Abstract
Most excitatory neurotransmission in the mammalian brain is mediated by a family of plasma membrane–bound signaling proteins called ionotropic glutamate receptors (iGluRs). iGluRs assemble at central synapses as tetramers, forming a central ion-channel pore whose primary function is to rapidly transport Na+ and Ca2+ in response to binding the neurotransmitter l-glutamic acid. The pore of iGluRs is also accessible to bulkier cytoplasmic cations, such as the polyamines spermine, spermidine, and putrescine, which are drawn into the permeation pathway, but get stuck and block the movement of other ions. The degree of this polyamine-mediated channel block is highly regulated by processes that control the free cytoplasmic polyamine concentration, the membrane potential, or the iGluR subunit composition. Recently, an additional regulation by auxiliary proteins, most notably transmembrane AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor regulatory proteins (TARPs), cornichons, and neuropilin and tolloid-like proteins (NETOs), has been identified. Here, I review what we have learned of polyamine block of iGluRs and its regulation by auxiliary subunits. TARPs, cornichons, and NETOs attenuate the channel block by enabling polyamines to exit the pore. As a result, polyamine permeation occurs at more negative and physiologically relevant membrane potentials. The structural basis for enhanced polyamine transport remains unresolved, although alterations in both channel architecture and charge-screening mechanisms have been proposed. That auxiliary subunits can attenuate the polyamine block reveals an unappreciated impact of polyamine permeation in shaping the signaling properties of neuronal AMPA- and kainate-type iGluRs. Moreover, enhanced polyamine transport through iGluRs may have a role in regulating cellular polyamine levels.
Keywords: ionotropic glutamate receptor; α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor, AMPAR); polyamine; ion channel; electrophysiology; channel pore; kainate receptor; membrane potential
Introduction
The first documented evidence linking polyamines to an experimental observation can probably be attributed to the work of pioneering Dutch microscopist, Antonie Van Leeuwenhoek (1), who in 1678, while observing spermatozoa, inadvertently noted crystal formation of polyamines in a specimen of drying semen (2). The three naturally occurring polyamines, namely spermine (Spm)2 (3, 4), spermidine (Spd) (5), and putrescine (Put), would not be isolated nor formally identified until several centuries later with much of the early seminal contributions, especially in the area of the microbial biosynthetic pathways, made by Dr. Herbert (or Herb) Tabor and his wife, Dr. Celia Tabor (6–11). Dr. Herb Tabor, who will celebrate his 100th birthday in November, 2018, could not have envisaged the many areas of research impacted by polyamines, although he and his wife appreciated early on, while working with Dr. Sanford Rosenthal at the National Institutes of Health, the ubiquitous cytoplasmic expression of polyamines and their potential role in disease (12–14). Dr. Herb Tabor also contributed significantly to the growth of the Journal of Biological Chemistry over many decades (6, 11); therefore, it is fitting that the Journal has commissioned a series of minireviews on the topic of polyamines to celebrate and reflect upon his life and career.
Our understanding of the impact of polyamines, particularly on membrane excitability, has advanced significantly in the last 2 decades beginning with a series of observations showing that cytoplasmic polyamines block the pore regions of several voltage- (15–18) and ligand-gated (17, 19–21) ion channel families that are all cation-selective. Because Spm, Spd, and Put are positively charged at physiological pH, they are preferentially attracted into the water-filled pore regions of cation-selective channels where, due to their larger cross-sectional diameter (cf. Refs. 22, 23), they obstruct the transport of smaller permeating ions, such as Na+, K+ and/or Ca2+ (24, 25). The degree of channel block is voltage-dependent; and as a result, the action of cytoplasmic polyamines can finely tune cellular excitability within the range of the resting membrane potential by regulating the number of channels available for activation. This property of cytoplasmic polyamines is exemplified by their modulation of G-protein–gated inward rectifier K+ (GIRK) channels, which slows the cardiac action potential (26, 27). Additional studies have revealed other dynamic aspects of polyamine channel block that include the following: (i) the regulated synthesis of cellular polyamine levels through the activity of ornithine decarboxylase (28); (ii) the relief of channel block by activity-driven unblocking of polyamines (29–31); and (iii) by trafficking polyamine-sensitive and -insensitive channels into and out of the plasma membrane (32–34). More recent work has identified an additional level of regulation of polyamine block of ionotropic glutamate receptors (iGluRs) through their association with several families of auxiliary proteins called transmembrane AMPA receptor regulatory proteins (or TARPs), cornichons (CNIHs), and neuropilin and tolloid-like proteins (NETOs) (35–39).
In this Minireview, I will examine what we have learned about the mechanism of polyamine block of iGluRs in the last 2 decades. A detailed treatise on the biophysical mechanism(s) of channel block has been reviewed elsewhere (25, 40–42). Consequently, more emphasis will be placed on recent work examining the structural make-up of the AMPAR and KAR ion channel pore and how different mechanisms, particularly via auxiliary proteins, modify their architecture to shape ion permeation and polyamine block of native channels.
What has emerged from this work is that most native AMPARs and KARs have evolved distinct mechanisms to circumvent polyamine channel block. The two distinct mechanisms that prevail in AMPARs include (i) the formation of an electrostatic repulsion site at the apex of the pore, called the Q/R site, and (ii) the co-assembly of AMPAR subunits with auxiliary proteins, such as TARPs and CNIHs. Kainate receptors have evolved three distinct mechanisms to attenuate polyamine block that include (i) electrostatic repulsion at the Q/R site, (ii) structural instability of pore helices via proline residues, and (iii) the modulatory effect of NETO proteins. Given the persistent presence of polyamines in the cytoplasm of almost all cells, these mechanisms ensure unfettered signaling by AMPARs and KARs in the mammalian CNS.
Ionotropic glutamate receptors are differentially sensitive to polyamine channel block
Ionotropic glutamate receptors mediate the vast majority of fast excitatory signaling in the mammalian brain through the activity of three major ion channel families that include AMPAR receptors (AMPARs), N-methyl-d-aspartic acid receptors (NMDARs), and kainate receptors (KARs) (Fig. 1) (42, 43). The iGluR family also includes an additional orphan-class family composed of δ1 and δ2 subunits (Fig. 1) that signal via a metabotropic pathway, rather than an ion channel pore (42), through the formation of a trans-synaptic scaffolding complex (44). Whereas AMPARs and KARs can be strongly regulated by cytoplasmic polyamines, NMDARs are relatively insensitive to intracellular channel block (25). Curiously, however, NMDARs are antagonized in a voltage-dependent manner by external Spm and polyamine spider toxins (45–50). Why NMDARs are insensitive to cytoplasmic polyamines is unclear, although it may relate to the narrower diameter (5.5 Å) and the multi-ion occupancy of the NMDAR pore (51) compared with the larger diameter (7–7.8 Å) (52) and the single ion-pore of AMPARs and KARs (50, 53) that may be more suitable for the blocker. Given the estimated radii of a sodium ion and a water molecule to be 1.02 and 2.75 Å, respectively (54), and the internuclear distance between sodium and oxygen to be 2.35 Å (55), these dimensions suggest that the 7–7.8 Å pore of AMPARs and KARs is capable of transporting hydrated alkali metal ions, such as Na+. As discussed later, subtle changes in the geometry of the pore may be a critical factor in governing the degree of block of iGluRs by polyamines especially as it relates to the regulatory effect of auxiliary subunits on AMPARs and KARs.
Figure 1.
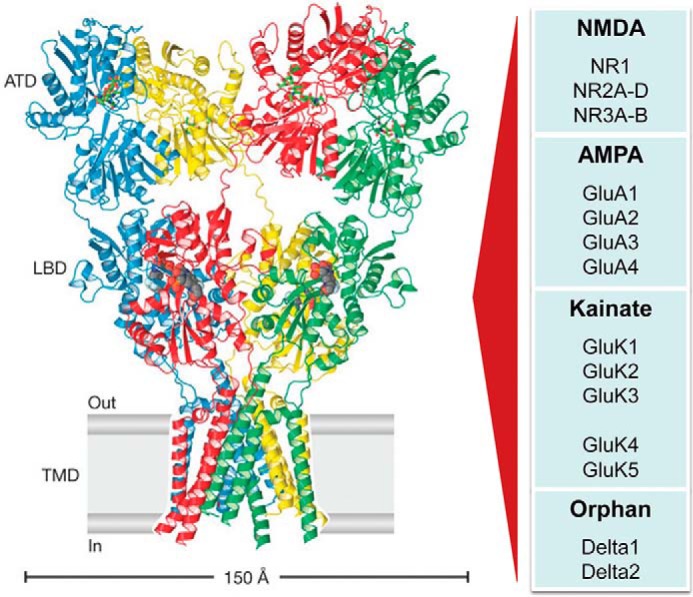
Ionotropic glutamate receptor families share a common tetrameric architecture. Left, X-ray crystal structure showing the tetrameric subunit arrangement of the homomeric GluA2 AMPAR, which consists of four distinct domains that include (i) an N-terminal domain (ATD), which directs subunit assembly and receptor clustering at synapses (189); (ii) four clamshell-like ligand-binding pockets (LBD) (190); (iii) a TMD that forms a central ion channel pore that transports Na+ and Ca2+ ions in response to neurotransmitter binding (42, 43); and (iv) a cytoplasmic C-terminal domain (not shown) that directs receptor trafficking and synaptic anchoring (191). Image of structure was adapted from Ref. 192. Right, table of the four classes of iGluR arranged with each of their respective subunits.
Native AMPA receptor heteromers either possess or lack the GluA2 subunit
AMPARs are found at almost all glutamatergic synapses and in all brain regions (42, 43). Consequently, understanding how they are regulated, including block by cytoplasmic polyamines, has been an area of active investigation.
AMPARs are primarily responsible for the rapid millisecond response rise time to the neurotransmitter, l-glutamate (l-Glu) (40, 42). However, they also strengthen or weaken glutamatergic transmission by cycling into and out of synapses during periods of sustained patterned activity or altered homeostasis (56–59). AMPARs can, in principle, assemble as homomeric and/or heteromeric tetramers from any one of four possible receptor subunits, namely GluA1 to GluA4, that were cloned in the 1990s (60, 61). However, in situ hybridization (62) and single-cell PCR studies (63–65) established during that time that most brain regions and individual neurons express more than one AMPAR subunit that usually includes the GluA2 subunit. The current consensus is that neurons express a preferred family of AMPARs (40, 57, 66). For example, studies in the hippocampal and cortical brain regions suggest that most native AMPARs assemble as GluA1/GluA2 heteromers or GluA2/GluA3 heteromers (67–69). GluA1/A2 heteromers are proposed to traffic into central synapses during periods of patterned activity triggered by the activation of Ca2+/calmodulin-dependent protein kinase II, whereas GluA2/A3 receptors are thought to constitutively cycle into and out of synapses (69). Because EM studies estimate that the GluA3 subunit is expressed at about a tenth of the level of GluA1 and GluA2 (70), it suggests that a significant proportion of all AMPARs are earmarked for synaptic plasticity mechanisms (71). Other combinations are also possible, for example, migrating and mature cerebellar granule cells are thought to express GluA2/A4 heteromeric receptors (72, 73) as well as femtosiemens conductance channels that are consistent with GluA2 homomers (72).
