Abstract
Mammalian circadian rhythms are entrained by photic stimuli that are relayed by retinal projections to the core of the suprachiasmatic nucleus (SCN). Neuronal activation, as evidenced by expression of the immediate early gene c-fos, leads to transcription of the core clock gene per1. The duper mutation in hamsters shortens circadian period and amplifies light-induced phase shifts. We performed two experiments to compare the number of c-FOS immunoreactive (ir) and PER1-ir cells, and the intensity of staining, in the SCN of wild type (WT) and duper hamsters at various intervals after presentation of a 15-minute light pulse in the early subjective night. Light induced c-FOS-ir within 1h in the dorsocaudal SCN of duper, but not WT hamsters. In cells that express vasoactive intestinal peptide (VIP), which plays a critical role in synchronization of SCN cellular oscillators, light-induced c-FOS-ir was greater in duper than WT hamsters. After the light pulse, PER1-ir cells were found in more medial portions of the SCN than FOS-ir, and appeared with a longer latency and over a longer time course, in VIP cells of duper than wild type hamsters. Our results indicate that the duper allele alters SCN function in ways that may contribute to changes in free running period and phase resetting.
Graphical Abstract
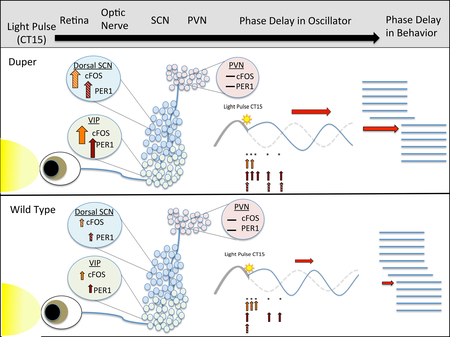
Duper mutant hamsters have a fast circadian clock and enhanced phase delays in response to a light pulse delivered in the early subjective night (Circadian Time 15). Top, the light signal is relayed from the eye to the suprachiasmatic nucleus (SCN), which serves as a master pacemaker. Subordinate circadian oscillators in other brain areas, including the paraventricular nucleus (PVN), adjust their phase in response to SCN input. As a result, behavioral rhythms show a phase delay (horizontal red arrow) that is greater in duper (middle panel) than wild type (bottom panel) hamsters. Resetting of the pacemaker of mutant hamsters differs from that in wild type: dupers show increased activation (as reflected by greater cFOS immunoreactivity) and clock gene expression (as reflected by PER1 immunoreactivity) in the ventral core (green) and dorsal shell (blue) neurons of the SCN at various time points sampled after the light pulse (vertical arrows). Activation of VIP neurons of the core SCN is specifically affected by the duper mutation.
Introduction
Although the role of the SCN as the circadian pacemaker is well established, its daunting complexity has complicated attempts to understand the generation and entrainment of biological rhythms. The Syrian hamster offers many advantages for circadian studies. Not only does it have a precise and regular clock, but the segregation of cell phenotypes between the ventrolateral “core” and dorsomedial “shell” regions is particularly clear within its SCN. The former receives input from the retina that is critical to entrainment by light, the principal zeitgeber. This region contains a group of neurons that co-express calbindin-D28K and gastrin releasing peptide (GRP). As in mice and rats, these cells overlap with a population of vasoactive intestinal polypeptide (VIP)-expressing neurons (Silver, et al., 1996; Aioun et al., 1998; Kawamoto et al., 2003; Karatsoreos, et al., 2004). Both the Calbindin/GRP and the VIP populations likely participate in communication of entraining signals throughout the SCN and coupling of cellular oscillators (Harmar et al., 2002; Antle et al., 2005; Kriegsfeld et al., 2008; Maywood, et al, 2011; Taylor et al., 2016). VIP efferents also project to other brain areas and may coordinate circadian outputs (van der Beek et al., 1994; Munch et al., 2002; Kriegsfeld et al., 2004). Antle et al. (2007) have proposed that non-rhythmic light–inducible, retinorecipient “gate” cells maintain phase coherence among autonomous oscillators, which in turn regulate the gate. The shell of the SCN receives sparse retinal input and is dominated by vasopressinergic neurons, which have a major (but not exclusive) role in output to other brain areas and may also couple to the core (Kalsbeek et al., 2010, Evans et al., 2015, Mieda et al., 2015).
The molecular basis of the circadian oscillator is a feedback loop in which BMAL1:CLOCK heterodimers bind to E-box promoter elements and activate transcription of per and cry. Within the SCN, the protein products of these genes undergo phosphorylation, translocate to the nucleus, and block the induction of per and cry expression at the start of the subjective night. Glutamatergic input from terminals of retinal ganglion cells induces c-fos transcription in SCN neurons via multiple signal transduction pathways (McNulty et al., 1998; Guido et al., 1999; Schwartz et al., 2000; Yamamoto et al., 2001; Yan and Silver 2002, 2004; Hamada et al., 2004; Butcher et al., 2005; Irwin and Allen 2007). Transcription of per1 is also induced by light exposure during subjective night and is an early event in phase shifts of the circadian oscillator (Akiyama et al., 1999; Wakamatsu et al., 2001).
An important objective for circadian research is to understand the relationship between molecular oscillators (Kuhlman et al., 2003; Brancaccio et al., 2013) and the physiological events (e.g., depolarization, Calcium influx) required to set the circadian clock. Comparing the dynamic expression of per and c-fos in specific cell types provides a powerful strategy to understand the relationships between molecular and functional mechanisms of circadian entrainment. In mice, acute depolarization in response to light leads to activation of per1-driven reporter expression in cells of the ventral core of the SCN, many of which express VIP. This is followed by spread of the PER1 signal to the dorsal shell with a latency of several hours (Kuhlman et al., 2003). Relatively few of the GRP cells of the core contain either PER protein, however, suggesting a complex circuitry of the ventral portion of the SCN through which light signals are relayed to the oscillator. In contrast, cells of the shell show strong autonomous rhythms of per mRNA that persist in constant conditions (Hamada et al., 2004).
Mutant strains have provided valuable insights into circadian mechanisms. The tau hamster was the first mammalian period mutant to be discovered. In these animals, a transversion in the coding sequence of casein kinase 1ε leads to hyperphosphorylation of PER2 and consequently shortens circadian period (Lowrey et al., 2000; Meng et al., 2008). A second hamster circadian mutant, duper, was discovered in our laboratory (Monecke et al., 2011). Like tau, the duper allele shortens free running period. However, duper is not a change in the coding sequence of CK1ε or CK1δ. A striking feature of the duper phenotype is the amplification of the response to light. Unlike tau hamsters, duper mutants entrain to a 14:10LD cycle and show a high amplitude phase response curve (Krug et al., 2011; Manoogian et al., 2015). The duper phenotype is not attributable to a change in retinal sensitivity, as the intensity threshold for light-induced phase shifts is similar to the wild type (Bittman, 2014). The duper mutation speeds oscillations of a Bmal1 luminescent reporter in SCN slices, but does not alter the period of embryonic fibroblasts or adult liver cell cultures (Kumar and Bittman, unpublished data). This suggests that duper affects cell networks of the master pacemaker, but not the cell autonomous function of transcriptional-translational feedback loops.
We aimed to determine whether the behavioral phenotype of the duper mutant is correlated with alterations in the time course or amplitude of cell activation and clock gene expression, particularly in specific regions and cell populations of the SCN. Thus we examined the temporal relationships between induction of c-FOS-ir and PER1-ir in the SCN, especially in VIP cells of wild type and duper mutant hamsters whose free run was undisturbed, or which were subjected to a light pulse in the early subjective night. To gain insight into SCN function during free runs and phase shifts, we quantified immunostaining in order to follow expression of c-fos and per1 with 2h resolution across the subjective night. In order to begin an analysis of effects of duper on communication of circadian phase in nearby targets of SCN efferents, we also examined cFOS-ir and PER1-ir in the sub-PVN and PVN. We find that light induces c-fos and per1 expression in different cell populations with different latencies, and that duper alters SCN function in ways that may contribute to both changes in free running period and phase resetting.
