Abstract
Objective:
To examine the association between self-reported racial discrimination and allostatic load, and whether the association differs by socioeconomic position.
Methods:
We recruited a purposive cross-section of midlife (ages 30–50) African American women residing in four San Francisco Bay area counties (n=208). Racial discrimination Was measured using the Experience of Discrimination scale. Allostatic load was measured as a comPosite of 15 biomarkers assessing cardiometabolic, neuroendocrine, and inflammatory activity. We calculated four composite measures of allostatic load and three system-specific measures of biological dysregulation. Multivariable regression was used to examine associations, while adjusting for relevant confounders.
Results:
In the high education group, reporting low (b=−1.09, P=.02, 95% CI=−1.99,−0.18) and very high (b=−1.88, P=.003, 95% CI=−3.11,−0.65) discrimination was associated with lower allostatic load (reference=moderate). Among those with lower education, reporting low (b=2.05, P=.008, 95% CI=0.55,3.56) discrimination was associated with higher allostatic load. Similar but less consistent associations were found for poverty status. Associations were similar for cardiometabolic functioning, but not for neuroendocrine or inflammatory activity.
Conclusions:
Racial discrimination may be an important predictor of cumulative physiologic dysregulation. Factors associated with educational attainment may mitigate this association for African American women and other groups experiencing chronic social stress.
Keywords: Racial Discrimination, African American, Race/ethnicity, Stress, Allostatic Load, Socioeconomic Position
1. INTRODUCTION
In the U.S., experiences of social stress associated with race and gender intersect to perpetuate and exacerbate poor health outcomes for women of color, particularly African Americans (Geronimus, 1996; Jackson, 2005; Jackson et al., 2001). African American (AA) women are disproportionately burdened by the simultaneous dysregulation of multiple physiologic systems (Chyu and Upchurch, 2011; Duru et al., 2012; Geronimus et al., 2006; Upchurch et al., 2015), commonly referred to as allostatic load or cumulative biological risk. Allostatic load has been associated with numerous negative health outcomes including decreased cognitive and physical function, heart disease, stroke, diabetes, and mortality (Beckie, 2012; Borrell et al., 2010; Habib et al., 2001; McEwen, 1998; Seeman et al., 1997). The association between social stress and cumulative biological risk is now well accepted. Though early studies focused on age-related changes in physiologic function (Crimmins et al., 2003; Karlamangla et al., 2002; Seeman et al., 2001; Seeman et al., 1997), more recent studies have demonstrated associations with explicit forms of social stress that appear to be unrelated to chronological age. In particular, numerous studies show an association between chronic (i.e., ongoing or repeated) social stress and allostatic load, which may help explain the heightened risk of poor health among AA women (Beckie, 2012; Borrell et al., 2010; Carlson and Chamberlain, 2005; Habib et al., 2001; Kessler et al., 1999; McEwen, 1998; Seeman et al., 1997).
Chronic stress plays a critical role in the progression of multiple disease states via prolonged activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympatho-adrenal-medullary (SAM) axis, the body’s primary stress response systems (Borrell et al., 2010; Karlamangla et al., 2002; McEwen, 1998; Seeman et al., 1997). Activation of these neuroendocrine systems results in a cascade of physiologic events including the secretion of pro-inflammatory cytokines, catecholamines, and acute phase proteins, all of which have deleterious effects on the body with sustained elevation (Karlamangla et al., 2002; McEwen, 1998; Seeman et al., 1997). When stress exposure is chronic, the negative feedback loops that typically shut-off the neuroendocrine stress response are dysregulated resulting in a chronic pro-inflammatory state (Karlamangla et al., 2002; Seeman et al., 1997), heightened and extended autonomic reactivity (Karlamangla et al., 2002; McEwen, 1998; Seeman et al., 1997), and a variety of other impairments (e.g., tissue damage, receptor desensitization) (Crimmins et al., 2003; McEwen, 1998; Miller et al., 2002). Although helpful in the short term (i.e., when responding to more mild and transient forms of stress) chronic hyperactivity of various systems can compromise the body’s ability to regulate the internal physiologic environment resulting in cumulative biological dysregulation (Karlamangla et al., 2002; McEwen, 1998; Seeman et al., 1997).
Perception of mistreatment has been associated with pronounced and sustained arousal across a number of physiologic systems including the HPA axis, autonomic nervous system, immune, metabolic and cardiovascular systems (Adam et al., 2015; Busse et al., 2017; Cooper et al., 2009; Cunningham et al., 2012; Dickerson et al., 2009; Friedman et al., 2009; Fuller-Rowell et al., 2012; Hill et al., 2017; Hoggard et al., 2015; Huynh et al., 2017; Lewis et al., 2010; Mays et al., 2007; Mendes et al., 2008; Myers, 2009; Wagner et al., 2015; Zeiders et al., 2014). Studies have also provided evidence of a more buffered stress response among those reporting chronic unfair treatment. For example, numerous studies have shown associations between reports of unfair treatment and a blunted cortisol response as well as overall poor HPA flexibility (e.g., flatter diurnal slope), especially among AAs (Adam et al., 2015; Busse et al., 2017; Fuller-Rowell et al., 2012; Hill et al., 2017; Huynh et al., 2016; Skinner et al., 2011; Zeiders et al., 2014). This heightened dysregulation observed among AAs may be at least partially explained by their histories of past exposures and their perception of the potential impact of these events (Zeiders et al., 2014)—getting hired and work settings, more generally, are among the most commonly reported sources of discrimination among AAs (Brondolo et al., 2011; Karlsen and Nazroo, 2002; Krieger et al., 2011).
Several studies show that the majority of AA women report racial discrimination as a unique and chronic social stressor (Cunningham et al., 2012; Fuller-Rowell et al., 2012; Krieger and Sidney, 1996; Lewis et al., 2010), evoking negative affective states that have been linked with biological alterations characteristic of the stress response (Nuru-Jeter et al., 2009; Tomfohr et al., 2016). Moreover, it is generally widely accepted that the nature of the threat matters for evoking particular stress responses (Dickerson et al., 2009; Kemeny, 2003; Miller et al., 2007; Plummer and Slane, 1996). Social evaluative threat, such as that experienced with racial discrimination, is a particular form of stress that studies show is associated with heightened inflammation via dysregulation of both the HPA and SAM axes (Kemeny, 2003). A strong body of research has shown adverse effects of discrimination on mental health outcomes including depression, anxiety, psychological distress, hypervigilance, and various health behaviors (Borrell et al., 2006; Brown et al., 2000; Landrine and Klonoff, 1996; Lorenzo-Blanco et al., 2011; Robinette et al., 2016; Sellers et al., 2006; Tomfohr et al., 2016; Williams and Mohammed, 2013; Williams et al., 1997), all of which have been linked with subsequent poor physical health outcomes via neurobiological stress responses (Brown et al., 2000; Chae et al., 2012b; Gee et al., 2006; Pieterse et al., 2012). Those neurobiological pathways include, among others, impaired hippocampal learning and activity, which has deleterious effects on HPA functioning; a hyperactive amygdala, which compromises memory and the body’s ability to predict threat and prepare the body for adaptive regulation (i.e., allostasis); and overall reduced neuroplasticity which promotes wear and tear due to the inability of the brain to respond adaptively to both anticipated and unanticipated stressors (Berger and Sarnyai, 2015; Carlson and Chamberlain, 2005; Fossion and Linkowski, 2007; Logan and Barksdale, 2008; Lucas et al., 2016; Lupien et al., 2015; McEwen, 2003; McEwen et al., 2015; Ziabreva et al., 2003). One of the amygdala’s primary roles is to regulate the fight or flight response. Notably, studies have shown enhanced amygdala activity—especially the left amygdala, which is associated with recall of events—among women than among men, suggesting that women may be more vulnerable to emotionally threatening events (Hamann, 2005).
Evidence suggests that rather than having uniform effects on physiology, stressors are met with integrated cognitive, affective, and behavioral responses that determine the psychobiological response to stress (Kemeny, 2003). In particular, studies suggest that how people appraise and cope with specific stressors differs by socioeconomic position (SEP). Lower socioeconomic groups are more likely to minimize attributions to discrimination and internalize their discrimination experiences (Crocker et al., 1991), both of which have been associated with poorer health (Chae et al., 2012a). Lower socioeconomic groups also report greater stress exposure, and hence may face depletion of health-protective resources and a lower sense of control over stressful life situations, increasing the likelihood of appraising stressors as threatening (Gallo and Matthews, 2003).
Geronimus proposed the “weathering hypothesis” to explain the accelerated physiologic deterioration experienced among black women due to the repeated stress associated with cumulative social disadvantage, including chronic experiences of racial discrimination (Geronimus, 1996; Geronimus et al., 2006). Several studies show heightened allostatic load among AA women relative to other race-gender groups (Chyu and Upchurch, 2011; Duru et al., 2012; Geronimus et al., 2006; Upchurch et al., 2015), which may help explain their elevated risk of poor health especially during midlife where differences are most pronounced (Borrell et al., 2010; Chyu and Upchurch, 2011). However, no studies to our knowledge have examined whether racial discrimination may account for the higher levels of allostatic load observed among this group. The purpose of this study was two-fold: To (1) examine the association between level of reported racial discrimination and allostatic load among a community-sample of midlife AA women, and (2) assess whether two commonly used indicators of SEP (i.e., educational attainment and poverty status) modify this association. We hypothesized that the association between racial discrimination and allostatic load would be greatest among lower socioeconomic groups.
