Abstract
Background:
Synthetic Cannabinoid (SC) intoxication has become difficult to diagnose and manage in the United States in part due to varying clinical effects within this heterogeneous group of compounds.
Case Report:
A 38 year-old male was admitted with altered mental status and bradycardia. He demonstrated progressive encephalopathy, seizure activity, second degree atrioventricular (AV) block, type I, respiratory failure, hypotension, hypothermia, and hypoglycemia. A computed tomography (CT) abdomen and pelvis revealed multiple packages in the patient’s stomach and rectum. Multiple attempts at gastrointestinal decontamination were unsuccessful. On hospital day eight the patient developed hypertensive emergency and was taken to the operating room for exploratory laparotomy. Twenty-two poorly wrapped packages were removed from the bowel. Post-operatively the patient demonstrated both generalized and focal seizure activity. His mental status slowly returned to baseline over the period of about one week and he was ultimately discharged without neurological sequelae after one month. Analysis of patient serum, urine, and plant matter from packages identified cannabis and 2.N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (ADB-FUBINACA).
Why should an emergency physician be aware of this?: The case presented demonstrates the suspected toxidrome associated with severe ADB-FUBINACA intoxication including mental status depression, bradycardia, autonomic instability, seizure, hypoglycemia, and hypothermia. Although the patient had simultaneous exposure to cannabis, his constellation of symptoms is not consistent with cannabis intoxication. A previous animal model supports the potential of this specific SC to cause the reported toxidrome.
Keywords: Synthetic cannabinoid, toxicology, body packing, bradycardia
Introduction
Synthetic cannabinoid (SC) intoxication continues to result in emergency department (ED) visits and intensive care unit stays. The presentations are ever changing due to the rapid turnover of specific compounds and the heterogeneity of the clinical effects of this group of novel psychoactive substances. Evidence suggests that recent SC intoxications result in higher severity of illness than previously reported (1).
Case Report
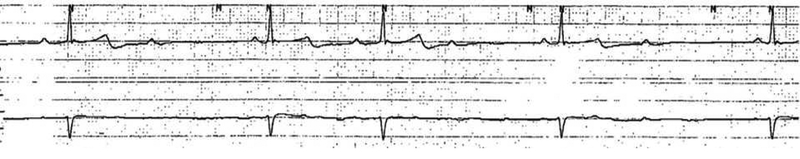
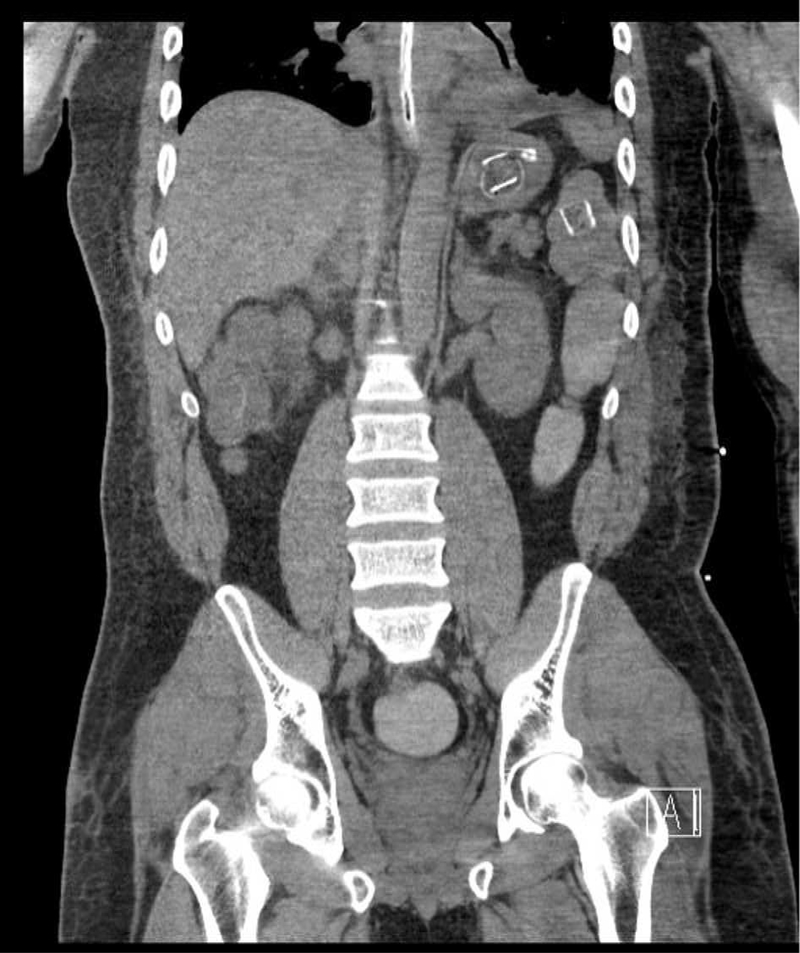
A 38 year-old male inmate of five years duration was transferred to an academic medical center after an initial seven day hospitalization for abnormal behavior. Upon arrival to the initial treatment facility, he was described as awake but answering questions inappropriately, complaining of shortness of breath and staring blankly into space. Past medical history included hypertension and asthma treated with amlodipine 5 mg daily, hydrochlorothiazide 25 mg daily, and albuterol. Initial vitals revealed temperature 35°C, heart rate 47 beats/minute, respiratory rate 12 breaths/minute, blood pressure 139/53 mmHg, and an oxygen saturation of 95% on room air. He was reportedly lethargic and unable to respond verbally to orientation questions; physical examination was otherwise unremarkable. A computed tomography (CT) scan of the head revealed no acute intracranial abnormality. An electrocardiogram (ECG) revealed sinus bradycardia. Laboratory investigations included undetectable serum acetaminophen and ethanol