Abstract
Background
Axitinib is an oral small molecule that inhibits receptor tyrosine kinases VEGFR1-3. A phase I and pharmacokinetic trial evaluating axitinib was conducted in children with refractory solid tumors.
Methods
Axitinib was administered orally, twice daily, in continuous 28-day cycles. Dose levels (2.4 and 3.2 mg/m2/dose) were evaluated using a rolling 6 design. Serial pharmacokinetics (PK) (cycle 1 days 1 and 8), and exploratory biomarkers were analyzed.
Results
Nineteen patients were enrolled; one patient was ineligible due to inadequate time elapsed from prior therapy. The median age was 13.5 years (range 5–17 years). Two of five patients treated at dose level 2 experienced DLT (palmar-plantar erythryodysesthesia syndrome (1); intratumoral hemorrhage (1)). Frequent (>20%) grade 1–2 toxicity during cycle 1 included anemia, anorexia, fatigue, diarrhea, nausea, and hypertension. Non-hematological grade ≥ 3 toxicity in subsequent cycles included hypertension and elevated serum lipase. PK analysis demonstrated variability in axitinib exposure, median time to peak plasma concentration was 2 hours (h) and half-life ranged from 0.7–5.2 h. Exposure and dose were not significantly associated with hypertension. Five patients had stable disease for ≥ 6 cycles as best response including patients with malignant peripheral nerve sheath tumor (1), Ewing sarcoma (1), hepatocellular carcinoma (1), and osteosarcoma (2). One patient with alveolar soft part sarcoma had a partial response. Kidney injury biomarkers were elevated at baseline; no trends were identified.
Conclusions
In children with refractory solid tumors, the maximum tolerated and recommended dose of axitinib is 2.4 mg/m2/dose, which provides pharmacokinetic exposures similar to adults.
Keywords: VEGFR, pediatric solid tumor, phase I, axitinib, INLYTA
Introduction
Angiogenesis plays a critical role in growth and metastases of cancer.1–3 Vascular endothelial growth factor (VEGF) is a pro-angiogenic factor important for formation of tumor blood vessels and modulating vascular permeability. VEGF activity is mediated by its receptors VEGFR1, VEGFR2 and VEGFR3.3 Inhibition of the VEGF receptor tyrosine kinases (RTKs) has emerged as an anticancer strategy in adults with renal and hepatic carcinomas as well as soft tissue sarcomas.4–9 VEGF RTK inhibitors, evaluated in the NCI pediatric preclinical testing program solid tumor panel, demonstrated tumor growth delay.10–12
Axitinib (INLYTA®), a potent and selective small molecule inhibitor of VEGFR1-3, binds to the inactive conformation of the catalytic domain of VEGF RTKs.13–15 Studies in adults16–24 established a maximum tolerated dose (MTD) of 5 mg PO BID, and provided guidelines for intra-patient dose titration to a maximum of 10 mg PO BID.22 Common adverse effects include diarrhea, hypertension, fatigue, anorexia, nausea, weight loss, dysphonia, palmar-plantar erythrodysaesthesia syndrome, proteinuria, and vomiting. Hypertension and diarrhea are the most common grade 3/4 events.15,25 In adults, the median time to onset of axitinib associated grade 1–2 and grade ≥ 3 hypertension is 16 days and 24 days, respectively. Axitinib related hypertension resulted in dose interruptions in 12%, dose modification in 5%, and discontinuation in < 1% of patients.26 Axitinib-treated patients with a diastolic blood pressure > 90 mm Hg23 or increased diastolic BP ≥ 10–15 mm Hg from baseline had longer progression-free survival (PFS).24
Pharmacokinetic parameters in adults receiving axitinib 5 mg PO BID were highly variable. Population PK analyses indicate that patients with higher axitinib exposures (AUC24h> 200 h•ng/mL) may have a higher objective response rate and trend toward improved PFS.22–24 However, there is insufficient data to recommend use of either pharmacokinetic parameters or blood pressure measurements as the exclusive guide to up-titration of the axitinib dose.24
We conducted a Phase 1 trial to estimate the MTD or recommended phase 2 dose (RP2D), describe the toxicities, and characterize the pharmacokinetics of axitinib administered orally twice daily in pediatric patients with refractory solid tumors. Secondary aims were to describe the antitumor activity of axitinib within the confines of a phase 1 study, and to investigate biomarkers of acute kidney injury (AKI) and nephrotoxicity.
