Abstract
In cultivated tomato (Solanum lycopersicum), increases in photosynthetically active radiation (PAR) induce type VI leaf glandular trichomes, which are important defensive structures against arthropod herbivores. Yet, how PAR affects the type VI trichome-associated leaf chemistry and its biological significance with respect to other photomorphogenic responses in this agronomically important plant species is unknown. We used the type VI trichome-deficient tomato mutant odorless-2 (od-2) and its wild type to investigate the influence of PAR on trichome-associated chemical defenses against thrips (Frankliniella occidentalis). High PAR increased thrips resistance in wild-type plants, but not in od-2. Furthermore, under high PAR, thrips preferred od-2 over the wild type. Both genotypes increased type VI trichome densities under high PAR. Wild-type plants, however, produced more trichome-associated allelochemicals, i.e. terpenes and phenolics, these being undetectable or barely altered in od-2. High PAR increased leaf number and thickness, and induced profound but similar metabolomic changes in wild-type and od-2 leaves. Enhanced PAR also increased levels of ABA in wild-type and od-2 plants, and of auxin in od-2, while the salicylic acid and jasmonate concentrations were unaltered. However, in both genotypes, high PAR induced the expression of jasmonic acid-responsive defense-related genes. Taken together, our results demonstrate that high PAR-mediated induction of trichome-associated chemical defenses plays a prominent role in tomato–thrips interactions.
Keywords: Abscisic acid, Frankliniella occidentalis, Jasmonic acid, Plant defenses, Tomato, Type VI
Introduction
Light intensity, i.e. levels of photosynthetically active radiation (PAR; 400–700 nm), is a potent regulator of plant growth and development and, consequently, has a great impact on plant–herbivore interactions (Gouinguen� and Turlings 2002, Roberts and Paul 2006, V�nninen et al. 2010, Ballar� 2014). PAR affects the photosynthetic capacity and CO2 fixation, altering the concentration of primary metabolites such as carbohydrates, and the fixation of nitrogen-based metabolites, e.g. amino acids (Nunes-Nesi et al. 2010), in plant tissues. These responses can affect the availability of nutrients for arthropod herbivores and, therefore, herbivore development and/or survival on the host plant. In addition, increased PAR induces the production of carbon-based compounds, such as phenolics and terpenes, that not only serve as ‘sunscreens’ and/or antioxidants (Agati et al. 2013), but also function as chemical defenses against biotic stressors (Roberts and Paul 2006, Leiss et al. 2009, Holopainen and Gershenzon 2010, Zavala et al. 2015). Similarly, leaf thickness, toughness and trichome densities all have been reported to increase under enhanced PAR conditions as part of the plant’s strategy to optimize the interaction of leaves with the incident light (Kennedy et al. 1981, P�rez-Estrada et al. 2000). In particular, trichomes, which are epidermal hairy structures, can reduce the absorbance of excess solar radiation by the mesophyll and facilitate the condensation of air moisture onto the leaf surface (Ehleringer et al. 1976, Vogelmann1993, Bickford2016). Altogether, these photomorphogenic responses can negatively affect consumption of plant tissues by arthropod herbivores (Kennedy et�al. 1981, Gianfagna et�al. 1992, Nihoul 1993, Martinez-Garza and Howe 2005, Schoonhoven et al. 2005). For example, a tough leaf can be harder to chew and more energetically costly to digest for chewing insects such as caterpillars (Caldwell et�al. 2016). Plant trichomes also contribute to plant resistance against herbivorous arthropods by physically hindering their movement or, in the case of glandular trichomes, by producing sticky, toxic and/or volatile substances that either restrain, harm or deter herbivores, or attract their natural enemies (van Dam et al. 1998, Weinhold and Baldwin 2011, Glas et�al. 2012). Thus, although trichomes are constitutively produced on leaves, plants can also modulate leaf trichome density in response to abiotic and biotic stresses (Gianfagna et al. 1992, Snyder et al. 1998, Traw and Dawson 2002, Escobar-Bravo et al. 2016, Escobar-Bravo et al. 2017).
In cultivated tomato (Solanum lycopersicum), type VI glandular trichomes are the most abundant trichome type on the leaves, contributing to important chemical and physical defenses against herbivores (Kang et al. 2010a, Tian et al. 2012, Kang et�al. 2014). They consist of a short multicellular stalk and a four-celled glandular head with the capacity to produce, store and secrete diverse specialized secondary metabolites, such as terpenes, acylsugars, phenolics and defensive proteins (Kennedy 2003, Glas et al. 2012, Balcke et al. 2017). Disruption of the type VI trichome’s head by arthropod movement and/or feeding releases these compounds, thus deterring herbivory, or priming for defense-related signaling pathways (Peiffer et al. 2009). In particular, some trichome-derived mono- and sesquiterpenes increase the antixenotic and antibiotic properties of the host plant against a wide array of herbivorous arthropods (Eigenbrode et al. 1994, Maluf et al. 2001, De Azebedo et al. 2003, Bleeker et al. 2009, Bleeker et al. 2012). Additionally, oxidation of phenolics by defensive polyphenol oxidase proteins after trichome rupture produces a rigid and sticky exudate that impedes the movement of small insects, or directly reduces herbivore performance upon ingestion (Kennedy 2003, Constabel and Barbehenn 2008).
Increased PAR has been reported to induce type VI leaf glandular trichome densities in cultivated tomato (S. lycopersicum), which was proposed to explain the enhanced physical entrapment of the spider mite Tetranychus urticae on the leaf surface (Nihoul 1993). However, to the best of our knowledge, the effect of light intensity on the trichome-associated leaf chemistry of cultivated tomatoes has not been studied before. Induction of tomato leaf trichome-associated allelochemicals by abiotic conditions has only been described for the wild species S. habrochaites f. glabratum and S. habrochaites f. hirsutum (Kennedy et�al. 1981, Gianfagna et�al. 1992). The first study reported on the effect of light intensity on trichome density and production of methylketones by type VI trichomes, and the latter on the effect of temperature and photoperiod on the production of a sesquiterpene that is not produced by the glandular trichomes of cultivated tomatoes. Hence, the trichome chemistry of cultivated and wild tomatoes is very different, in terms of which compounds are produced and in terms of quantities (McDowel et al. 2011). Furthermore, although the above-mentioned studies addressed the effect of light intensity on some of the trichome-associated features, the role of other plant photomorphogenic responses in tomato defenses against herbivores was mostly overlooked. Hence, it is unknown whether light intensity-mediated induction of type VI trichome density and associated chemistry might be responsible for an increased resistance against arthropod pests in cultivated tomatoes. Additionally, and most importantly, knowledge of the responses of these biochemical factories to increasing light intensities will be useful for improving tomato defenses against pests and pathogens in agriculture systems under changing climatic conditions.
Here we investigated the effect of changes in light intensity (i.e. PAR levels) on trichome-associated chemical defenses of cultivated tomato against the generalist Western flower thrips Frankliniella occidentalis [Pergande], a key agricultural pest that affects both crop and ornamental plant production (Mouden et al. 2017). To do so, we used the tomato mutant odorless-2 (od-2), which is deficient in type VI glandular trichome development and production of diverse trichome-associated metabolites, especially terpenes and flavonoids (Kang et al. 2010a). We analyzed how two contrasting PAR levels, low and high, affected type VI trichome density and their associated volatile and non-volatile allelochemicals, as well as tomato resistance against thrips, by performing non-choice and choice bioassays. In addition, we characterized tomato physiological responses to PAR by analyzing the leaf metabolome and concentrations of growth- and defense-related hormones, as well as the expression of jasmonic acid-related defense genes, a defense signaling pathway involved in tomato resistance against thrips (Li et�al. 2002, Escobar-Bravo et�al. 2017). Together, our study reveals how light intensity not only influences trichome densities in cultivated tomato, but also modulates the glandular trichome-associated leaf chemistry and other leaf tissue-associated defenses against an insect pest.
Results
High PAR increases tomato resistance to thrips in the wild type but not in od-2
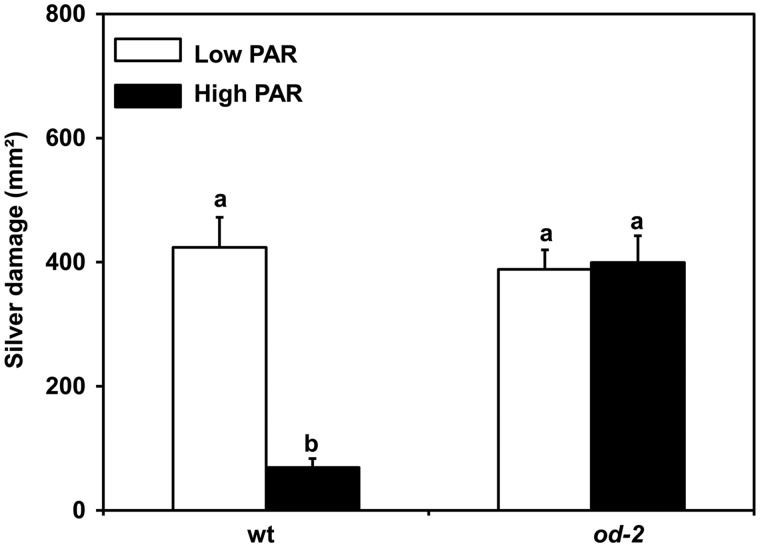
Under low PAR (∼56–65 �mol m–2 s–1), conditions both wild-type and od-2 plants showed similar levels of susceptibility to thrips, displaying equivalent amounts of thrips feeding damage, hereafter referred to as ‘silver damage’ (Fig.�1). However, under high PAR (∼200–300 �mol m–2 s–1), a significant reduction in silver damage was observed in wild-type plants when compared with low PAR-treated wild-type plants [analysis of variance (ANOVA): PAR, P = 0.024; plant genotype, P = 0.103; interaction, P = 0.007]. Conversely, no reduction in silver damage symptoms was observed for od-2.
Fig. 1.
