Abstract
In vitro investigations of tumor stem-like cells (TSC) isolated from human glioblastoma (GB) surgical specimens have been done primarily at an atmospheric oxygen level of 20%. To determine whether an oxygen level more consistent with in situ conditions affects their stem cell–like characteristics, we compared GB TSCs grown under conditions of 20% and 7% oxygen. Growing CD133+ cells sorted from three GB neurosphere cultures at 7% O2 reduced their doubling time and increased the self-renewal potential as reflected by clonogenicity. Furthermore, at 7% oxygen, the cultures exhibited an enhanced capacity to differentiate along both the glial and neuronal pathways. As compared with 20%, growth at 7% oxygen resulted in an increase in the expression levels of the neural stem cell markers CD133 and nestin as well as the stem cell markers Oct4 and Sox2. In addition, whereas hypoxia inducible factor 1α was not affected in CD133+ TSCs grown at 7% O2, hypoxia-inducible factor 2α was expressed at higher levels as compared with 20% oxygen. Gene expression profiles generated by microarray analysis revealed that reducing oxygen level to 7% resulted in the up-regulation and down-regulation of a significant number of genes, with more than 140 being commonly affected among the three CD133+ cultures. Furthermore, Gene Ontology categories up-regulated at 7% oxygen included those associated with stem cells or GB TSCs. Thus, the data presented indicate that growth at the more physiologically relevant oxygen level of 7% enhances the stem cell–like phenotype of CD133+ GB cells.
Introduction
Survival of patients with glioblastoma (GB) remains dismal, with the vast majority succumbing to disease within 1 to 2 years of diagnosis (1). Developing more effective therapies will depend on a better understanding of fundamental GB biology. Toward this end, recent studies have begun to focus on stem-like cells isolated from GB surgical specimens and grown in vitro as neurosphere cultures (2–4). Such tumor cells have a number of properties in common with neural stem cells, including continuous self-renewal, expression of stem cell markers, and at least partial differentiation along the neuronal and/or glial pathways (2–4). Moreover, in contrast to established human GB cell lines, these tumor stem-like cells (TSC) form brain tumors in immunocompromised mice that simulate the original primary tumor histology (5). Thus, consistent with the cancer stem cell model initially developed for other tumor types (6), TSCs are considered to play a critical role in initiating and maintaining GBs (2, 5). Consequently, the in vitro analysis of GB TSC cultures has generated considerable interest as an experimental approach for investigating not only the fundamental aspects of GB biology (2, 7–9) but also therapeutic response (10, 11).
As for in vitro studies in general, investigations using GB TSC cultures to date have been primarily done under atmospheric conditions of 20% O2. However, whereas alveolar oxygen concentration is ~14% (12), normal brain oxygen levels range from 5% to 10% (13, 14). Oxygen is a well-established mediator of a variety of signaling pathways as well as overall gene expression (15). It is also well established that O2 levels influence such fundamental processes as cell metabolism, proliferation, and survival (16). Finally, with respect to normal stem cells in vitro, reduction of O2 levels from 20% to 3–5% has been reported to enhance the survival of hematopoietic stem cells (17, 18) and neural precursors (19, 20). Thus, it would seem that O2 concentration needs to be taken into account in the in vitro analyses of GB TSCs.
Toward this end, the goal of the current study was to define the TSC phenotype in vitro under an O2 concentration that is representative of those that exist for the majority of GB cells in situ. Whereas O2 levels within GBs in situ range from 0.1% to 10% (13, 14, 21), studies using the nitroimidazole hypoxia marker EF5 indicate that although there is intertumor and intratumor heterogeneity, the vast majority of GB cells exist under O2 levels of 6% to 7% (13). Therefore, as an initial investigation into whether a more physiologically relevant O2 level affects their in vitro characteristics, we grew and maintained GB TSC cultures under conditions of 7% O2. The data presented indicate that as compared with the standard culture conditions of 20% O2, growth of GB TSCs at 7% results in increased self-renewal capacity, multilineage differentiation potential, and expression of stem cell–related genes/proteins. These data thus suggest that culturing at the reduced O2 concentration enhances the stem cell–like properties of GB TSCs.
Results
Enrichment of CD133+ Cells at 7% O2
MDNSC11, MDNSC20, and MDNSC23 neurosphere cultures, which were derived from surgical GB specimens, display the in vitro stem cell characteristics and are tumorigenic in immunodeficient mice (22). Whereas such neurosphere cultures contain a heterogeneous mixture of cell types, the critical marker denoting the stem cell–like subpopulation is considered to be CD133 (4, 11). To determine whether 7% O2 influences the percentage of CD133+ cells, neurospheres that had been grown under the standard O2 concentration of 20% were disaggregated and placed back into culture under conditions of 7% or 20% O2. Neurospheres reformed and, after 10 to 14 days, reached diameters of ~100 μm, at which time the percentage of CD133+ cells in each culture was determined. As shown in Fig. 1A, MDNSC23 contained >90% CD133+ cells when grown at 20% O2, which was not affected by growth at 7% O2. However, when MDNSC11 and MDNSC20 neurospheres were grown at 7% O2, the percentage of CD133+ cells in each culture increased by ~2-fold. These results suggest that formation and growth of the neurospheres at the more physiologically relevant O2 concentration led to an enrichment in the CD133+ population. Consistent with the increase in the percentage of CD133+ cells, after 5 days at 7% O2, the neurospheres in MDNSC11 (Fig. 1B) and MDNSC20 (data not shown) cultures were larger than those grown under standard in vitro conditions of 20% O2.
FIGURE 1.