Materials and Methods
Animal maintenance
Syrian hamsters (Mesocricetus auratus) were group housed in a 14:10 LD cycle (~150 lux) until they reached adulthood (3–6 months). Food and water were provided ad libitum and highly absorbent bedding (Bed-o-cob, Maumee, OH) was used at all times. As adults, hamsters were individually housed and provided a running wheel (17.5 cm), as previously described (Krug et al., 2011). Hamsters were then moved to DD to be phenotyped by assessing free running period and response to a 15’ light pulse during the subjective night. Animals remained in DD for 10–12 days before and after the pulse in order to measure the phase shift before being transferred back to LD for 10–12 days. All procedures were approved by the IACUC of the University of Massachusetts at Amherst.
Experimental Design
Experiment 1. The aim of this study was to examine the acute response to light of VIP cells in wild type and duper hamsters, whose phase shifting responses differ dramatically. Wild type hamsters were offspring of LAK-LVG animals purchased from Charles River laboratories. The duper mutation is recessive, and all mutant hamsters used in these studies were offspring of duper X duper crosses of animals whose phenotype was confirmed as previously described (Monecke et al., 2011). The duper or WT phenotype of the animals used in these experiments was confirmed by monitoring of locomotor activity in constant darkness and upon exposure to 15’ light pulses during the subjective night. When screened approximately 12 weeks prior to sacrifice for the ICC experiment, duper hamsters used in experiment 1 showed a mean τDD of 23.21+0.04h, and a 15’ light pulse at approximately CT18 elicited a phase advance of 4.0+0.10h. Wild types showed a τDD of 23.92+0.03h and a shift of 1.0+0.17h.
Hamsters were returned to 14L:10D until the start of the experiment. We performed immunocytochemistry for protein products (c-FOS and PER1) of the c-fos and per1 genes in order to assess effects of time, light exposure, and genotype on their expression. In order to examine c-FOS-ir and PER1-ir in the SCN during free runs and the acute response to light during the early-to-mid subjective night, animals were returned to DD for 10 days. We then exposed equal numbers of male duper and WT hamsters to a 15’ light pulse (as described by Krug et al. 2011) or performed a cage movement control at CT15. Tissue was collected 2h later.
Experiment 2. The aim of this study was to examine c-FOS-ir and PER1-ir in wild type and duper mutant hamsters over a more complete time course under free running conditions and after a phase-shifting light pulse. Adult male and female hamsters were phenotypically characterized as described above. The free running periods of duper and wild type animals were 22.57+0.30 and 24.06+0.02h, respectively. On day 10–12 of DD, groups of hamsters were sacrificed at CT12 or CT15, or received either a 15’ light pulse or cage handling (“jiggle” control) at CT 15. Tissue was collected 1, 3, 6, and 9 hours later (5–6 hamsters per group). A total of 148 hamsters were used.
Experiment 1 used 24 males. Experiment 2 used 67 male and 57 female hamsters (Duper: 39 male, 30 female, and WT: 28 male, 27 female). Each group included both males and females. No clear sex differences were observed.
Tissue collection
Hamsters were anesthetized with 0.3 ml of sodium pentobarbital (80mg/kg) and perfused with 100ml of 0.1M Phosphate buffer (PB) followed by 300ml of 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde overnight, and infiltrated in 20% sucrose in 0.1M PB at 4° C (~2 days). Frozen sections (40μ) were collected in a 1 in 4 series and stored in cryoprotectant at −20° until stained.
Antibodies
In experiment 1, triple label immunocytochemistry (ICC) was used to detect VIP, PER1, and c-FOS proteins in the SCN core. In experiment 2, double label immunocytochemistry (ICC) was used to detect VIP and PER1 protein in the core and triple label to detect PER1, c-FOS, and AVP associated neurophysin (AVP-NP) in the shell, the sub-PVN, and the PVN. Primary antibodies included: anti-VIP made in guinea pig (1:5000; Peninsula Labs, San Carlos, CA, T-5030); anti-PER1 made in rabbit (R43, 1:5000; a generous gift of David R. Weaver; LeSauter et al., 2012); anti-c-FOS made in goat (1:1000; Santa Cruz Biotech, Dallas, TX, sc-52-G); monoclonal anti-AVP associated neurophysin P45 made in mouse (1:50; ATC CRL 1798, kindly provided by Harold Gainer). Secondary antibodies, obtained from Jackson Immuno Research, West Grove, PA, were Alexa Fluor 488-conjugated AffiniPure Donkey anti Guinea Pig (DAGP; 1:300); Cy3 Donkey anti-Rabbit (DAR; 1:500); Alexa Fluor 488 Donkey anti-Goat (DAG; 1:300); and Cy5 Donkey anti-mouse (DAM; 1:300). Alexa Fluor 633 Donkey anti-Goat (DAG; 1:300) was obtained from Invitrogen.
Immunocytochemistry
4–6 sections containing mid-SCN, caudal-SCN, and PVN were chosen for each hamster. Only mid-SCN was analyzed for the PER1-VIP double label, as VIP cells were not located in caudal SCN or PVN. All sections in experiment 1 were stained simultaneously. Due to the large number of animals, experiment 2 was split into two runs, each with half of the animals from each group. Sections were rinsed 4 × 5’ in 0.1M Phosphate Buffer (PB), blocked for 1-hour in PB containing 0.4% Triton X-100 (Electrophoresis grade; Fisher Scientific, Pittsburg, PA) and 0.1% Bovine Serum Albumin, Factor V (Sigma, St. Louis, MO). Primary and secondary antibody incubations were conducted for approximately 18h and 2h, respectively, at room temperature. Between primary and secondary antibody incubations, sections were rinsed 4 × 5’ in 0.1M phosphate buffer. Upon completion of staining, tissue was rinsed 4x in 0.1M PB and mounted onto subbed slides. When dry, slides were cover-slipped using Aqua-Poly (Polysciences, Warrington, PA, 18606–20).
Image analysis
Images (snap shots and z-stacks) were taken at 10X and 20X respectively using a Zeiss 700 confocal microscope. FIJI (Fiji Is Just ImageJ; http://fiji.sc/Fiji; Schindelin et al., 2012) NIH imaging software was used for all image analysis. A researcher who was blind to the genotype and manipulation manually identified labeled VIP-ir cells. In addition, PER1 and c-FOS cells were counted using FIJI 3D automated cell counting as described in the supplemental materials. For manually identified VIP labeled cells, the intensity of PER1 was determined by automated identification. In order to determine the intensity of nuclear PER1 and c-FOS staining in VIP neurons we used a custom MATLAB (The MathWorks Inc., Natick, MA) script that used x,y coordinates of VIP cells marked by an experimenter blind to the condition and computed average intensity of PER1 or cFOS staining across a circular region with average radius of 7 microns.
The density of the AVP-NP-ir neuropil made it difficult to quantify cFOS-ir in dorsal SCN in sufficient neurons to allow statistical analysis. Thus staining was used mainly to delineate the extent of the dorsal SCN (Supplementary Fig. 1). Sections were categorized as mid-SCN, caudal SCN, or PVN based on expression of VIP and AVP fiber distribution, and ventral vs. dorsal SCN were determined by separating the SCN halfway between the rostral and caudal borders as defined by VIP or AVP fibers. Rostral SCN sections were not used, as they did not contain VIP or AVP cell bodies.
Statistical Analysis
In both experiments, effects of light pulse and genotype were evaluated both for the ventral and dorsal SCN as a whole, and specifically for VIP cells. As normality of distribution could not be assessed given the size of the groups, non-parametric tests were used. We used the Kruskal-Wallis test to assess effects of genotype and light exposure on numbers of cells stained > 2 standard deviations above background. The Kolmogorov-Smirnov test was used to evaluate the distribution of staining intensities of c-FOS and PER in cell nuclei of individual animals, and the median intensities for each animal were used to compute group means that were evaluated by the Kruskal-Wallis test. In experiment 2, effects of survival time were also assessed, using the Bonferroni correction for multiple comparisons. For the comparison of the effects of light within genotype the threshold was set at α=0.0125, and for effects of genotype at each time point at α=0.0045. Statistics were performed using GraphPad Prism 6 for Macintosh (GraphPad software, La Jolla, CA).