Effect of low and high PAR treatments on tomato resistance to the Western flower thrips F. occidentalis, tested in a whole plant no-choice bioassay, at 35 d after initial light treatments. Silver damage caused by thrips feeding was measured in low and high PAR-treated wild-type (wt) and odorless-2 (od-2) plants at 12 d after thrips infestation. Values represent the mean (� SEM) of 26 plants from three independent experiments. Different letters above bars denote significant differences among groups (ANOVA followed by Fisher’s LSD test, P ≤ 0.05).
High PAR increases antixenosis properties in wild-type and od-2 plants, but thrips preferred od-2 over the wild type
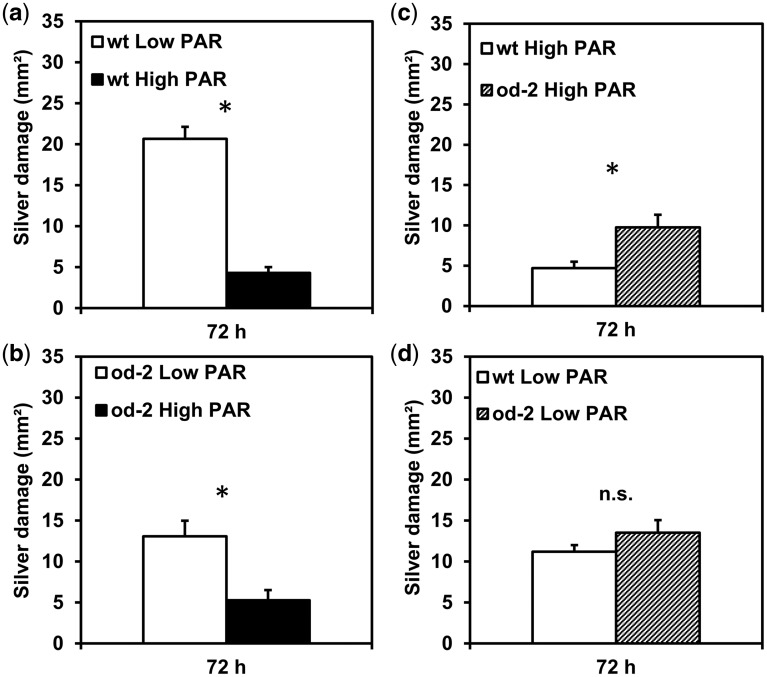
To investigate further whether PAR altered host plant suitability for thrips in od-2 and wild-type plants differently, thrips feeding preference for low vs. high PAR-treated wild-type or od-2 plants, and for wild-type vs. od-2 plants when both genotypes were subjected to low or high PAR conditions, were determined in leaf disc dual-choice assays. Thrips caused more silver damage in leaf discs taken from low PAR-treated wild-type plants than those taken from high PAR-treated wild-type plants (Z = –4.706, P < 0.001) (Fig.�2a). The same pattern was observed for od-2; thrips caused more silver damage in leaf discs taken from low PAR-treated plants than in those subjected to high PAR (Z = –2.83, P = 0.005) (Fig.�2b). Yet, when exposed to wild-type and od-2 leaf discs taken from plants subjected to high PAR, thrips showed preferential feeding for od-2 (Z = –2.962, P = 0.003) (Fig.�2c). Conversely, thrips did not discriminate between leaf discs taken from wild-type and od-2 plants both grown under low PAR (Z = –0.959, P = 0.338) (Fig.�2d).
Fig. 2.
Feeding preference of the Western flower thrips F. occidentalis for low and high PAR-treated wild-type (wt) and odorless-2 (od-2) plants tested in leaf disc dual-choice bioassays. Leaf discs were taken from leaflets belonging to the third/fourth youngest leaf at 35 d after initial light treatments. Silver damage (mean � SEM, n = 25–30) caused by thrips feeding was evaluated at 72 h after thrips release in the following pair-wise comparisons: (a) low PAR vs. high PAR-treated wild-type plants; (b) low PAR vs. high PAR-treated od-2 plants; (c) high PAR-treated wild-type vs. high PAR-treated od-2 plants; and (d) low PAR-treated wild-type vs. low PAR-treated od-2 plants. Pooled data from three independent experiments were analyzed. Asterisks denote significant differences tested by Wilcoxon signed rank test (P ≤ 0.05). n.s. = not significant.
High PAR increases type VI trichome densities in wild-type and od-2 plants, but trichome-associated volatiles are induced only in the wild type
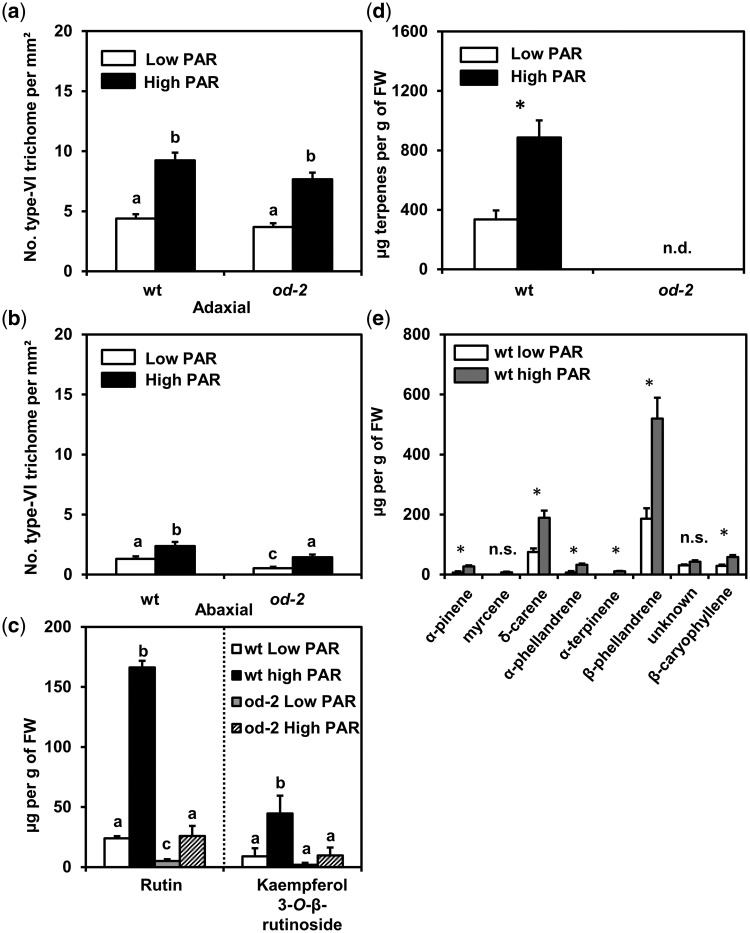
Light intensity had a significant effect on type VI trichome densities in wild-type and od-2 plants (Fig.�3a, b). High PAR increased type VI trichome densities on adaxial leaf sides (ANOVA: PAR, P = 0.002; genotype, P = 0.278; interaction, P = 0.988) (Fig.�3a), as well as on abaxial leaf sides (ANOVA: PAR, P = 0.001; genotype, P = 0.234; interaction, P = 0.628) (Fig.�3b) in both tomato genotypes. Despite the induction of type VI trichomes in od-2, the size of type VI glands was visibly smaller than those of the wild type (Supplementary Fig. S2). This agrees with prior observations by Kang et al. (2010a).
Fig. 3.
Effect of low and high PAR treatments on type VI leaf trichome-associated defenses in wild-type (wt) and odorless-2 (od-2) plants. Type VI trichome density was evaluated on (a) adaxial and (b) abaxial leaf sides of leaflets taken from the third/fourth youngest leaf at 35 d after initial light treatments. Values represent the mean (� SEM) of 28–29 plants from three independent experiments. (c) Main phenolic compounds identified in leaf exudates of low or high PAR-treated wild-type and od-2 plants. Values represent the mean (� SEM) of nine plants from two independent experiments. (d) Total terpene content (mean � SEM, n = 4–5) measured in leaf exudates of leaflets taken from the third/fourth youngest leaf and (e) levels (mean � SEM, n = 4–5) of individual terpene compounds. Asterisks denote significant differences between low and high PAR-treated wild-type plants analyzed by t-test or Mann–Whitney U tests (P ≤ 0.05). Different letters above bars denote significant differences among groups (ANOVA followed by Fisher’s LSD test, P ≤ 0.05). n.s. = not significant. n.d. = not detected.
To determine whether higher type VI trichome densities positively correlated with increased production of trichome-derived allelochemicals, levels of phenolic and terpene compounds reported to be produced by type VI glands (Kang et al. 2010a) were measured in leaf exudates of low and high PAR-treated wild-type and od-2 plants (Fig.�3c, e). Significantly higher levels of the flavonoid rutin were detected in the leaf exudates of wild-type and od-2 plants grown under high PAR (ANOVA: PAR, P = 0.043; genotype, P = 0.015; interaction, P = 0.025) (Fig.�3c). Yet, rutin content in leaf exudates of low and high PAR-treated od-2 plants was significantly lower than in the wild-type. The flavonoid kaempferol 3-O-β-rutinoside was also slightly induced in the wild-type under high PAR; however, the effect of PAR was not significant (ANOVA: PAR, P = 0.372; genotype, P = 0.411; interaction, P = 0.244). High PAR increased the total terpene content in leaf exudates of wild-type plants (t-test: P = 0.005) (Fig.�3d). Terpene compounds were not detected in the leaf exudates of od-2 under any of the light conditions. Similar results were observed in a second repetition of the experiment (Supplementary Fig. S3; Notes S1). Among the terpenes significantly induced by high PAR in wild-type plants, increased levels of α-pinene, δ-carene, α-phellandrene, α-terpinene, β-phellandrene and β-caryophyllene were observed (t-test: P < 0.05) (Fig.�3e).
