Abstract
Background:
Mammographic breast density (BD) is an independent risk factor for breast cancer. The effects of bariatric surgery on BD are unknown.
Objectives:
To investigate BD changes following sleeve gastrectomy (SG).
Setting:
University hospital, United States.
Methods:
Fifty women with mammograms before and after SG performed from 2009 to 2015 were identified after excluding patients with a history of breast cancer, hormone replacement, and/or breast surgery. Patient age, menopausal status, comorbidities, hemoglobin A1c, and body mass index (BMI) were collected. Craniocaudal mammographic views before and after SG were interpreted by a blinded radiologist and analyzed by software to obtain breast imaging reporting and data system (BI-RADS) density categories, breast area (BA), BD, and absolute dense breast area (ADA). Analyses were performed using Chi-squared, McNemar’s test, t-test, and linear regressions.
Results:
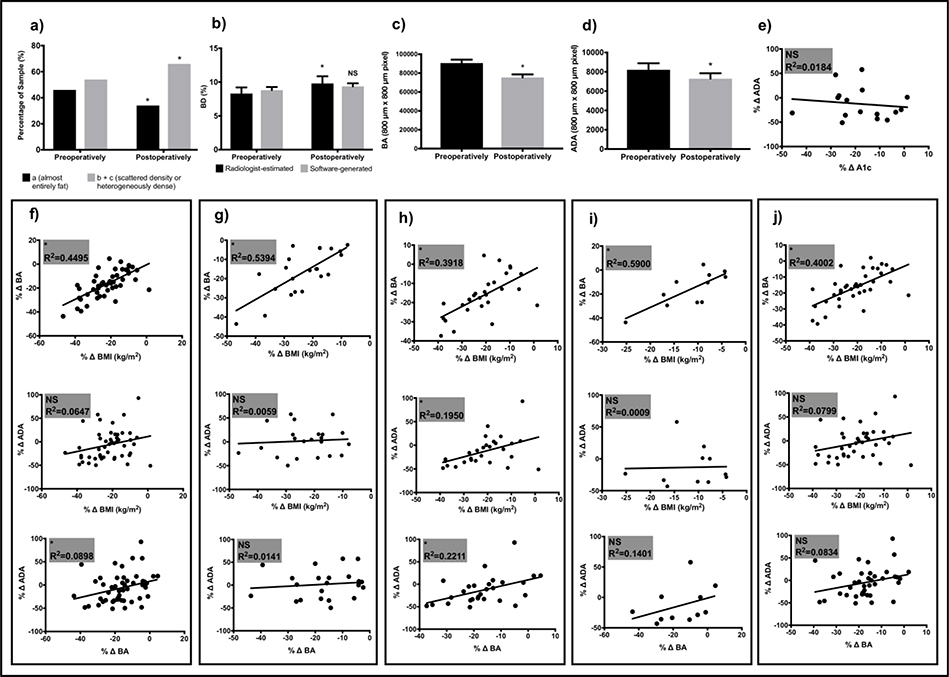
Radiologist’s interpretation revealed a significant increase in BI-RADS B+C category (68% vs. 54%; p=0.0095) and BD (9.8±7.4% vs. 8.3±6.4%; p=0.0006) after SG. Software analyses showed a postoperative decrease in BA (75398.9±22941.2 vs. 90655.9±25621.0 pixels; p<0.0001) and ADA (7287.1±3951.3 vs. 8204.6±4769.9 pixels; p=0.0314) with no significant change in BD. Reduction in ADA was accentuated in postmenopausal patients. Declining BA was directly correlated with BMI reduction (R2=0.4495; p<0.0001). Changes in breast rather than whole body adiposity better explained ADA reduction. Neither diabetes status nor changes in hemoglobin A1c correlated with changes in ADA.
Conclusions:
ADA decreases after SG, particularly in postmenopausal patients. Software-generated ADA may be more accurate than radiologist-estimated BD or BI-RADS for capturing changes in dense breast tissue after SG.
Keywords: Bariatric surgery, metabolic surgery, weight loss surgery, sleeve gastrectomy, SG, breast density, dense breast tissue, mammographic breast density, mammography, breast cancer, breast cancer risk, menopausal status, premenopausal, postmenopausal, glucose metabolism, diabetes
Graphical Abstract Legend
Absolute breast dense area may decrease after bariatric surgery. Preoperative (a) and postoperative (b) representative craniocaudal mammographic views (top) and corresponding software-processed images (bottom) showing ADA as white pixels within the breast boundary. ADA indicates absolute dense area; SG, sleeve gastrectomy; *, p < 0.05.