There are, of course, exceptions to this general rule of the inclusion of GluA2. For example, several studies using single-cell PCR and/or antibody staining to document the expression level of different AMPAR subunits have identified some cells that either lack the GluA2 subunit entirely or express it at low levels. Cerebellar Bergmann glia cells (63, 74) and inhibitory interneurons of the basolateral amygdala (75, 76) are apparently devoid of GluA2, whereas dentate gyrus basket cells and some interneurons of the hippocampus, such as parvalbumin-positive (PV+) basket cells (63, 65), and a fraction of neocortical fast-spiking nonpyramidal (64) and pyramidal layer V cells (77) all express low levels of GluA2. It is possible that other neurons and/or glial cells express GluA2 at diminished levels; however, their distribution has yet to be formally investigated. To complicate matters further, individual neurons may express both GluA2-lacking and GluA2-containing receptors which, in some cases, have been shown to be segregated to different synapses of the same neuron. For example, synapses of individual stratum lucidum hippocampal interneurons express GluA2-containing AMPARs when innervated by axon collaterals from CA3 pyramidal neurons and GluA2-lacking AMPARs when innervated by mossy-fiber axons of dentate gyrus granule cells (78). Interestingly, cells with reduced levels of GluA2 often express GluA1 and GluA4 receptor subunits. Whether GluA1 and GluA4 assemble as distinct populations of homomeric channels or heteromers in these cells is still not clear; although gene knockout studies reveal that both subunits contribute similarly to the ensemble AMPAR response of hippocampal PV+ basket cells (79). GluA4-containing AMPARs are also one of the most prominent receptors expressed in the developing brain especially in inhibitory interneurons (80–82). Compared with the delivery mechanisms described for GluA1/A2 heteromers, GluA4-containing AMPARs appear to follow a different set of trafficking rules that is independent of Ca2+/calmodulin-dependent protein kinase II and relies on spontaneous synaptic transmission rather than patterned neuronal activity (83). Consequently, receptor trafficking into and out of individual synapses is governed by the repertoire of AMPAR subunits that make up individual AMPAR heteromers. As explained below, the separation of native AMPAR populations into GluA2-containing or -lacking receptors not only affects receptor trafficking but also impacts the channel's functional behavior, including block by cytoplasmic polyamines.
GluA2 subunit determines ion-flow through the AMPA receptor pore
AMPARs have been grouped into two functionally distinct families based on the presence or absence of the GluA2 receptor subunit (25, 40, 41, 84). GluA2-lacking AMPARs possess ion channels with a large single-channel conductance (73), appreciable Ca2+ permeability (85–87), and high affinity to polyamine block (19, 88–90). In contrast, GluA2-containing receptors typically have much smaller unitary events (73), divalent impermeability (85–87), and insensitivity to polyamine block (19, 91). The exact copy number of GluA2 per AMPAR tetramer has been a matter of debate with some suggesting that the GluA2 content is variable (92) or fixed (93). However, a recent cryo-EM structure of the intact GluA2/A3 AMPAR suggests that they can assemble in a 2:2 fixed format with the GluA2 subunits occupying positions proximal to the pore (94). There is also evidence for the existence of a third class of AMPAR which, although similarly Ca2+-permeable, is characterized by its near-insensitivity to internal and external channel block by polyamines (40). Although its exact molecular composition still remains unknown, AMPARs with these characteristics have been observed in the retina (95, 96), brain stem (97), cerebellum (33, 98), and glia progenitor cells (40, 99).
The critical role of the GluA2 subunit in determining sensitivity to polyamine block is due to RNA editing of an exon that encodes a critical residue in the GluA2 AMPAR pore region, called the Q/R site (Fig. 2) (100–102). The resulting codon change in GluA2 RNA transcripts, from an adenosine to inosine due to adenosine deaminase acting on RNA type 2 (ADAR2) activity (103–105), results in a switch in the amino acid sequence from a glutamine (Gln) to an arginine (Arg) (106, 107). Almost all transcripts of GluA2 are fully edited (>99% efficiency), which means that the Q/R site of all GluA2 receptor subunits contains a positively charged Arg (85, 101). The activity of ADAR2 is particularly remarkable in this regard because RNA editing of most other signaling proteins, such as potassium channels and sodium pumps, is much less efficient (106). In contrast, transcripts of GluA1, -A3, and -A4 are not subject to editing at this site; consequently, these subunits contain a Gln at the Q/R site instead. The specificity of ADAR2's actions on GluA2 is due to the cis-acting intron downstream of the unedited exonic site that is absent from GluA1, -A3, and -A4 and forms a dsRNA structure needed for editing (100, 104, 105, 107).
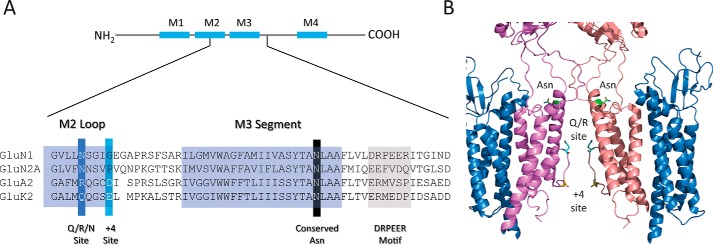
Figure 2.
Sequence alignment of M2 re-entrant loop and M3 helix of different iGluR families. A, sequence comparison of the GluN1 and GluN2A NMDAR subunits with GluA2(R) AMPAR and the GluK2(Q) KAR subunits. The apex of the M2 pore loop of all iGluR families is capped by the Q/R/N site. In AMPARs and KARs, the Q/R site together with the +4 site (Asp in AMPARs and Glu in KARs) govern the degree of polyamine block. All iGluR families possess a conserved Asn residue in the M3 helix that contributes to the high-divalent permeability of the pore. B, cryo-EM structure of the GluA2(Q) AMPAR pore adapted from Protein Data Bank code 5WEO.
To complicate matters, unedited AMPARs possess at least 3–4 subconductance levels that range in amplitude from 5 to 7, 10 to 15, 20 to 25, and 30 to 40 pS (73, 108–111). In conditions of reduced AMPAR desensitization, the distinct sublevels correspond to the fractional occupancy of each of the four ligand-binding sites with agonist molecule (72, 108, 112). However, when desensitization is intact, the subconductance levels have been proposed to correspond to the number of desensitized subunits per tetramer (113). Under these conditions, the sublevels have been linked to conformations occurring within the ligand-binding domain dimer interface (114, 115) and/or the extracellular portion of the α-helix of transmembrane domain 3 (M3) (112). Based on what we know about other tetrameric ion channels (116), it is unlikely that the distinct AMPAR open states of the channel correspond to significant structural re-arrangements of the pore region. It is possible, however, that each subconductance state may be distinguishable by their relative permeability to different cations. For example, in Shaker K+ channels where inactivation has been slowed, the different sublevels observed during sequential channel activation have different selectively for alkali metal ions (117, 118). The smaller and intermediate open states are more permeable to Rb+ ions, whereas the main open state is more permeable to K+ (117, 118). Whether the different AMPAR subconductance levels are similarly distinguishable in their ability to transport different cations remains to be investigated.
In contrast, AMPARs containing four positively-charged Arg residues at the Q/R site, as would occur with homomeric GluA2(R) homomers, exhibit a dramatically reduced unitary conductance (73) and lose their selectivity for cations (52). Unitary events mediated by GluA2(R) AMPARs are too small to be resolved by conventional single-channel recordings and thus have been estimated to be 300 femtosiemens using stationary noise analysis (73). Experiments on homomeric GluA2(R) channels suggest that the channel is not exclusively cation-selective but also exhibits anion permeability with the relative permeability of Cl− to Cs+ estimated to be 0.17 (52). The impact of the inclusion of an Arg at the Q/R site on unitary conductance and/or cation versus anion selectivity cannot be explained by differences in the cross-sectional diameter of the pore that are similar for both Q- and R-forms (52). Instead, the positively-charged Arg ring structure at the selectivity filter is proposed to attract anion binding while repelling divalent ions (52). The electropositive nature of the pore would also disfavor monovalent cation transport accounting for the much-reduced unitary channel conductance (73).
Less is known about the impact of the Q/R site on recombinant heteromeric AMPARs, such as GluA1/A2 or GluA2/A3 receptors, due to the difficulty of obtaining single-channel recordings that are not contaminated by the expression of homomeric channels. The recent GluA2/A3 AMPAR structure suggests that the pore region of heteromers has a 2Q:2R arrangement (94), which would be expected to impact cation transport. In keeping with this, earlier work by Swanson et al. (73) had shown that the single-channel events of GluA2(R)/A4(Q) heteromers had a main conductance of 4 pS when compared with the 8-pS main open state of GluA2(Q)/A4(Q) heteromers, which is similar to observations reported for GluA1(Q)/A2(R) heteromeric channels (93). Heteromeric channels are strictly cation-selective (52) suggesting that, unlike homomeric GluA2(R) channels, the presence of only two Arg residues at the Q/R site is insufficient to attract anions into the pore.
Molecular architecture and block of the AMPA receptor pore
Prior to any direct structural insight, the Q/R site was already recognized as being at the apex of a re-entrant loop facing the extracellular vestibule of the channel (119–121). This structural arrangement is comparable with the pore architecture of other ion channels, such as K+ channels, Na+ channels, Ca2+ channels, and cyclic nucleotide-gated ion channels, which are now collectively known as pore–loop channels (122–125). The commanding position of the Q/R site makes it suitable to act as a selectivity filter of cations entering the extracellular or intracellular vestibules of the pore. When the pore region of AMPARs contains four neutrally-charged glutamine (Gln) residues at the Q/R site, such as GluA1/A4 heteromers, they are thought to form a single ion–binding site at the narrowest region of the pore that controls the transport of both monovalent and divalent cations (53). Because the permeability of all alkali metal ions, such as Na+ and K+, is similar for unedited Q-form AMPARs (52), cations are thought to be transported without losing their inner water shells, which is probably not the case for the NMDAR pore that possesses multiple ion–binding sites (53, 126). As mentioned above, this property is in keeping with the cross-sectional diameter of the AMPAR and KAR pores that have been estimated to be 7–7.8 Å (52). Exactly how the electrostatic environment of the AMPAR pore is altered when the Q/R residues contain both Gln and Arg residues, as would occur with GluA1/A2 or GluA2/A3, is not known.