High PAR increases the number and thickness of tomato leaves
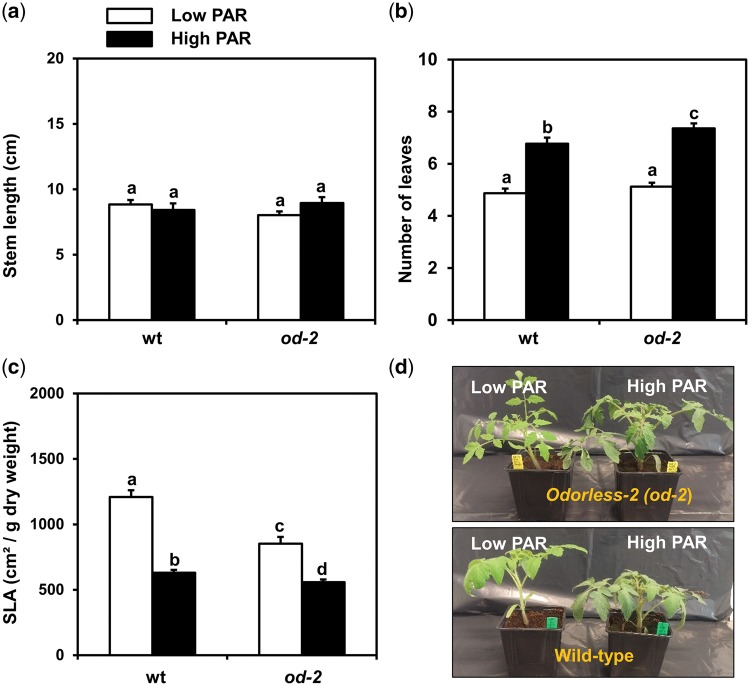
PAR conditions did not affect the length of the stems of wild-type and od-2 plants, and no differences between the two genotypes were observed either (ANOVA: PAR, P = 0.751; genotype, P = 0.848; interaction, P = 0.170) (Fig.�4a). Conversely, high PAR significantly increased the number of leaves in wild-type and od-2 plants (ANOVA: PAR, P = 0.018; genotype, P = 0.215; interaction, P = 0.081) (Fig.�4b). Specific leaf area (SLA) was significantly reduced in wild-type and od-2 plants grown under high PAR conditions (ANOVA: PAR, P = 0.023; genotype, P = 0.012; interaction, P = 0.261) (Fig.�4c). Notably, when compared with the wild type, od-2 plants displayed lower SLA values (i.e. thicker leaves) under both low and high PAR.
Fig. 4.
Effect of low and high PAR treatments on plant growth parameters. Graphs depict (a) stem length, (b) specific leaf area (SLA) and (c) number of leaves determined in low and high PAR-treated wild-type (wt) and odorless-2 (od-2) plants at 35 d after initial light treatments. Values of stem length and number of leaves represent the mean (� SEM) of 52–56 plants, while for SLA values represent the mean (� SEM) of 24–28 plants from three independent experiments. Different letters above bars denote significant differences among groups tested by ANOVA followed by Fisher’s LSD test (P ≤ 0.05). (d) Representative photographs of 5-week-old wild-type and od-2 plants exposed to low or high PAR conditions.
Wild-type and od-2 leaves experience similar metabolomic changes under high PAR conditions
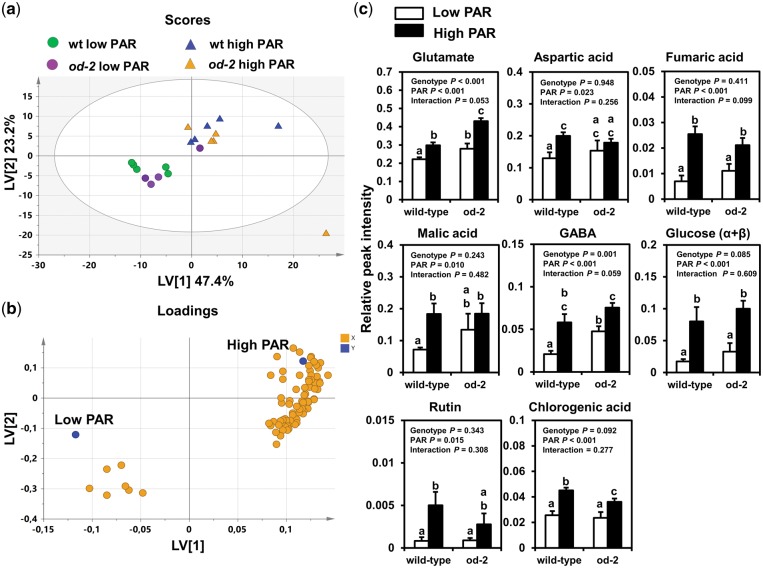
A total of 244 signals were detected in leaf extracts of low and high PAR-treated wild-type and od-2 plants by 1 H nuclear magnetic resonance (NMR). A multivariate partial least squares discriminant analysis (PLS-DA) of the detected signal profiles resulted in a model with three latent variables (LVs) explaining 82% of the total metabolomic variation and 84.6% of the light treatment response, with a 76.3% total model predictability [model statistics: R2X = 0.82, R2Y = 0.846 and Q2 = 0.763; CV-ANOVA (ANOVA of the cross-validated residuals), P < 0.001] (Fig.�5). The first LV explained 47.73% of the variance and separated low PAR-treated from high PAR-treated wild-type and od-2 plants (Fig.�5a). The second LV explained 23.4% and did not show a clear pattern of separation, suggesting a component related to the variability among samples. Differences between low and high PAR-treated wild-type and od-2 plants were mainly explained by 90 signals with variable importance for projection (VIP) scores >1 (Fig.�5b). Among these, 20 signals were identified, which corresponded to glutamate (δ 2.04), aspartic acid (δ 2.68, 2.80), fumaric acid (δ 6.56), malic acid (δ 2.48), γ-aminobutyric acid (GABA) (δ 2.32, 3.0), glucose (δ 4.56, 5.20), rutin (δ 5.04, 6.32, 7.0) and chlorogenic acid (δ 5.12, 6.40, 6.44, 6.48, 6.88, 7.08, 7.16, and 7.68). The effects of PAR and plant genotype were tested on the relative abundance (i.e. scaled to the internal standard) of these compounds (Fig.�5c). High PAR increased leaf content of the amino acids glutamate, aspartic acid and the non-protein amino acid GABA in wild-type and od-2 plants. Significant induction of aspartic acid, however, was only observed in the wild type. High PAR also increased levels of the organic acids fumaric and malic acid, though induction of malic acid was statistically significant only in the wild type. In both genotypes, higher levels of glucose, the flavonoid rutin and the phenylpropanoid chlorogenic acid were detected under high PAR.
Fig. 5.
Metabolomic responses in wild-type (wt) and odorless-2 (od-2) plants under low or high PAR conditions. Leaf metabolites were analyzed by NMR at 35 d after initial light treatments on leaflets collected from the third/fourth youngest leaf. Projection to latent structures-discriminant analysis (PLS-DA) was performed on the obtained 1H NMR spectra, and resulted in three latent variables (LVs) that cumulatively explained 82% of the total metabolomic variation and 84.6% of the light treatment response, with a 76.3% total model predictability. (a) Score plot showing the first two LVs. (b) Loading plot showing metabolites contributing most to the model (VIP score >1). (c) Relative spectral intensities (mean � SEM, n = 4–5), scaled to the internal standard, of selected metabolites (VIP score >1) identified in the 1H NMR spectra. Different letters above bars denote significant differences among groups (ANOVA followed by Fisher’s LSD test, P ≤ 0.05).
High PAR increases the leaf levels of ABA in both genotypes, and of auxin in od-2, but it does not affect the concentrations of SA, OPDA, JA and JA-Ile
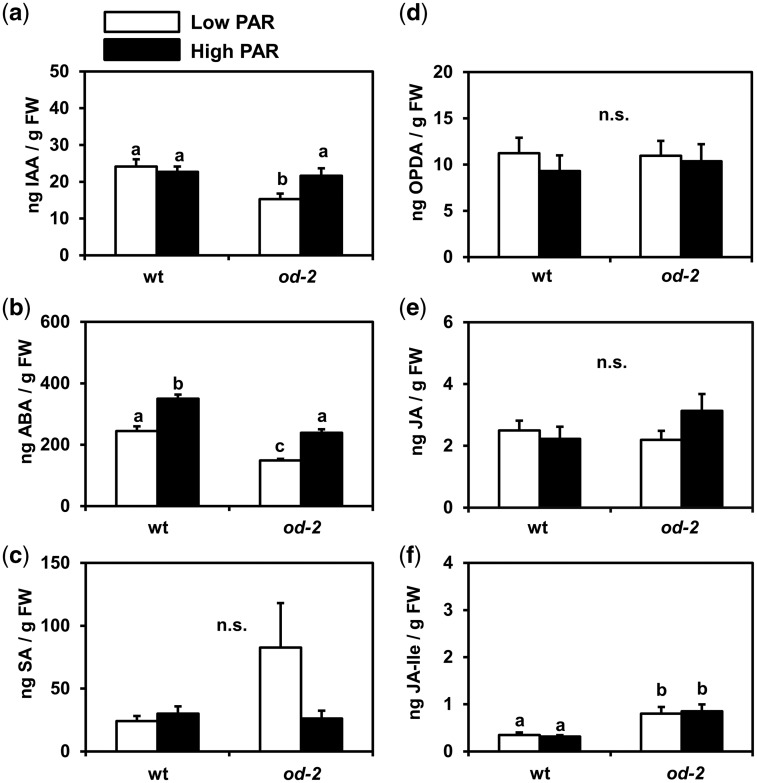
To obtain more insight into the physiological responses of tomato leaves to high PAR that might explain the differences in susceptibility to thrips, we determined the levels of growth- and defense-related hormones (Fig.�6). PAR did not affect the levels of IAA (auxin), but under low PAR the od-2 mutant contained significant lower concentrations than the wild type, while under high PAR both the wild type and od-2 had similar levels (ANOVA: PAR, P = 0.180; genotype, P = 0.012; interaction, P = 0.042) (Fig.�6a). In both genotypes, high PAR significantly increased the levels of ABA, these levels being higher in the wild type than in od-2 (ANOVA: PAR, P < 0.001; genotype, P < 0.001; interaction, P = 0.536) (Fig.�6b). High PAR did not affect the levels of salicylic acid (SA; ANOVA: PAR, P = 0.301; genotype, P = 0.259; interaction, P = 0.092) or the jasmonic scid (JA) precursor 12-oxo-phytodienoic acid (OPDA; ANOVA: PAR, P = 0.475; genotype, P = 0.821; interaction, P = 0.701) (Fig.�6c, d). JA levels were also not affected by PAR conditions (ANOVA: PAR, P = 0.414; genotype, P = 0.462; interaction, P = 0.150) (Fig.�6e). Similarly, PAR did not affect jasmonic acid-isoleucine (JA-Ile) levels, but these were significantly higher in od-2 plants than in the wild type irrespective of the treatment (ANOVA: PAR, P = 0.930; genotype, P < 0.001; interaction, P = 0.708) (Fig.�6f).