with a complete blood count and hepatic function panel both within normal limits. Initial serum chemistries were unremarkable including a glucose of 111 mg/dL. An immunoassay urine drug screen (UDS) including tetrahydrocannabinol and benzoylecgonine was negative. The patient received supportive care and intravenous (IV) fluid resuscitation while in the ED and was admitted to the medical floor. On hospital day (HD) one he demonstrated hypoglycemia, hypotension, and hypopnea with a respiratory rate of 8 breaths per minute. The patient underwent endotracheal intubation and was started on midazolam infusion. He subsequently demonstrated rhythmic motor activity and IV lorazepam was administered with cessation of seizure activity. The patient developed hypoglycemia, transiently responsive to boluses of 50% dextrose and ultimately requiring a continuous infusion of 10% dextrose. A repeat ECG demonstrated second degree atrioventricular (AV) block, type I with a rate around 30 beats/minute (figure 1). He was transiently treated with dopamine infusion for hypotension and bradycardia. A CT scan of the abdomen and pelvis revealed multiple ovoid packets throughout the gastrointestinal tract (figure 2) and the local poison control center was notified about the case. A repeat immunoassay UDS was positive for benzodiazepines which the patient was receiving for sedation but remained negative for tetrahydrocannabinol and benzoylecgonine. Whole bowel irrigation (WBI) was attempted via administration of polyethylene glycol (PEG) through a nasogastric tube at a rate of 100 mL/hour and he expelled two packets via rectum on HD seven. On HD seven on arrival to the academic medical center, a repeat CT scan revealed two oval packets in the stomach, three in the small bowel and two in the colon. A repeat UDS using Liquid Chromatography-Tandem Mass Spectrometry was positive for 11-nor-delta-9-tetrahydroxycannabinol-9-carboxylic acid (tetrahydrocannabinol metabolite) in addition to metoclopramide, atropine, and benzodiazepines, which the patient had received for WBI, bradycardia and seizure respectively. On HD eight, the two packets visualized in the stomach were removed by esophagogastroduodenoscopy. Another attempt at WBI was made with PEG-electrolyte solution at a rate of 1 L/hour after administration of 50 g activated charcoal to potentially bind any free drug leaking from packets. After 2 hours, WBI was discontinued due to 2 L of residual stomach content. The patient was noted to be intermittently hypertensive with systolic blood pressure above 200 mmHg. A serum troponin T was elevated at 0.19 ng/mL and trended up to 0.30 ng/mL over the course of 12 hours. The patient was taken to the operating room where 22 poorly wrapped packages of balloon and electrical tape containing green, leafy organic material were removed (figure 3). Post-operatively, he continued to demonstrate signs of toxicity with occasional seizure activity. Electroencephalogram identified subclinical left temporal focal seizure in addition to diffuse slowing consistent with toxic encephalopathy. He was extubated, but remained encephalopathic. Repeat CT scan on HD 16 demonstrated two residual packages in the patient’s rectum which eventually passed. His mental status slowly returned to baseline over one week and he was ultimately discharged without neurological sequelae after one month.