Results
Experiment 1
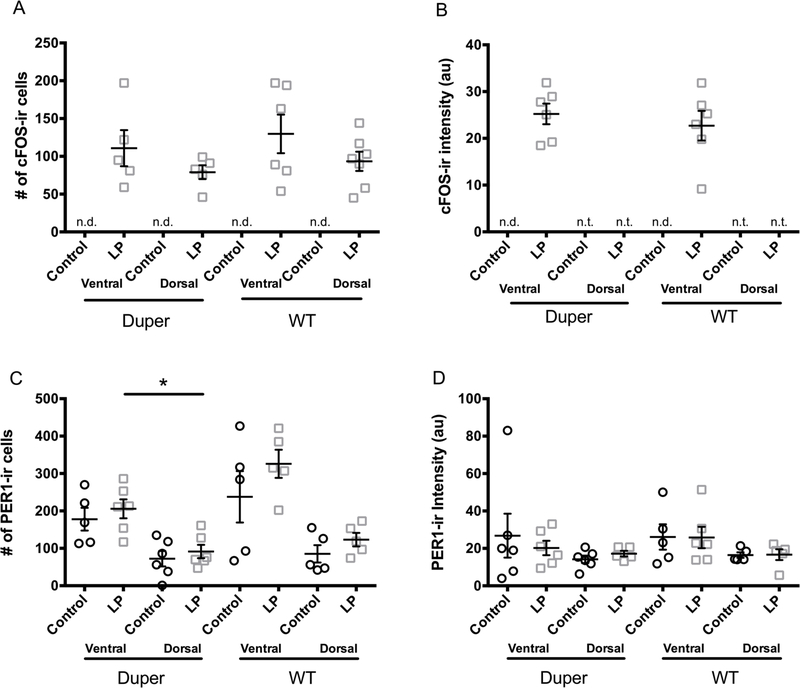
Movement of the cage, used as a control procedure, did not raise the number of cFOS-ir cells in SCN above surrounding regions of the hypothalamus (data not shown). cFOS labeling increased markedly 2h after a 15’ light pulse, but neither the number of cFOS-ir cells nor their staining intensity differed with genotype in either the ventral or the dorsal SCN (Fig 1 A and B). At the 2h survival time, light exposure had no effect on the number of PER1-ir cells of either genotype within the SCN as a whole. In duper hamsters exposed to light, more PER1-ir cells were found in ventral than dorsal SCN (Fig 1C). In wild types, this effect of light was not statistically significant. The intensity of PER1 staining was similar in the ventral and dorsal SCN and was not affected by genotype or light exposure (Fig. 1D).
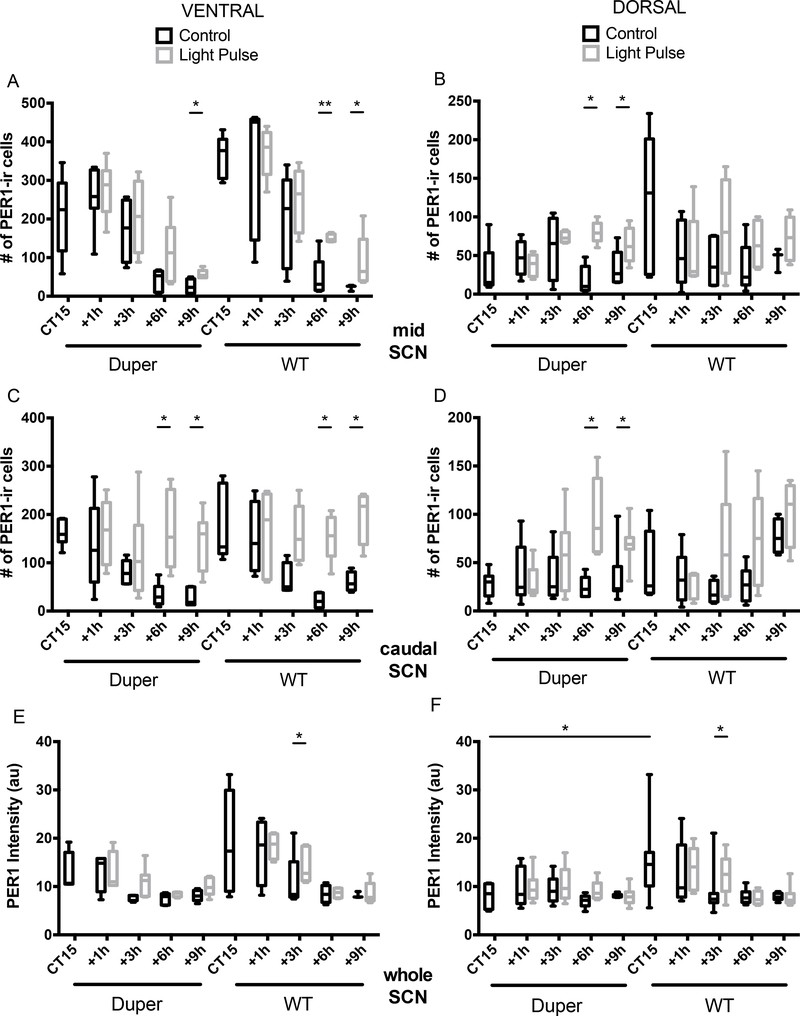
Figure 1.
Light exposure in early subjective night induced c-FOS-ir but not PER1-ir at the 2h survival point. (A) c-FOS-ir was not detected in the SCN of control hamsters 2h after cage movement, but after a 15’ light pulse at CT15, c-FOS-ir cells were found in both ventral and dorsal mid-SCN in similar numbers and intensities in wild type and duper mutant hamsters. (B) PER1 immunoreactivity in the SCN as a whole was similar in wild type and duper mutant hamsters. Neither the number of PER1-ir cells (C) nor PER1 staining intensity (mean±SEM, arbitrary units; D) was affected by the light pulse at this time point, although the light pulse resulted in a more ventral distribution of PER1-ir cells in duper hamsters [n.d. = not detectable; n.t. = not tested; Mann-Whitney test, *p=0.015].
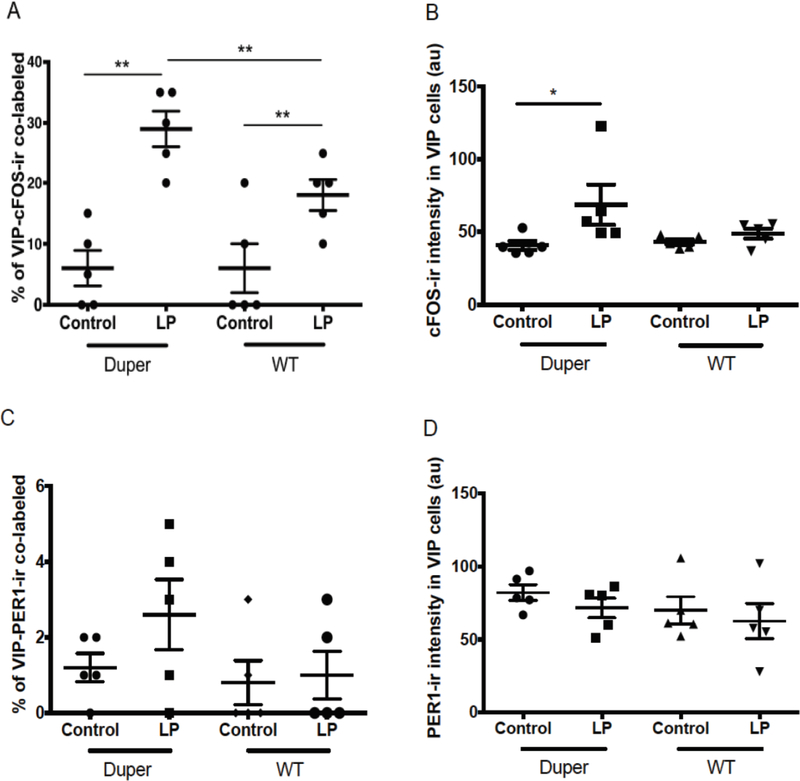
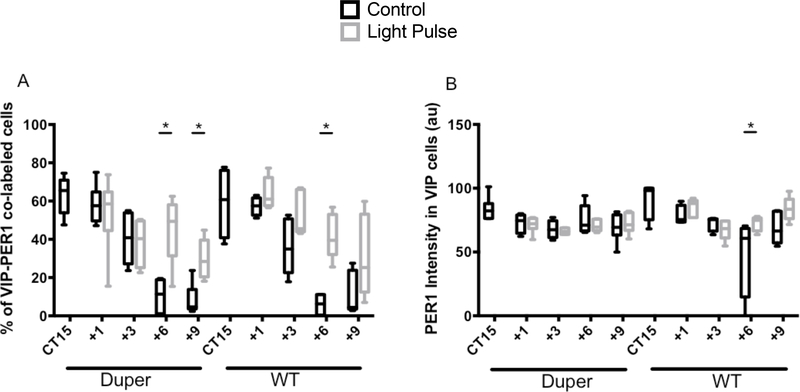
In light of the functional significance of VIP cells, we analyzed cFOS and PER-ir specifically in this population. VIP cell bodies were found only in ventral mid-SCN. VIP fibers were densely distributed through the mid-SCN, but were restricted to the dorsal portion of the caudal SCN. The number of VIP-ir cells and intensity of VIP staining was not affected by light. There were no significant differences between genotypes in number of VIP-ir cells (Supplementary Fig. 2A). A light pulse at CT15 induced a significant increase in the number of VIP-ir cells co-labeled with cFOS two hours later in both WT and duper hamsters (p=0.01 and p=0.04, respectively; Fig. 2A and 3A-D). The number of double-labeled VIP-ir/cFOS-ir cells was 29% greater in duper and 18% greater in WT hamsters than in their respective cage movement controls. This increase in percentage of VIP cells also expressing cFOS was greater in duper than in WT hamsters (p=0.02; Fig. 2A). Light exposure also evoked a significant increase in intensity of cFOS staining in VIP-ir cells in duper (p=0.03) but not WT hamsters (Fig. 2B).