Introduction
Obesity rates continue to rise in the United States and around the world. (1,2) Along with its contribution to the metabolic syndrome, obesity is also associated with many cancers including postmenopausal breast cancer. (3,4) Obesity and dense breast tissue are both established risk factors for breast cancer. (5–7) Bariatric surgery is safe and the most effective therapy for obesity and related metabolic comorbidities with long-lasting results. (8,9) Bariatric surgery may also decrease postmenopausal breast cancer risk. (10)
The impact of bariatric surgery on dense breast tissue remains largely unexplored. Moreover, the present method for estimating density-derived risk may lack accuracy in patients after bariatric surgery. Currently, density-derived risk is universally apprised through BI-RADS breast density categorization. According to this classification, breast density is defined as the relative amount of fibroglandular elements to fat on mammography as almost entirely fatty (A), scattered areas of fibroglandular density (B), heterogeneously dense (C), and extremely dense (D). Interestingly, given the strong positive correlation between body mass index (BMI) and breast fat, breast density assessed by current criteria may decrease with increasing body mass index (BMI) while absolute dense tissue may increase with rising BMI. (11) Therefore, rapid shifts in BMI such as those observed after bariatric surgery, may significantly alter BI-RADS breast density classification without meaningfully capturing changes in the absolute amount of dense breast tissue.
Our goals with this study were to better understand the relationship between bariatric surgery and dense breast tissue and the description of BI-RADS breast density categorization applied to patients following metabolic surgery. We obtained breast area (BA), breast density (BD), and absolute dense area (ADA) measurements in bilateral craniocaudal mammogram views before and after sleeve gastrectomy (SG) interpreted by a blinded breast radiologist and independently analyzed through software segmentation of dense area. Given the rapid decrease in breast size experienced by patients undergoing bariatric surgery and the inherent complexity this may add to breast density estimation by BI-RADS, we hypothesize that results between blinded breast radiologist interpretation and independent software imaging analysis will differ. We therefore expect that in patients following bariatric surgery, ADA by software segmentation rather than BI-RADS breast density category may more meaningfully capture breast cancer risk derived from the amount of dense breast tissue.
Materials and Methods
Design
This study is a retrospective analysis of craniocaudal digital mammogram views obtained from patients before and after SG. Our primary outcomes are BA, dense area as percentage of breast area or BD, and segmented dense tissue or ADA. This study was approved by the Institutional Review Board at our university and was in compliance with the Health Information Portability and Accountability Act.
Subjects and Characteristics
All female patients (n=474) undergoing SG from July 2009 to May 2015 at a single-center academic institution were identified. Exclusions included history of breast cancer (n=4) or breast surgery (n=19), and no preoperative (n=311) or postoperative (n=90) mammogram. The final study population comprised 50 women. All patients underwent SG performed by one of four bariatric surgeons. Collected patient characteristics included sex, age, race, baseline comorbidities, diabetes status at surgery and remission at two years collected from surgery and endocrinology clinic notes, hemoglobin A1c, BMI at surgery and two years postoperatively, date of surgery, and date of preoperative and postoperative mammograms. Menopausal status at surgery and throughout the study was extracted from primary care and gynecology clinic notes. Six patients resumed menstruation within the study period and were categorized as premenopausal while 2 patients discontinued menstruation and were included in the postmenopausal subgroup. When menopausal status was not available (11 patients or 22% of the study cohort) in the medical record, 51 years or older was used as the threshold for menopause with patients 50 years or younger included in the premenopausal subgroup.
Mammogram Collection, Image Analysis, and Outcomes
Full field digital mammograms (FFDMs) were acquired with a GE Senographe Essential FFDM system with a pixel size of 100 μm x 100 μm and 16 bits per pixel. The data set of bilateral craniocaudal view mammograms from 50 patients before and after SG were analyzed. The images were interpreted by a blinded radiologist specializing in breast imaging. Radiologist-estimated outcome variables included BD and BI-RADS density category.
Images were also analyzed using a previously validated, radiologist-assisted automated method for segmentation of the dense fibroglandular area on mammogram. (12–14) Raw FFDMs were used as input and enhanced by a multiresolution preprocessing scheme, resulting in 800 μm x 800 μm pixel size images. The breast boundary was tracked through a gradient-based edge-tracking algorithm. The breast region was then enhanced by an adaptive dynamic range compression technique. Histogram analysis was performed and gray-level thresholding was automatically calculated to segment the dense tissue from the breast region. The original mammogram and the segmented mammogram were displayed side-by-side on a graphical user interface and reviewed by a radiologist who could refine the gray-level threshold interactively as needed. Software-generated outcome variables included BA, BD, and ADA.
