the mock tumors were left untreated. The extraction of TILs is described previously.17, 43 Four animals per group were sacrificed after two virus treatments on day 16, and tumors, heart, lung, liver, spleen, and kidney were collected from each animal to detect biodistribution of the virus with qPCR. Tumor sizes on the rest of the animals (5–6 per group) were followed for 122 days.
Mouse Experiments
The Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland approved the animal experiments performed in this study. To investigate the effects of the adenovirally delivered transgenes, two B16-OVA tumors (2.5 × 105 cells each) were implanted into the flanks of C57BL/6 mice (Envigo, Cambridgeshire, UK). When the tumor size reached approximately 5 mm 10 days after the implantation, the animals were randomized into groups of 8–11 and treated intratumorally on days 1 and 4 with 1 × 109 VPs for single viruses or 0.5 × 109 VPs each when the virus coding for mIL-2 and mTNF-α were combined. The vehicle control group received PBS. CD8-enriched OVA-specific OT-I cells were processed as described previously40 and administered 1.4 × 106 cells per animal intraperitoneally on day 1. The animals were sacrificed a week after the treatments, and tumors were collected for detecting the spread of the virus and the immune cell contents.
To investigate OT-I cell trafficking in mice, we labeled the cells with Qtracker 565 Cell Labeling Kit (Thermo Fisher Scientific, Waltham, MA, USA). The animals received intraperitoneally 7.6 × 105 cells, out of which 14% showed a positive fluorescent signal. One of the two B16-OVA tumors was injected with 50 μL of PBS, 1 × 109 VPs of Ad5-Luc1 or Ad5-CMV-mTNF-α and Ad5-CMV-mIL-2 in a one-to-one ratio on days 1 and 3. Tumors were collected on day 6 and analyzed for fluorescein isothiocyanate (FITC)+ CD8+ cells, representing the transferred OT-I cells.
SPECT/CT Imaging
TILs were labeled with 111In-oxine and administered intraperitoneally into Syrian hamsters (5 × 107 cells, 5.82 ± 0.73 MBq) bearing two HapT1 tumors (n = 4/group). One of the two tumors received 1 × 108 VPs of Ad5/3-E2F-d24, Ad5/3-E2F-d24-TNF-α-IRES-IL-2, or 50 μL of PBS as a control on day 0, and the animals were imaged with NanoScan SPECT/CT (Mediso, Budapest, Hungary) at 48, 72, and 96 hr after the administration of the cells. The results are reported as standardized uptake values (SUVs), which were calculated using the average radioactivity concentration in the whole tumor normalized with the injected radioactivity dose and the animal weight. The tumors were delineated by using the co-registered CT images.
Gene Expression Profiling
To dissect the mechanisms of action on the gene expression level, we performed mRNA sequencing. Two of four subcutaneously established HapT1 tumors received virus and TIL treatments as described above. On day 10, 2 days after the last treatment, tumors were collected and stored in RNAlater (AM7020; Life Technologies, Thermo Fisher Scientific) before extracting RNA with RNeasy Mini Kit (74104; QIAGEN, Valencia, CA, USA). The library for sequencing was prepared with NEBNext Ultra Directional RNA Library Prep Kit 3 (E7420S; New England Biolabs, Ipswich, MA, USA) before performing single-end Illumina NextSeq High Output 1 × 75 bp sequencing (FC-404-2005; Illumina, San Diego, CA, USA).
The quality of the acquired data was analyzed with FastQ and summarized with MultiQC.45, 46 Light quality trimming was performed with Trimmomatic.47 The sample reads were aligned and annotated against RefSeq GCF_000349665.1_MesAur1.0_genomic reference and quantified with featureCounts.48 Expression profiles of injected and non-injected tumors in the treatment groups were compared with corresponding tumors in the vehicle group, and the statistics for differentially expressed genes were calculated with DESeq2.49 For each set of differentially expressed genes, obtained by comparing the treated group against the corresponding mock group (fold change > 1, p < 0.05), gene ontology analysis was performed against human orthologs with the WebGestalt toolkit.50 The human orthologs were retrieved from the NCBI database.51, 52
In addition, the expression of TGF-β and FoxP3 were assessed from the RNA samples by qRT-PCR. The RNA was transcribed to cDNA with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) according to manufacturer’s instructions. The primer and probe sequences were adopted from Zivcec et al.,53 and the results were normalized against GAPDH housekeeping gene as previously described.44
Flow Cytometry
Dendritic cells, macrophages, NK cells, and melanoma marker (Trp2, gp100, and OVA)-specific T cells were detected from mouse tumor samples with flow cytometry as described by Tähtinen et al.54
Virus Spread into Organs and Untreated Tumors
DNA was extracted from 25-mg tissue samples with QIAmp DNA Mini Kit (51326; QIAGEN), and the QIAcube machine according to the manufacturer’s protocol. Adenoviral E4 was detected by qPCR as described earlier and normalized against hamster GAPDH or mouse β-actin gene expression.42, 44
Statistics
Tumor growth curves were analyzed with mixed model analysis in IBM SPSS Statistics version 22.0.0.1 (IBM, Armonk, NY, USA). Other calculations were made with GraphPad Prism 7.03 (La Jolla, CA, USA). The survival benefit was evaluated with log rank test, and the other results were analyzed with ANOVA, Kruskal-Wallis test, or unpaired t test. All tests were performed as two-sided.