Materials and Methods
Patient eligibility
Patients > 12 months and < 18 years of age with a minimum body surface area (BSA) of 0.53 m2, and measurable or evaluable refractory/recurrent solid tumors, excluding primary brain tumors, were eligible. Patients may have received prior anti-VEGF targeting antibodies or blocking tyrosine kinase inhibitors but may not have received axitinib. Patients must have fully recovered from acute toxic effects of prior therapy.
Performance status of at least 50% (Karnofsky for patients > 16 years old, Lansky for ≤ 16 years) was required. Organ function requirements included absolute neutrophil count (ANC) ≥ 1000/mm3, platelet count ≥ 100,000/mm3, hemoglobin ≥ 8 gm/dL; creatinine clearance or radioisotope GFR ≥70mL/min/1.73 m2 or age-appropriate serum creatinine; bilirubin ≤ 1.5 times upper limit of normal (ULN) for age, SGPT (ALT) ≤ 110 U/L, SGOT (AST) ≤ 125 U/L, serum albumin ≥ 2 g/dL; lipase ≤ 1.5 x ULN; and shortening fraction ≥ 27% by echocardiogram or ejection fraction ≥ 50% by gated radionuclide study. Blood pressure ≤ 95th percentile for age, height and gender was required. Patients were required to swallow intact tablets.
Institutional Review Board approval was obtained prior to patient enrollment and written informed consent was obtained; assent was obtained according to institutional guidelines.
Trial design and definitions
A rolling 6 design was used for dose escalation27: twice daily administration of 2.4 mg/m2/dose (starting dose, equivalent to 4 mg in adults), 3.2 mg/m2/dose (equivalent to 5.5 mg in adults), and 4.2 mg/m2/dose (equivalent to 7.2 mg in adults) were planned. Intra-patient dose escalation or dose titration were not permitted. For patients experiencing dose-limiting axitinib related toxicity, their dose was decreased by one dose level or for patients on dose level 1, reduction to 1.8 mg/m2/dose (equivalent to 3 mg in adults). A dosing nomogram was used to prescribe doses based on BSA at each dose level. (Supplemental Table I). Axitinib (1 and 5 mg capsules) were supplied by Pfizer.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Hematological DLT was defined as grade 4 thrombocytopenia (platelet count < 25,000/mm3) or grade 4 neutropenia not due to malignant infiltration. Grade 3 or 4 fever of < 5 days duration with or without grade 1–3 neutropenia was not considered a DLT. Any grade 2 arterial thromboembolic events, any ≥ grade 3 venous thromboembolic events, any thrombotic events requiring systemic anti-coagulation, and any ≥ grade 2 hemorrhages were considered DLTs. Any grade 3 or 4 non-hematological toxicity attributable to axitinib was considered a DLT with the exclusion of: grade 3 proteinuria (urine protein/creatinine (UPC) ratio > 1.9) unless confirmed within 72 hours; grade 3 rapidly reversible hepatic function tests, asymptomatic lipase elevation that did not recur upon axitinib re-challenge; grade 3 electrolyte abnormalities responsive to oral supplementation; grade 3 diarrhea or nausea and vomiting ≤ 3 days duration; grade 3 infection < 5 days duration. Any grade 2 non-hematological toxicity that persisted for ≥ 7 days, and was sufficiently medically significant or intolerable to the patient that it required treatment interruption, was considered a DLT.
The MTD was defined as the dose level at which fewer than 33% of patients experienced a DLT in cycle 1. Once the MTD was defined, up to six additional patients could be enrolled to obtain tolerability and PK data on at least 3 patients less than 12 years of age. The RP2D would be the MTD, or in the absence of DLT, the highest dose level evaluated.
