Abstract
Simvastatin and CF680 dye encapsulated by stable nanodroplets has been developed for a drug delivery carrier. Simvastatin has previously been demonstrated as a potential degenerative disc disease (DDD) treatment drug. Multiple exposures of the nanodroplet to high-intensity focused ultrasound (HIFU) induced release of simvastatin. Each ultrasound exposure yielded a consistent concentration of the drug and dye released. B-mode ultrasound image analysis data and cavitation data clearly indicated the release mechanism is phase-transition of the liquid nanodroplets to gas bubbles. The nanodroplets were stably stored in ex vivo rabbit spine discs at least for 14 days and the contents responded to ultrasound exposure on demand. Lastly, nucleus pulpous cells harvested from rabbit spine discs and exposed to media with nanodroplets showed a decrease in cell viability (85%) relative to the cells only (96.7%) at 24 hr, but no difference at 48 hr. Thus, the system may be a potential DDD treatment.
Keywords: High-intensity focused ultrasound, On-demand drug release, Multiple releases, Degenerative disc disease
Introduction
Degenerative disc disease (DDD) refers to symptoms of back or neck pain caused by wear-and-tear on a spinal disc. Approximately 80% of adult population will experience low back pain associated with DDD throughout their lifetime (Health Quality 2006, Hicks, et al. 2009). Current therapeutic interventions for degenerated intervertebral discs (IVD) are intradiscal injections of nonsteroidal anti-inflammatory medications or epidural steroidal medications, which require repeated injections throughout the lifetime to reduce chronic pain (Mae T. Terada T 2012). These treatments last about 3 months depending on pain level. Open surgery is considered when these less invasive treatments, i.e. intradiscal injections, fail. Unfortunately, 20% of the patients are still in pain after surgery, and 7% to 15% develop the Failed Back Syndrome, resulting in for aminal stenosis, painful disc, and pseudarthrosis (Schofferman, et al. 2003, Volpentesta, et al. 2014).
Recent emphasis has been directed at the reversal of disc degeneration or replacement of the affected disc by utilizing biological materials, including growth factors, stem cells, and gene transplant (Taher, et al. 2012, Than, et al. 2014). We have previously shown that simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, enhances chondrogenesis of intervertebral disc cells, which in turn facilitates the repair of degenerative IVD in a developed animal model (Than, et al. 2014). However, the efficacy associated with local simvastatin injection can be transient if adequate vehicles are not used to provide sustainable release. There is a critical need to assess feasible options that can deliver hydrophobic simvastatin and control the release site-specifically, especially when the disease condition changes over time.
Ultrasound-mediated drug release is a potential solution. High intensity focused ultrasound (HIFU) generates pressure waves that can phase-transition liquid perfluorocarbon droplets to gas bubbles, a process called acoustic droplet vaporization (ADV) (Fabiilli, et al. 2009, Kripfgans, et al. 2000, Sheeran, et al. 2011). When the droplets undergo ADV, phase-transitioned gas bubbles can release the drug (Fabiilli, et al. 2010, Fabiilli, et al. 2010, Moncion, et al. 2017, O’Neill and Rapoport 2011, Rapoport, et al. 2010, Rapoport, et al. 2009, Sheeran, et al. 2011), and the bubbles can be detected by ultrasound imaging because of their echogenicity (Fabiilli, et al. 2009, Goldberg 1996, Park, et al. 2012). In this study, we investigated the potential of utilizing a double emulsion technique to stably encapsulate hydrophobic simvastatin in perfluorocarbon liquid droplets at body temperature and release the simvastatin at select times with consistent dosage using multiple temporally-spaced HIFU exposures. The cytotoxicity of the nanodroplets in physiological conditions and triggered release in ex-vivo rabbit discs was also investigated. The study addresses an important gap in knowledge of developing and quantifying hydrophobic drug delivery in vitro using HIFU for multiple uses.
Materials and Methods
Nanodroplet Materials and Synthesis
The nanodroplets were synthesized via a double emulsion process of drug-containing water droplets in perfluorocarbon, which was emulsified in water. The first emulsion of water droplet containing simvastatin powder at a concentration of 100 mg/mL and a total mass of 2.4 mg was dispersed in liquid perfluorpentane (1.55 mL) (Synquest Laboratories, Alachua, FL, USA) with Krytox (150 uL) (DuPont, USA) as polymer surfactant via probe sonication (Vibra Cell 400, Sonics, Newtown, CT, USA) at amplitude of 20%, 3 min with 10 s on and 20 s pause in an ice bath. It should be noted that simvastatin powder was used rather than solubilized simvastatin because of the low solubility of hydrophobic simvastatin in water. Care was taken to ensure that the simvastatin powder was uniformly dispersed in the water prior to emulsification to form water droplets in perfluoropentane. The second emulsion was then created by adding liposomes in DI water to the first emulsion and probe sonicating in an ice bath in the same manner. The liposome components, consisted of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (Avanti Polar Lipids, Inc., Alabaster, AL, USA) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000] (DSPE-PEG 5K) (Nanocs, Boston, MA, USA) at 85:15 mole ratio dissolved in chloroform (10 mg/ml). The chloroform was evaporated in a chemical hood. The dried film was hydrated with DI water at 5.62 μmol/mL and probe-sonicated at room temperature for 10 minutes to form liposomes. The resulting solution was washed three times by centrifugation (2700 rpm) and replacing the supernatant with DI water. The final product consists of water droplets in perfluoropentane droplets dispersed in water (W1/O/W2). Simvastatin was substituted with a dye (Rhodamine B or CF680), or co-encapsulated with a dye in the W1 phase.
