Abstract
This study has characterized a rat model with temporomandibular osteoarthritis (TMJ-OA) following a surgical anterior displacement of their articular disc (ADD). The well-established model of OA, induced by an intra-articular injection of complete Freund’s adjuvant (CFA) into the TMJ, was used for comparison purposes. Male Wistar rats were assigned into two surgical groups, namely, ADD (anterior disc displacement) and sham-operated (surgical access, without ADD). Additional groups received an intra-articular infiltration of CFA (50 μl/site; 1:1 oil/saline emulsion), or the vehicle (0.9% NaCl). The separate experimental subgroups were euthanized at 15, 30 or 60 days and their left TMJs were collected for histological, immunohistochemistry and micro-CT analyses. The serum levels of IL-1β, IL-6 and TNF were analyzed. The fibrocartilage thicknesses were increased in the ADD groups at all of the analyzed time-points. In the CFA group, fibrocartilage thickenings were seen only in the posterior thirds at 15 days. The ADD group displayed an increase of the proteoglycan contents and ADAMTS5 immunopositivity in the fibrocartilage at 30 and 60 days, without any variations of the collagen contents or the osteoclast activation. Upon the micro-CT evaluation, the ADD group presented increments of their trabecular separations and bone surfaces, with reduced trabecular thicknesses and bone volumes, plus osteophyte formations and condyle flattenings, from 30 to 60 days. The IL-1β, TNF or IL-6 serum levels were undetectable. The surgical ADD in the rats led to long-term OA-like alterations, with typical structural and morphological derangements of the TMJ, representing a reliable experimental model to investigate the TMJ-OA-related mechanisms.
Keywords: Osteoarthritis, TMJ, anterior disc displacement, CFA, rat
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is characterized by a degradation of the cartilage, with subchondral bone remodeling, and osteophyte formation, synovitis and pain [1,2]. OA commonly affects multiple joints, presenting a high prevalence worldwide. The occurrence of comorbidities and the elevated treatment charges are complicating factors for this medical condition [3]. Formerly, OA has been described as a non-inflammatory type of arthropathy. This concept has changed during the two last decades and OA is now currently defined as a low-grade inflammatory disease [3-5].
The temporomandibular joint (TMJ) is a complex structure that presents peculiar anatomic features and movements. The temporomandibular disorder (TMD) is a frequent alteration that is associated with this specific joint, inducing orofacial pain, articular noises and mandibular movement limitations [6,7]. TMJ-related OA is a frequent image finding that is correlated to the TMD, mainly being secondary to trauma, a functional overloading and internal derangements [6,8-10]. The anterior disc displacement (ADD) is a common internal disorder of the TMJ, which is characterized by its forward scape from the natural position [9]. The articular disc has an important role in the mechanical functionality of the TMJ, absorbing and redistributing the stress forces, contributing to the joint’s lubrication. Thus, the ADD can negatively affect the joint homeostasis, promoting an articular instability [11]. The clinical evidence has suggested a strong connection between the development of an ADD and the development of OA, but further studies on this matter are still required [8,12].
Mice submitted to a genetic manipulation have been widely used for studying the development of TMJ-OA [13], but these studies require sophisticated molecular biology facilities. In rats, most models of a TMJ-OA induction are limited to an intra-articular infiltration of the phlogistic agents, or they are induced by the occlusal abrupt alterations, which do not replicate the internal derangements that are seen in OA [7,14-18]. The induction of TMJ-OA in rabbits, following a surgical ADD, has been demonstrated as being useful in reproducing most of the clinical findings of TMJ-OA [19-21]. However, the utilization of rabbits is restricted due to economic and ethical concerns. Pertinently, the TMJ in rats presents anatomical and biomechanical features that resemble the human joint, indicating the applicability of this animal model for the study of TMJ-OA [22,23]. This present study has characterized an experimental model of TMJ-OA, following an ADD surgery in rats, comparing the main outcomes to those seen in the classical rat model of a CFA-induced TMJ inflammation.
Materials and methods
Experimental animals and groups
Male Wistar rats, 6-weeks-old, weighing from 170 to 190 g at the beginning of the experiments were used (a total number of 96 animals). They were obtained from the Central Animal House of the Pontifícia Universidade Católica do Rio Grande do Sul (CeMBE; PUCRS; Brazil). The animals were housed (four to a cage), under standard conditions of temperature (22 ± 2°C), light (12 h light-dark cycle) and humidity (50-70%), in individually ventilated cages, with an autoclaved wood chip bedding. The animals received standard rodent chow and tap water ad libitum. The Local Animal Ethics Committee (CEUA-PUCRS, CEUA 15/00465) approved all of the experimental procedures that are described in this study. The experiments were reported by following the ARRIVE Guidelines Checklist [24].
The animals were randomly divided into four groups, comprised of two surgical groups, namely, ADD and sham-operated, whilst two additional groups received an intra-articular infiltration of CFA or a saline solution (NaCl 0.9%). The different experimental subgroups were euthanized by using a sevoflurane inhalation at 15, 30 or 60 days. Either the surgery or the injections involved only the left TMJ, in order to avoid contralateral interferences.