The Q/R site is not the only residue that affects divalent permeability and polyamine block of AMPARs. Jatzke et al. (127) examined the role of several residues in the third transmembrane domain (M3) of AMPARs, including the conserved asparagine residue (N or Asn) of the SYTANLAAF motif and the highly-charged, extracellular vestibule formed by the DRPEER motif of the NR1 subunit, each of which have been linked to high Ca2+ permeability of NMDARs (Fig. 2) (128). As anticipated, mutation (Asn to Lys or Cys) of the conserved Asn in M3 of GluA2 AMPAR channels strongly attenuated divalent permeability establishing that this residue makes a significant contribution to Ca2+ influx in both AMPARs and NMDARs (127). Importantly, mutation of the Asn did not affect polyamine block (127) demonstrating that the ability of AMPARs to transport divalent ions can be uncoupled from their susceptibility to channel block. In contrast to NMDARs, the Glu and Arg residues C-terminal to M3 had only a minor effect on the divalent permeability of AMPARs and had no effect on KARs (127), although this region has been linked to regulation of KARs by auxiliary NETO proteins (129). Interestingly, another study also noted the uncoupling of Ca2+ permeability and polyamine block by mutating the negatively-charged Asp (to Asn) or Glu residue of AMPARs or KARs, respectively, which is four residues downstream of the Q/R site in the M2 (Fig. 2) (130). In this case, however, the +4 site attenuated polyamine channel block while apparently retaining high-divalent permeability (130). Panchenko et al. (121) explained this finding by proposing that there are multiple binding sites for the distributed charged amine groups in each polyamine molecule with the +4 site being located close to the cytoplasmic entrance of the channel pore. In keeping with this, mutation of the Q/R site (e.g. Gln to Asn (130)) was also shown to eliminate polyamine block while retaining divalent permeability (85, 130, 131) suggesting that polyamine molecules traverse multiple regulatory contact points in the pore.
To examine the structure of the ion-permeation pathway, Twomey et al. (132) compared the GluA2 AMPAR in conditions that promote the open or closed state of the channel. The closed conformation was favored by purifying the ZK200775 antagonist-bound GluA2 AMPAR in a covalent fusion construct with germline-specific gene 1-like (or GSG1L). GSG1L is an AMPAR auxiliary subunit that slows recovery from desensitization (133), and thus, the authors reasoned that it would promote the closed state of the channel (either resting and/or desensitized) (132, 134). The open conformation was achieved by purifying the agonist-bound GluA2 AMPAR in complex with the transmembrane AMPA receptor auxiliary protein (or TARP), stargazin, or γ2 (132), which, unlike GSG1l, promotes the channel open state (135, 136). The stability of the open state was further promoted by obtaining cryo-EM structures in the presence of the positive allosteric modulator, cyclothiazide (CTZ), which attenuates AMPAR desensitization (137). As elaborated below, these structural studies support the idea that the Q/R site, the +4 site, and conserved Asn of M3 are all ideally positioned to influence ion flow through the pore region of AMPARs (Figs. 3 and 4).
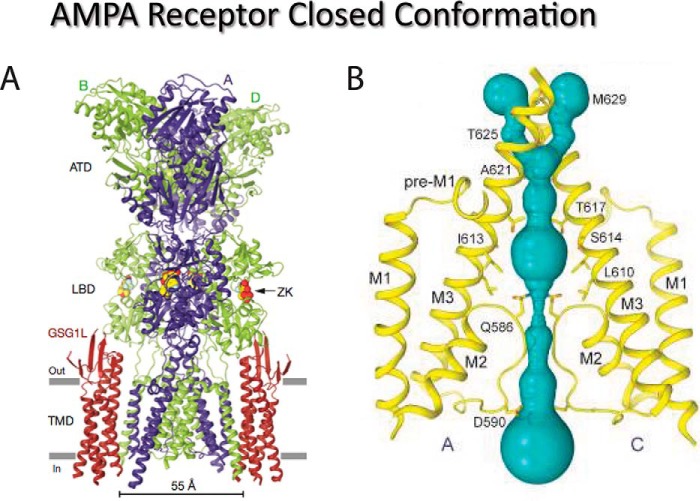
Figure 3.
Closed conformation of the GluA2 AMPAR. A, cryo-EM structure of the GluA2 AMPAR in complex with the auxiliary protein, GSG1L, and the antagonist, ZK200775, which promote channel closure. B, closer view of the ion-conduction pathway (violet) with pore-lining residues in the M2 and M3 segments of subunits A and C shown in yellow highlighting the Q/R site (Gln-586) and +4 site (Asp-590). Adapted from Ref. 132 with permission.
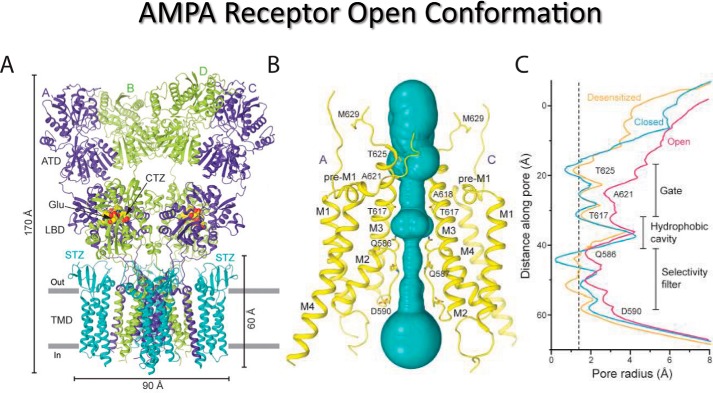
Figure 4.
Open conformation of the GluA2 AMPAR. A, cryo-EM structure of the GluA2 AMPAR in complex with the auxiliary protein, γ2, the agonist, l-Glu, and the positive allosteric modulator, CTZ, which promote channel opening. B, closer view of the ion-conduction pathway (violet) with pore-lining residues in the M2 and M3 segments of subunits A and C shown in yellow highlighting the Q/R site (Gln-586) and +4 site (Asp-590). C, pore radius calculated using HOLE highlighting differences between the open state (pink), closed state (blue), and desensitized state (orange). Adapted from Ref. 132 with permission.
As can be readily appreciated from these structures, access of ions to the Q/R site is regulated by movement of the M3 bundle crossing composed of the Thr-617, Ala-621, Thr-625, and Met-629 residues (Figs. 3 and 4). In the closed position, the bundle crossing occludes access to the Q/R site (i.e. Gln-586, Fig. 3) whereas in the open conformation, it kinks out into a hydrophobic cavity in the middle of the channel pore (Fig. 4). A second constriction is formed below this cavity by the re-entrant loop of M2, which may operate as a lower gate much like other tetrameric ion channels, such as K+ (138) and TRPV1 channels (139). In the closed conformation, the M2 helix below the Q/R site has an extensive network of hydrophobic interactions that are formed between M1 and M3 regions of the same subunit as well as M3 of the adjacent subunit (Figs. 3 and 4) (132). The open-pore dimensions shown in Fig. 4 fall short of the 7–7.8 Å measurement estimated for AMPARs and KARs (52), and therefore, our current model would not be expected to transport hydrated monovalent cations, such as Na+ (see text above). Although molecular dynamics simulations of the open channel structure (140) and electron density measurements of the selectivity filter (see extended data, Fig. 5, B and C, of Ref. 132) suggest that the pore is in an open configuration, it is likely that more information is still needed to understand the molecular architecture and dimensions of the fully open AMPAR pore. Twomey et al. (132) also note that the pore loop is apparently more flexible in closed-state structures but more ordered in the open state to facilitate ion transport through the pore. As discussed below, flexibility of the pore loop is a recurring theme in iGluRs. The loss of polyamine block in GluK2/K5 KAR heteromers has also been proposed to be due to the destabilizing effect of proline residues on the M2 helix of GluK5 subunits (39).
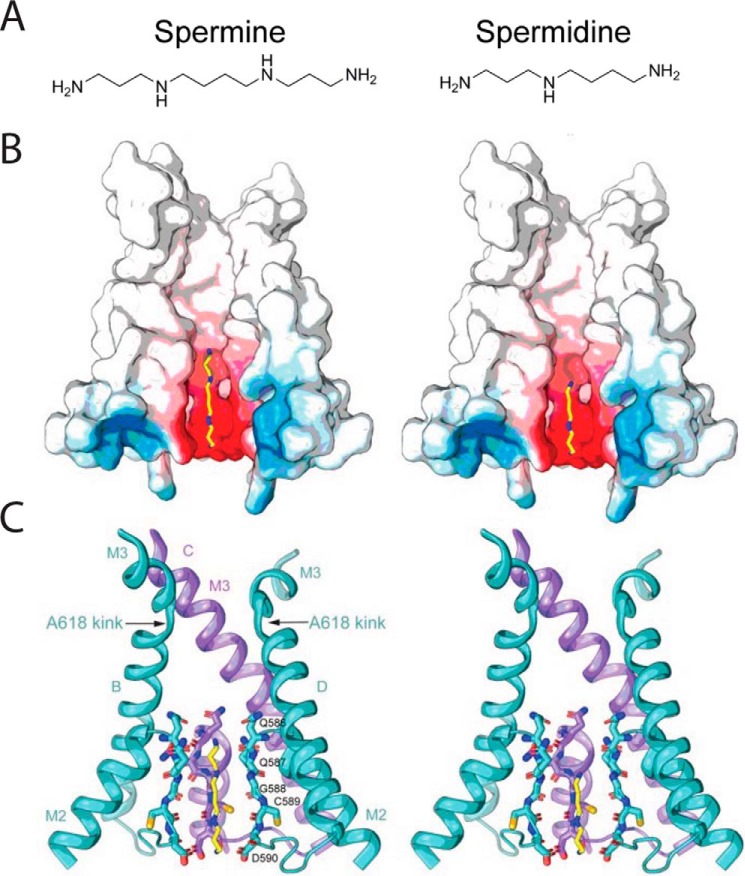
Figure 5.
AMPAR pore occupied by polyamine channel blocker. A, extended structures of the endogenous polyamines, spermine and spermidine. B, cross-section of the GluA2(Q) AMPAR pore highlighting the electroneutral cavity (white) above the Q/R site and electronegative cavity (red) of the inner pore where endogenous polyamines, Spm and Spd (yellow for carbon and blue for nitrogen), are proposed to bind. C, more detailed structure of the pore highlighting the residues in M2 that contribute to polyamine binding and the position of the M3 helices in the open state. Adapted from Ref. 144 with permission.
The position of the polyamine blocker in the ion-permeation pathway was recently resolved by imaging cryo-EM structures of the GluA2(Q) AMPAR pore occupied by several known channel blockers. Specifically, the authors used the orb weaver spider toxin, argiotoxin 636 (AgTx-636) (141), the spider toxin analog, 1-naphthyl acetyl spermine (NASPM) (142), and the adamantane derivative, 1-trimethylammonio-5-(1-adamantane-methyl-ammoniopentane dibromide) (IEM-1460) (143), each of which block Ca2+-permeable, GluA2(R)-lacking AMPARs in a use-dependent manner. To promote channel opening, Twomey et al. (132) tethered the AMPAR to the TARP, γ2, and bathed the samples in the agonist, l-Glu, and the positive allosteric modulator, CTZ (144). AgTX-636, NASPM, and IEM-1460 all contain extended polyamine tails and a bulky hydrophobic headgroup that gets trapped in the narrow constriction of the permeation pathway allowing the authors to identify which pore residues interact with the polyamine tail.