2. METHODS
2.1. Study Design
Data are from the African American Women’s Heart & Health Study, an observational cross-sectional study designed to examine associations between social-environmental stressors and cardiometabolic risk among a community sample of midlife (ages 30–50) AA women residing in the San Francisco Bay area (n=208). The SF Bay area is a major metropolitan area in the state of California. Study recruitment was conducted by the 1st and 5th author and took place March 2012 through March 2013. According to the 2010 U.S. Census, the nine-county San Francisco Bay area was: 52.5% white, 23.3% Asian, 6.7% AA, 16% other or two or more races, and 23.5% Latino (Census, 2010). Given their relatively low proportion compared to other groups, recruitment efforts targeted counties with the highest percent of AAs: Solano (14.7%), Alameda (12.6%), Contra Costa (9.3%), San Francisco (6.1%).
Purposive sampling was used to maximize variability on key exposures and covariates (e.g., racial discrimination, socioeconomic factors) and included: targeted neighborhood sampling (e.g., low vs. high percent AAs per census tract), venue-based (e.g., nail/hair salons, churches, transportation hubs), event-based (e.g., arts concerts/performances, festivals, black college expo) and organization-based sampling (e.g., non-profits serving either low- or high-income AA women). We also recruited via social media and traditional media outlets. Potential study participants were screened for the following eligibility criteria: 1) self-identified AA and female gender since birth, 2) ages 30–50, 3) US-born, 4) parent(s)/primary caregiver(s) US-born AA, 5) can read/write English. Women who were pregnant or lactating and/or self-reported a physician-diagnosed inflammatory (e.g., HIV) or auto-immune disease (e.g., Lupus) were excluded to avoid misclassification. Study participation consisted of two visits: 1) interviewer-administered questionnaire and computer-assisted self-interview, 2) physical exam (i.e., height, weight, resting blood pressure, waist and hip circumference, body fat percent and body mass index) and fasting venous blood draw. Participants incentives included a $70 gift card, t-shirt, 8 heart healthy cookbook, and a packet of health promotion materials. The study was approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley.
2.2. Measures
Allostatic Load (AL) was operationalized as a composite of 15 biomarkers assessing functioning of the HPA axis (cortisol), SAM axis (epinephrine and norepinephrine), inflammation (Interleukin-6 (IL-6) and C-reactive protein (hsCRP)), and cardiometabolic function (diastolic and systolic blood pressure, waist circumference, body mass index, glucose, glycosylated hemoglobin, high and low-density lipoprotein, triglycerides, and total cholesterol) (Borrell et al., 2010; Geronimus et al., 2006; McEwen, 1998; Seeman et al., 1997; Seplaki et al., 2005).
Resting diastolic and systolic blood pressure (DBP and SBP, respectively) were measured from the sitting position using an automated oscillometric blood pressure monitor, after an initial 5-minute rest. Four consecutive measures were taken in 1-minute intervals; the first measure was discarded and the average of the last 3 measures was recorded (Pickering et al., 2005). Waist circumference was measured just above the top of the iliac crest with a tape measure, arms/hands down at the sides after one normal exhalation (NHLBI Obesity Education Initiative, 2000). Body mass index was measured via bioelectrical impedance using a handheld body fat analyzer. Fasting venous blood specimens were collected and stored at −80°C. Assays for hsCRP, cortisol, epinephrine and norepinephrine were performed by Quest Diagnostics. All other labs were assayed by the Client Services Laboratory at the UC Davis Department of Pathology and Laboratory Medicine.
Cutpoints for each biomarker are displayed in Table 1. We used established clinically-based cutpoints where available (Crimmins et al., 2003). Most of the biomarkers with established cutpoints have thresholds for subclinical and clinical risk, permitting assessment of dysregulated systems that may not yet meet criteria for clinical diagnosis. For biomarkers without established cutpoints (IL-6, epinephrine, norepinephrine, and cortisol), risk assessment was based on the distribution of the study sample as described below (Geronimus et al., 2006; Karlamangla et al., 2002; Seeman et al., 1997; Seplaki et al., 2005).
Table 1.
Allostatic Load Biomarker Cutpoints
| Biomarker | Used | Cutpoints† | % Above Cutpoint for AL75‡ | Cutpoint§ | % Above Cutpoint for AL90‡ | ALIQR Cutpoints£ | ||
|---|---|---|---|---|---|---|---|---|
| Cardiometabolic System | Low Risk | Moderate Risk | High Risk | |||||
| HDL (mg/dL) | ATPIII | <50 | 41.6 | <40 | 12.6 | ≥50 | ≥40 & <50 | <40 |
| LDL (mg/dL) | ATPIII | ≥100 | 52.2 | ≥130 | 16.4 | <100 | ≥100 & <130 | ≥130 |
| Waist Circumference (in) | ATPIII | >35 | 72.3 | >49 | --- | <35 | ≥35 & ≤45 | >45 |
| Glucose (mg/dL) | ATPIII | ≥100 or <70 | 17.4 | ≥126 or <70 | 8.7 | ≥70 or <100 | ≥100 & <126 | ≥126 or <70 |
| HbA1c (mmol/mol) | ADA | ≥5.7 | 19.3 | ≥6.5 | 4.4 | <5.7 | ≥5.7& <6.5 | ≥6.5 |
| Total Cholesterol (mg/dL) | ATPIII | ≥160 | 66.2 | ≥200 | 22.7 | <160 | ≥160 & <200 | ≥200 |
| Triglycerides (mg/dL) | ATPIII | ≥150 | 7.3 | ≥200 | 2.9 | <150 | ≥150 & <200 | ≥200 |
| BMI (kg/m2) | ATPIII | ≥25 or<18.5 | 85.5 | ≥30 or <18.5 | 63.8 | <25 | ≥25 & <30 | ≥30 or <18.5 |
| Systolic BP (mm Hg)ұ | AHA | ≥120 | 47.3 | ≥140 | 15.0 | <120 | ≥120 & <140 | ≥140 |
| Diastolic BP (mm Hg)ұ | AHA | ≥80 | 47.8 | ≥90 | 26.6 | <80 | ≥80 & <90 | ≥90 |
| Neuroendocrine System | ||||||||
| Cortisol (pg/dL) | n/a | >12.7 | --- | >17.3 | --- | ≤7.4 | >7.4 & ≤12.7 | >12.7 |
| Epinephrine (pg/mL) | n/a | >77.7 | --- | >120 | --- | ≤47.4 | >47.4 & ≤77.7 | >77.7 |
| Norepinephrine (pg/mL) | n/a | >686.3 | --- | >849 | --- | ≤404.4 | >404.4 & ≤686.3 | >686.3 |
| Inflammatory System | ||||||||
| il-6 (pg/mL) | n/a | >7.9 | --- | >17.8 | --- | ≤1 | >1 & ≤7.9 | >7.9 |
| hsCRP (mg/L) | AHA | >3 | 49.3 | >9.6 | --- | <1 | >1 & <3 | >3 |
Notes:
75th percentile cutpoints used for biomarkers that don’t have established clinical guidelines (Il-6, epinephrine, norepinephrine and cortisol); Established subclinical cutpoints used for rest
Cells left empty intentionally because cutpoints are based off distributions, rendering % above cutpoint information redundant.
90th percentile cutpoints used for biomarkers that don’t have established clinical guidelines (Il-6, epinephrine, norepinephrine, cortisol, waist and hs-CRP); Established clinical (high risk) cutpoints used for rest.
IQR percentile cutpoints used for biomarkers that don’t have established clinical guidelines for assessing low, moderate, and high risk (Il-6, epinephrine, norepinephrine, cortisol and waist); Established clinical cutpoints used for the rest, where subclinical cutpoints=moderate risk and clinical cutpoints=high risk.
JNC7 used in AL75 and ALiqr; JNC8 used in AL90.
Abbreviations:
AL = Allostatic load
ATPIII = Adult Treatment Panel III
ADA = American Diabetes Association AHA = American Heart Association
JNC7 = Seventh Joint National Committee JNC8 = Eighth Joint National Committee
HDL = High-density lipoprotein. LDL = Low-density lipoprotein. BMI = Body mass index
Systolic BP = Systolic blood pressure. Diastolic BP = Diastolic blood pressure. Il-6 = Interlukin 6.
hsCRP = High-sensitivity C-reactive protein.
We calculated four composite measures of AL to assess predictive ability across varying assessments and degrees of dysregulation (Seplaki et al., 2005): 1) sum of biomarkers for which the respondent is above the established cutpoint for subclinical risk (for biomarkers with established criteria) or above the 75th-percentile (for biomarkers without established criteria), 2) sum of biomarkers for which the respondent is above the established cutpoint for clinical risk or the 90th-percentile to assess more extreme dysregulation, 3) summary score based on established cutpoints for subclinical and clinical risk respectively or, correspondingly, the interquartile range (IQR), and 4) sum of z-scores for each biomarker. For the 75th-percentile/subclinical and 90th-percentile/clinical risk measures, each of the 15 biomarkers was categorized at the specified cutpoint (0=not at risk, 1=at risk) and summed with scores ranging from 0 to 15 where higher scores reflect higher levels of AL. The summary measure based on the IQR categorizes biomarkers at the 25th-percentile/subclinical and 75th-percentile/clinical cutpoints to capture mor e of a gradient rather than establishing risk based only on the extremes of the distribution, and to avoid potential misclassification from combining all participants in the bottom 75th or 90th percentile as not at risk. Participants were categorized as low (0=bottom 25%), moderate (1=middle 50%), or high risk (2=top 25%); values were summed with scores ranging from 0 to 30 where higher values reflect higher levels of ALIQR. Last, z-scores were calculated for each biomarker and summed to represent risk in standard deviation units.
