Abstract
Phospholipids (PLs) are emerging as important factors that initiate signal transduction cascades at the plasma membrane. Their distribution within biological membranes is tightly regulated, e.g. by ATP-binding cassette (ABC) transporters, which preferably translocate PLs from the cytoplasmic to the exoplasmic membrane leaflet and are therefore called PL-floppases. Here, we demonstrate that a plant ABC transporter, Lr34 from wheat (Triticum aestivum), is involved in plasma membrane remodeling characterized by an intracellular accumulation of phosphatidic acid and enhanced outward translocation of phosphatidylserine. In addition, the content of phosphatidylinositol 4,5-bisphosphate in the cytoplasmic leaflet of the plasma membrane was reduced in the presence of the ABC transporter. When heterologously expressed in Saccharomyces cerevisiae, Lr34 promoted oil body formation in a mutant defective in PL-transfer in the secretory pathway. Our results suggest that PL redistribution by Lr34 potentially affects the membrane-bound proteome and contributes to the previously reported stimuli-independent activation of biotic and abiotic stress responses and neutral lipid accumulation in transgenic Lr34-expressing barley plants.
Keywords: ABC transporter, phosphatidylserine, phospholipid, lipid signaling, lipid metabolism, Lr34, flippase, floppase, oil bodies
Introduction
ATP-binding cassette (ABC)3 transporters constitute a large and diverse family of membrane proteins that use the energy provided by ATP hydrolysis to pump various compounds against steep concentration gradients. They occur as full-size or half-size transporters and form an active unit consisting of two transmembrane and two nucleotide-binding domains. The ubiquitous presence of ABC transporters in almost all organisms highlights their importance for cellular metabolism. Typically, they play a major role in extruding a multitude of cytotoxic compounds like heavy metal ions or xenobiotics (1, 2); however, their true physiological function goes far beyond as deduced from the fact that malfunction of certain ABC transporters can evoke drastic physiological effects. For instance, the human Tangier disease, characterized by the absence of high-density lipoprotein, can be traced back to a defect in HsABCA1, a member of the human A subfamily of ABC transporters. In addition, mutations in HsABCA4 result in delayed dark adaptation in the retina by a reduced all-trans-retinaldehyde clearance, a characteristic feature of Stargardt disease (2). The strong association with lipid metabolism suggests that certain lipids might directly serve as transport substrates. Indeed, several reports have demonstrated a role of numerous ABC transporters in the rearrangement of certain lipids within the membrane bilayer. Depending on the direction of movement, the translocator is either called flippase (from the exoplasmic to the cytoplasmic leaflet) or floppase (from the cytoplasmic to the exoplasmic leaflet). A prominent example is the pleiotropic drug resistance–type transporter PDR5 from Saccharomyces cerevisiae, which was characterized as a phosphatidylethanolamine (PE) floppase besides its function in extruding a diverse set of cytotoxic compounds (3). In addition, the human pathogen Leishmania contains a half-size ABCG transporter, which is responsible for the exposure of phosphatidylserine (PS) on its plasma membrane (PM) (4). The same effect has been observed in human gastric carcinoma cells, where the overexpression of the breast cancer resistance protein BCRP (ABCG2) leads to increased PS exposure (5). Moreover, the full size ABCG transporters CDR1–3p from the pathogenic fungus Candida albicans were characterized as phospholipid (PL) translocators with varying transport direction and broad substrate specificity (6).
The wheat ABC transporter Lr34, a member of the G subfamily, is known to confer partial, durable, and broad-spectrum resistance against several biotrophic fungi such as powdery mildew, leaf rust, or stem rust (7). The closest related orthologous genes can be found in Oryza sativa (OsABCG50) and Sorghum bicolor (SbABCG50) sharing 86 and 76% identity, respectively. By comparison, the corresponding genes in Arabidopsis thaliana only show intermediate similarity ranging from 50% identity (AtPEN/PDR8) to 56% identity (AtPDR5). In the wheat gene pool, the Lr34 gene is present in two different alleles, called Lr34sus and Lr34res, which are characterized by their susceptible and resistant phenotype, respectively, to biotrophic fungal pathogens in the corresponding wheat varieties. These two alleles differ at two positions within the coding region for the first transmembrane domain leading to a phenylalanine deletion and a conversion of tyrosine to histidine in Lr34res. It is still unclear whether the phenotypic differences are caused by varying protein levels or distinct biochemical functions of the two transporter versions. Recent reports have demonstrated that Lr34res can be functionally transferred to related crop species, like barley, rice, or maize, making them partially resistant against their specific pathogens (8–10). However, strong expression levels of the transgene correlate with the development of early leaf tip necrosis as an indicator of senescence (8) and strong triacylglycerol (TAG) accumulation in transgenic barley plants (11). Moreover, transcriptomic data of Lr34res-expressing barley have revealed the up-regulation of numerous stress- and pathogenicity-related (PR) genes in the absence of fungal infection (12, 13). To explain all these observations, it is essential to understand how the ABC transporter functions at the molecular level.
The fact that several stress-responsive genes are up-regulated in Lr34res-expressing plants in the absence of pathogens (12, 13) suggests that the activity of the transporter mimics stress exposure likely by modulating cellular metabolism. A common feature of hormone and pathogen defense signaling pathways is the activation of certain phospholipases (PLases) upon the perception of external signals (14). These enzymes convert membrane lipids into signaling compounds like phosphatidic acid (PA), diacylglycerol (DAG), or inositol triphosphate (IP3), which affects downstream PL-interacting factors in their subcellular localization and thus their activity. A prominent example in plants is the protein phosphatase ABI1 from A. thaliana, which binds to PA in the PM upon phospholipase D (PLD) activation and thereby loses its suppressor function in the abscisic acid–signaling pathway (15). Other reports showed that PLDβ1 has a major role in modulating defense responses against bacterial and fungal pathogens through the generation of PA (16). The aim of this work was to address whether the ABC transporter Lr34 affects membrane properties that might lead to the activation of PL-mediated stress signaling in the absence of external factors. We demonstrate that Lr34res promotes the translocation of PA and PS in transgenic barley and changes the distribution of PS and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in the PM of tobacco BY2 cells. When heterologously expressed in S. cerevisiae, both versions of the ABC transporter (Lr34sus and Lr34res) enhanced PS and reduced PE exposure at the PM. Finally, the presence of Lr34res promoted the accumulation of neutral lipids in oil bodies in an S. cerevisiae mutant strain defective in PL-transfer. Together, these data suggest that Lr34 plays a pivotal role in redistributing certain membrane PLs with impact on overall cellular lipid metabolism.
Results
Lr34res is present in the PM fraction when heterologously expressed in tobacco BY2 cells
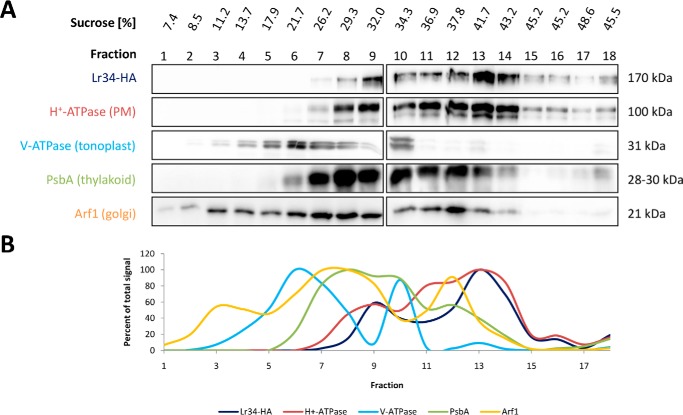
Pleiotropic drug resistance (PDR)-type transporters are usually found in the plasma membrane (17–19). As a prerequisite for subsequent functional studies, it was necessary to confirm the presence of Lr34res in the plant PM. Therefore, the total membrane fraction from tobacco BY2 cells expressing HA-tagged Lr34res was isolated and fractionated on a linear density gradient. Immunolabeling of the fractions with antibodies directed to specific proteins residing at different intracellular membrane compartments demonstrated that the distribution of HA-Lr34res correlated with the PM H+-ATPase marker (Fig. 1, A and B) indicating that Lr34res is indeed associated with the plant PM.
Figure 1.
Total membrane fractionation of BY2 cells expressing HA-tagged Lr34res protein. A, fractions ranging from 7.4 to 48.6% (w/v) sucrose were harvested after ultracentrifugation and tested for the presence of HA-Lr34res and marker proteins of the PM (H+-ATPase), the tonoplast (V-ATPase), thylakoid membranes (PsbA), and Golgi membranes (Arf1) by Western blotting using specific antibodies. Fractionation was repeated twice with the same outcome of HA-Lr34res distribution. B, quantification of signal intensities after blot development. For each protein, values are relative to the fraction with the strongest signal, which was set to 100%.