Toxicity Evaluation
Clinical history, physical exam and vital signs including protocol specific blood pressure measurement and laboratory assessment were required. A plain radiograph of tibia growth plate was required at the end of cycle 1, prior to cycle 5, and then every 6 months during study therapy. An algorithm for the management of axitinib-associated hypertension was provided.28
Pharmacokinetic and Correlative Studies
Blood samples for PK analyses were collected during cycle 1, day 1 and 8 (pre-dose and 1, 2, 4, 6, and 8 hours after the morning dose). Plasma concentrations of axitinib were measured by a validated liquid chromatography-mass spectrometry assay as previously described. 16 PK parameters were calculated using standard noncompartmental analysis (Phoenix WinNonlin 6.4; Pharsight Corporation, Mountain View, CA).29
Biomarkers of AKI included Kidney Injury Molecule-1 (KIM-1), measuring proximal tubular damage; Neutrophil Gelatinase-Associated Lipocalin (NGAL) discriminating pre-renal from intrinsic AKI; and interleukin-18 (IL-18), a mediator of ischemic tubular necrosis. Blood and urine were collected during cycle 1, prior to the first dose and 2 to 4 hours after the morning dose on days 1, 8, 15, 22. AKI Biomarkers were processed and assayed as previously described.30
Tumor Response Assessment
Response based on revised Response Evaluation Criteria In Solid Tumor (RECIST, version 1.1),31 was assessed prior to cycles 2, 4, 6, and every 3rd cycle thereafter. Radiographic objective responses (Complete Response (CR) or Partial Response (PR)) or stable disease greater than 6 months were reviewed centrally.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics, toxicities, pharmacokinetics, and correlative studies. Pharmacokinetic parameters were compared between age groups (<12 vs. ≥12 years), dose levels, and gender using the Wilcoxon rank sum test. The Wilcoxon rank sum test was also used to compare PK parameters between patients with and without hypertension. General linear mixed models were used to estimate the change in urinary biomarkers over time. The models included fixed effects for course number, and the matrix of correlated error terms assumed a first-order autoregressive structure to model repeated measures within the same subject. The UPC ratio was log transformed (natural logarithm) for the analysis. The regression models for the change in biomarkers over time were also adjusted for baseline values. Statistical significance was assessed at the 0.05 level for all hypotheses tested.
Results
Patients
Nineteen patients were enrolled; one patient was ineligible due to inadequate time elapsed from prior therapy. Patient characteristics are summarized in Table I. All patients enrolled had at least one prior therapy including chemotherapy, radiation or both.
Table I.
Patient Characteristics for eligible Patients (n=18)
| Characteristic | |
|---|---|
|
| |
| Age (years), Median (Range) | 13.5 (5 – 17) |
|
| |
| Sex, n(%) | |
| Male | 10 (56) |
| Female | 8 (44) |
|
| |
| Race, n(%) | |
| White | 13 (72) |
| Asian | 1 (6) |
| American Indian or Alaska Native | 0 (0) |
| Black or African American | 3 (17) |
| Unknown | 1 (6) |
|
| |
| Ethnicity, n (%) | |
|
| |
| Non-Hispanic | 14 (77.8) |
| Hispanic | 3 (16.7) |
| Unknown | 1 (5.6) |
|
| |
| Diagnosis, n(%) | |
|
| |
| Sarcoma | |
| Alveolar rhabdomyosarcoma | 1 (5.6) |
| Alveolar soft part sarcoma | 2 (11.1) |
| Epithelial-myoepithelial carcinoma | 1 (5.6) |
| Epitheliod sarcoma | 1 (5.6) |
| Ewing sarcoma/Primitive Neuroectodermal Tumor | 3 (16.7) |
| Malignant Peripheral Nerve Sheath Tumor | 2 (11.1) |
| Osteosarcoma | 2 (11.1) |
| Rhabdomyosarcoma (NOS) | 1 (5.6) |
|
| |
| Hepatoblastoma | 1 (5.6) |
|
| |
| Hepatocellular carcinoma, fibrolamellar | 1 (5.6) |
|
| |
| Renal Medullary carcinoma, | 1 (5.6) |
|
| |
| Nephroblastoma, NOS | 1 (5.6) |
|
| |
| Neuroblastoma, NOS | 1 (5.6) |
|
| |
| Prior Therapy | |
|
| |
| Number of chemotherapy regimens (N=17 patients) Median (Range) | 3 (1 – 8) |
|
| |
| Radiation Therapy Courses (N=14 patients) Median (Range) | 1 (1 – 3) |
Toxicities
Sixteen patients were evaluable for DLT assessment. Two patients were not evaluable because they did not receive 85% of the prescribed study drug and did not experience a DLT. Toxicities are summarized in Tables II and III. No DLTs occurred in 11 evaluable patients (6 in dose escalation, 5 in PK expansion cohort) at dose level 1 (2.4 mg/m2/dose). At dose level 2 (3.2 mg/m2/dose), five patients were evaluable and two DLTs occurred: Grade 2 palmar-plantar erythrodysesthesia syndrome (n=1), and Grade 3 intratumoral hemorrhage (n=1) in a 10 year old girl with a thoracic MPNST. A second patient on study with MPNST did not have hemorrhage. Thus, dose level 1 was defined as the MTD. There were three grade 3 toxicities (increased hemoglobin, hypertension, increased lipase) and no grade 4 toxicities (Table III). Grade 1–2 non-hematological toxicity that occurred in ≥ 20% of patents during cycle 1 included anorexia, diarrhea, fatigue, nausea and hypertension.