Characterization of Nanodroplets - Optical Imaging, Spectroscopy
Differential interference contrast (DIC) and fluorescence microscopy with an oil-immerse objective lens x63 (Carl Zeiss, Inc., Oberkochen, Germany) were used to determine drug/dye encapsulation of the nanodroplets optically. Dynamic light scattering (DLS) (NanoBrook Omni, Brookhaven Instruments Cooperation, Holtsville, NY, USA) was used to determine the hydrodynamic diameter of the nanodroplets. A UV-vis/fluorescence spectrometer (SpectraMax, Molecular Devices, LLC San Jose, CA, USA) was used to detect simvastatin and CF680 dye by observing peaks at 237 nm (Alvarez-Lueje, et al. 2005)’ (Merienne, et al. 2017) for UV and 680 nm for fluorescence, respectively.
Stability Test
Nanodroplets in DI water or cell culture medium (Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin-Streptomycin Solution) were stored in an incubator at 37 °C for two weeks. Every three days, 10 μL of droplets from each vial was withdrawn, and diluted 1000 times. Diluted droplets (10 μL) were put into a hemocytometer and counted using a microscope with an objective lens x40 in phase-contrast mode (Axio Observer A1, Carl Zeiss, Inc., Oberkochen, Germany). Due to the diffraction limit in resolution, particle sizes less than 400 nm were not considered. Dynamic light scattering (DLS) was used to monitor the stability of the nanodroplets against aggregation, rupture, or dissolution by size measurement. (Rovers, et al. 2015) When the nanodroplets undergo phase-transition, multiple peaks in the size distribution appear corresponding to droplets, bubbles (from droplets that phase transition), and shell fragments after dissolution. Bubbles are anticipated to be greater than 1 μm (Bardin, et al. 2011), shell fragments less than 100 nm (Rovers, et al. 2015), and droplets near 400~500 nm. The particle size range that the DLS can measure is from < 0.3 nm to 10 μm. Nanodroplets (150 μl) in water solution was diluted in 1.35 ml of DI water for the measurements. Three measurements were taken for each sample and averaged over time for 21 days.
Ultrasound exposure of nanodroplets
The nanodroplets encapsulating dye or drug were placed in a 1.5 mL conical tube. The conical tube was placed in the focal volume of an ultrasound transducer (H104, Sonic Concepts, Bothell, Washington, USA) using pulse-echo alignment and exposed to ultrasound in a tank of degassed water at 37 °C. The ultrasound had a center frequency of 500 kHz, peak negative pressure of 3 MPa (free field), pulse duration of 10 cycles, and pulse repetition frequency of 100 Hz. Each sample was insonified using the following exposure schemes.
Single exposure
Each sample vial had a volume of 2 mL, and was exposed to ultrasound for 2 minutes. The control sample (sham exposure) was placed inside of the tank, but was not exposed to ultrasound. Each sample was centrifuged at 4000 g for 3 minutes to collect released dye/drug in the supernatant.
Multiple exposures
Each sample underwent the same procedure as a single exposure. However, after centrifugation and removal of 300 μL of the supernatant, 300 μL of DI water was added to re-suspend the pellet. Ultrasound exposure, centrifugation, and reservation of the supernatant were repeated three more times. Control trials (sham exposure) were performed by repeating the above procedure, but setting the ultrasound exposure to 0 MPa.
UV-Vis absorbance for drug release analysis
The supernatant from ultrasound exposure was mixed with the same volume of acetonitrile (i.e. 1:1 (v/v) ratio of water and acetonitrile) to dissolve simvastatin possibly in the lipid shell debris and determine the concentration of simvastatin. Acetonitrile was used to disrupt the lipid aggregation structure because it is a commonly used solvent in HPLC (High Performance Liquid Chromatography) analysis which uses UV-Vis for concentration quantification (Reis, et al. 2013). The mixture was transferred into a well of a UV transparent 96-well plate, and its optical absorbance was measured at 237 nm using a UV-Vis plate reader (SpectraMax, Molecular Devices, LLC, San Jose, CA, USA). The concentration of simvastatin was calculated from the measured optical absorbance based on a standard calibration (Supporting Information).
IVIS (In vivo imaging system) for dye release analysis
A Bruker MultiSpectral FX (Bruker, Carestream) Fluorescence/X-ray Multimodality Imaging system was used to visualize dye CF680 release from the nanodroplets by ultrasound exposure. MATLAB was used to quantitatively analyze the average intensity within each vial.
B-mode ultrasound imaging & cavitation imaging
B-mode and passive cavitation data were acquired using an L7-4 linear array (Philips, Bothell, WA, USA) and Vantage 256 ultrasound research scanner (Verasonics, Kirkland, WA, USA). The L7-4 linear array provides millimeter to sub-millimeter lateral resolution (Haworth, et al. 2017). Additionally, because the fundamental insonation frequency (500 kHz) is well outside the bandwidth of the L7-4 linear array, the receive gain can be increased without saturating the analog to digital converters. The increased receive gain improves the signal-to-noise ratio of the measurement. The average echogenicity of a region of interest (ROI) in the B-mode image of each centrifuge tube containing droplets before, during, and after ultrasound exposure was computed for each of the 4 ultrasound exposures performed and for the 4 sham (no ultrasound) exposures. For each exposure, 5 samples were insonified (i.e., n=5). In preliminary experiments, harmonics were observed from 1.5 MHz to 8.5 MHz, however a drop in broadband emissions was observed at approximately 2 MHz and 8 MHz. Therefore passive cavitation images were produced (Haworth, et al. 2017) to quantify the magnitude of cavitation activity integrated over all frequency between 2 and 8 MHz during each of the ultrasound exposures (and the sham exposures). The total power in an ROI in the passive cavitation image of each centrifuge tube was computed.