Lr34res promotes PA inward and PS outward translocation in barley protoplasts
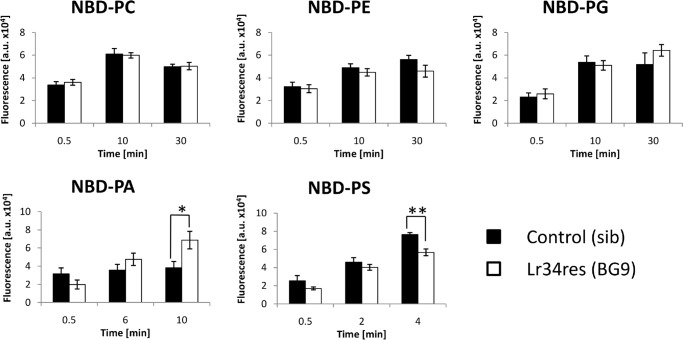
Next, the activity of the ABC transporter was investigated that might be responsible for the Lr34res-mediated resistance against various biotrophic pathogens. Based on numerous reports demonstrating a role of ABC transporters in PL metabolism (3–6), as well as the observed phenotypes in plants expressing the resistance gene (8, 9), we hypothesized that Lr34res might have a PL flippase or floppase activity with impact on the distribution of certain PLs within the membrane. To study this putative function, Lr34res-expressing (BG9) and the corresponding nontransgenic (sib) barley plants (8) were used to isolate protoplasts. These were then incubated with fluorescence-labeled (NBD) PLs to investigate their incorporation in a time-dependent manner. Overall, the uptake rates of NBD-PC, NBD-PE, and NBD-PG were similar between sib and BG9 protoplasts, and no statistically significant difference could be found at any time point (Fig. 2). By contrast, NBD-PA strongly accumulated in transgenic BG9 protoplasts after 10 min, while NBD-PS uptake was significantly reduced.
Figure 2.
Time-dependent incorporation of different NBD-labeled PLs in barley protoplasts expressing Lr34res. Leaf mesophyll protoplasts were isolated from Lr34res-expressing (BG9) and the corresponding nontransgenic (sib) barley plants and incubated with NBD-PA, NBD-PE, NBD-PG, NBD-PC, or NBD-PS. At the indicated time points, samples were taken, and the incorporated fluorescence was quantified. Values are means of arbitrary units (a.u.) ± S.E. (n = 4–6), *, p ≤ 0.05,**, p ≤ 0.01, according to Student's t test.
Lr34res reduces PS and PI(4,5)P2 levels in the cytoplasmic PM leaflet of tobacco BY2 protoplasts
The advantage of using NBD-labeled PLs is the easy detection and quantification of the emitted fluorescence. However, their physical properties slightly differ from natural lipids, what potentially affects their behavior in the hydrophobic membrane environment. To avoid this possible problem, protoplasts from control and transgenic Lr34res-expressing tobacco BY2 cells were transformed with PL-specific biosensors to visualize the intracellular distribution of selected native membrane lipids.
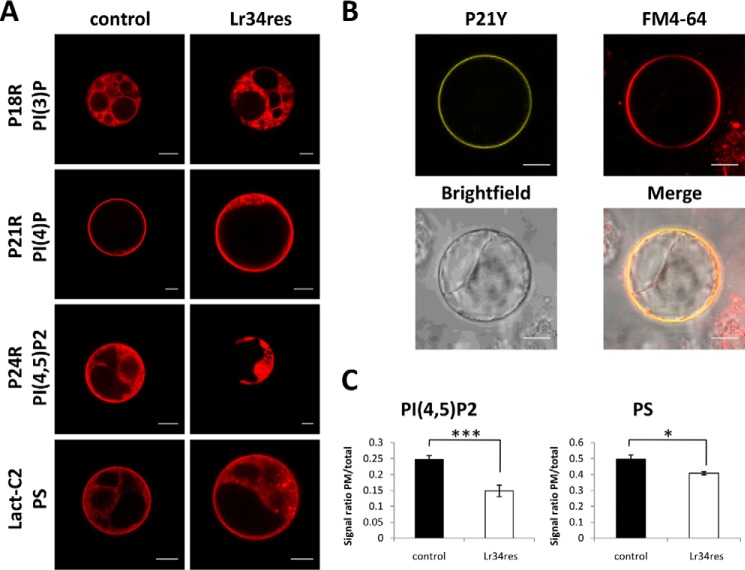
In agreement with previous reports (20), the phosphatidylinositol 3-phosphate (PI(3)P)-binding, red fluorescent protein (RFP)-tagged biosensor P18R was enriched in intracellular membranes both in the presence and absence of Lr34res (Fig. 3A). The phosphatidylinositol 4-phosphate (PI(4)P)-binding biosensor P21R and the corresponding citrine-tagged version (P21Y) showed the strongest signal at a membrane that could be stained with FM4-64 at the periphery of the protoplast (Fig. 3, A and B). This is consistent with the normal localization of PI(4)P at the PM (20). Although the ABC transporter did not affect the subcellular destination of the two monophosphorylated inositides, the PI(4,5)P2-binding biosensor P24R did not label the PM anymore under Lr34res expression but was instead restricted to intracellular, vacuole-like structures. A PS biosensor (Lact-C2) was present at the PM and endomembranes independently of Lr34res expression. However, an accumulation of this biosensor in dot-like structures was observed in Lr34res-expressing protoplasts in contrast to the control.
Figure 3.
Subcellular localization of fluorescent PL biosensors in BY2 protoplasts. A, RFP-tagged biosensors for PS (derived from the C2 domain of lactadherin), PI(4)P (P21R) (20), PI(4,5)P2 (P24R) (20), and PI(3)P (P18R) (20) were transiently expressed in transgenic control and Lr34res transformed BY2 protoplasts. Pictures were taken 16–18 h after transfection. B, colocalization of the citrine-tagged version of the PI(4)P biosensor (P21Y) with FM4-64 in BY2 control protoplasts. In this case, P21Y was selected instead of P21R to avoid the overlap of RFP and FM4-64 fluorescence emission spectra. C, calculation of the ratio of PM-derived to total signal for PI(4,5)P2 and PS biosensors in control and Lr34res BY2 protoplasts. Values are means ± S.E. (n ≥8), *, p ≤ 0.05; ***, p ≤ 0.001, according to Student's t test. Scale bars, 10 μm.
For a verification of these observations, the ratio of the PM/total signal was calculated for the PI(4,5)P2 and PS biosensors. This indeed confirmed a significantly lower fluorescence signal at the PM for the PI(4,5)P2 and PS biosensors in the presence of Lr34res (Fig. 3C) suggesting that these lipids accumulate less at the cytoplasmic PM leaflet.
Lr34res and Lr34sus enhance PS and reduce PE exposure at the PM in S. cerevisiae
To confirm the role of Lr34 as a translocator for certain PLs and to investigate potential differences in activity between the two transporter versions, Lr34sus and Lr34res, the protein was heterologously expressed in a WT S. cerevisiae strain in order to perform growth assays that use toxins to probe for the translocation of specific endogenous PLs at the PM. In addition to the two native Lr34 proteins, Lr34sus and Lr34res, inactive versions bearing mutations of the critical amino acids in all four Walker domains (Lr34sus/resW, Table 1) were used as controls besides the empty vector because these domains are essential for the hydrolysis of ATP in all ABC transporters.
Table 1.
Mutagenesis sites for generation of inactive Lr34 versions
| Motif (position in Lr34r) | Original sequence | Mutated sequence |
|---|---|---|
| Walker A1(168–175) | GPPGCGKS | GPPGCAAS |
| Walker B1(327–332) | AYFMDE | AYFMAE |
| Walker A2(846–853) | GVSGAGKT | GVSGAAAT |
| Walker B2(973–978) | IILMDE | IILMAE |
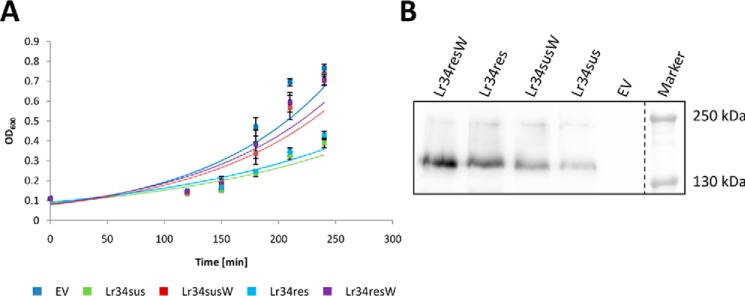
Under standard conditions, the expression of an active Lr34 transporter impaired growth in yeast cells, whereas the inactive Lr34 versions did not cause any effect even when slightly higher protein expression levels were achieved (Fig. 4, A and B).