Rat TMJ-OA ADD model
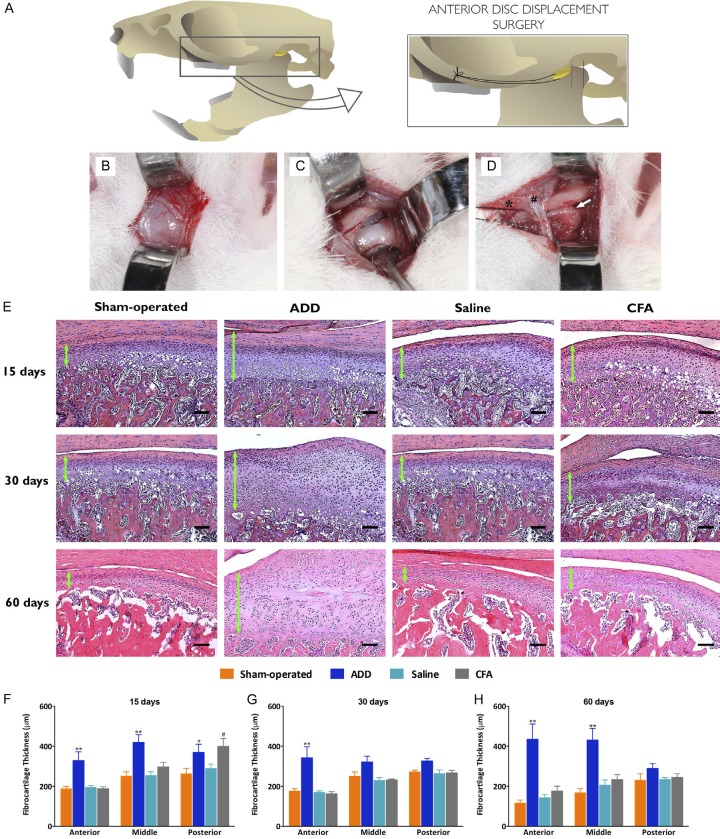
The method that was used in the present study for the ADD induction was adapted for the rats and it was based on the methodologies previously described for rabbits [19,20,25]. The animals received an oral prophylactic dose of cephalexin (60 mg/kg; 30 min before). The rats were anesthetized with xylazine (10 mg/kg) and ketamine (100 mg/kg), given intraperitoneally (i.p.). The area corresponding to the left pre-auricular region was shaved and disinfected with a 2% chlorhexidine solution. A local infiltration of 2% lidocaine plus 1:100.000 epinephrine was performed, in order to provide a further analgesia and hemostasis. A linear incision was made along the zygomatic arch (~1.2 cm), extending from the temporal root of the zygomatic arch, nearly to the lateral canthus of the eye. A capsular incision near to the zygomatic arch was performed, in order to expose the superior space of the joint. The anterior portion of the articular disc was localized and sutured with a 6-0 nylon wire. The suture wire was double-tied anteriorly into the maxillary process of the zygomatic bone, crossing inferiorly through the facial nerve, distending the attached disc forward, until reaching the maximal tension (~2 mm from the rest position). The lateral attachment of the disc to the condyle was incised, in order to facilitate the anterior disc displacement. The surgical site was irrigated with a saline solution (0.9% NaCl) and the incision was closed by a layered suture. The animals received oral acetaminophen (60 mg/kg) for the control of the pain for 48 h. All of these procedures were conducted in the sham-operated groups, except for the suture for stretching the articular disc forward. The surgery sequence is depicted in Figure 1A-D.
Figure 1.
(A) Representative illustration of the surgery procedure, showing the nylon suture anchorage in the zygomatic arch while stretching the disc. (B) Horizontal skin access between the lateral canthus of the eye and pre-auricular region. Special care was taken with the facial nerve that could easily be dissected. (C) A capsular incision exposed the articular disc and the superior space of the TMJ, so as to see all of the extensions of the disc attachments. A down-traction of the mandible facilitated this step of the procedures. (D) The anterior portion of the disc was transfixed by a nylon wire, allowing for the forward stretching and the suture was double-fixed in the anterior aspect of the zygomatic arch, in order to ensure no unlace. At this moment, an incision into the lateral attachment of the disc allowed for its traction. White star, articular disc. Hash, facial nerve. Black star, nylon suture. White arrow, the posterior zone of the disc and the retrodiscal ligament. (E) Analysis of the fibrocartilage thickness in the H&E-stained slides: the mid-sagittal sections of the TMJ, showing the fibrocartilage in the condyle from the anterior to the posterior zone. The three-thirds of the fibrocartilage were measured at three points, equally divided in each third from the fibrous layer to the hypertrophic layer. (F-H) The ADD group showed an increment in the thickness of their fibrocartilages, at the experimental times, when compared to the sham-operated control group and the infiltration groups, mainly in the anterior thirds. The thickening of this region showed a disorganization in the fibrocartilage layers, especially of the proliferative and the pre-hypertrophic layers. In the other groups, a time-dependent thinning was observed in the fibrocartilages and the posterior portion was generally thicker than the anterior. The thickness of the fibrocartilage (μm) at 15 (F), 30 (G) and 60 (H) days, as measured at the anterior, middle and posterior regions of the condyle. The columns show the mean and the vertical lines the show SEM of the 8 independent experiments. *P < 0.05; **P < 0.01 when comparing ADD to sham-operated rats. #P < 0.05 when comparing the CFA- to the saline injected animals. Bar = 100 μm.
TMJ inflammation elicited by the CFA injection
The animals were anesthetized as described above and the left TMJ was localized by a palpation of the zygomatic arch [14,26]. The animals received a single intra-articular injection containing 50 μl of CFA (1 mg/ml; heat-killed and dried Mycobacterium tuberculosis each milliliter of vehicle contained 0.85 ml paraffin oil plus 0.15 ml mannide monooleate; Sigma, St Louis, MO, USA), prepared as a 1:1 oil/saline emulsion. The CFA solution was injected over a 2-min period, by introducing a 30-gauge needle into the superior space of the joint [27]. The control group received an intra-articular injection of 50 μl of the vehicle.
Tissue collection and preparation
After the euthanasia, the incisor teeth were splinted by a dental composite in an occlusion position. The region corresponding to the left TMJ was separated by using a diamond disc at a low-speed rotation. After a fixation in 10% formaldehyde, the samples were decalcified in a 17% ethylenediaminetetraacetic acid (EDTA) solution, over 8 weeks, under a constant agitation, with a solution renewal every two days. For the histological procedures, consecutive mid-sagittal 4-μm-thick sections were obtained from paraffin blocks. The sections were mounted on poly-L-lysine-coated glass slides for the posterior analyses.
