Abstract
Background:
The inhibitory deficits in the motor cortex in schizophrenia have been well demonstrated using short-interval intracortical inhibition (SICI) by transcranial magnetic stimulation (TMS). However, it remains unknown whether these deficits originate from dysfunction of motor cortex itself or reflect abnormal modulations of the motor cortex by other schizophrenia-related brain areas.
Methods:
Twenty-four patients with schizophrenia spectrum disorders and 30 healthy controls completed the study. SICI was obtained by delivering TMS over the left motor cortex. Resting-state functional magnetic resonance imaging and diffusion tensor imaging fractional anisotropy were used to measure functional connectivity (FC) and white matter microstructures, respectively. Stimulation sites for SICI at the motor cortex were used as the seeds to obtain the whole-brain FC maps. The clinical symptoms were assessed with Brief Psychiatric Rating Scale (BPRS).
Results:
In schizophrenia, left prefrontal cortex (PFC) – motor cortex FC was inversely associated with SICI but positively associated with the underlying white matter microstructure at the left corona radiata (CR), and also associated with overall symptoms (all corrected p<0.05). Mediation analysis showed that the PFC-motor cortex FC significantly mediates the corona radiata white matter effects on SICI (p=0.007).
Conclusions:
Higher resting-state left PFC-motor cortex functional connectivity, accompanied by a higher FA of left corona radiata, predicts less inhibitory deficits, suggesting the inhibitory deficits in the motor cortex in schizophrenia may in part be mediated by a top-down prefrontal influence. SICI may serve as a robust biomarker indexing inhibitory dysfunction at anatomic as well as circuitry levels in schizophrenia.
Keywords: schizophrenia, TMS, resting, DTI, motor inhibition, connectivity
Introduction
Disinhibited motor behaviors, ranging from subtle odd movements and postures, to gross automatisms and disorganized behaviors, have been described in schizophrenia for as long as the disorder has been characterized(1–3). Modern brain stimulation research using transcranial magnetic stimulation(TMS) can directly document motor cortical inhibition dysfunctions in schizophrenia by delivering TMS over the motor cortex, using various paradigms such as short-interval intracortical inhibition(SICI), long-interval intracortical inhibition(LICI), cortical silent period(CSP) and others(4–7). Among them, SICI is the strongest biomarker candidate for inhibition dysfunction in schizophrenia because reduced SICI is the most replicated in schizophrenia(6) and is present in first-episode(8,9) and chronic patients(10–12), and even in individuals at high risk for developing schizophrenia(13). Abnormal SICI is typically thought of as reflecting a motor cortex or motor pathway dysfunction(14,15). However, the motor cortex is closely inter-connected with prefrontal and other brain regions(16), many of which show abnormalities in schizophrenia(17,18). Our goal is to test a novel hypothesis that SICI deficit in schizophrenia is not simply indexing motor pathway dysfunctions, but it may reflect motor cortex functional and structural connectivity abnormalities associated with other brain regions impacted by schizophrenia.
The cortico-motor circuits, such as the medial prefrontal-motor circuit, are related to psychomotor modulation(19). Since the medial prefrontal cortex has been implicated in the pathophysiology of schizophrenia(20), the top-down regulation to motor regions may play a role in motor circuitry abnormalities in schizophrenia. Besides the prefrontal–motor circuitry, other motor circuitries such as the cerebello-thalamo-motor circuit may also be relevant as it controls motor learning and postural control(21,22). Furthermore, altered sensorimotor and basal ganglia circuit abnormalities and their related motor behavioral problems have also been shown in schizophrenia(23–27). The dopamine-sensitive basal ganglia circuit in particular is known to be associated with both psychosis and spontaneous dyskinesia, suggesting overlapping mechanisms in psychosis and certain motor dysfunctions in schizophrenia(28–31). Our goal was to understand whether motor inhibition deficit as indexed by SICI is related to prefrontal and other motor circuitries in schizophrenia.
In SICI, a subthreshold stimulation is followed by a suprathreshold stimulation with short interstimulus intervals(ISIs)(32,33). Previous studies suggested that SICI is a marker reflecting motor cortex GABAA-receptor function(34). Besides the neurochemical explanation of SICI, our previous research hinted another important mechanism: by exploring the association between SICI and all major white matter tracts, we found corticospinal tract, internal capsule, and corona radiata was associated with SICI in schizophrenia patients(35). Corona radiata in particular connects the frontal and other cortical areas to motor cortex, implying a possible cortico-cortical fronto-motor modulation in schizophrenia. Therefore, we hypothesized that SICI might also be a network function biomarker indexing motor-frontal cortico-cortical circuitry functions. The limitation of our previous finding was that corona radiata also connects to many other cortical areas and it is not a functional measurement, and thus lacks the anatomic and functional specificity needed for confirming a fronto-motor involvement for SICI.
Therefore, the present study used resting-state functional connectivity(rsFC) to examine whether SICI would be associated the functional connectivity between motor and prefrontal (and other cortical and subcortical) areas using motor cortex seed based whole-brain rsFC analysis. Our approach is to identify functional circuitry that is not only related to SICI, but also related to symptoms in schizophrenia, followed by evaluating whether such rsFC mediates the previous finding of white matter contributions to SICI. As SICI is emerging as one of the most robust and replicable inhibitory biomarkers in schizophrenia(5), defining its underlying mechanism is important for supporting its development and implementation for future disease mechanism and treatment research.
Materials and methods
Participants
Patients with schizophrenia spectrum disorders (n=24) and healthy controls (n=30) participated in the study(Table 1). Patients were recruited from Maryland Psychiatric Research Center and neighboring mental health clinics in the Baltimore area. Controls were recruited from local media advertisement. The Structured Clinical Interview for DSM-IV was used to confirm the diagnoses of schizophrenia or schizoaffective disorder in patients and the absence of current DSM-IV Axis I diagnoses in controls. Exclusion criteria were major medical and neurological illnesses, history of head injury with loss of consciousness, substance abuse (except nicotine) and taking clozapine more than 400mg/day(36). Four schizophrenia patients were not on antipsychotic medications, 19 were on atypical, and 2 were on typical including 1 on both atypical and typical medications(Table 1). No patients took benzodiazepines at the time of scanning. Brief Psychiatric Rating Scale(BPRS)(37) was administered to patients for assessing their overall clinical symptoms (BPRS total score). Motor retardation was represented by item 13 in BPRS. All raters were master level clinicians and received training by a standing committee and must achieve intraclass correlation 0.9 or above with the gold standard. All subjects gave their written informed consent approved by local Institutional Review Board.
