This article reports a study using electronic health records from oncology practices in the Flatiron Health Network to assess race‐ and sex‐based disparities in treatment with immune checkpoint inhibitors of programmed death protein 1.
Abstract
Amid growing excitement for immune checkpoint inhibitors of programmed death protein 1 (anti‐PD1 agents), little is known about whether race‐ or sex‐based disparities exist in their use. In this observational study, we constructed a large and mostly community‐based cohort of patients with advanced stage cancers, including melanoma, non‐small cell lung cancer (NSCLC), and renal cell carcinoma, to compare the odds of receiving systemic treatment with or without anti‐PD1 agents by race and by sex. In multivariable models that adjusted for age, stage, and number of prior anticancer therapies, we found no significant race‐based disparities in anti‐PD1 treatment. However, among patients with NSCLC, males had significantly higher odds of receiving anti‐PD1 treatment compared with females (odds ratio 1.13, 95% confidence interval 1.02–1.24, p = .02). This finding suggests that as anti‐PD1 agents enter the market to transform patient care, it will be critical to monitor for disparities in the use of these drugs.
There is great enthusiasm for immune checkpoint inhibitors of programmed death protein 1 (anti‐PD1 agents), which have led to dramatic and durable responses to treatment [1], [2]. Because of impressive results in early clinical trials, the U.S. Food and Drug Administration (FDA) first approved anti‐PD1 treatment for clinical use in melanoma, non‐small cell lung cancer (NSCLC), and renal cell carcinoma (RCC). Yet despite evidence that treatment disparities are common in clinical practice [3], [4], it is uncertain whether race‐ or sex‐based disparities exist in the use of these drugs. We therefore assessed for race‐ or sex‐based disparities in anti‐PD1 treatment.
We conducted a retrospective cohort study using electronic health records (EHRs) from more than 200 oncology practices in the Flatiron Health Network. We used EHRs that were deidentified and then processed to capture elements from unstructured sources, such as provider notes, via technology‐enabled abstraction techniques. Our study sample included patients who received systemic treatment for advanced‐stage cancer between January 1, 2013, and August 31, 2016, for 1 of 3 cancers for which FDA had approved anti‐PD1 agents for use: melanoma, NSCLC, and RCC. We used medication orders and administrations to identify patients treated with anti‐PD1 agents (nivolumab or pembrolizumab) and to exclude patients treated in clinical trials. For each cancer type, we used chi‐square tests to compare the unadjusted distributions of anti‐PD1 treatment by race and by sex. In multivariable models, we determined the odds of anti‐PD1 treatment by race and by sex, adjusting for patient age, disease stage, and number of prior lines of therapy, as well as histologic subtype and smoking history (for those with NSCLC).
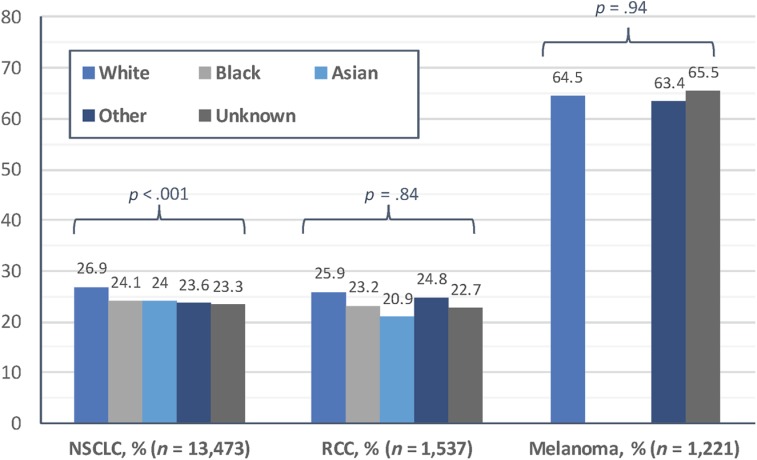
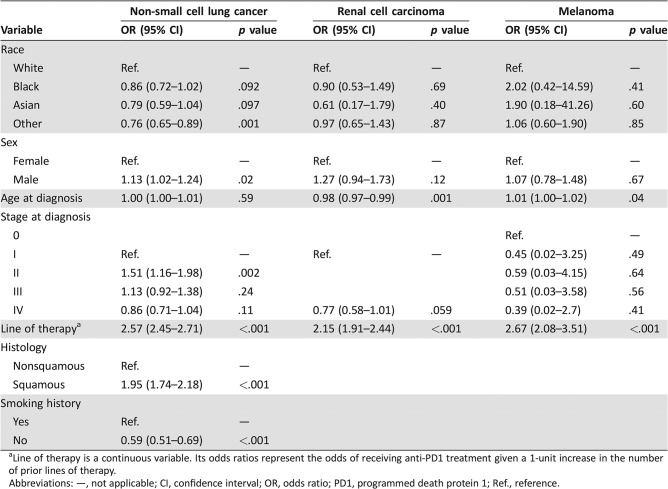
From a sample of 16,231 total patients treated for melanoma (n = 1,221), NSCLC (n = 13,473), or RCC (n = 1,537), we identified 4,643 (28.6%) receiving anti‐PD1 treatment. In bivariate analysis, there were small but statistically significant differences according to patient race and sex in the proportion receiving anti‐PD1 treatment for NSCLC, with 26.9% of White patients receiving anti‐PD1 treatment, compared with 24.1% and 24.0% of Black and Asian patients (p < .001; Fig. 1), and 27.2% of male patients receiving anti‐PD1 treatment, compared with 24.3% of female patients (p < .001). In multivariable models, differences in anti‐PD1 treatment among patients with NSCLC were not significant when black patients (odds ratio [OR] 0.86, 95% confidence interval [CI] 0.72–1.01, p = .09) or Asian patients (OR 0.79, 95% CI 0.59–1.04, p = .10) were compared with white patients (Table 1). However, male patients had higher odds than female patients of receiving anti‐PD1 treatment for NSCLC (OR 1.13, 95% CI 1.02–1.24, p = .02; Table 1). There were no significant disparities by race or by sex in receipt of anti‐PD1 treatment among patients melanoma or RCC (all p > .05; Table 1).