Author Contributions
Conceptualization, R.H., M.S., S.S., S.T., and A.H.; Methodology, R.H., T.R., D.L., and A.J.A.; Investigation, R.H., J.M.S., S.S., D.L., and V.C.-C.; Formal Analysis, R.H. and T.R.; Writing – Original Draft, R.H.; Writing – Review & Editing, R.H., J.M.S., S.S., T.R., D.L., M.S., A.J.A., V.C.-C., S.T., A.K., and A.H.; Funding Acquisition, A.K. and A.H.; Resources, A.J.A. and A.H.; Supervision, A.H.
Conflicts of Interest
A.H. is a shareholder in Targovax ASA. A.H. is an employee and shareholder in TILT Biotherapeutics Ltd. R.H., S.S., M.S., J.M.S., and V.C.-C. are employees of TILT Biotherapeutics Ltd.
Acknowledgments
The authors thank Minna Oksanen and Susanna Grönberg-Vähä-Koskela for expert assistance. FACS Core Facility (Biomedicum Helsinki, Finland) provided the facilities and the technical assistance for flow cytometry, and Functional Genomics Unit FuGU (Biomedicum Helsinki, Finland) performed the mRNA sequencing and the related data analysis. SPECT/CT Imaging Core at Centre for Drug Research (Helsinki, Finland) is acknowledged for providing the facilities and expertise for imaging. The study was supported by University of Helsinki Doctoral Programme in Clinical Research, Cancer Foundation Finland, Jane and Aatos Erkko Foundation, HUCH Research Funds (EVO), Sigrid Jusélius Foundation, Finnish Cancer Organizations, University of Helsinki, TILT Biotherapeutics Ltd., and European Commission Marie Curie Innovative Training Network (ITN) grant VIRION (H2020-MSCA-ITN-2014 project number 643130).
Footnotes
Supplemental Information includes six figures and two tables and can be found with this article online at https://doi.org/10.1016/j.omto.2018.10.005.
Supplemental Information
References
- 1.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18:43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gedye C., van der Westhuizen A., John T. Checkpoint immunotherapy for cancer: superior survival, unaccustomed toxicities. Intern. Med. J. 2015;45:696–701. doi: 10.1111/imj.12653. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz R.N., Stover L., Dutcher J.P. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- 4.Howells A., Marelli G., Lemoine N.R., Wang Y. Oncolytic viruses-interaction of virus and tumor cells in the battle to eliminate cancer. Front. Oncol. 2017;7:195. doi: 10.3389/fonc.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimäki A. Phase I study with ONCOS-102 for the treatment of solid tumors—an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman H.L., Amatruda T., Reid T., Gonzalez R., Glaspy J., Whitman E., Harrington K., Nemunaitis J., Zloza A., Wolf M., Senzer N.N. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J. Immunother. Cancer. 2016;4:12. doi: 10.1186/s40425-016-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl. Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., Mu Z., Rasalan T., Adamow M., Ritter E. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vacchelli E., Bloy N., Aranda F., Buqué A., Cremer I., Demaria S., Eggermont A., Formenti S.C., Fridman W.H., Fucikova J. Trial Watch: immunotherapy plus radiation therapy for oncological indications. OncoImmunology. 2016;5:e1214790. doi: 10.1080/2162402X.2016.1214790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koski A., Bramante S., Kipar A., Oksanen M., Juhila J., Vassilev L., Joensuu T., Kanerva A., Hemminki A. Biodistribution analysis of oncolytic adenoviruses in patient autopsy samples reveals vascular transduction of noninjected tumors and tissues. Mol. Ther. 2015;23:1641–1652. doi: 10.1038/mt.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesonen S., Diaconu I., Kangasniemi L., Ranki T., Kanerva A., Pesonen S.K., Gerdemann U., Leen A.M., Kairemo K., Oksanen M. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 13.Santos J.M., Havunen R., Siurala M., Cervera-Carrascon V., Tähtinen S., Sorsa S., Anttila M., Karell P., Kanerva A., Hemminki A. Adenoviral production of interleukin-2 at the tumor site removes the need for systemic postconditioning in adoptive cell therapy. Int. J. Cancer. 2017;141:1458–1468. doi: 10.1002/ijc.30839. [DOI] [PubMed] [Google Scholar]