Table 1. Demographic and clinical characteristics and group differences.
| Schizophrenia
n=24 |
Healthy control
n=30 |
Statistics t, F or χ2 |
p value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) (±SD) | 36.51±13.51 | 42.22±13.59 | 1.54 | 0.13 | ||||||||
| Male/Female | 17/7 | 16/14 | 1.72 | 0.19 | ||||||||
| BPRS | 41.13±11.23 | - | - | - | ||||||||
| Illness duration (years) | 14.59±14.75 | - | - | - | ||||||||
| Education (years) | 12.38±1.61 | 13.83±2.35 | 2.59 | 0.01* | ||||||||
| Smoker/Non-smoker | 7/17 | 6/24 | 0.61 | 0.43 | ||||||||
| Antipsychotic medication | ||||||||||||
| Typical | 2a | - | - | - | ||||||||
| Atypical | 19a | - | - | - | ||||||||
| Medication-free | 4 | - | - | - | ||||||||
| CPZ (mg) | 568.06±704.06 | - | - | - | ||||||||
| RMT (%) | 47.52±8.29 | 47.68±6.58 | 0.08 | 0.94 | ||||||||
| TS alone MEP (mV) | 0.84±0.62 | 1.13±0.74 | 1.53 | 0.13 | ||||||||
| SICI | 0.41±0.31 | 0.25±0.16 | -2.49 | 0.02* | ||||||||
| FA of left CRb | 0.48±0.03 | 0.49±0.02 | 10.59 | 0.002* | ||||||||
BPRS: Brief Psychiatric Rating Scale total score. Resting motor threshold (RMT) was reported as a percentage of the maximum stimulator output. CPZ: Chlorpromazine; SICI: short-interval intracortical inhibition; TS alone MEP: Test stimulation evoked peak-to-peak amplitude of the EMG motor-evoked potential (MEP) amplitude when single test pulse delivered at 120% of RMT. FA: fractional anisotropy; CR: corona radiata.
Statistically different between schizophrenia spectrum disorder patients and healthy controls.
one patient took both typical and atypical antipsychotic medications.
age was used as a covariate due the large effect of age on white matter microstructure (103).
TMS and electromyography procedure
A figure-of-eight coil with Magstim stimulators (Whitland, UK) was utilized to deliver the stimulations. Subject’s structural images were used for precise positioning of the coil through Brainsight™ (Montreal, Canada). The stimulus target was left motor cortex(M1) where TMS induced the maximum response from right first dorsal interosseous muscle. Peak-to-peak amplitude of the motor-evoked potentials(MEP) was measured.
Resting motor threshold(RMT) was defined as the minimum intensity needed to elicit a MEP of>50μV in at least 5 out of 10 consecutive stimuli(38). Paired-pulse TMS(ppTMS) with 1 and 3 ms ISIs(35,39) were used to induce short-interval intracortical inhibition(SICI). For each SICI trial, a subthreshold conditioning stimulus (80% RMT) was followed by a suprathreshold stimulation (120% RMT). Single pulse at 120% RMT was the test stimulation(TS) that serves as a control. SICI was defined as the ratio between responses of ppTMS and TS alone. Ratios less than 1 indicate inhibition and the smaller the ratio, the stronger the cortical inhibition. There were 24 trials for SICI and 24 trials for TS. Previous research demonstrated that SICI, but usually not RMT or MEP of TS alone, can robustly separate schizophrenia patients from healthy controls, supporting that the SICI deficit was likely inhibitory rather than deficit in motor responses(6).
Imaging data acquisition
Before the SICI session, we assessed structural, resting-state fMRI and diffusion tensor imaging(DTI) in a separate session. All imaging was performed using a Siemens 3T TRIO MRI system (Erlangen, Germany) equipped with a 32-channel head coil. Structural images were acquired using a fast spoiled gradient-recalled sequence (TR/TE=11.08/4.3ms, flip angle=45°, FOV=256mm, 256×256 matrix, 172 slices, 1mm3 spatial resolution). Resting-state functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence (TR/TE =2s/27ms; flip angle=90°; FOV=220mm; 64×64 matrix; 1.7mm2 in-plane resolution; 4mm slice thickness; 37 axial slices, 15 minutes scan for 450 volumes). Participants were asked to keep their eyes closed, relax and not to think about anything in particular. Post-scan questions confirmed that participants did not fall asleep during the scan. DTI data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence at 1.7×1.7×3.0mm, TE/TR=87/8000ms, FOV=200mm, 50 slices and no gaps, five b=0 images and 64 isotropically distributed diffusion weighted directions with b=700s/mm2.
Imaging data preprocessing
Standard resting-state functional MRI data processing was carried out using Analysis of Functional NeuroImages(AFNI)(40) software (Version 16.3.17). After discarding the first two TRs, the preprocessed data were spatially smoothed to a full width at half maximum of 4mm. The linear trend, 6 motion parameters (3 rotational and 3 translational directions), their 6 temporal derivatives (rate of change in rotational and translational motion) and time courses from the white matter and cerebral spinal fluid(CSF) were removed as regressors of no interest. Time points with excessive motion (>0.2mm) and their neighboring time points were censored from statistical analysis (details in Supplementary material). Finally, for group analysis, images were spatially normalized to the Talairach space(41).
Individual statistical maps were then calculated using a seed-based correlation analysis to infer the functional connectivity of the seed with the rest of the brain. The seed region of interest(ROI) was left M1 defined based on TMS site of SICI for each subject. A 10mm radius sphere was placed on each subject’s structural images with individual SICI site as the center(Figure 3A). White matter and CSF were removed from the seed ROI using the masks obtained from FreeSurfer(42). Then, the mean time-series within seed ROI was correlated with the time course of each voxel in the brain for each subject. Pearson’s correlation coefficients were converted to z values using Fisher’s r-to-z transform.
The details of DTI data preprocessing were described in Supplementary Material and our previous studies(35, 43). Based on a tract-based spatial statistics method(44), fractional anisotropy(FA) images were obtained, spatially normalized to the JHU atlas(45) which separated white matter into different tracts. The white matter directly under the left motor cortex and connecting motor cortex to the frontal area is the left corona radiata(CR). In our previous research, by exploring SICI-related white matter tracts (25 tracts), we found left CR was also the only tract whose association with SICI in schizophrenia survived Bonferroni correction(35). Therefore, we included only left CR in the study(Figure 3A). Some of the SICI and DTI data were reported in our previous work(35,38). None of the resting-state fMRI data were previously reported.