Using this approach, the overall GluA2 AMPAR structure with the pore blocker in place was revealed to be comparable with previous structures of the open-channel tetramer (132). The upper portion of the pore was characterized by an electroneutral surface and central cavity that sits atop a narrowing caused by the glutamines of the Q/R site, which then opens into the lower electronegative portion of the channel (Fig. 5). The electronegativity of this area most likely contributes to the cation selectivity of AMPARs, which occurs primarily through the backbone carbonyl oxygens of Gln-586, Gly-588, Cys-589, and Asp-590 (Fig. 5). These backbone residues presumably contribute to the membrane electric field that gives rise to the steep voltage dependence of polyamine block through the exit rates (unblock and permeation) of polyamine blocker from the pore (31, 38, 39). In keeping with this, Twomey et al. (132) suggest that the endogenous polyamines, Spm and Spd, block the pore at this point in the selectivity filter between residues Gln-586 and Asp-590. Because polyamines acting on AMPAR–TARP complexes induce both channel block and appreciable blocker permeation, it has been speculated that the polyamine molecule may adopt two conformations in the pore, a linear structure, as noted here, that would favor permeation and a kinked structure that would lead to channel block (38). Whether polyamines entering the pore adopt two distinct structures, as also proposed for channel block of cyclic nucleotide-gated channels (21) or K+ channels (145), awaits future investigation.
AMPA and kainate receptor auxiliary proteins attenuate polyamine channel block
An important development in the iGluR field has been the identification of a diverse family of auxiliary proteins that bind and modulate both AMPARs and KARs (146–151). TARP, stargazin (γ2), was the first auxiliary protein to be identified that was shown to be critical for the transport and synaptic targeting of native AMPARs (152). Additional studies uncovered that stargazin and other TARP isoforms modulate the gating properties of AMPARs (153) as well as their responsiveness to allosteric modulators (154) and antagonists (155, 156). Subsequent proteomic analyses then identified the cornichon family (157) as well as other auxiliary proteins such as GSG1L and CKAMP44 (158) that differentially associate with AMPARs in a developmental- and regional-specific manner (159). At about the same time, the neuropilin and tolloid-like proteins, NETO1 and NETO2, were also identified and revealed to bind to native KARs and to modulate their trafficking and gating behavior (149, 160–164). A feature common to both TARP, CNIH, and NETO auxiliary protein families has been their ability to attenuate the degree of polyamine block of AMPARs and KARs.
Both TARPs and CNIHs attenuate the polyamine block of AMPARs by causing a reduction in the onset of block and a greater relief of block observed at both negative and positive membrane potentials, respectively (35, 37, 38). NETOs attenuate the polyamine block of KARs in a comparable manner (36, 39) suggesting that the underlying mechanism may be similar (Fig. 6). Unlike block of the multi-ion pore of K+ channels (24, 27, 165, 166), the onset of block of AMPARs and KARs is caused by a single polyamine molecule entering and occluding the pore from the cytoplasm (31), whereas relief of block is due to polyamine permeation to the extracellular side of the pore (Fig. 6) (22, 25). At 0 mV, the apparent Spm affinity for unedited GluA2(Q) AMPARs is reduced by about 3- or 10-fold by association with CNIH3 or stargazin (38), respectively, whereas block of GluK2 KARs is attenuated by 8–20-fold through NETO1 and NETO2 association (36, 39). Because the rate of polyamine binding to the open AMPAR or KAR pore is unaffected by auxiliary proteins, the attenuation of block is caused almost entirely by faster exit rates of the blocker from the pore (Fig. 6) (36, 38, 39). Spm resides less time at its block site, and consequently, the blocker returns to the cytoplasm or permeates all the way through the pore more readily. In keeping with this, Brown et al. (38, 39) demonstrated that Spm permeates the AMPAR and KAR pores to a much greater extent when bound to auxiliary proteins. For example, the permeability of Spm relative to Na+ was estimated to be 4–16-fold more permeable in GluA2 AMPARs associated with TARPs or CNIHs than GluA2 AMPAR alone (38). A similar observation was also noted when comparing Spm permeability of GluK2 KARs in the presence and absence of NETOs (39).
Figure 6.
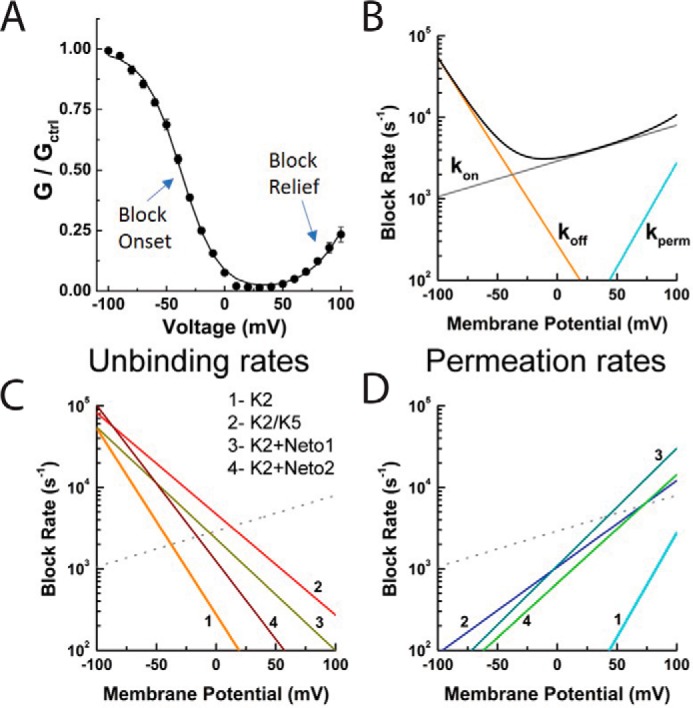
Auxiliary proteins speed up polyamine exit rates from the pore. A, conductance voltage plot of the voltage-dependent Spm block of GluK2 KARs. Note that the onset of block occurs at negative membrane potentials, whereas relief of block occurs at positive membrane potentials. B, plot showing how the rate of Spm binding (kon), unbinding (koff), and permeation (kperm) to the GluK2 KAR pore changes at different membrane potentials. Note that although Spm-binding rate is fairly voltage-insensitive, exit rates from the pore (koff and kperm) are steeply voltage-dependent. The solid black lines correspond to the sum of all block rates at different membrane potentials. C and D, plots showing how the rate of Spm unbinding (C) and permeation (D) is shifted by NETO1 and NET2 auxiliary proteins as well as by heteromerization. Adapted from Ref. 39.
The structural mechanism by which AMPAR and KAR auxiliary proteins attenuate polyamine block is still unresolved. However, because their primary effect is to curtail the time the blocking molecule resides at its binding site (38, 39), it is likely that TARPs, CNIHs, and NETOs alter the pore architecture. An attractive possibility is that auxiliary proteins re-shape the electronegative cavity between the Q/R and +4 sites where Spm and other endogenous polyamines are proposed to reside (144). In keeping with this, Perrais et al. (167) have shown that the high nanomolar block affinity of GluK3 KARs is due to methionine (Met) and serine (Ser) residues in the M2 helix that are absent from GluK1 and GluK2 KARs, which contain Val and Ala residues instead (Fig. 7). The loss of nanomolar Spm affinity by replacement of the Met and Ser residues of GluK3 with Val and Ala, respectively, of GluK1 and GluK2 would not be expected to change the electrostatic environment of the pore; however, it may alter the structural flexibility of the M2 helix. In support of this, attenuation of polyamine block in GluK2/K5 KAR heteromers has been attributed to a conserved proline residue also found in the M2 helix, in this case of the GluK5 (and GluK4) subunit (Fig. 7) (39). Molecular dynamic simulations suggest that the two proline residues, which are arranged on opposing subunits of the GluK2/K5 tetramer (168), increase structural flexibility in the pore making it less favorable for polyamine block and more favorable for permeation (Fig. 7) (39). This is an important point because it reveals that GluK4- and/or GluK5-containing KAR heteromers attenuate polyamine block, not by an electrostatic repulsion mechanism, as is proposed for GluA2-containing AMPAR heteromers, but rather through a different mechanism that introduces structural flexibility in the pore helices. The fact that the near loss of polyamine block in GluK2/K5 heteromers is not accompanied by changes in divalent permeability (39) again suggests that the primary disruption in the ion-permeation pathway is at the level of the Q/R site and M2 helix rather than in the extended M3 helix where the conserved Asn residue contributes to Ca2+ permeability (127). In keeping with this, TARPs acting on GluA4 AMPARs (37) and NETOs acting on GluK2 KARs (36) attenuate polyamine block without having any effect on divalent permeability.
Figure 7.
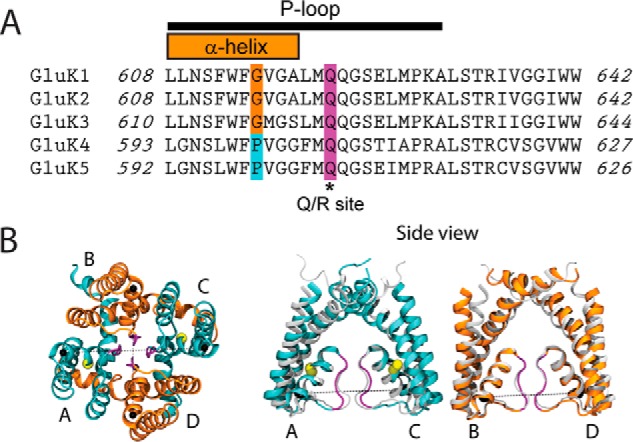
Asymmetric pore of heteromeric KAR channels. A, sequence alignment of the five KAR subunits highlighting the Gly residue of GluK1–3 subunits and the Pro of GluK4 and GluK5 found in the M2 helix. High nanomolar affinity block of GluK3 is conferred by the Met and Ser residues that are Val or Ala/Gly residues, respectively, in the other KAR subunits. B, left, NaK open channel structure (193) was used as a template for GluK2/K5 heteromers introducing the Pro residues on subunits A and C. B, right, side view of the A/C (cyan) and B/D (orange) subunits of the inverted NaK pore before (gray) and after (colored) 257-ns simulations reveal that the Pro residues introduce asymmetry into the pore. Adapted from Ref. 39.
The situation, however, is likely to be more complicated for several reasons. First, an Arg scan of the M3 helix atop of the Q/R site in GluK2 KARs identified residues that attenuate polyamine block (169) suggesting that alterations in this electroneutral cavity by auxiliary proteins may also affect the degree of channel block. A potential caveat, however, is that Arg replacement in the extracellular vestibule may create a local electrostatic environment that alters permeant ion flux in the pore, which will indirectly attenuate polyamine block (see Fig. 4D of Ref. 31), a possibility that may be in keeping with the enhanced anion permeability reported for the Arg mutants (169). Second, both CNIHs and TARPs attenuate polyamine block of GluA1 AMPARs while increasing divalent permeability (35) suggesting that auxiliary proteins may have a broader structural impact on both the inner electronegative cavity of the pore and the outer extracellular vestibule formed by the M3-S2 linker. The fact that the γ2 TARP enhances Ca2+ permeability of GluA1 (35) but not GluA4 (37) homomers is not understood, but it may suggest that the ability of TARPs to enhance divalent transport through the pore is AMPAR subunit-dependent. GSG1L also affects both ion permeation and channel block (133, 170). In this case, however, GSG1L acts to diminish AMPAR divalent permeability and to enhance polyamine block (albeit at membrane potentials >+50 mV) (170), which, although contrary to the effect of TARPs and CNIHs, is in keeping with auxiliary subunits having a broader impact on the structural integrity of the ion-permeation pathway. Third, and finally, two separate studies have identified that the cytoplasmic tails of NETOs and TARPs can attenuate polyamine block of KARs (36) and AMPARs (171), respectively. Fisher and Mott (36) observed that neutralization of three positively charged residues, namely Arg–Arg–Lys, which lie close to the transmembrane region of NETOs, eliminated the attenuation of polyamine block of KARs. Given this, the authors speculated that the C-tail of NETOs may act by a charge-screening mechanism to prevent polyamines entering the pore. A charge-screening mechanism, however, is unlikely because auxiliary subunits do not affect the rate of polyamine binding and therefore do not hinder their ability to access the pore (38, 39). Neutralization of the homologous residues in γ2 TARP, namely Arg–His–Lys, had no effect on polyamine block of AMPARs (171) suggesting that auxiliary proteins probably impact polyamine block differently between AMPARs and KARs and that other mechanisms are likely to be at play.