Table II.
Dose Limiting Toxicity Summary
| Dose Level | Stratum | Number of Patients Entered | Number of Patients Evaluable | Number of pts with DLT |
|---|---|---|---|---|
| 2.4 mg/m2 | A | 6 | 6 | 0 |
| 2.4 mg/m2 | PK | 6 | 5 | 0 |
| 3.2 mg/m2 | A | 6 | 5 | 2* |
DLTs include moderate Palmar-plantar erythrodysesthesia syndrome (1) and severe Intratumoral hemorrhage (1).
Table III.
Hematologic and non-Hematologic* non-dose limiting toxicities (possibly, probably or definitely attributed to axitinib) in evaluable patients (n=16)
| Cycle 1 (n=16) | Subsequent Cycles(Total, 47 patient-cycles) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Hematologic | Anemia | 3 | 2 | 1 | |||||
| Hemoglobin increased | 3 | 1 | 2 | 1 | |||||
| Lymphopenia | 1 | 1 | 1 | ||||||
| Neutropenia | 1 | 2 | |||||||
| Thrombocytopenia | 1 | ||||||||
| Leukopenia | 1 | 1 | |||||||
| Non-Hematologic | Abdominal pain | 1 | 1 | 3 | |||||
| Alanine aminotransferase increased | 1 | 1 | 4 | 1 | |||||
| Anorexia | 1 | 3 | 1 | 1 | |||||
| Aspartate aminotransferase increased | 3 | 5 | |||||||
| Creatinine increased | 3 | 1 | |||||||
| Diarrhea | 5 | 1 | 3 | 2 | |||||
| Fatigue | 3 | 1 | |||||||
| Headache | 2 | 1 | 1 | 1 | |||||
| Hypertension | 3 | 4 | 1 | 3 | 1 | ||||
| Hypophosphatemia | 2 | 1 | |||||||
| Hypothyroidism | 1 | 2 | 2 | ||||||
| Lipase increased | 1 | 1 | 2 | 1 | |||||
| Nausea | 8 | 1 | 2 | ||||||
| Palmar-plantar erythrodysesthesia syndrome | 2 | 1 | 1 | ||||||
| Proteinuria | 2 | 2 | 1 | ||||||
| Rash acneiform | 2 | ||||||||
| Sore throat | 2 | ||||||||
| Vomiting | 3 | 1 | |||||||
Non-hematological toxicities occurring in >10% of evaluable patients
Fourteen patients (14/18, 78%) had open growth plates at baseline; 12 were assessed in subsequent cycles. No growth plate abnormalities were observed, and all growth plates remained open through the course of the study.
Systolic (SBP) and diastolic (DBP) blood pressure did not significantly change over subsequent cycles. On average, SBP increased by 0.67 mm Hg/cycle (95% CI: −0.17, 1.52) and DBP decreased by 0.34 mm Hg/cycle (95% CI: −1.00, 0.33).