Emulsion stability and ultrasound response in ex-vivo rabbit discs
A 9-10 week old female New Zealand White rabbit was sacrificed, and the spine was extracted. All the surgery procedures followed an approved animal IACUC protocol from the University of Cincinnati, Laboratory Animal Medical Services. Dye CF680R-encapsulated nanodroplets were injected in the intervertebral discs. After 14 days of incubation, ultrasound with the same setup described in Section 2.4 was applied to the nanodroplets within the disc space. IVIS was performed to confirm injection of the nanodroplets within the disc space and the feasibility for storage of the nanodroplets within the space overtime.
Cytotoxicity in in vitro rabbit nucleus pulposus cells
The lumbar vertebral column from a female Sprague Dawley rat (250-300 grams) was removed and placed in an ice cold PBS (10 mM PO43−, 137 mM NaCl, and 2.7 mM KCl). All surgical procedures followed an approved animal IACUC protocol at the University of Cincinnati, Laboratory Animal Medical Services. The vertebral column was transferred to a 100 mm petri dish with PBS on ice. The vertebral bodies L1/2, L2/3, L3/4, and L4/5 were dissected and the intervertebral discs removed from the surrounding dense tissue, cartilaginous endplates, and outer annulus fibrosis from each vertebral body. Under sterile conditions, the discs were transferred to a 100 mm petri dish on ice containing 25 mL of equal parts of Dulbecco’s Modified Eagle Medium and Ham’s F-12 medium (DMEM/F12) containing 5% heat-inactivated fetal bovine serum (FBS) with 0.2% pronase and 0.004% deoxyribonuclease II Type IV. Using a scalpel, discs were minced into pieces of approximately 2 mm3 in volume. The samples contained within the solution were poured into a 50 mL centrifuge tube and placed into a 37 °C CO2 incubator for 1 hour under gentle agitation. A small pellet was formed at the bottom of the tube and the solution was carefully aspirated so as to not disturb the tissue pellet. The pellet was re-suspended in 25 mL of DMEM/F12 containing 5% heat-inactivated fetal bovine serum (FBS) with bacterial 0.02% collagenase Type II and 0.004% deoxyribonuclease II Type IV. The sample tube was placed into a 37 °C CO2 incubator overnight. After incubation, the pellet formed at the bottom of the tube was gently mixed then poured over a sterile 70 μm nylon mesh filter into a new sterile 50 mL Falcon tube. 20 mL of DMEM/F12 medium, 10% FBS, and 1% antibiotics was added to the 50 mL tube. The media containing filtered NP cells was gently mixed then seeded into a 75 cm2 flask at a density of 2.5 × 104 cells/cm2, and then placed into a 37°C CO2 incubator for 24 hours.
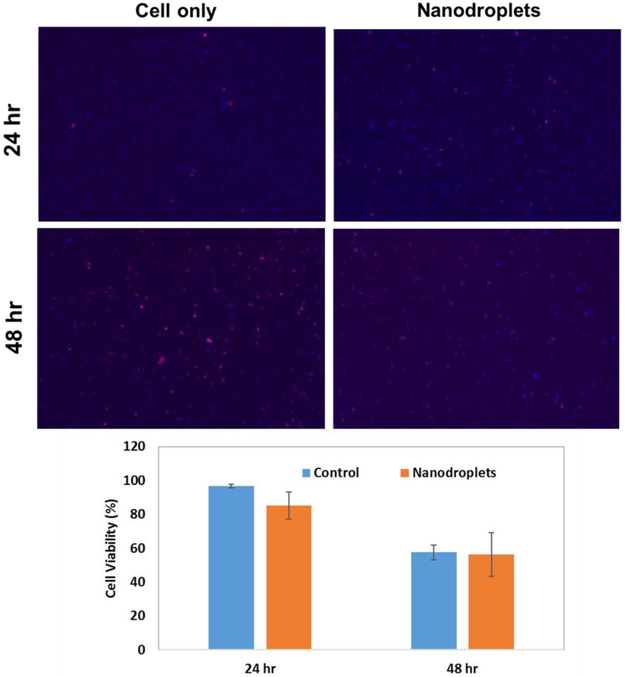
ReadyProbes™ Cell Viability Imaging Kit, Blue/Green was used to assay cytotoxicity (cell viability) of the nanodroplets. Hoechst 33342 (blue filter) and NucGreen® Dead reagent (green filter) were used for live and dead cell staining, respectively. Red color In Figure 6 was used instead of green for dead cells for color contrast using ImageJ software (National Institute of Health, Bethesda, MD). Cell viability was determined by counting the number of live or dead cells using ImageJ. Cell viability (%) was determined as 100 times the number of live cells divided by the total number of cells (live cells plus dead cells). The assay was performed in triplicate.
Figure 6.
Cytotoxicity of the nanodroplets compared to control (cells only) measured after 24 h and 48 h of incubation following cell harvest. The top images are representative images of the Live (blue)/Dead (red) assay. The bottom plots provide the average cell viability across all trials (n=3).