Histological analysis
The histological images were taken with a microscope (Axio Imager A1) that was coupled to an image capture system (Axio Vision Rel. 4.4 Software Multimedia) - both from Carl Zeiss (Hallbergmoos, Germany) - and the images were assessed by using NIH Image J 1.50 g Software. The fibrocartilage thicknesses were measured (in μm) as the mean of three points, equally divided at each third of the condyles, by using hematoxylin-eosin-stained slides [15]. For each sample, a sequence of images under × 100 magnification was merged in order to include the complete joint. Toluidine blue staining (0.04% in an acetate buffer; pH 4.0) was used for qualitatively evaluating the fibrocartilage proteoglycan contents. The collagen-rich blue-colored regions were analyzed in the deep layer of the subchondral bones, when considering two portions of 300 × 300 pixels, in Masson’s trichrome-stained sections (Accustain Mallory’s stain kit, Sigma-Aldrich, USA). Tartrate-resistant acid phosphate (TRAP) staining (Leukocyte TRAP kit, Sigma-Aldrich, USA) was performed in order to assess the number of the active osteoclasts, in four representative areas, beneath the hypertrophic layers, under × 400 magnification.
Immunohistochemistry for ADAMTS5
A standard, three-step, avidin-biotin complex staining procedure was performed for the immunohistochemical analyses. The polyclonal rabbit anti-ADAMTS5 (1:200; sc-83186, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a primary antibody. The immunopositivity was assessed by the relative values of the marked areas within the proliferative, the pre-hypertrophic and the hypertrophic layers, in relation to the total area, in each of the three thirds of the condyles, under × 400 magnification (NIH Image J 1.50 g Software; color deconvolution plug-in).
Micro-CT analysis
A microtomography scanning analysis was performed (SkyScan 1172, Bruker Micro-CT, Belgium) before the decalcification, at 89 Kv and 112 μA, with a 13-μm thickness, in a resolution of 1336 × 2000 pixels. In each third of the center-sagittal position of the condyles, a volume-of-interest (VOI; 0.3 × 0.3 × 0.5 mm) was selected, for a 3D-morphometric assessment, when using CTAn V.1.16 Software (Bruker), as illustrated in Figure 5A. The bone fractions (BV/TV) and the bone surface densities (BS/TV) were taken for the bone analyses. In order to evaluate the trabecular microstructures, the parameters of the trabecular numbers (Tb.N), the trabecular thicknesses (Tb.Th) and the trabecular separations (Tb.Sp) were selected. The angles between the coronal center of the condyles and the lateral projections of the condylar surfaces were measured, in order to evaluate the osteophyte formations.
Figure 5.
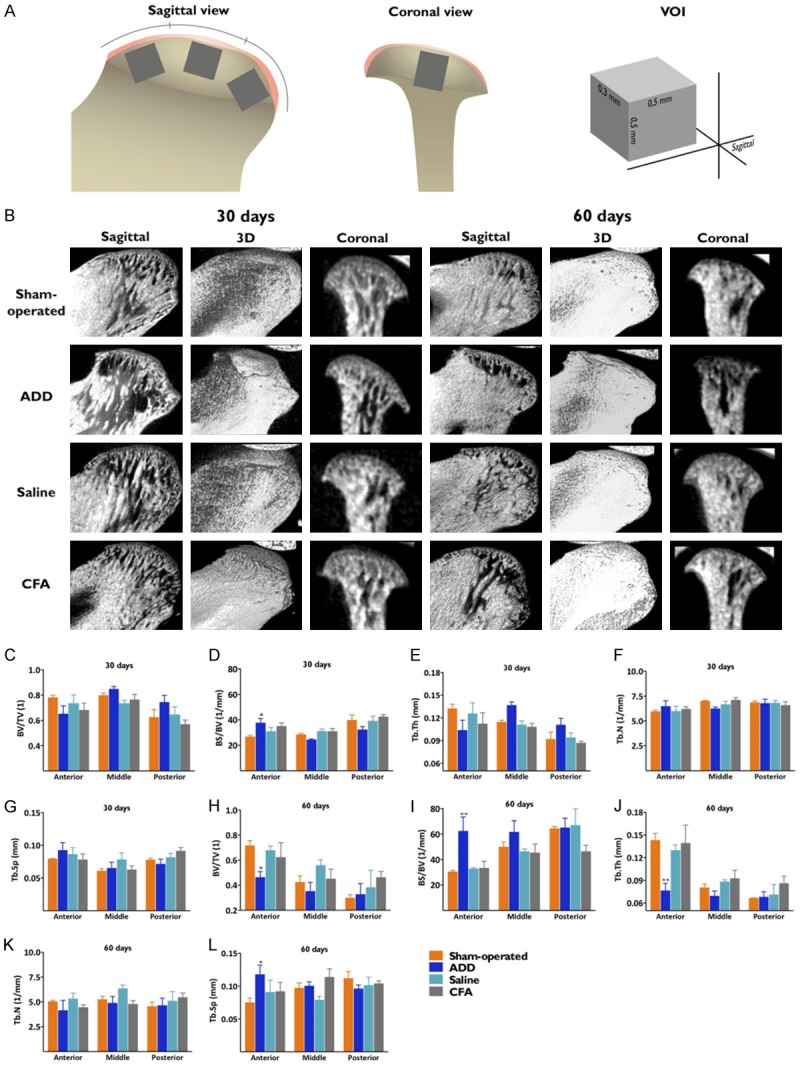
Microstructural evaluation of the TMJ. (A) An illustrative figure of the condyle region taken for the micro-CT analysis. From the center-sagittal plane, a volume of interest (VOI) was taken from each third of the subchondral bone region, respecting that the coronal anatomy could be thicker in some of the regions. (B) Micro-CT images in the sagittal, the 3D-reconstruction and the coronal views. The ADD group showed great morphological differences when at the coronal view at 30 days, as observed by lateral projection of the condylar surfaces. Trabecular morphology near the surface at 30 days, showing no differences between the groups (C-G). At 60 days, an increased surface flatness defined a difference among the groups. At this time-point, the ADD group displayed significant differences in all of the analyzed parameters, in the anterior portions of the condyles, showing specific patterns of morphology (H-L). *P < 0.05; **P < 0.01 when comparing the ADD to the sham-operated rats.