Figure 2.
Effects of the duper mutation upon activation depends upon cell type. (A) A light pulse at CT15 increased the number of cFOS-ir/VIP-ir co-labeled cells 2h later in both duper (p=0.008) and WT (p=0.04) hamsters. The inductive effect of light on this population was greater in duper than WT hamsters (**, p= 0.02). (B) cFOS-ir intensity in VIP-ir cells increased in duper but not WT hamsters 2h after a light pulse at CT15 (*, p=0.05). (C) Light pulse at CT15 did not influence the percentage of VIP-ir cells containing PER1 2h later in either WT (p=0.81) or duper (p=0.57) hamsters. (D). Intensity of PER1-ir staining in VIP cells was not affected by light exposure or genotype.
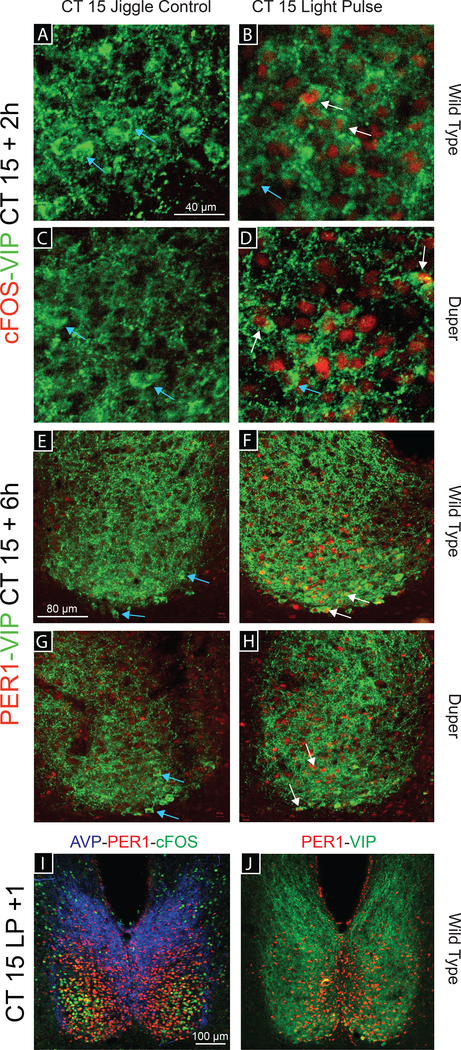
Figure 3.
Photomicrographs illustrating immunostaining for cFOS-VIP, PER1-VIP, and AVP-cFOS-PER1. (A-D) Representative images of a double labeled SCN in Experiment 1. cFOS-ir (red) and VIP-ir (green) are shown in WT (A, B) and duper (C, D) hamsters 2h after either a cage movement (control procedure, A, C) or a 15-min light pulse (B, D) at CT15 (40X). (E-J) Double labeled SCN in Experiment 2. PER1-ir (red) and VIP-ir (green) are detected in WT (E, F) and duper (G, H) hamsters 6h after either a cage movement (E, G) or a 15-min light pulse (F, H) at CT15 (20X). White arrows indicate VIP co-labeled cells; light blue arrows indicate cells stained for VIP only. (I, J) Representative 10X images of WT SCN 1h after a light pulse at CT15 triple labeled (I) for AVP (blue), cFOS (green) and VIP (red) or double labeled (J) for PER1 and VIP. Note that PER1-ir cells are medial to cFOS-ir cells.
The number of VIP cells stained for PER1 at the 2h survival point was similar in control and light pulsed hamsters of both genotypes (Fig. 2C). The intensity of PER1 staining in VIP cells also did not differ with genotype (Fig. 2D).
Experiment 2
The number of activated cellsdid not differ ase incedure, did not alter the ifts in amygdaloid and hypothalamic regulation of emotion d by linking oxytocinerThenumber of activated cells did not differ between duper and WT hamsters at CT12 or 15. As in experiment 1, we found c-FOS-ir cells throughout the SCN and PVN, with the greatest concentration in the ventral SCN, of light-exposed duper and WT hamsters. In general, c-FOS staining was found sooner after light exposure than was PER1-ir (Fig. 4 and 5) and was evident in more lateral regions of the SCN (Fig. 3I and J).
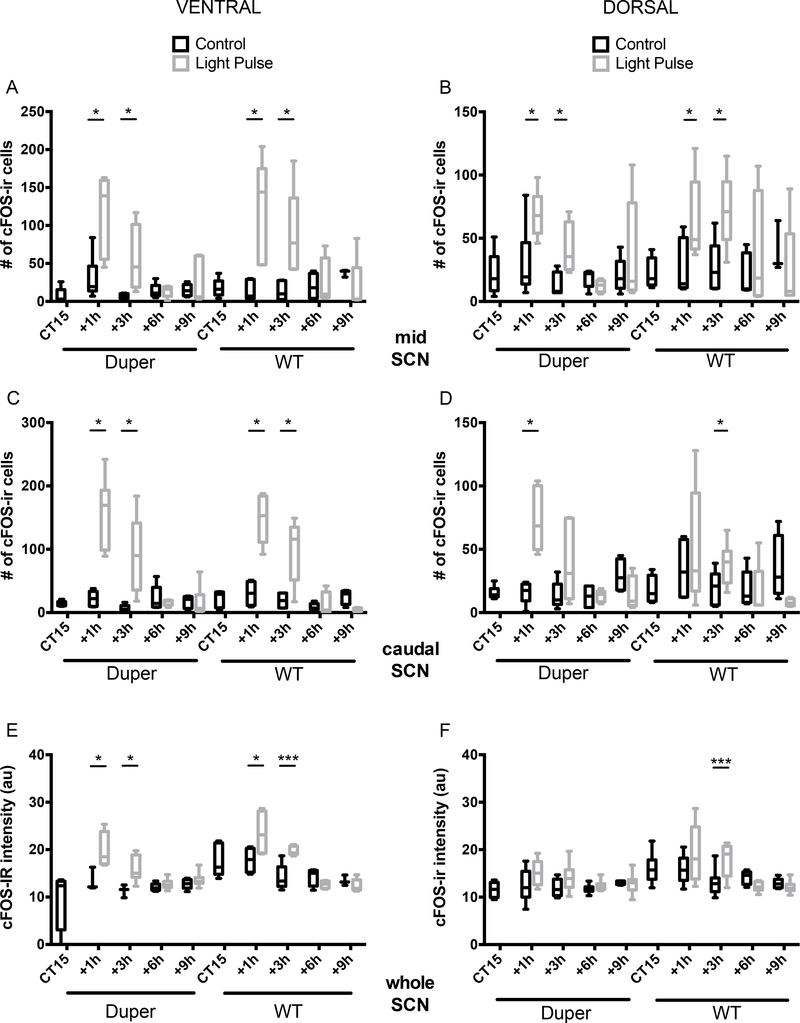
Figure 4.
