Abstract
At the intersection of the newly emerging fields of optoacoustic imaging and theranostic nanomedicine, promising clinical progress can be made in dismal prognosis of ovarian cancer. An acidic pH targeted wormhole mesoporous silica nanoparticle (V7-RUBY) was developed to serve as a novel tumor specific theranostic nanoparticle detectable using multispectral optoacoustic tomographic (MSOT) imaging. We report the synthesis of a small, < 40 nm, biocompatible asymmetric wormhole pore mesoporous silica core particle that has both large loading capacity and favorable release kinetics combined with tumor-specific targeting and gatekeeping. V7-RUBY exploits the acidic tumor microenvironment for tumor-specific targeting and tumor-specific release. In vitro, treatment with V7-RUBY containing either paclitaxel or carboplatin resulted in increased cell death at pH 6.6 in comparison to drug alone (p<0.0001). In orthotopic ovarian xenograft mouse models, V7-RUBY containing IR780 was specifically detected within the tumor 7X and 4X higher than the liver and >10X higher than in the kidney using both multispectral optoacoustic tomography (MSOT) imaging with secondary confirmation using near infrared fluorescence imaging (p<0.0004). The V7-RUBY system carrying a cargo of either contrast agent or an anti-neoplastic drug has the potential to become a theranostic nanoparticle which can improve both diagnosis and treatment of ovarian cancer.
1. Inroduction
Ovarian cancer remains a malignancy with a high fatality case rate. Clinical application of recent scientific advances which have markedly improved outcomes in some malignancies have had limited impact on the diagnosis and management of ovarian cancer. Unlike other gynecologic cancers, multiple factors contribute for delayed diagnosis of the ovarian cancer, such as; absence of the accurate serum biomarkers; non-specific signs and symptoms; anatomical location of the ovary, deep within the abdominal cavity; rare dissemination of the carcinoma through the vasculature.[1–4] While the overexpression of many factors, i.e. CA-125, folate receptor, and CA IX, represents a reasonable approach to identify and target ovarian cancer, the lack of specificity and sensitivity of these target molecules for ovarian tumors indicates that alternative tumor targets are required. An alternative ovarian tumor characteristic, extracellular acidic pH, represents a unique physical factor within the tumor microenvironment that can be exploited as a tumor target. The extracellular acidic pH of ovarian cancer results from several factors, i.e. altered cellular metabolism, tumor hypoxia, cancer antigens such as CA IX, and is associated with both inactivation of some chemotherapeutic agents as well as an immune suppressor [5–11]. To date, the development of ovarian tumor specific nanoparticles that identify early stage ovarian tumors remains unrealized. The lack of tools for early disease detection leads to frequent later state diagnosisand contributes to the high mortality rate of this malignancy.
To overcome these limitations, we have evaluated a newly emerging imaging modality, Multispectral photoacoustic imaging (MSOT) for the diagnosis of the ovarian cancer. MSOT is a powerful diagnostic tool in biomedical research which facilitates deep-tissue optical imaging beyond the penetration limits of the conventional methods with its capacity to form optoacoustic images at least 5 cm within the tissue.[12] The optoacoustic effect is the conversion of the absorbed electromagnetic energy (e.g., NIR light) into longer range, less scattered, high accuracy acoustic signals.[13] The selective absorption of the light at multiple wavelengths and slices enables 3D volumetric spectrally enriched imaging from deep within the living tissues in real time, and at high spatial resolution. The fine resolution of the MSOT allows for detection of the structures 78 μm and small vessels at <150μm in the organs including hemoglobin-rich structures.[14–16]
Recent estimates of the American Cancer Society indicated that in 2017, 22,440 women were diagnosed with ovarian carcinomas, and 14,080 women died from the disease; the association between newly diagnosed cases and deaths secondary to ovarian carcinomas underscores the lack of effective therapies.[17] Nanodelivery systems, both for diagnostic and therapeutic purposes, could meaningfully impact care for patients with ovarian cancer. However, successful translation of the nanotherapies into the clinic has been limited by lack of quantitative imaging systems to track these particles. Techniques which allow for greater image resolution and organ specificity and thereby enable longitudinal tracking of biodistribution/accumulation, pharmacokinetics, and efficacy in in vivo would represent a significant clinical advance.[18–20]
When imaging ovarian cancer high-contrast resolution of MRI helps to assess complex ovarian tumors, which frequently are obscured with other imaging modalities such as computed tomography and ultrasound; nonetheless, currently available imaging modalities remain largely ineffectual in identifying the early stages of ovarian cancer.[21, 22] Imaging modalities which capture nanoparticle that arrive at the tumor in a targeted fashion would enable early stage in vivo diagnosis as well as clinical management of the ovarian cancer. Previous work has found that multispectral optoacoustic tomography (MSOT) provides contrast-enhanced tomographic imaging of tumors at several centimeters of depth in clinical testing[12, 23], underscoring the promise of this modality.
By utilizing tumor-specific tags, nanodelivery systems can exploit the unique tumor microenvironment for highly specific tumor targeted drug delivery. [24–28] A number of design parameters govern the performance and behavior of nanoparticles in vivo for diagnostic or therapeutic use include; the choice of material, particle size and surface chemistry, particle shape, active vs passive targeting. In addition, evaluation of the nanodelivery systems in the context of more clinically revelant mouse models of cancer, e.g. orthotopic implantation, genetically modified mouse models, or patient derived tumors, further increases the potential for translation of the findings into the clinic in comparison to the more commonly utilized subcutaneously implanted tumors [29, 30]. The variety of nanosystems that have been developed to limit off-target toxicity, act as photothermal agents, and hold the promise for delivery of higher drug concentrations to tumors.[31–38] Of the many types of materials which are suitable for development as nanoparticles, a promising material, mesoporous silica, results in a nanoparticle that is characterized by their alterable pore topology. This permits them to be readily tunable based on the method of construction of the mesoporous silica nanoparticle.[39] Wormhole pores are a new approach to pore design within mesoporous silica nanoparticles; these can be visualized as a group of hollow, interconnected tubes with a lopsided worm-like appearance The wormhole pore structure creates a larger surface for higher adsorption of the cargo as compared to the more common hexagonally ordered pores. Additionally, the asymmetric conformation of the wormhole pores reduces the exit velocity for released cargo.[40] Nanoparticles with wormhole pores have a longer half-life in the blood, entrap more cargo per size, and have more favorable release kinetics than mesoporous nanoparticles with hexagonally ordered pores.[41] The MSNs of 15 – 50 nm in diameter are considered optimal for cellular uptake with the least possible toxicity.[42, 43] Several reports have demonstrated the biotolerability of mesoporous silica particles in various cancer cell lines [39, 44], establishing their strong potential for delivery of imaging dyes, and therapeutic agents.[45, 46]
The passive delivery of nanoparticles faces several key challenges, such as enhanced reticuloendothelial system uptake and a reduced permeability and retention effects and, to date, not demonstrated improvement in survival compared to standard drug, i.e. paclitaxel, and exhibit greater neurologic and hematologic toxicity.[47, 48] However, improving tumor specific targeting, optimizing the size and shape of nanoparticle and optimizing the tumor specificity of the gatekeeper may overcome these obstacles.[49, 50] Further, by exploiting tumor surface-specific features, an active targeting strategy can be developed which in turn enables the specific targeting of malignant cells. This approach optimizes dose delivery of anti-neoplastic agents and reduces the off-target toxicity compared to nonspecific targeting. Several studies have utilized pH based active targeting of the cancer microenvironment to enhance local drug concentrations at the target tissue and to minimize systemic exposures.[51] Chitosan-capped nanoparticles and with acidic extracellular pH targeting such as pH (low) insertion peptides, pHLIP V7, are considered as the most promising pH sensitive gatekeeping and targeting agents, respectively.
We report the synthesis of RUBY, a 25 nm particle with wormhole pores to allow for large capacity and favorable cargo release kinetics, which has a dual extracellular pH targeting system to improve tumor specificity. The IR780 imaging dye is carried as cargo and chitosan controls the release of the dye to orthotopic ovarian tumors. The size and shape of the biocompatible V7-RUBY have essential features including the ability to translocate into the cytoplasmic compartment for the release of the IR780 dye. The asymmetric morphology of wormhole pores provides a higher surface area for more cargo and results in slower cargo release. We also report on the anti-neoplastic efficacy of chemotherapy delivered with this nanosystem on ovarian cancer cell lines. We further report that MSOT detects the pH controlled release by chitosan of IR780 imaging dye from V7-RUBY in a murine mode with orthotopically implanted ovarian tumors.
2. Results and Discussion
2.1. Synthesis and characterization of biocompatible wormhole pore mesoporous silica core <40 nm.
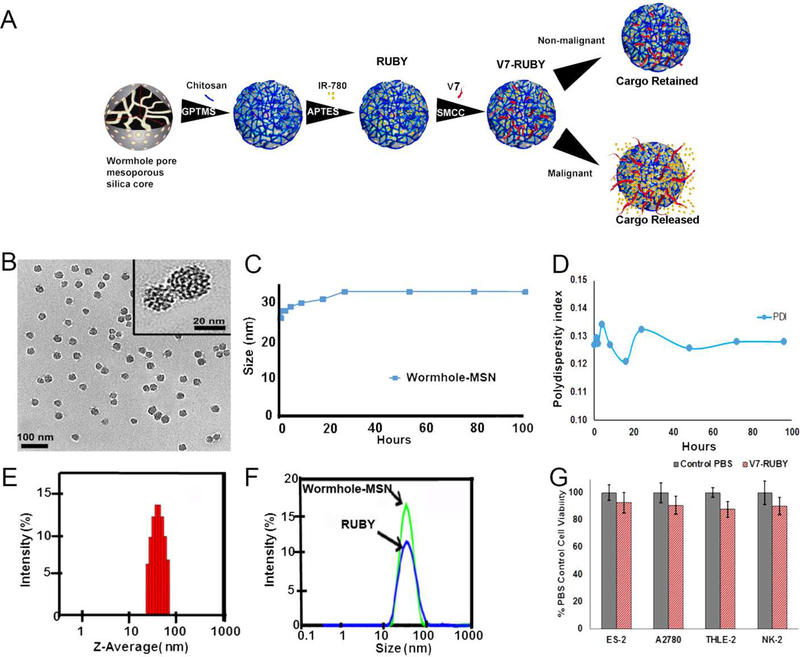
In this study, we have described the synthesis of <40 nm V7-RUBY nanoparticle featuring a wormhole pore structure within a mesoporous silica nanoparticle core (Figure 1A). The V7-RUBY demonstrated the capacity to deliver either IR780 dye or chemotherapeutic agents, paclitaxel or carboplatin, to ovarian cancer and serve as a theranostic nanoparticle. The geometry of the nanoparticles (e.g., size, pore architecture, shape, and aspect ratio) are critical factors which dictate the fate of the nanoparticle in biological systems. [40, 52, 53] The present study explores the use of surface modified biodegradable V7-RUBY with IR780 dye to identify ovarian cancer using optoacoustic imaging. Of note, tetramethyl orthosilcate (TMOS) was the silica component within the core silica component of the particle. The average particle diameter of Wormhole-MSN was 25 ± 3.1 nm with average pore diameter of 1.3 ± 0.2 nm based upon transmission electron microscopy (TEM) (Figure 1B). The size of base Wormhole-MSN particles was further characterized by dynamic light scattering (DLS) which indicated that at 24 h, Wormhole-MSNs reached their largest size (33.0 ± 1.94 nm), and further substantial increases in size were not observed. The mean Polydispersity Index (PDI) shows narrow polydispersed distribution as the PDI ranged from 0.12–0.135 over the course of 100 h. (Figure 1A-D). Brunauer-Emmett-Teller (BET) was calculated using BJH (Barrett-Joyner-Halenda) methodology.[54] BET analysis demonstrated that the total surface area of the particle was 749 m2 g−1 with pore diameter (1.359 nm) and pore volume (0.23 cm3 g−1.RUBY surface area is smaller than the recently reported calcined wormhole mesostructure (MSU-that had a surface area of 1030 m2/g with a pore diameter of 8.2 nm and pore volume of 0.5–2.0 cm3 g−1due in part to the use of TEOS and differing synthesis temperature (25–65 °C).[55] In contrast, Honeycomb-MSN particles have a reported surface area of 705 m2 g−1 with an average pore diameter of 6.3nm and a pore volume of 0.84 cm3 g−1.[56] Of note, the Honeycomb-MSN particles only contain one dimensional cylindrical mesopores, in contrast to the multi-dimensional wormhole pores (Supplemental Figure 1).