Statistical Analysis
GraphPad Prism statistical software 7.0c (La Jolla, CA) was used to analyze data. The results are expressed as means ± standard error (SE). Analyses were performed using Chisquared, McNemar’s test, t-test, and linear regressions as appropriate. A P value <0.05 was considered to be statistically significant.
Results
Patient Characteristics and Imaging Outcomes for Entire Cohort
Clinical characteristics including detailed comorbidity listing and imaging outcomes for the entire cohort are offered in Table 1 and Figure 1a-d. Mean age was 50.82 (±6.18) years, 44% of women were premenopausal, 88% of patients were non-Hispanic white/Caucasian, and mean number of comorbidities per patient was 6.62 (±2.51). BMI significantly decreased from 46.2 (±6.8) kg/m2 before surgery to 35.7 (±5.7) kg/m2 postoperatively. Preoperative mammograms were performed on average 5.68 (±3.81) months before the date of surgery (DOS) while postoperative studies were obtained 24.74 (±2.91) months after DOS. Software analyses showed a postoperative decrease in BA (75398.9±22941.2 vs. 90655.9±25621.0 pixels; p<0.0001) and ADA (7287.1±3951.3 vs. 8204.6±4769.9 pixels; p=0.0314) with no change in BD. Radiologist interpretation revealed a significant postoperative increase in B+C category (68% vs. 54%; p=0.0095) and BD (9.8±7.4% vs. 8.3±6.4%; p=0.0006).
Table 1.
Characteristics and Imaging Outcomes for All Patients (n = 50)
| Baseline Characteristic | Mean (±SD) or % | |||
|---|---|---|---|---|
| Months from preoperative study to DOS | 5.7 (3.8) | |||
| Months from DOS to postoperative study | 24.7 (2.9) | |||
| Age at surgery | 50.8 (6.2) | |||
| Premenopausal | 44.0 | |||
| White or Caucasian, non-Hispanic | 88.0 | |||
| Private insurance | 90.0 | |||
| Baseline Comorbidity | ||||
| Asthma | 18.0 | |||
| Cholelithiasis | 28.0 | |||
| CVD (angina, CAD, CHF, PCI, rhythm disorder and PVD) | 64.0 | |||
| Diabetes | 38.0 | |||
| GERD | 54.0 | |||
| Hyperlipidemia | 56.0 | |||
| Hypertension | 56.0 | |||
| Liver disease | 24.0 | |||
| Musculoskeletal | 76.0 | |||
| Psychological | 78.0 | |||
| Respiratory (including COPD, lung disease) | 20.0 | |||
| Sleep apnea | 62.0 | |||
| History of smoking | 42.0 | |||
| Urinary disease | 46.0 | |||
| Total number of comorbidities | 6.6 (2.5) | |||
| Change | Preoperatively | Postoperatively | ||
| BMI and Imaging Outcome | Mean (±SD) | Mean (±SD) | Mean (±SD) | P |
| BMI; kg/m2 | 46.2 (6.8) | 35.7 (5.7) | <0.0001 | |
| BMI change; kg/m2 | -10.4 (5.5) | |||
| BA$ | 90655.9 (25621.0) | 75398.9 (22941.2) | <0.0001 | |
| BA change$ | -15256.9 (10773.5) | |||
| ADA$ | 8204.6 (4769.9) | 7287.1 (3951.3) | 0.0314 | |
| ADA change$ | -917.4 (2929.0) | |||
| BD (software) | 8.8 (3.2) | 9.3 (3.2) | 0.1848 | |
| BD change (software) | 0.5 (2.8) | |||
| BD (radiologist) | 8.3 (6.4) | 9.8 (7.4) | 0.0006 | |
| BD change (radiologist) | 1.5 (2.9) | |||
SD indicates standard deviation; DOS, date of surgery; CVD, cardiovascular disease; CAD, coronary artery disease; CHF, congestive heart failure; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary disease; BMI indicates body mass index; BA, breast area
, area measured as number of pixels 800 μm x 800 μm; ADA, absolute dense area; BD, breast density.
Figure 1.