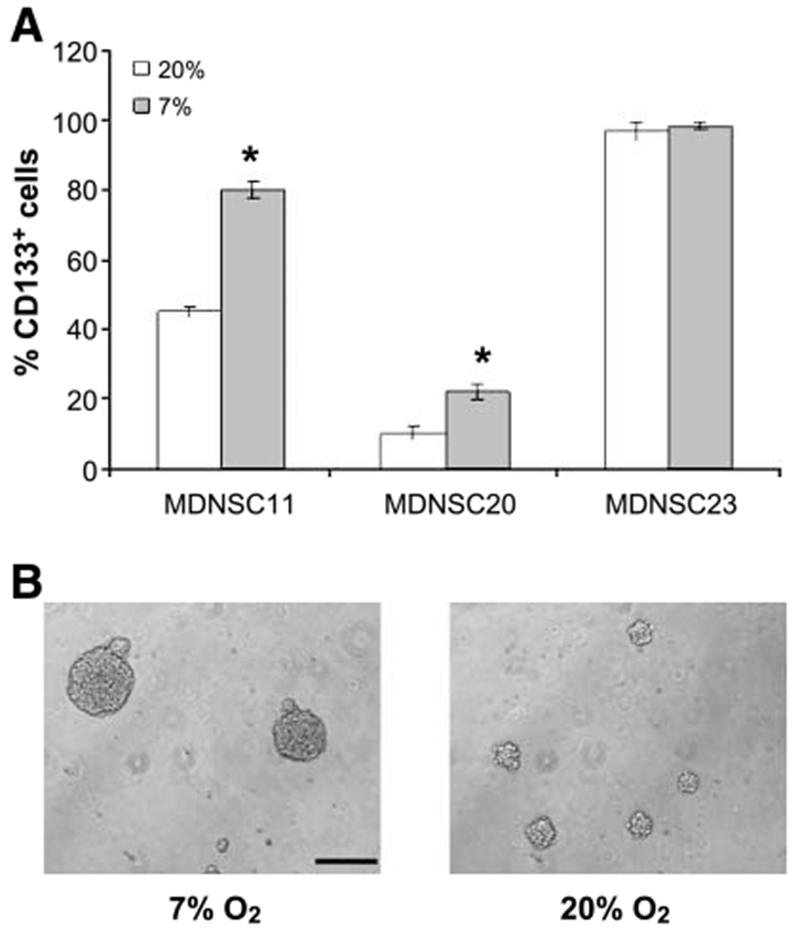
Influence of reduced O2 on the percentage of CD133+ cells in GB neurosphere cultures. A. MDNSC11, MDNSC20, and MDNSC23 neurosphere cultures were grown at 7% or 20% O2 for 10 to 14 d (~100 μm in diameter) and the percentage of CD133+ cells in each culture was determined by flow cytometry. Columns, mean of three independent experiments; bars, SE. *, P < 0.003. B. Representative photomicrographs of MDNSC11 cultures after 5 d of growth at 7% or 20% O2 (20× bar, 50 μm).
CD133+ TSCs
Given its significance as a marker for GB TSCs (4, 11), subsequent studies focused on the CD133+ cells of each neurosphere culture. Toward this end, fluorescence-activated cell sorting was used to isolate CD133+ cells from MDNSC11 and MDNSC20 cultures that were grown under standard conditions of 20% O2; this procedure was not necessary for MDNSC23, which already contained >90% CD133+ cells (Fig. 1A). CD133+ cells corresponding to each culture were then used to initiate neurospheres, which were maintained for 7 days at 20 or 7% O2 before use in an experiment. It should be noted that whereas some CD133-negative cell cultures isolated from GBs have been reported to show stem cell–like qualities in vitro (23), in our hands the CD133-negative cells sorted from MDNSC11 and MDNSC20 cultures did not reform neurospheres or proliferate in vitro (data not shown).
Proliferation Rate and Self-Renewal Potential of CD133+ TSCs Are Increased at 7% O2
To determine whether O2 level affects proliferation rate, CD133+ MDNSC11, MDNSC20, and MDNSC23 neurospheres, which had been maintained at 7% and 20% O2, were disaggregated and placed back into neurosphere-forming conditions at their respective O2 levels. The number of cells in each culture was then determined at times out to 14 days, and the mean population doubling time calculated as described (24). As shown in Table 1, as compared with 20%, the 7% O2 environment significantly reduced the doubling time of each of the CD133+ cultures. These data indicate that the reduced O2 level enhanced the proliferation rate of the CD133+ TSCs.
Table 1.
Mean Population Doubling Time (Hours) of CD133+ TSCs
| % O2 | MDNSC11 | MDNSC20 | MDNSC23 |
|---|---|---|---|
| 20% | 117.70 ± 4.16 | 141.27 ± 13.44 | 35.46 ± 1.91 |
| 7% | 80.36 ± 6.51* | 76.07 ± 1.98* | 26.29 ± 1.65* |
NOTE: CD133+ cells from MDNSC11, MDNSC20, and MDNSC23 were cultured at 7% or 20% O2 for 7 d, and then disaggregated and cultured in T25 flasks under neurosphere-forming conditions. The neurospheres were disaggregated every 3 d, cells were counted, and mean population doubling time (hours) was determined. Values represent the mean of three independent experiments ± SE.
P < 0.02.
A characteristic of stem or stem-like cells is continuous self-renewal (2, 4), which is reflected in vitro by colony forming efficiency (clonogenicity). To determine whether O2 level influences the clonogenic potential of GB TSCs, the CD133+ neurosphere cultures grown at 7% or 20% O2 were disaggregated into single-cell suspensions and seeded onto poly-l-lysine–coated tissue culture plates at clonogenic densities (i.e., 100-1,000 cells). Plates were returned to their respective O2 condition and the number of adherent colonies was determined after 14 to 20 days. As shown in Fig. 2, colony formation for each of the CD133+ TSC lines was significantly greater at 7% O2 as compared with 20%. It should be noted that the vast majority of cells with the colonies formed for each TSC cell line at 20% and 7% O2 continued to express CD133 (data not shown). These results suggest that the reduced O2 level modifies the phenotype of CD133+ cells such that their self-renewal potential is enhanced.
FIGURE 2.
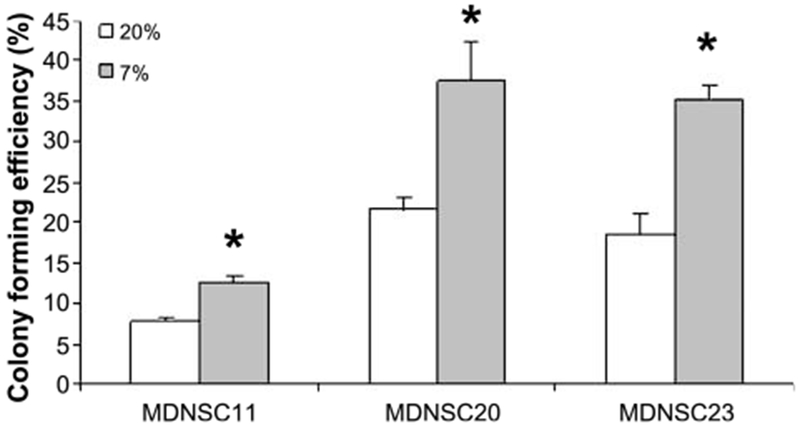
Influence of O2 on the clonogenicity of CD133+ cells. CD133+ cells from MDNSC11, MDNSC20, and MDNSC23 cultures were grown at 7% or 20% O2 as neurospheres for 7 d and subjected to clonogenic analysis (*, P < 0.02).