Figure 1. Characterization of the wormhole mesoporous silica nanoparticles.
A) Schematic illustration of the formation of RUBY. B) Transmission electron micrograph (TEM) images with an average particle size of 25 ± 3.1 nm. C) Dynamic light scattering (DLS) of the Wormhole-MSN at multiple timepoints during the synthesis.D) Polydispersity Index (PDI) shows narrow polydispersed distribution as the PDI ranged from 0.12–0.135 over the course of 100 h. E) Dynamic light scattering indicates the average size of the Wormhole-MSN is 33.03 nm at 24 h. F) Chitosan conjugation increased the size of the (RUBY) particle to 38.87 ±1.5 nm. G) In vitro toxicity evaluation of V7-RUBY in ovarian tumor cell lines, non-malignant hepatocytes, and proximal tubule kidney cells was evaluated.Treatment with 0.66 mM empty V7-RUBY particles did not exhibit cytotoxicity on ovarian tumor cells, non-malignant hepatocytes, or proximal tubule kidney cells in comparison to PBS treated controls (p=0.99,0.99,0.98,0.99).
When forming RUBY, the conjugation of the chitosan further increased the size of the Wormhole-MSN to 38.87 ±1.5 nm (Figure 1E-F). Before chitosan bio conjugation, the wormhole pore, mesoporous silica core had a positive potential of 25.45 mV± 5.62; however, after bioconjugation, the wormhole pore, mesoporous silica core displayed a higher positive mean potential value of 32 mV±1.24. Thus, the transition from lower to higher positive potential provides additional evidence for bioconjugation of chitosan to the Wormhole-MSN. Furthermore, 3-aminopropyl triethoxysilane (APTES) surface modification introduces amino groups on the surface of the particle by its silanization. However, when physiological pH was maintained at pH 7.4, the potential of RUBY decreased from 32 mV ±1.24 mV to 6.86 mV ±1.41 mV. 4-(N-maleimidomethyl) cyclohexane carboxylic acid N-hydroxysuccinimide ester (SMCC) was utilized to bind V7 peptide to RUBY particles.
V7-RUBY consists of a low pH insertion peptide (pHLIP) called V7 and a chitosan gatekeeper coating on the surface of the wormhole-MSN. At acidic pH, V7 undergoes a conformational change due to the protonation of Asp residues; the V7 structure changes to a helix that inserts into the lipid bilayer of the cellular membranes of cells in the low pH (6.6) microenvironment [57]. V7 does not enter the cell membrane via endocytosis or interactions with cell surface receptors; rather, it intercalates into the cell membrane due to the formation of a helical structure in the acidic microenvironment surrounding cancer cells. The intercalation of the V7 peptide causes minimal disruption of the phospholipid bilayers of the cellular membranes.[57–61]. The primary amine of the chitosan has a unique pKa of 6.5, which matches the weakly acidic pH surrounding malignant cells.[62] In this weak acidic microenvironment, amine groups of the chitosan are protonated which facilitates the release into the target tissue of the IR780 encapsulated inside the wormhole mesoporous silica nanoparticles. Of note, areas of inflammation have a much lower pH (< 6.0) and hence, V7-RUBY does not release IR780 in this setting. In the current study, we have developed V7-RUBY to target specifically the weak acidic tumor microenvironment surrounding cancer cells in order to identify using MSOT early stage ovarian epithelial cancers.
V7-RUBY particles were evaluated in both ovarian tumor cells as well as non-malignant hepatocytes and proximal tubule kidney cells.
Due to the very limited data available on potential toxicity of chitosan capped mesoporous silica particles, we treated both malignant and non-malignant cells with V7-RUBY for 24h and evaluated cell viability. No significant differences in cell viability were observed in any cell line after treatment with the particles when compared to PBS treated controls (p=0.99, 0.99, 0.98, 0.99) (Figure 1G). The non-cytotoxic effect of V7-RUBY nanoparticles were mainly due to the complete removal of the CTAB surfactant and is visually evident from the TEM images of the clean RUBY wormhole pores.
Entrapment efficiency % or loading capacity % of RUBY vs C-Honeycomb-MSN nanoparticles.
When RUBY and C-Honeycomb-MSN are evaluated using spectrophotometry at a concentration of less than 50 μg mL−1, RUBY and C-Honeycomb-MSN both have an encapsulation efficiency % or loading capacity % of essentially 100%; however, based on a constant pattern of the chitosan gatekeeper, in a concentration of 50 μg ml−1 of either RUBY or C-Honeycomb-MSN, both demonstrated a decrease in entrapment efficiency % or loading capacity %. The results for Ruby are 86.67±0.005% and for C-Honeycomb-MSN, 82.7±0.005%. TMOS was used as a substrate in RUBY synthesis to form a small nanoparticle (25 nm) with a larger surface area. In contrast, C-Honeycomb-MSN synthesis used TEOS as a substrate to form a larger nanoparticle (130 nm) with unrestricted pores. Our results indicate that the smaller size RUBY and larger C-Honeycomb-MSN have similar entrapment efficiency % or loading capacity %. With similar concentrations of RUBY or C-Honeycomb-MSN, the release kinetics of IR780 varied significantly due to different structures of the respective pores. A possible explanation may be that RUBY has a complex, possibly interconnected, asymmetric pore architecture which improves the pattern of IR780 release. Similarly, the burst release kinetics observed in C-Honeycomb-MSN is likely due to larger unrestricted symmetric pores. Also, as discussed, the relatively small size of RUBY is advantageous for specific in vivo targeting of tumor cells compared to the larger C-Honeycomb-MSN.
2.2. Release kinetics and Stability of the RUBY theranostic nanoparticle
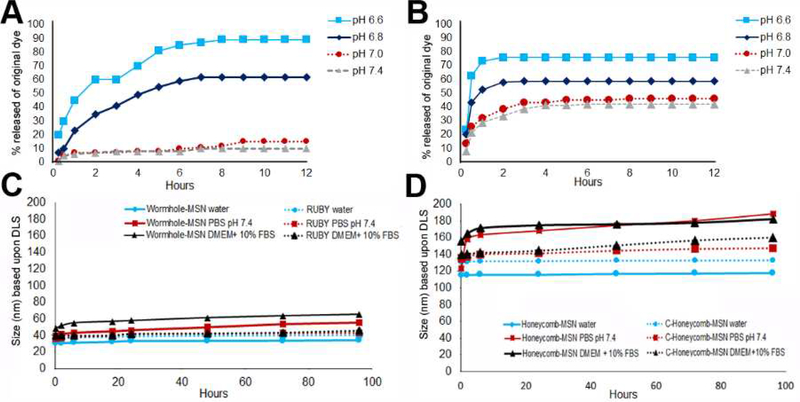
The major advantage of the asymmetric wormhole pore within the core structure of RUBY is the controlled release of cargo. While it should be noted that there is a small quantity of IR780 released from RUBY under physiological conditions (pH 7.0 −7.4), the IR780 release from RUBY gradually increased with the decrease in pH and time, supporting a higher tumor specificity. RUBY released IR780 dye at slow rate with 20% released within 30 minutes, increasing to 80% by 5 h, and 90% by 8–12 h (Figure 2A). This indicated that the wormhole pores within mesoporous silica core prolonged the release time of the entrapped IR780 dye via the gatekeeper. In contrast, prior studies that utilized symmetrical hexagonal (Honeycomb), pores within mesoporous silica coated particles demonstrated a burst release 62% within 30 min, increasing 73% at 1 h, and ultimately plateaued to 76% at 2 h (Figure 2B).[27] Of critical importance, C-Honeycomb-MSN released 21–38% within 2 h and 43–50% over 12 h at pH 7.4, indicating a poor retention of cargo at pH of normal tissues (pH 7.4).
Figure 2. Comparison of release kinetics and particle stability of RUBY in comparison to C-Honeycomb-MSN nanoparticles.
A) RUBY nanoparticles slowly releases a higher percentage, 90%, of the IR780 encapsulated in the RUBY nanoparticle at pH 6.6 (p<0.0001) B) C-Honeycomb-MSN, had a burst release kinetic profile with 62% released within 30 min. C) Wormhole-MSN and RUBY stability. D) C-Honeycomb-MSN with Honeycomb-MSN stability.
In the context of using theranostics in oncology, RUBY had a slower release profile (80 % release in 5 h) that is due to the wormhole asymmetric pore arrangement which reduces the exit velocity of the IR780 dye. This wormhole structure was also a benefit of minimumal release, 10%, of dye at pH 7.4. RUBY demonstrated delayed release kinetics achieved with wormhole pores at pH values associated with malignancies, i.e. pH 6.8–6.6, and retained the cargo under healthy physiological pH 7.4. This could significantly improve the release kinetics of cargo, i.e. chemotherapy drug or dye.
Over the course of 100 h at pH 7.4, the stability of the Wormhole-MSN (core particle) and RUBY was compared with the Honeycomb-MSN and chitosan capped Honeycomb-MSN (C-Honeycomb-MSN) in water, PBS, and DMEM + 10% FBS (Figure 2C-D). Chitosan capping for both the Wormhole-MSN and Honeycomb-MSN protected the particles from large increases in size. After 100 h pH 7.4, RUBY increased in size to 43 nm in PBS and 45 nm in DMEM + 10% FBS. After 100 h at pH 7.4, C-Honeycomb-MSN increased in size to 147 nm in PBS 7.4 and 161 nm in DMEM + 10% FBS. As anticipated, all nanoparticles were stable in water. While the Wormhole-MSN was relatively stable based on size, the addition of the chitosan cap to RUBY resulted in improved particle stability and reduced large changes in diameter.