Summary of Imaging Outcomes Over Time and Relationship of Weight loss and Hemoglobin A1c with Breast Area, and Absolute Dense Area. BI-RADS density categorization over time (a). Radiologist-estimated and software-calculated BD (b). BA (c) and ADA (d) given by the numbers of 800 μm x 800 μm pixels over time. Linear regression of percent average hemoglobin A1c change and percent change in ADA (e). Linear regression of percent change in BMI to percent change in BA and ADA and percent change in BA to percent change in ADA for entire cohort (f), premenopausal (g), postmenopausal (h), patients with persistent diabetes (i), and patients without or remitted diabetes (j). * indicates statistical significance with p value < 0.05; NS, non-significance; BD, breast density; BA, breast area; ADA, absolute dense area; BMI, body mass index.
Subgroup Analysis by Menopausal Status
The cohort was stratified by menopausal status and subgroup analyses were performed (Table 2). Twenty-two women or 44% of the cohort were identified as being premenopausal. Except for age at surgery which, as expected, was significantly different between pre and postmenopausal patients, variables including race, pre and postoperative mammograms months from DOS, and BMI reduction did not differ between subgroups. Software analyses revealed a significant and equivalent postoperative decrease in BA in both subgroups. However, ADA was noted to significantly decrease for patients in the postmenopausal subgroup only. Consequently, BD increased in premenopausal patients and was unchanged in postmenopausal women. Radiologist interpretation revealed a significant postoperative increase in BD regardless of menopausal status.
Table 2.
Characteristics and Imaging Outcomes by Menopausal Status
| Premenopausal(n=22) | Postmenopausal (n=28) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preopera tive | Postopera tive | ||||||
| Mean (±SD or % | Mean (±SD) | Mean (±SD) | Pa | Mean (±SD) or % | Mean (±SD) | Mean (±SD) | Pa | Pb | |
| White or Caucasian, non-Hispanic | 86.3 | 89.2 | 0.754 | ||||||
| Months from preoperative study to DOS | 6.4 (2.4) | 6.7 (2.5) | 0.5998 | ||||||
| Months from DOS to postoperative study | 24.2 (2.6) | 25.1 (3.1) | 0.3163 | ||||||
| Age at surgery | 46.1(3.6) | 54.4 (5.2) | <0.0001 | ||||||
| BMI; kg/m2 | 48.4(7.9) | 36.8 (5.3) | <0.0001 | 44.4 (5.1) | 34.9 (6.0) | <0.0001 | |||
| BMi change; kg/m2 | -11.6(6.2) | -9.4 (4.8) | 0.1591 | ||||||
| 94816.4 (24359.1) | 79119.6 (22759.5) | <0.0001 | 87387.0 (26544.6) | 72475.5 (23067.4) | <0.0001 | ||||
| BA change$ | -15696.7 (11631.5) | -14911.4 (10253.5) | 0.801 | ||||||
| ADA$ | 8103.0 (3616.3) | 7823.6 (3733.9) | 0.61 31 | 8284.4 (5577.1) | 6865.6 (4131.7) | 0.0243 | |||
| ADA change$ | -279.4 (2553.8) | -1418.7 (3147.2) | 0.1747 | ||||||
| BD (software) | 8.3 (2.6) | 9.7 (3.6) | 0.02 72 | 9.2 (3.7) | 9.0(2.8) | 0.793 | |||
| BD change (software) | 1.4 (2.7) | -0.1 (2.7) | 0.0561 | ||||||
| BD (radiologist) | 7.5 (5.2) | 8.8 (6.3) | 0.03 03 | 8.9 (7.2) | 10.5 (8.3) | 0.0098 | |||
| BD change (radiologist) | 1.3 (2.7) | 1.6 (3.0) | 0.7657 | ||||||
SD indicates standard deviation; DOS, date of surgery; BMI indicates body mass index; BA, breast area
indicates area measured as number of pixels 800 μm x 800 μm; ADA, absolute dense area; BD, breast density given as percentage ADA/BA
comparison between preoperative and postoperative values
comparison by menopausal status.
Subgroup Analysis by Diabetes Status
We also evaluated the potential association of glucose metabolism and ADA by stratifying the cohort by diabetes status (Table 3). The prevalence of diabetes preoperatively was 38%. Eight of 19 patients or 42% experienced remission of diabetes, defined as hemoglobin A1c in the normal range in the absence of antidiabetic medication including oral agents and insulin, following SG. Patient with diabetes that persisted postoperatively (n=11) were compared to a second subgroup (n=39) that included patients without diabetes (n=31) and those experiencing remission of diabetes (n=8) after SG. Characteristics including age, race, pre and postoperative mammograms months from DOS, and BMI reduction did not differ between subgroups. Software analyses showed a significant and equivalent postoperative decrease in BA after stratification by diabetes status. However, postoperative ADA did not significantly differ from preoperative values neither for patients with persistent diabetes nor for those with absent or remitted diabetes. Similar findings were appreciated for BD. Radiologist interpretation showed a significant postoperative increase in BD for patients with absent or remitted diabetes only. Lastly, no correlation was appreciated between average hemoglobin A1c percent change and percent change in ADA (R2=0.0184; p=0.6168) (Figure 1e).