Figure 1. Rhythm Strip.
Second degree AV block, type I with a rate around 30 beats/minute.
Figure 2. Packets Identified on CT Scan.
Initial CT of the abdomen demonstrated0 twopackets in the body of the stomach, one in the small bowel, and two in the colon.
Figure 3. Packets Removed During Exploratory Laparotomy.
Contents removed included 22 poorly wrapped packages of balloon and electrical tape containing green, leafy organic material.
Initial testing of serum and urine specimens for SCs was conducted using routine validated methods (NMS Labs, Willow Grove, PA). 2.N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (ADB-FUBINACA) was confirmed in serum sample collected on day 6 of hospitalization and urine specimen contained MDMB-FUBINACA 3,3-dimethyl-butanoic acid, which is a metabolite of ADB-FUBINACA. Specimens were screened by Liquid Chromatography-Quadrupole Time of Flight Mass Spectrometry (LC-QTOF) using a Sciex TripleTOF 5600+ and processed against an extensive database containing more than 450 drugs, including novel psychoactives. Presumptive positive results included diphenhydramine, metoclopramide, cocaine, scopolamine, midazolam, and ADB-FUBINACA. Scopolamine and diphenhydramine were suspected to be adulterants. Quantification of ADB-FUBINACA using standard addition was performed at The Center for Forensic Science Research and Education (Willow Grove, PA). Serum concentration of ADB-FUBINACA was 34 ng/mL on HD 6 and 17 ng/mL on HD 8. Cocaine was not confirmed at a reporting limit of 20 ng/mL. The discrepancy between the screen and confirmation results for cocaine are likely due to the difference in the detection limits used for screening and confirmation.
Discussion
We report a case of toxicity secondary to body packing with large amounts of ADB-FUBINACA and cannabis. Though THC was identified in packages removed from the patient’s bowel and trace levels of cocaine were identified in the serum, it is likely that the presentation demonstrates toxicity associated with ADB-FUBINACA. It is noteworthy that benzoylecogonine was not detected on serial urine screening in the context of the hypertensive crises.
To our knowledge, this represents the highest serum concentration of ADB-FUBINACA reported in the literature. A previous case report of a patient with somnolence, agitation, and brief supraventricular tachycardia detected a serum concentration of 15.6 ng/mL; ABFUBINACA was also confirmed in the serum of this patient (2). Additionally, an ADBFUBINACA serum concentration of 7.3 ng/mL was identified on autopsy of a patient with history of pre-mortem SC inhalation and resultant agitation and unresponsiveness (3). AB-FUBINACA, differing from ADB-FUBINACA by a single methyl group, has produced hypothermia and bradycardia in a murine model, an effect that is blunted by the administration of the CB1 antagonist rimonabant (4). Interestingly, the clinical presentation in our patient is markedly different than the toxidrome described after a 2016 outbreak of AMB-FUBINACA, a similarly structured SC (5). Though no mechanism has been elucidated, hypoglycemia has been reported with SC intoxication (6,7). Seizure activity has also been associated with SC intoxication; however, the focality identified in this patient is atypical of toxin-induced seizures. It is possible that our patient had an underlying seizure focus and the SC lowered the seizure threshold.