Differentiation Pattern of TSCs
An additional characteristic of GB TSCs is the potential to differentiate along the neuronal and glial pathways. To determine whether the reduction in O2 influences this differentiation pattern, CD133+ MDNSC11, MDNSC20, and MDNSC23 neurospheres were cultured at 7% or 20% O2 for 7 days, disaggregated, and seeded onto slides in growth factor–free medium containing 10% fetal bovine serum to induce differentiation. After an additional 7 days at the corresponding O2 level, the percentages of cells expressing glial fibrillary acidic protein (GFAP), a glial marker, or βIII tubulin, a neuronal marker, were determined. At 20% O2, the CD133+ TSCs differentiated primarily toward the astrocyte lineage, with relatively few cells expressing the neuronal marker (Fig. 3), which is consistent with results from other GB TSCs (25). However, at 7% O2, more cells were positive for βIII tubulin, indicating an increase in the number of cells differentiating along the neuronal lineage. Thus, as compared with 20%, the CD133+ cells at 7% O2 seem to have a more diverse differentiation potential, consistent with an enhancement of the stem cell–like properties.
FIGURE 3.
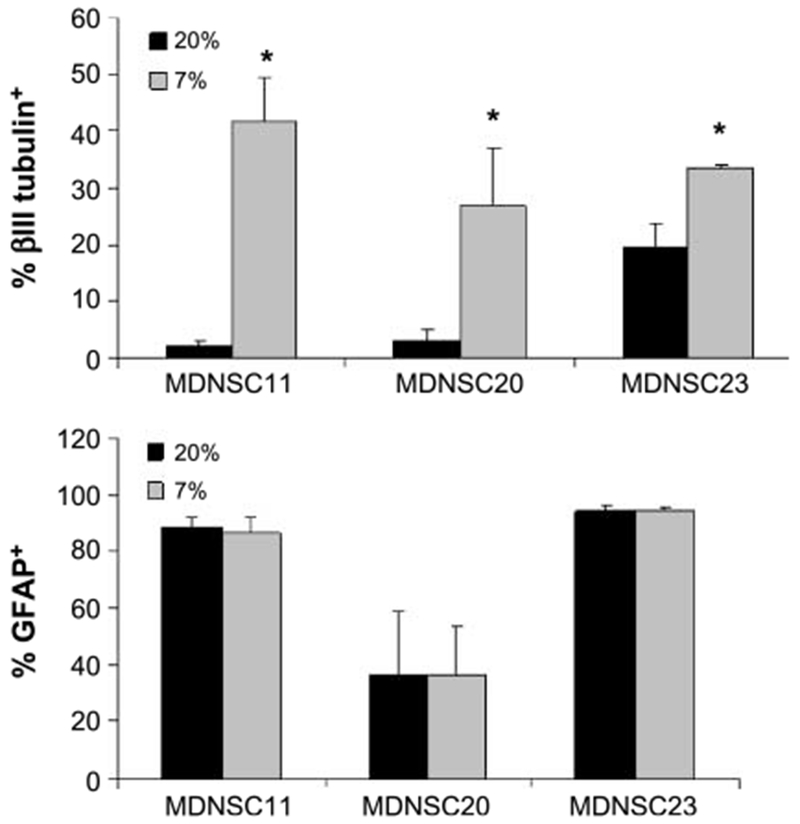
Influence of O2 on CD133+ cell differentiation. CD133+ cells from MDNSC11, MDNSC20, and MDNSC23 were grown at 7% and 20% O2 for 7 d, then subjected to differentiation and stained for βIII tubulin or GFAP. Cells were scored as positive or negative for either marker. Columns, mean of three experiments; bars, SE.
Increased Expression of Stem Cell Markers in CD133+ TSCs at 7% O2
The cell level studies described above suggest that growth of CD133+ TSCs under conditions of 7% O2 enhances their stemlike phenotype. To pursue this idea at a molecular level, the effects of the reduced O2 level on the expression of proteins previously implicated as stem cell markers were determined. As for the data described in Table 1 and Figs. 2 and 3, these analyses were done on CD133+ neurosphere cultures. Whereas under both 20% and 7% O2, >90% of cells expressed CD133, as shown in Fig. 4A, the median fluorescence intensity corresponding to CD133 expression was consistently greater for each of the TSC cultures at 7% as compared with 20% O2, consistent with an increase in the level of CD133 expression. This increase was most apparent for the MDNSC11 and MDNSC23 lines; although relatively minor in MDNSC20, the increase in CD133 expression was consistent throughout the experiments. Immunoblot analysis was then used to determine whether 7% O2 influences the expression of other stem cell–associated proteins (Fig. 4B). As compared with 20%, after 7 days in 7% O2, nestin expression was increased in each of the CD133+ neurosphere cultures. In contrast to the uniform increase in CD133 and nestin expression across the 3 CD133+ TSC cultures, the expression of stem cell markers Oct4 and Sox2 was enhanced at 7% O2 only in MDNSC11 and MDNSC23. In MDNSC20 culture, Oct4 protein was not detectable at either O2 level; Sox2 protein expression was essentially the same at both O2 levels. Thus, although there is some heterogeneity between CD133+ neurosphere cultures, which is consistent with the intertumor heterogeneity of primary GB in situ (26), these results indicate that growth at 7% O2 enhances the expression of proteins previously associated with a stem cell–like phenotype.
FIGURE 4.