The pore architecture and shape related factors such as aspect ratio or edge geometrical effects interact with the movement of nanoparticles, cell-nanoparticle interactions, and release kinetics. The release kinetics of RUBY demostrated that its asymmetric wormhole pore arrangement delayed release of cargo at the pH associated with tumor, i.e. pH 6.8–6.6,[63–66] while retaining the cargo at non-malignant physiological pH 7.4. Due to the symmetric ordered pores of the Honeycomb-MSN which resulted in a burst release pattern of cargo within 1h (pH 6.6), the delayed release associated with the wormhole pore structure has greater potential impact within a clinical setting. In addition, the retention of cargo within the wormhole structure in comparison to the honeycomb pore structure indicated that pore architecture of the nanoparticle had a substantial impact on the particle release kinetics (Figure 2). In order to improve the lack of exposure of healthy cells to the drug and to expose the malignant cells to cytotoxic therapy, the release of cargo after the initial few hours is preferred.[67]
2.3. Propidium iodide (PI), a cell membrane impermeable dye, was utilized as a cargo to demonstrate the ability of V7-RUBY to deliver cargo into tumor cells within acidic environment based upon its differential fluorescence when bound to nucleic acids.
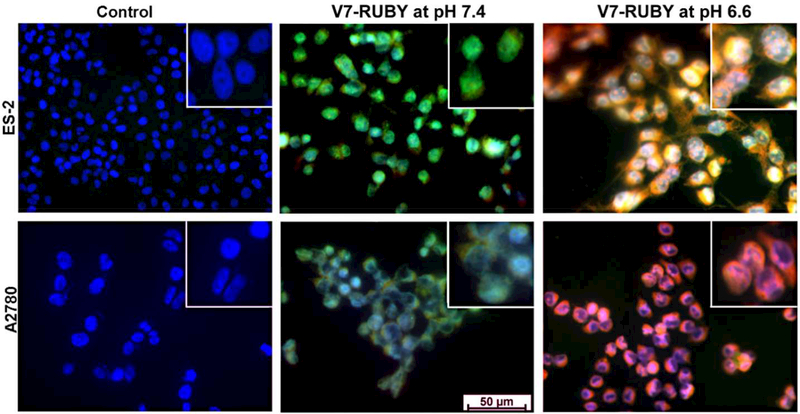
The V7-RUBY nanoparticle was able to release propidium iodide imaging dye into ovarian cancer cells grown at pH 6.6 which closely mimics the acidic extracellular tumor microenvironment. Usually, PI can only penetrate into dead cells resulting in red fluorescence upon binding to nucleic acids. However, nanoencapsulation of the PI within the V7-RUBY nanoparticle permitted transport across the live cell membrane so that PI was released in ovarian cancer cells with intact cell membranes. Both the ES-2 and A2780 ovarian cancer cell lines treated with pH 6.6 (Figure 3) had significant uptake of the PI released from the V7-RUBY as compared to the matching cell line treated with free PI not contained as cargo in V7-RUBY. Similarly, ES-2 and A2780 ovarian cancer cells exposed to pH 7.4 had less cellular uptake of PI (Figure 3). The extracellular accumulation of PI is indicated by green fluorescence whereas the bright yellow and red areas indicate the accumulation of the PI in the cytoplasm and nucleus. White areas are caused by the co-localization of the red, green and blue fluorescent signals and are interpreted to correlate with the binding of the PI to DNA. The PI displayed maximum red fluorescence values when bound to nucleic acids. The movements of V7-RUBY with PI cargo were tracked by the release and accumulation of PI. The above results demonstrate the pH based targeting and controlled release of PI from these nanoparticles. Specifically, the greatest release of PI was detected in the ovarian cancer cells maintained at pH 6.6 which mimics the in vivo acidic extracellular microenvironment of cancers.
Figure 3. Demonstration of V7-RUBY to specifically release cargo within tumor cells at extracellular pH of 6.6.
Cells treated at pH 6.6 had significant cell uptake and PI release from particle (red fluorescence) as compared to the matching cell line treated with free PI not contained as cargo in the V7-RUBY at pH 7.4. Similarly, ES-2 and A2780 ovarian cancer cells exposed to pH 7.4 had less cellular uptake of PI as identified by green fluorescence and lack of red fluorescence. Note that PI is cell membrane impermeable and fluoresces green when outside of the cell membrane, but fluoresces red when inside the cell membrane and bound to nucleic acid. All nuclei were stained with DAPI (blue).
2.4. In vitro assay determined the ability of V7-RUBY with IR780 as cargo to target ovarian cancer cells.
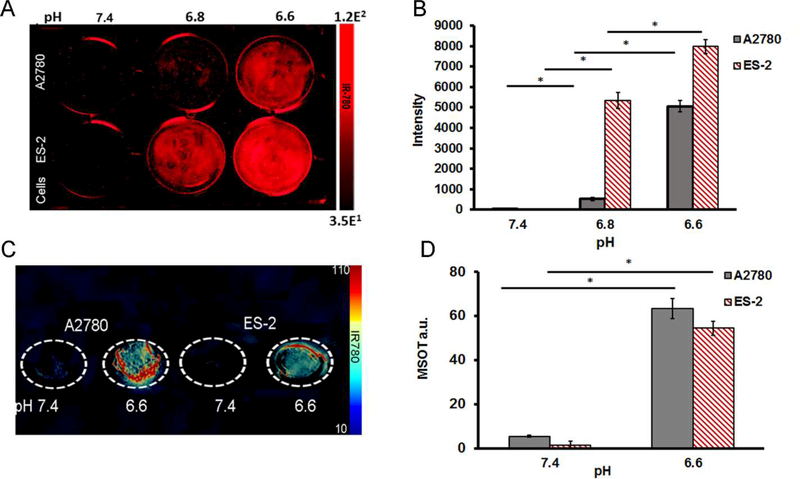
Both the ES-2 and A2780 ovarian cancer cell lines were treated with V7-RUBY with IR780 for 2 h under pH 7.4, 6.8, or 6.6 conditions. Subsequently, before imaging with the Odyssey Infrared Imaging System, these nanoparticles were washed five times in PBS with appropriate respective pH adjustments. Our NIR fluorescence results showed that both the A2780 and ES-2 cell lines took up a higher amount of V7-RUBY with IR 780 cargo at pH 6.8 and 6.6 as compared to pH 7.4 (23X and 222X increase for A2780 both p ≤ 0.0001), and (750X and 1122X for ES-2 both p ≤ 0.00007) (Figure 4 A-B). We further confirmed the ability of the V7-RUBY with IR780 cargo to release the IR780 dye in response to the acidic extracellular pH of the cancer cells. As the extracellular pH becomes more acidic, up to pH 6.6, higher amounts of IR780 were detected in both cell lines in tissue mimicking phantoms using MSOT (Figure 4 C-D) with the IR780 spectrum (Supplement Figure 2). The A2780 and ES-2 ovarian cancer cell lines were treated with V7-RUBY with IR780 cargo for 2h at pH 7.4 or 6.6; the cells were scraped, inserted into tissue mimicking phantoms and imaged by MSOT. The optoacoustic signal observed in the tissue phantoms confirmed increasing cellular uptake of the IR780 dye corresponding to increasing extracellular acidity. In A2780 and ES-2 cells grown in pH 6.6 media had significantly greater uptake of V7-RUBY and IR780 cargo 12X A2780 and 43X ES-2 (both p<0.0001) compared to the similar treatment at pH 7.4 (Figure 4 C-D).
Figure 4. Secondary confirmation of pH specificity of V7-RUBY within cells based upon NIR and MSOT imaging. A-B,
Odyssey Infrared Imaging results showed that both the A2780 and ES-2 cell lines took up a higher amount of V7-RUBY with IR780 at pH 6.8 and 6.6 as compared to pH 7.4. In A2780 cells, V7-RUBY uptake increased in pH 6.8 23X and pH 6.6 increased 222X compared to pH 7.4 both p≤0.0001. Likewise, ES-2 cell uptake of V7-RUBY at pH 6.8 increased 750X and pH 6.6 increased 1122X compared to pH 7.4 with p≤0.00007. C-D, Multispectral Optoacoustic Tomography (MSOT) imaging results showed that A2780 and ES-2 cells grown in pH 6.6 media had significantly greater uptake of V7-RUBY and IR780 12X A2780 and 43X ES-2 (both p<0.0001) compared to the similar treatment at pH 7.4. *p<0.0001.
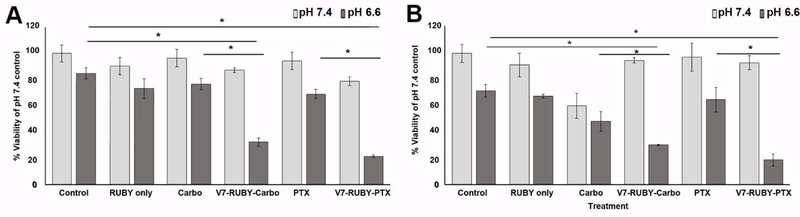
2.5. Acidic pH targeted V7-RUBY delivered chemotherapeutic drugs causing cell death.
Treatment with V7-RUBY loaded with carboplatin resulted in cytotoxicity at pH 6.6 in both ES-2 (32% viability) and A2780 (30% viability) cells in comparison to carboplatin only (76% and 48% viability) (p=0.018, p=0.034) (Figure 5A-B). Treatment with V7-RUBY loaded with PTX resulted in cytotoxicity at pH 6.6 in both ES-2 (21% viability) and A2780 (18% viability) cells in comparison to PTX only (69% and 64% viability) (p=0.007, p=0.008). Treatment with RUBY alone at pH 7.4 did not induce cytotoxicity (90% and 92%) in the ES2 and A2780 ovarian cancer cells respectively (p=0.89, p=0.92). Similarly, treatment with RUBY alone at pH 6.6 did not induce any greater toxicity as compared to the pH 6.6 PBS treatment (p=0.85, p=0.97). The lack of cytotoxicity after V7-RUBY containing drug treatment at pH 7.4 along with lack of cell death after RUBY treatment only suggests this particle has the ability to retain drug in the context of non-malignant pH in addition to its ability to deliver a therapeutic cargo to result in increased cytotoxicity at malignant pH 6.6.
Figure 5. V7-RUBY containing carboplatin or paclitaxel successfully killed tumor cells at pH 6.6 and retained drug at pH 7.4. A).