We also calculated system-specific measures of physiologic dysregulation to examine whether certain systems were more or less responsive to stress. Scores for inflammation included CRP and IL-6, and were based on the IQR specification described above due to the greater range of the summary scores (0–4 vs. 0–2) and to minimize the misclassification inherent in dichotomous risk assessment. Scores from the component biomarkers were summed and then categorized to reflect risk: none (both inflammatory markers in normal range), low (one biomarker in normal range and one in moderate range), moderate (two in moderate range, or one in high range and one in normal range), high (one biomarker in moderate range and one in high range, or both in high range). Scores for neuroendocrine dysregulation included cortisol, epinephrine and norepinephrine and was based on the 75th-percentile scoring (0/1) since the majority of the biomarker values fell within the laboratory reference range and there was no distinction in level of risk between the 25th- and 75th-percentile cutpoints. Scores from the component biomarkers were summed and categorized to reflect low (all biomarkers in normal range), moderate (one elevated biomarker), and high risk (at least two elevated biomarkers). For cardiometabolic function, each of the 10 remaining biomarkers was categorized used the coding scheme described above for low, moderate, and high based on subclinical and clinical cut-points, respectively. Next, a summary score ranging from 0–20 was generated.
We decided to collapse cardiovascular and metabolic functioning into one cardiometabolic system-specific measure for several reasons: (1) blood glucose and various measures of lipid balance vary together and are determined by a common set of lifestyle factors (Basaranoglu et al., 2015; Sacks et al., 2014), (2) models of allostasis include many of the biomarkers reflected in our CV and metabolic system measures but do not separate them (McEwen and Rasgon, 2018) (3) the synergy of the metabolic and cardiovascular systems leave decisions about which biomarkers belong to which system equivocal and dependent upon the study aims. High density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides, and total cholesterol could easily be moved to the CV system measure leaving the metabolic system focused on carbohydrate metabolism (glucose, hemoglobin A1c (HbA1c), body mass index (BMI), waist circumference). Some studies have separated cardiovascular, lipid metabolism, and glucose metabolism (e.g., Ong et al., 2017; Robinette et al., 2016), which would also be acceptable for our study aims, (4) the metabolic syndrome (MS) has been characterized as a critical pathway to CVD as well as allostatic load (McEwen & Rasgon, 2018), (5) MS includes biomarkers from both our cardiovascular and metabolic system measures, and (6) recent studies are starting to link allostatic load and the stress response with cardiometabolic health (Nobel et al., 2017; Picard et al., 2014).
Racial discrimination was measured using a modified version of the widely used and previously validated Experiences of Discrimination (EOD) scale (Krieger et al., 2005), consisting of eight items asking whether respondents have ever experienced discrimination because of their race, ethnicity, or skin color in any of eight different domains (i.e., at school, getting hired/getting a job, at work, getting housing, getting medical care, getting credit/bank loans/mortgage, from police/courts, on the street/public setting). Reliability of the scale in our sample (α=.92) was higher than in the original validation study (α=0.81–0.87 among AA respondents). Responses were scored on a 5-point likert scale ranging from 1=“Never” to 5=“6 or more times”, summed across items, and categorized into: none, low, moderate, high, and very high levels of racial discrimination, given previous evidence of non-linear associations (Table 2).
Table 2.
Study Sample Characteristics (n=207), San Francisco Bay Area
| Variable | Mean (SD) or n (%) |
|---|---|
| COVARIATES (n=207) | |
| Age, mean (SD) | 42 (6) |
| Educational Attainment A | |
| > High school diploma | 138 (66.7) |
| ≤ High school diploma | 69 (33.3) |
| Poverty Status, n (%) | |
| > 100% Federal poverty level (FPL) | 168 (81.2) |
| ≤ 100% Federal poverty level (FPL) | 39 (18.8) |
| Employment Status | |
| Employed | 114 (55.1) |
| Not employed | 93 (44.9) |
| Health Insurance | |
| Insured | 152 (73.4) |
| Not insured | 55 (26.6) |
| Marital/Domestic Partnership Status | |
| Married/domestic partnership | 61 (29.5) |
| Not married | 146 (70.5) |
| Smoking Status | |
| Non-smoker | 118 (57) |
| Smoker % | 89 (43) |
| Alcohol Use | |
| < 3 drinks per day | 169 (81.6) |
| ≥ 3 drinks per day | 38 (18.4) |
| Exercise | |
| ≥ 5 times per week | 74 (35.8) |
| < 5 times per week | 133 (64.3) |
| Currently Taking Cardiovascular (CV) Medication | |
| No | 164 (79.2) |
| Yes | 43 (20.8) |
| Currently Taking Diabetes Medication | |
| No | 195 (94.2) |
| Yes | 12 (5.8) |
| Neuroticism-fleiprSD | 3.08 (0.8) |
| RACIAL DISCRIMINATION | |
| Experience of discrimination (EOD) Categories | |
| None (EOD score: 8) | 22 (10.6) |
| Low (EOD score: 9–16) | 71 (34.3) |
| Moderate (EOD score: 17–24) | 63 (30.4) |
| High(EOD score: 25–32) | 29 (14.0) |
| Very High (EOD score: 33–40) | 22 (10.6) |
| ALLOSTATIC LOAD MEASURES | |
| AL75, mean (SD) | 6.10 (2.2) |
| AL90, mean (SD) | 2.32 (1.6) |
| ALIQR, mean (SD) | 11.45 (3.8) |
| SYSTEM-SPECIFIC MEASURES | |
| Inflammatory System a | |
| Very low risk | 33 (15.0) |
| Low risk | 39 (18.8) |
| Moderate risk | 77 (37.2) |
| High risk | 60 (29.0) |
| Neuroendocrine System b | |
| Low risk | 86 (41.6) |
| Moderate risk | 93 (44.9) |
| High risk | 28 (13.5) |
| Cardiometabolic System, mean (SD) c | 6.79 (3.10) |
Notes:
Interlukin 6 (Il-6 and High-sensitivity C-reactive protein (hsCRP)
Cortisol, epinephrine, and norepinephrine.
Systolic blood pressure, diastolic blood pressure, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, cholesterol, BMI, waist, glucose, and HbA1c.
We used moderate as the referent group given previous evidence indicating that moderate stress exposure is self-regulatory and provides optimal psychological and biological functioning (Aschbacher et al., 2013; Bush et al., 2011; Chatkoff et al., 2010; Del Giudice et al., 2011; Dooley et al., 2017; Gunnar et al., 2015; Seery et al., 2010). The standard approach of modeling racial discrimination continuously, rather than categorically, precludes an assessment of the potentially protective/buffering effects of moderate levels of racial discrimination. One exception is Krieger & Sidney (1996) who used moderate discrimination (experiencing 1 or 2 situations vs. none or 3+) as the referent category in a study of the association between EOD and blood pressure among young Black and White adults in the CARDIA study. Findings showed moderate discrimination to be protective against high blood pressure in most study subgroups defined jointly by race, gender, and class. In another study, O’Brien and colleagues (2017) examined hair cortisol and lifetime discrimination in a multi-ethnic sample of 180 adults. They found the mean level of lifetime discrimination (moderate) to be associated with lower hair cortisol concentration compared to 1 standard deviation (SD) above (high) or below (low) the mean. Based on this evidence, we treated moderate discrimination as the referent category in all analyses.
Educational attainment and poverty status were both dichotomous variables. Participants were asked to report the highest level of education completed. Responses ranged from “some grade school but did not graduate” to a “doctoral degree”. Due to cell size, educational attainment was dichotomized as > versus ≤ a high school diploma (0/1). Participants were also asked to report their total monthly household income and the number of persons in the household. Poverty status was then operationalized as ≤ versus > 100% of the Federal Poverty Threshold (FPT) according to the 2011 FPT guidelines. Potential covariates included age in years, health insurance (0=yes, 1=no), marital status (married/domestic partner=0 vs. not married/no domestic partner=1), neuroticism based on a summary score from the 8-item neuroticism subscale of the Big 5 Inventory of personality traits, and medication use. For medication use, participants were asked if they were currently taking medication for “high blood pressure, hypertension, stroke, heart attack, blood circulation problems, hardening of the arteries, or any other heart or blood problem” and whether they were “currently taking medication to manage diabetes” (no/yes).
2.3. Statistical Analysis
Multivariable regression was used to quantify the change in AL per unit change in racial discrimination. Ordinary least squares and ordered logistic models were used, according to the specification of the dependent variable. First, we estimated the independent effect of racial discrimination on each outcome. Next, we performed two different sets of marginal effects models to examine effect modification: first by educational attainment, then by poverty status. Following recommendations outlined by Selvin (2004), we used a threshold of P<0.2 for interactions to prevent threats to validity resulting from false negatives or “wrong model bias”. For all other estimates, significance was based on P<.05 and 95% confidence intervals (CIs). Models were adjusted for age, educational attainment and medication use regardless of significance to account for known confounders and ensure accurate risk assessment. Health insurance, poverty status adjusted for household size, marital status, and neuroticism were included in final models if significant at P<.10 to account for potential confounding and reporting bias (see Table 2). Previous studies show associations between lifestyle behaviors and allostatic load. However, there is also literature suggesting that lifestyle behaviors may mediate the association between racial discrimination and allostatic load. Hence, we conceptualized lifestyle behaviors as mediators rather than moderators and therefore consistent with epidemiologic modeling (directed acyclical graph), did not include these variables as covariates in our analysis in order to avoid over-controlling and due to the cross-sectional nature of our data. Employment status was also conceptualized as a potential mediator and not included in our analysis.
Multiple imputation by chained equations was used to account for missing data (missing at random). Several biomarkers were log-transformed prior to imputation to correct for skewness and meet the normality assumptions of multiple imputation. Monte Carlo estimates were examined to confirm reproducibility of effect estimates across imputations. There were no significant departures from established criteria. Relative efficiency ranged from 98–100%, and relative variance increase and percent increase in standard error were both within 5%. One observation was excluded because it had missing data on both the right- and left-hand side of the imputation model, and therefore had missing data post-imputation (n=207). One additional outlying observation (> 3 SD beyond the IQR) was excluded from models estimating inflammation and neuroendocrine dysregulation (n=206). Additional post-estimation diagnostics included variance inflation factors to assess multicolinearity and tests of leverage and influence (Cook’s D), multivariate normality (Doornik-Hansen omnibus test) and heteroskedasticity (Breusch-Pagan and Cook-Weisberg). Analyses were conducted using Stata/SE 14.0.