Fig. 6.
Concentrations of (a) IAA, (b) ABA, (c) salicylic acid (SA), (d) 12-oxo-phytodienoic acid (OPDA), (e) jasmonic acid (JA) and (f) jasmonic acid-isoleucine (JA-Ile) determined in low and high PAR-treated wild-type (wt) and odorless-2 (od-2) plants at 35 d after the initial light treatments. The analysis was performed on leaflets collected from the third/fourth youngest leaf. Values represent the mean (� SEM) of five individual plants. Different letters above bars denote significant differences among groups (ANOVA followed by Fisher’s LSD test, P ≤ 0.05). n.s. not significant.
High PAR induces the expression of JA-associated defense genes in both wild-type and od-2 plants
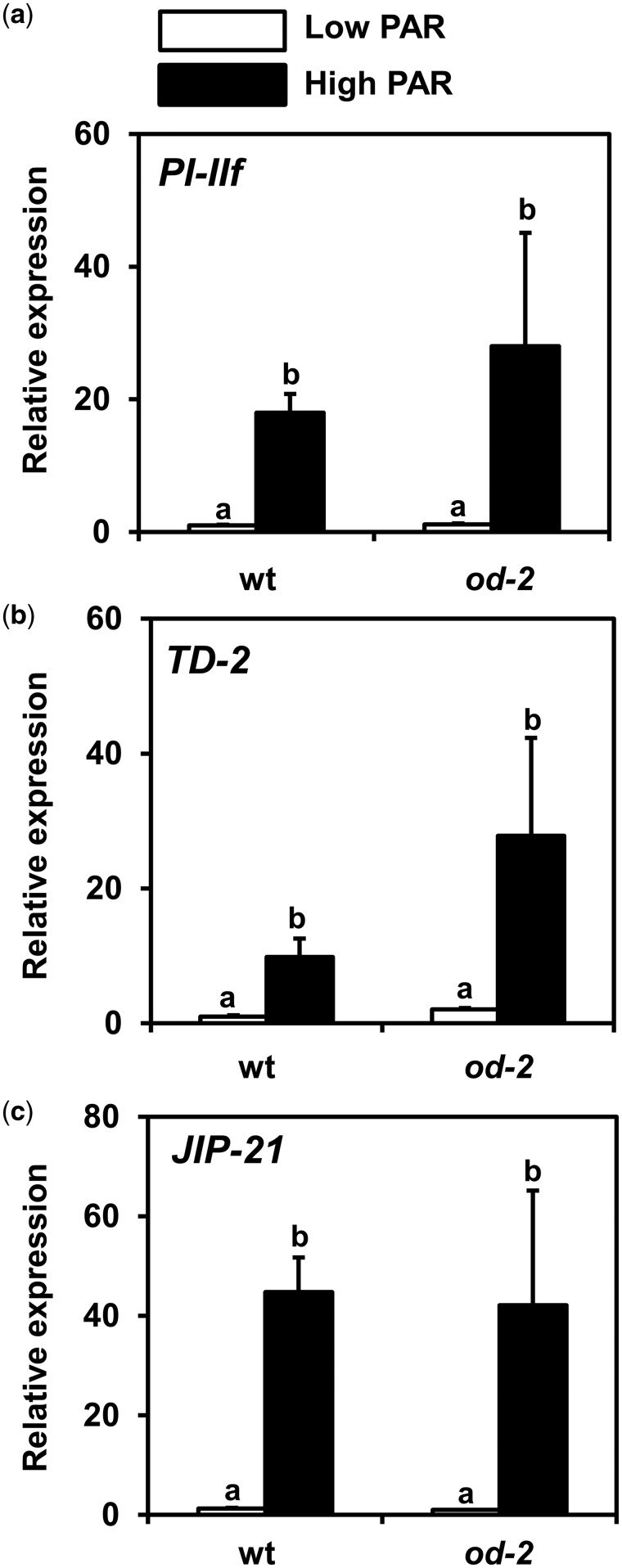
To investigate whether increased PAR altered basal levels of JA-associated defenses, which are important for tomato defenses against thrips (Li et�al. 2002, Escobar-Bravo et�al. 2017), expression of the JA-responsive genes PI-IIf (proteinase inhibitor-IIf), TD-2 (threonine deaminase-2) and JIP-21 (jasmonate inducible protein-21J) was analyzed in low and high PAR-treated wild-type and od-2 plants (Fig.�7). High PAR significantly induced the expression of PI-IIf in wild-type and od-2 plants (ANOVA: PAR, P < 0.001; genotype, P = 0.985; interaction, P = 0.608) (Fig.�7a). Likewise, expression of TD-2 (ANOVA: PAR, P < 0.001; genotype, P = 0.117; interaction, P = 0.612) (Fig.�7b) and JIP-21 (ANOVA: PAR, P < 0.001; genotype, P = 0.234; interaction, P = 0.592) (Fig.�7c) was significantly induced under high PAR in both genotypes.
Fig. 7.
Relative transcript levels of the JA-responsive genes (a) proteinase inhibitor-IIf (PI-IIf), (b) threonine deaminase-2 (TD-2) and (c) jasmonate inducible protein-21 (JIP-21) measured in low and high PAR-treated wild-type (wt) and odorless-2 (od-2) plants at 35 d after the initial light treatment. The analysis was performed on leaflets collected from the third/fourth youngest leaf. Values represent the mean (� SEM) of relative expression of each treatment group (n = 5 biological replicates, two technical replicates). Different letters above bars denote significant differences among groups (ANOVA followed by Fisher’s LSD test, at P ≤ 0.05.
Discussion
Here we have demonstrated that increased PAR affects not only trichome densities but also the accumulation of trichome-derived allelochemicals in the leaf, JA-associated defenses, ABA concentrations, as well as leaf number and thickness in cultivated tomato. Moreover, the use of two tomato genotypes differing in the presence of functional trichomes, but displaying similar physiological responses when exposed to two contrasting PAR levels, allowed us to demonstrate that PAR-mediated induction of type VI trichome-associated allelochemicals significantly increases resistance of cultivated tomatoes to Western flower thrips.
First, we found that wild-type tomato plants grown under high PAR suffered less thrips damage than those grown under low PAR in whole plant no-choice bioassays. This difference, however, was not observed in the tomato mutant od-2, deficient in type VI trichome-associated compounds (Kang et al. 2010a) (Fig.�1). Consistent with these results, thrips caused more damage to leaf discs taken from wild-type plants grown under low PAR conditions in dual-choice assays (Fig.�2a). Surprisingly, thrips showed a similar preference in od-2, i.e. they caused less damage on leaf discs from plants grown under high PAR than on those from the low PAR treatment (Fig.�2b). However, when compared with the wild type, a 4-fold reduction in silver damage was observed in leaf discs of high vs. low PAR-treated wild-type plants, while only a 2-fold reduction in silver damage was detected in leaf discs taken from high vs. low PAR-treated od-2 plants (Fig.�2a, b). To investigate further the level of susceptibility of od-2 with respect to the wild type under high PAR, we determined thrips feeding preference between high PAR-treated wild-type and od-2 plants. Our results showed that thrips preferred leaf discs of high PAR-treated od-2 plants over those taken from high PAR-treated wild-type plants (Fig.�2c). Thus, we concluded that od-2 plants grown under high PAR were more susceptible to thrips than the wild type.
Our results also showed that although type VI trichome densities were increased in the wild type and od-2 under high PAR, only wild-type plants produced significantly more trichome-associated allelochemicals. Both terpenes and flavonoids were strongly induced under high PAR in the wild type, but not detected or hardly altered in od-2 trichome-derived extracts. This agrees with results previously reported by Kang et al. (2010a), where lower levels of trichome-derived monoterpenes, sesquiterpenes and flavonoids were described for od-2 and associated with higher susceptibility to diverse arthropod herbivores. The lack of induction of these trichome-associated allelochemicals might explain the differences in thrips susceptibility between the wild type and od-2 under high PAR conditions, as well as the higher vulnerability to thrips in wild-type plants subjected to low PAR conditions (Fig.�1). Terpenes are well known for their significant role in direct and indirect plant defenses against herbivorous arthropods (Kant et al. 2009). Multiple trichome-derived mono- and sesquiterpenes have been identified as potent repellent compounds against arthropod pests in wild tomato species (De Azevedo et al. 2003, Gon�alves et al. 2006, Bleeker et�al. 2009, Bleeker et al. 2012). For instance, the monoterpenes p-cymene, α-terpinene and α-phellandrene have repellent properties against whiteflies in tomato (Bleeker et al. 2009). Notably, higher levels of α-phellandrene and α-terpinene were detected in leaf exudates of high PAR-treated wild-type plants. Additionally, the amount of β-phellandrene was strongly increased in the trichome-derived exudates of wild-type plants grown under high PAR. However, β-phellandrene has not been associated with repellent properties against insects. Finally, a 4.2-fold induction of the flavonoid rutin (quercetine 3-O-rutinoside) was observed in the trichome-derived exudates of wild-type plants under high PAR. Interestingly, the magnitude of this induction was higher when compared with the 2.5-fold increase in terpene levels. This might be explained by the fact that flavonoids can function as photo-protective ‘sunscreens’ and antioxidants by inhibiting and/or reducing high light stress-induced reactive oxygen species levels (Agati et al. 2012). Accordingly, quercetine glycosides have been reported to increase in various plant species (Agati et al. 2009, L�vdal et�al. 2010), as well as in the secretory products of Phillyrea latifolia leaf glandular trichomes, upon high irradiance (Tattini et al. 2000). Furthermore, the enhanced content of flavonoids in the leaf exudates of high PAR-treated wild-type plants was higher than the increase in type VI trichome densities (i.e. 2.2-fold increase), suggesting a higher production of these compounds per trichome. To our knowledge, the induction of flavonoids in type VI glandular trichomes by environmental cues has not been previously reported. In addition to their photo-protective and antioxidant properties, flavonoids and other phenolics might play an important role in plant defenses against insects. Enzymatic oxidation and browning reaction of phenolics have been proposed to increase entrapment of small arthropods, impede their feeding or act as anti-nutritive defenses (Duffey1986). This might explain the increased physical entrapment of T. urticae mites in tomato plants exposed to higher light intensities described by Nihoul (1993).