Table 3.
Characteristics and Imaging Outcomes by Diabetes Status
| Diabetes (n=11) | Diabetes Absent/Remitted (n=39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preopera tive | Postopera tive | ||||||
| Mean (1SD) or % | Mean (±SD) | Mean (±SD) | Pa | Mean (±SD) or % | Mean (±SD) | Mean (±SD) | Pa | Pb | |
| white or Caucasian, non-Hispanic | 81.8 | 89.7 | 0.8035 | ||||||
| Months from preoperative study to DOS | 7.6 (4.4) | 5.1 (3.5) | 0.0555 | ||||||
| Months from DOS to postoperative study | 25.0(3.8) | 24.6(2.7) | 0.7360 | ||||||
| Age at surgery | 51.7 (6.9) | 50.6 (6.0) | 0.5846 | ||||||
| Premenopaus al | 54.5 | 41.0 | 0.4297 | ||||||
| BMI; kg/m2 | 44.2 (4.8) | 32.7 (4.2) | <0.0001 | 46.8 (7_2) | 36.7 (5.9) | <0.0001 | |||
| BMi change; kg/m2 | -11.4 (6.3) | -10.1(5.4) | 0.5005 | ||||||
| BA$ | 98372.2 (37495.6) | 80627.1 (28011.7) | <0.0040 | 88479.5 (21316.2) | 73924.4 (21494.6) | <0.0001 | |||
| BA change$ | -17745.1 (15808.5) | -14555.2 (9033.6) | 0.3913 | ||||||
| ADA$ | 10292.0 (8141.0) | 8271.27 (5689.62) | 0.1642 | 7615.9 (3206.3) | 7009.6 (3354.7) | 0.1104 | |||
| ADA change$ | -2020.73 (4464.9) | -606.31 (2316.2) | 0.1594 | ||||||
| BD (software) | 9.9 (5.1) | 9.9 (4.9) | 0.9994 | 8.5 (2.6) | 9.2 (2.6) | 0.0840 | |||
| BD change (software) | 0.0 (4.1) | 07(2.5) | 0.4795 | ||||||
| BD (radiologist) | 9.6 (9.6) | 11.4 (10.0) | 0.1039 | 8.0 (5.4) | 9.4 (6.7) | 0.0032 | |||
| BD change (radiologist) | 1.8 (3.4) | 1.4 (2.8) | 0.6835 | ||||||
SD indicates standard deviation; DOS, date of surgery; BMI indicates body mass index; BA, breast area
indicates area measured as number of pixels 800 μm x 800 μm; ADA, absolute dense area; BD, breast density given as percentage ADA/BA
comparison between preoperative and postoperative values
comparison by diabetes status values.
Relationship of BMI and BA reduction with Changes in ADA
Finally, we evaluated the dose effect of percent BMI and BA reduction on percent change in ADA for the entire cohort and by subgroups based on menopausal and diabetes status (Figure 1f-j). Linear regression of percent change in BA by percent change in BMI showed a strong and significant direct correlation for the entire cohort and regardless of subgroup. On the other hand, a significant direct correlation between percent BMI reduction and percent decrease in ADA was only noted for patients with postmenopausal status (R2=0.1950; p=0.0186). Linear regression of percent change in ADA by percent change in BA showed a significant direct correlation for all patients (R2=0.0898; p=0.0346) and those with postmenopausal status (R2=0.2211; p=0.0116).
Discussion
This is the first study to our knowledge exploring the impact of the SG on BD measured by radiologist-estimated and software-generated methods. In this cohort of patients experiencing rapid weight loss after SG, different results were noted between radiologist-estimated and software-generated methods with the former showing an increase in BD whereas no change was noted with the latter (Table 1 and Figure 1b-c). Radiologist’s interpretation revealed a significant increase in BI-RADS B+C density category also contrasting with BD values generated by software analysis (Figure 1a). Notably, our data show a reduction in ADA for the entire cohort after SG which was accentuated in postmenopausal patients (Tables 1 and 2 and Figure 1d). While weight loss was significantly correlated to reduction in BA regardless of menopausal or diabetes status, changes in BMI correlated to changes in ADA for postmenopausal patients only (Figure 1f-j). Changes in BA were directly correlated to changes in ADA for the entire cohort and postmenopausal subgroup (Figure 1f,h). Finally, neither diabetes status (Table 3) nor hemoglobin A1c changes (Figure 1e) impacted ADA following SG. These data describe the impact that bariatric surgery has on dense breast tissue while acknowledging the limitations of utilizing BI-RADS density categorization in this patient population.