Statistical Analysis
2-way ANOVA with either Tukey’s Honest Significant Difference or Sidak’s multiple comparison test was used to statistically analyze data for drug/dye release, B-mode echogenicity, and cavitation activity. Student’s t-test was used for the cytotoxicity data. The error bar values are one standard deviation.
Results
Characterization and Stability of Nanodroplets
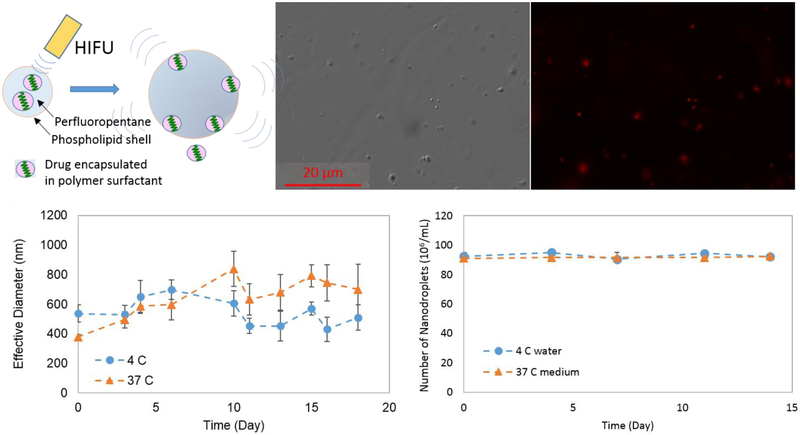
The average size of the nanodroplets measured by dynamic light scattering was ~500 nm in diameter (Figure 1C at Day 0). The nanodroplets observed in the DIC optical image show the size 300 ~ 500 nm in diameter.
Figure 1.
(A) Schematic of drug release from the nanodroplet via phase-transition by HIFU trigger. (B) DIC (Differential Interference Contrast) and fluorescence optical images (63x magnification). (C) Effective (hydrodynamic) diameter measured by DLS over time at two different temperatures. (D) The number of nanodroplets counted over time at 4 °C and at 37 °C in the medium using phase-contrast.
When Rhodamine B dye was co-encapsulated with simvastatin, the encapsulation inside the nanodroplets was visualized by fluorescence images (Figure 1B, red color in the nanodroplets). UV-vis data also proved presence of simvastatin and Rhodamine B associated with the nanodroplets by showing peaks at a wavelength of 237 nm and 555 nm, respectively (Merienne, et al. 2017, Stobiecka and Hepel 2011).
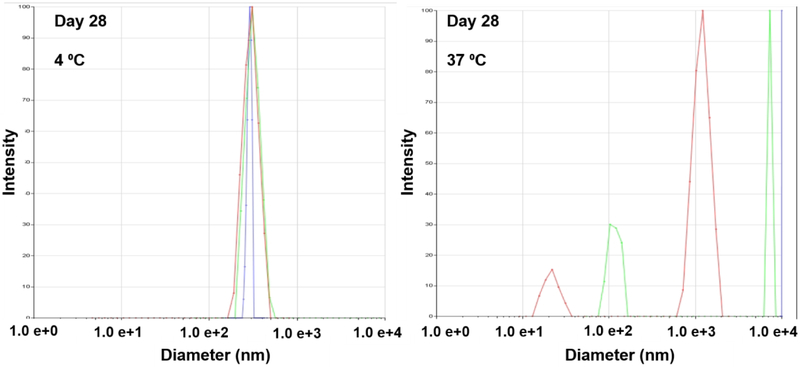
Only a single peak around 400 nm was observed in the size distribution measured by DLS after storing the droplets in 4 °C deionized water for 28 days. The lack of larger and smaller peaks indicate that no aggregation, dissolution, or fragmentation occurred (Figure 2). A single peak was observed at 18 days when the droplets were stored in 37 °C deionized water (data not shown). In addition, the number of nanodroplets greater than 400 nm did not change for at least 14 days at 4 °C in water and 37 °C in the medium, determined optically (Figure 1D). The size distribution of the nanodroplets stored at 37°C on day 28 showed peaks at greater than 1000 nm and at 100 nm and smaller (Figure 2), suggesting that the nanodroplets undergo a phase-transition showing debris and bubbles.
Figure 2.
Dynamic light scattering data: size distribution of the nanodroplets stored at 4 °C and 37 °C for 28 days. Different colored curves indicate each measurement (n=3).