Figure 4.
Growth behavior of S. cerevisiae WT strain BY4741 expressing different Lr34 versions. A, cells were either transformed with an empty vector (EV) or plasmids for the expression of Lr34sus, inactive Lr34sus (Lr34susW), Lr34res, or inactive Lr34res (Lr34resW) and grown in selective SD media under standard growth conditions. A600 of freshly inoculated overnight grown cultures (starting A600 = 0.1) was measured at the indicated time points during the exponential growth phase. Values are means ± S.E. (n ≥5). B, immunoblot analysis of microsomal yeast fractions expressing different HA-tagged versions of Lr34.
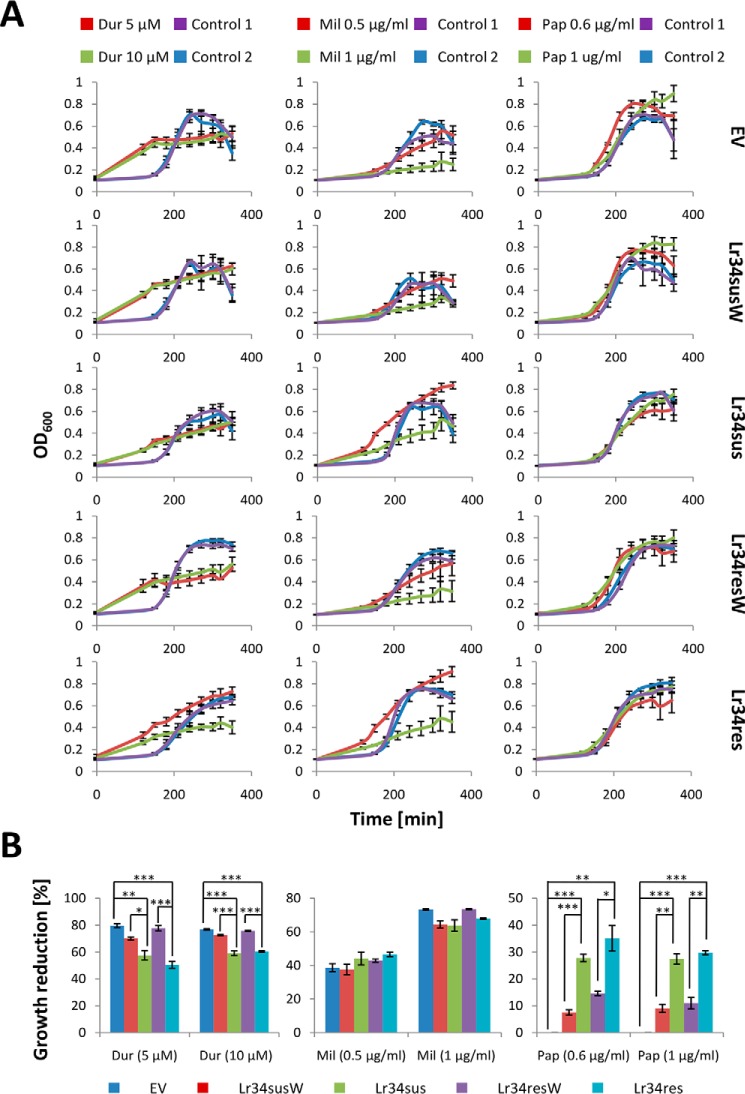
Next, the growth behavior of Lr34-expressing yeast cells was investigated in the presence of specific toxins. The cyclic depsipeptide duramycin specifically binds PE upon exposure to the outer leaflet of the PM and forms pores in the membrane that induces cell death (21). Therefore, any floppase activity resulting in increased PE exposure should enhance the drug sensitivity of the cells, whereas a PE flippase would evoke the opposite effect. Interestingly, duramycin application altered the general growth behavior of yeast cells, indicated by a constant linear growth and loss of clearly distinguishable growth phases (Fig. 5A). Moreover, when growth inhibition was calculated, cells expressing Lr34sus and especially Lr34res were more resistant to the drug and consequently grew significantly better than the corresponding controls at 5 and 10 μm duramycin (Fig. 5B), pointing to a PE flippase activity of both Lr34 versions.
Figure 5.
Drug sensitivity test of yeast BY4741 cells expressing different Lr34 versions. A, growth pattern of freshly inoculated overnight grown yeast cells (starting A600 = 0.1) containing an empty vector (EV) or expressing inactive Lr34sus (Lr34susW) or Lr34res (Lr34resW) or the active protein versions Lr34sus or Lr34res was monitored. Cells were grown in media containing either the cytotoxic drugs duramycin (Dur), miltefosine (Mil), or papuamide A (Pap) at the indicated concentrations or DMSO (Dur, Pap) or ethanol (Mil) as the corresponding controls (1, low concentration; 2, high concentration). A600 was measured at the indicated time points. Values are means ± S.E. (n = 4–6). B, calculation of growth inhibitory effects for the respective drug concentrations. Values are means ± S.E. (n = 4–6), *, p ≤ 0.0167; **, p ≤ 0.0033; ***, p ≤ 0.00033, according to one-way ANOVA and post hoc t tests with Bonferroni corrections.
Similarly, cells were tested for growth in the presence of the PS-binding cyclic depsipeptide papuamide A, which develops its toxic effect by the same mechanism as duramycin (22, 23). The growth of Lr34sus- and Lr34res-expressing cells was significantly impaired compared with cells expressing the corresponding inactive transporter versions or containing the empty vector already at 0.6 μg/ml papuamide A. This effect persisted when increasing the drug concentration to 1 μg/ml.
To get further insights on the PL specificity of the ABC transporter, the growth of cells expressing the different Lr34 versions was also tested in media containing miltefosine. This compound is a structural analogue of lyso-PC and develops its toxic effect upon transport into the cell (24). If Lr34 were able to extrude miltefosine, yeast cells expressing the ABC transporter should be less sensitive to this drug. Apparently, even small toxin concentrations altered the growth behavior in a similar way as duramycin. However, when comparing the growth inhibition rates, it was asserted that in this case neither Lr34sus nor Lr34res expression significantly altered the drug sensitivity of the yeast cells, suggesting that no import or export of miltefosine occurred through the PM.
The papuamide and duramycin sensitivity assays probe the distribution of lipids at the PM, which is affected both by the presence of specific transporters at this membrane and by the distribution of lipids at the membrane of vesicles fusing to the PM from internal compartments (25). In contrast, the miltefosine sensitivity assay requires the presence of the transporter at the PM, as miltefosine needs to be transported inside the cell to exert its action. Therefore, a lack of miltefosine sensitivity could be related to a trafficking problem, rather than reflect the substrate transport preference of the transporter. Indeed, fluorescent GFP-tagged versions of the Lr34 transporters could be visualized in internal membranes in yeast, but no PM localization was detected (Fig. S1).
Nevertheless, we aimed at performing lipid uptake and export assays using NBD-PLs, to directly test the lipid transport capabilities of the Lr34 transporters in yeast. A number of different growth parameters (inducible/constitutive expression, agitation, and temperature) were tested, but we were unable to identify reproducible growth conditions leading to the presence of the transporters at the PM.
In summary, Lr34sus and Lr34res enhanced the yeast tolerance to duramycin, suggesting that PE is translocated to the inner side of the yeast PM. The opposite effect was observed for papuamide A, indicating a role of Lr34sus and Lr34res in PS exposure. In accordance with the protein expression levels (Fig. 4B), these effects were slightly more pronounced for Lr34res than for Lr34sus.