- 14.Hemminki O., Parviainen S., Juhila J., Turkki R., Linder N., Lundin J., Kankainen M., Ristimäki A., Koski A., Liikanen I. Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6:4467–4481. doi: 10.18632/oncotarget.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg A.D., Galluzzi L., Apetoh L., Baert T., Birge R.B., Bravo-San Pedro J.M., Breckpot K., Brough D., Chaurio R., Cirone M. Molecular and translational classifications of DAMPs in immunogenic cell death. Front. Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaconu I., Cerullo V., Hirvinen M.L., Escutenaire S., Ugolini M., Pesonen S.K., Bramante S., Parviainen S., Kanerva A., Loskog A.S. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 17.Havunen R., Siurala M., Sorsa S., Grönberg-Vähä-Koskela S., Behr M., Tähtinen S., Santos J.M., Karell P., Rusanen J., Nettelbeck D.M. Oncolytic adenoviruses armed with tumor necrosis factor alpha and interleukin-2 enable successful adoptive cell therapy. Mol. Ther. Oncolytics. 2016;4:77–86. doi: 10.1016/j.omto.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siurala M., Havunen R., Saha D., Lumen D., Airaksinen A.J., Tähtinen S., Cervera-Carrascon V., Bramante S., Parviainen S., Vähä-Koskela M. Adenoviral delivery of tumor necrosis factor-α and interleukin-2 enables successful adoptive cell therapy of immunosuppressive melanoma. Mol. Ther. 2016;24:1435–1443. doi: 10.1038/mt.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tähtinen S., Blattner C., Vähä-Koskela M., Saha D., Siurala M., Parviainen S., Utikal J., Kanerva A., Umansky V., Hemminki A. T-cell therapy enabling adenoviruses coding for IL2 and TNFα induce systemic immunomodulation in mice with spontaneous melanoma. J. Immunother. 2016;39:343–354. doi: 10.1097/CJI.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 20.Thomas M.A., Spencer J.F., La Regina M.C., Dhar D., Tollefson A.E., Toth K., Wold W.S. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 21.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 22.Schiller J.H., Storer B.E., Witt P.L., Alberti D., Tombes M.B., Arzoomanian R., Proctor R.A., McCarthy D., Brown R.R., Voss S.D. Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Res. 1991;51:1651–1658. [PubMed] [Google Scholar]
- 23.Eggermont A.M., de Wilt J.H., ten Hagen T.L. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–437. doi: 10.1016/s1470-2045(03)01141-0. [DOI] [PubMed] [Google Scholar]
- 24.Itzhaki O., Levy D., Zikich D., Treves A.J., Markel G., Schachter J., Besser M.J. Adoptive T-cell transfer in melanoma. Immunotherapy. 2013;5:79–90. doi: 10.2217/imt.12.143. [DOI] [PubMed] [Google Scholar]
- 25.Dudley M.E., Yang J.C., Sherry R., Hughes M.S., Royal R., Kammula U., Robbins P.F., Huang J., Citrin D.E., Leitman S.F. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramante S., Kaufmann J.K., Veckman V., Liikanen I., Nettelbeck D.M., Hemminki O., Vassilev L., Cerullo V., Oksanen M., Heiskanen R. Treatment of melanoma with a serotype 5/3 chimeric oncolytic adenovirus coding for GM-CSF: results in vitro, in rodents and in humans. Int. J. Cancer. 2015;137:1775–1783. doi: 10.1002/ijc.29536. [DOI] [PubMed] [Google Scholar]
- 28.Kanerva A., Zinn K.R., Chaudhuri T.R., Lam J.T., Suzuki K., Uil T.G., Hakkarainen T., Bauerschmitz G.J., Wang M., Liu B. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol. Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 29.Moesta A.K., Cooke K., Piasecki J., Mitchell P., Rottman J.B., Fitzgerald K., Zhan J., Yang B., Le T., Belmontes B. Local delivery of OncoVEXmGM-CSF generates systemic antitumor immune responses enhanced by cytotoxic T-lymphocyte-associated protein blockade. Clin. Cancer Res. 2017;23:6190–6202. doi: 10.1158/1078-0432.CCR-17-0681. [DOI] [PubMed] [Google Scholar]
- 30.Geller M.A., Miller J.S. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3:1445–1459. doi: 10.2217/imt.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seliger B., Wollscheid U., Momburg F., Blankenstein T., Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61:1095–1099. [PubMed] [Google Scholar]