ES-2 and B) A2780 cells treated with V7-RUBY delivered carboplatin at pH 6.6 to significantly killed with ES-2 (32% viability) and A2780 cells (30% viability) at higher levels than carboplatin only (76% viability) and 48% viability (p=0.018,p=0.034). V7-RUBY delivered PTX at pH 6.6 which significantly resulted in significant cytotoxicity in ES-2 (21% viability) and A2780 (18% viability) cells in comparison to PTX only (69% and 64% viability) respectively, (p=0.007, p=0.008). In addition, at pH 7.4, V7-RUBY delivered carboplatin or PTX did not result in cell death in either ES-2 or A2780 cells. Likewise, RUBY only did not result in significantly increased cytotoxicity when compared to matched control pH 7.4 or 6.6 PBS (p=0.89, p=0.92, p=0.85, p=0.97). * p<0.05
2.6. V7-RUBY with IR780 cargo detects very small ovarian orthotopic xenograft models.
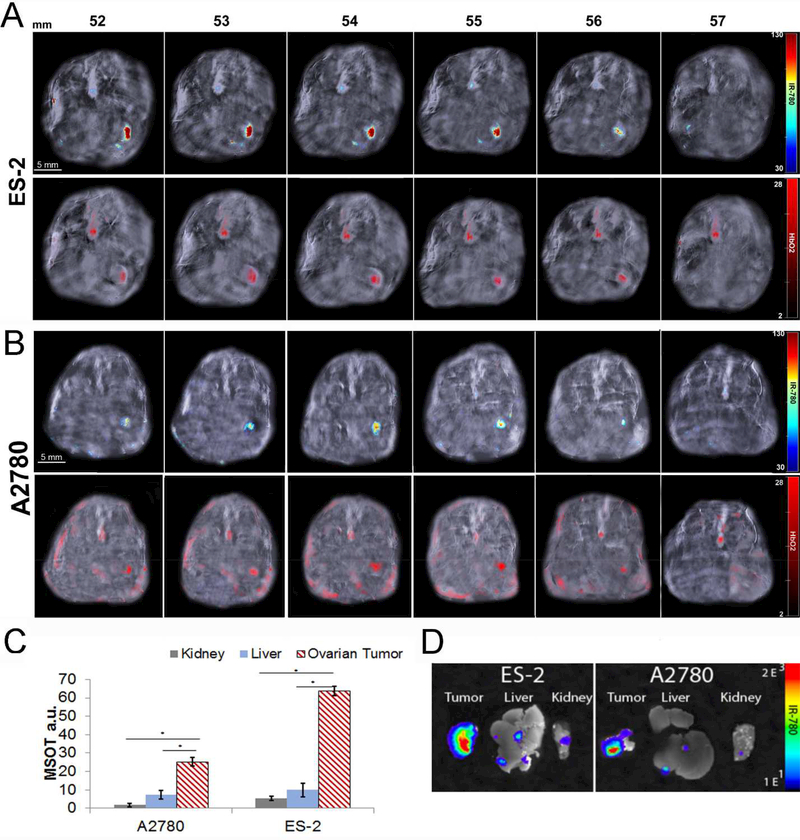
Athymic mice were orthotopically implanted with 2X105 (ES-2 or A2780) ovarian cancer cells at the bursa of the ovary. Ten days following the implantation, the mice were tail vein injected with V7-RUBY with IR780 cargo. After 4h, significant accumulation of the IR780 dye in the ES-2 (Figure 6A) and A2780 (Figure 6B) ovarian orthotopic xenografts was observed via MSOT. Mice injected with IR780 dye only did not have detectable signal at 4 h as observed with MSOT imaging (data not shown). MSOT imaging of V7-RUBY containing IR780 cargo indicated signal accumulated primarily within the tumor of both the ES-2 and A2780 models. IR780 signals were observed in serial sections with tumor confirmed using oxy-hemoglobin (Figure 6A-B). The results for the ES-2 model indicated that tumor accumulation was 63.8 a.u, liver 9.8 a.u., and kidneys 1.1 a.u. (Figure 6C). Tumor accumulation in the ES-2 model also was significantly increased compared to both liver and kidneys (p=0.00001 and p=0.00003). The A2780 tumors measured 25.2 a.u., liver 9.2 a.u. and kidneys 0.8 a.u. resulting in a significantly increased accumulation within the tumor compared to either the liver or kidneys (p=0.0004 and p=0.00001) (Figure 6C). The in vivo results indicated that these nanoparticles primarily and specifically accumulated in the targeted orthotopic xenograft. The organs collected at animal sacrifice also were analyzed using NIR fluorescence imaging and confirmed the results obtained with MSOT (Figure 6D).
Figure 6. V7-RUBY with IR780 cargo specifically accumulated within small ovarian orthotopic tumors as detected using MSOT with secondary NIR fluorescent confirmation.
Athymic mice were orthotopically implanted with (A) ES-2 or (B) A2780 ovarian cancer cells 2X105 at the bursa of the ovary. Ten days following the implantation, the mice were tail vein injected with V7-RUBY with IR780 (500 nM based upon dye/100μL). After 4h, significant accumulation of the IR780 dye in the (A) ES-2 or (B) A2780 ovarian orthotopic xenografts was observed via MSOT. Selected serial axial slices are shown (for orientation, the spleen is to the reader’s left and the vertebral column to the top) to visualize the tumor specific accumulation of V7-RUBY containing IR780. The bottom row of A and B indicates that oxyhemoglobin also has accumulated in the tumor secondary to its increased neovascularity (red signal). Note IR780 (top) and oxyhemoglobin (bottom) are seen in the aorta. C) Biodistribution and accumulation of V7-RUBY nanoparticles containing IR780 cargo was calculated using Region of Interest and reported as arbitrary units (a.u). There was accumulation of V7-RUBY containing IR780 cargo in the ES-2 and A2780 xenografts. The results for the ES-2 model indicates that tumor accumulation was (63.8 a.u), liver (9.8 a.u.), and kidneys (1.1 a.u.). Tumor accumulation in the ES-2 model was significantly increased compared to both liver and kidneys (p=0.00001 and p=0.00003). The data for A2780 tumors measure (25.2 a.u.), liver (9.2 a.u.) and kidneys (0.8 a.u.) resulting in a significantly increased accumulation within the tumor (p=0.0004 and p=0.00001). D) Ex vivo conformation of V7-RUBY was conducted using near infrared fluorescence imaging. The organs collected from ES-2 and A2780 cell xenografted animlas were collected for IR780 detection using NIR fluorescence imaging. The results for the ES-2 and A2780 model indicates that the IR780 accumulation were significantly higher in the tumor (p<0.0001) than the kidney and liver.
Because of the high sensitivity and specificity of MSOT for imaging of V7-RUBY nanoparticles carrying IR780 dye, ovarian cancers could be imaged only a few days after implantation (Figure 6A-D). Currently, the identification of early stage ovarian carcinomas is complicated by both the lack of clinical symptoms and sensitive and specific screening tests. This is the first report to demonstrate that the pH-triggered controlled release of imaging dye from biocompatible MSN with asymmetric wormhole pore nanoarchitecture can systematically target orthotopically implanted ovarian tumors. We further demonstrated the feasibility of high resolution MSOT based deep tissue imaging technology as a modality for detecting IR780 imaging dye for spatiotemporal identification of submillimeter ovarian carcinomas.
Currently, the identification of small ovarian carcinomas is difficult due to the asymptomatic features of ovarian carcinomas and the lack of adequate screening tests to identify this cancer. At present there is no reliable imaging method that identifies small ovarian tumors and the blood-based test, i.e. CA-125 have not proven to be prognostic.[68] Because of the high sensitivity and specificity of MSOT for imaging of IR780 dye carrying V7-RUBY nanoparticles, ovarian cancers could be imaged only a few days after implantation (Figure 6) providing essential data which could be translated into the clinic. While feasibility of the tumor targeted honeycomb-MSN has been demonstrated in various xenograft tumor models,[69] The large size and sub-optimal release kinetics of these particles makes them less attractive for clinical applications. In contrast, we describe V7-RUBY, a theranostic particle which has the capabilty to serve as a contrast agent for MSOT detection of ovarian cancer as well as the ability to improve tumor specific delivery of anti-neoplastic therapies in the context of the acidic extracellular tumor microenvironment. Utilizing MSOT, we further show tumor specific uptake of V7-RUBY with contrast agent cargo in orthotopically transplanted ovarian carcinoma.
Histopathological examination to identify in vivo toxicity of the V7-RUBY nanoparticle.
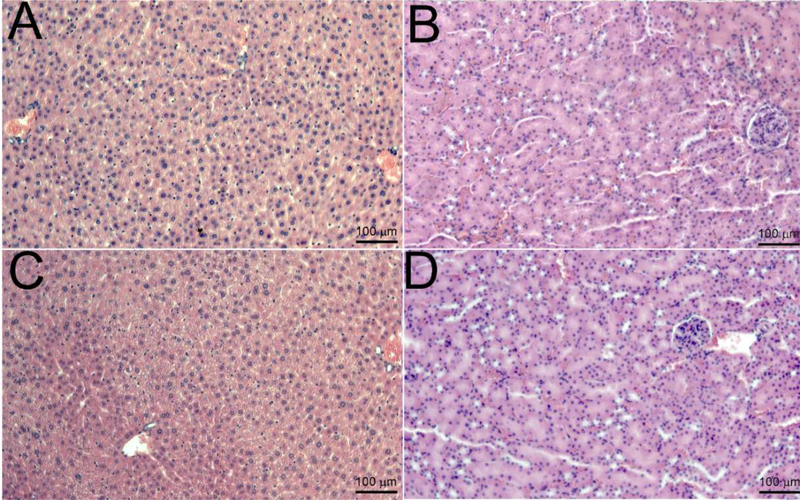
After 24 hrs of the treatment with V7-RUBY nanoparticle, H&E sections of the liver and kidney were analyzed to identify inflammatory infiltrates (Figure-7). Notably, our results indicate that after the V7-RUBY treatment, hepatocytes appeared to be viable and the liver did not exhibit an apparent change in inflammation compared to the liver from untreated mice (Figure 7). Similarly, the V7-RUBY treatment did not cause any apparent abnormalities in the kidney compared to untreated controls (Figure 7).
Figure 7: Histopathological examination to identify in vivo toxicity of the V7-RUBY.
Histological evaluation of the liver and kidney samples were analyzed (24hrs) after intravenous administration of the V7-RUBY. The tissue samples were fixed with paraformaldehyde and paraffin embedded followed by staining of sections from the paraffin block with hematoxylin & eosin. The tissue sections were evaluated at X 200. The scale bars represent 100 μm for all images. The pictures are representative of at least 4 independent sections. Notably, after the V7-RUBY treatment, our results indicate that hepatocytes appeared viable and the liver had no apparent changes in inflammation (Figure 7). Similarly, treatment with V7-RUBY did not cause detectable abnormalities in the kidney samples compared to untreated kidney controls (Figure 7).