Expression of Lr34res promotes oil body formation in a PL-transfer-deficient S. cerevisiae mutant strain
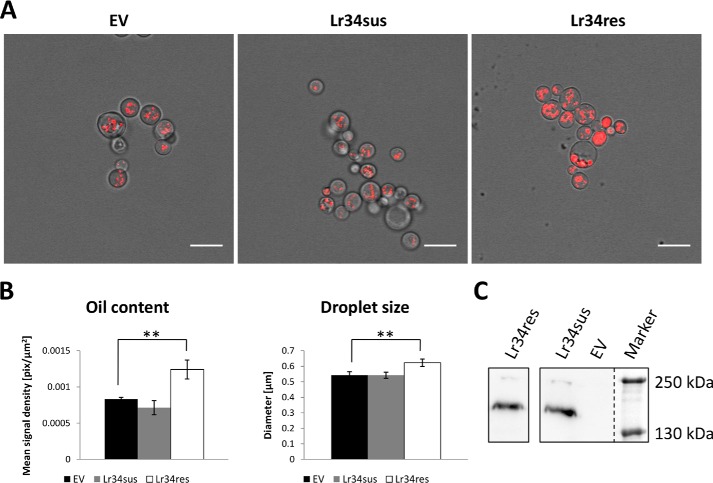
The previously reported triacylglycerol accumulation in leaves of Lr34res-expressing barley plants (11) suggests a potential link between the PL flippase or floppase activity of Lr34res and overall lipid metabolism. As Lr34res apparently not only translocates PLs in the PM of plants but also in the PM of yeast, it could be assumed that enhanced neutral lipid storage might also be induced by the ABC transporter in fungo. Instead of using WT S. cerevisiae strains that hardly accumulate any lipids under standard growth conditions, the Sec14ts PL-transfer protein mutant CTY1-1A showing constitutively enhanced oil body formation for the storage of accumulating neutral lipids was selected for the following studies. This mutant is characterized by a defect in the PL-transfer protein Sec14, which is necessary for efficient vesicle budding and fusion events between the trans-Golgi network and the PM. Because a deletion of the Sec14 gene is lethal, a mutation was introduced in the coding sequence that renders the yeast cells temperature-sensitive due to impaired PL-transfer activity by the mutated Sec14 protein (26). To visualize oil bodies and quantify the intracellular oil content, the lipophilic dye Nile red was applied, which is only fluorescent when embedded in a hydrophobic environment typically found in oil bodies (27). Expression of Lr34res but not Lr34sus significantly increased the number and the size of these lipid reservoirs (Fig. 6, A and B). Because expression levels of both Lr34 versions were similar in this mutant (Fig. 6C), a different activity of the two transporters can be assumed to be the cause of the accumulation of neutral lipids.
Figure 6.
Oil body quantification in the S. cerevisiae Sec14ts mutant CTY1-1A. A, yeast cells transformed with an empty vector or plasmids for the expression of Lr34sus and Lr34res, respectively, were grown to stationary phase and stained with Nile red for the microscopic visualization of oil bodies. Scale bars, 10 μm. B, determination of Nile red–derived fluorescence signal density as a measure of oil content and determination of average oil droplet size in transformed yeast cells. Values are means ± S.E. (n = 6–10 for oil content, n = 16–33 for droplet size). **, p ≤ 0.01, according to Student's t test. C, protein levels of HA-tagged Lr34sus and Lr34res in CTY1-1A microsomal fractions.
Discussion
Resolving the molecular mechanism of disease resistance in cereals is of great importance, especially in view of the challenges in sustainable food supply in the future. The ABC transporter Lr34 from wheat is known to confer partial, durable, and broad-spectrum disease resistance, which makes it a very interesting candidate for molecular investigations. It was demonstrated that the Lr34res gene can be functionally transferred into other grass species like rice, barley, or maize, resulting in resistance against all tested species-specific biotrophic and hemibiotrophic pathogens, such as Magnaporthe oryzae, Blumeria graminis f. sp. hordei, or Exserohilum turcicum (8–10). However, strong transgene expression can cause side effects, such as triacylglycerol accumulation in barley leaves (11) or early senescence in barley and rice (8–9). The aim of this study was to characterize the activity of the ABC transporter Lr34 in an attempt to find a common cause for the observed diverse physiological effects.
Before investigating the activity of Lr34 in planta, the subcellular localization of the ABC transporter was elucidated. The absence of signaling peptides and the localization studies from related PDR-type transporters (17–19) suggested that Lr34 is most likely present in the PM. Fractionation of total membranes from transgenic BY2 cells indeed revealed that the HA-tagged Lr34res protein showed a signal correlation with the plasma membrane H+-ATPase. Although it cannot be ruled out that Lr34res has additional subcellular destinations, this result confirmed that at least a major part of the expressed protein is present in the plant PM fraction.
Next, the transporter activity was determined. The lipid translocation assay in barley protoplasts suggested that Lr34res might be capable of internalizing PA, while exporting PS. Consequently, Lr34res, on the one hand, most likely acts as PS floppase and thereby reduces the amount of cytoplasmic PS and, on the other hand, might either directly flip PA from the exoplasmic to the cytoplasmic membrane leaflet or indirectly promote the accumulation of negatively charged PA to compensate for the reduced PS levels. The resulting elevation of the PA concentration at the inner PM leaflet by the activity of Lr34res could explain the up-regulation of PR genes in transgenic barley (12) and further stress responsive genes in Lr34res containing wheat varieties (13) through PA-mediated activation of the corresponding signaling pathways (14–16). Numerous reports have demonstrated that these cellular responses are highly dependent on the activity of specific phospholipases generating PA as a signaling molecule (28–30). Moreover, the loss of PS in the cytoplasmic membrane leaflet could cause a detachment of intracellular proteins with PS-binding motifs from the PM and thereby alter their activity. For instance, the evolutionarily highly conserved copines contain calcium-dependent PS-binding domains and play important roles in development and disease resistance, even though their precise molecular function is still unclear (31–33).
The investigation on the distribution of natural membrane lipids with specific PL-biosensors showed that a smaller portion of the expressed PS-biosensor (Lact-C2) is bound to the PM in Lr34res-expressing BY2 protoplasts compared with the control; instead, it accumulated in dot-like structures. Possibly, the fraction of Lr34res present in the Golgi and Golgi-derived vesicles on their way to the PM along the secretory pathway promotes PS translocation to the luminal membrane leaflet. Newly synthesized PS-binding biosensor molecules would therefore be able to bind these vesicles, explaining these intracellular dots. Interestingly, not only PS but also PI(4,5)P2 concentration was lowered in the cytoplasmic PM leaflet in Lr34res-expressing protoplasts, indicated by an enrichment of the corresponding biosensor in the cytosol and vacuoles. Reasons for this observation could either be a putative PI(4,5)P2 floppase activity of the transporter or altered PI(4,5)P2 metabolism either through enhanced enzymatic conversion by phospholipase C (PLC) or through reduced biosynthesis by PI(4)P-kinase, because the activity of these two enzymes depends on the cytoplasmic PS and PA concentrations, respectively, of the PM (34, 35). Without further investigations on the PI(4,5)P2 content and distribution in the PM, none of these possibilities can be excluded. However, an enhanced PLC-mediated conversion of PI(4,5)P2 to DAG and IP3 could explain several of the phenotypes associated with a strong expression of Lr34res in plant cells. As PIPs constitute only a minor portion of membrane lipids, it seems unlikely that PIP–PLC-derived DAG can be considered as the main cause of the observed lipid accumulation profile in transgenic Lr34res-expressing barley plants (11). Instead, PIPs and their degradation products such as IP3 play crucial roles as signaling molecules that among other things regulate intracellular Ca2+ levels (36), although the precise role of inositol phosphates in plants remains controversial (37). Nevertheless, PIP–PLC-induced Ca2+ signaling could take part in the activation of stress responses in Lr34res-expressing plants considering that numerous plant protein kinases working as activators of intracellular stress signaling pathways are calcium-dependent (38, 39). At the same time, the portion of DAG remaining intact in the membrane attracts C1 domain-containing proteins and thereby modulates their activity (40, 41). The most prominent example is protein kinase C (PKC), a universal regulator of signal transduction and cell proliferation in mammals that needs PS and DAG for its full activity (42). Interestingly, no PKC isoform has been found in plants, but evidence exists that other protein kinases compensate for its absence. For instance, phosphoinositide-dependent kinase PDK1 mediates responses to reactive oxygen species during root hair development and pathogen attack, and its activity is stimulated by PA, the phosphorylated form of DAG (43).
Further support for a possible connection of Lr34res with TAG homeostasis can be gathered from the yeast experiments. When heterologously expressed in an S. cerevisiae mutant that is defective in PL-transfer, Lr34res promoted the accumulation of neutral lipids in oil bodies. Oil body formation is not unique to yeast but is thought to occur in all eukaryotes and starts with the formation of microdomains at the endoplasmic reticulum (ER) and the local recruitment of lipid-biosynthesis enzymes. An increased production of neutral lipids, such as DAG, steryl esters, or TAG, at these sites promotes the extension and finally the conversion of these microdomains into oil bodies after their release from the ER (44). A defect in PL-transfer in the Sec14ts mutant per se causes the formation of oil bodies, which under our experimental conditions hardly accumulate in WT yeast cells. This observation could, for example, be explained by a reduction of PL delivery along the secretory pathway resulting in increased oil body formation for the storage of membrane lipid precursor molecules. Interestingly, the expression of Lr34res but not Lr34sus enhanced the density and size of oil bodies in this mutant. Because of similar protein expression levels, it seems likely that a different activity of the two transporter versions is responsible for the increased oil body content and size in Lr34res-expressing yeast cells. Presumably, Lr34res further impedes lipid transfer along the secretory pathway caused by its PL floppase activity and thereby enhances the phenotype of the Sec14ts mutant by promoting oil body formation. Alternatively, the enhanced lipid content in the yeast mutant might be derived from bulk membrane PL degradation by nonspecific PLCs indirectly stimulated by Lr34res-mediated PL redistribution, in analogy to the observations in Lr34res-expressing barley plants.