3. RESULTS
Sample characteristics are shown in Table 2. Mean age was 42 years. Two-thirds of the study sample had more than a high school diploma. Approximately 20% were below the federal poverty level, a little over half were employed (55%), 30% were married, and 73% had health insurance. Mean levels of AL were in the low to moderate range. Approximately 11% of the sample reported no experiences of racial discrimination, the majority (64%) reported low to moderate levels of discrimination (i.e., between 1 to 3 experiences of discrimination in at least 1 domain), and 25% reported high to very high levels of racial discrimination (i.e., 5 to 6+ experiences in at least 1 domain). Discrimination experienced “on the street or in a public setting” was the most common form reported followed by “from the police or in the courts”, “at work”, “getting hired or getting a job”, and “at school”: 73%, 67%, 67%, 65%, and 61% respectively.
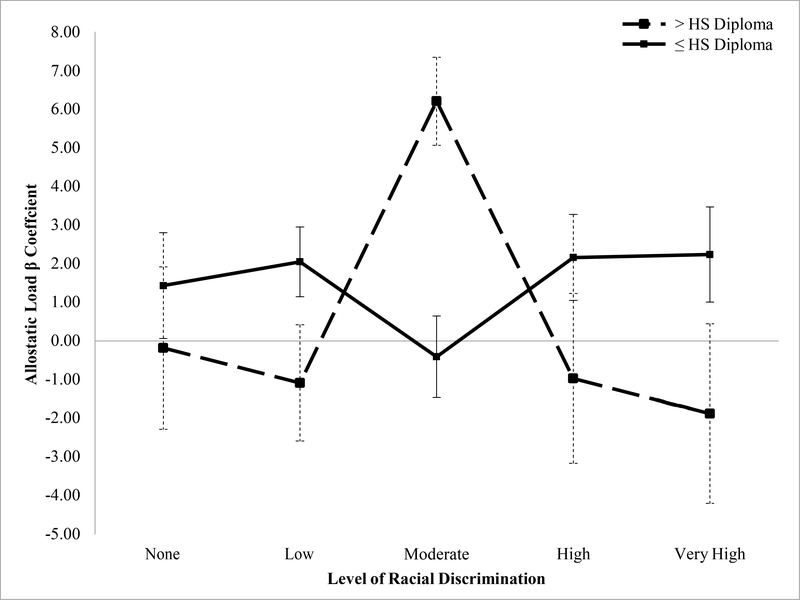
Results showing the adjusted association between racial discrimination and AL are shown in Table 3. For three of the four composite measures of AL, associations varied by education (all except ALz_sum). For simplicity, we display results for AL75 only (results for all composite measures is available online, supplementary Table 1). For AL75: among those with higher education (> high school diploma), those reporting low (b=−1.09, P=.02, 95% CI= −1.99, −0.18), and very high (b=−1.88, P=.003, 95% CI= - 3.11, −0.65) levels of racial discrimination had lower levels of AL (ref=moderate). Among those with lower education, we found the opposite (Figure 1): those reporting low (b=2.05, P<.01, 95% CI= 0.55, 3.56) racial discrimination had the highest levels of AL75. Associations for high and very high racial discrimination did not reach statistical significance but are suggestive of a U-shaped relationship among those with lower education. For ALIQR, a similar and statistically significant U-shaped pattern emerged among those with lower education, but with effect sizes roughly 1.5–2× greater than observed for AL75. Among those with higher education, reporting very high levels of discrimination was associated with lower levels of ALIQR (supplementary Figure 1). Fewer significant associations emerged for AL90: among those with higher education, those reporting very high discrimination had lower AL. The opposite was observed among those with lower education, although the joint test of coefficients for the interaction did not reach statistical significance (P>.2) (Selvin, 2004). There were no significant differences by education for ALz_sum and no significant main effects, although the direction of effect estimates is consistent with those observed using the other AL measures for the high education group. Interaction results for poverty were less consistent.
Table 3:
Risk of Allostatic Load by Racial Discrimination and Educational Attainment (n=207), San Francisco Bay Area
| Variable | b | AL75†‡ 95% CI | P |
|---|---|---|---|
| Discrimination X > HS Diploma | |||
| None | −0.18 | −1.56, 1.19 | 0.79 |
| Low | −1.09 | −1.99, −0.18 | 0.02 |
| Moderate (ref) | 6.21 | 5.16, 7.27 | <.001 |
| High | −0.97 | −2.08, 0.14 | 0.09 |
| Very high | −1.88 | −3.11, −0.65 | <.001 |
| Discrimination X ≤ HS Diploma | |||
| None | 1.44 | −0.67, 3.54 | 0.18 |
| Low | 2.05 | 0.55, 3.56 | 0.01 |
| Moderate (ref) | −0.41 | −1.55, 0.73 | 0.50 |
| High | 2.16 | −0.04, 4.36 | 0.05 |
| Very high | 2.24 | −0.08, 4.57 | 0.06 |
| Covariates∫ | |||
| ≤ HS diploma∂ | --- | --- | --- |
| Age | 0.07 | 0.02, 0.12 | 0.01 |
| Not insured | −0.10 | −0.78, 0.59 | 0.78 |
| ≤ 100% FPL | 0.58 | 0.16, 1.32 | 0.12 |
| Taking CV medication | 0.99 | 0.25, 1.73 | 0.01 |
| Taking diabetes medication | 1.59 | 0.36, 2.81 | 0.01 |
Notes:
One outlying observation removed, n = 206.
Finteraction = 2.21 (p<0.0696), where p<.2 is statistically significant52
Finteraction = 0.78 (p<0.5382), where p<.2 is statistically significant52
Finteraction = 2.27 (p<0.0637), where p<.2 is statistically significant52
Finteraction = 1.33 (p<0.2588), where p<.2 is statistically significant52
Empty cells due to statistically non-significant interaction between discrimination and education level for the model. Main effect of education reported as a covariate.
Referent groups: moderate discrimination, >HS diploma, and the dichotomized low-risk counterpart for all covariates.
Empty cells because among the ≤ HS diploma group, educational attainment is reported as an interaction term.
Abbreviations:
CV medication = cardiovascular medication
HS diploma = high school diploma
FPL = federal poverty level
Figure 1:
Allostatic load (AL75) by Racial Discrimination and Educational Attainment (n=206), San Francisco Bay Area
Note: Reference group is moderate. *p<0.10, **p<0.05, ***p<0.01; HS Diploma=High School Diploma
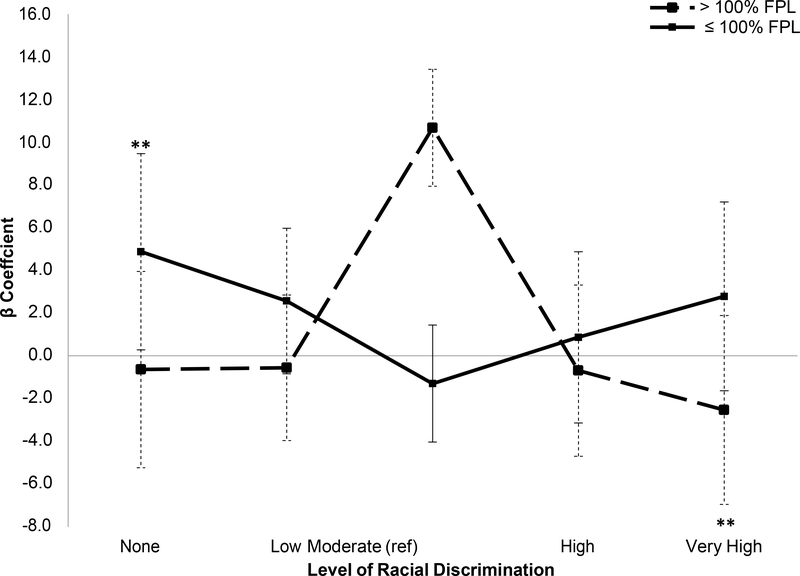
Only one of the composite AL measures, AL30, showed significant differences between poverty-level groups (supplementary Table 2). Findings were consistent with the results for educational attainment (Figure 2): among those not in poverty, reporting very high (vs. moderate) racial discrimination was associated with lower levels of AL (b=−2.54, P<.05, 95% CI= −4.56, −0.52). Among those at or below 100% of the FPL, reporting no racial discrimination (vs. moderate) was associated with higher levels of AL (b=4.88, P<.04, 95% CI= 0.28–9.48).
Figure 2:
Linear Regression of Allostatic Load (ALIQR) and Racial Discrimination by Poverty Level (n=206)
Note: Reference group is moderate. FPL is Federal Poverty Line. *p<0.10, **p<0.05, ***p<0.01
Table 4 shows results for the interaction of racial discrimination and educational attainment in relation to each of our system-specific measures of physiologic dysregulation. Findings for cardiometabolic function mirrored those reported for AL75 and ALIQR above (supplementary Figure 2). For neuroendocrine and inflammatory activity, no differences by education emerged. Instead, reporting very high levels of racial discrimination was associated with blunted physiologic activity, regardless of educational attainment. There were no significant interactions for poverty status: reporting very high levels of racial discrimination was associated with blunted cardiometabolic activity after adjusting for model covariates including education and poverty status (b=−1.61, P=0.03, 95% CI=−3.06, −0.15). For neuroendocrine activity, those reporting very high racial discrimination had lower odds of AL. However, these results did not reach statistical significance (OR=0.32, P=.06, 95% CI= 0.10, 1.05).