Exposure to light at CT15 induced c-fos expression in SCN of hamsters within 3 hours. (A-B) Light induced an increase in the number of c-FOS-ir cells in both dorsal (A) and ventral (B) mid-SCN of both duper and WT hamsters at the 1 and 3h survival intervals (ventral SCN: duper, p=0.02 and p=0.03 at 1 and 3h, respectively; WT, p=0.008 and 0.004, respectively. Dorsal SCN: duper, p=0.05 at 1 and 3h; WT, p=0.01 and 0.03, respectively). Horizontal lines within the bars indicate mean, bars show 5%−95% confidence intervals, and vertical lines indicate range of individual points. See Supplementary Fig 1 for micrograph illustrating plane in which staining was quantified. Light induced an increase in the number of c-FOS-ir cells in dorsal (C) and ventral (D) caudal SCN at 1h and 3h survival intervals in both genotypes. (ventral SCN: duper, p=0.002 and p=0.008 at 1 and 3h, respectively; WT, p=0.02 and 0.04, respectively. Dorsal SCN: duper, p=0.0005 at 1h; WT, p=0.006 at 3h). (E) Staining intensity of cFOS-ir was increased in ventral SCN of both duper and WT hamsters at the 1 and 3h survival intervals. Ventral SCN: duper, p=0.0004 and p=0.004 at 1 and 3h, respectively; WT, p=0.0005 and 0.0001, respectively. Dorsal SCN: duper, p=0.05 at 1 and 3h; WT, p=0.01 and 0.03, respectively). (F) Staining intensity of cFOS-ir in dorsal SCN was increased only at 3h in WT hamsters (p=0.0001)
Figure 5.
Exposure to light at CT15 induced PER1-ir in SCN above cage movement control levels with a latency of many hours, and this effect differed with genotype. (A) The number of PER1-ir cells increased in the ventral mid-SCN of duper hamsters exceeded that of cage movement controls 9h after the light pulse (p=0.004). In WT hamsters, the light pulse increased the number of PER1-ir cells at latencies of both 6h (p=0.008) and 9h (p=0.006). (B) The number of PER1-ir cells increased in the dorsal mid-SCN of duper hamsters exposed to light at CT15 exceeded that of cage movement controls both 6h (p=0.0001) and 9h (p=0.04) after treatment, but no such effect was evident in WT hamsters. (C) At both 6h and 9h after the light pulse, the number of PER1-ir cells was increased in the ventral portion of the caudal SCN of both duper (p=0.004 and p=0.0003) and WT (p=0.0004 and p=0.004) hamsters. (D) The number of PER1-ir cells was increased in the dorsal portion of the caudal SCN of duper hamsters 6h and 9h after light exposure at CT15 (p=0.002 and p=0.04), but no statistically significant effect was evident in WT hamsters. (E, F) 3h after treatment, the intensity of PER1-ir staining was greater in light-exposed than control WT hamsters in both ventral (p=0.04, E) and dorsal (p=0.03, F) SCN, but the light pulse had no significant effect at any latency in duper hamsters. At CT15, PER1-ir intensity of free running WT hamsters exceeded that of duper mutants in dorsal SCN (p=0.02).
Activational effects of light were evident in the mid-SCN of both duper and WT hamsters: the number of c-FOS-ir cells increased significantly over control values at 1 and 3 hours after the light pulse in both the ventral and the dorsal portions; Fig. 4A and B). In the ventral part of the caudal SCN, light exerted significant activational effects at both 1 and 3h in both duper and WT hamsters (Fig. 4C). In the dorsal portion of the caudal SCN, light induced a dramatic increase in the number of FOS-ir cells within 1h (Fig. 4D). In WT hamsters the effect of light to induce cFOS-ir was not statistically significant at the 1h survival interval in the dorsal portion of the caudal SCN but the number of c-FOS-ir cells increased at the 3h survival interval (Fig. 4D). In no region of the SCN was cFOS-ir induction sustained beyond 3h in either genotype.
The intensity of c-FOS-ir staining was greater in the ventral SCN of both duper and WT hamsters that received a light pulse at CT15 than in controls at survival intervals of 1 and 3 hours, but not 6 or 9 hours (Fig. 4E). In dorsal SCN, WT hamsters had increased c-FOS-ir staining intensity only 3h after the light pulse compared to controls (Fig. 4F). No increase in c-FOS staining was evident in duper hamsters at any survival interval.
Light induced PER1-ir occurred only after c-FOS-ir had subsided. In the ventral portion of the mid-SCN, the first significant change in the number of PER1-ir cells compared to controls was found at 6h after the light pulse in WT hamsters (Fig. 5A). A similar trend in duper hamsters was not statistically significant, but PER1-ir cell numbers were increased at the 9h survival interval in both genotypes. In the dorsal portion of the mid-SCN, induction of this core clock gene differed with genotype: duper hamsters showed higher PER1-ir cell counts 6h and 9h after light pulse (Fig. 5B). No difference was found in WT hamsters at either survival interval. In the ventral portion of the caudal SCN, PER1-ir cell numbers showed a similar pattern in both genotypes, with an increase in cell numbers at 6h and 9h survival intervals in both duper and WT hamsters (Fig. 5C). In the dorsal portion of the caudal SCN, the induction of PER1 was statistically significant at 6 and 9 h survival points in duper hamsters Fig. 5D) but not in WT. Although PER1-ir staining intensity was not significantly altered by light exposure in duper hamsters, WT hamsters had higher PER1-ir intensity at CT15 compared to duper hamsters in dorsal SCN and showed a treatment effect at the 3h survival interval in both ventral and dorsal SCN (Fig. 5E and F).
In light of the functional importance of VIP cells, we analyzed PER1-ir in this population in the mid-SCN. There were no significant differences in the number of VIP-ir cells across survival intervals (Supplementary Fig. 2). The total number and percentage of VIP cells co-labeled with PER1 after light exposure increased in both genotypes, but only after FOS-ir had subsided. We sought to determine how long Per1 expression is sustained in VIP-ir neurons, and whether this might be affected by genotype. Six hours after a light pulse, the percentage of PER1-ir VIP cells was elevated compared to cage movement controls in both dupers and WTs (Fig 6A). Nine hours after a light pulse, duper mutants continued to show a greater percentage of VIP cells expressing PER1 than controls (Fig 6A). WT hamsters showed a similar sustained increase in PER1-ir expressing VIP cells, but this trend was not statistically significant at 9h (Fig. 6A). The effect of light exposure to increase the intensity of PER1-ir in VIP cells was also generally similar in WT and duper hamsters, but WT hamsters showed significantly higher PER1 intensity in VIP cells 6h after a light pulse than cage movement controls (Fig 6B).
Figure 6.
Time course of PER1-ir in VIP cells in SCN of hamsters exposed to light at CT15 or subjected to a cage movement control. (A) PER1-ir expression in VIP-ir cells increased 6h after a light pulse in subjective night in both duper (p=0.002) and WT (p=0.001) hamsters, but is sustained until 9h only in duper hamsters (p=0.004). (B) PER1-ir intensity in VIP cells was increased 6h after the light pulse in WT hamsters (p=0.001), but not in dupers.