Statistical analysis
The demographic data were compared using independent-sample t tests for continuous values and Chi-squared test for categorical values. Although many demographic characters did not significantly differ between groups, their potential effects on the results were evaluated. Group-level brain-behavioral correlations were evaluated using Pearson’s correlation with age as a covariate. Results were also evaluated by adding gender, education level, and chlorpromazine equivalent dose(CPZ) as extra covariates; only significant findings would be reported. The primary analysis was to test whether there are M1-seeded rsFC associated with both motor inhibition (i.e., SICI) and overall symptoms of schizophrenia (i.e., BPRS total score). Brain-SICI correlation maps were calculated by correlating behavioral variables with z values in rsFC maps. We first identified regions showing significant M1 rsFC related to SICI in patients and controls separately, and schizophrenia symptoms in patients. The significant clusters were determined by estimating the cluster-size threshold (cluster size>202 voxels) using the updated 3dClustSim with the Spatial AutoCorrelation Function(46–48) to obtain corrected p<0.017 (0.05/3; Bonferroni correction for 3 comparisons). We further used leave-one-out cross-validation(LOOCV) to evaluate the validities of clusters identified above (details in Supplementary material)(49). Clusters with validity of 80% or higher were considered as valid clusters. The same procedures were repeated for BPRS score. The overlapping region(s) of significant rsFC-SICI clusters and significant rsFC-BPRS clusters was extracted to represent M1 rsFC that were significantly related to both SICI and clinical symptoms of schizophrenia. The relationship between these clusters and motor retardation was examined to confirm their role in motor abnormality. The group-difference of rsFC was also assessed. The rsFC from those clusters was then used to examine for their mediation relationship with FA of left CR with age as a covariate using PROCESS(50,51) in SPSS 23.0.
Results
The demographic and clinical information of participants are in Table 1. Patients showed reduced SICI (t(52)=−2.49, p=0.02)compared with healthy controls. There was no significant difference in age (t(52)=1.54, p=0.13), gender (χ2=1.72, p=0.19), smoking status (χ2=0.61, p=0.43), but patients had less education (t(52)=2.59, p=0.01). RMT (t(52)=0.08, p=0.94) and TS alone MEP (t(52)=1.53, p=0.13) were not significantly different between the two groups, suggesting no significant motor response impairment in the patients. No association was found between smoking status and SICI in neither group (SZ: t(22)=−0.63, p=0.54; HC: t(28)=0.48, p=0.63).
SICI related M1-seeded rsFC in schizophrenia
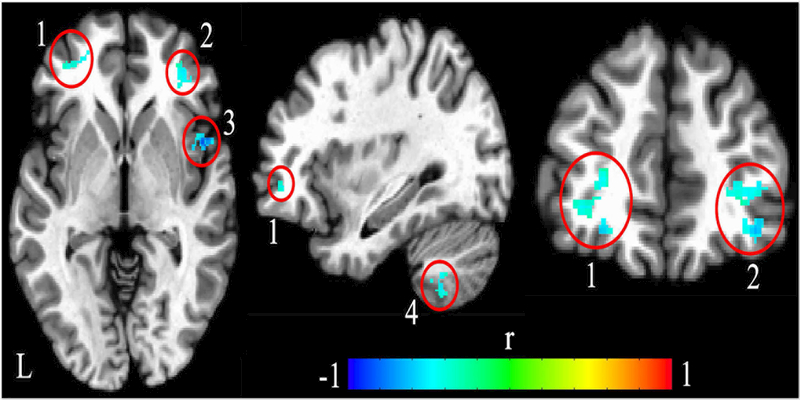
In patients with schizophrenia, there were four brain areas whose rsFC with left M1 significantly associated with SICI (corrected p<0.05, see Table 2 for coordinates, cluster size, and statistics, and Figure 1 for anatomic locations): left middle prefrontal gyrus, right middle prefrontal gyrus, right insula, and left cerebellum (VIII area). Higher rsFCs between those areas and left M1 predicted smaller SICI ratio (stronger cortical inhibition). All clusters except left cerebellum had high cluster validities (>80%). Similar results were obtained when adding medication dosage [converted to chlorpromazine equivalent dose(CPZ)], education, and gender as additional covariates. In addition, comparing M1-seeded rsFC between healthy controls and schizophrenia patients, two of these four SICI-related rsFC, i.e., the left M1 - left middle prefrontal gyrus (reduced threshold) and left M1 - right insula rsFC (significant), were reduced in patients compared with controls (details in Supplementary Material and Figure S4). We also ‘reversed’ the analysis by selecting the left middle PFC seed, and found that its rsFC with M1 remained significantly correlated with SICI (Figure S5). Finally, to test for anatomic specificity, we selected a left occipital seed and repeated the analyses, and found no significant rsFC that was associated with SICI, suggesting that the correlation between M1- middle PFC was unlikely due to a diffuse global rsFC effect (details in Supplementary material).
Table 2. Coordinates of significant brain areas where resting-state functional connectivity with left motor cortex were significantly related with cortical inhibition and overall symptoms in schizophrenia spectrum disorder patients.
| Talairach (x, y, z) mm |
Brodmann area |
Cluster size |
r | |
|---|---|---|---|---|
| SICI related rsFC in schizophrenia | ||||
| Left middle prefrontal gyrus | (–25, 47, –2) | 10/11 | 206 | −0.81* |
| Left middle Cerebellum (VIII) | (–15, –66, –34) | n/a | 222 | -0.75* |
| Right middle prefrontal gyrus | (39, 39, –6) | 10/11 | 427 | -0.87* |
| Right Insula | (46, 9, –5) | 13 | 219 | -0.77* |
| BPRS related rsFC in schizophrenia | ||||
| Left superior occipital gyrus | (–17, –85, 41) | 19 | 656 | 0.79* |
| Left postcentral gyrus | (–35, –29, 47) | 3/4 | 601 | 0.83* |
| Left middle prefrontal gyrus | (–20, 54, –4) | 10/11 | 546 | -0.84* |
| Left precentral gyrus | (–30, –14, 60) | 4/6 | 256 | 0.73* |
| Left middle occipital gyrus | (–18, –97, 9) | 18 | 246 | 0.78* |
| Left inferior frontal gyrus | (–50, 6, 24) | 9/44 | 210 | 0.73* |
| Left cerebellum (VI) | (–30, –43, –27) | n/a | 204 | -0.81* |
| Right superior occipital gyrus | (19, –86, 33) | 7/19 | 808 | 0.74* |
| Right superior parietal lobule | (28, –65, 52) | 7 | 754 | 0.81* |
| Right postcentral gyrus | (33, –30, 48) | 3/4 | 535 | 0.86* |
| Right anterior cingulate | (10, 36, 25) | 32 | 358 | -0.83* |
| Right cerebellum (VI) | (22, –76, –12) | n/a | 328 | 0.75* |
| Right precentral gyrus | (40, –18, 60) | 4/6 | 251 | 0.81* |
r: correlation coefficients between behavioral, i.e., Brief Psychiatric Rating Scale total score (BPRS) and short-interval intracortical inhibition (SICI), and the mean resting-state functional connectivities (rsFCs) between motor cortex and each cluster.
corrected p<0.05. Data for healthy controls are in Supplementary Table S1.