Figure 1.
Proportion of patients receiving anti‐programmed death protein 1 (anti‐PD1) treatment, January 1, 2013, to August 31, 2016. Anti‐PD1 treatment was assessed across categories of race using chi‐square tests, with p values listed by cancer type. Because of small sample sizes (<5 patients) among those with melanoma, we tested black patients and Asian patients with melanoma in the “Other” race group, which lowers the risk of patient identification.
Abbreviations: NSCLC, non‐small cell lung cancer; RCC, renal cell carcinoma.
Table 1. Multivariable models of factors associated with anti‐PD1 treatment for each cancer type.
Line of therapy is a continuous variable. Its odds ratios represent the odds of receiving anti‐PD1 treatment given a 1‐unit increase in the number of prior lines of therapy.
Abbreviations: —, not applicable; CI, confidence interval; OR, odds ratio; PD1, programmed death protein 1; Ref., reference.
In this study of patients treated in a network of US oncology practices, we found no significant race‐based disparities in receipt of anti‐PD1 treatment. We found a modest but statistically significant sex‐based disparity, however, in the receipt of anti‐PD1 treatment among patients with NSCLC.
Notably, even after adjusting for age, race, and stage, males still had higher odds than females of receiving anti‐PD1 treatment for NSCLC. This finding needs further study, as it builds upon prior works that found sex disparities in surgical treatment of patients with NSCLC and in clinical trial participation of patients with NSCLC [4], [5]. Although women are more likely than men to have tumors with certain mutations (i.e., epidermal growth factor receptor) that might influence treatment [6], it is unclear whether these differences might account for sex disparities in anti‐PD1 treatment. Our findings are somewhat reassuring with regard to race. However, racial disparities in anti‐PD1 treatment may exist in settings that were outside the scope of this study, such as in safety‐net practices with limited access to expensive new drugs. Because the association between anti‐PD1 treatment and race neared significance in our sample of patients with NSCLC, further studies may be warranted to monitor for race‐based disparities in the treatment of patients with NSCLC.
Fortunately, there are several ways to reduce disparities in the use of new drugs. First, it is critical to ensure that there is equity in access to clinical sites that offer new treatments—such as checkpoint inhibitors—as soon as the treatments are shown to be safe and effective. Second, because of the high and rising costs of cancer care, it is important to mitigate the financial barriers to cancer treatment and to support policies that reduce prices. Third, there is a need for future studies to understand patient perceptions of new treatments and to determine how to facilitate informed decision‐making. Finally, it is critical to assess for disparities in a variety of clinical contexts—including in academic and community practices as well as safety‐net clinics—and to monitor for disparities during the initial period of time when novel therapies enter the market to transform cancer care.
Our study has limitations, including the fact that our sample was largely restricted to community‐based practices (>90% of practices in our sample) and that it did not assess socioeconomic factors that may influence treatment. In addition, we did not assess for disparities in programmed death ligand 1 testing because of evidence that testing had rarely been done in clinical practice at the time of our study [7]. Our findings, however, should be a reminder that as novel therapeutic agents enter the market to transform cancer care, it will be imperative to identify and eliminate disparities among patients who might benefit from paradigm‐shifting drugs.
Acknowledgments
We thank Emily Yin, Carolyn Presley, Anne Chiang, Kristen Fessele, Nate Nussbaum, Kerin Adelson, Katie Darius, and Amy Abernethy for their contributions to earlier iterations of this work. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. This work was made possible by grant TL1 TR001864 from the National Center for Advancing Translational Science, a component of the NIH, as well as the Yale Center for Clinical Investigation at the Yale University School of Medicine. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Kathi Seidl‐Rathkopf: Flatiron Health (E, OI); Aracelis Z. Torres: Flatiron Health (E, OI); Paul You: Flatiron Health (E, OI); Kenneth R. Carson: Flatiron Health (E, OI); Joseph S. Ross: Medtronic, Johnson & Johnson, Blue Cross Blue Shield Association (RF); Cary P. Gross: Pfizer, Johnson & Johnson, 21st Century Oncology (RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Jacob JA. Cancer immunotherapy researchers focus on refining checkpoint blockade therapies. JAMA 2015;314:2117–2119. [DOI] [PubMed] [Google Scholar]
- 2. Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross CP, Smith BD, Wolf E et al. Racial disparities in cancer therapy. Cancer 2008;112:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shugarman LR, Mack K, Sorbero ME et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care 2009;47:774–781. [DOI] [PubMed] [Google Scholar]
- 5. Pang HH, Wang X, Stinchcombe TE et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol 2016;34:3992–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchetti A, Martella C, Felicioni L et al. EGFR mutations in non–small‐cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857–865. [DOI] [PubMed] [Google Scholar]
- 7. O'Connor J, Fessele K, Steiner J et al. Speed of adoption of immune checkpoint inhibitors of programmed death protein 1 and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA 2017;318:1647–1648. [DOI] [PubMed] [Google Scholar]