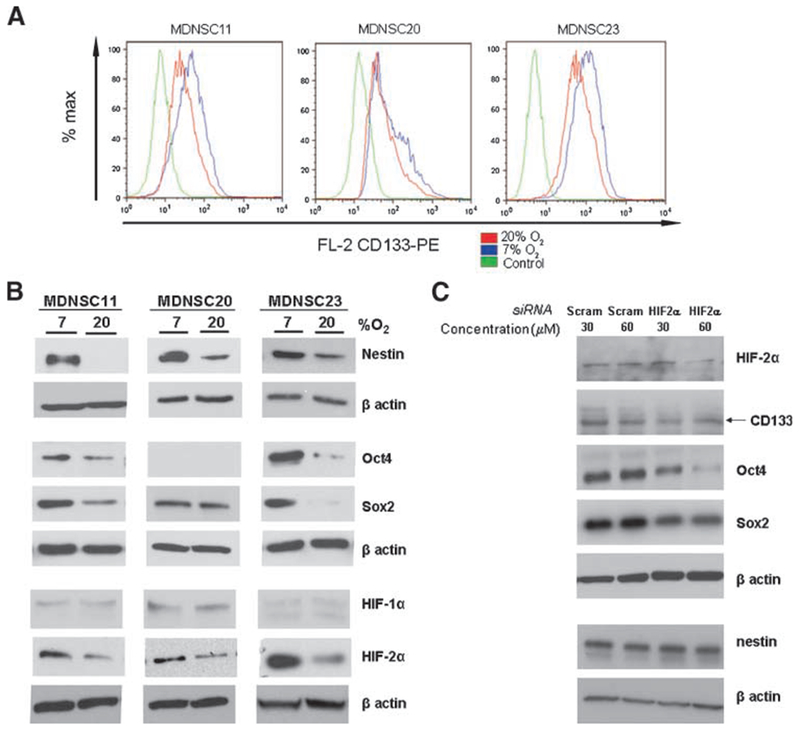
Influence of O2 on the expression of stem cell markers in CD133+ cells. CD133+ cells from MDNSC11, MDNSC20, and MDNSC23 were cultured at 7% or 20% O2 for 7 d, then subjected to flow cytometry for CD133 expression using a mouse IgG1 as a staining control (green; A) or immunoblot analysis for stem cell–associated proteins using β-actin as a loading control (B). C. CD133+ MDNSC11 were maintained at 7% O2 and transfected with a scrambled control (scram) or siRNA for HIF-2α at the indicated concentration for 24 h, and immunoblots were done. Blots are representative of two independent experiments.
Hypoxia-Inducible Factor-2α Expression Is Enhanced at 7% O2 and Drives the Expression of Oct4 and Sox2
Hypoxia-inducible factors (HIF-1α and HIF-2α) are critical transcription factors mediating changes in gene expression in response to hypoxia and have recently been implicated in the regulation of the stem cell phenotype (16). However, most investigations of HIF expression have focused on O2 levels of <5%. Therefore, HIF-1α and HIF-2α protein levels were determined in the CD133+ MDNSC11, MDNSC20, and MDNSC23 neurospheres grown at 20% and 7% O2 (Fig. 4B). HIF-1α protein was expressed at low levels in each of the CD133+ cultures with no difference between the O2 levels. In contrast, HIF-2α levels were clearly detectable in each of CD133+ cultures and, moreover, were increased by growth at 7% O2 as compared with 20%. These data suggest that at the more physiologic O2 condition, HIF-2α may play a role in regulating gene expression in TSCs.
Given that Oct4 has been identified as a putative HIF-2α target gene (27), we investigated whether the expression of stem cell markers that were up-regulated at 7% O2 was dependent on HIF-2α activity. Specifically, because Oct4 and Sox2 are part of the same transcriptional network (28, 29), we hypothesized that these two proteins would be down-regulated if HIF-2α activity was inhibited. To assess the role of HIF-2α in regulating the expression of these stem cell markers, we grew CD133+ MDNSC11 at 7% O2 for 1 week, then transfected the cells with siRNA specific for the gene that codes for HIF-2α (EPAS-1) or a nonspecific scrambled siRNA. After 24 h, cells were assessed for knockdown of EPAS-1, and levels of nestin, CD133, Oct4, and Sox2 were determined by immunoblot (Fig. 4C). As expected, HIF-2α levels were reduced after siEPAS-1 transfection. Oct4 and Sox2 protein levels were also markedly decreased after knockdown of EPAS-1. These data provide a putative HIF-2α–dependent pathway by which Oct4 and Sox2 are up-regulated at physiologic O2. However, CD133 and nestin levels were not affected, suggesting that HIF-2α–independent pathways also play a role in the stem cell phenotype of TSCs.
Influence of 7% O2 on Gene Expression Profiles Generated from CD133+ Cells
The data presented indicate that the reduction in O2 to a more physiologic level affects the phenotype of CD133+ TSCs, suggesting modifications in the global gene expression pattern. Therefore, microarray analysis was done on the CD133+ MDNSC11, MDNSC20, and MDNSC23 neurospheres grown at 7% and 20% O2. To identify the genes whose expression was either up-regulated or down-regulated at 7% O2, biological replicates were averaged for each cell line and compared with the gene expression profile generated from their corresponding 20% O2 culture using SAM (false discovery rate of <0.1). As shown in the Venn diagrams in Fig. 5A, each of the CD133+ neurosphere cultures contained a significant number of genes up-regulated and down-regulated as a result of growth at 7% O2. Moreover, a significant number of genes were commonly affected at 7% O2 among the three TSC lines, suggesting a general response to the more physiologic O2 condition. To compare the gene expression response elicited by 7% O2 to established GB cell lines, the same experiment was done using U251 and U87 cells. As shown in the Venn diagrams (Fig. 5A, bottom), there were considerably fewer genes whose expression was modified at 7% O2 and fewer genes commonly affected in the two established GB cell lines. These results suggest that the TSCs are more susceptible to O2-mediated changes in gene expression than the traditional established GB cell lines. To determine whether the genes affected by 7% O2 in the CD133+ TSCs correspond to specific biological/biochemical processes, a gene set analysis was done according to Gene Ontology (GO) category enrichment. For this analysis, to account for the heterogeneity between the CD133+ TSC cultures, genes significantly up-regulated and down-regulated at 7% O2 in any two of the three cultures were distributed into GO categories, with the enriched GO categories used to generate a heat map (Fig. 5B). The number of enriched GO categories (P < 0.05) that were up-regulated and down-regulated were 39 and 57, respectively. Of interest with respect to TSCs, the enriched up-regulated GO categories included the frizzled-2 signaling pathway, Notch2, transforming growth factor β, and angiogenesis, all of which have been associated with brain tumor stem cells (30).