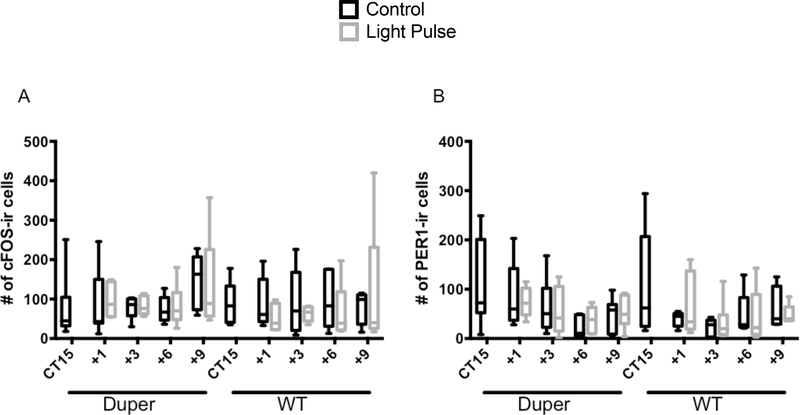
AVP-NP-ir cells were located in the dorsal SCN shell and PVN (Supplementary Fig. 1). A dense AVP-NP fiber network in the SCN shell extended into the ventral SCN, and through the subPVN and PVN. We detected no effects of either genotype or light exposure on c-FOS-ir or PER1-ir cell counts in the subPVN or PVN (Fig 7). Duper hamsters tended to have more c-FOS-ir cells in PVN than WTs 9h after cage movement, but this trend was not statistically significant (p=0.07).
Figure 7.
Neither light nor genotype had a significant influence on the number of cFOS-ir or PER1-ir cells in PVN.
Discussion
Our results provide insight into the time course of cell activation in different SCN compartments, and the specific response of VIP cells within the core, upon light exposure during the subjective night. In addition, they address the question of how the duper mutation, which not only accelerates the circadian clock but also amplifies and speeds phase resetting, impacts neuronal activation and cell-specific levels of core clock proteins within the SCN.
The activation of retinorecipient and second order cells of the SCN by light during the subjective night are early steps in the process of entrainment. c-FOS and other Fos-related antigens likely participate in light-induced phase shifts (Wollnik et al., 1995; Honrado et al., 1996; Schwartz et al., 2000). Regional differences between wild type and duper mutants in the extent of cFOS-ir in dorsal vs. ventral regions of mid- vs. caudal SCN suggest an influence of the mutation on specific stages of the multi-step process of shifting circadian phase.
Best et al. (1999) found that c-fos mRNA returns to baseline within 2h in the SCN of hamsters given a light pulse at CT14. Our findings that induction of c-FOS-ir in the SCN occurs within 1h and lasts until at least 3h after light exposure is consistent with this time course. When we examined cFOS staining specifically in VIP cells two hours after the light pulse at CT15, we found that duper hamsters showed more FOS-ir cells, and more intense cFOS staining, than WT. As duper amplifies and extends activation by light of the VIP population, recruitment of neurons in the dorsal SCN may increase, oscillator coupling may strengthen, and resetting of the intrinsically rhythmic population may be enhanced. While confocal microscopy reveals appositions of retinal terminals on VIP cells in mouse SCN (Lokshin et al., 2015), electron microscopy indicates little direct synaptic input from retinal terminals to VIP cells in hamsters (Aioun et al., 1998). Thus light activation of c-fos expression in this cell population is likely indirect. Pharmacological studies suggest that GRP can instigate phase shifts in hamsters (Chan et al., 2016). This action may be mediated by relay from GRP to VIP cells, and these cell types may have overlapping roles as both cell types are activated and both peptides are released in response to light (Romijn et al., 1996; Francl et al., 2010a and b). Furthermore, both GRP and VIP cells communicate to a dorsolateral SCN population to induce expression of c-fos and per1/2 (Antle et al., 2005).
Through dimerization with CRY and upon appropriate post-translational modifications, PERIOD proteins play a principal role in the negative limb of the circadian transcriptional-translational feedback loop. Thus they have an important effect in determination of free running period, which is shortened in duper mutants. Although we found no effects of genotype on PER1 levels at CT12, PER1 intensity was lower in duper hamsters at CT15 (Fig. 5). Furthermore, induction of per1 is believed to be a critical step in the initiation of phase shifts (Akiyama et al., 1999; Wakamatsu, et al., 2001; Hamada et al., 2004). Thus, we sought to determine whether light might elicit greater PER1-ir in the SCN of duper mutants, whose phase shift amplitude greatly exceeds that of the WT (Krug et al., 2011, Manoogian et al., 2015). The appearance of PER1-ir in mid- and caudal-SCN lagged that of c-FOS-ir by at least an hour in all hamsters exposed to light at CT15. As with c-FOS-ir, these observations conform to the expectation that the protein peak is delayed relative to appearance of the Per1 transcript (Yamamoto et al., 2001; Yan and Silver, 2002, 2004). Differences between duper and WT hamsters in PER1-ir in the ventral SCN and the PVN of were not evident in experiment 1. However, PER1-ir was more sustained in the dorsal SCN of duper than WT hamsters in experiment 2. In earlier work, we found no effect of duper on levels of either Per1 or Per2 mRNA in hamsters at 30 to 120 minutes after light exposure at CT15 (Krug et al., 2011), although baseline Per2 transcript was lower in mutant than WT hamsters (Krug et al., 2011). Thus the present immunocytochemical results are consistent with findings from in situ hybridization. This method allowed us to identify genotype effects on activation of VIP cells, which we were not able to do in our earlier work.
The difference in the latency and time course with which light induced c-FOS-ir and PER1-ir is of interest, as both c-fos and per1 expression depend upon activation of CRE promoters. We also found an anatomical distinction in that the PER1 population lies medial to the cell group in which c-FOS-ir is induced by light. Post-transcriptional and post-translational regulation of period gene expression likely contributes to delayed appearance, complex pattern, and persistence of its protein product (Cao et al., 2015; Yoo et al., 2017). Our observation that a lateral population dominates the early wave of c-FOS-ir, followed by appearance of PER1-ir in a more medial region, points to a need for more detailed analysis of cell types and interactions in the ventral core of the SCN. To the extent that synaptic relays increase latency of activation, this finding is consistent with ultrastructural observations that retinal input to hamster SCN is far more extensive to GRP-ir perikarya and dendrites than to VIP neurons (Aioun et al., 1998). Although our study describes PER1-ir over the course of the subjective night in free running and light-exposed hamsters, sampling at more frequent time points is needed to increase precision of analysis. As tools such as bioluminescent reporters become available we may be able to monitor events in mutant vs. wild type SCN continuously either ex vivo or in vivo, and to examine regional differences in signal propagation. Better yet would be development of methods to identify the peptidergic phenotype of cells expressing the reporter in order to gain an accurate picture of the chain of events by which different cell types convey light’s perturbation of phase from one region of the SCN to another.
The finding that the duper allele amplifies the phase response curve (Krug et al., 2011; Bittman, 2014; Manoogian et al., 2015) and alters the latency with which hamsters entrain upon a shift of photoperiod (Kumar et al., unpublished) suggests that this mutation alters communication between the SCN core and shell. VIP coordinates the phase of cell autonomous oscillators throughout the SCN (Harmar et al., 2002; Maywood et al., 2011), exerting concentration-dependent effects upon SCN cell synchrony and entrainment (An et al., 2013). Overexpression of VPAC2 can speed phase resetting in rats and decrease circadian period in mice (Shen et al., 2000). Thus, an impact of the duper allele upon this population could contribute to the effect of the duper mutation to amplify phase responses to light.
In experiment 1, induction of c-FOS-ir in VIP cells was more robust in the mutant than WT hamsters. Furthermore, the intensity of c-FOS staining in VIP cells was greater in dupers. Despite similarities in pattern, the time course of PER1-ir differed between WT and duper hamsters in experiment 2. Upon application to slice cultures in early subjective night, both GRP and VIP can excite mouse SCN neurons through cAMP pathways that regulate potassium currents (Gamble et al., 2011; Kudo et al. 2013). Any of these steps may be altered by the duper mutation. By extending our analysis as long as 9h after the light pulse (Fig. 5 and 6), we identified increases in PER1-ir that occur beyond the 3–5h window of “delayed response” of the mouse SCN identified largely by electrophysiological methods (Kuhlman et al., 2003). It is difficult to distinguish whether these changes reflect events in the phase resetting process or longer-term expression of the phase shift. While time- and region-dependent effects of the duper allele on SCN function may contribute to alterations in period and phase resetting, it is also possible that responses of cells that receive VIP input are affected by the mutation. A mathematical model indicates that changes in coupling can account for the duper phenotype (Manoogian et al., 2015). These observations are consistent with the hypothesis that VIP cells are interposed between a Calbindin/GRP “gate” which is the direct recipient of photic input and the vasopressinergic shell population whose phase corresponds most closely to the hands of the clock we use to assess phenotype (Aioun et al., 1998; Antle et al., 2005; Karatseoros et al., 2004; Evans et al., 2015).