Herein we report a reduction in ADA after SG which was emphasized in postmenopausal patients (Tables 1 and 2 and Figure 1d). Our results parallel those noted by Vohra who described decreased fibroglandular volume for the entire sample and postmenopausal subgroup. (15) Contrastingly and despite showing lower postoperative ADA for the entire cohort, the study by Williams did not demonstrate a postsurgical difference in this outcome in their subgroup analysis by menopausal status. (16) Differing results could have resulted from a variety of factors including criteria utilized for menopausal status categorization and the software employed for image analysis. Regardless, a reduction in ADA or fibroglandular volume following metabolic surgery is consistently appreciated across the literature and herein. Absolute measurements of dense breast tissue rather than BD may more accurately reflect breast cancer risk particularly after correction for BMI. (11) This appears particularly important after metabolic surgery. Moreover, the relationship of obesity with postmenopausal breast cancer is well-documented and bariatric surgery may reduce this risk. (4,10) It is therefore plausible that metabolic surgery may decrease breast cancer risk broadly through weight loss but also more specifically via reduction in dense breast tissue. The underlying mechanisms whereby weight loss surgery may result in diminished dense breast tissue remain unknown and notable interactions may exist between reduced adiposity and declining dense breast tissue.
Several mechanisms including metabolic and endocrine changes have been linked to dense breast tissue including cycling estrogen, elevated glucose and insulin resistance, and insulin-like growth factor 1 (IGF-1). (17–19) Interestingly, reduced adiposity following metabolic surgery may be the link between these two groups of phenomena. Not surprisingly, estrogens and inflammatory cytokines produced by adipose tissue have been proposed as a mechanism whereby obesity may be associated with postmenopausal breast cancer. (20) Moreover, reduction in dense breast tissue such as that observed with endocrine therapy in patients diagnosed with receptor-positive breast cancer has been associated with improved prognosis. (21,22) Derangements of glucose metabolism including high blood glucose, insulin resistance, and IGF-1 have also been associated with increased dense breast tissue. (18,23) Vohra and coauthors reported a reduction in fibroglandular volume in the subgroup of patients without diabetes preoperatively (n=45; p=0.001) with a trend towards significance in the smaller subgroup of patients who experienced diabetes remission (n=21; p=0.08) and no change in patients who continued to suffer from diabetes. In our study, we did not observe such association by diabetes status (Table 3) nor hemoglobin A1c changes (Figure 1e). Contrasting results may reflect differences in power, timing of collected endpoints, and or different methods for image analysis. An ongoing trial exploring the relationship of components of the metabolic syndrome including insulin resistance and growth factors such as IGF-1 and dense breast tissue may more conclusively address these contrasting results. (24)
Because of the proposed association of adiposity and dense breast tissue via the production of estrogens and inflammatory cytokines, (20) we explored the relationship between these outcomes in this cohort of patients experiencing substantial weight loss after SG. We used BMI as a surrogate for whole body adiposity or fat mass as these tend to parallel each other following SG (25,26) and BA as a proxy for breast adiposity in order to study the relationship of these outcomes with ADA. As expected, weight loss significantly correlated to reduction in BA regardless of menopausal or diabetes status (Figure 1f-j). Changes in local adiposity given by reduction in BA rather than BMI as a proxy for whole body fat mass better explained changes in ADA and were emphasized in the postmenopausal subgroup (Figure 1f,h). Though others have reported consistently on the correlation of BMI reduction with lower ADA after metabolic surgery, (16) to our knowledge this is the first report showing a correlation between changes in BA and ADA.