- 32.Balasa B., Yun R., Belmar N.A., Fox M., Chao D.T., Robbins M.D., Starling G.C., Rice A.G. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol. Immunother. 2015;64:61–73. doi: 10.1007/s00262-014-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deauvieau F., Ollion V., Doffin A.C., Achard C., Fonteneau J.F., Verronese E., Durand I., Ghittoni R., Marvel J., Dezutter-Dambuyant C. Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. Int. J. Cancer. 2015;136:1085–1094. doi: 10.1002/ijc.29087. [DOI] [PubMed] [Google Scholar]
- 34.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccioli D., Sbrana S., Melandri E., Valiante N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorner B.G., Smith H.R., French A.R., Kim S., Poursine-Laurent J., Beckman D.L., Pingel J.T., Kroczek R.A., Yokoyama W.M. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J. Immunol. 2004;172:3119–3131. doi: 10.4049/jimmunol.172.5.3119. [DOI] [PubMed] [Google Scholar]
- 37.Kratochvill F., Neale G., Haverkamp J.M., Van de Velde L.A., Smith A.M., Kawauchi D., McEvoy J., Roussel M.F., Dyer M.A., Qualls J.E., Murray P.J. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12:1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannenmann S.R., Thielicke J., Stöckli M., Matter C., von Boehmer L., Cecconi V., Hermanns T., Hefermehl L., Schraml P., Moch H. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. OncoImmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa S., Brion R., Lintunen M., Kronqvist P., Sandholm J., Monkkonen J., Kellokumpu-Lehtinen P.L., Lauttia S., Tynninen O., Joensuu H. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101. doi: 10.1186/s13058-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tähtinen S., Grönberg-Vähä-Koskela S., Lumen D., Merisalo-Soikkeli M., Siurala M., Airaksinen A.J., Vähä-Koskela M., Hemminki A. Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol. Res. 2015;3:915–925. doi: 10.1158/2326-6066.CIR-14-0220-T. [DOI] [PubMed] [Google Scholar]
- 41.Patel S.J., Sanjana N.E., Kishton R.J., Eidizadeh A., Vodnala S.K., Cam M., Gartner J.J., Jia L., Steinberg S.M., Yamamoto T.N. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–542. doi: 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanerva A., Wang M., Bauerschmitz G.J., Lam J.T., Desmond R.A., Bhoola S.M., Barnes M.N., Alvarez R.D., Siegal G.P., Curiel D.T., Hemminki A. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol. Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 43.Siurala M., Vähä-Koskela M., Havunen R., Tähtinen S., Bramante S., Parviainen S., Mathis J.M., Kanerva A., Hemminki A. Syngeneic syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1136046. e1136046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koski A., Kangasniemi L., Escutenaire S., Pesonen S., Cerullo V., Diaconu I., Nokisalmi P., Raki M., Rajecki M., Guse K. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews, S. (2010). FastQC—a quality control tool for high throughput sequence data. Babraham Bioinformatics. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 46.Ewels P., Magnusson M., Lundin S., Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 49.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B., Kirov S., Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2017;45(D1):D12–D17. doi: 10.1093/nar/gkw1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown G.R., Hem V., Katz K.S., Ovetsky M., Wallin C., Ermolaeva O., Tolstoy I., Tatusova T., Pruitt K.D., Maglott D.R., Murphy T.D. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 2015;43:D36–D42. doi: 10.1093/nar/gku1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zivcec M., Safronetz D., Haddock E., Feldmann H., Ebihara H. Validation of assays to monitor immune responses in the Syrian golden hamster (Mesocricetus auratus) J. Immunol. Methods. 2011;368:24–35. doi: 10.1016/j.jim.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tähtinen S., Kaikkonen S., Merisalo-Soikkeli M., Grönberg-Vähä-Koskela S., Kanerva A., Parviainen S., Vähä-Koskela M., Hemminki A. Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS ONE. 2015;10:e0131242. doi: 10.1371/journal.pone.0131242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.