When heterologously expressed in an S. cerevisiae WT strain, Lr34sus and Lr34res but not the inactive transporter versions impaired growth, demonstrating that this effect is not caused by protein expression per se but by the activity of the transporters. On the one hand, a strongly enhanced ATP consumption in yeast cells could reduce the availability of metabolic energy required for growth, and on the other hand, an Lr34-mediated redistribution of certain PLs within the PM might affect cell division as cell cycle regulation is known to be closely connected to lipid metabolism (45). Possibly, PL-specific toxins interfere with cell cycle regulation in a similar manner resulting in a loss of clearly distinguishable lag, exponential, and stationary phases of growing yeast cells.
The functional analysis of Lr34 in yeast revealed that the sensitivity to papuamide A, which specifically binds exposed PS, was increased by the expression of both Lr34 versions. Consequently, Lr34sus and Lr34res could work as PS floppases and would both be able to expose PS in the yeast PM supporting the data of the lipid translocation assay using barley protoplasts. Even if (at least the major part of) the expressed Lr34 protein accumulates in intracellular vesicles (Fig. S1), PS distribution remains altered after fusion of these vesicles with the PM and therefore increases yeast sensitivity to papuamide A. This intracellular accumulation of Lr34 would further explain why no changes in sensitivity to miltefosine were observed as this cytotoxic compound needs to be actively exported at the PM. As a consequence, the question whether Lr34sus/res might be able to translocate lyso-PC remains open. However, differential growth effects were also observed for the PE-binding toxin duramycin because the expression of both native Lr34 versions decreased yeast sensitivity to the drug, pointing to a reduction of the PE level in the exoplasmic PM leaflet. Interestingly, NBD-PE was not found to be a potential substrate of Lr34res in barley protoplasts, which might be due to the different membrane PL composition in yeast and barley that potentially influences the flipping rate of certain PLs so that PE is not a preferred substrate in barley compared with yeast. Another reason might be the difference in the fatty acid moieties of NBD-labeled PE compared with natural PE species, which potentially affects its intrabilayer translocation.
In summary, our results of the PL translocation assays in plants and in yeast indicate that Lr34res (and Lr34sus) most likely work as PL floppases specific for PS, which is a known function of numerous related ABCG transporters associated with PS exposure in the PM in various organisms (4, 5). The putative translocation of PA and PE to the inner leaflet of the PM is thought to be a secondary compensatory effect either due to increased spontaneous diffusion (PA) or due to stimulation of P-type ATPases that transfer PE and PS to the inner side of the PM. This hypothesis is supported by the fact that ABC transporters have so far not been described as bidirectional PL translocators but instead as unidirectional PL flippases or floppases with more or less narrow substrate spectra (3–6).
Despite the strong evidence that Lr34 is a translocator for certain PLs (especially PS), a detailed analysis of the substrate spectrum can only be performed in proteoliposomes with pure Lr34 protein that is reconstituted in liposomes of a defined lipid composition. In this system, differences between Lr34sus and Lr34res in the affinity for certain PLs could also be determined, which would finally allow a putative functional discrimination of the two transporter versions for the elucidation of the exact molecular mechanism behind the Lr34res-mediated pathogen resistance. Given that many ABCG transporters can transport very diverse compounds, this system would also allow us to define whether Lr34 might have further transport activities.
Nevertheless, this work shows that the wheat ABC transporter Lr34 changes the lipid environment in the PM by exposing PS on the outer membrane leaflet and by redistributing further PLs in a direct or indirect manner. The resulting membrane rearrangement could be a crucial factor for the pathogen resistance mechanism, the activation of stress responses, the accumulation of neutral lipids, and the early induction of senescence in Lr34res-expressing plants.
Experimental procedures
Material
All chemicals were purchased from Sigma unless otherwise stated.
DNA cloning and constructs
The genomic DNA of Lr34 together with the 35S promotor and the N-terminal HA tag coding sequences was integrated into the p6U vector, as described previously (8), to obtain constructs for Agrobacterium tumefaciens-mediated transformation of BY2 cells (see below). The p6U vector contains an hpt cassette for hygromycin selection in target organisms. The phosphoinositide biosensor constructs P18R, P21R, P21Y, and P24R were obtained from NASC and were used directly for BY2 protoplast transfection. The PS biosensor Lact-C2 was created by PCR amplification of the C2 domain of lactadherin derived from the Lact-C2-GFP plasmid, which was a gift from Sergio Grinstein (Addgene plasmid 22852 (46)), for cloning into the GATEWAYTM (Invitrogen)-compatible pDONR207 vector. Plant expression constructs were obtained by subcloning into the destination vector pUBC-RFP (47).
For expression in yeast, NotI-restricted Lr34 cDNA, including a C-terminal coding sequence for an HA tag, was integrated into the pNEV-N vector (48) at the NotI restriction site to enable constitutive expression under the PMA1 promotor. Inactive Lr34 versions were generated by four consecutive PCR-based mutations of both Walker A and Walker B domains (Table 1) using the “Muta” primers listed in Table 2. N-terminal GFP fusion constructs of Lr34 for localization studies in yeast were created according to the FX cloning system protocol published by Geertsma and Dutzler (49). Briefly, Lr34 cDNA was amplified using the recommended primer design, integrated into the pINITIALKan donor vector, and subcloned into the destination vector pYEXNHG3. All DNA constructs were verified by Sanger sequencing.
Table 2.
Primers used in this study
fwd is forward, and rev is reverse.
| Primer | Sequence (5′-3′) | Experiment |
|---|---|---|
| Lact-C2-attB1_fwd | GGGGACAAGTTTGTACAAAAAAGCAGGCTCAATGTGCACTGAACCCCTAGGCCTG | PS-biosensor |
| Lact-C2-attB2_rev | GGGGACCACTTTGTACAAGAAAGCTGGGTCACAGCCCAGCAGCTCCAC | PS-biosensor |
| Lr34-Not1_fwd | GCGGCCGCATGGAGGGCCTCGCAAGAGA | Lr34 expression in yeast |
| Lr34-Not1_rev | GCGGCCGCTTACCTCTTCTGGAAATTAAGTTTCT | Lr34 expression in yeast |
| Lr34-pINKan_fwd | ATATATGCTCTTCTAGTGAGGGCCTCGCAAGAGAGACCAACCCA | Lr34-GFP localization in yeast |
| Lr34-pINKan_rev | TATATAGCTCTTCATGCCCTCTTCTGGAAATTAAGTTTCTCAAT | Lr34-GFP localization in yeast |
| Muta-WA1(Lr34)_fwd | CTGGATGTGCCGCTAGCACTCTGTTGCGAGCT | Inactive Lr34 versions |
| Muta-WA1(Lr34)_rev | GAGGTCCCAGTAGAAGAGTCAATCTGCAGGGTTTG | Inactive Lr34 versions |
| Muta-WA2(Lr34)_fwd | GAGCTGCCGCCACAACTCTACTAGATGTATTAGCAG | Inactive Lr34 versions |
| Muta-WA2(Lr34)_rev | CACTAACACCCATTAGTGCAGAAAGAACACCG | Inactive Lr34 versions |
| Muta-WB1(Lr34)_fwd | CATACTTTATGGCTGAAATATCAAATGGTCTGGATAG | Inactive Lr34 versions |
| Muta-WB1(Lr34)_rev | CACTTGCGGGGCCCACAATCATCTCGGCTGTG | Inactive Lr34 versions |
| Muta-WB2(Lr34)_fwd | CAACAACAGGTTTAGATACAAGG | Inactive Lr34 versions |
| Muta-WB2(Lr34)_rev | GTTCAGCCATTAGTATGATTGATGG | Inactive Lr34 versions |
Plant material
Tobacco BY-2 cells (Nicotiana tabacum L. cv. Bright Yellow 2) were grown in liquid LS-medium (Murashige-Skoog medium supplemented with 30 g/liter sucrose, 0.37 g/liter KH2PO4, 1 mg/liter thiamine-HCl, and 0.2 mg/liter 2,4-dichlorophenoxyacetic acid) at 25 °C under continuous dark conditions. For protein extraction and protoplast generation, cells were harvested 14 and 4 days, respectively, after sub-culturing. Transgenic barley plants (lines BG9 and BG9 sib (8)) were grown in standard soil under 12-h/day illumination (300 μmol m−2 s−1) at 25 °C.