Serum cytokine levels
The serum pro-inflammatory cytokines, interleukin-1β (IL-1β), tumor necrosis factor (TNF) and interleukin-6 (IL-6), were measured when using specific enzyme-linked immunosorbent assay (ELISA) kits, according to the supplier’s recommendations (R&D Systems, Minneapolis, Minnesota, USA).
Statistical analysis
The measurements were carried out in a blinded manner. All of the data is expressed as the mean ± SEM. Bartlett’s test was employed, revealing a Gaussian distribution for the data, permitting the use of a parametric analysis. The results were evaluated by a one-way or a two-way analysis of variance (ANOVA), followed by the Bonferroni post-hoc test. P values less than 0.05 were considered statistically significant. All of the statistical tests and the production of the graphs were performed by using GraphPad 6 Software (San Diego, CA, USA). The intra-examiner concordance was evaluated by the intra-class correlation coefficient, reaching a value of 0.99 (0.8 to 1.0 being considered ideal).
Results
The CFA injections or the ADD surgery did not alter their body weight gains and the animals fed normally throughout all of the experimental period. The serum levels of the pro-inflammatory cytokines (IL-1β, TNF or IL-6) were undetectable in all of the experimental groups and at all of the time-points (data not shown).
The ADD group showed higher thicknesses in their fibrocartilage layers, according to the evaluations at the three time-points, when compared with the sham-operated and the infiltration groups (Figure 1E). The anterior third of the condyles exhibited the highest values of thickness in all of the layers, most expressively in the proliferative layers. A statistical difference was observed between the ADD and sham-operated groups at 15 days, in all of the analyzed thirds (Figure 1F). At 30 days, these differences were observed only in the anterior thirds (Figure 1G). At 60 days, the increases in the fibrocartilage thicknesses were prominent, with statistical differences in the anterior and the middle thirds, when comparing the ADD and the sham-operated groups (Figure 1H). The CFA group showed significantly enlarged thicknesses in their posterior thirds at 15 days (Figure 1F).
The anterior thirds in the ADD group showed an expressive presence of proteoglycans, as revealed by the toluidine blue staining (Figure 2A). The proteoglycans were mainly distributed in the pre-hypertrophic and the hypertrophic layers, in all of the experimental groups. Masson’s trichrome was used in order to assess the collagen presence in the subchondral layers, as evaluated by the blue staining of the bones (Figure 2B). All of the experimental groups presented similar collagen levels in their subchondral bones, without any significant differences among the groups (Figure 2C-E).
Figure 2.
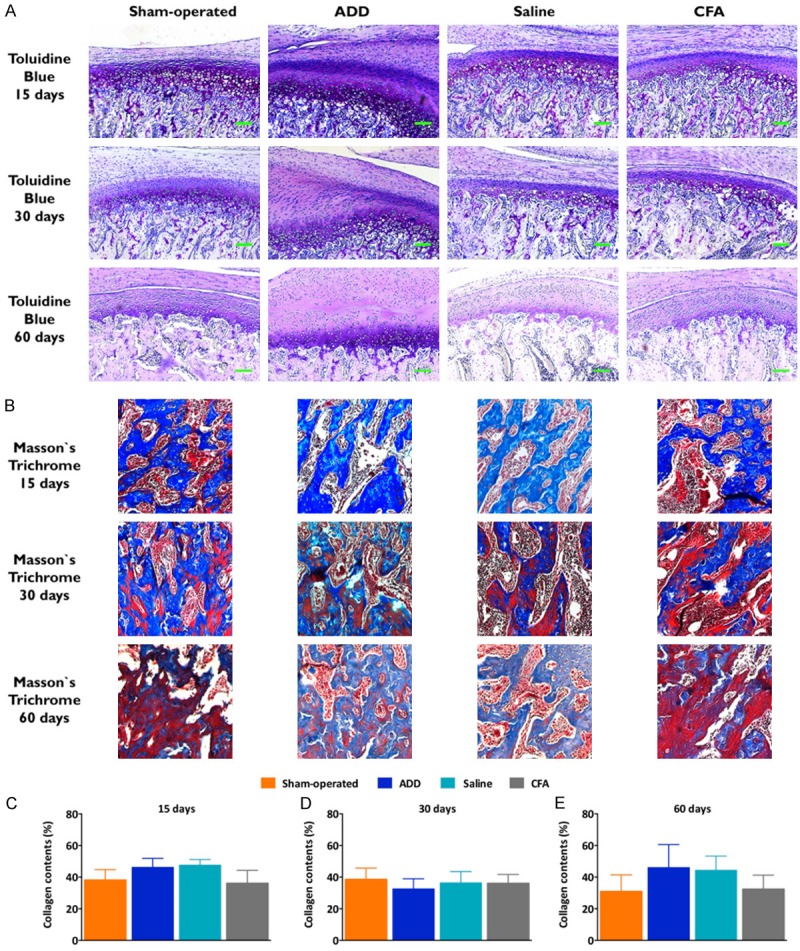
Histological analysis of the proteoglycan and the collagen contents in TMJ. (A) Toluidine blue staining from the anterior third of the fibrocartilages. The qualitative evaluation revealed the presence of proteoglycans in the pre-hypertrophic and the hypertrophic layers. A time-dependent loss of the proteoglycan contents was observed in all of the groups; however, the ADD group showed a higher presence of the proteoglycans, when compared to the other groups, mainly in the anterior thirds, at the evaluated time-points. In the ADD group, the increased proteoglycan staining was proportional to the augmented pre-hypertrophic and hypertrophic layers. (B) The collagen presence, as indicated by Masson’s trichrome staining, can be observed in the subchondral bone. The collagen contents were measured as the percentages of the blue-colored regions at 15 (C), 30 (D) and 60 (E) days. No statistical differences were observed. The columns show the mean and the vertical lines show the SEM of the eight independent experiments.