Pharmacokinetic and Correlative Studies
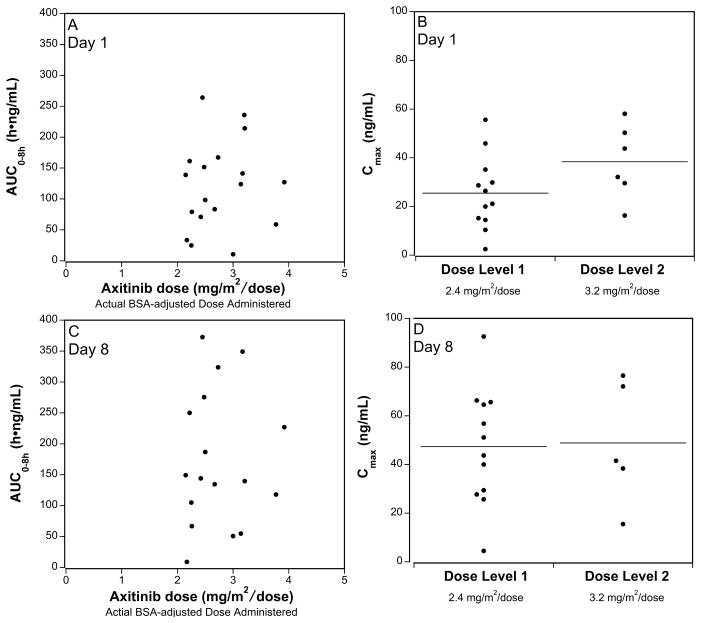
PK samples were obtained on Day 1 and Day 8 after the first dose (Table IV). Peak plasma concentrations were achieved at a median (range) of about 2 (0–4) hours. Peak concentration (Cmax) and exposure (Area Under Concentration versus time Curve, AUC) were highly variable and did not appear different between the dose levels on day 1 (p=0.10 and 0.34) or day 8 (P=0.88 and 1.00) (Table IV; Figure 1). The correlation between Cmax (rday 1=0.36, p=0.14 and rday 8=0.15, p=0.57) or AUC0–8h (rday 1=0.20, p=0.43 and rday 8=0.10, p=0.71) and dose was not statistically significant. On Day 1, median oral clearance was similar in males and females (CL/F= 24.2 and 19.7 L/h/m2; p=0.36) and did not differ in children <12 years compared with children ≥12 years (CL/F= 28.6 and 19.7 L/h/m2; p=0.36). The median half-life of axitinib was 2.4 hours (Day 1) and 2.5 hours (Day 8). The median (range) accumulation ratio based on comparison of day 8 AUC0–8h versus day 1 AUC0–8h, was 1.8 (0.3 – 4.7).
Table IV.
Axitinib pharmacokinetic (PK) parameters (median (range))
| Pharmacokinetic Parameter | Dose Level 2.4 mg/m2 | Dose Level 3.2 mg/m2 | ||
|---|---|---|---|---|
| Day 1 (n=12) | Day 8 (n=12) | Day 1 (n=6) | Day 8 (n=5) | |
| Tmax (h) | 2.0 (1.0–4.0) | 1.5 (0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.1–4.0) |
| Cmax (ng/mL) | 23.8 (2.5–55.6) | 47.4 (4.5–92.6) | 38.0 (16.3–58.1) | 41.5 (15.5–76.5) |
| Half life (h) | 2.2 (0.7–5.2) | 2.5 (0.9–631.5) | 2.6 (1.1–3.2) | 1.8 (1.1–4.3) |
| AUC0–8h (h•ng/mL) | 91.0 (10.7–264.1) | 146.7 (8.9–372.7) | 134.3 (59.0–236.0) | 139.9 (54.9–349.1) |
| Accumulation (AUC Day8/Day1) | 1.7 (0.3–4.7) | 1.8 (0.4–2.5) | ||
| Cl/F (L/h) | 28.0 (9.8–230.1) | 32.6 (14.2–65.2) | ||
| Cl/m2 (L/h/m2) | 21.4 (6.0–230.1) | 19.9 (11.4–61.5) | ||
| V/F (L) | 74.9 (43.6–888.7) | 99.4 (48.9–150.6) | ||
| V/m2 (L/m2) | 67.4 (39.1–888.7) | 65.5 (39.1–101.6) | ||
Figure 1. Axitinib Pharmacokinetics in Children and Adolescents with Refractory Solid Tumors.
The axitinib exposure (AUClast) for each patient is plotted as function of the patient’s dose (mg administered/patient’s actual BSA) on Day 1 (A) or Day 8 (C). Peak plasma concentration (Cmax) after the first dose on Day 1 (B) or Day 8 (D), for each dose level (mg/m2/dose), is plotted, with horizontal annotation of medians.