Multiple exposure drug/dye release by ultrasound
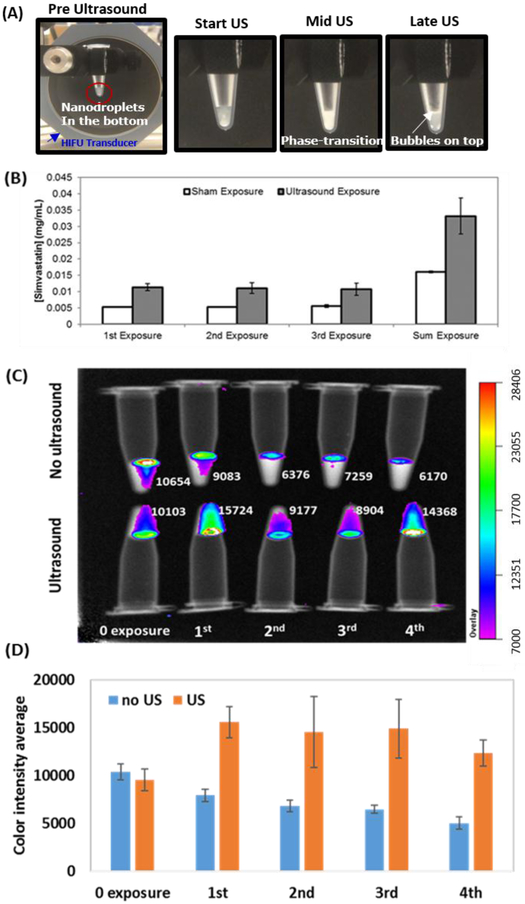
When the nanodroplets were exposed to HIFU, the optical turbidity of the solution increased (Figure 3A) suggesting acoustic droplet vaporization occured(Kripfgans, et al. 2000, Zhang and Porter 2010). Bubbles were also observed at the top of the fluid after insonation. Each of the multiple ultrasound exposures resulted in an increase in the measured simvastatin (Figure 3B) or CF680 dye (Figure 3C) relative to sham (no ultrasound) exposures. Figure 3B shows that approximately 11 μg/ml of simvastatin was released following each exposure. The sham exposure had a release of approximately 5 μg/ml. The difference in the concentration of simvastatin released between the sham and US exposure groups was significant (ANOVA, p-value of 0.0026). The difference in the concentration between the two groups for each individual US insonation was also determined to be significant (p-values of 0.0019, 0.0054, and 0.0073 for 1st, 2nd, and 3rd exposure, respectively). Each exposure released approximately 1% of the total amount of encapsulated simvastatin (1.24 mg/mL).
Figure 3.
Release of drug and dye following multiple US exposures (A) Photographs of nanodroplets triggered by HIFU (B) simvastatin concentrations in the supernatant measured after HIFU exposure. Sham exposure (n=2), Ultrasound exposure (n=4). (C) Representative IVIS images and color intensity analysis for dye release. The numbers next each sample represent relative dye concentrations. (D) Average color intensity within each tube (n=3).
IVIS measurements indicate that more CF680 dye was released upon US exposure relative to sham exposure (Figure 3C and D). Similar to the simvastatin results, consistent amounts of dye was released after each ultrasound (US) exposure. The p-values between the US and sham groups for each individual exposure were 0.1944, 0.1846, 0.1261, and 0.2252 for 1st, 2nd, 3rd, and 4th exposure, respectively. Large standard deviations may explain why the p-values were not significant despite nearly twice as much simvastatin being released with ultrasound relative to no ultrasound exposure. The color intensity decreases with increasing sham exposures, likely due to the dilution that occurs when the supernatant is replaced with DI water.
B-mode echogenicity and cavitation activity
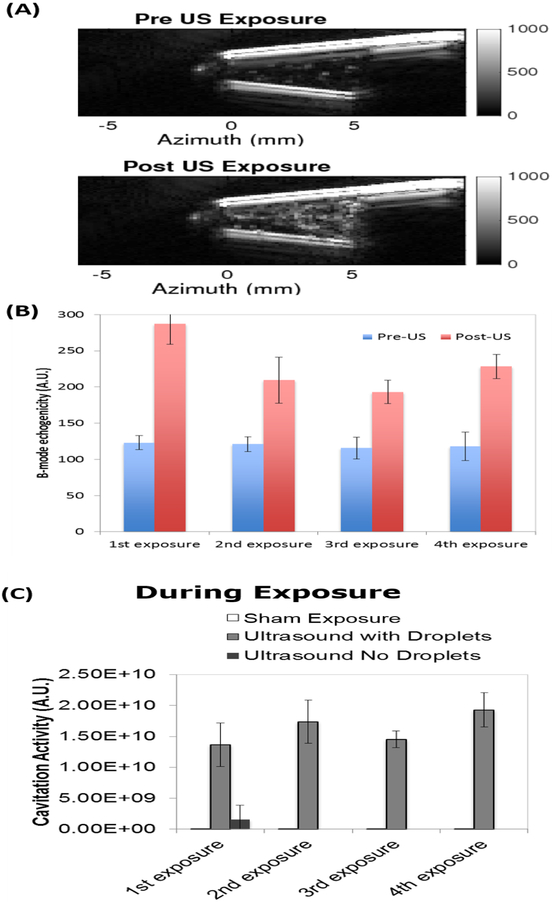
After ultrasound exposure, the echogenicity was significantly higher than before US exposure (p ≤ 0.0002), based on ultrasound B-mode analysis (Figure 4A and B), indicating that microbubbles were produced by phase-transitioning the liquid perfluorocarbon core of the nanodroplets. The echogenicity was also significantly greater after ultrasound exposure as compared to after sham treatment (p < 0.0001). Even after four exposures, the post-US showed consistently increased echogenicity, indicating that not all of the droplets had been converted into bubbles. No change in echogenicity was observed in deionized water without nanodroplets.
Figure 4.
(A) Ultrasound B-mode images pre and post US exposure; (B) The average B-mode echogenicity within the tube is shown before (blue) and after (red) US exposure. (C) Cavitation activity of the nanodroplets before, during and after US and sham exposures. Measurements were made with and without droplets.