Plant physiology and the metabolome of wild-type and od-2 plants were profoundly affected by PAR. In greenhouse conditions, PAR fluctuates during the day. For instance, G�mez and Mitchell (2015) described that this can result in daily light integrals (DLIs) of 2–10 mol m–2 d–1 during the winter and of 25–35 mol m–2 d–1 during the summer in a humid continental climate (data collected in Indiana, USA). It should be noted that the DLI measured inside greenhouses is generally lower than outside levels (i.e. DLI values in June and July of the northern hemisphere are close to 46 mol m–2 d–1), as the greenhouse infrastructure might reduce the DLI (Bugbee 1994, Hanan1998). In our study, plants were exposed to 65 or 300 �mol m–2 s–1 for 16 h per day, resulting in a DLI of approximately 2.8 and 17 mol m–2 d–1, respectively. Notably, DLIs of 13–16 mol m−2 d−1 are reported to increase the net photosynthesis rate and are considered the optimal growth conditions for young tomato plants (Fan et al. 2013). Thus, we expected that increases in light intensity from 50 to 300 �mol m–2 s–1 would induce strong photomorphogenetic responses in young tomato plants (de Groot et al. 2001, Fan et�al. 2013). Accordingly, plants grown under high PAR had significantly more and thicker leaves, which can result from both increased tissue density and cell wall thickness, as described in Fan et�al. (2013) for tomato leaves. These responses were similar to those described for tomato (Fan et al. 2013) and other plant species (Kitaya et al. 1998, Oguchi et al. 2003, Chang et al. 2008) under high PAR. Yet, leaf thickness was markedly higher in od-2 than in the wild type under both PAR conditions. This might be explained by a larger investment of resources in plant growth in the tomato mutant, but also by the reduced levels of auxin in comparison with the wild type under low PAR (Fig.�6a) (Deng et al. 2012). Hence, the lack of PAR-mediated induction of trichome-associated metabolites in od-2 might have conferred certain growth-related advantages (i.e. more and thicker leaves) over the wild type (Neilson et al. 2013, Z�st and Agrawal 2017). Thicker leaves can affect the leaf mechanical properties and, therefore, defenses against insect herbivores (Hanley et al. 2007, Caldwell et al. 2016). Increased leaf thickness might have reinforced the mechanical resistance of od-2 leaves against thrips feeding. However, although this might explain the preferential feeding of thrips on PAR-treated od-2 leaf discs over those from high PAR-treated plants in the dual-choice assays, the level of susceptibility in the tomato mutant was still higher than in the wild type under high PAR (Fig.�1).
Untargeted NMR metabolomic analysis of wild-type and od-2 leaves further revealed that both genotypes experienced similar responses when grown under increased PAR conditions. High PAR increased the content of amino acids, organic acids, glucose and phenolics in wild-type and od-2 young leaves. These results are in line with previous studies reporting the increased production of soluble sugars, and organic acids such as fumaric acid, under enhanced light irradiance (Chia et al. 2000, Cou�e et al. 2006). Light also controls the nitrate assimilation, providing the reducing power for the incorporation of nitrate into amino groups in plants (Foyer and Noctor 2006). This higher photosynthetic capacity might explain the increase in glutamate, a C and N source for the biosynthesis of most other amino acids, as well as the non-protein amino acid GABA (Forde and Lea 2007). Accumulation of GABA is a common plant response to biotic and abiotic stresses (Ramesh et�al. 2015), and its induction might be involved in plant defenses against herbivores (Ramputh and Bown 1996, McLean et al. 2003, Scholz et al. 2015, Bown and Shelp 2016). However, the detected variations in GABA, amino acid and glucose levels cannot explain the differences in tomato susceptibility because they experienced the same variations in both genotypes. On the contrary, an increased production of some of these photo-assimilates was expected to increase the nutritional quality of tomato plants for thrips. This reinforces the hypothesis that food requirements of generalist insects might not be a limiting factor for host plant selection, but that the latter mostly relies on differences in secondary metabolites and other chemical plant defenses (Fraenkel 1959, Chen et al. 2005, K�hler et al. 2015), such as the reinforcement of trichome-associated defenses described here. Yet, under high PAR, the increases in phenolic compound levels, such as rutin and chlorogenic acid, observed in both genotypes might have contributed to the increased repellency against thrips observed in the two-choice leaf disc bioassays. Enhanced rutin levels can deter insect feeding (reviewed by Simmonds 2001), and chlorogenic acid has been positively associated with thrips resistance in chrysanthemum (Leiss et al. 2009).
Plant hormones are central regulators of light acclimation (Kazan and Manners 2011, Dietz et al. 2015) and defenses against herbivorous arthropods (Pieterse et al. 2012). In particular, ABA plays a fundamental role in the regulation of plants’ water status (Christmann et�al. 2006), and its production is required for an effective physiological response of leaves to a fluctuating light environment (Galvez-Valdivieso et�al. 2009). Leaf trichomes can affect the plant water use efficiency when exposed to high light irradiances (Bickford 2016). The lack of functional trichomes in od-2 was expected to increase the water- and high light-associated stress and, accordingly, ABA levels in comparison with the wild type. However, our results showed that high PAR similarly affected the levels of ABA in wild-type and od-2 plants. Yet, these levels were significantly higher in the wild type irrespective of the light treatment (Fig.�6b), which might be associated with a stronger response to high irradiances and water stress. Notably, ABA is also required to activate fully JA-dependent defense responses against herbivores in systemic tissues, and this synergistic interaction is suggested to occur upstream and downstream of JA signaling (see review by Nguyen et�al. 2016). To what extent the higher levels of ABA detected in wild-type tomato plants are involved in resistance against thrips is unknown. Future experiments using ABA- and JA-deficient tomato genotypes might extend our knowledge of the molecular mechanisms of high PAR-mediated induction of plant resistance to arthropod herbivores.
The development and chemical content of type VI trichomes have been described to be controlled by JA (Li et�al. 2004, Boughton et al. 2005, Van Schie et al. 2007, Escobar-Bravo et�al. 2017). Notably, levels of the jasmonates OPDA, JA and JA-Ile were not altered in plants exposed to 35 d of low or high PAR. Increases in these plant hormones have been described during light acclimation of Arabidopsis thaliana plants transferred from low to high light conditions (Alsharafa et al. 2014). Yet, those changes were reported to occur within hours, and JA and JA-Ile returned to basal levels at 6 h after the transfer to high-light conditions. Possibly, fluctuations in jasmonate levels prior to our sampling moment might explain the induction of type VI trichomes in wild-type and od-2 plants. Interestingly, although no differences in jasmonate concentrations were detected, JA-responsive genes were significantly induced in wild-type and od-2 plants under high PAR. The similar levels of induction of JA-associated responses in od-2 and the wild type confirmed previous results described by Kang et al. (2010a), where responses to mechanical wounding were comparable in both genotypes. Activation of JA defenses is important for tomato resistance against thrips (Li et�al. 2002, Escobar-Bravo et�al. 2017). Thus, reinforcement of these defenses in wild-type and od-2 plants would be expected to increase tomato resistance to thrips in both genotypes. In whole-plant bioassays, however, high PAR increased resistance against thrips in the wild type but in not in od-2. Moreover, under high PAR, od-2 showed higher susceptibility than the wild type in dual-choice assays. We hypothesize that PAR-mediated enhancement of JA signaling might have indeed increased od-2 defenses, but they were insufficient to reach the resistance levels observed in the wild type. Taken together, these results suggest that high PAR-mediated induction of type VI trichome-associated chemical defenses in the wild type, but absent in od-2, indeed play an important role in tomato resistance against thrips.
Until recently, research on type VI trichomes of cultivated tomatoes has focused on unraveling their development and the genetic control of their associated metabolites (McDowell et al. 2011, Balcke et al. 2014, Bergau et al. 2015, Balcke et�al. 2017). We have previously described that trichome density and overall production of their volatiles per leaf are affected by herbivory in cultivated tomato (Escobar-Bravo et�al. 2017). Here we provide novel insights into the modulation and the biological significance of trichome-associated leaf chemistry under variable abiotic conditions, thus bringing a new perspective for future studies on chemistry and genetic engineering of these biochemical epidermal factories. These novel insights have important implications for agriculture under changing climate conditions.
Materials and Methods
Plant material and light treatments
Seeds of Solanum lycopersicum Mill. cv. ‘Castlemart’ (wild type) and the trichome-deficient mutant odorless-2 (od-2) (kindly provided by Professor Gregg Howe from Michigan State University, USA) were sown in plastic trays filled with potting soil and placed in one of the two climate cabinets provided with either low or high PAR conditions. Fifteen days after germination, plantlets were transplanted to 11 cm diameter plastic pots. Wild-type and od-2 plants were kept under low or high PAR conditions for a total period of 35 d from sowing. To generate low and high PAR levels, the number of incandescent light tubes (Sylvania T8, F30W/830) and distance to the light source were adjusted in each chamber at the beginning of the experiment. Light intensity at the level of the apical tomato leaves increased progressively along with plant height during the experiment, ranging from about 56 �mol m–2 s–1 (at day 1) to about 65 �mol m–2 s–1 (at day 35) under low PAR, and from approximately 200 �mol m–2 s–1 (at day 1) to about 300 �mol m–2 s–1 (at day 35) under high PAR. PAR was measured by a light meter sensor (Eijkelkamp), and the spectral composition by a spectrometer equipped with a cosine corrector (Flame-S, Ocean Optics) (Supplementary Fig. S1). Both cabinets were provided with a photoperiod of 16 h light:8 h dark, 20�C and 70% relative humidity. At day 35, plants were used for non-choice whole-plant and two-choice thrips preference bioassays, trichome density determination, assessment of plant growth parameters, and chemical and gene expression analyses.