BY2 cell culture and transformation
Suspension cultures of tobacco BY2 cells were maintained by weekly dilution (1:10) of cells into fresh LS medium according to Nagata et al. (50) and cultured at 25 °C with shaking at 130 rpm in the dark. The p6U vector containing Lr34res (genomic DNA) and N-terminal HA tag under the 35S promotor and the empty vector, respectively, were introduced by electroporation into the A. tumefaciens strain GV3101. Tobacco cells obtained from 50 ml of a 3-day-old culture were co-cultivated with 100 μl of an overnight culture of the transformed A. tumefaciens strain (adjusted to A600 = 1) on LS plates supplemented with acetosyringone (6.6 mg/liter) and hygromycin (30 mg/liter) for selection. After 2 days, cells were collected and washed three times with BY2 culture medium and were transferred to LS plates supplemented with cefotaxime (200 mg/liter) and hygromycin (30 mg/liter). After 4–6 weeks, the transformed BY2 cells developed calli that were checked for protein presence and used as starting material for liquid cultures.
Protoplast isolation and transfection
Leaf mesophyll protoplasts were prepared from 8-day-old transgenic (Lr34) and nontransgenic (sib) barley plants according to Kaiser (51) for NBD-lipid uptake experiments. Protoplasts of BY2 suspension cultured cells were prepared and transfected with phospholipid biosensor constructs as described in Miao and Jiang (52).
NBD-lipid uptake in barley protoplasts
Dipalmitoyl-PC (DPPC) and fluorescence-labeled 7-nitro-2–1,3-benzoxadiazol-4-yl)amino (NBD) palmitoyl-(NBD-hexanoyl)-PS (NBD-PS), palmitoyl-(NBD-hexanoyl)-PE (NBD-PE), palmitoyl-(NBD-hexanoyl)-phosphocholine (NBD-PC), palmitoyl-(NBD-hexanoyl)-phosphatidylglycerol (NBD-PG), and palmitoyl-(NBD-hexanoyl)-phosphatidic acid (NBD-PA) were obtained from Avanti Polar Lipids (Birmingham, AL). NBD-lipid stocks were prepared as vesicles by sonication consisting of 60 mol % DPPC and 40 mol % NBD-lipid in buffer A (25 mm Tris-HCl, pH 7.5, 5 mm EDTA, 0.25 m sucrose). Leaf mesophyll protoplasts from transgenic and nontransgenic barley plants were normalized by adjusting their chlorophyll content to 200 μg/ml and incubated in W5 buffer (160 mm KCl, 125 mm CaCl2, 5 mm glucose, and 2 mm MES, pH 5.7) containing lipid vesicles to a final concentration of 2.5 μm (NBD-PC/NBD-PG), 5 μm (NBD-PA/NBD-PS) or 10 μm (NBD-PE) at 25 °C and under mild agitation (300 rpm). Samples were taken at the indicated time points, and protoplasts were washed twice by resuspending and centrifuging (100 × g) in ice-cold W5 buffer supplemented with 3% (w/v) BSA to remove excessive lipids. All samples were boiled at 90 °C for 30 s after adding hot isopropyl alcohol for heat inactivation of phospholipases. The phospholipid extraction was continued according to Bligh and Dyer (53), and the lipid-containing chloroform/methanol phase was collected, dried, and resuspended in a small volume of chloroform before separation by thin-layer chromatography (TLC glass plates, prod-no: 100390, Merck) using chloroform/ethanol/water/trimethylamine (30:35:7:35, v/v/v/v (54)). NBD-lipid standards were chromatographed on the same plate. Fluorescent lipid spots were visualized with an Imager (Fusion FX6) and signal intensities were quantified with ImageJ.
Membrane fractionation of BY2 microsomes
Protoplasts were isolated from 14-day-old transgenic Lr34res-expressing BY2 suspension cells according to Miao and Jiang (52) in 2× cell volume enzyme solution for 2.5 h. The resulting protoplasts were collected by centrifugation (100 × g, 6 min), resuspended in 1× cell volume ice-cold buffer 1 (25 mm Tris-HCl, pH 7.5, 5 mm EDTA, 1× protease inhibitor mixture (PIC) (Roche Applied Science, catalog no. 11836170001)), and immediately homogenized with glass beads (1× cell volume) by vortexing (four times for 10 s, cooling in between). After removal of cell debris and glass beads (4000 rpm, 10 min, 6 °C) the supernatant was transferred to ultracentrifugation tubes to obtain the total membrane fraction by centrifugation (100,000 × g; 6 °C; 45 min). The pellet was resuspended in STED 10 buffer (10% (w/v) sucrose, 10 mm Tris-HCl, pH 7.5, 0.2 mm EDTA, 0.2 mm DTT) and loaded on a linear sucrose gradient (10–50% sucrose in STED buffer). After ultracentrifugation (16 h; 4 °C; 100,000 × g) 18 fractions were harvested, and their sucrose concentration was measured by refractometry before Western blotting.
Yeast strains and growth conditions
All growth assays were performed with the S. cerevisiae WT strain BY4741 (MATa his3 leu2 ura3 met15; EUROSCARF), and the S. cerevisiae mutant CTY1-1A (ura3 his3 lys2 sec14ts (25)) was used for investigations on oil body formation. Yeast cells were transformed by electroporation as described by Thompson et al. (55).
Yeast WT cultures were grown overnight (14–18 h) in selective SD medium containing glucose (0.7% yeast nitrogen base, 2% glucose, 1× synthetic dropout media lacking uracil (Sigma)) and freshly inoculated in the next morning at A600 = 0.1 in selective SD medium supplemented with 2% galactose and 1% raffinose instead of 2% glucose to induce protein expression. These cultures were grown to reach the exponential growth phase (A600 = 1–2 and 4–6 h) and then used for fluorescence microscopy.
For toxicity assays papuamide A (Flintbox, Lynsey Huxham) and duramycin (Sigma) were prepared as 1 mg/ml and 2 mm stock solutions, respectively, dissolved in DMSO. Miltefosine (hexadecylphosphocholine; Calbiochem) was prepared as 30 mg/ml stock solution dissolved in ethanol. Yeast cells were grown overnight in liquid SD medium containing 2% glucose allowing constitutive protein expression. These cultures were inoculated in the next morning at A600 = 0.1 in fresh medium and grown in 96-well plates at 30 °C and shaking at 260 rpm. When indicated, toxins or DMSO/ethanol as solvent controls was added to the samples at the respective concentrations. All experiments were repeated independently at least four times. The growth inhibitory effects of the toxins were defined as the percentage of growth reduction under toxin application using the formula (1 −(A600tox(t2)/A600tox(t1))/(A600con(t2)/A600con(t1)))·100 with A600tox(t2) defined as the A600 value at the end of the exponential growth phase (240 min) with toxin application, whereas t1 indicates the beginning of the exponential growth phase (120 min). The corresponding values were determined for the solvent controls (con).
Nile red staining of yeast cells
Cells were grown in selective SD medium for 16 h and harvested in the stationary phase. The A600 was adjusted to 1.0 before cells were stained for 15 min with Nile red (dissolved in DMSO, final concentration 5 μg/ml).
Yeast microsomal membrane preparation
Fresh yeast transformants were inoculated in selective SD medium and grown overnight at 30 °C with 160 rpm shaking, before inoculation into SD medium at A600 = 0.2. After another 6–7-h incubation period under the same conditions, cells were harvested by centrifugation at 3000 × g at 4 °C for 5 min, washed in ice-cold water, and resuspended 1:1 (w/v) in buffer 1 (see above). Cells were lysed by the addition of 1 volume of ice-cold acid-washed glass beads (0.5 mm), followed by three cycles of 30 s homogenization with the fastprep® cell disruptor (MP Biomedicals, Santa Ana, CA) and 3 min rest on ice between cycles. Samples were centrifuged at 3000 × g at 4 °C for 10 min to eliminate glass beads and cell debris. After a washing step, supernatants were combined, and total microsomal membrane fractions were collected by centrifugation (100,000 × g; 4 °C; 45 min) and homogenized in buffer 2 (10 mm MES, pH 7.8, 250 mm sucrose, 1× PIC).
Immunodetection
A rat monoclonal anti-HA–HRP-coupled antibody (Roche Applied Science, catalog no. 12013819001, anti-HA-peroxidase, high affinity, dilution, 1:1000) was used for Lr34-HA detection. BY2 organelle marker proteins were visualized by the consecutive development with primary antibodies from Agrisera (catalog nos. AS07 260-100, AS07 213, AS05 084A, and AS08 325) according to the manufacturer's recommendations and secondary HRP-coupled goat anti-rabbit IgG antibody (Santa Cruz Biotechnology, catalog no. sc-2400).