Linking neurodevelopmental disorders and cancer to polyamines and their transport
One of the most unexpected effects of auxiliary proteins is their ability to enhance the transport of polyamines across the plasma membrane (38, 39). This is particularly evident at AMPARs where the permeability of Spm relative to Na+ at GluA2(Q) AMPARs (PSpm/PNa = 0.3) increased about 4- and 10-fold when GluA2 was co-assembled with γ2 TARP (PSpm/PNa = 1) and CNIH-3 (PSpm/PNa = 4), respectively (38). Given that other endogenous polyamines, such as Spd and Put, have fewer amine groups, they would be expected to be transported at a greater rate than Spm, as has already been shown at KARs (22, 39). Although it is not immediately clear how enhanced polyamine transport might be important to the mammalian CNS, it is worth noting that the developing brain is thought to express a greater abundance of GluA2-lacking AMPARs (172, 173), where polyamine transport may play a role. Outside the brain, polyamines are recognized for fulfilling many roles in cells, such as regulating protein and nucleic acid synthesis and structure, cell proliferation, differentiation, and apoptosis (174, 175). Consequently, it is likely that polyamines contribute to similar roles in the developing CNS. Interestingly, disrupted polyamine levels, due to the inactivation of the Spm synthase gene, give rise to the X-linked neurodevelopmental disorder called Snyder-Robinson syndrome that is associated with mental retardation (176). Although the potential role of polyamine-sensitive K+ channels and AMPARs has been speculated upon (176), it is still not clear how deficits in cellular polyamines account for the CNS defects.
Polyamines and their transport by AMPARs may be more compellingly linked to their proposed roles in cancer (177). Cell growth and cancer have long been associated with elevated levels of polyamines (178) and with more recent studies establishing a connection to AMPAR activity. For example, pancreatic (179, 180) and kidney (181, 182) cancers have each been associated with the activation of AMPARs with tumor growth in breast and lung carcinoma, colon adenocarcinoma, and neuroblastoma cells shown to be diminished by AMPAR antagonists (183, 184). The elevated levels of extracellular polyamine levels reported in many cancers coupled with the prolonged activation of AMPARs (185) would provide the appropriate environment to permit the slow but steady transport of polyamines into cells. Although the mechanisms regulating polyamine biosynthesis and catabolism are well-understood (186, 187), the molecular events that lead to polyamine transport in mammalian cells are less clear (188). Consequently, whether the AMPAR in complex with auxiliary proteins, such as CNIHs, could contribute to this function would be an important question to consider in future studies.
Conclusion
Much has been learned about polyamine channel block of iGluRs since it was first identified over 2 decades ago. The discovery of auxiliary proteins and their regulation of iGluRs as well as breakthroughs in structural biology open new opportunities for the development of clinically-relevant compounds that might exploit these novel protein–protein interactions. Recent advances in gene-editing techniques, such as CRISPR, also make it possible to pinpoint how distinct families of auxiliary proteins shape iGluR signaling within neuronal circuits and how they may give rise to the aberrant activity that underlines neurological disease. Consequently, there is still much to be uncovered about how polyamine channel block contributes to membrane excitability and its potential role in disease.
Acknowledgments
I thank the present and former members of the Bowie lab for their ongoing discussions and careful reading of this manuscript. I also thank Yuhao Yan for providing the pore structure shown in Fig. 2. I am grateful to the two reviewers for their thoughtful critique that has helped improve the overall clarity and readability of the text.
This work was supported by grants from the Canadian Institutes of Health Research (to D. B.). This article is part of a series on “Polyamines,” written in honor of Dr. Herbert Tabor's 100th birthday. The author declares that he has no conflicts of interest with the contents of this article.
- Spm
- spermine
- Spd
- spermidine
- iGluR
- ionotropic glutamate receptor
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- NMDAR
- N-methyl-d-aspartic acid receptor
- TARP
- transmembrane AMPA receptor regulatory protein
- NETO
- neuropilin and tolloid-like protein
- Put
- putrescine
- KAR
- kainate; receptor
- NASPM
- 1-naphthyl acetyl spermine
- CNIH
- cornichon
- CNS
- central nervous system
- pS
- picosiemen
- CTZ
- cyclothiazide.
References
- 1. Anderson D. (2014) Still going strong: Leeuwenhoek at eighty. Antonie Van Leeuwenhoek 106, 3–26 10.1007/s10482-014-0152-1 [DOI] [PubMed] [Google Scholar]
- 2. Lewenhoeck A. (1678) Observationes D. Anthonii Lewenhoeck, de natis è semine genitali animalculis. Philo. Trans. R. Soc. Lond. 12, 1040–1042 [Google Scholar]
- 3. Ladenburg A., and Abel J. (1888) Ueber das aethylenimin (Spermin?). Ber. Ditsch. Chem. Ges. 21, 758–766 [Google Scholar]
- 4. Dudley H. W., Rosenheim M. C., and Rosenheim O. (1924) The chemical constitution of spermine. I. The isolation of spermine from animal tissues, and the preparation of its salts. Biochem. J. 18, 1263–1272 10.1042/bj0181263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dudley H. W., Rosenheim O., and Starling W. W. (1927) The constitution and synthesis of spermidine, a newly discovered base isolated from animal tissues. Biochem. J. 21, 97–103 10.1042/bj0210097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kresge N. S., Simoni R. D., and Hill R. L. (2007) The biosynthesis of polyamines: the work of Herbert Tabor and Celia White Tabor. J. Biol. Chem. 282, e26 [Google Scholar]
- 7. Tabor H., and Tabor C. W. (1964) Spermidine, spermine, and related amines. Pharmacol. Rev. 16, 245–300 [PubMed] [Google Scholar]
- 8. Tabor C. W., and Tabor H. (1976) 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu. Rev. Biochem. 45, 285–306 10.1146/annurev.bi.45.070176.001441 [DOI] [PubMed] [Google Scholar]
- 9. Tabor C. W., and Tabor H. (1984) Polyamines. Annu. Rev. Biochem. 53, 749–790 10.1146/annurev.bi.53.070184.003533 [DOI] [PubMed] [Google Scholar]
- 10. Tabor C. W., and Tabor H. (1985) Polyamines in microorganisms. Microbiol. Rev. 49, 81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabor C. W., and Tabor H. (1999) It all started on a streetcar in Boston. Annu. Rev. Biochem. 68, 1–32 10.1146/annurev.biochem.68.1.1 [DOI] [PubMed] [Google Scholar]
- 12. Rosenthal S. M., and Tabor C. W. (1956) The pharmacology of spermine and spermidine; distribution and excretion. J. Pharmacol. Exp. Ther. 116, 131–138 [PubMed] [Google Scholar]
- 13. Tabor C. W., and Rosenthal S. M. (1956) Pharmacology of spermine and spermidine; some effects on animals and bacteria. J. Pharmacol. Exp. Ther. 116, 139–155 [PubMed] [Google Scholar]
- 14. Tabor H., Rosenthal S. M., and Tabor C. W. (1958) The biosynthesis of spermidine and spermine from putrescine and methionine. J. Biol. Chem. 233, 907–914 [PubMed] [Google Scholar]
- 15. Fu L. Y., Cummins T. R., and Moczydlowski E. G. (2012) Sensitivity of cloned muscle, heart and neuronal voltage-gated sodium channels to block by polyamines: a possible basis for modulation of excitability in vivo. Channels 6, 41–49 10.4161/chan.19001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomez M., and Hellstrand P. (1995) Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 430, 501–507 10.1007/BF00373886 [DOI] [PubMed] [Google Scholar]
- 17. Kerschbaum H. H., Kozak J. A., and Cahalan M. D. (2003) Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys. J. 84, 2293–2305 10.1016/S0006-3495(03)75035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopatin A. N., Makhina E. N., and Nichols C. G. (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 10.1038/372366a0 [DOI] [PubMed] [Google Scholar]
- 19. Bowie D., and Mayer M. L. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 10.1016/0896-6273(95)90049-7 [DOI] [PubMed] [Google Scholar]
- 20. Haghighi A. P., and Cooper E. (1998) Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J. Neurosci. 18, 4050–4062 10.1523/JNEUROSCI.18-11-04050.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu Z., and Ding L. (1999) Blockade of a retinal cGMP-gated channel by polyamines. J. Gen. Physiol. 113, 35–43 10.1085/jgp.113.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bähring R., Bowie D., Benveniste M., and Mayer M. L. (1997) Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J. Physiol. 502, 575–589 10.1111/j.1469-7793.1997.575bj.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo D., and Lu Z. (2000) Mechanism of cGMP-gated channel block by intracellular polyamines. J. Gen. Physiol. 115, 783–798 10.1085/jgp.115.6.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nichols C. G., and Lopatin A. N. (1997) Inward rectifier potassium channels. Annu. Rev. Physiol. 59, 171–191 10.1146/annurev.physiol.59.1.171 [DOI] [PubMed] [Google Scholar]
- 25. Bowie D., Bahring R., and Mayer M. L. (1999) in Handbook of Experimental Pharmacology, Ionotropic Glutamate Receptors in the CNS (Jonas P., and Monyer H., eds) pp. 251–273, Springer, Berlin [Google Scholar]
- 26. Sakmann B., Noma A., and Trautwein W. (1983) Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature 303, 250–253 10.1038/303250a0 [DOI] [PubMed] [Google Scholar]
- 27. Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., and Kurachi Y. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- 28. Aizenman C. D., Muñoz-Elias G., and Cline H. T. (2002) Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34, 623–634 10.1016/S0896-6273(02)00674-8 [DOI] [PubMed] [Google Scholar]
- 29. Rozov A., Zilberter Y., Wollmuth L. P., and Burnashev N. (1998) Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J. Physiol. 511, 361–377 10.1111/j.1469-7793.1998.361bh.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rozov A., and Burnashev N. (1999) Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401, 594–598 10.1038/44151 [DOI] [PubMed] [Google Scholar]
- 31. Bowie D., Lange G. D., and Mayer M. L. (1998) Activity-dependent modulation of glutamate receptors by polyamines. J. Neurosci. 18, 8175–8185 10.1523/JNEUROSCI.18-20-08175.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu S. J., and Cull-Candy S. G. (2002) Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J. Neurosci. 22, 3881–3889 10.1523/JNEUROSCI.22-10-03881.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu S. Q., and Cull-Candy S. G. (2000) Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458 10.1038/35013064 [DOI] [PubMed] [Google Scholar]
- 34. Plant K., Pelkey K. A., Bortolotto Z. A., Morita D., Terashima A., McBain C. J., Collingridge G. L., and Isaac J. T. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 9, 602–604 10.1038/nn1678 [DOI] [PubMed] [Google Scholar]