3. Conclusion
Advances in both early detection and therapeutic options would represent meaningful clinical advances for the care of pateints with ovarian cancer. Nanodelivery systems hold the potential to address both these critical needs. V7-RUBY described here demonstrated the capacity to deliver either IR780 dye or chemotherapeutic agents, paclitaxel or carboplatin, to ovarian cancer and serve as a theranostic nanoparticle. This is the first report to demonstrate that the pH-triggered controlled release of imaging dye from biocompatible MSN with asymmetric wormhole pore nanoarchitecture can systematically target orthotopically implanted ovarian tumors. We further demonstrated the feasibility of high resolution MSOT based deep tissue imaging technology as a modality for detecting IR780 imaging dye for spatiotemporal identification of submillimeter ovarian carcinomas.
We have developed V7-RUBY, a <40 nm particle, that has dual extracellular acidic pH specificity via the chitosan gatekeeper and V7 peptide targeting. Although, the MSN geometry can contribute to optimal binding and in-vivo drug release, it is critical to incorporate additional mechanisms of controlled drug release to improve target specificity; thus, the surface of the wormhole mesoporous silica core was modified to doubly target acidic pH. Targeting wormhole pore mesoporous silica core with the V7 low pH insertion V7 peptide allowed for active targeting and anchoring within the membrane of orthotopic xenograft ovarian carcinoma cells within the acidic tumor microenvirnment. The wormhole pore architecture within the particle allowed for delayed release of cargo. The control the release of imaging dye from V7-RUBY at the acidic cancer sites permited the MSOT-based detection of small foci of the orthotopically-implanted ovarian tumors. Our data demonstrate the utility of the wormhole mesoporous silica core nanoarchitecture in improved release kinetics and the efficiency of the pH-release into ovarian carcinoma cells. This is analogous to the detection of the early primary ovarian carcinomas in women. The paucity of the presently available modalities for early detection of ovarian carcinomas, and of novel highly effective, low toxicity therapies, makes this approach worthy of rapid development.
4. Experimental Methods
Chemicals.
Cetrimonium bromide (CTAB, ≥99%molecular biology), tetramethyl orthosilicate (TMOS,≥99%), triethanolamine (TEA,≥99%), 3-glycidoxypropylmethyldiethoxysilane (GPTMS,≥98%), 3-aminopropyl triethoxysilane (APTES,99%), IR780(>99%), sodium phosphate monobasic, sodium phosphate dibasic, agar, ammonium nitrate, chitosan polymer, N-succinimidyl (maleimidomethyl) cyclohexanecarboxylate (SMCC), acetic acid, sodium hydroxide (NaOH,5M), methanol (99.8%) and ethanol (99.5%) were purchased from Sigma-Aldrich (St.Louis, MO,USA). V7 pHLIP was synthesized by and purchased from CS Bio Company (Menlo Park, CA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), L-glutamine and Roswell Park Memorial Institute (RPMI 1640) medium were purchased from Life Technologies (New York, NY, USA) and dissolved in autoclaved PBS.
Cell lines.
ES-2(CRL-1978) and A2780 (93112519) human ovarian carcinoma cell lines which were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and the European Collection of Authenticated Cell Cultures (ECACC) (Salisbury, UK), were used in studies as indicated. Additional non-malignant controls for hepatocytes (THLE-2 cells (CRL-2706)) and proximal tubule kidney cells (NK-2 cells (CRL-2190)) were obtained from ATCC. These were tested for cell viability after treatment with V7-RUBY.
Synthesis procedures for asymmetric wormhole pore mesoporous silica nanoparticle (Wormhole-MSN).
Wormhole pore mesoporous silica core synthesis reaction was initiated by the preparation of silica sol by mixing methanol (88 mmol L−1,100%), triethanolamine (TEA, 2.2 mL L−1, ≥99.0%), and Milli-Q-water (18.2 M·cm at 25 °C). The use of the b ase triethanolamine (TEA) has been reported to result in wormhole pore architecture.[70] Cetrimonium bromide (CTAB) (8 g/L) was added to the solution, and the temperature gradually increased and maintained at 800C (30 min) with constant stirring.[71] The alkoxide precursor tetramethyl orthosilicate (TMOS 11 mmol L−1, ≥99.0%) was added dropwise to the sol mixture. The solution turns turbid immediately after the reaction starts. The mixture was condensed by stirring vigorously for 24 h at 80°C. The excess of the CTAB from te TMOS-based particles was removed by dialysis with ethanol (100%) and Milli-Q-water (1:1 ratio).
Chitosan capping of Wormhole-MSN to form RUBY.
Wormhole pore mesoporous silica core was surface modified with pH responsive gatekeeper, chitosan. Chitosan (1%) was dissolved in 5% acetic acid (200 mL) under constant stirring for 24 h at room temperature. Wormhole-MSNs were dispersed in ethanol (100%) by ultrasonication (3 min). Addition of acetic acid decreased the pH of the Wormhole-MSN from 3.8 to 3.5. 0.1 g of the organic silane hybrid material fabricator, 3-glycidoxypropylmethyldie-thoxysilane (GPTMS) and 80 μL of the 3-aminopropyl triethoxysilane (APTES, 99%)was coupled with the wormhole mesoporous silica core particles in ethanol (100%) for 3 h under constant magnetic stirring to bind the chitosan to the particle. The combination of the GPTMS and APTES enhanced the binding efficiency of the chitosan on the surface of the wormhole mesoporous silica particle. Twenty mL of chitosan solution (1%) was added to the above mixture with constant stirring for 24 h resulting in chitosan capped Wormhole-MSN (RUBY). RUBY was washed with ethanol (70%) and stored at 4 °C.
RUBY conjugation with the V7 peptide (V7-RUBY).
100μg (0.66mM) of the RUBY (2 mL) were dissolved in 400 μM N-succinimidyl (maleimidomethyl) cyclohexanecarboxylate(SMCC) peptide binder (Sigma-Aldrich, St. Louis, MO, USA) and stirred for 10 min. To the above mixture, 667 μg of the V7 pHLIP peptide was added (V7 pHLIP sequence ACEEQNPWARY LEWLFPTETLLLEL, CS Bio, Menlo Park, CA, USA) with constant stirring for 16h.[60] V7-RUBY was washed 5X in PBS and collected by centrifugation at 13,776 g for 10 min. The supernatant was collected and the binding efficiency of the V7 peptide to RUBY was determined using a Bio-Rad protein assay. The protein assay indicated 440 μg of the total V7 was bound to the 100 μg of the RUBY.
Pore volume and specific surface area for Wormhole-MSN.
Wormhole silica nanoparticles were dialyzed with the Milli-Q-water for 24 hours and were dried in the Eppendorf vacuum at 30o C to obtain 100 mg of the particle. One hundred mg of powder was dried at 120°C under vacuum overnight using the vacuum pump of the TriStar analyzer (TriStar 3000, Norcross, Georgia, U.S. A). The seven-point BET isotherm and a 50-point adsorption/desorption isotherm in a liquid nitrogen bath were obtained, using pure nitrogen gas. Specific surface area (SSABET) was calculated using BET (Brunauer-Emmett-Teller) method. Pore diameter and volume were calculated using BJH (Barrett-Joyner-Halenda) methodology.[54]
Characterization of Wormhole-MSN
The wormhole pore mesoporous silica core was characterized by Dynamic Light Scattering (DLS).[72, 73] he synthesized TMOS-based particles were suspended in double distilled water (1.5 mL/L of synthesis). The size measurements were performed with a Zetasizer Nano ZS (Malvern Instruments, UK). The scattered light was detected at 1730 nm using a 5 mW HeNe laser at 633 nm wavelength. The ultrapure water preserved at room temperature was used in the data analysis with a refractive index of 1.33 and viscosity 0.89 in automatic mode using the DTS 6.0 instrument software. The morphological characterization of the wormhole pore mesoporous silica core was performed with transmission electron microscopy (TEM). Morphological characteristics such as average size, shape, and structure were qualitatively analyzed with a Tecnai-F20 transmission electron microscope (FEI, Hillsboro, Oregon, USA). Wormhole-MSN particles were analyzed by placing 10 μL of the sample on a 200-mesh lacey-carbon grid and before viewing, wick dried using filter paper. The average pore size of the Wormhole-MSN particles was calculated based on analysis of 22 core particles using the Image J software.
Assessment of potential toxicity of empty V7-RUBY on malignant and non-malignant cell lines.
pH specific media was utilized for evaluation of pH-specificity of particle uptake and cell viability of tumor cells. Briefly, phosphate buffer (25 mM) at the desired pH (7.4, 6.6) was prepared by dissolving sodium phosphate monobasic and dibasic solution dissolved in distilled water (Sigma-Aldrich, St. Louis, MO, USA). The phosphate buffer (25 mM) was autoclaved and the sterilized solution at the desired pH (7.4 or 6.6) was dissolved in 13.6 g of DMEM powder. The solution was enriched with 10% fetal bovine serum and 1% L-glutamine and filtered through grade-1 Whatman qualitative filter paper (Sigma-Aldrich, St. Louis, MO, USA). The pH of the solution was determined by pH meter (Denver Instrument Ultrabasic, Bohemia, NY, USA). The acidity and basicity adjustments of the pH solutions were performed with sterilized sodium hydroxide (1 M) or hydrochloric acid (1 M). For evaluation of potential toxicity of V7-RUBY on non-malignant cells, THLE-2 cells and NK-2 cells were grown in standard DMEM with 10% FBS and supplemented with the Lonza Bullet Kit (CC3170) without the addition of gentamycin, Amphotericin, and epinephrine. Ovarian cancer cells, ES-2 and A2780, along with non-malignant hepatocytes, THLE-2, and non-malignant proximal tubule kidney cells, NK-2, were plated into 96 well plates at 5 × 103 cells/ well for 24 h in standard DMEM media with 10% FBS. Both THLE-2 and NK-2 cells received extra supplements from the Lonza Bullet kit as indicated above. The cells were treated with 0.66 mM of V7-RUBY in a 96 well plate for 24 and 48 h at 37oC with 5% CO2. Cell viability was measured using ATPlite according to the manufacturer’s instructions (Perkin Elmer, Waltham, MA, USA). Adenosine triphosphate (ATP) levels were measured using Advanced Molecular Imager (AMI, Spectral Imaging Instruments, Tucson, AZ, USA) and normalized to phosphate-buffered saline (PBS) treated control.
Synthesis of Honeycomb mesoporous silica nanoparticles (Honeycomb-MSN).
To compare the importance of the mesoporous structure, i.e. Honeycomb vs Wormhole, we synthesize Honeycomb-MSNs in a similar manner as described previously.[74] The surfactant cetrimonium bromide (CTAB, 1.0 g) was added to a solution of deionized (DI) water (500 mL) and NaOH (4.0 mL, 5 M), then stirred vigorously for 4 h at 80 °C. Next, the silica monomer TMOS (5.0 mL ) was added dropwise to the solution and stirred for an additional 4 h. The resulting white precipitate was collected via centrifugation (1000 g, 25 min) and rinsed with ethanol three times. The sample was then dried under vacuum (45 °C) overnight. The resultant dry solid was then dissolved in a solution of ethanol (95%) and ammonium nitrate (10 mg/mL) and refluxed at 80°C for 6 h. Refluxing removed all CTAB from the solution, resulting in the honeycomb, mesoporous silica structure of the Honeycomb-MSNs. The precipitated Honeycomb-MSNs were collected via centrifugation and dried under vacuum (45 °C, 2 4 h) as described above.