Our data also suggest that for patients following bariatric surgery, software-generated ADA may be better than BD and BI-RADS density categorization at capturing postoperative changes in dense breast tissue. Others have explored changes in BD following metabolic surgery with differing results. (15,16,27) Mokhtari et al. retrospectively reported on mammographic outcomes by radiologist interpretation including BI-RADS for 10 patients with at least 50% excess weight loss following laparoscopic adjustable gastric banding (LAGB), SG, and Roux-en-Y gastric bypass (RYGB). The authors noted an overall decrease in breast size along with higher postoperative BD at 8.7 months. (27) Vohra and colleagues studied mammograms by conventional radiologist’s interpretation and volumetric software for 80 patients before and 10.1 moths after metabolic surgery (LAGB, SG, and RYGB) resulting in a 12.3 kg/m2 BMI reduction. (15) They reported no postoperative change in BI-RADS density categories while noticing an increase in volumetric BD by software analysis. The third study by Williams et al. reported a reduction in BD by BI-RADS and software analysis after examining preoperative and postoperative mammograms at 21.4 months for 76 patients undergoing SG or RYGB resulting in a 9.9 kg/m2 BMI reduction. (16) Our data show increased BD by BI-RADS with no change in software-generated BD at an average of 24.7 months after SG resulting in 10.4 kg/m2 BMI reduction. Discrepancies in the literature could be attributed to several methodologic differences including the metabolic operation(s) utilized, magnitude of weight loss, software used, and length of time between surgery and postoperative mammogram. Additionally, concerns about the intra- and inter-observer reproducibility of BI-RADS density estimation in the general population have been noted by others. (28,29) More importantly, rapid weight loss such as that observed with metabolic surgery may compromise the clinical meaningfulness of BI-RADS density categorization in this population. BI-RADS is based on mammographic BD which represents the ratio of ADA to BA. Following bariatric surgery, a drastic decline in BA is obvious. However, postoperative reduction in ADA may be harder to detect, particularly in patients with low preoperative ADA. This translates, as seen in this study, into higher postoperative BI-RADS density categorization giving the impression of higher breast cancer risk when in fact a reduction in ADA, a proxy for dense breast tissue at risk for malignant transformation, is observed. In this scenario, software-generated ADA or absolute dense volume could more meaningfully predict dense breast tissue-derived cancer risk.
Several limitations of the current data should be considered. Our patient population is derived from a single academic center which may limit generalizability. However, this allowed us to collect imaging data and menopausal status otherwise not available through larger registries. Although menopausal status was collected by closely and longitudinally examining primary care and gynecology clinic records, it was self-reported and may misclassify some patients. Changes in dense breast tissue are dynamic and influenced by factors in addition to those captured by our study. Moreover, we acknowledge that dense breast tissue intrinsically changes over time and therefore future case-control and or clinical trial designs are needed to conclusively explore the associations described here.
Conclusions
Notable changes in breast tissue are appreciated after bariatric surgery. In this patient population experiencing significant weight loss following SG, software-generated ADA may be more accurate than BD and BI-RADS density categorization at capturing breast cancer risk derived from dense breast tissue. Moreover, we offer evidence supporting a reduction in ADA after SG which was accentuated in postmenopausal patients. Additionally, changes in breast rather than whole-body adiposity may better explain postsurgical changes in ADA. Contrasting with others, an association between ADA was neither observed with diabetes status nor with hemoglobin A1c. Overall, these data offer important insight into the changes in dense breast tissue which may follow metabolic surgery and support additional efforts further describing these relationships and exploring their underlying mechanisms.
Highlights.
Absolute dense area (ADA) may decrease after sleeve gastrectomy (SG).
Reduction in ADA was accentuated in postmenopausal patients.
Software analysis may more accurately capture changes in dense tissue after SG.
Changes in breast rather than whole-body adiposity may better explain changes in ADA.
Neither diabetes status nor hemoglobin A1c were associated with changes in ADA.
Acknowledgments
Acknowledgements and sources of support: Supported by a T32 grant (1T32DK108740) awarded to Dr. Justin B. Dimick and by P30 (DK089503) and R01 (DK107652) grants awarded to Dr. Randy J. Seeley through the National Institutes of Health.