Thrips
Western flower thrips (Frankliniella occidentalis) were obtained from a colony reared on chrysanthemum flowers maintained in a climate room at 16 h light:8 h dark, 25�C and 70% relative humidity.
Whole-plant no-choice thrips bioassay
Low and high PAR-treated wild-type and od-2 plants were individually placed into thrips-proof cages consisting of a clear plastic cylinder (80 cm height, 20 cm diameter) closed at the top end with a lid made of thrips-proof gauze (Leiss et�al. 2009). Cages with plants were randomly placed in a climate room provided with 113.6 �mol m–2 s–1 of PAR, a photoperiod of 16 h light:8 h dark, 25�C and 70% relative humidity. Each plant was infested with 20 adult thrips (18 females and two males). After 12 d, thrips feeding damag (‘silver damage’) was evaluated for the whole plant and expressed as mm2 of total damaged leaf area. This bioassay was replicated three times with 6–10 plant replicates per treatment.
Two-choice leaf disc thrips bioassay
A dual-choice assay (Leiss et�al. 2009) was used to test thrips preference for leaf discs taken from low vs. high PAR-treated wild-type or od-2 plants, and for wild-type vs. od-2 plants subjected to low or high PAR conditions. Leaf discs (diameter of 10 mm), each corresponding to an individual plant, were punched from the third/fourth youngest leaf and placed on a thin layer of 1% agar in a 90 mm diameter Petri dish. Ten starved female F. occidentalis adults were briefly anesthetized with CO2 and placed on a filter paper positioned between the discs. The Petri dishes were then sealed with parafilm and placed in a climate room provided with 110 �mol m–2 s–1 of PAR, 25�C and a 16 h light:8 h dark light regime. Silver damage was determined at 72 h after thrips release. This bioassay was performed three times with 6–10 replicates (i.e. Petri dishes) per pair-wise comparison.
Trichome density determination
Type VI glandular trichome density was measured on the adaxial and abaxial surfaces of leaflets taken from the third/fourth youngest as described in Escobar-Bravo et�al. (2017). A Leica MZ16 stereomicroscope (Leica Microsystems) equipped with a Leica DFC420 digital camera was used to take two pictures of an area of 12 mm2 at both leaf sides of the main vein. Type VI trichomes were counted using the ImageJ software (http://imagej.nih.gov/ij/) and density was expressed as number of type VI trichomes per mm2. Trichome density measurements were taken over three independent experiments with 9–10 plant replicates per treatment.
LC/MS analysis of phenolics
Production of the most abundant phenolic compounds by type VI glandular trichomes was analyzed in trichome-derived leaf exudates collected from the third/fourth youngest leaf following the protocol described by Kang et�al. (2010b, 2014) with some modifications. Fresh weight was measured before extraction. Leaflets were placed in 1 ml of 80% methanol aqueous solution and gently shaken for 2 min. The extracts were filtered through a 45 mm syringe filter and 5 μl was used for liquid chromatography–mass spectrometry (LC/MS) analysis. LC/MS was performed using a micrOTOF-QII (Bruker Daltonics GmbH) coupled to an Ultimate 3000 RS (ThermoScientific) UHPLC quaternary pump with a diode array detector. Detection was carried out using electrospray ionization in negative mode over the mass range of m/z 120–1200. Reverse-phase liquid chromatographic separation was performed using a Kinetex C18 column (100�2.10 mm, 2.6 μm particles) (Phenomenex) maintained at 30�C. An elution gradient with a solvent system consisting of 0.1% formic acid (Optima, Fisher Scientific) in MilliQ water (solvent A) and methanol (Merck Millipore) + 0.1% formic acid (solvent B) was used. The gradient profile used an initial condition of 10% solvent B, a 12 min linear gradient to 65% solvent B, a 4 min ramp to achieve 90% solvent B, a 1 min hold at 90% solvent B and return to 10% solvent B over 1.1 min, resulting in a run time of 18.1 min per sample. The flow rate was set at 0.35 ml min–1. Quantification of the main flavonoids detected in leaf exudates was performed in UV light (280–340 nm) using calibration curves derived from the external standards rutin and kaempferol 3-O-β-rutinoside (Sigma-Aldrich). Phenolic content was expressed as μg g-1 FW. This analysis was performed in two independent experiments with 4–5 plant replicates per treatment.
GC/MS analysis of terpenes
Terpene production by type VI glandular trichomes was analyzed in leaf exudates collected from two leaflets, belonging to the same leaf used for trichome density measurement, by using the leaf dip method (Kang et al. 2010a,b, Sallaud et�al. 2012, Kang et al. 2014). This protocol was chosen because the terpenoid profile detected in individually collected type VI glands has been shown to be nearly identical to that observed with the leaf dip procedure (Kang et al. 2010b, Kang et al. 2014). Leaf fresh weight was measured before extraction. Leaf exudates were obtained by dipping the leaf tissue in 2 ml of pentane (Sigma-Aldrich) containing 10 μg of tetradecane (Sigma-Aldrich) as internal standard. Following an incubation period of 2 min with gentle shaking, the leaflets were removed. A 1 μl aliquot of the resulting pentane leaf extract was injected into an Agilent model 7890 gas chromatograph fitted with a 5975C inert XL MSD Triple Axis Detector using a split ratio of 20:1. The initial column (30 m�0.25 mm, 0.25 μm film thickness, DB-5MS, Agilent Technologies) temperature was set at 40�C, then ramped to 150�C at 15�C min–1 and finally to 220�C at 6�C min–1. The helium carrier gas flow was 1.6 ml min–1. Terpenes were identified by comparison with authentic standards when possible, or by comparison with retention times and spectral information available in Agilent GC/MSD ChemStation. Compounds were quantified on the basis of the internal standard procedure described in Escobar-Bravo et al. (2017). For this, α-pinene and β-caryophyllene (Sigma-Aldrich) were used as external standards. Terpene content was expressed as μg g–1 FW. This analysis was performed in two independent experiments with 4–5 plant replicates per treatment.
Plant growth parameters
The number of leaves and stem length were measured in all the plants that were also used for trichome density and SLA determination, chemical analysis and thrips bioassays. Stem length was assessed above the cotyledons. SLA, a parameter used to estimate leaf thickness (Vile et al. 2005), was determined in one leaflet taken from the third/fourth youngest leaf from the apex. This leaflet was also used for trichome density measurement prior to SLA determination. For SLA calculation, the leaflet was scanned and the leaf area was determined by using ImageJ software. Then, the leaflet was dried in an oven at 60�C for 2 d, and dry leaf material was weighed. SLA was expressed as cm2 g–1 of dry mass. Plant growth parameters were determined in three independent experiments with 12–27 plant replicates per treatment.
Nuclear magnetic resonance (NMR) analysis
NMR metabolic analysis was performed on leaflets taken from the third/fourth youngest leaf from the apex. For this, 10 mg of freeze-dried plant material were extracted with 1 ml of KH2PO4 buffer in D2O (pH 6) containing 0.05% trimethylsilane propionic acid sodium salt (TMSP) and CH3OH-d4 (1:1). Plant extracts were vortexed, sonicated for 20 min and centrifuged at 13,000 r.p.m. for 10 min at room temperature. A 300 μl of the supernatant was transferred to NMR tubes for the spectral analysis. 1H NMR spectra were recorded at 25�C on a 600 MHz Bruker AV 600 spectrometer equipped with cryo-probe operating at a proton NMR frequency of 600 MHz, following the procedure described in L�pez-Gresa et al. (2012). The resulting spectra were manually phased, baseline corrected and calibrated to TMSP at 0.0 p.p.m., using Topspin (version 2.1, Bruker). 1H NMR spectra were reduced to ASCII files using AMIX (v. 3.7, Bruker Biospin). Spectral intensities were scaled to the intensity of the internal standard TMSP and reduced to integrated regions of equal width (0.04 p.p.m.) corresponding to the region of δ 0.4–10. Regions in the range of δ 4.7–4.9 and δ 3.28–3.34 corresponding to residuals signals of water and methanol, respectively, were excluded from the analysis.
Hormone analysis
The concentration of the phytohormones OPDA, JA, JA-Ile, SA, ABA and auxin (IAA) was analyzed in leaflets taken from the third/fourth youngest leaf from the apex by means of LC-MS/MS following the procedures described in Machado et al. (2013) and Sch�fer et al. (2016) with minor modifications (Supplementary Methods S1).
Gene expression analysis
Total RNA was isolated as described in Verwoerd et al. (1989) and treated with DNase (Ambion). cDNA was synthesized from 4 �g of total RNA using M-MuLV Reverse Transcriptase (Fermentas) in a 20 �l reaction. Quantitative reverse transcription–PCR (qRT–PCR) was performed in the CFX96™ Optics Module (Bio-Rad) using iQ™ SYBR� Green Supermix (Bio-Rad) following the procedure described in Escobar-Bravo et�al. (2017). Five biological replicates with two technical replicates were analyzed per treatment. Actin was used as a reference gene. The normalized expression (NE) data were calculated by the ΔCt method NE = –(PEtarget∧Cttarget)/ (PEreference∧Ctreference) (PE = primer efficiency; Ct = cycle threshold). The PEs were determined by fitting a linear regression on the Ct values of a standard cDNA dilution series. To plot the relative expression, NE values were scaled, with the lowest average NE within the plot being set to 1. Transcript levels of the JA marker genes PI-IIf (formerly known as WIPI-II, wound-inducible proteinase inhibitor-II, in Farmer et al. 1992), JIP-21 and TD-2 (Alba et al. 2015) were analyzed. Gene-specific primers used for the qRT–PCRs are shown in Supplementary Table S1.