The relationship between axitinib exposure and hypertension was assessed. A cut-off AUClast of 130 h·ng/mL was selected based on the adult cut-off of AUC0–12h 200 h·ng/mL,24 terminal elimination rate constant, and difference in the sample collection interval of 0–8h used in our trial versus the 0–12h collection interval used in the adult trial. There appeared to be no association between hypertension and PK parameters [Day 1 Cmax (P=0.43), Day 8 AUClast (P=0.16), or Day 8 AUClast ≥ 130 h•ng/mL (P=0.30)].
The urine UPC ratio did not significantly change during axitinib therapy, increasing 4% per cycle (P=0.30), even after adjusting for baseline values (P=0.10). Biomarkers of AKI are presented in Supplemental Table II. The normal ranges for urine AKI biomarkers in healthy children are dependent on sex and age. At baseline, urine KIM-1 (median: 4 ng/mL; range: 0.2 – 14.4 ng/mL) and IL-18 (median: 88 ng/mL; range: 5 – 445 ng/mL) were elevated compared to normal ranges for healthy children (KIM-1 (median: 0.41 ng/ml; range: 0.226 – 0.703); IL-18 (median: 0.0216 ng/ml; range: 0.0136 – 0.0329); NGAL (median: 6.6 ng/ml; range 2.8 – 17)), 32 indicating possible kidney dysfunction prior to enrollment. During axitinib therapy, AKI biomarkers were highly variable and no trends were identified during cycle 1 or between dose levels.
Response
Sixteen patients were evaluable for response; 15 patients had sufficient evaluations to report a response (1 patient did not have all required evaluations). One patient with alveolar soft part sarcoma had a confirmed partial response that was durable for 6 months prior to clinical progression of disease and discontinuation of protocol therapy. Five patients had stable disease for ≥ 6 cycles including 2 patients with osteosarcoma and 1 each with malignant peripheral nerve sheath tumor, Ewing sarcoma, and hepatocellular carcinoma.
Discussion
VEGF has been demonstrated to be overexpressed or biologically active in pediatric cancers including Wilms tumor,28,33 hepatocellular carcinoma,34 hepatoblastoma,35 nonrhabdomyosarcoma soft tissue sarcomas,9,36 rhabdomyosarcomas,28,37 Ewing sarcoma,38 osteosarcoma,39 and neuroblastoma.40 Targeting VEGF in pediatric solid tumors has been of interest to the pediatric oncology community.28, 41–43
We have established the RP2D of axitinib (2.4 mg/m2/dose PO BID) for patients > 12 months and < 18 years of age. This dose is approximately 80% of the adult recommended starting dose. The toxicity and PK profiles are similar in children and adolescents to those reported in adults. The most common grade 1 and 2 toxicities were gastrointestinal (nausea, diarrhea), fatigue, anorexia, anemia and hypertension. Grade 3 and 4 toxicities were rare. Two DLTs at dose level 2 (3.2 mg/m2/dose, equivalent to the adult dose of 5.5 mg PO BID) were palmar-plantar erythrodysesthesia and intratumoral hemorrhage. Intratumoral hemorrhage may not be dose related, but we defined this event as a DLT to err on the side of patient safety. As in adults, PK parameters were variable, and there was no correlation between hypertension or response and axitinib exposure.16, 44
In adults, dose titration is utilized to maximize potential clinical benefit. Adults treated with axitinib 5 mg PO BID, without adverse events greater than Grade 2 for consecutive 2 week periods and with blood pressure ≤ 150/90 mm Hg, are permitted to increase their dose to 7 mg BID, then to a maximum of 10 mg BID. We did not permit intra-patient dose titration in this study, but it is possible that some patients would have tolerated higher doses utilizing similar criteria. We observed preliminary evidence of efficacy, one patient with alveolar soft part sarcoma experiencing a partial response and 5 patients had stable disease for ≥ 6 cycles. Intra-patient dose titration in future studies might increase the likelihood of disease response.