Footnotes
Disclosures
One of the authors receives financial research support from Ethicon Endo- Surgery/Johnson & Johnson, Novo Nordisk, Janssen/Johnson & Johnson, Zafgen, MedImmune, Sanofi, and Kallyope and serves as a consultant for Ethicon Endo-Surgery/Johnson & Johnson, Orexigen, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Kallyope, and Scohia. One of the authors receives salary support from Blue Cross Blue Shield for leadership and participation in statewide quality improvement initiatives. The remaining authors have no commercial associations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond Engl. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele CB, Thomas CC, Henley SJ, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–1058. doi:10.15585/mmwr.mm6639e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1(5):611–621. doi:10.1001/jamaoncol.2015.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2006;15(6):1159–1169. doi:10.1158/1055-9965.EPI-060034 [DOI] [PubMed] [Google Scholar]
- 7.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi:10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. doi:10.1056/NEJMoa1401329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376(7):641–651. doi:10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–662. doi:10.1016/S14702045(09)70159-7 [DOI] [PubMed] [Google Scholar]
- 11.Schetter SE, Hartman TJ, Liao J, et al. Differential impact of body mass index on absolute and percent breast density: implications regarding their use as breast cancer risk biomarkers. Breast Cancer Res Treat. 2014;146(2):355–363. doi:10.1007/s10549-014-3031-6 [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Chan HP, Petrick N, et al. Computerized image analysis: estimation of breast density on mammograms. Med Phys. 2001;28(6):1056–1069. doi:10.1118/1.1376640 [DOI] [PubMed] [Google Scholar]
- 13.Wei J, Sahiner B, Hadjiiski LM, et al. Computer-aided detection of breast masses on full field digital mammograms. Med Phys. 2005;32(9):2827–2838. doi:10.1118/1.1997327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Chan H-P, Helvie MA, et al. Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Med Phys. 2004;31(4):933–942. doi:10.1118/1.1668512 [DOI] [PubMed] [Google Scholar]
- 15.Vohra NA, Kachare SD, Vos P, et al. The Short-Term Effect of Weight Loss Surgery on Volumetric Breast Density and Fibroglandular Volume. Obes Surg. 2017;27(4):1013–1023. doi:10.1007/s11695-016-2415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AD, So A, Synnestvedt M, et al. Mammographic breast density decreases after bariatric surgery. Breast Cancer Res Treat. June 2017. doi:10.1007/s10549-017-4361-y [DOI] [PubMed] [Google Scholar]
- 17.Frydenberg H, Flote VG, Iversen A, et al. Insulin-like growth factor-1, growth hormone, and daily cycling estrogen are associated with mammographic density in premenopausal women. Cancer Causes Control CCC. 2014;25(7):891–903. doi:10.1007/s10552-014-0389-z [DOI] [PubMed] [Google Scholar]
- 18.Kim B-K, Chang Y, Ahn J, et al. Metabolic syndrome, insulin resistance, and mammographic density in pre- and postmenopausal women. Breast Cancer Res Treat. 2015;153(2):425–434. doi:10.1007/s10549-015-3544-7 [DOI] [PubMed] [Google Scholar]
- 19.Woolcott CG, Courneya KS, Boyd NF, et al. Longitudinal changes in IGF-I and IGFBP-3, and mammographic density among postmenopausal women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013;22(11):2116–2120. doi:10.1158/1055-9965.EPI-13-0401 [DOI] [PubMed] [Google Scholar]
- 20.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(22):6074–6083. doi:10.1158/1078-0432.CCR-12-2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyante SJ, Sherman ME, Pfeiffer RM, et al. Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J Natl Cancer Inst. 2015;107(3). doi:10.1093/jnci/dju425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(18):2249–2256. doi:10.1200/JCO.2012.44.5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Leon DD, Wilson DM, Powers M, Rosenfeld RG. Effects of insulin-like growth factors (IGFs) and IGF receptor antibodies on the proliferation of human breast cancer cells. Growth Factors Chur Switz. 1992;6(4):327–336. [DOI] [PubMed] [Google Scholar]
- 24.Martinez JA, Chalasani P, Thomson CA, et al. Phase II study of metformin for reduction of obesity-associated breast cancer risk: a randomized controlled trial protocol. BMC Cancer. 2016;16:500. doi:10.1186/s12885-016-2551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubnov-Raz G, Inge TH, Ben-Ami M, Pienik R, Vusiker I, Yardeni D. Body composition changes in adolescents after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2016;12(2):322–329. doi:10.1016/j.soard.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Strain GW, Gagner M, Pomp A, et al. Comparison of weight loss and body composition changes with four surgical procedures. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2009;5(5):582–587. doi:10.1016/j.soard.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Mokhtari TE, Rosas US, Downey JR, Miyake KK, Ikeda DM, Morton JM. Mammography before and after bariatric surgery. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2017;13(3):451–456. doi:10.1016/j.soard.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 28.Ciatto S, Houssami N, Apruzzese A, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast Edinb Scotl. 2005;14(4):269–275. doi:10.1016/j.breast.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 29.Kerlikowske K The mammogram that cried Wolfe. N Engl J Med. 2007;356(3):297–300. doi:10.1056/NEJMe068244 [DOI] [PubMed] [Google Scholar]