Statistical analysis
Data were analyzed using the SPSS software package (version 21; SPSS Inc.). Residuals were tested for normality and heteroscedasticity of variance. The effects of PAR, plant genotype and their interaction on silver damage, trichome density, phenolic compounds identified in leaf exudates, stem length, number of leaves, SLA, normalized gene expression and hormone levels were analyzed by two-way ANOVA. For this, ‘plant genotype’ and ‘light treatment/PAR’ were considered as fixed factors, and ‘experimental replicate’ as the random factor when pooled data from independent replicated experiments were analyzed. Differences among groups were tested by Fisher’s least significant difference (LSD) post-hoc test. Data on silver damage symptoms from whole-plant bioassays, trichome density in the adaxial leaf side, SLA, normalized expression of PI-IIf, TD-2 and JIP-21 were log transformed prior to analysis to meet ANOVA assumptions. Data on trichome density in the abaxial leaf side and individual phenolic compounds were log (x + 1) and square root (x + 1) transformed, respectively, prior to analysis. Differences in total terpene content and levels of individual terpene compounds in leaf exudates of low and high PAR-treated wild-type plants were analyzed by t-test or, when transformation was not possible, by non-parametric Mann–Whitney U tests. Thrips feeding preference tested in leaf disc dual-choice bioassays obtained from three independent experiments were pooled and analyzed by Wilcoxon signed rank test. For this, data from the three independent experiments were tested for heterogeneity using contingency tables and associated χ2 test. Patterns of chemical signals detected by NMR in leaf extracts of low and high PAR-treated wild-type and od-2 plants were subjected to multivariate analysis using the SIMCA-P 13 software package (Umetrics). A supervised PLS-DA was used to determine the variation in X variables (metabolites) modeled by the Y explanatory variable corresponding to PAR levels. The cumulative variations in X and Y explained by the model are reported as R2X and R2Y, respectively. The resulting model was fit to the minimum number of latent variables showing the highest value of predicted variation (Q2). The important X variables were selected based on a VIP score >1. Effect of plant genotype, PAR and their interaction on the relative peak intensity of identified compounds with VIP score >1 was then tested using a two-way ANOVA followed by LSD post-hoc test.
Funding
This work was supported by Rijk Zwaan, Dummen Orange, Deliflor, Dekker Chrysanten and Incotec [companies involved in the STW Perspective program ‘Green Defense against Pests’ (GAP) (Ref. 13553]; and the German Research Foundation [FZT 118, German Center for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funding to K.G and N.M.V.D.].
Disclosures
The authors have no conflicts of interest to declare.
Supplementary Material
Glossary
Abbreviations
- ANOVA
analysis of variance
- DLI
daily light integral
- GABA
γ-aminobutyric acid
- GC/MS
gas chromatography–mass spectrometry
- JA
jasmonic acid
- JA-Ile
jasmonic acid-isoleucine
- JIP-21
jasmonate inducible protein-21
- LC/MS
liquid chromatography–mass spectrometry
- LSD
least significant difference
- LV
latent variable
- NE
normalized expression
- NMR
nuclear magnetic resonance
- od-2
odorless-2
- OPDA
12-oxo-phytodienoic acid
- PAR
photosynthetically active radiation
- PE
primer efficiency
- PI-IIf
proteinase inhibitor-IIf
- PLS-DA
partial least squares discriminant analysis
- qRT–PCR
quantitative reverse transcription–PCR
- SA
salicylic acid
- SLA
specific leaf area
- TD-2
threonine deaminase-2
- TMSP
trimethylsilane propionic acid sodium salt
- VIP
variable importance in projection
References
- Agati G., Azzarello E., Pollastri S., Tattini M. (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196: 67–76. [DOI] [PubMed] [Google Scholar]
- Agati G., Brunetti C., Di Ferdinando M., Ferrini F., Pollastri S., Tattini M. (2013) Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol. Biochem. 72: 35–45. [DOI] [PubMed] [Google Scholar]
- Agati G., Stefano G., Biricolti S., Tattini M. (2009) Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 104: 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba J.M., Schimmel B.C., Glas J.J., Ataide L., Pappas M.L., Villarroel C.A., et al. (2015) Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 205: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharafa K., Vogel M.O., Oelze M.-L., Moore M., Stingl N., K�nig K., et al. (2014) Kinetics of retrograde signalling initiation in the high light response of Arabidopsis thaliana. Philos. Trans. R. Soc. B: Biol. Sci. 369: 20130424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcke G.U., Bennewitz S., Bergau N., Athmer B., Henning A., Majovsky P., et al. (2017) Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell 29: 960–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcke G.U., Bennewitz S., Zabel S., Tissier A. (2014) Isoprenoid and metabolite profiling of plant trichomes. Plant Isoprenoids 189–202. [DOI] [PubMed] [Google Scholar]
- Ballar� C.L. (2014) Light regulation of plant defense. Annu. Rev. Plant Biol. 65: 335–363. [DOI] [PubMed] [Google Scholar]
- Bickford C.P. (2016) Ecophysiology of leaf trichomes. Funct. Plant Biol. 43: 807–814. [DOI] [PubMed] [Google Scholar]
- Bleeker P.M., Diergaarde P.J., Ament K., Guerra J., Weidner M., Sch�tz S., et al. (2009) The role of specific tomato volatiles in tomato–whitefly interaction. Plant Physiol. 151: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker P.M., Mirabella R., Diergaarde P.J., VanDoorn A., Tissier A., Kant M.R., et al. (2012) Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 109: 20124–20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton A.J., Hoover K., Felton G.W. (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 31: 2211–2216. [DOI] [PubMed] [Google Scholar]
- Bown A.W., Shelp B.J. (2016) Plant GABA: not just a metabolite. Trends Plant Sci. 21: 811–813. [DOI] [PubMed] [Google Scholar]
- Bergau N., Bennewitz S., Syrowatka F., Hause G., Tissier A. (2015) The development of type VI glandular trichomes in the cultivated tomato Solanum lycopersicum and a related wild species S. habrochaites. BMC Plant Biol. 15: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugbee B. (1994) Effects of radiation quality, intensity, and duration on photosynthesis and growth. InInternational Lighting in Controlled Environments Workshop, NASA-CP-95-3309. Edited Tibbitts T.W. pp. 39–50. Kennedy Space Center, National Aeronautics and Space Administration (NASA), FL. [Google Scholar]
- Caldwell E., Read J., Sanson G.D. (2016) Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Ann. Bot. 117: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Alderson P.G., Wright C.J. (2008) Solar irradiance level alters the growth of basil (Ocimum basilicum L.) and its content of volatile oils. Environ. Exp. Bot. 63: 216–223. [Google Scholar]
- Chen H., Wilkerson C.G., Kuchar J.A., Phinney B.S., Howe G.A. (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 102: 19237–19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia D.W., Yoder T.J., Reiter W.-D., Gibson S.I. (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751. [DOI] [PubMed] [Google Scholar]
- Christmann A., Moes D., Himmelbach A., Yang Y., Tang Y., Grill E. (2006) Integration of abscisic acid signalling into plant responses. Plant Biol. (Stuttg.) 8: 314–325. [DOI] [PubMed] [Google Scholar]
- Constabel C.P., Barbehenn R. (2008) Defensive roles of polyphenol oxidase in plants InInduced Plant Resistance to Herbivory. Edited by Schaller A. pp. 253–270. Springer, Dordrecht, The Netherlands [Google Scholar]
- Cou�e I., Sulmon C., Gouesbet G., El Amrani A. (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 57: 449–459. [DOI] [PubMed] [Google Scholar]
- de Azevedo S.M., Faria M.V., Maluf W.R., De Oliveira A.C.B., de Freitas J.A. (2003) Zingiberene-mediated resistance to the South American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica 134: 347–351. [Google Scholar]
- De Groot C.C., Marcelis L.F., Van Den Boogaard R., Lambers H. (2001) Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light. Plant. Cell Environ. 24: 1309–1317. [Google Scholar]
- Deng W., Yang Y., Ren Z., Audran-Delalande C., Mila I., Wang X., et al. (2012) The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 194: 379–390. [DOI] [PubMed] [Google Scholar]
- Duffey S. (1986) Plant glandular trichomes: their partial role in defence against insects InInsects and the Plant Surface. Edited by Juniper B., Southwood T.R.E. pp. 151–172. Edward Arnold, London. [Google Scholar]
- Ehleringer J., Bj�rkman O., Mooney H.A. (1976) Leaf pubescence: effects on absorptance and photosynthesis in a desert shrub. Science 192: 376–377. [DOI] [PubMed] [Google Scholar]
- Eigenbrode S.D., Trumble J.T., Millar J.G., White K.K. (1994) Topical toxicity of tomato sesquiterpenes to the beet armyworm and the role of these compounds in resistance derived from an accession of Lycopersicon hirsutum f. typicum. J. Agric. Food Chem. 42: 807–810. [Google Scholar]
- Escobar-Bravo R., Alba J.M., Pons C., Granell A., Kant M.R., Moriones E., et al. (2016) A jasmonate-inducible defense trait transferred from wild into cultivated tomato establishes increased whitefly resistance and reduced viral disease incidence. Front. Plant Sci. 7: 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Bravo R., Klinkhamer P., Leiss K. (2017) Induction of jasmonic acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol. 58: 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.-X., Xu Z.-G., Liu X.-Y., Tang C.-M., Wang L.-W., Han X.-L. (2013) Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 153: 50–55. [Google Scholar]
- Farmer E.E., Johnson R.R., Ryan C.A. (1992) Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 98: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B.G., Lea P.J. (2007) Glutamate in plants: metabolism, regulation, and signalling. J. Exp. Bot. 58: 2339–2358. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. (2006) Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism. Springer Science & Business Media, Dordrecht, The Netherlands. [Google Scholar]
- Fraenkel G.S. (1959) The raison d’etre of secondary plant substances. Science 129: 1466–1470. [DOI] [PubMed] [Google Scholar]