The pre-hypertrophic and the hypertrophic fibrocartilage layers presented the majority of the immunopositive cells for ADAMTS5, as a result of the nucleus and cytoplasm staining (Figure 3A). No statistical differences were observed among the groups, although a trend toward an increased ADAMTS5 immunopositivity was noted in the ADD group, at 30 and 60 days (Figure 3B-D).
Figure 3.
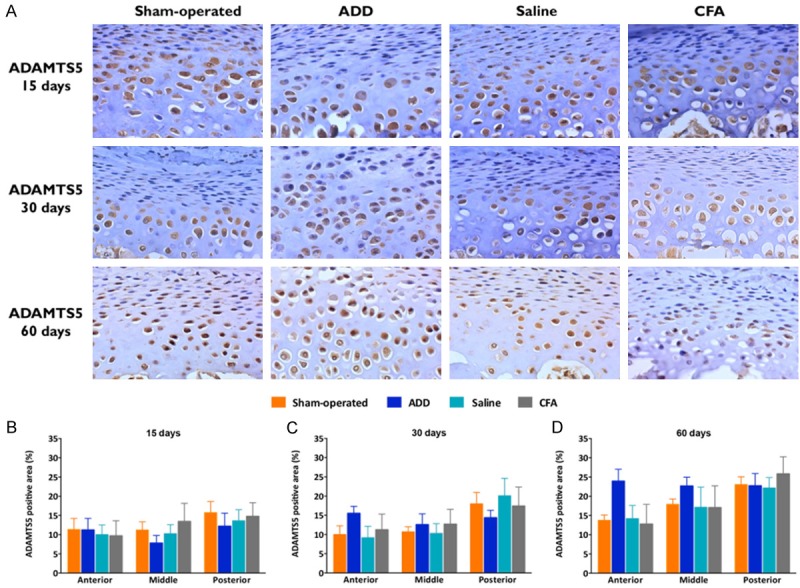
Immunopositivity for the metalloproteinase ADAMTS5. (A) Immunohistochemistry for ADAMTS5 in the TMJ fibrocartilages under x400 magnification. The ADAMTS5 gene was highly expressed in the condylar tissues, mainly in the pre-hypertrophic and in the hypertrophic layers, being only partially expressed in the proliferative layers. Most of the cells of the pre-hypertrophic and the hypertrophic layers were immunopositive for ADAMTS5. (B-D) Marked areas/total area of the ADAMTS5 immunopositive cells in the proliferative, the pre-hypertrophic and the hypertrophic layers, expressed in percentages at 15 (B), 30 (C) and 60 (D) days. No statistical differences were observed, despite that the ADD group showed an increased relative immunopositive area at 30 and 60 days, in their anterior thirds, especially at 60 days. The columns represent the mean of 5-8 independent experiments and the vertical lines indicate the standard error of the mean.
The TRAP-positive osteoclasts were counted beneath the hypertrophic layers. In most of the samples, the osteoclasts were predominantly located in the interface between the hypertrophic layers and the subchondral bones (Supplementary Figure 1A). No statistical differences among the groups were observed (Supplementary Figure 1B-D).
The 3D reconstructions showed an important view of the condylar surfaces and the osteophyte formation (Figure 4A, 4B). The samples from the ADD group had an increased projection of the lateral pole of their condyles, at 30 days; this finding was observed in all of the condyles for this group. The angles between the lateral projections of the condyles and the center-coronal positions were lower in the ADD group, when compared to the other groups (Figure 4C). At 60 days, a trend toward an increase of the projection angle was observed, with a flattening of the condyles (Figure 4D). There were no marked differences in the sham-operated or the injected groups. At 30 days, only the ADD group showed significant increases of their bone surfaces (BS/BV) (Figure 5C-G). Remarkable differences featuring the TMJ-OA were observed at 60 days for the ADD group, with a reduction of bone volume (BV/TV), an increment of bone surface (BS/BV), a decreased trabecular thickness (Tb.Th) and an augmented trabecular space (Tb.Sp), but only in the anterior portion of the condyles (Figure 5H-L). This data was compatible with the 3D-observations (Figure 5B).
Figure 4.