Table 4:
System-Specific Physiologic Dysregulation by Racial Discrimination and Educational Attainment (n=207), San Francisco Bay Area
| Variable | Cardiometabolic † | Neuroendocrine§ | Inflammatory£ұ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Discrimination Category | b | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Discrimination X > HS Diploma | |||||||||
| None | 0.027921 | 1.88, 1.94 | 0.977 | 1.45 | 0.52, 4.06 | 0.48 | --- | --- | --- |
| Low | −1.190299 | −2.43, 0.05 | 0.060 | 1.05 | 0.51, 2.19 | 0.89 | 0.89 | 0.34, 2.29 | 0.81 |
| Moderate (ref) | 6.261993 | 4.82, 7.71 | 0.000 | 0.75 | 0.28, 2.04 | 0.58 | |||
| High | −1.382565 | −2.86, 0.10 | 0.067 | 1.87 | 0.70, 4.96 | 0.21 | 0.37 | 0.12, 1.08 | 0.07 |
| Very high | −2.636509 | −4.32, −0.96 | 0.002 | 0.32 | 0.10, 1.05 | 0.06 | 0.71 | 0.22, 2.32 | 0.57 |
| Discrimination X ≤ HS Diploma¶ | |||||||||
| None | 0.4411431 | −1.23, 2.11 | 0.603 | --- | --- | --- | --- | --- | --- |
| Low | 1.419099 | 0.19, 2.64 | 0.024 | --- | --- | --- | --- | --- | --- |
| Moderate (ref) | −0.5645661 | −1.49, 0.36 | 0.228 | --- | --- | --- | --- | --- | --- |
| High | 1.973987 | 0.23, 3.72 | 0.027 | --- | --- | --- | --- | --- | --- |
| Very high | 2.158017 | 0.25, 4.06 | 0.027 | --- | --- | --- | --- | --- | --- |
| Covariates∫ | |||||||||
| ≤ HS diploma∂ | --- | --- | --- | 1.5 | 0.78, 2.88 | 0.22 | 1.11 | 0.55, 2.23 | 0.77 |
| Age | 0.1044384 | 0.03, 0.18 | 0.005 | 1.01 | 0.96, 1.06 | 0.78 | 3.01 | 1.54, 5.91 | <.001 |
| Not insured | 0.3999438 | −0.53, 1.33 | 0.398 | 0.94 | 0.49, 1.80 | 0.86 | 1 | 0.95, 1.05 | 0.94 |
| ≤ 100% FPL | 0.3776245 | −0.66, 1.42 | 0.475 | 1.46 | 0.69, 3.09 | 0.33 | 1.11 | 0.61, 2.02 | 0.73 |
| Taking CV medication€ | 1.419316 | 0.38, 2.46 | 0.008 | --- | --- | --- | 0.57 | 0.30, 1.10 | 0.09 |
| Taking diabetes medication€ | 2.773564 | 1.04, 4.51 | 0.002 | --- | --- | --- | 1.46 | 0.76, 2.79 | 0.25 |
Notes:
Finteraction = 2.46 (p<0.0469)
Finteraction = 1.08 (p<0.3626)
Fintraction = 0.67 (p<0.6128)
Referent group: no discrimination.
Empty cells due to statistically non-significant interaction between discrimination and education level for the model. Main effect of education reported as a covariate.
Referent groups: >HS diploma, and the dichotomized low-risk counterpart for all covariates.
Empty cells because among the ≤ HS diploma group, educational attainment is reported as an interaction term.
Empty cells because cardiovascular medication usage not relevant for neuroendocrine dysregulation.
Empty cells because diabetes medication usage not relevant for cardiovascular or neuroendocrine dysregulation.
Abbreviations:
OR = Odds Ratio reported for logistic regression
B = Beta reported for ordinary least squares regression
CV medication = cardiovascular medication
HS diploma = high school diploma
FPL = federal poverty level
To determine if our results were being driven by particular biomarkers, we performed separate regressions on each individual biomarker. Given the direction, magnitude and statistical significance of associations, it does not appear that a few biomarkers are driving the results (supplementary Tables 3 and 4). For educational attainment, there were significant effects for two of the individual biomarkers: epinephrine and waist circumference. Among those in the low education group: reporting a high level of racial discrimination was associated with higher levels of circulating epinephrine (b=.63, P=.03, 95% CI=0.05, 1.2) and higher waist circumference (b=0.52, P=.03, 95% CI=0.05, 0.98). Among those in the higher education group: reporting low levels of racial discrimination was associated with lower waist circumference (b= −0.21, P=.04, 95% CI= −0.41, −0.01). For poverty status, there were no significant interaction effects for any of the individual biomarkers. We also examined independent main effects for the remaining 13 biomarkers (excluding epinephrine and waist circumference). HbA1c was the only biomarker showing a significant main effect for racial discrimination: reporting very high racial discrimination was associated with lower HbA1c (b= −0.21, P=.04, 95% CI=−0.42, −.01).
4. DISCUSSION
We examined the association between racial discrimination and cumulative biological dysregulation among a community sample of midlife AA women in the San Francisco Bay Area. This is the first study to examine racial discrimination and AL among this group. This is also the first study to show that the association between racial discrimination and AL is moderated by educational attainment, and quite dramatically. This is not only a novel finding but a very informative one that points to potential differences in the mechanisms linking racial discrimination and AL between socioeconomic groups. The buffered pattern observed among both the higher education and higher income groups highlights the need to better understand the factors that may safeguard against the negative health outcomes associated with racial discrimination and potentially other forms of chronic social stress. The U-shaped pattern observed among the lower socioeconomic groups, particularly the inverse association among those reporting low (vs. high) levels of racial discrimination, raises questions that may help inform future research in this area such as potential reporting bias (i.e., underreporting) and the role of coping, racial identity and internalized racism (Chae et al., 2014; Krieger, 1990; Sellers et al., 2006), among other potential explanations. Additionally, although our findings do not appear to be driven by particular individual biomarkers, cardiometabolic function may be a primary driver and warrants further consideration as a mechanism underlying this association.
The buffered pattern observed among the higher socioeconomic groups is consistent with prior work suggesting that acknowledging racism is protective compared to internalizing those experiences and potentially engaging in self-blame (Chae et al., 2012a; Chae et al., 2014; Krieger and Sidney, 1996; LaVeist et al., 2001; Major et al., 2002). The U-shaped relationship observed among the lower socioeconomic groups is also consistent with previous research. Reporting lower levels of racial discrimination has been associated with worse cardiovascular health among low socioeconomic black women who respond to unfair treatment by accepting it and keeping it to themselves, suggesting that coping may influence how discrimination impacts biological functioning (Krieger and Sidney, 1996). This may reflect a tendency to deny, suppress, or otherwise passively cope with racial discrimination (Krieger and Sidney, 1996; Nuru-Jeter et al., 2009), which has been previously associated with poor health outcomes (Krieger and Sidney, 1996), and may suggest some degree of reporting bias among lower socioeconomic groups. Literature on stigma and attributions to discrimination reflect the need to better understand the factors that determine variability in responses to discrimination. It is now generally understood that vulnerability and resilience are both common responses to prejudice and discrimination (Major et al., 2002).
Other studies have, similarly, noted non-linearities in the racial discrimination-biology relationship (Fuller-Rowell et al., 2012; Krieger and Sidney, 1996; Skinner et al., 2011). Skinner (2011) found that AAs reporting low (vs. high) levels of racial discrimination have a flatter diurnal cortisol rhythm, characteristic of poor HPA flexibility (i.e., dysregulation) (Skinner et al., 2011). Using data from the National Study of Midlife in the United States (MIDUS), Fuller-Rowell et al. examined associations by race and found that among African Americans, reporting low (vs.) high levels of discrimination (where 90% of AAs reported their discrimination experiences as due to race) was associated with lower waking cortisol (i.e., less HPA flexibility), and that this effect was greatest among lower socioeconomic groups (Fuller-Rowell et al., 2012).
The lack of consistent results for poverty are not surprising given previous findings that higher income does not protect blacks from higher levels of AL (Geronimus et al., 2006). Further studies suggest that neighborhood poverty may be a stronger predictor of AL than individual-level income. (Robinette et al., 2016; Schulz et al., 2012; Tan et al., 2017) Additionally, studies suggest that higher educated, but not necessarily higher income, AAs are more likely to live and work in settings where they are the minority, thereby increasing opportunities for experiencing racial discrimination (NPR/Robert Wood Johnson Foundation/Harvard T.H. Chan School of Public Health, 2017; Pew Research Center, 2016; Wilson et al., 2017). Hence, higher educated AAs may not only experience certain types of discrimination more but may also be better equipped to recognize it. Howard and Sparks (2015) found that among AAs, those with a college degree or higher had significantly lower AL compared to those with less than a high school diploma. Our study findings complement these results and provide preliminary evidence suggesting that reports of racial discrimination may be one factor helping to explain this disparity. Further research is needed to explicitly test this hypothesis.
Consistent with the concept of subclinical dysregulation, the association between racial discrimination and AL was strongest for measures capturing more moderate (i.e., AL75, ALIQR) vs. extreme levels of dysregulation (AL90), which is especially salient given our sample of mostly healthy women not yet diagnosed with serious medical conditions
Several methodological considerations should be noted. This was an exploratory study intended to generate preliminary data and hypotheses about the potential role of social stress, in particular racial discrimination, for heightened biological dysregulation among AA women. However, we are unable to infer causality due to the cross-sectional nature of the study. We used a non-probability purposive sample intended to maximize variability in the primary exposure and ensure stable estimates across subgroups (Rothman et al., 2014). Compared to AA women in the 2013 American Community Survey ages 30–50 residing in the same Bay Area counties, our sample had a similar distribution of educational attainment, employment and marital status but a higher percent not insured and lower percent in poverty (data available upon request) (Ruggles et al., 2010). Although we used validated scales, our self-report measures are subject to the typical reporting bias inherent in all survey research. Biomarkers were assessed via one fasting venous blood draw and therefore do not capture circadian rhythm. Resting, rather than ambulatory, blood pressure was measured but taken to capture a true average and minimize white coat effect. A few of our models had power below 80% (i.e., inflammation=34%, neuroendocrine=65%). Hence, observed differences are likely under-estimates of the true effects. Educational attainment was dichotomized at high school diploma, also biasing our results toward the null vs. dichotomizing at college degree. Last, detailed information about medication use was not available. Hence, we were unable to control for medications prescribed off-label.