All blots were developed with luminol and enhancer solution (WesternBrightTM ECL HRP substrate, advansta), and signals were detected using a Fusion-FX6 imaging device. The PageRulerTM Plus Prestained protein ladder (ThermoFisher Scientific, catalog no. 26619) was used as standard for protein size estimation.
Fluorescence microscopy and signal quantification
A Leica SP5 II spectral confocal laser-scanning microscope (Leica Microsystems, Heidelberg, Germany) was used for fluorescence microscopy. For visualization of plant tissues, a ×40/1.2 N.A. oil immersion objective was used, and yeast imaging was done with a ×63/1.40 N.A. oil immersion objective. For Nile red visualization in yeast cells, the dye was excited with an argon laser at 488 nm, and emission was captured between 565 and 585 nm. Fluorescence signals were evaluated using the Leica software LAS X (version 1.1.0.12420). For the quantification of Nile red fluorescence as a measure of oil body content, individual yeast cells were defined as regions of interest (ROIs), and the pixel intensity sum of the ROI stack was calculated and divided by the number of pictures to obtain the mean pixel intensity. For the determination of the mean oil body size, the maximum oil body diameter in the stack was measured for each individual organelle in several yeast cells. GFP-tagged Lr34 protein was visualized in yeast by excitation at 488 nm and emission capture between 500 and 560 nm.
In plants, biosensor proteins with RFP were excited at 532 nm, and emission spectra were recorded between 580 and 630 nm. The citrine-tagged version P21Y was excited at 488 nm, and emission was captured between 510 and 570 nm. The middle planes (maximum diameter) of BY2 protoplasts, including PM signal, were defined as concentric circles (ROI1) to obtain the total fluorescence signal. ROI2 as a measure of the intracellular signal was defined as the inner concentric circle (ROI1) after subtraction of the PM signal from ROI2. The ratio of PM/total signal was calculated using the following formula: (ROI1 − ROI2)/ROI1. At least eight protoplasts showing comparable signal intensities were analyzed to compensate for fluctuations in organelle distribution. The dye FM4-64 was added to protoplasts at 17 μm for PM staining. FM4-64 was excited at 488 nm, and emission was captured between 660 and 710 nm.
Statistical analyses
Statistical analyses for NBD-lipid uptake in barley protoplasts, biosensor quantification in BY2 protoplasts, and oil body investigations in yeast were performed using a Student's t test with a two-tailed distribution and two-sample unequal variance. The significance of growth differences in the yeast toxicity assay was evaluated using one-way ANOVA analysis and post hoc t-tests (Bonferroni-corrected).
Author contributions
J .P. D. conceptualization; J. P. D., S. H., E. M., and R. L. L.-M. data curation; J. P. D. and R. L. L.-M. formal analysis; J. P. D. validation; J. P. D. and R. R. investigation; J. P. D. visualization; J. P. D., E. M., and R. L. L.-M. methodology; J. P. D. writing-original draft; J. P. D. and B. K. project administration; S. H., B. K., E. M., and R. L. L.-M. writing-review and editing; B. K. and R. L. L.-M. supervision; B. K. funding acquisition.
Supplementary Material
Acknowledgments
We thank Dr. Joohyun Kang for helpful advice regarding the generation of stable transgenic tobacco BY2 cell lines and Dr. Rainer Böni for providing transgenic Lr34res-expressing barley seeds. We also thank Dr. Undine Krügel for providing the S. cerevisiae Sec14ts mutant.
This work was supported by University of Zürich and ERC Advanced Grant Durable Resistance 249996. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1.
- ABC
- ATP-binding cassette
- PL
- phospholipid
- PM
- plasma membrane
- PDR
- pleiotropic drug resistance
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- TAG
- triacylglycerol
- PR
- pathogenicity related
- PLase
- phospholipase
- PA
- phosphatidic acid
- DAG
- diacylglycerol
- IP3
- inositol trisphosphate
- PLD
- phospholipase D
- ER
- endoplasmic reticulum
- HA
- human influenza hemagglutinin
- BY2
- bright yellow 2
- HRP
- horseradish peroxidase
- NBD
- 7-nitro-2–1,3-benzoxadiazol-4-yl
- PC
- phosphatidylcholine
- PG
- phosphatidylglycerol
- PIP
- phosphatidylinositol phosphate
- PLC
- phospholipase C
- PKC
- protein kinase C
- RFP
- red fluorescent protein
- ROI
- region of interest
- DPPC
- dipalmitoyl-PC
- ANOVA
- analysis of variance
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PI(3)P
- phosphatidylinositol 3-phosphate.
References
- 1. Higgins C. F. (2001) ABC transporters: physiology, structure and mechanism–an overview. Res. Microbiol. 152, 205–210 10.1016/S0923-2508(01)01193-7 [DOI] [PubMed] [Google Scholar]
- 2. Borst P., and Elferink R. O. (2002) Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71, 537–592 10.1146/annurev.biochem.71.102301.093055 [DOI] [PubMed] [Google Scholar]
- 3. Decottignies A., Grant A. M., Nichols J. W., de Wet H., McIntosh D. B., and Goffeau A. (1998) ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273, 12612–12622 10.1074/jbc.273.20.12612 [DOI] [PubMed] [Google Scholar]
- 4. Campos-Salinas J., León-Guerrero D., González-Rey E., Delgado M., Castanys S., Pérez-Victoria J. M., and Gamarro F. (2013) LABCG2, a new ABC transporter implicated in phosphatidylserine exposure, is involved in the infectivity and pathogenicity of Leishmania. PLoS Negl. Trop. Dis. 7, e2179 10.1371/journal.pntd.0002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woehlecke H., Pohl A., Alder-Baerens N., Lage H., and Herrmann A. (2003) Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2). Biochem. J. 376, 489–495 10.1042/bj20030886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smriti, Krishnamurthy S., Dixit B. L., Gupta C. M., Milewski S., and Prasad R. (2002) ABC transporters Cdr1p, Cdr2p, and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19, 303–318 10.1002/yea.818 [DOI] [PubMed] [Google Scholar]
- 7. Krattinger S. G., Lagudah E. S., Spielmeyer W., Singh R. P., Huerta-Espino J., McFadden H., Bossolini E., Selter L. L., and Keller B. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363 10.1126/science.1166453 [DOI] [PubMed] [Google Scholar]
- 8. Risk J. M., Selter L. L., Chauhan H., Krattinger S. G., Kumlehn J., Hensel G., and Lagudah E. S. (2013) The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol. J. 11, 847–854 [DOI] [PubMed] [Google Scholar]
- 9. Krattinger S. G., Sucher J., Selter L. L., Chauhan H., Zhou B., Tang M., Upadhyaya N. M., Mieulet D., Guiderdoni E., Weidenbach D., Schaffrath U., Lagudah E. S., and Keller B. (2016) The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnol. J. 14, 1261–1268 10.1111/pbi.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sucher J., Boni R., Yang P., Rogowsky P., Büchner H., Kastner C., Kumlehn J., Krattinger S. G., and Keller B. (2017) The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol. J. 15, 489–496 10.1111/pbi.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bucher R. R. (2017) Mass Spectrometry-based Metabolomic Approaches to Characterize Plant Pathogen Resistance in Cereals and the Flavonols Modifying Development in Arabidopsis thaliana. Doctoral dissertation, Universität of Zürich, Zürich [Google Scholar]
- 12. Chauhan H., Boni R., Bucher R., Kuhn B., Buchmann G., Sucher J., and Glauser G. (2015) The wheat resistance gene Lr34 results in the constitutive induction of multiple defense pathways in transgenic barley. Plant J. 84, 202–215 [DOI] [PubMed] [Google Scholar]
- 13. Hulbert S. H., Bai J., Fellers J. P., Pacheco M. G., and Bowden R. L. (2007) Gene expression patterns in near isogenic lines for wheat rust resistance gene Lr34/Yr18. Phytopathology 97, 1083–1093 10.1094/PHYTO-97-9-1083 [DOI] [PubMed] [Google Scholar]
- 14. Xue H., Chen X., and Li G. (2007) Involvement of phospholipid signaling in plant growth and hormone effects. Curr. Opin. Plant Biol. 10, 483–489 10.1016/j.pbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 15. Mishra G., Zhang W., Deng F., Zhao J., and Wang X. (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312, 264–266 10.1126/science.1123769 [DOI] [PubMed] [Google Scholar]