The passive cavitation spectra acquired during US exposure with the nanodroplets showed no harmonics, potentially indicative of strong inertial cavitation (data not shown). Passive cavitation images acquired during US exposure had five orders of magnitude more cavitation in the centrifuge tube as compared to images acquired during the sham exposure (Figure 4C), which was statistically significant (p<0.0001 for all comparisons). The sham exposure values were similar to the pre and post ultrasound exposures, and the ultrasound exposure of the centrifuge tube without droplets (p>0.9999 for all time points within each exposure). The measured cavitation activity pre- and post-exposure are indicative of the noise floor of the system. This data indicates that microbubbles were formed and cavitated during insonation of the nanodroplets. In Figure 4C, a relatively high cavitation activity was observed for the pre-US measurement for the first exposure. Manual observation of the ultrasound B-mode image shows a few sparse bubbles were present and may have been nuclei for the increased cavitation activity.
Emulsion stability and the ultrasound response in ex vivo rabbit discs
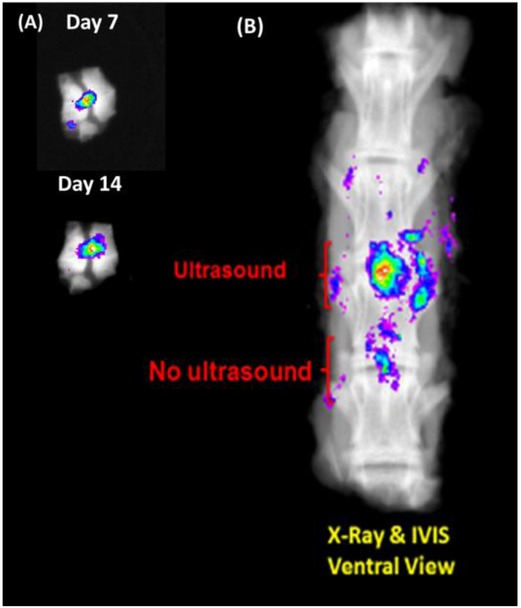
Dye-encapsulated nanodroplets injected in ex-vivo rabbit discs were incubated at 37 °C and monitored using IVIS 7 and 14 days after incubation began (Figure 5A). The results demonstrate that the dye was stored in the discs for at least 14 days. The nanodroplets injected in the whole ex-vivo spine with ultrasound exposure and no exposure imaged using IVIS after 14 days of incubation also demonstrate the storage stability in the ex-vivo disc at least for 14 days (Figure 5B). The dye remained active after ultrasound exposure.
Figure 5.
(A) IVIS images of dye-encapsulated nanodroplets in ex-vivo rabbit disc at day 7 and 14 of incubation. (B) Ex-vivo test of HIFU-trigger of dye-encapsulated nanodroplets 14 days of incubation after injection in the disc.
Cytotoxicity
Cytotoxicity of the nanodroplets was assessed on nucleus pulpous (NP) cells harvested from the rabbit intervertebral discs. At 24 h, cell viability with and without nanodroplets was 85% ± 4.3% and 96.7% ± 1.1%, respectively (n=3). The difference was statistically significant (p-value = 0.011). After 48 hr of incubation, the cell viability was not significantly different between cells incubated with and without nanodroplets (56.3% ± 12.9% and 57.5% ± 7.9%, respectively; p-value=0.463) (Figure 6).
Discussion
Overall, the results from this study demonstrate that a stable nanodroplet emulsion can release drug as a result of multiple ultrasound exposures. According to Antoine equation with elevated pressure inside the nanodroplet due to Laplace pressure (Sheeran, et al. 2011), the boiling point of the perfluoropentane nanodroplet is 32.7 °C. The temperature value does not fully explain why the nanodroplets did not go through a phase-transition during the first 14 days of incubation at 37 °C. Thus, we believe the stability of nanodroplets (i.e., reduced spontaneous vaporization) is in part due to metastability of the superheated perfluorocarbon against bubble nucleation (Delale, et al. 2003, Mountford, et al. 2015). Over time, if droplets aggregate and merge, it is possible that the boiling point of the larger particles decreases to 37 °C or less, and the nanodroplets are no longer stable. Furthermore, we have noticed that mechanical forces such as pipetting, stirring and centrifugation also affected the stability. These phenomena are consistent with observations from other studies (Rapoport, et al. 2009), demonstrating that shear stresses due to pipetting can cause a phase-transition. For clinical applications for DDD, the nanodroplets will not be affected by mechanical forces except during injection because they will be isolated in the discs.
Both B-mode and passive cavitation imaging provided feedback of the liquid nanodroplet phase-transition into gas microbubbles. Ultrasound imaging has been adopted to monitor needle-based injections of therapeutics and also to confirm the phase-transition of nanodroplets to bubbles (Kopechek, et al. 2013, Rapoport, et al. 2011, Rapoport, et al. 2009). If a strong correlation between drug release and the number of phase-transitioned droplets exists, then the B-mode imaging could be used as a non-invasive metric for drug release.
Each ultrasound exposure demonstrated consistent release of both a drug and a dye. Approximately twice as much drug release occurred for ultrasound exposures relative to sham exposures (Figure 3B). A similar ratio was observed for dye release (Figure 3D). The dye released before the first ultrasound exposure (Figure 3D) could be partly due to passive leakage from the droplets or imperfect separations between the supernatant and the nanodroplets. A previous study has shown that degenerative discs treated with 5 mg/mL of simvastatin in hydrogel demonstrated higher gene expression of aggrecan and collagen type II than control (Than, et al. 2014). (Dosage lower than 5 mg/mL of simvastatin has not been tested for chondrogenesis yet.) In this study, 0.012 mg/mL of simvastatin per each insonation was released, which is 1/100th of the sample. Although the concentration is lower than 5 mg/mL, we have shown consistent amount of simvastatin was released by each ultrasound exposure, meaning multiple exposures will lead to higher dosage effective for chondrogenesis. Please also note that in the cited report, 5 mg/mL of simvastatin was loaded in a biodegradable hydrogel and released in a controlled manner over a 12-week time span. The transient, effective dose to obtain chondrogenesis would be lower than 5 mg/mL. Since, further in vivo investigation will be warranted to evaluate the required ultrasound exposure of the proposed delivery for an efficacious outcome. Furthermore, modification of the droplet properties and ultrasound insonation parameters may lead to a greater droplet vaporization efficiency, resulting in more drug release (Fabiilli, et al. 2010)).