- 35. Coombs I. D., Soto D., Zonouzi M., Renzi M., Shelley C., Farrant M., and Cull-Candy S. G. (2012) Cornichons modify channel properties of recombinant and glial AMPA receptors. J. Neurosci. 32, 9796–9804 10.1523/JNEUROSCI.0345-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher J. L., and Mott D. D. (2012) The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J. Neurosci. 32, 12928–12933 10.1523/JNEUROSCI.2211-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soto D., Coombs I. D., Kelly L., Farrant M., and Cull-Candy S. G. (2007) Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat. Neurosci. 10, 1260–1267 10.1038/nn1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown P. M. G. E., McGuire H., and Bowie D. (2018) Stargazin and cornichon-3 relieve polyamine block of AMPA receptors by enhancing blocker permeation. J. Gen. Physiol. 150, 67–82 10.1085/jgp.201711895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown P. M., Aurousseau M. R., Musgaard M., Biggin P. C., and Bowie D. (2016) Kainate receptor pore-forming and auxiliary subunits regulate channel block by a novel mechanism. J. Physiol. 594, 1821–1840 10.1113/JP271690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bowie D. (2012) Redefining the classification of AMPA-selective ionotropic glutamate receptors. J. Physiol. 590, 49–61 10.1113/jphysiol.2011.221689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cull-Candy S., Kelly L., and Farrant M. (2006) Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 16, 288–297 10.1016/j.conb.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 42. Dingledine R., Borges K., Bowie D., and Traynelis S. F. (1999) The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 [PubMed] [Google Scholar]
- 43. Traynelis S. F., Wollmuth L. P., McBain C. J., Menniti F. S., Vance K. M., Ogden K. K., Hansen K. B., Yuan H., Myers S. J., and Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 10.1124/pr.109.002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elegheert J., Kakegawa W., Clay J. E., Shanks N. F., Behiels E., Matsuda K., Kohda K., Miura E., Rossmann M., Mitakidis N., Motohashi J., Chang V. T., Siebold C., Greger I. H., Nakagawa T., Yuzaki M., and Aricescu A. R. (2016) Structural basis for integration of GluD receptors within synaptic organizer complexes. Science 353, 295–299 10.1126/science.aae0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Priestley T., Woodruff G. N., and Kemp J. A. (1989) Antagonism of responses to excitatory amino acids on rat cortical neurones by the spider toxin, argiotoxin636. Br. J. Pharmacol. 97, 1315–1323 10.1111/j.1476-5381.1989.tb12594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reynolds I. J. (1991) The spider toxin, argiotoxin636, binds to a Mg2+ site on the N-methyl-d-aspartate receptor complex. Br. J. Pharmacol. 103, 1373–1376 10.1111/j.1476-5381.1991.tb09796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raditsch M., Ruppersberg J. P., Kuner T., Günther W., Schoepfer R., Seeburg P. H., Jahn W., and Witzemann V. (1993) Subunit-specific block of cloned NMDA receptors by argiotoxin636. FEBS Lett. 324, 63–66 10.1016/0014-5793(93)81533-6 [DOI] [PubMed] [Google Scholar]
- 48. Benveniste M., and Mayer M. L. (1993) Multiple effects of spermine on N-methyl-d-aspartic acid receptor responses of rat cultured hippocampal neurones. J. Physiol. 464, 131–163 10.1113/jphysiol.1993.sp019627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams K. (1997) Interactions of polyamines with ion channels. Biochem. J. 325, 289–297 10.1042/bj3250289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huettner J. E. (2015) Glutamate receptor pores. J. Physiol. 593, 49–59 10.1113/jphysiol.2014.272724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villarroel A., Burnashev N., and Sakmann B. (1995) Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys. J. 68, 866–875 10.1016/S0006-3495(95)80263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burnashev N., Villarroel A., and Sakmann B. (1996) Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J. Physiol. 496, 165–173 10.1113/jphysiol.1996.sp021674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wollmuth L. P., and Sakmann B. (1998) Different mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. J. Gen. Physiol. 112, 623–636 10.1085/jgp.112.5.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Briand C. L., and Burton J. J. (1976) Molecular dynamics study of the effects of ions on water microclusters. J. Chem. Phys. 64, 14 [Google Scholar]
- 55. Marcus Y. (1988) Ionic radii in aqueous solutions. Chem. Rev. 88, 24 [Google Scholar]
- 56. Lisman J. (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160260 10.1098/rstb.2016.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Henley J. M., and Wilkinson K. A. (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17, 337–350 10.1038/nrn.2016.37 [DOI] [PubMed] [Google Scholar]
- 58. Turrigiano G. G. (2017) The dialectic of Hebb and homeostasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160258 10.1098/rstb.2016.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herring B. E., and Nicoll R. A. (2016) Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol. 78, 351–365 10.1146/annurev-physiol-021014-071753 [DOI] [PubMed] [Google Scholar]
- 60. Seeburg P. H. (1993) The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol. Sci. 14, 297–303 10.1016/0165-6147(93)90047-N [DOI] [PubMed] [Google Scholar]
- 61. Hollmann M., and Heinemann S. (1994) Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 10.1146/annurev.ne.17.030194.000335 [DOI] [PubMed] [Google Scholar]
- 62. Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., and Seeburg P. H. (1990) A family of AMPA-selective glutamate receptors. Science 249, 556–560 10.1126/science.2166337 [DOI] [PubMed] [Google Scholar]
- 63. Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., and Monyer H. (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204 10.1016/0896-6273(95)90076-4 [DOI] [PubMed] [Google Scholar]
- 64. Angulo M. C., Lambolez B., Audinat E., Hestrin S., and Rossier J. (1997) Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J. Neurosci. 17, 6685–6696 10.1523/JNEUROSCI.17-17-06685.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bochet P., Audinat E., Lambolez B., Crépel F., Rossier J., Iino M., Tsuzuki K., and Ozawa S. (1994) Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron 12, 383–388 10.1016/0896-6273(94)90279-8 [DOI] [PubMed] [Google Scholar]
- 66. Jacobi E., and von Engelhardt J. (2017) Diversity in AMPA receptor complexes in the brain. Curr. Opin. Neurobiol. 45, 32–38 10.1016/j.conb.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 67. Lu W., Shi Y., Jackson A. C., Bjorgan K., During M. J., Sprengel R., Seeburg P. H., and Nicoll R. A. (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268 10.1016/j.neuron.2009.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wenthold R. J., Petralia R. S., Blahos J. II., and Niedzielski A. S. (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 10.1523/JNEUROSCI.16-06-01982.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shi S., Hayashi Y., Esteban J. A., and Malinow R. (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 10.1016/S0092-8674(01)00321-X [DOI] [PubMed] [Google Scholar]
- 70. Sans N., Vissel B., Petralia R. S., Wang Y. X., Chang K., Royle G. A., Wang C. Y., O'Gorman S., Heinemann S. F., and Wenthold R. J. (2003) Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J. Neurosci. 23, 9367–9373 10.1523/JNEUROSCI.23-28-09367.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kessels H. W., Kopec C. D., Klein M. E., and Malinow R. (2009) Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat. Neurosci. 12, 888–896 10.1038/nn.2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith T. C., and Howe J. R. (2000) Concentration-dependent substate behavior of native AMPA receptors. Nat. Neurosci. 3, 992–997 10.1038/79931 [DOI] [PubMed] [Google Scholar]
- 73. Swanson G. T., Kamboj S. K., and Cull-Candy S. G. (1997) Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci. 17, 58–69 10.1523/JNEUROSCI.17-01-00058.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., and Sakmann B. (1992) Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science 256, 1566–1570 10.1126/science.1317970 [DOI] [PubMed] [Google Scholar]
- 75. Mahanty N. K., and Sah P. (1998) Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394, 683–687 10.1038/29312 [DOI] [PubMed] [Google Scholar]
- 76. McDonald A. J. (1996) Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci. Lett. 208, 175–178 10.1016/0304-3940(96)12585-4 [DOI] [PubMed] [Google Scholar]
- 77. Kumar S. S., Bacci A., Kharazia V., and Huguenard J. R. (2002) A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J. Neurosci. 22, 3005–3015 10.1523/JNEUROSCI.22-08-03005.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tóth K., and McBain C. J. (1998) Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat. Neurosci. 1, 572–578 10.1038/2807 [DOI] [PubMed] [Google Scholar]
- 79. Fuchs E. C., Zivkovic A. R., Cunningham M. O., Middleton S., Lebeau F. E., Bannerman D. M., Rozov A., Whittington M. A., Traub R. D., Rawlins J. N., and Monyer H. (2007) Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53, 591–604 10.1016/j.neuron.2007.01.031 [DOI] [PubMed] [Google Scholar]
- 80. Bernard V., Somogyi P., and Bolam J. P. (1997) Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J. Neurosci. 17, 819–833 10.1523/JNEUROSCI.17-02-00819.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pelkey K. A., Barksdale E., Craig M. T., Yuan X., Sukumaran M., Vargish G. A., Mitchell R. M., Wyeth M. S., Petralia R. S., Chittajallu R., Karlsson R. M., Cameron H. A., Murata Y., Colonnese M. T., Worley P. F., and McBain C. J. (2015) Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85, 1257–1272 10.1016/j.neuron.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Akgül G., and McBain C. J. (2016) Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. J. Physiol. 594, 5471–5490 10.1113/JP271764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu J. J., Esteban J. A., Hayashi Y., and Malinow R. (2000) Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat. Neurosci. 3, 1098–1106 10.1038/80614 [DOI] [PubMed] [Google Scholar]
- 84. Isaac J. T., Ashby M. C., and McBain C. J. (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871 10.1016/j.neuron.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 85. Burnashev N., Monyer H., Seeburg P. H., and Sakmann B. (1992) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8, 189–198 10.1016/0896-6273(92)90120-3 [DOI] [PubMed] [Google Scholar]
- 86. Hollmann M., Hartley M., and Heinemann S. (1991) Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science 252, 851–853 10.1126/science.1709304 [DOI] [PubMed] [Google Scholar]
- 87. Hume R. I., Dingledine R., and Heinemann S. F. (1991) Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253, 1028–1031 10.1126/science.1653450 [DOI] [PubMed] [Google Scholar]
- 88. Donevan S. D., and Rogawski M. A. (1995) Intracellular polyamines mediate inward rectification of Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc. Natl. Acad. Sci. U.S.A. 92, 9298–9302 10.1073/pnas.92.20.9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kamboj S. K., Swanson G. T., and Cull-Candy S. G. (1995) Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J. Physiol. 486, 297–303 10.1113/jphysiol.1995.sp020812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koh D. S., Burnashev N., and Jonas P. (1995) Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J. Physiol. 486, 305–312 10.1113/jphysiol.1995.sp020813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., and Sakmann B. (1991) Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252, 1715–1718 10.1126/science.1710829 [DOI] [PubMed] [Google Scholar]