Figure 1.
Locations showed significant associations between left motor cortex-seeded resting-state functional connectivity (rsFC) with short-interval intracortical inhibition (SICI) in the patients. Significant negative correlations with SICI were found at four areas: bilateral middle prefrontal gyrus (cluster 1 and 2), right insula (cluster 3) and cerebellum VIII (cluster 4). Higher rsFC between these four areas and motor cortex predicted smaller SICI, i.e., stronger cortical inhibition. Color bar indicates correlation coefficient (r). L, left hemisphere.
BPRS related M1-seeded rsFC in schizophrenia
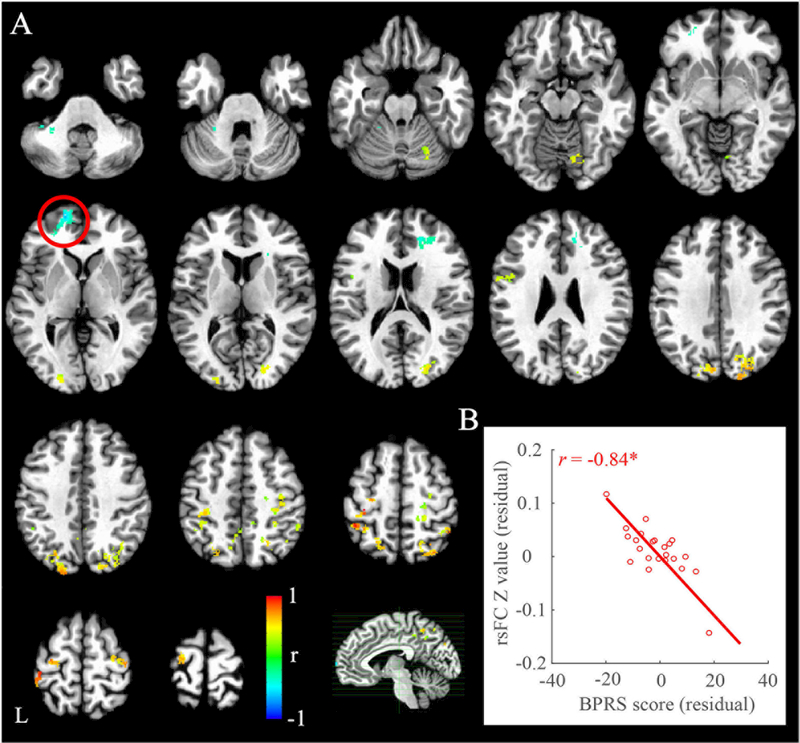
There were thirteen brain clusters showing significant BPRS-related rsFC with left M1 in schizophrenia patients (corrected p<0.05, Table 2 and Figure 2A). Among them, three were negatively associated with BPRS score, including left M1 rsFC with left middle prefrontal gyrus (Figure 2B), left cerebellum (VI area), and right anterior cingulate (Figure 2A), suggesting higher rsFC predicted fewer clinical symptoms in patients. In addition, left M1 rsFC with another 12 brain regions showed positive correlation with BPRS score (Table 2). All those clusters except left postcentral gyrus had high cluster validities (>80%). Similar results were obtained when adding CPZ, education, and gender as additional covariates.
Figure 2.
Significant associations between left motor cortex-seeded resting-state functional connectivity (rsFC) with overall symptoms as measured by Brief Psychiatric Rating Scale (BPRS) in schizophrenia patients. The significant cluster at left middle prefrontal gyrus was highlighted in the circle (A) and its rsFC with left motor cortex was negatively associated with BPRS in patients (B). Color bar indicates correlation coefficient (r). L, left hemisphere. *corrected p<0.05.
Overlapping SICI and BPRS related rsFC
When overlapping SICI-related rsFC with BPRS-related rsFC, only the rsFC between left M1 and left middle prefrontal gyrus remained in the patient-group (x=−33, y=43, z=−6; overlapped voxels: 73; Figure 3A). The left PFC-M1 rsFC was negatively associated with SICI (Figure 3B) and BPRS: higher left PFC-M1 rsFC predicted both stronger cortical inhibition and fewer clinical symptoms in patients. The association between left PFC-M1 rsFC and motor retardation was also significant (r=−0.47, p=0.02; Figure S3).
Although the SICI-related rsFC map and BPRS-related rsFC map did not overlap in cerebellum, it is worth noting that two adjacent cerebellar sub-regions (VIII and VI) were significantly associated with SICI and BPRS, respectively (Figure S1).
Correlation with corona radiata
Schizophrenia patients had reduced FA at the left corona radiata(CR) (Table 1). The SICI-derived left PFC-M1 rsFC showed significant positive correlation with the FA of left corona radiata (CR) in the patients (r=0.54, p=0.006) (Figure 3B). By comparison, no such correlation was observed in healthy controls (SICI: r=0.02, p=0.90; left CR: r=0.13, p=0.50).
Mediation by left PFC-M1 rsFC in schizophrenia
Mediation analysis was conducted with left CR as the independent variable, SICI as the outcome variable, left PFC-M1 rsFC as the potential mediator and age as a covariate. As shown in Figure 3C, the total effect of left CR on SICI was significant (path C; t=−4.86, p=0.0001). Adding left PFC-M1 rsFC as the mediator, the direct effect from left CR to SICI was no longer significant (path C’; t=−1.89, p=0.07) while the indirect path via left PFC-M1 rsFC was significant (Sobel test, p=0.007). Therefore, the left CR effect on SICI was largely mediated by left PFC-M1 rsFC.
Figure 3.