It should be noted that the nature of the released dye is different from simvastatin, likely due to the relative hydrophobicity of simvastatin as compared to the dye. Solubilities of simvastatin and CF680 dye in water are −0.03 mg/mL (Sigma-Aldrich) and >100 mg/mL (Biotum), respectively. When released, the simvastatin likely precipitated. The occurrence of precipitation is supported by the fact that the post-US simvastatin concentration matched the sham concentration when the ultrasound exposed sample was centrifuged at 10,000 g for 5 min. However the dye concentration was significantly higher following ultrasound exposure and centrifugation at 10,000 g at 5 min than the sham conditions. The molecular weight of the dye and simvastatin are 3,000 Da and 419 Da, respectively.
A limitation associated with this study is that the difference in molecular weight and hydrophobicity of the drug and dye may require different centrifugation parameters to isolate the released drug or dye in the supernatant versus drug and dye in the droplets. Additionally, it is possible that the hydrophobic drug molecules may have been associated with lipid monolayers following ultrasound exposure, which would affect the ability to separate the released drug from the encapsulated drug using centrifugation and may affect the drug’s bioavailability. To better understand this study limitation, we measured the size of particles in the supernatant using dynamic light scattering after 2 min of centrifugation at 2,000, 4,000, 6,000, 8,000, and 10,000 g. A peak around 70 to 80 nm was observed following 2,000 g and 4,000 g centrifugation. The peak disappeared when using 6,000 g. At 2,000 g and 4,000 g more drug was measured using UV absorbance in the supernatant following US exposure relative to sham exposure. The results imply some drug molecules are still encapsulated in debris after phase-transition and depending on the size of the debris, different centrifugation speeds and times may be needed to isolate these drug-loaded debris. This study used 4,000 g as a balance between ensuring that nanodroplets accrued in the pellet while any drug-loaded debris remained in the supernatant.
The ex-vivo disc study suggests the feasibility of injecting the nanodroplets into the discs. The dye remained in the disk for 14 days at 37 °C. It also implies that the fluorescent nanodroplets can be monitored after ultrasound exposure using IVIS techniques for future in vivo animal experiments.
The in vitro data with the harvested nucleus pulpous (NP) cells demonstrated that the nanodroplets are not highly cytotoxic. Minimal cytotoxicity was observed after 24 h of incubation with the nanodroplets. The live cell population naturally decreases because in vitro conditions are not favorable for NP cells. Another limitation of this study is the uncertainty of the bioavailability of free simvastatin versus simvastatin that may be associated with lipid particles following droplet vaporization. It has previously been observed that drugs encapsulated in lipid micelles can be delivered to cells (Park, et al. 2008). However, because NP cells in culture may not behave identically to in vivo NP cells, the bioavailability should be determined in vivo experimentally in future studies.
Conclusion
Overall, we have developed stable nanodroplet emulsion that can be activated multiple times by ultrasound (HIFU). The activation can be monitored by ultrasound imaging by analyzing B-mode ultrasound and passive cavitation images. The nanodroplets were demonstrated to be used potentially as a long-term drug carrier for disc diseases along with great stability in ex-vivo conditions, low cytotoxicity, and controlled triggered release. This new drug delivery system will be applied to a degenerative disc disease model in the future.