Recently, the combination of anti-angiogenic therapy with immune checkpoint inhibitor therapy has been evaluated in adults with renal cell carcinoma (RCC). Nivolumab in combination with sunitinib with each agent administered at full dose is tolerable and demonstrated anti-tumor activity and a manageable safety profile.45 Axitinib in combination with pembrolizumab was tolerable when each agent was administered at its MTD/RP2D.46 Translocation-associated RCC (tRCC), accounts for 48% of all pediatric RCC,47 and both children and adults with tRCC have achieved objective responses and durable complete remissions with VEGF RTK inhibitors.48–50 The Children’s Oncology Group, along with other NCI-affiliated cooperative groups, plans to conduct a 3-arm randomized trial to evaluate axitinib vs axitinib in combination with nivolumab vs nivolumab in tRCC across all age groups. The present study establishes the recommended starting axitinib dose for children for the upcoming trial. Future exploration of strategies to permit intra-patient dose titration of the axitinib in children who do not experience toxicity at the starting doses is warranted.
Supplementary Material
Acknowledgments
Funding Sources: National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number UM1 CA097452, Mayo Clinic Comprehensive Cancer Center Grant P30 CA15083-43, Cookies for Kids’ Cancer Foundation, Champions FORE Children, and the Children’s Oncology Group Foundation.
Footnotes
Clinical Trials Registry: NCT02164838
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Author Contribution: All authors have read, were involved with planning and conduct of the trial and manuscript, contributed to data analysis and assisted in writing, and approved the manuscript. Drs. Geller and Weigel are responsible for the overall content as guarantors.
References
- 1.de Castro G, Junior, Puglisi F, de Azambuja E, et al. Angiogenesis and cancer: A cross-talk between basic science and clinical trials (the “do ut des” paradigm) Crit Rev Oncol Hematol. 2006;59:40–50. doi: 10.1016/j.critrevonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 9.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 10.Keir ST, Maris JM, Lock R, et al. Initial testing (stage 1) of the multi-targeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55:1126–33. doi: 10.1002/pbc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maris JM, Courtright J, Houghton PJ, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51:42–8. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 12.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–7. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 13.Kania RS. Structure-Based Design and Characterization of Axitinib, Kinase Inhibitor Drugs. John Wiley & Sons, Inc; 2009. pp. 167–201. [Google Scholar]
- 14.Solowiej J, Bergqvist S, McTigue MA, et al. Characterizing the effects of the juxtamembrane domain on vascular endothelial growth factor receptor-2 enzymatic activity, autophosphorylation, and inhibition by axitinib. Biochemistry. 2009;48:7019–31. doi: 10.1021/bi900522y. [DOI] [PubMed] [Google Scholar]
- 15.Pfizer Inc. Investigator’s Brochure for Axitinib (AG-013736) Dec, 2013. [Google Scholar]
- 16.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 17.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–84. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 18.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27:3836–41. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–8. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 21.Fruehauf J, Lutzky J, McDermott D, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17:7462–9. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 22.Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol. 2013;14:1233–42. doi: 10.1016/S1470-2045(13)70464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rini BI, Schiller JH, Fruehauf JP, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–9. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 24.Rini BI, Melichar B, Fishman MN, et al. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann Oncol. 2015 Jul;26(7):1372–7. doi: 10.1093/annonc/mdv103. [DOI] [PubMed] [Google Scholar]
- 25.Matias M, Le Teuff G, Albiges L, et al. Real world prospective experience of axitinib in metastatic renal cell carcinoma in a large comprehensive cancer centre. Eur J Cancer. 2017 Jul;79:185–192. doi: 10.1016/j.ejca.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Rini BI, Quinn DI, Baum M, et al. Hypertension among patients with renal cell carcinoma receiving axitinib or sorafenib: analysis from the randomized phase III AXIS trial. Targ Oncol. 2015;10:45–53. doi: 10.1007/s11523-014-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skolnik JM, Barrett JS, Jayaraman B, et al. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 28.Kim A, Widemann BC, Krailo M, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015 Sep;62(9):1562–6. doi: 10.1002/pbc.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibaldi M, Perrier D. Series on Drugs and the Pharmaceutical Sciences. Marcel Dekker Inc; NY: 1982. Pharmacokinetics (ed2) [Google Scholar]