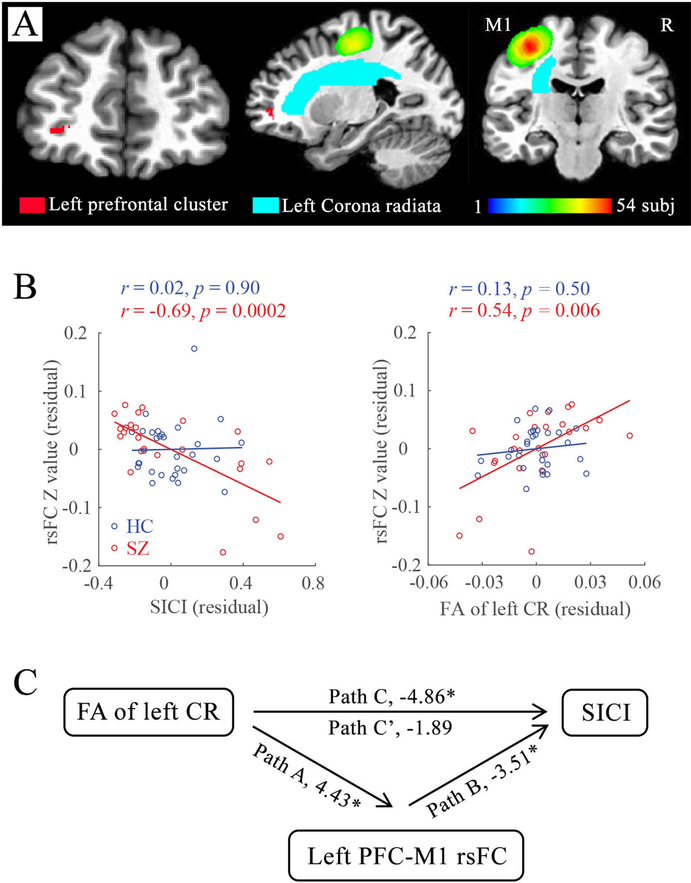
Associations and mediation analyses between PFC-motor cortex (M1) resting-state functional connectivity (rsFC), short-interval intracortical inhibition (SICI) and white matter microstructure of left corona radiata (CR). A: The anatomic relationships of left prefrontal gyrus (red cluster), left CR (cyan; extract from JHU white matter atlas), individualized left M1 region-of-interest. The color spectrum scale indicates the number of overlaps on the TMS stimulation site with the 10 mm sphere among the 54 subjects. B (left): Significant association between PFC-M1 rsFC and SICI in patients, but not healthy controls. B (right): Significant correlations between PFC-M1 rsFC and fractional anisotropy (FA) of left CR in patients but not healthy controls. C: Mediation analysis in the patient group suggested that the impacts of left CR microstructure on SICI in patients were strongly mediated by the left PFC-M1 rsFC. The t value for each path was presented. * p<0.05.
SICI related rsFC in healthy controls
There were three brain regions whose rsFC with left M1was significantly correlated with SICI in healthy controls (corrected p<0.05; Figure S2 and Table S1). Negative associations were found between SICI and M1 rsFC with left inferior parietal lobule and right middle occipital gyrus, while positive association was observed between SICI and M1 rsFC with right middle frontal gyrus.
Discussion
Motor-related dysfunctions are some of the important clinical features in schizophrenia(52). We hypothesized that the motor inhibition deficit in schizophrenia may reflect abnormal connectivity between motor cortex and other brain regions impacted by schizophrenia. We examined this hypothesis by using SICI as the motor inhibition marker and explore its associations with motor cortex seeded resting-state functional connectivity(rsFC). The results showed that left middle prefrontal gyrus - motor cortex rsFC was significantly associated with both cortical inhibition and symptoms of schizophrenia: stronger the rsFC, stronger the motor cortical inhibition (indicated by smaller SICI values) and fewer symptoms. This left PFC-M1 rsFC largely mediated the effects of left corona radiata structural connectivity on SICI in patients.
The left PFC-M1 rsFC was associated with the overall symptom of schizophrenia(Figure 2B). Defective PFC in schizophrenia has been well demonstrated in neuroimaging studies(53–55) and used as a therapeutic target(56,57). Aberrant top-down effects from PFC on motor-related cortical and sub-cortical regions has been suggested to contribute to symptoms of schizophrenia(27,58). For example, catatonic patients not only exhibited prefrontal hypoactivation and frontal gray matter loss(17,59), but also had altered connections between PFC and premotor/motor cortex(60). In a recent study of Walther and colleagues, catatonia was associated with higher perfusion in supplementary motor area and prominent GM loss in frontal and insular cortices(61), which resemble the SICI-related PFC and insula clusters observed in our study. Schizophrenia patients with severe gesture deficits showed poor frontal lobe function(62,63). A middle PFC-motor cortico-cortical circuit(MPMCCC) that links high-order functions and movements allowing for psychomotor modulation has in fact been proposed by Northoff and colleagues(27,60) to be the core circuitry for motor dysfunction is schizophrenia. This circuit is highly consistent with the PFC-M1 rsFC found to be associated with both the cortical inhibition biomarker and overall clinical symptoms here. Previous studies have also demonstrated top-down regulation of motor cortex by ipsilateral PFC, and the importance of connectivity between these areas in guiding actions(64,65). RsFC did not provide directional information, therefore, we cannot determine whether the influences of PFC on M1 were top-down or interactive regulations. However, the correlation between SICI and PFC-M1 rsFC may represent a modulation from PFC to M1, instead of M1 to PFC, because SICI measurement depended on directly probing M1. As we used actual M1 site as the seed for rsFC calculation. The relationship with non-M1 networks were not directly assessed. For example, dopamine-related substantia nigra(SN)-thalamo-sensorimotor functional hyper-connectivity was significantly associated with psychopathological symptoms of schizophrenia(66), suggesting additional motor circuitries are also likely involved in motor dysfunctions and overall symptoms of schizophrenia. Overall, our data suggest that cortical motor inhibition deficit in schizophrenia was likely driven in part by the aberrant modulation between the ipsilateral motor cortex and PFC, and the SICI over the motor cortex may have efficiently indexed this circuitry dysfunction.
It is important to understand the neural underpinnings of SICI deficits in schizophrenia from both functional and structural perspectives. The corona radiata provides the majority of the ipsilateral fibers connections between frontal and motor cortical areas(67). This white matter structure has shown the largest effect size of white matter microstructural deficits in schizophrenia(68) and is also highly heritable(69). It is not clear why this SICI and white matter relationship was strongly mediated by the connections between ipsilateral prefrontal cortex and motor cortex(Figure 3C). The underlying mechanisms are difficult to fully explain as our current knowledge of the underlying mechanisms of both rsFC from fMRI and FA from DTI are limited. Considering that functional connectivity usually reflects the corresponding structural connectivity(70,71), one plausible interpretation is that the left corona radiata is the “infrastructure” for a prefrontal-to-motor interaction and the strong mediating effect of the rsFC is indexing the functional interaction of PFC with the motor cortex inhibition functions carried by this infrastructure(72, 73). Therefore, these findings further supported the notion that the motor inhibition biomarker in schizophrenia originates from a circuitry-level deficit.