FIGURE 5.
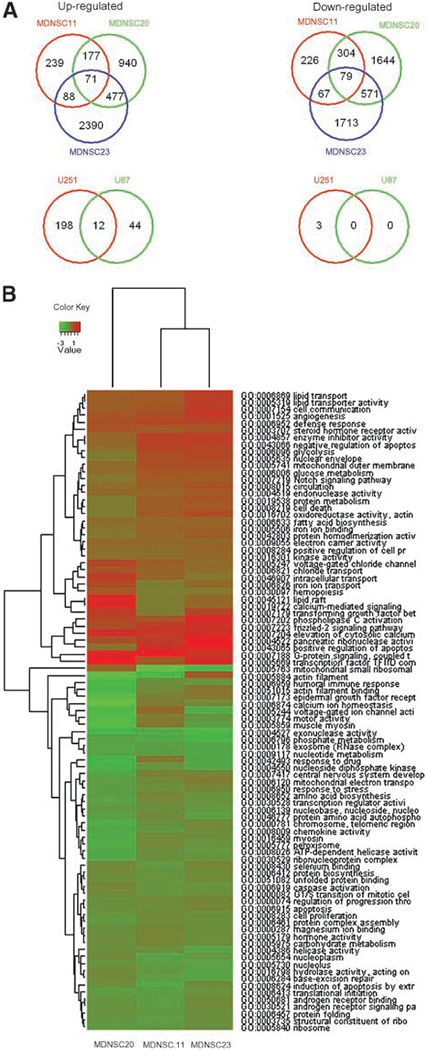
Effects of 7% O2 on the gene expression profiles of CD133+ cultures. CD133+ cells from MDNSC11, MDNSC20, and MDNSC23 were grown at 7% and 20% O2 for 7 d and then subjected to microarray analysis. A. Venn diagrams depicting the number of genes that were commonly up-regulated (left) or down-regulated (right) by growth at 7% O2 as compared with 20% O2. The same experiment was also done for monolayer cultures of U87 and U251 (bottom). For each culture, the genes whose expression was affected by 7% O2 were defined by SAM (<0.10 false discovery rate). B. Gene set enrichment analysis according to GO functional categories. Genes identified as being significantly affected by SAM in two of the three CD133+ TSC cultures were submitted to enrichment analysis, with the corresponding GO categories displayed as a heat map.
Oxygen-Dependent Enhancement of Stem-Like Phenotype Is Reversible
Whereas CD133 identifies TSCs, the CD133+ cells most likely include a number of subpopulations (31, 32). This potential heterogeneity suggested that it was also possible that the survival data in Fig. 2 corresponded to the selection of a CD133+ subpopulation with greater clonogenic potential. If such a selection process was operative, the enhanced survival induced by 7% O2 would be expected to be irreversible. Therefore, to test the issue of reversibility, MDNSC11, MDNSC20, and MDNSC23 CD133+ neurosphere cultures were initiated at 7% O2 and, 7 days later, moved to 20% O2 for an additional 7 days (7→20) followed by clonogenic analysis. Colony formation was also determined for accompanying cultures maintained at 7% O2 or 20% O2. Consistent with the results in Fig. 2, the colony formation at 7% O2 was significantly greater than at 20% (Fig. 6A). However, when CD133+ TSCs were moved from 7% to 20% O2 (7→20), the colony formation returned to essentially the same values as for cultures maintained at 20% O2. These data indicate that the enhancement in CD133+ TSC clonogenicity induced at 7% O2 is a reversible process. Moreover, these results are suggestive of an epigenetic modification in the TSC phenotype and not the selection of a subpopulation of CD133+ cells with increased colony forming efficiency.
FIGURE 6.
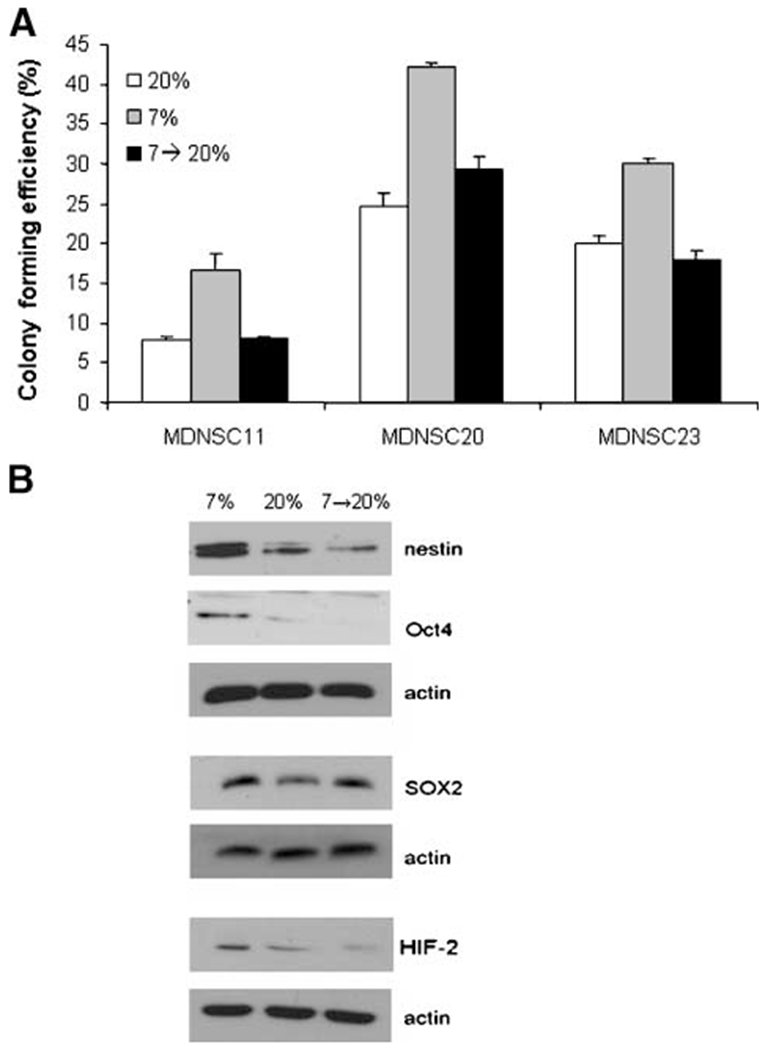
Reversibility of 7% O2 effects on stem cell phenotype. CD133+ MDNSC11, MDNSC20, and MDNSC23 cultures were maintained at 7% O2 for 7 d and then returned to 20% O2 for 7 d (7→20); separate cultures were maintained at 7% or 20% for 7 d. These cells were then subjected to clonogenic analysis (A). Columns, mean of three independent experiments; bars, SE. *, P < 0.02. B. CD133+ MDNSC11 were maintained at 7%, 20%, or (7→20) O2 condition, then subjected to immunoblot analysis for nestin, Oct4, Sox2, and HIF-2α. Representative of three independent experiments.