- 30.Du Y1, Zappitelli M, Mian A, et al. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol. 2011 Feb;26(2):267–74. doi: 10.1007/s00467-010-1673-0. Epub 2010 Oct 27. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed]
- 32.Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol. 2015 Apr;30(4):677–85. doi: 10.1007/s00467-014-2989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki M, Ostalska-Nowicka D, Kaczmarek M, et al. The significance of VEGF-C/VEGFR-2 interaction in the neovascularization and prognosis of nephroblastoma (Wilms’ tumour) Histopathology. 2007;50:358–64. doi: 10.1111/j.1365-2559.2007.02613.x. [DOI] [PubMed] [Google Scholar]
- 34.Sun XY, Wu ZD, Liao XF, et al. Tumor angiogenesis and its clinical significance in pediatric malignant liver tumor. World J Gastroenterol. 2005;11:741–3. doi: 10.3748/wjg.v11.i5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eicher C, Dewerth A, Kirchner B, et al. Treatment effects of the multikinase inhibitor sorafenib on hepatoblastoma cell lines and xenografts in NMRI-Foxn1 nu mice. Liver Int. 2012;32:574–81. doi: 10.1111/j.1478-3231.2011.02729.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Yu D, Hu M, et al. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 2000;60:3655–61. [PubMed] [Google Scholar]
- 37.Armistead PM, Salganick J, Roh JS, et al. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma: correlation with overall survival in 105 patients. Cancer. 2007;110:2293–303. doi: 10.1002/cncr.23038. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin Cancer Res. 2004;10:1344–53. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 39.Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–7. [PubMed] [Google Scholar]
- 40.Shusterman S, Maris JM. Prospects for therapeutic inhibition of neuroblastoma angiogenesis. Cancer Lett. 2005;228:171–9. doi: 10.1016/j.canlet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 41.Kieran MW, Chi S, Goldman S, et al. A phase I trial and PK study of cediranib (AZD2171), an orally bioavailable pan-VEGFR inhibitor, in children with recurrent or refractory primary CNS tumors. Childs Nerv Syst. 2015 Sep;31(9):1433–45. doi: 10.1007/s00381-015-2812-5. Epub 2015 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DuBois SG, Shusterman S, Reid JM, et al. Tolerability and pharmacokinetic profile of a sunitinib powder formulation in pediatric patients with refractory solid tumors: a Children’s Oncology Group study. Cancer Chemother Pharmacol. 2012 Apr;69(4):1021–7. doi: 10.1007/s00280-011-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuk Meredith K, Widemann Brigitte C, Ahern Charlotte H, et al. A phase I study of cabozantinib (XL184) in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: A Children’s Oncology Group phase I consortium trial. J Clin Oncol. 2014;32(5s) suppl abstr 10078. [Google Scholar]
- 44.Chen Y1, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK. Clinical pharmacology of axitinib. Clin Pharmacokinet. 2013 Sep;52(9):713–25. doi: 10.1007/s40262-013-0068-3. [DOI] [PubMed] [Google Scholar]
- 45.Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) Journal of Clinical Oncology. 2014;32:5010–5010. [Google Scholar]
- 46.Atkins MB, Gupta S, Choueiri TK, et al. Phase Ib dose-finding study of axitinib plus pembrolizumab in treatment-naïve patients with advanced renal cell carcinoma. Journal for Immunotherapy of Cancer. 2015;3:P353–P353. [Google Scholar]
- 47.Geller JI, Ehrlich PF, Cost NG, et al. Characterization of adolescent and pediatric renal cell carcinoma: A report from the Children’s Oncology Group study AREN03B2. Cancer. 2015 Jul 15;121(14):2457–64. doi: 10.1002/cncr.29368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choueiri TK, Lim ZD, Hirsch MS, et al. Vascular endothelial growth factor-targeted therapy for the treatment of adult metastatic Xp11.2 translocation renal cell carcinoma. Cancer. 2010;116:5219–25. doi: 10.1002/cncr.25512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury T, Prichard-Jones K, Sebire NJ, et al. Persistent complete response after single-agent sunitinib treatment in a case of TFE translocation positive relapsed metastatic pediatric renal cell carcinoma. J Pediatr Hematol Oncol. 2013;35:e1–3. doi: 10.1097/MPH.0b013e318266bf34. [DOI] [PubMed] [Google Scholar]
- 50.Malouf GG, Camparo P, Oudard S, et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol. 2010;21:1834–8. doi: 10.1093/annonc/mdq029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.