- 16. Zhao J. (2015) Phospholipase D and phosphatidic acid in plant defence response: from protein–protein and lipid–protein interactions to hormone signalling. J. Exp. Bot. 66, 1721–1736 10.1093/jxb/eru540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang J., Hwang J. U., Lee M., Kim Y. Y., Assmann S. M., Martinoia E., and Lee Y. (2010) PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. U.S.A. 107, 2355–2360 10.1073/pnas.0909222107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobae Y., Sekino T., Yoshioka H., Nakagawa T., Martinoia E., and Maeshima M. (2006) Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol. 47, 309–318 10.1093/pcp/pcj001 [DOI] [PubMed] [Google Scholar]
- 19. Crouzet J., Roland J., Peeters E., Trombik T., Ducos E., Nader J., and Boutry M. (2013) NtPDR1, a plasma membrane ABC transporter from Nicotiana tabacum, is involved in diterpene transport. Plant Mol. Biol. 82, 181–192 10.1007/s11103-013-0053-0 [DOI] [PubMed] [Google Scholar]
- 20. Simon M. L. A., Platre M. P., Assil S., Wijk R., Chen W. Y., Chory J., and Jaillais Y. (2014) A multi-colour/multi-affinity marker set to visualize phosphoinositide dynamics in Arabidopsis. Plant J. 77, 322–337 10.1111/tpj.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Märki F., Hänni E., Fredenhagen A., and van Oostrum J. (1991) Mode of action of the lanthionine-containing peptide antibiotics duramycin, duramycin B and C, and cinnamycin as indirect inhibitors of phospholipase A2. Biochem. Pharmacol. 42, 2027–2035 10.1016/0006-2952(91)90604-4 [DOI] [PubMed] [Google Scholar]
- 22. Andjelic C. D., Planelles V., and Barrows L. R. (2008) Characterizing the anti-HIV activity of papuamide A. Mar. Drugs 6, 528–549 10.3390/md20080027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parsons A. B., Lopez A., Givoni I. E., Williams D. E., Gray C. A., Porter J., and Wang J. (2006) Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell 126, 611–625 [DOI] [PubMed] [Google Scholar]
- 24. Hanson P. K., Malone L., Birchmore J. L., and Nichols J. W. (2003) Lem3p is essential for the uptake and potency of alkylphosphocholine drugs, edelfosine and miltefosine. J. Biol. Chem. 278, 36041–36050 10.1074/jbc.M305263200 [DOI] [PubMed] [Google Scholar]
- 25. Alder-Baerens N., Lisman Q., Luong L., Pomorski T., and Holthuis J. C. (2006) Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol. Biol. Cell 17, 1632–1642 10.1091/mbc.e05-10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bankaitis V. A., Malehorn D. E., Emr S. D., and Greene R. (1989) The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108, 1271–1281 10.1083/jcb.108.4.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen W., Zhang C., Song L., Sommerfeld M., and Hu Q. (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 77, 41–47 10.1016/j.mimet.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 28. de Jong C. F., Laxalt A. M., Bargmann B. O., de Wit P. J., Joosten M. H., and Munnik T. (2004) Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J. 39, 1–12 10.1111/j.1365-313X.2004.02110.x [DOI] [PubMed] [Google Scholar]
- 29. Wang X. (2005) Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 139, 566–573 10.1104/pp.105.068809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Testerink C., and Munnik T. (2011) Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J. Exp. Bot. 62, 2349–2361 10.1093/jxb/err079 [DOI] [PubMed] [Google Scholar]
- 31. Creutz C. E., Tomsig J. L., Snyder S. L., Gautier M. C., Skouri F., Beisson J., and Cohen J. (1998) The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J. Biol. Chem. 273, 1393–1402 10.1074/jbc.273.3.1393 [DOI] [PubMed] [Google Scholar]
- 32. Zou B., Hong X., Ding Y., Wang X., Liu H., and Hua J. (2016) Identification and analysis of copine/BONZAI proteins among evolutionarily diverse plant species. Genome 59, 565–573 10.1139/gen-2016-0015 [DOI] [PubMed] [Google Scholar]
- 33. Jambunathan N., Siani J. M., and McNellis T. W. (2001) A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13, 2225–2240 10.1105/tpc.13.10.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ananthanarayanan B., Das S., Rhee S. G., Murray D., and Cho W. (2002) Membrane targeting of C2 domains of phospholipase C-δ isoforms. J. Biol. Chem. 277, 3568–3575 10.1074/jbc.M109705200 [DOI] [PubMed] [Google Scholar]
- 35. Moritz A., De Graan P. N., Gispen W. H., and Wirtz K. W. (1992) Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J. Biol. Chem. 267, 7207–7210 [PubMed] [Google Scholar]
- 36. Dawson A. P. (1997) Calcium signalling: how do IP3 receptors work? Curr. Biol. 7, R544–R547 10.1016/S0960-9822(06)00277-6 [DOI] [PubMed] [Google Scholar]
- 37. Munnik T., and Testerink C. (2009) Plant phospholipid signaling: in a nutshell. J. Lipid Res. 50, S260–S265 10.1194/jlr.R800098-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung E., Park J. M., Oh S. K., Joung Y. H., Lee S., and Choi D. (2004) Molecular and biochemical characterization of the Capsicum annuum calcium-dependent protein kinase 3 (CaCDPK3) gene induced by abiotic and biotic stresses. Planta 220, 286–295 10.1007/s00425-004-1372-9 [DOI] [PubMed] [Google Scholar]
- 39. Ludwig A. A., Saitoh H., Felix G., Freymark G., Miersch O., Wasternack C., Boller T., Jones J. D., and Romeis T. (2005) Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. U.S.A. 102, 10736–10741 10.1073/pnas.0502954102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dries D. R., Gallegos L. L., and Newton A. C. (2007) A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J. Biol. Chem. 282, 826–830 10.1074/jbc.C600268200 [DOI] [PubMed] [Google Scholar]
- 41. Colón-González F., and Kazanietz M. G. (2006) C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim. Biophys. Acta 1761, 827–837 10.1016/j.bbalip.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 42. Johnson J. E., Giorgione J., and Newton A. C. (2000) The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry 39, 11360–11369 10.1021/bi000902c [DOI] [PubMed] [Google Scholar]
- 43. Anthony R. G., Henriques R., Helfer A., Meszaros T., Rios G., Testerink C., and Bögre L. (2004) A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J. 23, 572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy D. J., and Vance J. (1999) Mechanisms of lipid-body formation. Trends Biochem. Sci. 24, 109–115 10.1016/S0968-0004(98)01349-8 [DOI] [PubMed] [Google Scholar]
- 45. Jackowski S. (1994) Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 269, 3858–3867 [PubMed] [Google Scholar]
- 46. Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A., and Grinstein S. (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- 47. Grefen C., Donald N., Hashimoto K., Kudla J., Schumacher K., and Blatt M. R. (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 64, 355–365 10.1111/j.1365-313X.2010.04322.x [DOI] [PubMed] [Google Scholar]
- 48. Sauer N., and Stolz J. (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 6, 67–77 10.1046/j.1365-313X.1994.6010067.x [DOI] [PubMed] [Google Scholar]
- 49. Geertsma E. R., and Dutzler R. (2011) A versatile and efficient high-throughput cloning tool for structural biology. Biochemistry 50, 3272–3278 10.1021/bi200178z [DOI] [PubMed] [Google Scholar]
- 50. Nagata T., Nemoto Y., and Hasezawa S. (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int. Rev. Cytol. 132, 1–30 10.1016/S0074-7696(08)62452-3 [DOI] [Google Scholar]
- 51. Kaiser W. M. (1982) Correlation between changes in photosynthetic activity and changes in total protoplast volume in leaf tissue from hygro-, meso-, and xerophytes under osmotic stress. Planta 154, 538–545 10.1007/BF00402997 [DOI] [PubMed] [Google Scholar]
- 52. Miao Y., and Jiang L. (2007) Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2, 2348–2353 10.1038/nprot.2007.360 [DOI] [PubMed] [Google Scholar]
- 53. Bligh E. G., and Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 54. Weingärtner A., Kemmer G., Müller F. D., Zampieri R. A., Gonzaga dos Santos M., Schiller J., and Pomorski T. G. (2012) Leishmania promastigotes lack phosphatidylserine but bind annexin V upon permeabilization or miltefosine treatment. PLoS One 7, e42070 10.1371/journal.pone.0042070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thompson J. R., Register E., Curotto J., Kurtz M., and Kelly R. (1998) An improved protocol for the preparation of yeast cells for transformation by electroporation. Yeast 14, 565–571 10.1002/(SICI)1097-0061(19980430)14:6%3C565::AID-YEA251%3E3.0.CO%3B2-B [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.