- 92. Washburn M. S., Numberger M., Zhang S., and Dingledine R. (1997) Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J. Neurosci. 17, 9393–9406 10.1523/JNEUROSCI.17-24-09393.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mansour M., Nagarajan N., Nehring R. B., Clements J. D., and Rosenmund C. (2001) Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron 32, 841–853 10.1016/S0896-6273(01)00520-7 [DOI] [PubMed] [Google Scholar]
- 94. Herguedas B., García-Nafría J., Cais O., Fernández-Leiro R., Krieger J., Ho H., and Greger I. H. (2016) Structure and organization of heteromeric AMPA-type glutamate receptors. Science 352, aad3873 10.1126/science.aad3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Osswald I. K., Galan A., and Bowie D. (2007) Light triggers expression of philanthotoxin-insensitive Ca2+-permeable AMPA receptors in the developing rat retina. J. Physiol. 582, 95–111 10.1113/jphysiol.2007.127894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mørkve S. H., Veruki M. L., and Hartveit E. (2002) Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. J. Physiol. 542, 147–165 10.1113/jphysiol.2002.020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Otis T. S., Raman I. M., and Trussell L. O. (1995) AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. J. Physiol. 482, 309–315 10.1113/jphysiol.1995.sp020519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Soler-Llavina G. J., and Sabatini B. L. (2006) Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat. Neurosci. 9, 798–806 10.1038/nn1698 [DOI] [PubMed] [Google Scholar]
- 99. Meucci O., Fatatis A., Holzwarth J. A., and Miller R. J. (1996) Developmental regulation of the toxin sensitivity of Ca2+-permeable AMPA receptors in cortical glia. J. Neurosci. 16, 519–530 10.1523/JNEUROSCI.16-02-00519.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Higuchi M., Single F. N., Köhler M., Sommer B., Sprengel R., and Seeburg P. H. (1993) RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell 75, 1361–1370 10.1016/0092-8674(93)90622-W [DOI] [PubMed] [Google Scholar]
- 101. Sommer B., Köhler M., Sprengel R., and Seeburg P. H. (1991) RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 10.1016/0092-8674(91)90568-J [DOI] [PubMed] [Google Scholar]
- 102. Melcher T., Maas S., Higuchi M., Keller W., and Seeburg P. H. (1995) Editing of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. J. Biol. Chem. 270, 8566–8570 10.1074/jbc.270.15.8566 [DOI] [PubMed] [Google Scholar]
- 103. Rueter S. M., Burns C. M., Coode S. A., Mookherjee P., and Emeson R. B. (1995) Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science 267, 1491–1494 10.1126/science.7878468 [DOI] [PubMed] [Google Scholar]
- 104. Yang J. H., Sklar P., Axel R., and Maniatis T. (1995) Editing of glutamate receptor subunit B pre-mRNA in vitro by site-specific deamination of adenosine. Nature 374, 77–81 10.1038/374077a0 [DOI] [PubMed] [Google Scholar]
- 105. Higuchi M., Maas S., Single F. N., Hartner J., Rozov A., Burnashev N., Feldmeyer D., Sprengel R., and Seeburg P. H. (2000) Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406, 78–81 10.1038/35017558 [DOI] [PubMed] [Google Scholar]
- 106. Rosenthal J. J., and Seeburg P. H. (2012) A-to-I RNA editing: effects on proteins key to neural excitability. Neuron 74, 432–439 10.1016/j.neuron.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Seeburg P. H. (2002) A-to-I editing: new and old sites, functions and speculations. Neuron 35, 17–20 10.1016/S0896-6273(02)00760-2 [DOI] [PubMed] [Google Scholar]
- 108. Rosenmund C., Stern-Bach Y., and Stevens C. F. (1998) The tetrameric structure of a glutamate receptor channel. Science 280, 1596–1599 10.1126/science.280.5369.1596 [DOI] [PubMed] [Google Scholar]
- 109. Banke T. G., Bowie D., Lee H., Huganir R. L., Schousboe A., and Traynelis S. F. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20, 89–102 10.1523/JNEUROSCI.20-01-00089.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dawe G. B., Musgaard M., Aurousseau M. R. P., Nayeem N., Green T., Biggin P. C., and Bowie D. (2016) Distinct structural pathways coordinate the activation of AMPA receptor-auxiliary subunit complexes. Neuron 89, 1264–1276 10.1016/j.neuron.2016.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang W., Cho Y., Lolis E., and Howe J. R. (2008) Structural and single-channel results indicate that the rates of ligand-binding domain closing and opening directly impact AMPA receptor gating. J. Neurosci. 28, 932–943 10.1523/JNEUROSCI.3309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang W., Eibl C., Weeks A. M., Riva I., Li Y. J., Plested A. J. R., and Howe J. R. (2017) Unitary properties of AMPA receptors with reduced desensitization. Biophys. J. 113, 2218–2235 10.1016/j.bpj.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bowie D., and Lange G. D. (2002) Functional stoichiometry of glutamate receptor desensitization. J. Neurosci. 22, 3392–3403 10.1523/JNEUROSCI.22-09-03392.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Daniels B. A., Andrews E. D., Aurousseau M. R., Accardi M. V., and Bowie D. (2013) Crosslinking the ligand-binding domain dimer interface locks kainate receptors out of the main open state. J. Physiol. 591, 3873–3885 10.1113/jphysiol.2013.253666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Musgaard M., and Biggin P. C. (2016) Steered molecular dynamics simulations predict conformational stability of glutamate receptors. J. Chem. Inf. Model. 56, 1787–1797 10.1021/acs.jcim.6b00297 [DOI] [PubMed] [Google Scholar]
- 116. Root M. J., and MacKinnon R. (1994) Two identical noninteracting sites in an ion channel revealed by proton transfer. Science 265, 1852–1856 10.1126/science.7522344 [DOI] [PubMed] [Google Scholar]
- 117. Zheng J., and Sigworth F. J. (1997) Selectivity changes during activation of mutant Shaker potassium channels. J. Gen. Physiol. 110, 101–117 10.1085/jgp.110.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zheng J., and Sigworth F. J. (1998) Intermediate conductances during deactivation of heteromultimeric Shaker potassium channels. J. Gen. Physiol. 112, 457–474 10.1085/jgp.112.4.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kuner T., Beck C., Sakmann B., and Seeburg P. H. (2001) Channel-lining residues of the AMPA receptor M2 segment: structural environment of the Q/R site and identification of the selectivity filter. J. Neurosci. 21, 4162–4172 10.1523/JNEUROSCI.21-12-04162.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bennett J. A., and Dingledine R. (1995) Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron 14, 373–384 10.1016/0896-6273(95)90293-7 [DOI] [PubMed] [Google Scholar]
- 121. Panchenko V. A., Glasser C. R., Partin K. M., and Mayer M. L. (1999) Amino acid substitutions in the pore of rat glutamate receptors at sites influencing block by polyamines. J. Physiol. 520, 337–357 10.1111/j.1469-7793.1999.t01-1-00337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. MacKinnon R. (1995) Pore loops: an emerging theme in ion channel structure. Neuron 14, 889–892 10.1016/0896-6273(95)90327-5 [DOI] [PubMed] [Google Scholar]
- 123. Panchenko V. A., Glasser C. R., and Mayer M. L. (2001) Structural similarities between glutamate receptor channels and K+ channels examined by scanning mutagenesis. J. Gen. Physiol. 117, 345–360 10.1085/jgp.117.4.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen G. Q., Cui C., Mayer M. L., and Gouaux E. (1999) Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402, 817–821 10.1038/45568 [DOI] [PubMed] [Google Scholar]
- 125. Kuner T., Seeburg P. H., and Guy H. R. (2003) A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci. 26, 27–32 10.1016/S0166-2236(02)00010-3 [DOI] [PubMed] [Google Scholar]
- 126. Schneggenburger R., and Ascher P. (1997) Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron 18, 167–177 10.1016/S0896-6273(01)80055-6 [DOI] [PubMed] [Google Scholar]
- 127. Jatzke C., Hernandez M., and Wollmuth L. P. (2003) Extracellular vestibule determinants of Ca2+ influx in Ca2+-permeable AMPA receptor channels. J. Physiol. 549, 439–452 10.1113/jphysiol.2002.034413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Watanabe J., Beck C., Kuner T., Premkumar L. S., and Wollmuth L. P. (2002) DRPEER: a motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J. Neurosci. 22, 10209–10216 10.1523/JNEUROSCI.22-23-10209.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Griffith T. N., and Swanson G. T. (2015) Identification of critical functional determinants of kainate receptor modulation by auxiliary protein Neto2. J. Physiol. 593, 4815–4833 10.1113/JP271103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dingledine R., Hume R. I., and Heinemann S. F. (1992) Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J. Neurosci. 12, 4080–4087 10.1523/JNEUROSCI.12-10-04080.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Curutchet P., Bochet P., Prado de Carvalho L., Lambolez B., Stinnakre J., and Rossier J. (1992) In the GluR1 glutamate receptor subunit a glutamine to histidine point mutation suppresses inward rectification but not calcium permeability. Biochem. Biophys. Res. Commun. 182, 1089–1093 10.1016/0006-291X(92)91843-F [DOI] [PubMed] [Google Scholar]
- 132. Twomey E. C., Yelshanskaya M. V., Grassucci R. A., Frank J., and Sobolevsky A. I. (2017) Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 549, 60–65 10.1038/nature23479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shanks N. F., Savas J. N., Maruo T., Cais O., Hirao A., Oe S., Ghosh A., Noda Y., Greger I. H., Yates J. R. 3rd., and Nakagawa T. (2012) Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 1, 590–598 10.1016/j.celrep.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Twomey E. C., Yelshanskaya M. V., Grassucci R. A., Frank J., and Sobolevsky A. I. (2017) Structural bases of desensitization in AMPA receptor-auxiliary subunit complexes. Neuron 94, 569–580.e5 10.1016/j.neuron.2017.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tomita S., Adesnik H., Sekiguchi M., Zhang W., Wada K., Howe J. R., Nicoll R. A., and Bredt D. S. (2005) Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058 10.1038/nature03624 [DOI] [PubMed] [Google Scholar]
- 136. Priel A., Kolleker A., Ayalon G., Gillor M., Osten P., and Stern-Bach Y. (2005) Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 25, 2682–2686 10.1523/JNEUROSCI.4834-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Patneau D. K., Vyklicky L. Jr., and Mayer M. L. (1993) Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J. Neurosci. 13, 3496–3509 10.1523/JNEUROSCI.13-08-03496.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., and MacKinnon R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- 139. Cao E., Liao M., Cheng Y., and Julius D. (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118 10.1038/nature12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yelshanskaya M. V., Mesbahi-Vasey S., Kurnikova M. G., and Sobolevsky A. I. (2017) Role of the ion channel extracellular collar in AMPA receptor gating. Sci. Rep. 7, 1050 10.1038/s41598-017-01146-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Herlitze S., Raditsch M., Ruppersberg J. P., Jahn W., Monyer H., Schoepfer R., and Witzemann V. (1993) Argiotoxin detects molecular differences in AMPA receptor channels. Neuron 10, 1131–1140 10.1016/0896-6273(93)90061-U [DOI] [PubMed] [Google Scholar]
- 142. Koike M., Iino M., and Ozawa S. (1997) Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci. Res. 29, 27–36 10.1016/S0168-0102(97)00067-9 [DOI] [PubMed] [Google Scholar]