This study also has several strengths. First, only one other study has, to our knowledge, examined the relationship between racial discrimination and AL among any population. Brody et. al. examined the association among AA adolescents ages 16–18 in the rural south (Brody et al 2014). Other studies have examined racial discrimination and health more broadly (e.g., birth outcomes, mental health outcomes, cardiovascular disease) (Dominguez et al., 2008; Lewis et al., 2014; Pieterse et al., 2012) and in relation to specific biomarkers (e.g., cortisol, CRH, CRP, catecholamines) (Albert et al., 2008; Braveman et al., 2015; Fuller-Rowell et al., 2012; Lewis et al., 2010; Wagner et al., 2013), though findings are mixed (Cohen et al., 2006; Huynh et al., 2016; Schmitt et al., 2014; Skinner et al., 2011; Wagner et al., 2013). However, previous studies indicate the need to study simultaneous dysregulation across multiple systems to better understand how psychosocial factors contribute to stress-related illness, given the importance of integrative biology for disease outcomes. In fact, some studies have shown significant associations between composite AL scores and subsequent poor health outcomes whereas individual biomarkers are not significant independent risk factors (Ong et al., 2017). Hence, studies point to the need to understand the “interconnected nature of biological stress systems including their co-occurring responses to social stressors” (Lucas et al., 2016).
There have also been studies examining more general unfair treatment (not attributed to race, ethnicity, skin color) in relation to specific biomarkers (e.g., IL-6, CRP, cortisol, coronary artery calcification) and one in relation to AL (Upchurch et al., 2015). These studies support previous findings suggesting that experiencing discrimination is more salient than the type of discrimination experienced (Huynh et al., 2016; Kershaw et al., 2016; Ong et al., 2017; Zeiders et al., 2014). However, other studies suggest that racial discrimination has particularly pernicious health effects among racial minority groups for whom such experiences are more frequent, more severe and occur across a wider range of situations; and among those with a particularly strong racial group identity (Crocker and Major, 1989; Major et al., 2002; Sellers et al., 2003). Racial discrimination is commonly reported by AA women, has been reported as a particularly salient form of social stress (Carter, 2007; Nuru-Jeter et al., 2009; Williams et al., 2012), and as distinct from other forms of social stress including other forms of unfair treatment. Our study is the first to examine whether racial discrimination is associated with multi-system physiologic dysregulation among AA women.
Prior research has shown considerable variation in reports of discrimination by race both in terms of frequency of reports and to what the discrimination was attributed (e.g., race, gender). The majority of AAs attribute their discrimination experiences to race and report more chronic experiences, whereas whites report non-racial forms of discrimination more frequently (Cozier et al., 2006; Karlamangla et al., 2002; Krieger and Sidney, 1996; Plummer and Slane, 1996; Williams et al., 2012). African Americans’ reports of racial discrimination have been further described as a form of historical trauma characterized by rumination, heightened vigilance, and the processing of contemporary experiences as re-experiencing historical oppression (Borders and Liang, 2011; Carter, 2007; Hicken et al., 2013; Nuru-Jeter et al., 2009; Williams et al., 2012). On the contrary, whites commonly attribute their experiences to reverse racism such as being denied opportunities due to Affirmative Action policies (i.e., policies intended to protect and provide opportunities to groups that have faced historical discrimination) (Williams et al., 2012). Further work is needed to better understand differences in how different groups recognize and understand discrimination. However, these qualitative differences suggest the importance of not conflating the racial discrimination experiences of AAs and whites, and the importance of distinguishing between racial discrimination and other forms of unfair treatment. Taken together, this evidence was a primary motivation for limiting our study to African American women, in addition to evidence documenting significant gender disparities in AL by race (Chyu and Upchurch, 2011; Deuster et al., 2011; Duru et al., 2012).
Additionally, acknowledging the heterogeneity in reports of racial discrimination among AA women, our within group study was an attempt to better isolate racial discrimination as the exposure of interest. By restricting the sample to AA women, we avoid the misclassification and biases inherent in multi-ethnic studies vis a vis incommensurability of the exposure across groups (Howard and Sparks, 2015). Testing this association among AA women provides evidence suggesting that reported racial discrimination may be one risk factor associated with higher AL among this group; but that importantly, this association varies by level of educational attainment. Further, given that AAs report racial discrimination at a much higher frequency than whites, it follows that racial discrimination may help explain the higher rates of AL observed among this group. However, future studies examining differences between AA men and women is warranted. It is unclear whether this association is unique to AA women and thus explains the disparity observed among this group. If the association persists for AA women and men, it would suggest that racial discrimination is not an adequate explanation for heightened risk among AA women alone but rather may help explain why AAs, as a group, experience accelerated biological weathering relative to whites.
This is also the first study to examine the racial discrimination-AL association in adulthood, the period of life where race-gender disparities in AL become more apparent. Geronimus showed the gap in AL scores between race-gender groups becomes “especially pronounced after age 30” with AA women having the highest probability. Notably, although AA and white men and women start with the same intercept at age 18, the slope of the association between age and AL is greatest among AA women, suggesting that AA women experience biological “weathering” at an accelerated pace relative to other race-gender groups. We believe this distinction—the racial discrimination-AL association among midlife AA women—is an important one that may help explain the higher rates of AL observed in previous studies. Thus, while several studies have now shown that AA women have higher levels of AL relative to other groups, no previous studies to our knowledge, have specifically investigated the factors that may account for this particular vulnerability (heightened multi-system physiologic dysregulation during midlife among this group).
5. CONCLUSIONS
Racial discrimination may be an important predictor of biological dysregulation, increasing risk for poor health among AA women. Results suggest mechanisms that may buffer or exacerbate the effect of racial discrimination; and the need to further explore differences by socioeconomic position, particularly educational attainment. Findings provide new insights that may help explain the role of social determinants for disease pathogenesis, and offer direction for future research among socially disadvantaged groups and other groups experiencing more general forms of chronic stress. In the US, disparities by race have persisted over time and across health outcomes. African Americans are among the most socially disadvantaged groups in the US. However, after adjustment for socioeconomic factors, health behaviors, and access to and utilization of health care, health disparities persist. The role of social stress for adverse health outcomes is now widely accepted. Although de jure racial discrimination no longer exists, AAs report various forms of de facto racial discrimination (structural, institutional, personally-mediated) as a salient and ongoing source of life stress (Carter, 2007; Guyll et al., 2001; Nuru-Jeter et al., 2009; Williams et al., 2012; Williams and Mohammed, 2013). Geronimus’ “weathering” hypothesis was developed as a framework for examining the early health deterioration observed among AA women, and suggests that this decline is a physical manifestation of the stress associated with being a member of a socially marginalized group and contending with various forms of recurring racial discrimination (Geronimus et al., 2006). Our findings have implications for AA women in the US and for socially marginalized groups more broadly. Studies show associations between racial discrimination and mental and physical health among many socially marginalized groups; for example, indigenous people of Australia, Native Hawaiians, Turkish immigrants in Germany, minority and immigrant women in New Zealand, Southeast Asian refugees and Korean immigrants in Canada, and “blacks and browns” in Brazil (Bastos et al., 2014; Berger et al., 2015; Fischer et al., 2017; Kaholokula et al., 2012; Larson et al., 2007; Thayer and Kuzawa, 2015). Importantly, these reports have included both observational and laboratory- based assessments, though attention to multi-system physiologic dysregulation is limited.
Further research using larger probability-based samples is needed to provide more precise risk estimates, improve generalizability, and determine causation. Additionally, studies examining lifestyle behaviors as potential mediators is also warranted. Though further research is needed, discrimination remains a unique and important stressor and may have relevance for identifying at-risk groups and help inform strategies to prevent, detect, and treat disease progression.
Supplementary Material
Highlights.
There was a non-linear association between discrimination and allostatic load
The association between discrimination and allostatic load varied by education
A U-shaped relationship emerged among those with lower education
High discrimination had a buffering effect among those with higher education
Associations were found for allostatic load and in system-specific analyses
ACKNOWLEDGEMENTS
This work was supported by research grants from: University of California, Berkeley (UCB) Hellman Fund, UCB Population Center, UCB Research Bridging Grant, UCB Experimental Social Science Laboratory, Robert Wood Johnson Health and Society Scholars Program (UCB site), UC Center for New Racial Studies, and the UCB Institute for the Study of Societal Issues. A Allen was also partially supported by NIMHD grant P60MD006902; MT was partially supported by NIGMS grant UL1GM118985. Study sponsors did not participate in study design, data collection, data analysis, interpretation of study results, or drafting of the manuscript.
We wish to thank Holly Berryman Stern from the UCB Tang Center for support related to laboratory specimens, Roger Hoffman of Westportal for in-kind support in building the CASIC platform, Miguel Dorta from StataCorp and Maureen Lahiff for comments on statistical analysis, and the many undergraduate, graduate, and postdoctoral students working as volunteer research assistants. We also thank Nancy Krieger and John Lynch as well as four anonymous reviewers for comments on previous drafts of this manuscript.
A Allen, Principal Investigator, conceptualized the study, coordinated all logistics related to recruitment and data collection, designed and performed all statistical analyses (and takes responsibility for the integrity of the data analyzed), and took primary responsibility for drafting the manuscript; M Thomas assisted with the design of the analysis, specifically operationalizing study variables, as well as data interpretation, and drafting the manuscript; E Michaels assisted with design of the analysis, reviewing statistical code, data interpretation, and drafting the manuscript; AN Reeves assisted with data interpretation and drafting the manuscript; U Okoye assisted with data interpretation and editing of the manuscript; M Price was responsible for management of data collection, made contributions to the intellectual content of the manuscript and assisted with manuscript revisions; R Hasson assisted with laboratory data collection and manuscript revisions; SL Syme helped provided assistance with interpreting the study results and drafting the manuscript. DH Chae helped conceptualize the study and provided assistance with data interpretation and drafting the manuscript.