To determine whether the reversible effect of 7% O2 on stem cell phenotype extended to the up-regulation of stem cell markers, the CD133+ MDNSC11 cells were grown as above at 7, 20, and 7→20 oxygen conditions, after which levels of the proteins nestin, Oct4, Sox2, and HIF-2α were determined by immunoblot (Fig. 6B). After reintroduction to 20% O2, the expression levels of nestin, HIF-2α, and Oct4 returned to control levels (20%). However, the expression of Sox2 was not reversed after 7 days at 20% O2. The implications of reversibility of only two of the three stem cell markers are unclear and warrant further studies. The reversibility of most of the stem cell markers is consistent with the reversible increase in clonogenicity and supports the hypothesis that the 7% O2 condition does not simply select for a subpopulation of TSCs.
Discussion
According to the cancer stem cell hypothesis, TSCs exist as a small percentage of the total cells within a GB, and yet play a significant role in tumor growth and response to therapy. Given the difficulties of in vivo investigations on such a minor subpopulation of cells, in vitro cultures provide an experimentally expedient model system essential for defining the fundamental biology of GB TSCs. Whereas interpreting data generated from any cell culture approach must be tempered by the inability to recapitulate the tumor microenvironment, there are parameters that can be modified to better simulate in situ conditions. One such parameter involves O2, a critical signaling molecule that has been implicated in regulating the neoplastic phenotype (15) as well as that of normal stem and progenitor cells (18–20, 33, 34). In this report, we tested the hypothesis that a more physiologically relevant O2 concentration modifies the in vitro phenotype of GB TSCs.
CD133 serves as the marker protein typically used to identify and isolate TSCs from the heterogeneous neurosphere cultures initiated from GB surgical specimens (4, 5). As few as 100 such CD133+ cells have been reported to form brain tumors in immunocompromised mice (4). Platet et al. (35) reported that culturing neurosphere-forming cells from GB surgical specimens at 3% O2 increased the percentage of CD133+ cells as compared with conditions of 20% O2. As shown here, a less severe reduction of O2 levels to 7% also results in a significant increase in the percentage of CD133+ cells within GB neurosphere cultures. This enrichment in CD133-expressing cells is consistent with subsequent analyses of CD133+ cells sorted from each neurosphere culture, indicating that their doubling time was reduced and their clonogenic potential was increased at 7% O2. At this reduced O2 level, the capacity of the CD133+ cells to differentiate along both the glial and neuronal pathways was also enhanced. A similar increase in neuronal differentiation has been reported for normal neural stem cells maintained at in vitro oxygen levels of 3% to 5% (19, 20, 34), as well as increased astrocytic death in primary rat cultures at 20% O2 (36). Whether the enhancement of stem cell phenotype at 7% O2 is a result of an increase in stem cell traits or a release of an inhibition of stem cell traits resulting from 20% O2 remains to be determined. However, the increases in proliferation rate, clonogenicity (self-renewal), and multilineage differentiation potential of the CD133+ cells are consistent with an enhancement of their stem-like properties at the more physiologic O2 level of 7%.
Within a GB in situ, the O2 level to which a given cell or group of cells are exposed is likely to be somewhat transient, varying according to not only tumor growth dynamics but also treatment response. As shown here, with respect to clonogenic potential and expression of stem cell markers, most of the changes elicited at 7% O2 were completely reversible, suggesting that if a similar variation occurs in vivo, the stem cell–like properties of TSCs may also vary. Whether such an oscillation in phenotype actually occurs in vivo and whether it would have any treatment significance are clearly speculative. However, this in vitro reversibility is consistent with the plasticity generally associated with TSCs as well as normal stem cells and their ability to respond to environmental signals. These results lend support to the hypothesis that “sternness” in the context of tumor-initiating cells may be an environmentally inducible state (16).
Further support for CD133+ TSCs assuming a more stem cell–like phenotype at 7% O2 can be derived from the analyses of stem cell markers. The expression of nestin, a well-established marker for stem cells of neural origin (37), was elevated at 7% O2, as was the absolute level of CD133. However, although established as markers, the specific function, if any, of nestin and CD133 in stem cell biology remains undefined. In contrast, reducing O2 levels to 7% was also found to enhance the expression of the transcription factors Sox2 and Oct4, which are two of the four transgenes necessary to convert normal fibroblasts to embryonic stem-like cells (38) and play critical roles in regulating genes mediating normal stem cell behavior (28, 39, 40). HIF proteins have been implicated in the hypoxia-mediated regulation of cancer stem cells, contributing to the maintenance of their undifferentiated state (41). The mechanism was reported to involve the HIF-2α-mediated, but not the HIF-1α-mediated, induction of Oct4 expression (27). As described here, in CD133+ cells HIF-2α was expressed at a higher level than HIF-1α and its level was increased at 7% O2. Specific knockdown of HIF-2α at 7% O2 resulted in a reduction in Sox2 and Oct4 proteins. Although clearly requiring further investigation, these results suggest that HIF-2α may play a role in regulating the stem cell–like phenotype of CD133+ GB TSCs.
