Introduction
Stroke survivors with language difficulties (aphasia) vary: some recover quickly while others suffer long-term impairments, and different patients respond differently to the same speech and language therapies.1 In recent years, we and others have shown that much of the variability in language outcomes after stroke can be explained by reference to the details of the brain damage that individual patients have suffered.2 Here, we show that this same information can be used to predict responses of patients who had stroke to treatment.
Methods
Detailed methods are provided in online supplementary material: here, we summarise the key points. Our focus is on a novel treatment for Central Alexia (CA): an acquired reading disorder in the context of a general language impairment (aphasia). Patients with CA are slow to read, make frequent errors and have additional problems with spoken and written language. Our intervention is a computerised therapy embodied in an application called ‘iReadMore’, which uses multimodal cueing and massed practice to improve patients’ single-word reading skills.3
jnnp-2017-317446supp001.pdf (375.9KB, pdf)
Our study included 23 participants with CA after left hemisphere stroke (see online supplementary table), recruited through both the PLORAS project2 and the outpatient speech and language therapy services at the National Hospital for Neurology and Neurosurgery, University College London Hospitals. Before the treatment began, each participant’s cognitive skills were assessed with an extensive protocol including linguistic and non-linguistic tests, yielding a total of 28 pretreatment behavioural variables per patient. We also acquired structural MRI from each patient, extracting lesion images using the Automatic Lesion Identification toolbox.4 The outputs (binary lesion images) were encoded as the proportion that each lesion destroyed, of a series of 69 anatomically defined regions of interest. With four demographic variables (age at therapy onset, time since stroke, sex and lesion volume), we had a total of 101 pretreatment variables to consider; our dependent variable was absolute change in the participants’ single-word reading skills.
We ran two analyses with these data. Our first, ‘explanatory’ (in-sample) analysis quantified the relative utility of: (1) behavioural and demographic data and (2) lesion location data, in explaining individual patients’ responses to treatment (ie, improvement in single-word reading accuracy). We did this by fitting linear models using each set of variables, both separately and in combination, using the Automatic Linear Modelling (ALM) facility distributed with the SPSS software package. Our second, ‘predictive’ (out-of-sample) analysis asked whether the pretreatment data could be used to predict responses in new patients. We did this by embedding the ALM process within a cross-validation process.
Results
Analysis 1: explanatory (in-sample) analysis
The Aikaike Information Criterion (AIC) for the model derived from behavioural and demographics data alone was higher (worse) than that selected from lesion data alone (81.75 vs 69.35), and the model selected from all of the data together was better still (AIC=47.98). Bayes factors corresponding to these differences were 493 (behavioural and demographics vs lesions) and 43 696, respectively (all data together vs lesions alone): that is, very strong evidence both (1) that lesion data explain more of the variance in the patients’ treatment responses than the behavioural and demographic data alone and (2) that the combination of the data yields a better explanation than either set alone. Only the ‘neuroimaging only’ and ‘combined data’ models were significant, as assessed relative to null distributions of regression coefficients derived when the same models were regressed against 1000 permutations of the treatment responses (ie, actual R2>95% of null statistics).
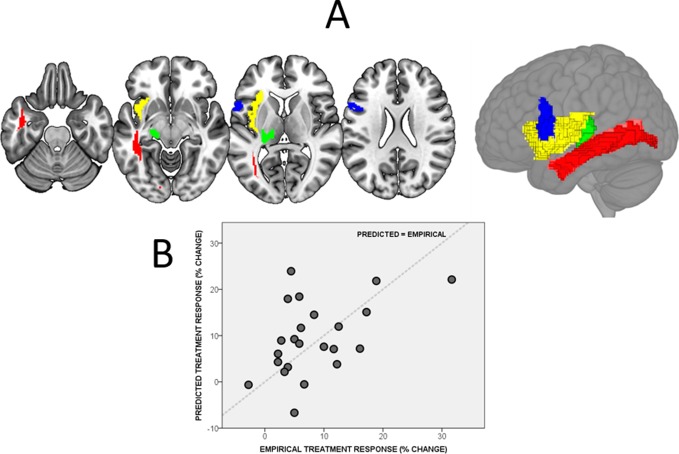
The best, explanatory model that we found included: (1) age at therapy onset; (2) accuracy in the Neale reading test; (3) comprehension in the Neale reading test; (4) accuracy in the written semantic matching task; (5) damage to the white matter connecting the thalamus to the parietal cortex and damage to left (6) Broca’s area; (7) insula and (8) inferior longitudinal fasciculus (adjusted R2=0.94). Figure 1A displays the brain regions from this model.
Figure 1.
(A) The brain regions implicated in our combined model, displayed both on axial slices of the brain (left) at Z = −24, –8, 7 and 23 and on a rendered whole brain (right). The regions are: (a) the white matter connecting the thalamus to the parietal cortex (green); (b) the inferior longitudinal fasciculus (red); (c) Broca’s area 44 (blue) and (d) the left insula (yellow). (B) A scatter plot of predicted versus empirical treatment responses from our out-of-sample analysis. The dashed line is at y=x: perfect predictions would fall along this line.