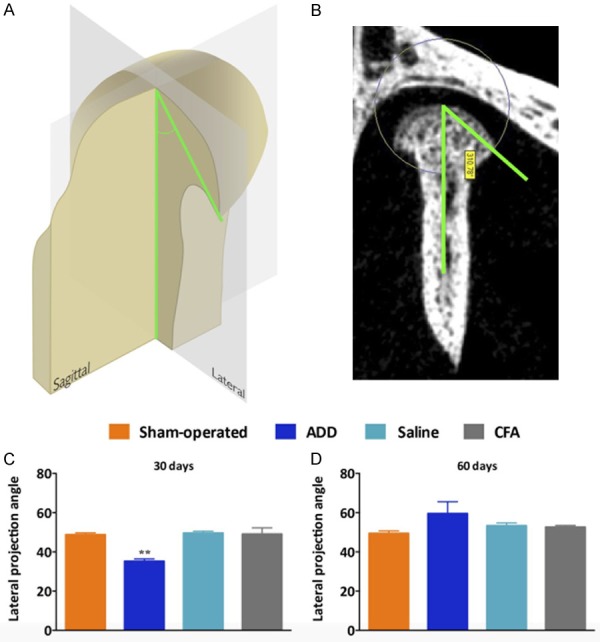
3D reconstruction showing the condylar surfaces and the osteophyte formations. A and B. Representative image and the quantitative analysis of the angle that was formed between the lateral projection of the condyles and the center-coronal axis. C. The ADD group showed a significant reduction of the lateral angle projections when compared with the other groups. All of the samples from the ADD showed the closest angles of projection, associated with the formation of the osteophytes, at 30 days. D. At 60 days, these angles of projection became more open, with no statistical differences among the groups. This was probably correlated with the bone remodeling at the surfaces from the ADD condyles. **P < 0.01 when comparing the ADD to the sham-operated rats.
Discussion
The experimental OA induced chemically represents an acute model for the study of the cartilage degradation and joint pain [13,28]. In the protocols, when using the CFA without any dilution, or with those employing a bilateral CFA injection into the TMJ, the disease development was associated with a severe inflammatory response that did not mimic the OA-related low inflammation grade [29,30]. This led us to adopt a protocol for using a diluted CFA (1:1 oil/saline solution), injected unilaterally, for a comparison with the ADD TMJ-OA model, aiming to induce a mild inflammatory response. Herein, the CFA infiltration or the ADD surgery did not alter the body weight gains, indicating that the animals were able to feed normally. There were no visible behavioral changes or the occurrence of stress-related signs, such as chromodacryorrhea or piloerection, discarding the grave systemic alterations [30]. Besides, the animals in the CFA or the ADD groups presented undetectable serum levels of the IL-1β, TNF or IL-6 cytokines, further indicating the absence of systemic inflammatory changes in both models [31].
The histological analysis revealed that ADD elicited an imbalance of the fibrocartilage dynamics. A remarkable finding was the increase in the fibrocartilage thicknesses in the ADD group. A clear disorganization among the fibrocartilage layers, accompanied by an increased cell proliferation in the proliferative layers at 30 days, were associated with the fibrocartilage thickening. At 60 days, the ADD group presented higher fibrocartilage thicknesses, with increases of the extracellular matrix space. The articular chondrocyte proliferation was an important characteristic during the process of the OA, accompanied by marked hypertrophic differentiation, with elevated collagen X and MMP-13 expression and with a subsequent apoptotic death and a mineralization of the diseased cartilage [32]. These increments were mainly localized in the anterior third of the condyles, likely due to the interference of the ADD procedures. Kuang et al. [17] described similar thickenings of the cartilage layer in a rat model with disordered occlusions that were induced by an orthodontic device, suggesting the occurrence of adaptative responses. Another study showed thicknesses of the fibrocartilages according to a qualitative histological analysis, after the intra-articular injections of iodoacetate (0.5 mg/site) into the rats’ TMJ [33]. Nonetheless, this finding is somewhat in contrast to some previous publications on rat models with CFA-induced TMJ-OA, which have shown a thinning of the fibrocartilage layers over the course of time [16,29]. Herein, the CFA group also displayed a thickening of their fibrocartilages, although this feature was restricted to the posterior third of their condyles, being observed only at 15 days.
Regarding the disc changes, the evaluations of the H&E-stained slides at 60 days showed that most of the samples displayed ruptured/perforations of their articular discs, with thickness alterations and morphological deformities, probably due to the altered function that was inherent to this surgical model. At the same time point, a further inflammatory alteration that was observed for the ADD group was a hyperplasic response in their synovial tissues. This finding could be a consequence of the disc displacements that were associated with the osteoarthritic changes in the fibrocartilages of the condyles. In the other groups, at this same time point, no visible alterations were observed. This was also expected with the OA progresses [29], supporting the relevance of the researchers’ ADD experimental rat model.
Toluidine blue staining was employed, in order to evaluate the proteoglycan contents, which are essential components for a fibrocartilage functionality [34]. The qualitative analyses revealed increased proteoglycan levels in the ADD groups, along with the experimental times. The increased proteoglycan contents might be a consequence of the hypertrophic proliferation of the fibrocartilages. Noteworthy, the TMJ proteoglycan levels in the CFA-injected groups were similar to those that were seen in the saline-treated control animals. Based upon the histological data, it was possible to infer that the ADD- and the CFA-induced TMJ-OA clearly displayed distinct patterns of a disease progression for up to 60 days.
ADAMTS are members of the super-family of metalloproteinases that have been widely implicated in the mechanisms underlying an OA progression [35,36]. The gene deletion of the catalytic domain of ADAMTS5 prevented the cartilage destructions in the mice, clearly suggesting the importance of this enzyme in an OA condition [37]. The immunohistochemical analyses revealed a marked immunopositivity for ADAMTS5 in most of the cartilage layers, except in the fibrous layers, in all of the experimental groups. The surgical ADD groups showed increased immunopositive areas at 30 and 60 days in their anterior thirds, despite the absence of any statistical differences. The expression of this aggrecanase may be under an uncontrolled production in the OA cartilage, resulting in a destruction of the cartilage matrices [32]. Probably, in the ADD group, this increment of ADAMTS5 was related to the fibrocartilage thickening and the disorganization of the cartilage layers. An increase of the ADAMTS5 expression, that was associated with an upregulation of MMP-13 and collagen X, was observed in the TMD rat model that was induced by the intra-articular bilateral injections of 100 μl undiluted CFA, according to the evaluations by western-blotting [38]. However, this present study has shown, for the first time, the distribution of ADAMTS5 throughout the different regions of the TMJ, by using immunohistochemistry.