Our case highlights the limitations of CT scan for identification of swallowed foreign bodies. It is of note that after laparotomy, the patient was found to have two additional packets in the rectum almost one week later. Importantly, our case also emphasizes several drawbacks of WBI. Although generally recommended for body packers, the goal rate is 1–2 L/hr in adults, a dose that is frequently not used as was the case in our patient when initially attempted at an outside facility. Additionally, it can be challenging to achieve the goal rate of PEG-electrolyte solution, as evidenced by the high residuals documented in our patient.
Why should an emergency physician be aware of this?
In this single patient case report, ADB-FUBINACA intoxication was associated with encephalopathy, hypoglycemia, hypothermia, hypotension, bradycardia, seizures, and hypertensive emergency.
Acknowledgments
Author Acknowledgements: The authors would like to acknowledge Cayman Chemical Company (Ann Arbor, MI) for analytical support and use of analytical standard reference material.
Funding Source: These studies were supported in part by funds provided by NIH/NIDA award no. DA039143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
Jeffery Moran is an employee of PinPoint Testing, LLC.
Sherri Kacinko is an employee of NMS Labs
The remaining authors have nothing to disclose
Contributor Information
Nicholas Nacca, Department of Emergency Medicine, University of Rochester Medical Center, Rochester NY Upstate Poison Center, Syracuse NY, Twitter handle: @nicholasenacca.
Schult Rachel, Department of Pharmacy, University of Rochester Medical Center, Rochester NY; Department of Emergency Medicine, University of Rochester Medical Center, Rochester NY; Upstate Poison Center, Syracuse NY.
Loflin Robert, Emergency and Critical Care Medicine, Martin Health System, Stuart FL.
Weltler Adam, Department of Emergency Medicine, University of Rochester Medical Center, Rochester NY.
Gordetsky Rachel, D’Youville College School of Pharmacy, Buffalo NY; Department of Emergency Medicine, University of Rochester Medical Center, Rochester NY; Upstate Poison Center, Syracuse NY.
Kacinko Sherri, NMS Labs, Willow Grove, PA.
Moran Jeffery, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, AR; Pinpoint Testing, LLC, Little Rock, AR.
Krotulski Alex, The Center for Forensic Science Research and Education, Willow Grove, PA.
Wiegand Timothy, Department of Emergency Medicine, University of Rochester Medical Center, Rochester NY; Upstate Poison Center, Syracuse NY.
References:
- 1.Papadopoulos EA, Cummings KR, Marraffa JM, et al. Reports of adverse health effects related to synthetic cannabinoid use in New York State. Am J Addict 2017;26:772–775. [DOI] [PubMed] [Google Scholar]
- 2.Lam RPK, Tang MHY, Leung SC, et al. Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: a case report with quantitative analysis of serum drug concentrations. Clin Toxicol (Phila) 2017;55:662–667. [DOI] [PubMed] [Google Scholar]
- 3.Shanks KG, Clark W, Behonick G. Death Associated With the Use of the Synthetic Cannabinoid ADB-FUBINACA. J Anal Toxicol 2016;40:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banister SD, Moir M, Stuart J, et al. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADBPINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci 2015;6:1546–1559. [DOI] [PubMed] [Google Scholar]
- 5.Adams AJ, Banister SD, Irizarry L, et al. “Zombie” Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N Engl J Med 2017;376:235–242. [DOI] [PubMed] [Google Scholar]
- 6.Hermanns-Clausen M, Müller D, Kithinji J, et al. Acute side effects after consumption of the new synthetic cannabinoids AB-CHIMNACA and MDMB-CHMICA. Clin Toxicol (Phila) 2018;56:404–411. [DOI] [PubMed] [Google Scholar]
- 7.Seywright A, Torrance HJ, Wylie FM, et al. Analysis and clinical findings of cases positive for the novel synthetic cannabinoid receptor agonist MDMB-CHMICA. Clin Toxicol (Phila) 2016;54:632–637. [DOI] [PubMed] [Google Scholar]