Abbreviations:
- CV medication
cardiovascular medication
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Levy DJ, Kemeny M, Brodish AB, Malanchuk O, 2015. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology 62, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Ravenell J, Glynn RJ, Khera A, Halevy N, de Lemos JA, 2008. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. American heart journal 156, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E, 2013. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaranoglu M, Basaranoglu G, Bugianesi E, 2015. Carbohydrate intake and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction. Hepatobiliary Surgery and Nutrition 4, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos JL, Barros AJ, Celeste RK, Paradies Y, Faerstein E, 2014. Age, class and race discrimination: their interactions and associations with mental health among Brazilian university students. Cadernos de saude publica 30, 175–186. [DOI] [PubMed] [Google Scholar]
- Beckie TM, 2012. A systematic review of allostatic load, health, and health disparities. Biological research for nursing 14, 311–346. [DOI] [PubMed] [Google Scholar]
- Berger M, Juster R-P, Sarnyai Z, 2015. Mental health consequences of stress and trauma: allostatic load markers for practice and policy with a focus on Indigenous health. Australasian Psychiatry 23, 644–649. [DOI] [PubMed] [Google Scholar]
- Berger M, Sarnyai Z, 2015. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress 18, 1–10. [DOI] [PubMed] [Google Scholar]
- Borders A, Liang CT, 2011. Rumination partially mediates the associations between perceived ethnic discrimination, emotional distress, and aggression. Cultural Diversity and Ethnic Minority Psychology 17, 125. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Dallo FJ, Nguyen N, 2010. Racial/Ethnic Disparities in All-Cause Mortality in US Adults: The Effect of Allostatic Load. Public Health Rep. 125, 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, Gordon-Larsen P, 2006. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Soc. Sci. Med 63, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Heck K, Egerter S, Marchi KS, Dominguez TP, Cubbin C, Fingar K, Pearson JA, Curtis M, 2015. The role of socioeconomic factors in black–white disparities in preterm birth. Am. J. Public Health 105, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E, Hausmann LR, Jhalani J, Pencille M, Atencio-Bacayon J, Kumar A, Kwok J, Ullah J, Roth A, Chen D, 2011. Dimensions of perceived racism and self-reported health: examination of racial/ethnic differences and potential mediators. Ann. Behav. Med 42, 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TN, Williams DR, Jackson JS, Neighbors HW, Torres M, Sellers SL, Brown KT, 2000. “Being black and feeling blue”: The mental health consequences of racial discrimination. Race and Society 2, 117–131. [Google Scholar]
- Bush NR, Obradović J, Adler N, Boyce WT, 2011. Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Development and psychopathology 23, 1089–1106. [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, Campos B, Marshburn CK, 2017. Discrimination and the HPA axis: current evidence and future directions. J. Behav. Med 40, 539–552. [DOI] [PubMed] [Google Scholar]
- Carlson E, Chamberlain R, 2005. Allostatic load and health disparities: a theoretical orientation. Research in nursing & health 28, 306–315. [DOI] [PubMed] [Google Scholar]
- Carter RT, 2007. Racism and psychological and emotional injury: Recognizing and assessing race-based traumatic stress. The Counseling Psychologist 35, 13–105. [Google Scholar]
- Census BA, 2010. Metropolitan Transportation Commission and the Association of Bay Area Governments Library. http://www.bayareacensus.ca.gov/index.html accessed 5/22/2013.
- Chae DH, Nuru-Jeter AM, Adler NE, 2012a. Implicit racial bias as a moderator of the association between racial discrimination and hypertension: a study of Midlife African American men. Psychosom. Med 74, 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, Epel ES, 2014. Discrimination, Racial Bias, and Telomere Length in African-American Men. Am. J. Prev. Med 46, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Lincoln KD, Arriola KRJ, 2012b. Racial Discrimination, Mood Disorders, and Cardiovascular Disease Among Black Americans. Ann. Epidemiol 22, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatkoff DK, Maier KJ, Klein C, 2010. Nonlinear associations between chronic stress and cardiovascular reactivity and recovery. International Journal of Psychophysiology 77, 150–156. [DOI] [PubMed] [Google Scholar]
- Chyu L, Upchurch DM, 2011. Racial and Ethnic Patterns of Allostatic Load Among Adult Women in the United States: Findings from the National Health and Nutrition Examination Survey 1999–2004. J. Womens Health 20, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T, 2006. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med 68, 41–50. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE, 2009. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. American journal of hypertension 22, 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier Y, Palmer JR, Horton NJ, Fredman L, Wise LA, Rosenberg L, 2006. Racial discrimination and the incidence of hypertension in US black women. Ann. Epidemiol 16, 681–687. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Johnston M, Hayward M, Seeman T, 2003. Age differences in allostatic load: an index of physiological dysregulation. Experimental gerontology 38, 731–734. [DOI] [PubMed] [Google Scholar]
- Crocker J, Major B, 1989. Social stigma and self-esteem: The self-protective properties of stigma. Psychological Review 96, 608. [Google Scholar]
- Crocker J, Voelkl K, Testa M, Major B, 1991. Social stigma: The affective consequences of attributional ambiguity. Journal of Personality and Social Psychology 60, 218. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF, 2012. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc. Sci. Med 75, 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA, 2011. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews 35, 1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuster PA, Kim-Dorner SJ, Remaley AT, Poth M, 2011. Allostatic Load and Health Status of African Americans and Whites. Am. J. Health Behav 35, 641–653. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME, 2009. Social-evaluative threat and proinflammatory cytokine regulation: An experimental laboratory investigation. Psychol. Sci 20, 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA, 2008. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 27, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley LN, Slavich GM, Moreno PI, Bower JE, 2017. Strength through adversity: Moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors. Stress and Health 33, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Harawa NT, Kermah D, Norris KC, 2012. Allostatic Load Burden and Racial Disparities in Mortality. J. Natl. Med. Assoc 104, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Nater UM, Strahler J, Skoluda N, Dieterich L, Oezcan O, Mewes R, 2017. Psychobiological impact of ethnic discrimination in Turkish immigrants living in Germany. Stress 20, 167–174. [DOI] [PubMed] [Google Scholar]
- Fossion P, Linkowski P, 2007. The relevance of the concept of resiliency in the field of psychiatry. Revue medicale de Bruxelles 28, 33–38. [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD, 2009. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain Behav. Immun 23, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, Eccles JS, 2012. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology 37, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA, 2003. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol. Bull 129, 10. [DOI] [PubMed] [Google Scholar]
- Gee GC, Ryan A, Laflamme DJ, Holt J, 2006. Self-reported discrimination and mental health status among African descendants, Mexican Americans, and other Latinos in the New Hampshire REACH 2010 Initiative: the added dimension of immigration. Am. J. Public Health 96, 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, 1996. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc. Sci. Med 42, 589–597. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J, 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Doom JR, Esposito EA, 2015. Psychoneuroendocrinology of stress: Normative development and individual differences. Handbook of child psychology and developmental science, 1–46. [Google Scholar]
- Guyll M, Matthews KA, Bromberger JT, 2001. Discrimination and unfair treatment: Relationship to cardiovascular reactivity among African American and European American women. Health Psychol. 20, 315–325. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP, 2001. Neuroendocrinology of stress. Endocrinology and Metabolism Clinics 30, 695–728. [DOI] [PubMed] [Google Scholar]
- Hamann S, 2005. Sex differences in the responses of the human amygdala. The Neuroscientist 11, 288–293. [DOI] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR, 2013. “Every shut eye, ain’t sleep”: The role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race and social problems 5, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Hoggard LS, Richmond AS, Gray DL, Williams DP, Thayer JF, 2017. Examining the Association Between Perceived Discrimination and Heart Rate Variability in African Americans. Cult. Divers. Ethn. Minor. Psychol 23, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard LS, Hill LK, Gray DL, Sellers RM, 2015. Capturing the cardiac effects of racial discrimination: Do the effects “keep going”? International Journal of Psychophysiology 97, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JT, Sparks P, 2015. The role of education in explaining racial/ethnic allostatic load differentials in the United States. Biodemography and Social Biology 61, 19–39. [DOI] [PubMed] [Google Scholar]
- Huynh VW, Guan S-SA, Almeida DM, McCreath H, Fuligni AJ, 2016. Everyday discrimination and diurnal cortisol during adolescence. Hormones and behavior 80, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh VW, Huynh Q-L, Stein M-P, 2017. Not just sticks and stones: Indirect ethnic discrimination leads to greater physiological reactivity. Cultural Diversity and Ethnic Minority Psychology 23, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FM, 2005. The Development of a Race and Gender-Specific Stress Measure for African- American Women: Jackson, Hogue, Phillips Contextualized Stress Measure. Ethnicity & Disease 15, 594–600. [PubMed] [Google Scholar]
- Jackson FM, Phillips MT, Hogue CJR, Curry-Owens TY, 2001. Examining the Burdens of Gendered Racism: Implications for Pregnancy Outcomes Among College-Educated African American Women. Maternal and Child Health Journal 5, 95–107. [DOI] [PubMed] [Google Scholar]
- Kaholokula J.K.a., Grandinetti A, Keller S, Nacapoy AH, Mau MK, 2012. Association between perceived racism and physiological stress indices in Native Hawaiians. J. Behav. Med 35, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE, 2002. Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. Journal of clinical epidemiology 55, 696–710. [DOI] [PubMed] [Google Scholar]
- Karlsen S, Nazroo JY, 2002. Relation between racial discrimination, social class, and health among ethnic minority groups. Am. J. Public Health 92, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME, 2003. The psychobiology of stress. Current directions in psychological science 12, 124–129. [Google Scholar]