The collagen contents or the osteoclast activity did not variate among the experimental groups. This prompted us to carry out a micro-CT analysis of the subchondral bone morphology, in order to assess any possible microstructural changes. At 30 days, the 3D images showed lateral projections of the condyles in the ADD group, which were presumably compatible with the osteophyte formations. This was consistently observed in all of the ADD samples and it was confirmed by the measurements of the projection angles. Anatomically, the zygomatic arches were more lateralized in the center-sagittal view, in relation to the condyles and they were used as an anchorage for the nylon wire. Consequently, the stretching forces moved the disc anterolaterally, leading to the formation of the osteophytes. This event probably stimulated an initial proliferation in the fibrocartilages, as indicated by the histological findings, demonstrating a remodeling response after the traumatic dislocation of the discs. At 60 days, the bones and the trabecular structures suffered intense modifications in the ADD group. The changes were consistent with the OA-like findings, such as increased trabecular separations and bone volumes, allied to reductions of the trabecular thicknesses and the bone surfaces [29,39,40]. These alterations were evidenced in the anterior thirds, likely because of the disc dislocations. A flattening occurred in the condyle surfaces, with a decrease in the projection angles, indicating that the ADD TMJ-OA model led to chronic alterations. An overall analysis of the histological and the micro-CT images allowed for suggesting that the disc displacements lead to cartilage hypertrophy circumstances, accompanied by the OA-compatible bone changes.
All of the surgical procedures were also conducted in the sham-operated groups, except for the suture stretching forward of the articular discs. Thus, the incisions in the lateral capsular ligaments of TMJ did not appear to cause the OA-like alterations. Of note, the CFA group showed a fibrocartilage thickening only at 15 days, displaying a resolution tendency. Based upon the previous literature data, there was a great variability concerning the number of injections, the methods of infiltration, or the concentrations of CFA that were used to induce the TMJ-OA in the rodents [18,29,41]. As discussed above, we decided to use a single unilateral injection of CFA into their TMJs, which might explain the mild inflammatory changes. In relation to the CFA protocol that was used here in this study, the surgical ADD procedures caused time-dependent TMJ changes, consistent with the OA features that were related to the internal joint derangements.
Acknowledgements
This work was supported by a FINEP research grant “Implantação, Modernização e Qualificação de Estrutura de Pesquisa da PUCRS” (PUCRSINFRA) # 01.11.0014-00 and by PUCRS. M.M.C. (CNPq, 303842-2014-8) is Research Career Awardee of the National Research Council of Brazil (CNPq). L.T. and R.B.M.S. are the recipients of scholarships awarded by CAPES (Financial Code: 001). The authors would like to thank Mrs. Janaína Pasetti Nunes and Mrs. Raquel M. Oliveira for their excellent technical assistance with the histological techniques. This research was also supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Brazil.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bertuglia A, Lacourt M, Girard C, Beauchamp G, Richard H, Laverty S. Osteoclasts are recruited to the subchondral bone in naturally occurring post-traumatic equine carpal osteoarthritis and may contribute to cartilage degradation. Osteoarthritis Cartilage. 2016;24:555–566. doi: 10.1016/j.joca.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Wen ZH, Tang CC, Chang YC, Huang SY, Lin YY, Hsieh SP, Lee HP, Lin SC, Chen WF, Jean YH. Calcitonin attenuates cartilage degeneration and nociception in an experimental rat model of osteoarthritis: role of TGF-beta in chondrocytes. Sci Rep. 2016;6:28862. doi: 10.1038/srep28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81–90. doi: 10.1016/j.bone.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94:666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 7.Kuroki Y, Honda K, Kijima N, Wada T, Arai Y, Matsumoto N, Iwata K, Shirakawa T. In vivo morphometric analysis of inflammatory condylar changes in rat temporomandibular joint. Oral Dis. 2011;17:499–507. doi: 10.1111/j.1601-0825.2010.01782.x. [DOI] [PubMed] [Google Scholar]
- 8.Bertram S, Moriggl A, Neunteufel N, Rudisch A, Emshoff R. Lateral cephalometric analysis of mandibular morphology: discrimination among subjects with and without temporomandibular joint disk displacement and osteoarthrosis. J Oral Rehabil. 2012;39:93–99. doi: 10.1111/j.1365-2842.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Cai X, Wang S, Yang C, Song H, Huang L. Disc positions and condylar changes induced by different stretching forces in the model for anterior disc displacement of temporomandibular joint. J Craniofac Surg. 2014;25:2112–2116. doi: 10.1097/SCS.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 10.Asakawa-Tanne Y, Su S, Kunimatsu R, Hirose N, Mitsuyoshi T, Okamoto Y, Tanaka E, Tanne K, Tanimoto K. Effects of enzymatic degradation after loading in temporomandibular joint. J Dent Res. 2015;94:337–343. doi: 10.1177/0022034514560588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XD, Cui SJ, Liu Y, Luo Q, Du RJ, Kou XX, Zhang JN, Zhou YH, Gan YH. Deterioration of mechanical properties of discs in chronically inflamed TMJ. J Dent Res. 2014;93:1170–1176. doi: 10.1177/0022034514552825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emshoff R, Innerhofer K, Rudisch A, Bertram S. The biological concept of “internal derangement and osteoarthrosis”: a diagnostic approach in patients with temporomandibular joint pain? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:39–44. doi: 10.1067/moe.2002.117451. [DOI] [PubMed] [Google Scholar]