To further define the influence of oxygen on the phenotype of CD133+ GB TSCs, microarray analysis was used to compare the gene expression profiles generated under conditions of 7% and 20% O2. As compared with the traditional established GB cell lines, the CD133+ TSC lines were more susceptible to changes in gene expression resulting from growth at 7% O2, consistent with the putative heightened ability of TSCs to respond to their environment. As illustrated by the gene set enrichment analysis, a number of the GO categories up-regulated in TSCs at 7% O2 have previously been associated with brain tumor stem cells. Bao et al. (7) and Calabrese et al. (42) have suggested that TSCs play a major role in driving angiogenesis, consistent with the up-regulation of the angiogenesis GO category shown here. Notch and frizzled-2 signaling pathway genes, which have been associated with embryonic stem cells as well as TSCs (30), were up-regulated by growth at 7%. Moreover, the frizzled-2 pathway includes many of the WNT genes, which have been implicated in regulating the radioresistance of normal mammary stem cells (43). Finally, transforming growth factor β, which was up-regulated at 7% O2, has been associated with the regulation of stem cell differentiation and interacts with other developmental pathways such as WNT and BMP (30). Thus, the changes in gene expression profiles induced by 7% O2 are consistent with an enhanced stem cell phenotype.
The cells within a GB in situ can be exposed to a wide range of O2 levels (21) from the severely hypoxic (<0.1%) to that of well-oxygenated tissue (~10%). The studies described here addressed only a single O2 level (7%), which likely reflects the O2 level to which the majority of GB cells are exposed. Whether lower O2 levels affect CD133+ cells in a similar or more dramatic manner remains to be determined. However, as shown, the relatively modest reduction in O2 has a significant effect on stem cell–like characteristics of CD133+ TSCs. Thus, investigations of CD133+ TSCs at physiologically relevant O2 levels may provide a model system for generating additional insight into GB biology.
Materials and Methods
Isolation and Culture of GB Tumor Stem Cells
Neurosphere-forming cultures MDNSC11, MDNSC20, and MDNSC23 were isolated from three human GB surgical specimens as described previously (2). These cells exhibit the in vitro stem cell characteristics of self-renewal and multipotent differentiation and were tumorigenic in immunodeficient mice (22). Neurospheres were maintained in medium consisting of DMEM/F-12 (Invitrogen), B27 supplement (0.5×; Invitrogen), and human recombinant basic fibroblast growth factor and epidermal growth factor (50 ng/mL each; R&D Systems). To dissociate neurospheres into single cells, spheres were treated with TryplE Express (Invitrogen) for 5 min at 37°C, then subjected to mechanical disaggregation and strained through a 40-μm cell strainer (BD). For the standard in vitro condition of 20% O2, cultures were maintained in a Forma Series II CO2 incubator at 5% CO2/95% air. For culture at 7% O2, cultures were maintained in a Forma Series II multi-gas incubator in which CO2 and N2 were continuously supplied to achieve a balance of 5% CO2/7% O2.
Clonogenic Analysis
Clonogenicity was defined using a colony forming efficiency assay. Neurospheres were disaggregated into single-cell suspensions as described above. A specified number of cells were then seeded into poly-l-lysine–coated six-well plates, which allows for adherent colony formation, containing the serum-free growth medium noted above. After 14 to 20 d, colonies, defined as >25 cells, were fixed and stained with 0.5% crystal violet and colony forming efficiency was determined.
Flow Cytometry and Fluorescence-Activated Cell Sorting
For determination of CD133-expressing cells by flow cytometry, spheres were disaggregated into a single-cell suspension, washed twice in PBS, and incubated with a phycoerythrin-conjugated anti-CD133 (1:10; Miltenyi) or an isotype control (mouse IgG1, Miltenyi) and human Fc blocking reagent (1:10, Miltenyi). Cells were analyzed on a FACScan (BD). For sorting of CD133+ cells, spheres were processed as above under sterile conditions and sorted on a FACSVantage cell sorter. Only cells positive for CD133 were collected. Purity was determined post-sort as >90% positive.
Immunoblot Analysis
Cell lysates were prepared as previously described for in vitro cultures (44). For immunoblot analysis, lysates (25 μg) were electrophoresed on SDS-polyacrylamide gels and electrophoretically transferred at 100 V for 2 h to Immobilon-P membranes. Membranes were blocked with 5% nonfat dry milk in 500 mmol/L NaCl, 20 mmol/L Tris (pH 7.5), and 0.1% Tween 20 (TBST) for 1 h followed by incubation with primary antibody at the following concentrations: anti-nestin (1:2,500; R&D Systems), anti-Oct4 (1:500; Abnova), anti-Sox2 (1:1,250; Millipore), anti–HIF-1α (1:1,000; Abcam), anti–HIF-2α (1:500; Abcam), and anti–β-actin (1:5,000; Sigma). Blots were washed in TBST and incubated with horse-radish peroxidase–conjugated secondary antibody (1:2,500 dilution in 5% nonfat dry milk/TBST; Santa Cruz Biotechnology). Blots were developed using Western blotting ECL detection kit (Pierce Biotechnology) according to instructions from the manufacturer.
Differentiation Assay
To define differentiation potential, neurospheres were disaggregated into single cells, which were then seeded onto CC2-coated glass slides (Nalge Nunc Int.) in growth factor–free medium containing 10% FCS (5). After 7 d, slides were fixed in 4% paraformaldehyde in PBS for 10 min, washed with PBS, and permeabilized with 0.2% NP40. Slides were blocked in 5% goat serum in 1% bovine serum albumin for 1 h at room temperature and incubated with primary antibodies diluted in 1% bovine serum albumin [anti-GFAP, 1:200 (Millipore); anti–βIII tubulin, 1:500 (Abcam)] for 2 h, and then washed and incubated with secondary antibodies (goat anti-mouse conjugated to AlexaFluor 488, Invitrogen) for 1 h at room temperature followed by washing and mounting in anti-fade with 4′,6-diamidino-2-phenylindole (Invitrogen). Cells were analyzed on a Zeiss upright fluorescent microscope.
siRNA Transfection for Silencing of HIF-2α
MDNSC11 were seeded at subconfluency in 24-well plates and grown for 48 h. Transfections were done with Lipofectamine RNAimax (Invitrogen) according to the manufacturer’s directions for forward transfection. siRNA for EPAS-1 (Santa Cruz Biotechnology) or a scrambled control (10 or 20 pmol) was added per well. Cells were harvested after 24 h and immunoblots were done as described.