Although their functions may overlap to some degree, the PER1 and 2 proteins may be expressed in different cells and/or different circadian phases (Riddle et al., 2017), suggesting further complexity in the organization of the SCN core that may be important in trans-synaptic communication affected by duper. The region of peak expression of a per2 reporter changes over circadian time (Brancaccio et al., 2013), and this spatial pattern of gene expression may reflect oscillator coupling that is altered in circadian mutants. Deletion of per1 and per2 have opposite effects on free running period and phase responses in mice (Pendergast et al., 2010), and protein products of these genes likely contribute differently to phase advances and delays (Romijn et al., 1996; Yan and Silver, 2004; Schwartz et al., 2011).
Given that duper shortens free running period and exaggerates both the phase advancing and delaying effects of light, a more detailed comparison of free running and light-induced patterns of expression of both PER1 and PER2 in mutant vs WT hamsters is called for. Molecular events subsequent to the induction of per1 expression could be critically affected by the duper phenotype, and contribute to both the shortening of free running period and the amplification of responses to light. For example, shifts in expression of Cry and other core clock genes may occur with different latencies upon advances or delays of the LD cycle (Reddy et al., 2002). Duper amplifies levels of Dbp mRNA in the SCN, reflecting integration of effects of the mutation at the level of expression of clock controlled genes (Krug et al 2011). Determination of the duper sequence may ultimately prove the most efficient way to gain insight into the mechanism by which the mutation alters free running period and phase resetting.
In light of the dramatic shifts of locomotor activity rhythms elicited by light pulses in duper hamsters, we anticipated that c-FOS-ir or PER1-ir in the dorsal SCN or its immediate projection targets might differ with genotype in light-exposed animals. Thus we were surprised to observe no major effects of genotype on PER1 induction in subPVN or PVN, regions that receive efferent projections from the pacemaker. Although some duper hamsters displayed more PER1-ir cells in the PVN at 6 and 9 hours after a light pulse at CT 15 compared to WTs, this response was variable and did not reach statistical significance. Thus effects of the duper mutation to enhance signaling to extra-SCN regions that control locomotor output remain to be identified. Our findings in SCN suggest that it may be important to focus analysis on particular cell phenotypes rather than these regions as a whole. Retinal input to the ventral anterior hypothalamus extends into the shell of the SCN (Lokshin et al., 2015; Fernandez et al., 2016) and beyond it (Youngstrom et al., 1991; Canteras et al., 2011). Light may have direct effects on c-fos and per1 expression in sub-PVN and PVN independent of SCN activation and circadian genotype.
In conclusion, our results point to subtle but significant impact of duper on SCN function. They provide insight into the time course of cell activation in different SCN compartments, and the specific response of VIP cells within the core, upon light exposure during the subjective night. Additionally, they address the question of how the duper mutation, which dramatically amplifies and accelerates phase resetting, impacts neuronal activation and cell-specific levels of core clock proteins within the SCN. These findings clarify molecular events and cell groups that constrain and determine not only acute responses to photic shifts but also longer-term behavioral responses to changes in the light cycle.
Supplementary Material
S1. Representative composite 10X image of triple label for AVP-NP (blue), PER1 (red), and cFOS (green) caudal-SCN defining regions of ventral and dorsal SCN. (B) Low power image depicting definition of PVN and sPVN stained for AVP-NP (blue). [3V: Third ventricle; OC: Optic chiasm].
S2. The number of VIP cells did not differ between WT and duper hamsters at any of the circadian times examined, and was unaffected by light exposure.
Acknowledgements
The authors thank Amanda Hageman for animal care, Julia Napolitano for assistance with ICCs in experiment 2, and David Weaver for generous donation of antibodies. This work was supported by NIH R21NS099473 to E.L.B.
Abbreviations
- AVP
Vasopressin
- AVP-NP
Vasopressin associated neurophysin
- CT
Circadian Time
- DD
Constant Darkness
- GRP
Gastrin Releasing Peptide
- LD
Light Dark cycle
- PVN
Paraventricular Nucleus
- SCN
Suprachiasmatic Nucleus
- VIP
Vasoactive Intestinal Peptide
- VPAC2
Vasoactive Intestinal Peptide receptor type 2
- WT
Wild Type
Footnotes
Competing Interests
The authors have no competing interests to declare.
Data Accessibility
Supplies and data from current study are available from corresponding author upon request.
Contributor Information
Emily N.C. Manoogian, University of Massachusetts at Amherst - Program in Neuroscience and Behavior, Amherst, Massachusetts, United States
Ajay Kumar, University of Massachusetts at Amherst - Program in Neuroscience and Behavior, Amherst, Massachusetts, United States.
Doha Obed, University of Massachusetts Amherst Department of Biology Ringgold standard institution – Biology, Amherst, Massachusetts, United States.
Joseph Bergan, University of Massachusetts at Amherst - Psychological and Brain Sciences and Program in Neuroscience and Behavior, Amherst, Massachusetts, United States.
Eric L Bittman, University of Massachusetts Amherst Department of Biology Ringgold standard institution - Biology and Program in Neuroscience and Behavior Amherst, Massachusetts, United States.
References
- Aioun J, Chambille I, Peytevin J & Martinet L (1998). Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tiss Res., 291, 239–253. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Kouzu Y, Takahahsi S, Wakamatsu H, Moriua T, Maetani M, Watanabe S, Tei H, Sakaki Y, & Shibata S (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci., 19, 1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Harang R, Meeker, Granados-Fuentes D, Tsai CA, Mazuski C., Kim J., Doyle FJ., Petzold LR. & Herzog ED (2013). A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci., 110, E4355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Kriegsfeld LJ & Silver R (2005). Signaling within the master clock of the brain: Localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci., 25, 2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Foley NC, Foley DK & Silver R (2007). Gates and oscillators II: Zeitgebers and the network model of the brain clock. J Biol Rhythms, 22, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Coplwell CS, Harmar AJ, Waschek J & Herzog ED (2005) Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci., 8, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM & Weaver DR (2001) Differential functions of mPer1, mPer2 and mPer3 in the SCN circadian clock. Neuron, 30, 525–536. [DOI] [PubMed] [Google Scholar]
- Best JD, Maywood ES, Smith KL & Hastings MH (1999) Rapid resetting of the mammalian circadian clock. J Neurosci., 19, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL (2012) Does the precision of a biological clock depend upon its period? Effects of the duper and tau mutations in Syrian hamsters. PLoS One, 7(5), e36119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL (2014) Effects of the duper mutation on responses to light: parametric and nonparametric responses, range of entrainment, and masking. J Biol Rhythms, 29, 97–109. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon AS & Hastings MH (2013) A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron, 78, 714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Cheng HY & Obrietan K (2005) Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J Neurosci., 25, 5305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, & Swanson LW (2011). The retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain Res Rev., 65(2), 150–183. [DOI] [PubMed] [Google Scholar]
- Cao R, Gkogkas CG, de Zavalia N, Blum ID, Yanagiya A, Tsukumo Y, Xu H, Lee C, Storch KF, Liu AC, Amir S, & Sonenberg N (2015). Light-regulated translational control of circadian behavior by eIF4E phosphorylation. Nat. Neurosci, 18(6), 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Sterniczuk R, Enkhbold Y, Jeffers RT, Basu P, Duong B, Chow S-L, Smith VM & Antle MC (2016) Phase shifts to light are altered by antagonists to neuropeptide receptors. Neuroscience, 327, 115–124. [DOI] [PubMed] [Google Scholar]
- Evans JA, Suen TC, Callif BL, Mitchell AS, Castanon-Cervantes O, Baker KM, Kloehn I, Baba K, Teubner BJ, Ehlen JC, Paul KN, Bartness TJ, Tosini G, Leise T, & Davidson AJ (2015). Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC biology, 13(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Chang YT, Hattar S & Chen SK (2016) Architecture of retinal projections to the central circadian pacemaker. Proc Natl Acad Sci., 113, 6047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francl JM, Kauer G, & Glass JD (2010). Roles of light and serotonin in the regulation of gastrin-releasing peptide and arginine vasopressin output in the hamster SCN circadian clock. Eur J. Neurosci, 32, 1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francl JM, Kauer G, & Glass JD (2010). Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport, 21:1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Kudo T, Colwell CS, & McMahon DG (2011). Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J. Biol. Rhythms 26,99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido ME, Goguen D, DeGuido L, Robertson HA & Rusak B (1999) Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neuroscience, 90, 555–71. [DOI] [PubMed] [Google Scholar]