Chitosan was added to Honeycomb-MSN surface (C-Honeycomb-MSN) to allow for pH responsive retention and release of a payload. To conjugate the Honeycomb-MSNs with chitosan, a stock solution of chitosan was first prepared. Preparation consisted of placing chitosan (2.0 g) in an aqueous solution of acetic acid (5 wt. %, 200 mL) and stirring at room temperature for 24 h. In a separate flask, the previously synthesized Honeycomb-MSNs (0.1 g) were dispersed in ethanol (10 mL) using sonication for 15 minutes. The pH of the mixture was lowered to 3.5 – 4.5 by the drop wise addition of acetic acid. (3-glycidoxypropyl) methyldiethoxysilane (GPTMS, 0.1 g) was added to the dispersion and stirred at room temperature for 3 h. Finally, the stock solution of chitosan was added to the Honeycomb-MSN mixture (20 mL) and stirred at room temperature for 24 h. Chitosan conjugated Honeycomb-MSNs (C-Honeycomb-MSN) were collected via centrifugation (5000 g) and rinsed using water and ethanol three times. The sample was then dried under vacuum overnight.
Characterization of Honeycomb-MSN.
The wormhole pore mesoporous silica core was characterized by Transmission electron microscopy and Dynamic Light Scattering (DLS) using the same procedures as for characterizing Wormhole-MSN. Briefly, the synthesized Honeycomb-MSN particles were suspended in double distilled water (1.5 mL/L of synthesis). Morphological characteristics such as average size, shape, and structure were qualitatively analyzed with a Tecnai-F20 transmission electron microscope with size measurements also conducted using a Zetasizer Nano ZS as described above (Supplemental Figure 1).
Determination of the Entrapment efficiency % and Loading Capacity % of the RUBY and C-Honeycomb-MSN.
IR780 imaging probe was dissolved in 100% ethanol with a concentration of 50 μg mL-1. 50 μg mL-1 of the RUBY or C-Honeycomb-MSN was encapsulated with the 50 μg mL-1 as described above. Both the IR 780 dye encapsulated RUBY or C-Honeycomb-MSN were centrifuged at 13,776g for 30minutes. The pellet was collected for the IR780 loaded nanoparticles and the supernatant for calculating the unabsorbed dye in the nanoparticle. In order to remove the IR780 from the exterior surface of the RUBY or C-Honeycomb-MSN, the IR780 loaded RUBY or C-Honeycomb-MSN were washed with distilled water for twice and collected after centrifugation at 13,776g for 30minutes. The supernatant in the washing process was combined with the previous supernatant solution. The mass of the IR780 loaded into RUBY or C-Honeycomb-MSN was calculated by subtracting the mass of IR780 in the supernatant from the total mass of IR780 in the initial solution [75, 76]. The amount of IR 780 adsorbed was analyzed with UVvis at the wavelength of 780 nm. The IR780 loading capacity (LC%) and entrapment efficiency were calculated using the eqn (1) and (2), respectively:
| (1) |
| (2) |
Fluorescent evaluation of particles
Synthesis of propidium iodide encapsulated V7-RUBY nanoparticles.
2 mg of the RUBY were added to the aqueous solution of the PI dye. The pH of the solution was maintained at 3.5 by the addition of 3 M HCl. The solution was stirred at room temperature for 24 h, an increase in the pH (pH8.0) of the solution using NaOH (5 M) locked in the PI dye loading in the RUBY. The PI dye loaded RUBY was stirred for an additional 3 h at room temperature to remove the unbound dye. Dialysis was performed using autoclaved Milli-Q water. RUBY was functionalized with V7 peptide (V7-RUBY).
Evaluation of pH specific propidium iodide cargo release from V7-RUBY was conducted via fluorescent microscopy.
ES-2 and A2780 cells were seeded at a density of 5 × 10 5 cells/well into 6-well plates containing 12-mm coverglasses. Both cell lines were grown overnight in DMEM with 10 % FBS and 1 % l-glutamine in 5 % CO2 at 37 °C. The cells were then serum-starved for 3 h. pH-specific DMEM that was buffered with PBS pH 7.4 or pH 6.6 (Fisher) was added to cells at 37 °C in 0 % CO 2. Following the pH change of the extracellular microenvironment, cells were treated with 80 μL PBS with pH 6.6 or 7.4 (control) or 80 μL of the PI encapsulated within V7-RUBY for 1 h. ES-2 and A2780 cell lines were fixed with 4% formaldehyde for 5 min at room temperature (Electron Microscopy Sciences). The cells were washed with PBS with pH values 7.4 or 6.6. After fixing, the coverglasses were mounted with ProLong with DAPI (Invitrogen, Waltham, MA, USA). The samples were dried overnight in darkness. The images were taken at a magnification of 400X using Zeiss Image Photomicroscope 2 (Carl Zeiss, Oberkochen, Germany). The filters used were DAPI, FITC, and Texas Red with the exposure time 20 ms (DAPI), 200 ms (FITC and Texas Red).
Assessment of V7-RUBY as a theranostic nanoparticle to deliver carboplatin or paclitaxel resulting in tumor cell death.
Ovarian cancer cells ES-2 and A2780 cells were seeded in 24 well plates at 5 × 10 5 cells/well and allowed to attach for 24 h. Prior to the drug treatment, the cells were pretreated with pH specific medium (pH 6.6 or 7.4) for 1 h. The cells were treated with pH-specific PBS, RUBY only (0.66 mM), carboplatin 30 μM, V7-RUBY containing carboplatin 30 μM (carboplatin dose), paclitaxel (PTX 600 nM), or V7-RUBY containing PTX (600 nM) for 3 h. The treatments were removed from all the wells after 3h and all media was replaced with 1 mL of the complete media (DMEM with 10%FBS, pH7.4). The cells were incubated for a total of 24 h. Cell viability was measured using the ATPlite assay detected using the Advanced Molecular imager (AMI, Spectral imaging instruments, Tucson, AZ). The results of the 3 replicate readings were normalized to controls at pH 7.4. The data were compared using a Wilcoxon non-parametric evaluation using SAS 9.3. Differences were considered significant at p < 0.05.
Secondary confirmation of V7-RUBY particle uptake within cells using Near Infrared and Optoacoustic imaging
Evaluation of ovarian cancer cell uptake of V7-RUBY containing IR780 using NIR fluorescent imaging and tissue mimicking phantoms.
5 × 105 ovarian cancer cells (ES-2 and A2780) were grown in 6 well plates with the pH media 7.4 or 6.6 respectively. The cells were incubated for 2 h with either RUBY or V7-RUBY, no peptide or conjugated with V7 peptide, respectively, containing IR780 (50 μg/mL). The cells were washed (3 X) with the pH specific PBS containing 10% FBS. The uptake of IR780 dye from the particles was measured in two ways: 1) by analyzing the fluorescence intensity using an Odyssey Infrared System (LI-COR) in both the cell lines with dosimetry measured using LI-COR software; 2) tissue mimicking phantoms were prepared for multispectral optoacoustic tomography (MSOT) analysis.[27] Cell pellets were added to fixed cylindrical tissue mimicking phantoms of 2 cm diameter. Phantoms were prepared using a gel made from distilled water containing Agar (Sigma Aldrich, St. Louis, MO, USA) for jellification (1.3% w/w) and an intralipid 20% emulsion (Sigma Aldrich, St. Louis, MO, USA) for light diffusion (6% v/v), resulting in a gel presenting a reduced scattering coefficient. ES-2 and A2780 cells treated with either RUBY or V7-RUBY containing IR780 were added to the 3 mm diameter cylindrical opening in the tissue phantoms. All samples were evaluated using MSOT with multiple wavelengths (680, 710, 730, 740, 760, 760, 770, 780, 800, 850 and 900nm) as previously described using the absorbance of IR780 (Supplemental Figure 2).[27, 74]
Assessment of V7-RUBY as a theranostic nanoparticle to deliver carboplatin or paclitaxel resulting in tumor cell death.
Ovarian cancer cells ES-2 and A2780 cells were seeded in 24 well plates at 5 × 10 5 cells/well and allowed to attach for 24 h. Prior to the drug treatment, the cells were pretreated with pH specific medium (pH 6.6 or 7.4) for 1 h. The cells were treated with pH-specific PBS, RUBY only (0.66 mM), carboplatin 30 μM, V7-RUBY containing carboplatin 30 μM (carboplatin dose), paclitaxel (PTX 600 nM), or V7-RUBY containing PTX (600 nM) for 3 h. The treatments were removed from all the wells after 3h and all media was replaced with 1 mL of the complete media (DMEM with 10%FBS, pH7.4). The cells were incubated for a total of 24 h. Cell viability was measured using the ATPlite assay detected using the Advanced Molecular imager (AMI, Spectral imaging instruments, Tucson, AZ). The results of the 3 replicate readings were normalized to controls at pH 7.4. The data were compared using a Wilcoxon non-parametric evaluation using SAS 9.3. Differences were considered significant at p < 0.05.
In vivo evaluation of biodistribution and tumor specificity of V7-RUBY Orthotopic (intrabursa) implantation of the human ovarian ES-2 and A2780 cancer cells in athymic mice.
Orthotropic implantation of the ovarian tumors in female athymic mice was developed based on previously demonstrated protocols (Supplement Figure 3).[77] Four weeks old female athymic mice were used in this study with strict adherence to the Wake Forest University Institutional Animal Care and Use Committee (IACUC) approved protocol. In order to reduce the background noise, a diet of 2920X alpha alfalfa-free feed (Harlan Laboratories, Indianapolis, IN, USA) was used. Mice were anesthetized with the Isoflurane and Betadine was used to prepare the right lower back. A small incision was made in the right lower back near the midline and the bursa of the ovary was externalized. ES-2 or A2780 ovarian cancer cells were suspended in a sterile tube containing DMEM media (serum free). Using a 30 gauge needle, 2.0 × 105 ES-2 or A2780 cells/ 10μL were injected into the bursa of the ovary. 10μL of PBS without cells were sham injected into two mice. A sterile cotton-tipped applicator was used to prevent peritoneal leakage of the ES-2 or A2780 tumor cells at the injection site. The ovary was returned to the abdomen. The skin and peritoneum in the incised region were sutured and mice were treated with Acetimenphen for pain. Underneath a warming blanket, mice recovered their full motility and then were returned to their cages with food and water ad libitum.
Detection of biodistribution and tumor specificity of V7-RUBY in vivo as detected using MSOT.