- 143. Magazanik L. G., Buldakova S. L., Samoilova M. V., Gmiro V. E., Mellor I. R., and Usherwood P. N. (1997) Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J. Physiol. 505, 655–663 10.1111/j.1469-7793.1997.655ba.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Twomey E. C., Yelshanskaya M. V., Vassilevski A. A., and Sobolevsky A. I. (2018) Mechanisms of channel block in calcium-permeable AMPA receptors. Neuron 99, 956–968.e4 10.1016/j.neuron.2018.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Miller C. (1982) Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J. Gen. Physiol. 79, 869–891 10.1085/jgp.79.5.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jackson A. C., and Nicoll R. A. (2011) The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199 10.1016/j.neuron.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Greger I. H., Watson J. F., and Cull-Candy S. G. (2017) Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94, 713–730 10.1016/j.neuron.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 148. Copits B. A., and Swanson G. T. (2012) Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat. Rev. Neurosci. 13, 675–686 10.1038/nrn3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Howe J. R. (2015) Modulation of non-NMDA receptor gating by auxiliary subunits. J. Physiol. 593, 61–72 10.1113/jphysiol.2014.273904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tomita S., and Castillo P. E. (2012) Neto1 and Neto2: auxiliary subunits that determine key properties of native kainate receptors. J. Physiol. 590, 2217–2223 10.1113/jphysiol.2011.221101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Haering S. C., Tapken D., Pahl S., and Hollmann M. (2014) Auxiliary subunits: shepherding AMPA receptors to the plasma membrane. Membranes 4, 469–490 10.3390/membranes4030469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schnell E., Sizemore M., Karimzadegan S., Chen L., Bredt D. S., and Nicoll R. A. (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. U.S.A. 99, 13902–13907 10.1073/pnas.172511199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kato A. S., Siuda E. R., Nisenbaum E. S., and Bredt D. S. (2008) AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron 59, 986–996 10.1016/j.neuron.2008.07.034 [DOI] [PubMed] [Google Scholar]
- 154. Tomita S., Sekiguchi M., Wada K., Nicoll R. A., and Bredt D. S. (2006) Stargazin controls the pharmacology of AMPA receptor potentiators. Proc. Natl. Acad. Sci. U.S.A. 103, 10064–10067 10.1073/pnas.0603128103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Menuz K., Stroud R. M., Nicoll R. A., and Hays F. A. (2007) TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science 318, 815–817 10.1126/science.1146317 [DOI] [PubMed] [Google Scholar]
- 156. Maclean D. M., and Bowie D. (2011) Transmembrane AMPA receptor regulatory protein regulation of competitive antagonism: a problem of interpretation. J. Physiol. 589, 5383–5390 10.1113/jphysiol.2011.219485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Schwenk J., Harmel N., Zolles G., Bildl W., Kulik A., Heimrich B., Chisaka O., Jonas P., Schulte U., Fakler B., and Klöcker N. (2009) Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319 10.1126/science.1167852 [DOI] [PubMed] [Google Scholar]
- 158. Schwenk J., Harmel N., Brechet A., Zolles G., Berkefeld H., Müller C. S., Bildl W., Baehrens D., Hüber B., Kulik A., Klöcker N., Schulte U., and Fakler B. (2012) High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633 10.1016/j.neuron.2012.03.034 [DOI] [PubMed] [Google Scholar]
- 159. Collins M. O. (2014) AMPA receptor complex dynamics in time and space. Neuron 84, 1–3 10.1016/j.neuron.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 160. Zhang W., St-Gelais F., Grabner C. P., Trinidad J. C., Sumioka A., Morimoto-Tomita M., Kim K. S., Straub C., Burlingame A. L., Howe J. R., and Tomita S. (2009) A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396 10.1016/j.neuron.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ng D., Pitcher G. M., Szilard R. K., Sertié A., Kanisek M., Clapcote S. J., Lipina T., Kalia L. V., Joo D., McKerlie C., Cortez M., Roder J. C., Salter M. W., and McInnes R. R. (2009) Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLos Biol. 7, e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Copits B. A., Robbins J. S., Frausto S., and Swanson G. T. (2011) Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J. Neurosci. 31, 7334–7340 10.1523/JNEUROSCI.0100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Straub C., Hunt D. L., Yamasaki M., Kim K. S., Watanabe M., Castillo P. E., and Tomita S. (2011) Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat. Neurosci. 14, 866–873 10.1038/nn.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tang M., Pelkey K. A., Ng D., Ivakine E., McBain C. J., Salter M. W., and McInnes R. R. (2011) Neto1 is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. 31, 10009–10018 10.1523/JNEUROSCI.6617-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kubo Y. (1996) Two aspects of the inward rectification mechanism. Effects of cytoplasmic blockers and extracellular K+ on the inward rectifier K+ channel. Jpn. Heart J. 37, 631–641 10.1536/ihj.37.631 [DOI] [PubMed] [Google Scholar]
- 166. Baronas V. A., and Kurata H. T. (2014) Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 5, 325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Perrais D., Coussen F., and Mulle C. (2009) Atypical functional properties of GluK3-containing kainate receptors. J. Neurosci. 29, 15499–15510 10.1523/JNEUROSCI.2724-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Reiner A., Arant R. J., and Isacoff E. Y. (2012) Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Rep. 1, 234–240 10.1016/j.celrep.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wilding T. J., Chen K., and Huettner J. E. (2010) Fatty acid modulation and polyamine block of GluK2 kainate receptors analyzed by scanning mutagenesis. J. Gen. Physiol. 136, 339–352 10.1085/jgp.201010442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. McGee T. P., Bats C., Farrant M., and Cull-Candy S. G. (2015) Auxiliary subunit GSG1L acts to suppress calcium-permeable AMPA receptor function. J. Neurosci. 35, 16171–16179 10.1523/JNEUROSCI.2152-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Soto D., Coombs I. D., Gratacòs-Batlle E., Farrant M., and Cull-Candy S. G. (2014) Molecular mechanisms contributing to TARP regulation of channel conductance and polyamine block of calcium-permeable AMPA receptors. J. Neurosci. 34, 11673–11683 10.1523/JNEUROSCI.0383-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Pellegrini-Giampietro D. E., Bennett M. V., and Zukin R. S. (1992) Are Ca2+-permeable kainate/AMPA receptors more abundant in immature brain? Neurosci. Lett. 144, 65–69 10.1016/0304-3940(92)90717-L [DOI] [PubMed] [Google Scholar]
- 173. Talos D. M., Fishman R. E., Park H., Folkerth R. D., Follett P. L., Volpe J. J., and Jensen F. E. (2006) Developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J. Comp. Neurol. 497, 42–60 10.1002/cne.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Miller-Fleming L., Olin-Sandoval V., Campbell K., and Ralser M. (2015) Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 427, 3389–3406 10.1016/j.jmb.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 175. Pegg A. E. (2016) Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912 10.1074/jbc.R116.731661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cason A. L., Ikeguchi Y., Skinner C., Wood T. C., Holden K. R., Lubs H. A., Martinez F., Simensen R. J., Stevenson R. E., Pegg A. E., and Schwartz C. E. (2003) X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur. J. Hum. Genet. 11, 937–944 10.1038/sj.ejhg.5201072 [DOI] [PubMed] [Google Scholar]
- 177. Casero R. A. Jr., Murray Stewart T., and Pegg A. E. (2018) Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 10.1038/s41568-018-0050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gerner E. W., and Meyskens F. L. Jr. (2004) Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 4, 781–792 10.1038/nrc1454 [DOI] [PubMed] [Google Scholar]
- 179. Herner A., Sauliunaite D., Michalski C. W., Erkan M., De Oliveira T., Abiatari I., Kong B., Esposito I., Friess H., and Kleeff J. (2011) Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int. J. Cancer 129, 2349–2359 10.1002/ijc.25898 [DOI] [PubMed] [Google Scholar]
- 180. Ripka S., Riedel J., Neesse A., Griesmann H., Buchholz M., Ellenrieder V., Moeller F., Barth P., Gress T. M., and Michl P. (2010) Glutamate receptor GRIA3–target of CUX1 and mediator of tumor progression in pancreatic cancer. Neoplasia 12, 659–667 10.1593/neo.10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hu H., Takano N., Xiang L., Gilkes D. M., Luo W., and Semenza G. L. (2014) Hypoxia-inducible factors enhance glutamate signaling in cancer cells. Oncotarget 5, 8853–8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. von Roemeling C. A., Radisky D. C., Marlow L. A., Cooper S. J., Grebe S. K., Anastasiadis P. Z., Tun H. W., and Copland J. A. (2014) Neuronal pentraxin 2 supports clear cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4. Cancer Res. 74, 4796–4810 10.1158/0008-5472.CAN-14-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rzeski W., Ikonomidou C., and Turski L. (2002) Glutamate antagonists limit tumor growth. Biochem. Pharmacol. 64, 1195–1200 10.1016/S0006-2952(02)01218-2 [DOI] [PubMed] [Google Scholar]
- 184. Stepulak A., Sifringer M., Rzeski W., Brocke K., Gratopp A., Pohl E. E., Turski L., and Ikonomidou C. (2007) AMPA antagonists inhibit the extracellular signal regulated kinase pathway and suppress lung cancer growth. Cancer Biol. Ther. 6, 1908–1915 10.4161/cbt.6.12.4965 [DOI] [PubMed] [Google Scholar]
- 185. Prickett T. D., and Samuels Y. (2012) Molecular pathways: dysregulated glutamatergic signaling pathways in cancer. Clin. Cancer Res. 18, 4240–4246 10.1158/1078-0432.CCR-11-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Casero R. A., and Pegg A. E. (2009) Polyamine catabolism and disease. Biochem. J. 421, 323–338 10.1042/BJ20090598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pegg A. E. (2009) Mammalian polyamine metabolism and function. IUBMB Life 61, 880–894 10.1002/iub.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Poulin R., Casero R. A., and Soulet D. (2012) Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 42, 711–723 10.1007/s00726-011-0987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. García-Nafría J., Herguedas B., Watson J. F., and Greger I. H. (2016) The dynamic AMPA receptor extracellular region: a platform for synaptic protein interactions. J. Physiol. 594, 5449–5458 10.1113/JP271844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mayer M. L., and Armstrong N. (2004) Structure and function of glutamate receptor ion channels. Annu. Rev. Physiol. 66, 161–181 10.1146/annurev.physiol.66.050802.084104 [DOI] [PubMed] [Google Scholar]
- 191. Shepherd J. D., and Huganir R. L. (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 10.1146/annurev.cellbio.23.090506.123516 [DOI] [PubMed] [Google Scholar]
- 192. Sobolevsky A. I., Rosconi M. P., and Gouaux E. (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462, 745–756 10.1038/nature08624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Alam A., and Jiang Y. (2009) High-resolution structure of the open NaK channel. Nat. Struct. Mol. Biol. 16, 30–34 10.1038/nsmb.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]