On the neurochemical level, SICI has been viewed as primarily a GABAA receptor function based on GABAergic drug challenge studies(34). In parallel, the MPMCCC has also shown to be modulated by GABAergic drugs(58,74). The GABAergic dysfunction hypothesis in schizophrenia also has extensive support from clinical, genetic and postmortem studies(75–78). Therefore, the finding that PFC-M1 rsFC played a fundamental role in SICI in schizophrenia may also index a common underlying GABAergic mechanism leading to the strong correlation and mediation effects. It is noteworthy that the critical role of left PFC-M1 rsFC on SICI was only observed in patients but not in controls. As healthy controls do not have deficits in these structural, functional or GABAergic mechanisms, the relationship may be difficult to extract using only a modest sample size. Only in patients with deficits in many of these measures and the putatively shared underlying mechanisms, the relationships between imaging and behavioral biomarkers may have become apparent.
Cerebellum VI, VIII are motor-related cerebellar areas(79,80). Moreover, cerebellum VI was also shown to be engaged along with prefrontal and parietal cortices in cognitively demanding tasks(81). Here we found M1 rsFCs with cerebellum VIII and VI were associated with SICI and BPRS, respectively(Table 2; Figure 1, 2 and S1). One parsimonious explanation would be that BPRS and SICI are not strongly related, and these findings may reflect the functional differentiations in these two cerebellar regions: the cerebellar VIII area and its connectivity with M1 is more related to aspects of the symptoms(BPRS), but the cerebellar VI area and its connectivity with M1 is more related to motor inhibition function(SICI). This is consistent with the literature. The cerebellar-motor circuit is linked to deficit of sensorimotor control in schizophrenia(17,61,82) and stimulation of cerebellum altered the size of SICI at motor cortex(83); and the involvement of cerebellum in the neuropathology of schizophrenia is well supported(82,84). However, it should be noted that the association between SICI and cerebellum did not pass LOOCV test and this finding need to be confirmed by future studies.
The M1 rsFC with right insula was also associated with cortical inhibition in schizophrenia(Figure 1). Reduced right insula activation has been linked to slower performance in a stop-signal reaction time task, suggesting failed engagement of the right insula may be associated with poorer inhibition in patients with schizophrenia(85). Insula dysfunction has also been implicated in defective gesture performance in schizophrenia(86). The results that M1-insula rsFC was associated with SICI in schizophrenia add to this line of literature and support the hypothesis that the right insula influences motor abnormality in schizophrenia through this M1-insula circuit.
Due to the close relationship between SICI and inhibitory control(87,88), SICI has been proposed reflecting an inhibitory network for minimizing unwanted movements(89,90). Reduced SICI has been documented in movement disorders(91) such as Parkinson’s disease(89), dystonia(92), myoclonus(93), and tourette syndrome(94). The SICI deficit in schizophrenia may reflect similar but much more subtle motor abnormalities driven more by the altered left PFC-M1 connections in schizophrenia. Walther and colleagues also demonstrated the close relationship between motor abnormalities and motor-related networks(17). Our current findings could be important complements to the current understandings of neural networks underlying of motor abnormalities in schizophrenia.
There are limitations in the present study. As the majority of patients here were currently treated with antipsychotics, we cannot rule out possible impacts of medication as antipsychotic drugs may alter functional connectivity(95–98) and motor abnormalities(99). The four cases of medication-free patients here were too few to conduct meaningful sub-analysis. However, similar results were obtained after adding CPZ, education, and gender as additional covariates, suggesting at least that the current medication dosage, education level and gender may not be the main factors driving the results. It should be noticed that we excluded patients with substance abuse, so generalizing the current findings to schizophrenia patients with substance abuse need to be cautious. The moderate sample size limited ability to fully examine effects from covariates such as gender and comorbidity. Only SICI was used as the cortical inhibition index in the study; whether our findings can be replicated in analogous TMS measures (e.g., LICI, CSP)(6) should be determined. Therefore, another important direction of future research is to assess various behavioral markers of motor inhibition in schizophrenia and explore their associations with SICI and/or related brain regions/circuits.
In summary, the study provided integrated structural and functional brain imaging evidence to identify the left middle prefrontal-motor cortex circuitry as an underlying circuitry mechanism for the SICI deficit in schizophrenia. Schizophrenia has been associated with several inhibition deficits measured at brain stimulation, brain imaging, electrophysiological and behavioral levels(7,100–102). Identifying reliable functional biomarkers with sufficiently delineated underlying mechanisms is critical for supporting future efforts that utilize these inhibition-related biomarkers for etiology and intervention research aiming to remediate these deficits.
Supplementary Material
Acknowledgements
LEH has received or plans to receive research funding or consulting fee on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Sound Pharma, Regeneron, and Takeda. All other authors report no biomedical financial interests or potential conflicts of interest.
Funding
Support was received from National Institutes of Health (grant U01MH108148, R01MH112180, P50MH103222, and T32MH067533), the Brain and Behavior Research Foundation, a State of Maryland contract (M00B6400091), and a generous private philanthropic donation from the Clare E. Forbes Trust.