- Hamada T, Antle MC & Silver R (2004) Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci., 19, 1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, & Hastings MH (2002) The VPAC 2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell, 109(4), 497–508. [DOI] [PubMed] [Google Scholar]
- Honrado GI, Johnson RS, Golombek DA, Spiegelman BM, Papaioannou VE, & Ralph MR (1996) The circadian system of c-fos deficient mice. J Comp Physiol. A, 178(4), 563–570. [DOI] [PubMed] [Google Scholar]
- Irwin RP & Allen CN (2007) Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci., 27, 11748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Hofman MA, Swaab DF, & Buijs RM (2010) Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol., 22(5), 362–372. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, & Silver R (2004) Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci., 24(1), 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Nagano M, Kanda F, Chihara K, Shigeyochi Y, Okamura H (2003) Two types of VIP neuronal components in rat suprachiasmatic nucleus. J Neurosci Res., 74, 852–857. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Yan L, Witkovsky P, LeSauter J, Hamada T, Silver R (2008) Targeted mutation of the calbindin D28K gene disrupts circadian rhythmicity and entrainment. Eur J Neurosci., 27, 2907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J & Silver R (2004) Organization of surpachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol., 468, 361–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug S, Brewer JM, Bois AS, & Bittman EL (2011) Effects of the Duper mutation on circadian responses to light. J Biol Rhythms, 26(4), 293–304. [DOI] [PubMed] [Google Scholar]
- Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, & Colwell CS (2013). Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J. Neurophysiol, 110:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, LeSauter J, Bult-Ito A & McMahon DG (2003) Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci., 23, 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Lambert CM, Robotham MR, Model Z, Silver R & Weaver DR (2012) Antibodies for assessing circadian clock proteins in the rodent suprachiasmatic nucleus. PLoS One, 7(4), e35938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Burton KJ, Zhang C, Hu SB, & Zhou QY (2009) Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr and Comp Physiol, 296(3), 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokshin M, LeSauter J, & Silver R (2015). Selective distribution of retinal input to mouse SCN revealed in analysis of sagittal sections. J Biol. Rhythms, 30, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M & Takhashi JS (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutationtau. Science, 288, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA & Hastings MH (2011) A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci., 108, 14306–14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian EN, Leise TL & Bittman EL (2015) Phase resetting in duper hamsters: specificity to photic zeitgebers and circadian phase. J Biol Rhythms, 30, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S, Schurov IL, Sloper PJ & Hastings MH (1998) Stimuli which entrain the circadian clock of the neonatal Syrian hamster in vivo regulate the phosphorylation of the transcription factor CREB in the suprachiasmatic nucleus in vitro. Eur J Neurosci., 10(3), 1063–72. [DOI] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sladek M, Semikhodskii AS, Glossop NRJ, Piggins HD, Chesham JE, Bechtold DA, Yoo S-H, Takahashi JS, Virshup DM, Boot-Handford R, Hastings MH & Loudon ASI (2008) Setting clock speed in mammals: The CK1e tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron, 58, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K-I, Honma S & Sakurai T (2015) Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavioral rhythm. Neuron, 85, 1103–16. [DOI] [PubMed] [Google Scholar]
- Monecke S, Brewer JM, Krug S & Bittman EL (2011) Duper: A mutation that shortens hamster circadian period. J. Biol Rhythms, 26(4), 283–292. [DOI] [PubMed] [Google Scholar]
- Munch IC, Moller M, Larsen PJ & Vrang N (2002) Light-induced c-Fos expression in suprachiasmatic nuclei neurons targeting the paraventricular nucleus of the hamster hypothalamus: phase dependence and immunochemical identification. J Comp Neurol., 442, 48–62. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Leise TL, Kingsbury N & Welsh DK (2017) Calcium circadian rhythmicity in the suprachiasmatic nucleus: Cell autonomy and network modulation. eNeuro 4(4), 0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Friday RC & Yamazaki S (2010) Photic entrainment of Period mutant mice is predicted from their phase response curves. J Neurosci., 30, 12179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Field MD, Maywood ES & Hastings MH (2002) Differential resynchronization of circadian clock gene expression within suprachiasmatic nuclei of mice subjected to experimental jet lag. J Neurosci, 22, 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M, Mezia E, Foley D, LeSauter J & Silver R (2017) Differential localization of PER1 and PER2 in the brain master circadian clock. Eur J Neurosci., 45, 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Pool CW, Wortel J, & Buijs RM (1996) Differences in colocalization between Fos and PHI, GRP, VIP, and VP in neurons of the rat suprachiasmatic nucleus after a light stimulus during the phase delay versus the phase advance period of the night. J. Comp. Neuro, 372,1–8. [DOI] [PubMed] [Google Scholar]
- Rusak B, Robertson HA, Wisden W & Hunt SP (1990) Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science, 248(4960), 1237–1240. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saafield S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceri K, Tomancak P & Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods, 9, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Carpino A, de la Iglesia HO, Baler R, Klein DC, Nakabeppu Y & Aronin N (2000) Differential regulation of fos family genes in the ventrolateral and dorsomecial subdivisions of the rat suprachiasmatic nucleus. Neuroscience 98, 535–47. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Tavakoli-Nezhad M, Lambert CM, Weaver DR & de la Iglesia HO (2011) Distinct patterns of Period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc Natl Acad Sci, 108, 17219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM & Harmar AJ (2000) Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci., 97(21), 11575–11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM & LeSauter J (1996) Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport, 7(6), 1224. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Shimomura K & Kumar V (2008) Searching for genes underlying behavior: Lessons from circadian rhythms. Science, 322, 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Wang TJ, Granados-Fuentes D, Herzog& E.D. (2016) Resynchronization dynamics reveal that the ventral entrains the dorsal suprachiasmatic nucleus. J Biol Rhythms, 32, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden JC, Buijs RM, van der Donk HA, van den Hurk R & Wiegant VM (1994) Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons. During a steroid-induced luteinizing hormone surge in the female rat. Endocrinol, 134, 2636–44. [DOI] [PubMed] [Google Scholar]
- Wakamatsu H, Takahashi S, Moriuya T, Inouye ST, Okamura H, Akiyama M & Shibata S (2001) Additive effect of mPer1 and mPer2 antisense oligonucleotides on light-induced phase shift. Neuroreport, 12, 127–131. [DOI] [PubMed] [Google Scholar]
- Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen KH & Herdegen T (1995) Block of c-Fos and JunB Expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. European Journal of Neuroscience, 7(3), 388–393. [DOI] [PubMed] [Google Scholar]
- Yan L & Silver R (2002) Differential induction and localization of mPer1 and mPer2 during advancing an d delaying phase shifts. Eur J Neurosci., 16, 1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L & Silver R (2004) Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci., 19, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N & Okamura H (2001) Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. Journal of Comparative Neurology, 430(4), 518–532. [PubMed] [Google Scholar]
- Yoo S, Kojima S, Shimomura K, Koide N, Buhr ED, Furukawa T, Ko CH, Gloston G, Ayuoub C, Nohara K, Reyes BA, Tsuchiya Y, Yoo O-J, Yagita K, Lee C, Chen Z, Yamazaki S, Green CB, Takahashi JS (2017) Period2 3’UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci, 114(42), E8855–E8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom TG, Weiss ML, & Nunez AA (1991) Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain research bulletin, 26(3), 403–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Representative composite 10X image of triple label for AVP-NP (blue), PER1 (red), and cFOS (green) caudal-SCN defining regions of ventral and dorsal SCN. (B) Low power image depicting definition of PVN and sPVN stained for AVP-NP (blue). [3V: Third ventricle; OC: Optic chiasm].
S2. The number of VIP cells did not differ between WT and duper hamsters at any of the circadian times examined, and was unaffected by light exposure.