Supplementary Material
Acknowledgments
This study was partially supported by NIH/NIAMS R01AR056649.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Lueje A, Valenzuela C, Squella JA, Nunez-Vergara LJ. Stability study of simvastatin under hydrolytic conditions assessed by liquid chromatography. Journal of AOAC International 2005; 88:1631–6. [PubMed] [Google Scholar]
- Bardin D, Martz TD, Sheeran PS, Shih R, Dayton PA, Lee AP. High-speed, clinical-scale microfluidic generation of stable phase-change droplets for gas embolotherapy. Lab on a Chip 2011; 11:3990–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delale CF, Hruby J, Marsik F. Homogeneous bubble nucleation in liquids: The classical theory revisited. The Journal of Chemical Physics 2003; 118:792–806. [Google Scholar]
- Fabiilli ML, Haworth KJ, Fakhri NH, Kripfgans OD, Carson PL, Fowlkes JB. The role of inertial cavitation in acoustic droplet vaporization. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 2009; 56:1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiilli ML, Haworth KJ, Sebastian IE, Kripfgans OD, Carson PL, Fowlkes JB. Delivery of chlorambucil using an acoustically-triggered perfluoropentane emulsion. Ultrasound in medicine & biology 2010; 36:1364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiilli ML, Lee JA, Kripfgans OD, Carson PL, Fowlkes JB. Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions. Pharmaceutical research 2010; 27:2753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg BB. Ultrasound Contrast Agents: Taylor & Francis, 1996. [Google Scholar]
- Haworth KJ, Bader KB, Rich KT, Holland CK, Mast TD. Quantitative Frequency-Domain Passive Cavitation Imaging. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 2017; 64:177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Quality O. Artificial Discs for Lumbar and Cervical Degenerative Disc Disease –Update: An Evidence-Based Analysis. Ontario Health Technology Assessment Series 2006; 6:1–98. [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine 2009; 34:1301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopechek JA, Park E, Mei C-S, McDannold NJ, Porter TM. Accumulation of Phase-Shift Nanoemulsions to Enhance MR-Guided Ultrasound-Mediated Tumor Ablation In Vivo. Journal of healthcare engineering 2013; 4:109–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripfgans OD, Fowlkes JB, Miller DL, Eldevik OP, Carson PL. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound in medicine & biology 2000; 26:1177–89. [DOI] [PubMed] [Google Scholar]
- Mae T. Terada T HN, et al. Intradiscal pressurized physiologic saline injection drastically reduced pain from cervical and lumbar disc herniation. J. Pain 2012; 13:S89. [Google Scholar]
- Merienne C, Semely D, Wolff E, Roussiere A, Tafani M, Ramjaun Z, Caturla L. PP-046 A stability indicating hplc-uv method for quantification of simvastatin and detection of its impurities in oral capsules. European Journal of Hospital Pharmacy 2017; 24:A221–A22. [Google Scholar]
- Moncion A, Lin M, O'Neill EG, Franceschi RT, Kripfgans OD, Putnam AJ, Fabiilli ML. Controlled release of basic fibroblast growth factor for angiogenesis using acoustically-responsive scaffolds. Biomaterials 2017; 140:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford PA, Thomas AN, Borden MA. Thermal Activation of Superheated Lipid-Coated Perfluorocarbon Drops. Langmuir 2015; 31:4627–34. [DOI] [PubMed] [Google Scholar]
- O’Neill BE, Rapoport N. Phase-shift, stimuli-responsive drug carriers for targeted delivery. Therapeutic delivery 2011; 2:1165–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee S, Kim J-H, Park K, Kim K, Kwon IC. Polymeric nanomedicine for cancer therapy. Progress in Polymer Science 2008; 33:113–37. [Google Scholar]
- Park Y, Luce AC, Whitaker RD, Amin B, Cabodi M, Nap RJ, Szleifer I, Cleveland RO, Nagy JO, Wong JY. Tunable Diacetylene Polymerized Shell Microbubbles as Ultrasound Contrast Agents. Langmuir 2012; 28:3766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Christensen DA, Kennedy AM, Nam K. CAVITATION PROPERTIES OF BLOCK COPOLYMER STABILIZED PHASE-SHIFT NANOEMULSIONS USED AS DRUG CARRIERS. Ultrasound in medicine & biology 2010; 36:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Nam K-H, Gupta R, Gao Z, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Parker DL, Jeong E-K, Kennedy AM. Ultrasound-Mediated Tumor Imaging and Nanotherapy using Drug Loaded, Block Copolymer Stabilized Perfluorocarbon Nanoemulsions. Journal of controlled release : official journal of the Controlled Release Society 2011; 153:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble Generation in Phase-Shift Nanoemulsions used as Anticancer Drug Carriers. Bubble science engineering and technology 2009; 1:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release 2009; 138:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, Rudnitskaya A, Blackburn GJ, Fauzi NM, Pitt AR, Spickett CM. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. Journal of Lipid Research 2013; 54:1812–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovers TAM, Sala G, van der Linden E, Meinders MBJ. Disintegration of protein microbubbles in presence of acid and surfactants: a multi-step process. Soft Matter 2015; 11:6403–11. [DOI] [PubMed] [Google Scholar]
- Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O'Neill C. Failed back surgery: etiology and diagnostic evaluation. The Spine Journal 2003; 3:400–03. [DOI] [PubMed] [Google Scholar]
- Sheeran PS, Luois S, Dayton PA, Matsunaga TO. Formulation and Acoustic Studies of a New Phase-Shift Agent for Diagnostic and Therapeutic Ultrasound. Langmuir 2011; 27:10412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobiecka M, Hepel M. Multimodal coupling of optical transitions and plasmonic oscillations in rhodamine B modified gold nanoparticles. Physical Chemistry Chemical Physics 2011; 13:1131–39. [DOI] [PubMed] [Google Scholar]
- Taher F, Essig D, Lebl DR, Hughes AP, Sama AA, Cammisa FP, Girardi FP. Lumbar Degenerative Disc Disease: Current and Future Concepts of Diagnosis and Management. Advances in Orthopedics 2012; 2012:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than KD, Rahman SU, Wang L, Khan A, Kyere KA, Than TT, Miyata Y, Park Y-S, La Marca F, Kim HM, Zhang H, Park P, Lin C-Y. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. The Spine Journal 2014; 14:1017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpentesta G, De Rose M, Bosco D, Stroscio C, Guzzi G, Bombardier IC, Chirchiglia D, Plastino M, Romano M, Cristofalo S, Pardatscher K, Lavano A Lumbar percutaneous intradiscal injection of radiopaque gelified ethanol (Discogel) in patients with low back and radicular pain. J Pain Relief. 2014; 3: 145. [Google Scholar]
- Zhang P, Porter T. An in vitro study of a phase-shift nanoemulsion: a potential nucleation agent for bubble-enhanced HIFU tumor ablation. Ultrasound in medicine & biology 2010; 36:1856–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.