Ten days after orthotopic implantation (Supplemental Figure 3), mice (5 mice per group) containing ES-2 or A2780 tumors were intravenously injected with V7-RUBY containing IR780 (500 nM based upon dye/100μL) or 500 nM of IR780 dye only (data not shown). Mice were imaged using the MSOT InVision 512TF (iThera Medical, Munich, Germany) 4 h post injection. Multispectral optoacoustic tomographic (MSOT) imaging was performed as previously described.[16, 78] The mice were anesthetized with 1.6% isoflurane inhalant delivered in 0.8 L medical air and 0.1 L O2. All mice were evaluated at multiple wavelengths (680, 710, 730, 740, 760, 770, 780, 800, 850 and 900 nm) with 25 averages per wavelength and an acquisition time of 10 μs per frame. The water temperature was 33o C within the instrument during acquision. Mice were allowed to equilibrate within the instrument for 5 minutes prior to the start of imaging.
The raw data of the multispectral analyzed samples were reconstructed with the ViewMSOT software version 3.8 (Ithera Medical, Munich, Germany). Using ViewMSOT software wavelengths corresponding to the IR780 dye were reconstructed at a resolution of 75 μm. The multispectral processing was conducted using linear regression with ViewMSOT 3.8. The region of interest (ROI) was plotted over the tumor, liver, and kidney (all using 3.5 mm2 elipse) and the settings were kept constant for all the image slices obtained throughout the experiment. The signal intensity obtained from V7-RUBY containing IR780 at the tumor, liver, and kidney was represented in MSOT a.u units. The MSOT a.u. values for the nanoparticles were compared using SAS 9.3 Cary, NC, USA.
Ex vivo conformation of V7-RUBY was conducted using near infrared fluorescence imaging
Following MSOT imaging, mice were euthanized and organs, i.e. tumor, liver, and kidney, were removed and near infrared fluorescence was measured with AMI as in previous work.
Histological evaluation of liver and kidney
Athymic female mice were either PBS only or treated with V7-RUBY containing IR780 (500 nM based upon dye/100μL). Histological evaluation of the liver and kidney samples were analyzed (24hrs) after intravenous administration of the PBS or V7-RUBY. The tissue samples were fixed with paraformaldehyde and paraffin embedded followed by staining of sections from the paraffin block with hematoxylin & eosin. The tissue sections were evaluated at X 200 and were evaluated by a diagnostic pathologist (William Grizzle, MD, PhD). Four independent sections per organ were evaluated.
Supplementary Material
Acknowledgements
This work was supported by NIH grants R01CA205941, R01CA212350, R01EB020125, R01CA193437, R25CA134283, and P30CA012197. The authors would like to acknowledge the support of the Conn Center for Materials Characterization.
Footnotes
Ethics statement.
In this study, animal experiments were performed according to the guidelines set for the care and use of laboratory animals and with the rules formulated under the Animal Welfare Act by the United States Department of Agriculture (USDA). This protocol was approved by the IACUC Committee of the Comprehensive Cancer Center of Wake Forest University and animal studies performed at a facility accredited by AAALAC and USDA.
Notes
The author declares no competing financial interests
Supporting Information.
Honeycomb-MSN characterization, Spectrum for IR780 in V7-RUBY, Surgical method for orthotopic implantation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sasaroli D, Coukos G, Scholler N, Beyond CA125: the coming of age of ovarian cancer biomarkers. Are we there yet?, Biomark Med 3(3) (2009) 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jelovac D, Armstrong DK, Recent progress in the diagnosis and treatment of ovarian cancer, CA Cancer J Clin 61(3) (2011) 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Savelli L, Transvaginal sonography for the assessment of ovarian and pelvic endometriosis: how deep is our understanding?, Ultrasound Obstet Gynecol 33(5) (2009) 497–501. [DOI] [PubMed] [Google Scholar]
- [4].Lengyel E, Ovarian cancer development and metastasis, Am J Pathol 177(3) (2010) 1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y, Acidic extracellular microenvironment and cancer, Cancer Cell Int 13(1) (2013) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fang X, Yu S, Bast RC, Liu S, Xu H-J, Hu S-X, LaPushin R, Claret FX, Aggarwal BB, Lu Y, Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells, Journal of Biological Chemistry 279(10) (2004) 9653–9661. [DOI] [PubMed] [Google Scholar]
- [7].Cairns RA, Harris IS, Mak TW, Regulation of cancer cell metabolism, Nature reviews. Cancer 11(2) (2011) 85–95. [DOI] [PubMed] [Google Scholar]
- [8].Cairns R, Papandreou I, Denko N, Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment, Molecular Cancer Research 4(2) (2006) 61–70. [DOI] [PubMed] [Google Scholar]
- [9].Lardner A, The effects of extracellular pH on immune function, Journal of Leukocyte Biology 69(4) (2001) 522–530. [PubMed] [Google Scholar]
- [10].Fischer B, Muller B, Fischer KG, Baur N, Kreutz W, Acidic pH inhibits non-MHC-restricted killer cell functions, Clinical immunology (Orlando, Fla.) 96(3) (2000) 252–63. [DOI] [PubMed] [Google Scholar]
- [11].De Milito A, Fais S, Tumor acidity, chemoresistance and proton pump inhibitors, Future oncology (London, England) 1(6) (2005) 779–86. [DOI] [PubMed] [Google Scholar]
- [12].Knieling F, Neufert C, Hartmann A, Claussen J, Urich A, Egger C, Vetter M, Fischer S, Pfeifer L, Hagel A, Kielisch C, Gortz RS, Wildner D, Engel M, Rother J, Uter W, Siebler J, Atreya R, Rascher W, Strobel D, Neurath MF, Waldner MJ, Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity, The New England journal of medicine 376(13) (2017) 1292–1294. [DOI] [PubMed] [Google Scholar]
- [13].McNally LR, Mezera M, Morgan DE, Frederick PJ, Yang ES, Eltoum IE, Grizzle WE, Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology, Clin Cancer Res 22(14) (2016) 3432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burton NC, Patel M, Morscher S, Driessen WH, Claussen J, Beziere N, Jetzfellner T, Taruttis A, Razansky D, Bednar B, Ntziachristos V, Multispectral opto-acoustic tomography (MSOT) of the brain and glioblastoma characterization, Neuroimage 65 (2013) 522–8. [DOI] [PubMed] [Google Scholar]
- [15].Reber J, Willershauser M, Karlas A, Paul-Yuan K, Diot G, Franz D, Fromme T, Ovsepian SV, Beziere N, Dubikovskaya E, Karampinos DC, Holzapfel C, Hauner H, Klingenspor M, Ntziachristos V, Non-invasive Measurement of Brown Fat Metabolism Based on Optoacoustic Imaging of Hemoglobin Gradients, Cell Metab 27(3) (2018) 689–701 e4. [DOI] [PubMed] [Google Scholar]
- [16].Bhutiani N, Grizzle WE, Galandiuk S, Otali D, Dryden GW, Egilmez NK, McNally LR, Noninvasive Imaging of Colitis Using Multispectral Optoacoustic Tomography, Journal of nuclear medicine : official publication, Society of Nuclear Medicine 58(6) (2017) 1009–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC, Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening, CA Cancer J Clin 67(2) (2017) 100–121. [DOI] [PubMed] [Google Scholar]
- [18].Liu J, Wang P, Zhang X, Wang L, Wang D, Gu Z, Tang J, Guo M, Cao M, Zhou H, Liu Y, Chen C, Rapid Degradation and High Renal Clearance of Cu3BiS3 Nanodots for Efficient Cancer Diagnosis and Photothermal Therapy in Vivo, ACS Nano 10(4) (2016) 4587–98. [DOI] [PubMed] [Google Scholar]
- [19].Yong Y, Cheng X, Bao T, Zu M, Yan L, Yin W, Ge C, Wang D, Gu Z, Zhao Y, Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy, ACS Nano 9(12) (2015) 12451–63. [DOI] [PubMed] [Google Scholar]
- [20].Balasundaram G, Ho CJ, Li K, Driessen W, Dinish US, Wong CL, Ntziachristos V, Liu B, Olivo M, Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer, Int J Nanomedicine 10 (2015) 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alvarez Moreno E, Jimenez M de la Pena R Cano Alonso, Role of New Functional MRI Techniques in the Diagnosis, Staging, and Followup of Gynecological Cancer: Comparison with PET-CT, Radiol Res Pract 2012 (2012) 219546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sharma SK, Nemieboka B, Sala E, Lewis JS, Zeglis BM, Molecular Imaging of Ovarian Cancer, Journal of nuclear medicine : official publication, Society of Nuclear Medicine 57(6) (2016) 827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stoffels I, Morscher S, Helfrich I, Hillen U, Lehy J, Burton NC, Sardella TC, Claussen J, Poeppel TD, Bachmann HS, Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging, Science Translational Medicine 7(317) (2015) 317ra199–317ra199. [DOI] [PubMed] [Google Scholar]
- [24].Leung AW, Kalra J, Santos ND, Bally MB, Anglesio MS, Harnessing the potential of lipid-based nanomedicines for type-specific ovarian cancer treatments, Nanomedicine : nanotechnology, biology, and medicine 9(3) (2014) 501–522. [DOI] [PubMed] [Google Scholar]
- [25].Engelberth SA, Hempel N, Bergkvist M, Development of Nanoscale Approaches for Ovarian Cancer Therapeutics and Diagnostics, Critical reviews in oncogenesis 19(0) (2014) 281–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang Y, Cui D, Fang Y, Zhen X, Upputuri PK, Pramanik M, Ding D, Pu K, Amphiphilic semiconducting polymer as multifunctional nanocarrier for fluorescence/photoacoustic imaging guided chemo-photothermal therapy, Biomaterials 145 (2017) 168–177. [DOI] [PubMed] [Google Scholar]
- [27].Zeiderman MR, Morgan DE, Christein JD, Grizzle WE, McMasters KM, McNally LR, Acidic pH-Targeted Chitosan-Capped Mesoporous Silica Coated Gold Nanorods Facilitate Detection of Pancreatic Tumors via Multispectral Optoacoustic Tomography, Acs Biomaterials Science & Engineering 2(7) (2016) 1108–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hascicek C, Gun O, Nano drug delivery systems for ovarian cancer therapy.