- 13.Fang H, Beier F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat Rev Rheumatol. 2014;10:413–421. doi: 10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 14.do Nascimento GC, Leite-Panissi CR. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol Behav. 2014;125:1–7. doi: 10.1016/j.physbeh.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jiao K, Zhang M, Zhou T, Liu XD, Yu SB, Lu L, Jing L, Yang T, Zhang Y, Chen D, Wang MQ. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J Dent Res. 2013;92:253–259. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Wang H, Zhang J, Zhang H, Yang H, Wan X, Jing L, Lu L, Liu X, Yu S, Chang W, Wang M. Unilateral anterior crossbite induces aberrant mineral deposition in degenerative temporomandibular cartilage in rats. Osteoarthritis Cartilage. 2016;24:921–931. doi: 10.1016/j.joca.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuang B, Dai J, Wang QY, Song R, Jiao K, Zhang J, Tian XG, Duan YZ, Wang MQ. Combined degenerative and regenerative remodeling responses of the mandibular condyle to experimentally induced disordered occlusion. Am J Orthod Dentofacial Orthop. 2013;143:69–76. doi: 10.1016/j.ajodo.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Wang XD, Kou XX, Mao JJ, Gan YH, Zhou YH. Sustained inflammation induces degeneration of the temporomandibular joint. J Dent Res. 2012;91:499–505. doi: 10.1177/0022034512441946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills DK, Daniel JC, Herzog S, Scapino RP. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillofac Surg. 1994;52:1279–1292. doi: 10.1016/0278-2391(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Zhou Y, Zhang Y, Zhao S, Liu J, Hu J. An animal model for inducing anterior disc displacement of the temporomandibular joint. J Orofac Pain. 2006;20:166–173. [PubMed] [Google Scholar]
- 21.Berteretche MV, Foucart JM, Meunier A, Carpentier P. Histologic changes associated with experimental partial anterior disc displacement in the rabbit temporomandibular joint. J Orofac Pain. 2001;15:306–319. [PubMed] [Google Scholar]
- 22.Porto GG VB, Andrade ES, Silva-Junior VA. Comparison between human and rat TMJ: anatomic and histopathologic features. Acta Cirúrgica Brasileira. 2010;25:290–293. doi: 10.1590/s0102-86502010000300012. [DOI] [PubMed] [Google Scholar]
- 23.Orset E, Chaffanjon P, Bettega G. Temporomandibular joint model: anatomic and radiologic comparison between rat and human. Surg Radiol Anat. 2014;36:163–166. doi: 10.1007/s00276-013-1159-4. [DOI] [PubMed] [Google Scholar]
- 24.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Sharawy M, Ali AM, Choi WS, Larke V. Ultrastructural characterization of the rabbit mandibular condyle following experimental induction of anterior disk displacement. Cells Tissues Organs. 2000;167:38–48. doi: 10.1159/000016765. [DOI] [PubMed] [Google Scholar]
- 26.Fuentes R, Veuthey C, Arias A, Saravia D, Ottone NE. Injection in temporomandibular joint of rats. Description of technical protocol. Pol J Vet Sci. 2017;20:207–211. doi: 10.1515/pjvs-2017-0025. [DOI] [PubMed] [Google Scholar]
- 27.Miao X, Meng X, Wu G, Ju Z, Zhang HH, Hu S, Xu GY. Upregulation of cystathionine-beta-synthetase expression contributes to inflammatory pain in rat temporomandibular joint. Mol Pain. 2014;10:9. doi: 10.1186/1744-8069-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, Matyas J, McDougall J, Pritzker K, Rudolphi K, van den Berg W, Yaksh T. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(Suppl 3):S10–16. doi: 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Guo H, Li C, Xu J, Fang W, Long X. A time-dependent degeneration manner of condyle in rat CFA-induced inflamed TMJ. Am J Transl Res. 2016;8:556–567. [PMC free article] [PubMed] [Google Scholar]
- 30.Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol Biochem Behav. 2003;75:181–189. doi: 10.1016/s0091-3057(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 31.Figueroba SR, Desjardins MP, Ferreira LE, Berto LA, Valdrighi HC, Groppo FC. The influence of altered occlusion on pro-inflammatory cytokine levels in the TMJ synovial tissues of rats. Arch Oral Biol. 2014;59:1164–1171. doi: 10.1016/j.archoralbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson BG, Briggs MD. The aggrecanopathies; an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J Rare Dis. 2016;11:86. doi: 10.1186/s13023-016-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Wu M, Jiang S, Ding W, Luo Q, Shi J. Expression of ADAMTs-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol. 2014;59:524–529. doi: 10.1016/j.archoralbio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Won Y, Shin Y, Kim JH, Chun JS. Reciprocal activation of hypoxia-inducible factor (HIF)-2alpha and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthritis Cartilage. 2016;24:134–145. doi: 10.1016/j.joca.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 38.Xu T, Xu G, Gu Z, Wu H. Hedgehog signal expression in articular cartilage of rat temporomandibular joint and association with adjuvant-induced osteoarthritis. J Oral Pathol Med. 2017;46:284–291. doi: 10.1111/jop.12497. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HY, Liu YD, Yang HX, Zhang M, Liao LF, Wan XH, Wang MQ. Installing and thereafter removing an aberrant prosthesis elicited opposite remodelling responses in growing mouse temporomandibular joints. J Oral Rehabil. 2015;42:685–92. doi: 10.1111/joor.12304. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Zhang J, Cao Y, Zhang M, Jing L, Jiao K, Yu S, Wang M. Decreased bone marrow stromal cells activity involves in unilateral anterior crossbite-induced early subchondral bone loss of temporomandibular joints. Arch Oral Biol. 2014;59:962–969. doi: 10.1016/j.archoralbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Harper RP, Kerins CA, McIntosh JE, Spears R, Bellinger LL. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund’s adjuvant. Osteoarthritis Cartilage. 2001;9:619–624. doi: 10.1053/joca.2001.0461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.