Microarray Procedure
Total RNA was isolated from neurosphere cultures using TRIzol reagent (Invitrogen) following the manufacturer’s protocol and further purified using the RNeasy cleanup procedure (Qiagen, Inc.). The quality of total RNA was assessed by agarose gel electrophoresis and by analysis on the Agilent 2100 Bioanalyzer. Five micrograms of total RNA served as the mRNA source for microarray analysis. The polyadenylic acid RNA was specifically converted to cDNA and then amplified and labeled with biotin following the procedure initially described by Van Gelder et al. (45). Hybridization to U133A GeneChips (Affymetrix) was done according to the manufacturer’s instructions (46).
Microarray Data Analyses
Raw data analysis was done using code written in R4 and software from open-source Bioconductor Project (47). Preliminary data quality control assessments were done with affyQCReport package. The raw data were background adjusted, normalized, and converted to log 2 transformed expression level data by using a method implemented in the robust multiarray average method (48). Differences in gene expression between 7% and 20% O2 culture conditions for each of stem cell line in duplicate were done on most variable genes (SD > 0.2 across samples) using the SAM algorithm (49) with the false discovery rate set to 10%. Overlap of the significant gene lists from each of the tested samples was evaluated according to Venn diagrams; genes common to any two of the three cell lines were selected for gene set analysis. Pathway analysis was done using Gene Set Enrichment Analysis (50). Gene Set Enrichment Analysis takes a list of genes and tests whether, within that queried list of genes, there is statistically significant enrichment of predefined groups of genes or “gene sets.” An a priori gene set file was created from the cellular, molecular, and biological categories of Gene Ontology (GO) database. We have selected all categories with gene membership of <500 or >15 in each category. This resulted in 594 categories in total. False discovery rate and P value estimates were computed for each gene set based on 1,000 separate permutation distributions. Gene sets were deemed to be enriched when P < 0.05.
Acknowledgments
Grant support: Cancer Center Support Grant to Moffitt Cancer Center from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352: 987–96. [DOI] [PubMed] [Google Scholar]
- 2.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004; 64:7011–21. [DOI] [PubMed] [Google Scholar]
- 3.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 2003;100:15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821–8. [PubMed] [Google Scholar]
- 6.Kakarala M, Wicha MS. Cancer stem cells: implications for cancer treatment and prevention. Cancer J 2007;13:271–5. [DOI] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 2006; 66:7843–8. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006;9: 391–403. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006;444:761–5. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444: 756–60. [DOI] [PubMed] [Google Scholar]
- 12.Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 13.Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 2004;10:8177–84. [DOI] [PubMed] [Google Scholar]
- 14.Dings J, Meixensberger J, Jager A, Roosen K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998; 43:1082–95. [DOI] [PubMed] [Google Scholar]
- 15.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006;441:437–43. [DOI] [PubMed] [Google Scholar]
- 16.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 2007;17:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanovic Z, Hermitte F, Brunet de la Grange P, et al. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%). Stem Cells 2004;22:716–24. [DOI] [PubMed] [Google Scholar]
- 18.Kovacevic-Filipovic M, Petakov M, Hermitte F, et al. Interleukin-6 (IL-6) and low O2 concentration (1%) synergize to improve the maintenance of hematopoietic stem cells (pre-CFC). J Cell Physiol 2007;212:68–75. [DOI] [PubMed] [Google Scholar]
- 19.Studer L, Csete M, Lee SH, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 2000;20:7377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang CP, Zhu LL, Zhao T, et al. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1α. Neurosignals 2006;15:259–65. [DOI] [PubMed] [Google Scholar]
- 21.Evans SM, Judy KD, Dunphy I, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res 2004;64:1886–92. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst 2007;99:1410–4. [DOI] [PubMed] [Google Scholar]
- 23.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007;67:4010–5. [DOI] [PubMed] [Google Scholar]
- 24.Davis JM. Basic cell culture: a practical approach. In: Rickwood D, Homes BD, editors. Oxford (NY): Oxford University Press; 1994. [Google Scholar]
- 25.Zhang QB, Ji XY, Huang Q, et al. Differentiation profile of brain tumor stem cells: a comparative study with neural stem cells. Cell Res 2006;16:909–15. [DOI] [PubMed] [Google Scholar]
- 26.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 2008;26:2839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covello KL, Kehler J, Yu H, et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006;20:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003;17:126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomioka M, Nishimoto M, Miyagi S, et al. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res 2002;30:3202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark PA, Treisman DM, Ebben J, Kuo JS. Developmental signaling pathways in brain tumor-derived stem-like cells. Dev Dyn 2007;236:3297–308. [DOI] [PubMed] [Google Scholar]
- 31.Miki J, Furusato B, Li H, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res 2007;67:3153–61. [DOI] [PubMed] [Google Scholar]
- 32.Monzani E, Facchetti F, Galmozzi E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer 2007; 43:935–46. [DOI] [PubMed] [Google Scholar]
- 33.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun 2007;358:948–53. [DOI] [PubMed] [Google Scholar]
- 34.Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 2007;35:424–35. [DOI] [PubMed] [Google Scholar]
- 35.Platet N, Liu SY, Atifi ME, et al. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett 2007;258:286–90. [DOI] [PubMed] [Google Scholar]
- 36.Danilov CA, Fiskum G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia 2008;56:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 1990;60:585–95. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 39.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24: 372–6. [DOI] [PubMed] [Google Scholar]
- 40.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379–91. [DOI] [PubMed] [Google Scholar]
- 41.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007;129:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- 43.Woodward WA, Chen MS, Behbod F, et al. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A 2007;104:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res 2006;66:9211–20. [DOI] [PubMed] [Google Scholar]
- 45.Van Gelder RN, von Zastrow ME, Yool A, et al. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A 1990; 87:1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrington JA, Nair A, Mahadevappa M, Tsyganskaya M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics 2000;2:143–7. [DOI] [PubMed] [Google Scholar]
- 47.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4:249–64. [DOI] [PubMed] [Google Scholar]
- 49.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001;98:5116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efron B, Tibshirani R. On testing the significance of sets of genes. Annals of Applied Statistics 2007;1:107–29. [Google Scholar]