- [29].Kersten K, de Visser KE, van Miltenburg MH, Jonkers J, Genetically engineered mouse models in oncology research and cancer medicine, EMBO Molecular Medicine 9(2) (2017) 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].House CD, Hernandez L, Annunziata CM, Recent Technological Advances in Using Mouse Models to Study Ovarian Cancer, Frontiers in oncology 4 (2014) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang H, Lovell JF, Advanced Functional Nanomaterials for Theranostics, Adv Funct Mater 27(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li D, Qin W, Xu B, Qian J, Tang BZ, AIE Nanoparticles with High Stimulated Emission Depletion Efficiency and Photobleaching Resistance for Long-Term Super-Resolution Bioimaging, Adv Mater 29(43) (2017). [DOI] [PubMed] [Google Scholar]
- [33].Maher S, Kumeria T, Wang Y, Kaur G, Fathalla D, Fetih G, Santos A, Habib F, Evdokiou A, Losic D, From The Mine to Cancer Therapy: Natural and Biodegradable Theranostic Silicon Nanocarriers from Diatoms for Sustained Delivery of Chemotherapeutics, Adv Healthc Mater 5(20) (2016) 2667–2678. [DOI] [PubMed] [Google Scholar]
- [34].Lvov Y, Wang W, Zhang L, Fakhrullin R, Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds, Adv Mater 28(6) (2016) 1227–50. [DOI] [PubMed] [Google Scholar]
- [35].Deng R, Yi H, Fan F, Fu L, Zeng Y, Wang Y, Li Y, Liu Y, Ji S, Su Y, Facile exfoliation of MoS2 nanosheets by protein as a photothermal-triggered drug delivery system for synergistic tumor therapy, RSC Advances 6(80) (2016) 77083–77092. [Google Scholar]
- [36].Khanal A, Ullum C, Kimbrough C, Garbett N, Burlison J, McNally M, Chuong P, El-Baz A, Jasinski J, McNally L, Tumor targeted mesoporous silica-coated gold nanorods facilitate detection of pancreatic tumors using Multispectral optoacoustic tomography, Nano Research 8(12) (2015) 3864–3877. [Google Scholar]
- [37].Yin W, Yan L, Yu J, Tian G, Zhou L, Zheng X, Zhang X, Yong Y, Li J, Gu Z, Zhao Y, High-Throughput Synthesis of Single-Layer MoS2 Nanosheets as a Near-Infrared Photothermal-Triggered Drug Delivery for Effective Cancer Therapy, ACS nano 8(7) (2014) 6922–6933. [DOI] [PubMed] [Google Scholar]
- [38].Chen Q, Liang C, Sun X, Chen J, Yang Z, Zhao H, Feng L, Liu Z, H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay, Proceedings of the National Academy of Sciences (2017) 201701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yanes RE, Tamanoi F, Development of mesoporous silica nanomaterials as a vehicle for anticancer drug delivery, Ther Deliv 3(3) (2012) 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma X, Yuan S, Yang L, Li L, Zhang X, Su C, Wang K, Fabrication and potential applications of CaCO 3–lentinan hybrid materials with hierarchical composite pore structure obtained by self-assembly of nanoparticles, CrystEngComm 15(41) (2013) 8288–8299. [Google Scholar]
- [41].Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, Discher DE, Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage, Mol Pharm 6(5) (2009) 1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ma N, Ma C, Li C, Wang T, Tang Y, Wang H, Moul X, Chen Z, Hel N, Influence of nanoparticle shape, size, and surface functionalization on cellular uptake, Journal of nanoscience and nanotechnology 13(10) (2013) 6485–98. [DOI] [PubMed] [Google Scholar]
- [43].He X, Nie H, Wang K, Tan W, Wu X, Zhang P, In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles, Analytical chemistry 80(24) (2008) 9597–9603. [DOI] [PubMed] [Google Scholar]
- [44].He Q, Zhang Z, Gao Y, Shi J, Li Y, Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles, Small 5(23) (2009) 2722–9. [DOI] [PubMed] [Google Scholar]
- [45].Choi Y, Jeong JH, Kim J, Mechanically Enhanced Hierarchically Porous Scaffold Composed of Mesoporous Silica for Host Immune Cell Recruitment, Adv Healthc Mater 6(8) (2017). [DOI] [PubMed] [Google Scholar]
- [46].Tao Y, Ju E, Ren J, Qu X, Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications, Adv Mater 27(6) (2015) 1097–104. [DOI] [PubMed] [Google Scholar]
- [47].Chirgwin J, Chua SL, Management of breast cancer with nanoparticle albumin-bound (nab)-paclitaxel combination regimens: a clinical review, Breast (Edinburgh, Scotland) 20(5) (2011) 394–406. [DOI] [PubMed] [Google Scholar]
- [48].Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, Mayer EL, Naughton M, Toppmeyer D, Carey LA, Perez EA, Hudis C, Winer EP, Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance), J Clin Oncol 33(21) (2015) 2361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li L, Lu Y, Jiang C, Zhu Y, Yang X, Hu X, Lin Z, Zhang Y, Peng M, Xia H, Mao C, Actively Targeted Deep Tissue Imaging and Photothermal-Chemo Therapy of Breast Cancer by Antibody-Functionalized Drug-Loaded X-Ray-Responsive Bismuth Sulfide@Mesoporous Silica Core-Shell Nanoparticles, Adv Funct Mater 28(5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wan L, Song H, Chen X, Zhang Y, Yue Q, Pan P, Su J, Elzatahry AA, Deng Y, A Magnetic-Field Guided Interface Coassembly Approach to Magnetic Mesoporous Silica Nanochains for Osteoclast-Targeted Inhibition and Heterogeneous Nanocatalysis, Adv Mater (2018) e1707515. [DOI] [PubMed] [Google Scholar]
- [51].Kimbrough CW, Khanal A, Zeiderman M, Khanal BR, Burton NC, McMasters KM, Vickers SM, Grizzle WE, McNally LR, Targeting Acidity in Pancreatic Adenocarcinoma: Multispectral Optoacoustic Tomography Detects pH-Low Insertion Peptide Probes In Vivo, Clin Cancer Res 21(20) (2015) 4576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Farokhzad OC, Langer R, Impact of nanotechnology on drug delivery, ACS nano 3(1) (2009) 16–20. [DOI] [PubMed] [Google Scholar]
- [53].Lin YS, Haynes CL, Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity, Journal of the American Chemical Society 132(13) (2010) 4834–42. [DOI] [PubMed] [Google Scholar]
- [54].Lehman SE, Morris AS, Mueller PS, Salem AK, Grassian VH, Larsen SC, Silica nanoparticle-generated ROS as a predictor of cellular toxicity: mechanistic insights and safety by design, Environmental Science: Nano 3(1) (2016) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Park I, Pinnavaia TJ, Large-Pore Mesoporous Silica with Three-Dimensional Wormhole Framework Structures, Microporous and mesoporous materials : the official journal of the International Zeolite Association 118(1–3) (2009) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nakajima K, Tomita I, Hara M, Hayashi S, Domen K, Kondo JN, Triblock copolymer-assisted synthesis of a hybrid mesoporous ethenylene–silica with 2D hexagonal structure and large pores, Journal of Materials Chemistry 15(24) (2005) 2362–2368. [Google Scholar]
- [57].Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM, Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane, Proceedings of the National Academy of Sciences of the United States of America 105(40) (2008) 15340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA, pHLIP peptide targets nanogold particles to tumors, Proceedings of the National Academy of Sciences of the United States of America 110(2) (2013) 465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Musial-Siwek M, Karabadzhak A, Andreev OA, Reshetnyak YK, Engelman DM, Tuning the insertion properties of pHLIP, Biochimica et biophysica acta 1798(6) (2010) 1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Andreev OA, Engelman DM, Reshetnyak YK, pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents, Molecular membrane biology 27(7) (2010) 341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kimbrough CW, Khanal A, Zeiderman M, Khanal BR, Burton NC, McMasters KM, Vickers SM, Grizzle WE, McNally LR, Targeting acidity in pancreatic adenocarcinoma: multispectral optoacoustic tomography detects ph-low insertion peptide probes in vivo, Clinical Cancer Research 21(20) (2015) 4576–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lim C, Lee DW, Israelachvili JN, Jho Y, Hwang DS, Contact time-and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces, Carbohydrate polymers 117 (2015) 887–894. [DOI] [PubMed] [Google Scholar]
- [63].Tannock IF, Rotin D, Acid pH in Tumors and Its Potential for Therapeutic Exploitation, Cancer research 49(16) (1989) 4373–4384. [PubMed] [Google Scholar]
- [64].Gerweck LE, Seetharaman K, Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer, Cancer research 56(6) (1996) 1194–1198. [PubMed] [Google Scholar]
- [65].Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA, pH imaging. A review of pH measurement methods and applications in cancers, IEEE Eng Med Biol Mag 23(5) (2004) 57–64. [DOI] [PubMed] [Google Scholar]
- [66].Mahoney BP, Raghunand N, Baggett B, Gillies RJ, Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro, Biochem Pharmacol 66(7) (2003) 1207–18. [DOI] [PubMed] [Google Scholar]
- [67].Mohanraj V, Chen Y, Nanoparticles-a review, Tropical journal of pharmaceutical research 5(1) (2006) 561–573. [Google Scholar]
- [68].Xu X, Wang Y, Wang F, Jia L, Zhou Y, Deng F, Qu J, Zhou B, Meng A, Fu B, Chen X, Qian Z, Wang J, Nadir CA-125 level as prognosis indicator of high-grade serous ovarian cancer, J Ovarian Res 6 (2013) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zeiderman MR, Morgan DE, Christein JD, Grizzle WE, McMasters KM, McNally LR, Acidic pH-targeted chitosan capped mesoporous silica coated gold nanorods facilitate detection of pancreatic tumors via multispectral optoacoustic tomography, ACS Biomater Sci Eng 2(7) (2016) 1108–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Möller K, Kobler J, Bein T, Colloidal Suspensions of Nanometer-Sized Mesoporous Silica, Advanced Functional Materials 17(4) (2007) 605–612. [Google Scholar]
- [71].Trewyn BG, Giri S, Slowing II, Lin VS, Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems, Chemical communications (31) (2007) 3236–45. [DOI] [PubMed] [Google Scholar]
- [72].Hassan PA, Rana S, Verma G, Making sense of brownian motion: colloid characterization by dynamic light scattering, Langmuir 31(1) (2014) 3–12. [DOI] [PubMed] [Google Scholar]
- [73].Domingos RF, Baalousha MA, Ju-Nam Y, Reid MM, Tufenkji N, Lead JR, Leppard GG, Wilkinson KJ, Characterizing manufactured nanoparticles in the environment: multimethod determination of particle sizes, Environmental science & technology 43(19) (2009) 7277–7284. [DOI] [PubMed] [Google Scholar]
- [74].Gurka MK, Pender D, Chuong P, Fouts B, Sobelov A, McNally M, Mezera M, Woo S, McNally LR, Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography, Journal of Controlled Release (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chang B, Guo J, Liu C, Qian J, Yang W, Surface functionalization of magnetic mesoporous silica nanoparticles for controlled drug release, Journal of Materials Chemistry 20(44) (2010) 9941–9947. [Google Scholar]
- [76].Papadimitriou S, Bikiaris D, Novel self-assembled core-shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release, 2009. [DOI] [PubMed]
- [77].Nunez-Cruz S, Connolly DC, Scholler N, An orthotopic model of serous ovarian cancer in immunocompetent mice for in vivo tumor imaging and monitoring of tumor immune responses, J Vis Exp (45) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kimbrough CW, Hudson S, Khanal A, Egger ME, McNally LR, Orthotopic pancreatic tumors detected by optoacoustic tomography using Syndecan-1, Journal of Surgical Research 193(1) (2015) 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.