Analysis 2: predictive (out-of-sample) analysis
Predicted treatment responses from the cross-validation analysis, using the combined demographic, behavioural and lesion location data, were significantly correlated with the patients’ empirical treatment responses (r=0.48, 95% CI 0.08, to 0.75, p=0.02); see figure 1B. Predictions driven by models using either demographic and behavioural data or lesion location data, separately, were not significantly correlated with the empirical treatment responses (both p>0.1).
Discussion
Past studies have suggested that responses to treatment for word-finding deficits (anomia) after stroke may be related to pretreatment behavioural skills5 or lesions.6 7 Our results are consistent with that work and also extend it by: (1) quantifying the relative value of pretreatment structural MRI versus demographic and behavioural data, in explaining treatment responses and (2) demonstrating that, in combination (though not separately), these pretreatment data can be used to predict new patients’ responses to treatment.
We have only considered a single therapy (iReadMore), focused on a specific aphasic deficit (CA), in a study with specific inclusion criteria at both the lesion level and the behavioural level (see online supplementary material). And though large by the standards of other similar therapy studies, our sample is still too small either to properly constrain multivariable models or to measure their predictive power precisely. So our results are necessarily preliminary.
However, they are also plausible. Cognitive neuroanatomy is specialised: the lesion damage that patients have suffered should determine both their initial symptoms and their likely potential to recover. Our best model of those treatment responses is also reasonable. Initial reading accuracy is naturally relevant to patients’ likely gains after reading therapy, and preserved semantic skills have been associated with recovery from aphasia.8 The inferior longitudinal fasciculus, the insula and Broca’s area have all been associated with reading in neuroimaging experiments,9–12 and it is at least plausible that older patients responded less well to our treatment. Finally, our predictive analysis should penalise small samples, because these will tend to maximise the variability between folds of the cross-validation process. Indeed, our results demonstrate the gulf that separates prediction from in-sample analysis: we could ‘explain’ 94% of the variance in those treatment responses, but predict only 23%. The out-of-sample effect size should grow with increasing sample size, but the in-sample effect size is clearly inflated.
In any case, we hope that these results will encourage further attempts to characterise lesion-site-dependent treatment effects and to distinguish predictable variance from noise in this area—and that further results like this will drive the development of a more personalised medicine for stroke survivors with aphasia.
Footnotes
Contributors: APL, JC, ZVJW, MFC and TMHH: designed research. APL and CJP: funding acquisition. OMA and TMHH: performed research. APL and JC: handled patient recruitment. Y-HO: software development. OMA, TMHH, SJK and ZVJW: analysed data. OMA and TMHH: wrote the paper.
Funding: This study was supported by the Medical Research Council (MR/K022563/11, MR/M023672/1), Wellcome (091593/Z/10/Z, 097720/Z/11/Z and 205103/Z/16/Z), the COLCIENCIAS Administrative Department of Science, Technology and Innovation (programme 529, 2011) and the Stroke Association (TSA PDF 2017/02)
Disclaimer: The funders had no participation in the design and results of this study.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: London Queen Square Research Ethics Committee at University College London.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Brady MC, Kelly H, Godwin J, et al. . Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2016;6:CD000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seghier ML, Patel E, Prejawa S, et al. . The PLORAS Database: A data repository for Predicting Language Outcome and Recovery After Stroke. Neuroimage 2016;124:1208–12. 10.1016/j.neuroimage.2015.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodhead ZV, Penny W, Barnes GR, et al. . Reading therapy strengthens top-down connectivity in patients with pure alexia. Brain 2013;136:2579–91. 10.1093/brain/awt186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seghier ML, Ramlackhansingh A, Crinion J, et al. . Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage 2008;41:1253–66. 10.1016/j.neuroimage.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambon Ralph MA, Snell C, Fillingham JK, et al. . Predicting the outcome of anomia therapy for people with aphasia post CVA: both language and cognitive status are key predictors. Neuropsychol Rehabil 2010;20:289–305. 10.1080/09602010903237875 [DOI] [PubMed] [Google Scholar]
- 6. Naeser MA, Baker EH, Palumbo CL, et al. . Lesion site patterns in severe, nonverbal aphasia to predict outcome with a computer-assisted treatment program. Arch Neurol 1998;55:1438–48. 10.1001/archneur.55.11.1438 [DOI] [PubMed] [Google Scholar]
- 7. Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci 2010;30:11558–64. 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fucetola R, Connor LT, Perry J, et al. . Aphasia severity, semantics, and depression predict functional communication in acquired aphasia. Aphasiology 2006;20:449–61. 10.1080/02687030500390177 [DOI] [Google Scholar]
- 9. Broca P. Remarques sur le siége de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletin de la Société Anatomique 1861;6:330–57. [Google Scholar]
- 10. Klein M, Grainger J, Wheat KL, et al. . Early Activity in Broca’s Area During Reading Reflects Fast Access to Articulatory Codes From Print. Cereb Cortex 2015;25:1715–23. 10.1093/cercor/bht350 [DOI] [PubMed] [Google Scholar]
- 11. Dronkers NF. A new brain region for coordinating speech articulation. Nature 1996;384:159–61. 10.1038/384159a0 [DOI] [PubMed] [Google Scholar]
- 12. Ripamonti E, Aggujaro S, Molteni F, et al. . The anatomical foundations of acquired reading disorders: a neuropsychological verification of the dual-route model of reading. Brain Lang 2014;134:44–67. 10.1016/j.bandl.2014.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2017-317446supp001.pdf (375.9KB, pdf)