References
- 1.Peralta V, Cuesta MJ (2017): Motor Abnormalities: From Neurodevelopmental to Neurodegenerative Through “Functional” (Neuro)Psychiatric Disorders. Schizophr Bull 43:956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manschreck TC, Maher BA, Rucklos ME, Vereen DR (1982): Disturbed voluntary motor activity in schizophrenic disorder. Psychol Med 12:73–84. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS (2016): Phenomenology of Schizophrenia and the Representativeness of Modern Diagnostic Criteria. JAMA Psychiatry 73:1082–1092. [DOI] [PubMed] [Google Scholar]
- 4.Daskalakis ZJ, Fitzgerald PB, Christensen BK (2007): The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev 56:427–442. [DOI] [PubMed] [Google Scholar]
- 5.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ (2013): A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 124:1309–1320. [DOI] [PubMed] [Google Scholar]
- 6.Bunse T, Wobrock T, Strube W, Padberg F, Palm U, Falkai P, et al. (2014): Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimul 7:158–169. [DOI] [PubMed] [Google Scholar]
- 7.Rogasch NC, Daskalakis ZJ, Fitzgerald PB (2014): Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr Bull 40:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wobrock T, Schneider-Axmann T, Retz W, Rosler M, Kadovic D, Falkai P, et al. (2009): Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry 42:194–201.971. [DOI] [PubMed] [Google Scholar]
- 9.Wobrock T, Schneider M, Kadovic D, Schneider-Axmann T, Ecker UK, Retz W, et al. (2008): Reduced cortical inhibition in first-episode schizophrenia. Schizophr Res 105:252–261. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J (2002): A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res 114:11–22. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Leone A, Manoach DS, Birnbaum R, Goff DC (2002): Motor cortical excitability in schizophrenia. Biol Psychiatry 52:24–31. [DOI] [PubMed] [Google Scholar]
- 12.Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, et al. (2011): Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res 224:15–22. [DOI] [PubMed] [Google Scholar]
- 13.Hasan A, Wobrock T, Grefkes C, Labusga M, Levold K, Schneider-Axmann T, et al. (2012): Deficient inhibitory cortical networks in antipsychotic-naive subjects at risk of developing first-episode psychosis and first-episode schizophrenia patients: a cross-sectional study. Biol Psychiatry 72:744–751. [DOI] [PubMed] [Google Scholar]
- 14.Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. (1998): Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119:265–268. [DOI] [PubMed] [Google Scholar]
- 15.Di Lazzaro V, Rothwell J, Oliviero A, Profice P, Insola A, Mazzone P, et al. (1999): Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res 129:494–499. [DOI] [PubMed] [Google Scholar]
- 16.Bates JF, Goldman-Rakic PS (1993): Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 336:211–228. [DOI] [PubMed] [Google Scholar]
- 17.Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV (2017): Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull 43:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Preda A, Ford JM, Mathalon DH, Keator DB, van Erp TG, et al. (2015): Functional magnetic resonance imaging of motor cortex activation in schizophrenia. J Korean Med Sci 30:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northoff G (2002): What catatonia can tell us about “top-down modulation”: A neuropsychiatric hypothesis. Behav Brain Sci 25:555.-+. [DOI] [PubMed] [Google Scholar]
- 20.Hoftman GD, Datta D, Lewis DA (2017): Layer 3 Excitatory and Inhibitory Circuitry in the Prefrontal Cortex: Developmental Trajectories and Alterations in Schizophrenia. Biol Psychiatry 81:862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marvel CL, Turner BM, O’Leary DS, Johnson HJ, Pierson RK, Ponto LL, et al. (2007): The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology 21:761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolbecker AR, Steinmetz AB, Mehta CS, Forsyth JK, Klaunig MJ, Lazar EK, et al. (2011): Exploration of cerebellar-dependent associative learning in schizophrenia: effects of varying and shifting interstimulus interval on eyeblink conditioning. Behav Neurosci 125:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann T, Skatun KC, Alnaes D, Doan NT, Duff EP, Tonnesen S, et al. (2015): Disintegration of Sensorimotor Brain Networks in Schizophrenia. Schizophr Bull 41:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman RA, Gotts SJ, McAdams HM, Greenstein D, Lalonde F, Clasen L, et al. (2016): Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain 139:276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. (2015): Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. Jama Psychiatry 72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier-Baldelli A, Lunsford-Avery JR, et al. (2014): Cerebellar Networks in Individuals at Ultra High-Risk of Psychosis: Impact on Postural Sway and Symptom Severity. Hum Brain Mapp 35:4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal VA, Bernard JA, Northoff G (2017): What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull 43:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, et al. (2010): Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry 68:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R (2005): Relationship of neuromotor disturbances to psychosis symptoms in first-episode neurolepticnaive schizophrenia patients. Schizophr Res 75:65–75. [DOI] [PubMed] [Google Scholar]
- 30.Middleton FA, Strick PL (2002): Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex 12:926–935. [DOI] [PubMed] [Google Scholar]
- 31.Tekin S, Cummings JL (2002): Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 53:647–654. [DOI] [PubMed] [Google Scholar]
- 32.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. (1993): Corticocortical inhibition in human motor cortex. J Physiol 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziemann U, Rothwell JC, Ridding MC (1996): Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. (2015): TMS and drugs revisited 2014. Clin Neurophysiol 126:1847–1868. [DOI] [PubMed] [Google Scholar]
- 35.Du X, Kochunov P, Summerfelt A, Chiappelli J, Choa FS, Hong LE (2017): The role of white matter microstructure in inhibitory deficits in patients with schizophrenia. Brain Stimul 10:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedlund, Vieweg (1980): The brief psychiatric rating scale (BPRS): a comprehensive review. Journal of operational psychiatry 11:48–65. [Google Scholar]
- 38.Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE (2014): Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J Mot Behav 46:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Gennaro L, Marzano C, Veniero D, Moroni F, Fratello F, Curcio G, et al. (2007): Neurophysiological correlates of sleepiness: a combined TMS and EEG study. NeuroImage 36:1277–1287. [DOI] [PubMed] [Google Scholar]
- 40.Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- 41.Talairach J, Tournoux P (1988): Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging [Google Scholar]
- 42.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- 43.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, et al. (2017): Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry 74:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones DK, Horsfield MA, Simmons A (1999): Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42:515–525. [PubMed] [Google Scholar]
- 45.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 46.Mueller K, Lepsien J, Moller HE, Lohmann G (2017): Commentary: Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Front Hum Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017): FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL (2018): Network Mechanisms of Clinical Response to Transcranial Magnetic Stimulation in Posttraumatic Stress Disorder and Major Depressive Disorder. Biol Psychiatry 83:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preacher KJ, Hayes AF (2008): Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879–891. [DOI] [PubMed] [Google Scholar]
- 51.Preacher KJ, Rucker DD, Hayes AF (2007): Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivariate behavioral research 42:185–227. [DOI] [PubMed] [Google Scholar]