- Galvez-Valdivieso G., Fryer M.J., Lawson T., Slattery K., Truman W., Smirnoff N., et al. (2009) The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21: 2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfagna T.J., Carter C.D., Sacalis J.N. (1992) Temperature and photoperiod influence trichome density and sesquiterpene content of Lycopersicon hirsutum f. hirsutum. Plant Physiol. 100: 1403–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas J.J., Schimmel B.C., Alba J.M., Escobar-Bravo R., Schuurink R.C., Kant M.R. (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 13: 17077–17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G�mez C., Mitchell C.A. (2015) Growth responses of tomato seedlings to different spectra of supplemental lighting. HortScience 50: 112–118. [Google Scholar]
- Gon�alves L.D., Maluf W.R., Cardoso MdG., Resende J.D., Castro E.D., Santos N.M., et al. (2006) Rela��o entre zingibereno, tricomas foliares e repel�ncia de tomateiros a Tetranychus evansi. Pesq. Agropec. Bras. 41: 267–273. [Google Scholar]
- Gouinguen� S.P., Turlings T.C. (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 129: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanan J.J. (1998) Greenhouses: Advanced Technology for Protected Cultivation. CRC Press, Boca Raton, FL. [Google Scholar]
- Hanley M.E., Lamont B.B., Fairbanks M.M., Rafferty C.M. (2007) Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 8: 157–178. [Google Scholar]
- Holopainen J.K., Gershenzon J. (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15: 176–184. [DOI] [PubMed] [Google Scholar]
- Kang J.-H., Liu G., Shi F., Jones A.D., Beaudry R.M., Howe G.A. (2010a) The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 154: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.-H., McRoberts J., Shi F., Moreno J.E., Jones A.D., Howe G.A. (2014) The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 164: 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.-H., Shi F., Jones A.D., Marks M.D., Howe G.A. (2010b) Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 61: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant M.R., Bleeker P.M., Van Wijk M., Schuurink R.C., Haring M.A. (2009) Plant volatiles in defence. Adv. Bot. Res. 51: 613–666. [Google Scholar]
- Kazan K., Manners J.M. (2011) The interplay between light and jasmonate signalling during defence and development. J Exp Bot. 62: 4087–4100. [DOI] [PubMed] [Google Scholar]
- Kennedy G., Yamamoto R., Dimock M., Williams W., Bordner J. (1981) Effect of day length and light intensity on 2-tridecanone levels and resistance in Lycopersicon hirsutum f. glabratum to Manduca sexta. J. Chem. Ecol. 7: 707–716. [DOI] [PubMed] [Google Scholar]
- Kennedy G.G. (2003) Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annu. Rev. Entomol. 48: 51–72. [DOI] [PubMed] [Google Scholar]
- Kitaya Y., Niu G., Kozai T., Ohashi M. (1998) Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 33: 988–991. [Google Scholar]
- K�hler A., Maag D., Veyrat N., Glauser G., Wolfender J.L., Turlings T.C., et al. (2015) Within-plant distribution of 1,4-benzoxazin-3-ones contributes to herbivore niche differentiation in maize. Plant. Cell Environ. 38: 1081–1093. [DOI] [PubMed] [Google Scholar]
- Leiss K.A., Choi Y.H., Abdel-Farid I.B., Verpoorte R., Klinkhamer P.G. (2009) NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J. Chem. Ecol. 35: 219–229. [DOI] [PubMed] [Google Scholar]
- Leiss K.A., Maltese F., Choi Y.H., Verpoorte R., Klinkhamer P.G.L. (2009) Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 150: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Williams M.M., Loh Y.-T., Lee G.I., Howe G.A. (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 130: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhao Y., McCaig B.C., Wingerd B.A., Wang J., Whalon M.E., et al. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L�pez-Gresa M.P., Lis�n P., Kim H.K., Choi Y.H., Verpoorte R., Rodrigo I., et al. (2012) Metabolic fingerprinting of tomato mosaic virus infected Solanum lycopersicum. J. Plant Physiol. 169: 1586–1596. [DOI] [PubMed] [Google Scholar]
- L�vdal T., Olsen K.M., Slimestad R., Verheul M., Lillo C. (2010) Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 71: 605–613. [DOI] [PubMed] [Google Scholar]
- Machado R.A., Ferrieri A.P., Robert C.A., Glauser G., Kallenbach M., Baldwin I.T., et al. (2013) Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol. 200: 1234–1246. [DOI] [PubMed] [Google Scholar]
- Maluf W.R., Campos G.A., das Gra�as Cardoso M. (2001) Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121: 73–80. [Google Scholar]
- Martinez-Garza C., Howe H. (2005) Developmental strategy or immediate responses in leaf traits of tropical tree species? Int. J. Plant Sci. 166: 41–48. [Google Scholar]
- McDowell E.T., Kapteyn J., Schmidt A., Li C., Kang J.-H., Descour A., et al. (2011) Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 155: 524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M.D., Yevtushenko D.P., Deschene A., Van Cauwenberghe O.R., Makhmoudova A., Potter J.W., et al. (2003) Overexpression of glutamate decarboxylase in transgenic tobacco plants confers resistance to the northern root-knot nematode. Mol. Breeding 11: 277–285. [Google Scholar]
- Mouden S., Sarmiento K.F., Klinkhamer P.G., Leiss K.A. (2017) Integrated pest management in western flower thrips: past, present and future. Pest Manag. Sci. 73: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E.H., Goodger J.Q., Woodrow I.E., M�ller B.L. (2013) Plant chemical defense: at what cost? Trends Plant Sci. 18: 250–258. [DOI] [PubMed] [Google Scholar]
- Nguyen D., Rieu I., Mariani C., van Dam N.M. (2016) How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 91: 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihoul P. (1993) Do light intensity, temperature and photoperiod affect the entrapment of mites on glandular hairs of cultivated tomatoes? Exp. Appl. Acarol. 17: 709–718. [Google Scholar]
- Nunes-Nesi A., Fernie A.R., Stitt M. (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 3: 973–996. [DOI] [PubMed] [Google Scholar]
- Oguchi R., Hikosaka K., Hirose T. (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant. Cell Environ. 26: 505–512. [Google Scholar]
- Peiffer M., Tooker J.F., Luthe D.S., Felton G.W. (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol. 184: 644–656. [DOI] [PubMed] [Google Scholar]
- P�rez-Estrada L.B., Cano-Santana Z., Oyama K. (2000) Variation in leaf trichomes of Wigandia urens: environmental factors and physiological consequences. Tree Physiol. 20: 629–632. [DOI] [PubMed] [Google Scholar]
- Pieterse C.M., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S.C. (2012) Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28: 489–521. [DOI] [PubMed] [Google Scholar]
- Ramesh S.A., Tyerman S.D., Xu B., Bose J., Kaur S., Conn V., et al. (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 6: 7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramputh A.-I., Bown A.W. (1996) Rapid [gamma]-aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf-roller larvae. Plant Physiol. 111: 1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.R., Paul N.D. (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 170: 677–699. [DOI] [PubMed] [Google Scholar]
- Sallaud C., Giacalone C., T�pfer R., Goepfert S., Bakaher N., R�sti S., et al. (2012) Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 72: 1–17. [DOI] [PubMed] [Google Scholar]
- Sch�fer M., Br�tting C., Baldwin I.T., Kallenbach M. (2016) High-throughput quantification of more than 100 primary- and secondary-metabolites, and phytohormones by a single solid-phase extraction based sample preparation with analysis by UHPLC–HESI–MS/MS. Plant Methods 12: 30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz S.S., Reichelt M., Mekonnen D.W., Ludewig F., Mith�fer A. (2015) Insect herbivory-elicited GABA accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response. Front. Plant Sci. 6: 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven L.M., Van Loon J.J., Dicke M. (2005) Insect–Plant Biology. Oxford University Press, Oxford. [Google Scholar]
- Simmonds M.S. (2001) Importance of flavonoids in insect–plant interactions: feeding and oviposition. Phytochemistry 56: 245–252. [DOI] [PubMed] [Google Scholar]
- Snyder J.C., Simmons A.M., Thacker R.R. (1998) Attractancy and ovipositional response of adult Bemisia argentifolii (Homoptera: Aleyrodidae) to type IV trichome density on leaves of Lycopersicon hirsutum grown in three day-length regimes. J. Entomol. Sci. 33: 270–281. [Google Scholar]
- Tattini M., Gravano E., Pinelli P., Mulinacci N., Romani A. (2000) Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 148: 69–77. [DOI] [PubMed] [Google Scholar]
- Tian D., Tooker J., Peiffer M., Chung S.H., Felton G.W. (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Traw B.M., Dawson T.E. (2002) Differential induction of trichomes by three herbivores of black mustard. Oecologia 131: 526–532. [DOI] [PubMed] [Google Scholar]
- Van D., Nicole M., Hare D.J. (1998) Differences in distribution and performance of two sap-sucking herbivores on glandular and non-glandular Datura wrightii. Ecol. Entomol. 23: 22–32. [Google Scholar]
- V�nninen I., Pinto D., Nissinen A., Johansen N., Shipp L. (2010) In the light of new greenhouse technologies: 1. Plant-mediated effects of artificial lighting on arthropods and tritrophic interactions. Ann. Appl. Biol 157: 393–414. [Google Scholar]
- van Schie C.C., Haring M.A., Schuurink R.C. (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 64: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T.C., Dekker B., Hoekema A. (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17: 2362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile D., Garnier E., Shipley B., Laurent G., Navas M.-L., Roumet C., et al. (2005) Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 96: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann T.C. (1993) Plant tissue optics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44: 231–251. [Google Scholar]
- Weinhold A., Baldwin I.T. (2011) Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA 108: 7855–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J.A., Mazza C.A., Dillon F.M., Chludil H.D., Ballare C.L. (2015) Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant Cell Environ. 38: 920–928. [DOI] [PubMed] [Google Scholar]
- Z�st T., Agrawal A.A. (2017) Trade-offs between plant growth and defense: past, present, and future. Annu. Rev. Plant Biol. 68 :513–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.