- Kershaw KN, Lewis TT, Roux AVD, Jenny NS, Liu K, Penedo FJ, Carnethon MR, 2016. Self-reported experiences of discrimination and inflammation among men and women: The multi-ethnic study of atherosclerosis. Health Psychol. 35, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Williams DR, 1999. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav, 208–230. [PubMed] [Google Scholar]
- Krieger N, 1990. Racial and gender discrimination: risk factors for high blood pressure? Social science & medicine (1982) 30, 1273–1281. [DOI] [PubMed] [Google Scholar]
- Krieger N, Kosheleva A, Waterman PD, Chen JT, Koenen K, 2011. Racial discrimination, psychological distress, and self-rated health among US-born and foreign-born Black Americans. Am. J. Public Health 101, 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Sidney S, 1996. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. Am. J. Public Health 86, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM, 2005. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Soc. Sci. Med 61, 1576–1596. [DOI] [PubMed] [Google Scholar]
- Landrine H, Klonoff EA, 1996. The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology 22, 144–168. [Google Scholar]
- Larson A, Gillies M, Howard PJ, Coffin J, 2007. It’s enough to make you sick: the impact of racism on the health of Aboriginal Australians. Australian and New Zealand journal of public health 31, 322–329. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Sellers R, Neighbors HW, 2001. Perceived racism and self and system blame attribution: consequences for longevity. Ethnicity & disease 11, 711–721. [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL, 2010. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav. Immun 24, 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Williams DR, Tamene M, Clark CR, 2014. Self-Reported Experiences of Discrimination and Cardiovascular Disease. Current cardiovascular risk reports 8, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JG, Barksdale DJ, 2008. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs 17, 201–208. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Blanco EI, Unger JB, Ritt-Olson A, Soto D, Baezconde-Garbanati L, 2011. Acculturation, Gender, Depression, and Cigarette Smoking Among US Hispanic Youth: The Mediating Role of Perceived Discrimination. J. Youth Adolesc 40, 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Lumley MA, Flack JM, Wegner R, Pierce J, Goetz S, 2016. A preliminary experimental examination of worldview verification, perceived racism, and stress reactivity in African Americans. Health Psychol. 35, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet‐Morin I, Hupbach A, Tu MT, Buss C, Walker D, Pruessner J, Mcewen BS, 2015. Beyond the stress concept: Allostatic load—A developmental biological and cognitive perspective. Developmental Psychopathology: Volume Two: Developmental Neuroscience, 578–628. [Google Scholar]
- Major B, Quinton WJ, McCoy SK, 2002. Antecedents and consequences of attributions to discrimination: Theoretical and empirical advances, Advances in experimental social psychology. Elsevier, pp. 251–330. [Google Scholar]
- Mays VM, Cochran SD, Barnes NW, 2007. Race, race-based discrimination, and health outcomes among African Americans. Annu. Rev. Psychol 58, 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2003. Mood disorders and allostatic load. Biological psychiatry 54, 200–207. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C, 2015. Mechanisms of stress in the brain. Nature neuroscience 18, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Rasgon NL, 2018. The Brain and Body on Stress: Allostatic Load and Mechanisms for Depression and Dementa, in: Strain JJ, Blumenfield M (Eds.), Depression As a Systemic Illness. Oxford University Press, England, pp. 14–36. [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J, 2008. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of personality and social psychology 94, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 133, 25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK, 2002. Chronic psychological stress and the regulation of pro- inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21, 531. [DOI] [PubMed] [Google Scholar]
- Myers HF, 2009. Ethnicity-and socio-economic status-related stresses in context: an integrative review and conceptual model. J. Behav. Med 32, 9–19. [DOI] [PubMed] [Google Scholar]
- NHLBI Obesity Education Initiative, N.I.H., 2000. North American Association for the Study of Obesity, Expert Panel on the Identification Treatment of Overweight Obesity in Adults. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. [Google Scholar]
- Nobel L, Roblin DW, Becker ER, Druss BG, Joski PI, Allison JJ, 2017. Index of cardiometabolic health: a new method of measuring allostatic load using electronic health records. Biomarkers 22, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPR/Robert Wood Johnson Foundation/Harvard T.H. Chan School of Public Health, 2017. Discrimination in America: Experiences and Views of African Americans, Discrimination in America.
- Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, Jones CP, Braveman P, 2009. “It’s the skin you’re in”: African-American women talk about their experiences of racism. An exploratory study to develop measures of racism for birth outcome studies. Maternal and child health journal 13, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Williams DR, Nwizu U, Gruenewald TL, 2017. Everyday Unfair Treatment and Multisystem Biological Dysregulation in African American Adults. Cult. Divers. Ethn. Minor. Psychol 23, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center, 2016. On Views of Race and Inequality, Black and Whites are Worlds Apart, Social and Demographic Trends.
- Picard M, Juster R-P, McEwen BS, 2014. Mitochondrial allostatic load puts the’gluc’back in glucocorticoids. Nature Reviews Endocrinology 10, 303. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ, 2005. Recommendations for blood pressure measurement in humans and experimental animals. Circulation 111, 697–716. [DOI] [PubMed] [Google Scholar]
- Pieterse AL, Todd NR, Neville HA, Carter RT, 2012. Perceived racism and mental health among Black American adults: a meta-analytic review. American Psychological Association. [DOI] [PubMed] [Google Scholar]
- Plummer DL, Slane S, 1996. Patterns of coping in racially stressful situations. Journal of Black Psychology 22, 302–315. [Google Scholar]
- Robinette JW, Charles ST, Almeida DM, Gruenewald TL, 2016. Neighborhood features and physiological risk: an examination of allostatic load. Health & place 41, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K, Hatch E, Gallacher J, 2014. Representativeness is not helpful in studying heterogeneity of effects across subgroups. International journal of epidemiology 43, 633–634. [DOI] [PubMed] [Google Scholar]
- Ruggles S, Genadek K, Goeken R, Grover J, Sobek M, 2010. Integrated Public Use Microdata Series: Version 6.0 [Machine-readable database]. Minneapolis: Minnesota Population Center, University of Minnesota [producer and distributor]. [Google Scholar]
- Sacks FM, Carey VJ, Anderson CA, Miller ER, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, 2014. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. Jama 312, 2531–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MT, Branscombe NR, Postmes T, Garcia A, 2014. The consequences of perceived discrimination for psychological well-being: a meta-analytic review. Psychol. Bull 140, 921. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA, 2012. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am. J. Public Health 102, 1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH, 2001. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. U. S. A 98, 4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS, 1997. Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Archives of internal medicine 157, 2259–2268. [PubMed] [Google Scholar]
- Seery MD, Holman EA, Silver RC, 2010. Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience. Journal of personality and social psychology 99, 1025. [DOI] [PubMed] [Google Scholar]
- Sellers RM, Caldwell CH, Schmeelk-Cone KH, Zimmerman MA, 2003. Racial identity, racial discrimination, perceived stress, and psychological distress among African American young adults. J. Health Soc. Behav, 302–317. [PubMed] [Google Scholar]
- Sellers RM, Copeland‐Linder N, Martin PP, Lewis RH, 2006. Racial identity matters: The relationship between racial discrimination and psychological functioning in African American adolescents. Journal of Research on Adolescence 16, 187–216. [Google Scholar]
- Selvin S, 2004. Statistical analysis of epidemiologic data. Oxford University Press. [Google Scholar]
- Seplaki CL, Goldman N, Glei D, Weinstein M, 2005. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental gerontology 40, 438–449. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF, 2011. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and psychopathology 23, 1167–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Mamun A, Kitzman H, Mandapati SR, Dodgen L, 2017. Neighborhood Disadvantage and Allostatic Load in African American Women at Risk for Obesity-Related Diseases. Preventing chronic disease 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW, 2015. Ethnic discrimination predicts poor self-rated health and cortisol in pregnancy: Insights from New Zealand. Soc. Sci. Med 128, 36–42. [DOI] [PubMed] [Google Scholar]
- Tomfohr LM, Pung MA, Dimsdale JE, 2016. Mediators of the Relationship Between Race and Allostatic Load in African and White Americans. Health Psychol. 35, 322–332. [DOI] [PubMed] [Google Scholar]
- Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng CH, Huang MH, Lewis TT, Kravitz HM, Seeman T, 2015. A Longitudinal Investigation of Race, Socioeconomic Status, and Psychosocial Mediators of Allostatic Load in Midlife Women: Findings From the Study of Women’s Health Across the Nation. Psychosom. Med 77, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Lampert R, Tennen H, Feinn R, 2015. Exposure to discrimination and heart rate variability reactivity to acute stress among women with diabetes. Stress and Health 31, 255–262. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Tennen H, Finan PH, Ghuman N, Burg MM, 2013. Self-reported Racial Discrimination and Endothelial Reactivity to Acute Stress in Women. Stress and Health 29, 214–221. [DOI] [PubMed] [Google Scholar]
- Williams DR, John DA, Oyserman D, Sonnega J, Mohammed SA, Jackson JS, 2012. Research on discrimination and health: an exploratory study of unresolved conceptual and measurement issues. Am. J. Public Health 102, 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, 2013. Racism and Health I: Pathways and Scientific Evidence. Am. Behav. Sci 57, 1152–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yan Y, Jackson JS, Anderson NB, 1997. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol 2, 335–351. [DOI] [PubMed] [Google Scholar]
- Wilson KB, Thorpe RJ Jr, LaVeist TA, 2017. Dollar for Dollar: Racial and ethnic inequalities in health and health-related outcomes among persons with very high income. Preventive medicine 96, 149–153. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK, 2014. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial–ethnic minority status. Psychoneuroendocrinology 50, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I, Poeggel G, Schnabel R, Braun K, 2003. Separation-induced receptor changes in the hippocampus and amygdala of Octodon degus: influence of maternal vocalizations. Journal of Neuroscience 23, 5329–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.