- 52.Garvey MA, Cuthbert BN (2017): Developing a Motor Systems Domain for the NIMH RDoC Program. Schizophr Bull 43:935–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, et al. (2001): Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res 108:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, et al. (2010): Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry 15:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO (2011): Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. (2014): Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125:2150–2206. [DOI] [PubMed] [Google Scholar]
- 57.Cole JC, Green Bernacki C, Helmer A, Pinninti N, O’Reardon J P (2015): Efficacy of Transcranial Magnetic Stimulation (TMS) in the Treatment of Schizophrenia: A Review of the Literature to Date. Innov Clin Neurosci 12:12–19. [PMC free article] [PubMed] [Google Scholar]
- 58.Northoff G (2002): What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci 25:555–577. [DOI] [PubMed] [Google Scholar]
- 59.Northoff G, Steinke R, Nagel DC, Grosser O, Danos P, Genz A, et al. (2000): Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol Med 30:583–596. [DOI] [PubMed] [Google Scholar]
- 60.Northoff G, Kotter R, Baumgart F, Danos P, Boeker H, Kaulisch T, et al. (2004): Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull 30:405–427. [DOI] [PubMed] [Google Scholar]
- 61.Walther S, Schappi L, Federspiel A, Bohlhalter S, Wiest R, Strik W, et al. (2017): Resting-State Hyperperfusion of the Supplementary Motor Area in Catatonia. Schizophr Bull 43:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walther S, Stegmayer K, Sulzbacher J, Vanbellingen T, Muri R, Strik W, et al. (2015): Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull 41:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther S, Vanbellingen T, Muri R, Strik W, Bohlhalter S (2013): Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia 51:2674–2678. [DOI] [PubMed] [Google Scholar]
- 64.Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE (2004): The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex 15:85–95. [DOI] [PubMed] [Google Scholar]
- 65.Narayanan NS, Laubach M (2006): Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martino M, Magioncalda P, Yu H, Li X, Wang Q, Meng Y, et al. (2018): Abnormal Resting-State Connectivity in a Substantia Nigra-Related Striato-Thalamo-Cortical Network in a Large Sample of First-Episode Drug-Naive Patients With Schizophrenia. Schizophr Bull 44:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmahmann J, Pandya D (2009): Fiber pathways of the brain OUP; USA. [Google Scholar]
- 68.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2017): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kochunov P, Jahanshad N, Marcus D, Winkler A, Sprooten E, Nichols TE, et al. (2015): Heritability of fractional anisotropy in human white matter: a comparison of Human Connectome Project and ENIGMA-DTI data. NeuroImage 111:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. (2009): Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sui J, He H, Yu Q, Chen J, Rogers J, Pearlson GD, et al. (2013): Combination of Resting State fMRI, DTI, and sMRI Data to Discriminate Schizophrenia by N-way MCCA + jICA. Front Hum Neurosci 7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sui J, Huster R, Yu Q, Segall JM, Calhoun VD (2014): Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. NeuroImage 102 Pt 1:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richter A, Grimm S, Northoff G (2010): Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 25:55–62. [DOI] [PubMed] [Google Scholar]
- 75.Rowland LM, Summerfelt A, Wijtenburg A, Du XM, Chiappelli JJ, Krishna N, et al. (2016): Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. Jama Psychiatry 73:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis DA, Hashimoto T, Volk DW (2005): Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324. [DOI] [PubMed] [Google Scholar]
- 77.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009): Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65:1006–1014. [DOI] [PubMed] [Google Scholar]
- 78.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB (2005): Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 57:252–260. [DOI] [PubMed] [Google Scholar]
- 79.Stoodley CJ, Schmahmann JD (2009): Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage 44:489–501. [DOI] [PubMed] [Google Scholar]
- 80.O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stoodley CJ, Valera EM, Schmahmann JD (2012): Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage 59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO (2008): The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 34:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R (2004): Exploring the connectivity between the cerebellum and motor cortex in humans. Journal of Physiology-London 557:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andreasen NC, Pierson R (2008): The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes ME, Fulham WR, Johnston PJ, Michie PT (2012): Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol 89:220–231. [DOI] [PubMed] [Google Scholar]
- 86.Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Moor J, Wiest R, et al. (2016): Structural brain correlates of defective gesture performance in schizophrenia. Cortex 78:125–137. [DOI] [PubMed] [Google Scholar]
- 87.Coxon JP, Stinear CM, Byblow WD (2006): Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95:3371–3383. [DOI] [PubMed] [Google Scholar]
- 88.Kratz O, Diruf MS, Studer P, Gierow W, Buchmann J, Moll GH, et al. (2009): Effects of methylphenidate on motor system excitability in a response inhibition task. Behav Brain Funct 5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridding MC, Inzelberg R, Rothwell JC (1995): Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol 37:181–188. [DOI] [PubMed] [Google Scholar]
- 90.Stinear CM, Byblow WD (2003): Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol 89:2014–2020. [DOI] [PubMed] [Google Scholar]
- 91.Berardelli A, Abbruzzese G, Chen R, Orth M, Ridding MC, Stinear C, et al. (2008): Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul 1:183–191. [DOI] [PubMed] [Google Scholar]
- 92.Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V (2003): Remote changes in cortical excitability after stroke. Brain 126:470–481. [DOI] [PubMed] [Google Scholar]
- 93.Lefaucheur JP (2006): Myoclonus and transcranial magnetic stimulation. Neurophysiol Clin 36:293–297. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM (2005): Transcranial magnetic stimulation-evoked cortical inhibition: a consistent marker of attentiondeficit/hyperactivity disorder scores in tourette syndrome. Biol Psychiatry 57:1597–1600. [DOI] [PubMed] [Google Scholar]
- 95.Gass N, Schwarz AJ, Sartorius A, Cleppien D, Zheng L, Schenker E, et al. (2013): Haloperidol modulates midbrain-prefrontal functional connectivity in the rat brain. Eur Neuropsychopharmacol 23:1310–1319. [DOI] [PubMed] [Google Scholar]
- 96.Hutcheson NL, Sreenivasan KR, Deshpande G, Reid MA, Hadley J, White DM, et al. (2015): Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum Brain Mapp 36:1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. (2015): Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, ver Hoef L, et al. (2016): Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin 10:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peralta V, Cuesta MJ (2010): The effect of antipsychotic medication on neuromotor abnormalities in neuroleptic-naive nonaffective psychotic patients: a naturalistic study with haloperidol, risperidone, or olanzapine. Prim Care Companion J Clin Psychiatry 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosen AM, Spellman T, Gordon JA (2015): Electrophysiological endophenotypes in rodent models of schizophrenia and psychosis. Biol Psychiatry 77:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greenwood TA, Light GA, Swerdlow NR, Calkins ME, Green MF, Gur RE, et al. (2016): Gating Deficit Heritability and Correlation With Increased Clinical Severity in Schizophrenia Patients With Positive Family History. Am J Psychiatry 173:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Javitt DC, Freedman R (2015): Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kochunov P, Glahn DC, Rowland LM, Olvera RL, Winkler A, Yang YH, et al. (2013): Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry 73:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.