Abstract
MicroRNAs (miRNAs) are small noncoding RNAs that could regulate gene expressions transcriptionally or post-transcriptionally through binding to 3’ untranslated region (3’UTR) of target messenger RNAs (mRNAs), which were identified to be associated with tumorigenesis in various neoplasms. Among them, miR-101, encoded by two precursor transcripts (miR-101-1 and miR-101-2), was recognized to serve as a tumor suppressor via targeting critical oncogenes or anti-oncogenes. Additionally, studies have shown that miR-101 was participated in multiple cancer-related biological processes, including proliferation, apoptosis, angiogenesis, drug resistance, invasion and metastasis. In this review, we aim to summarize the function of miR-101 in different biological processes by figuring out the underlying target gene networks and explore its potential role as a biomarker in diverse neoplasms, which will provide a brand-new insight in molecular targeting cancer treatment.
Keywords: miR-101, cancers, biomarker, therapeutic targets
Introduction
In modern society, an increasing number of people are in great danger of malignant neoplasms under the pressure of fast-paced work and heavy-burdened life [1]. In spite of the application of traditional treatments, like surgery, chemotherapy and radiotherapy, many cancer patients are still suffering from limited effects, owing to metastasis, recurrence and drug resistance [2]. So it is urgent for us to identify molecular targets and develop effective agents for molecular targeting treatment. Fortunately, microRNAs (miRNAs), potential therapeutic targets, entered into the public view and brought hope for cancer patients.
MicroRNAs (miRNAs), 18-25 nucleotides in length, are a series of evolutionally conserved, single-stranded, small non-coding RNA molecules, which can modulate gene expressions at both transcriptional and post-transcriptional level via binding to the 3’ untranslated region (3’UTR) of target messenger RNAs (mRNAs), thus leading to mRNA degradation or translational inhibition [3]. Among them, miR-101, generating from two precursor transcripts: miR-101-1 on chromosome 1p31 and miR-101-2 on chromosome 9p24, was recently acknowledged to be a tumor suppressor in the incidence and development of various neoplasms [4]. Based on available researches, miR-101 was down-regulated in gastric cancer (GC) [5], intrahepatic cholangiocarcinoma (ICC) [6], osteosarcoma (OS) [7], hepatocellular carcinoma (HCC) [8], non-small-cell lung cancer (NSCLC) [9], oral squamous cell carcinoma (OSCC) [10], bladder transitional cell carcinoma (BTCC) [11], cervical cancer [12], intraductal papillary mucinous neoplasm of the pancreas (IPMN) [13], ERα-positive breast cancer [14] and so on. In detail, miR-101 was also reported to take part in many cancer-related biological processes, including proliferation, apoptosis, angiogenesis, drug resistance, invasion and metastasis [15-17]. What’s more, the gene network involved in multiple biological processes was found to be more complex than we could imagine. MiR-101 was influenced by many extrinsic and intrinsic factors, like atmospheric particles, viruses, proinflammatory cytokines and so on. Meanwhile, downstream targets of miR-101 were complicated, which means that miR-101 could modulate diverse target genes while a single target gene could be regulated by multiple microRNAs, including miR-101. Therefore, it could be seen that the distinct molecular mechanism deserves for further exploration in future. Apart from its role in cancers, miR-101 was claimed to be related with kinds of non-malignant diseases, such as multiple system atrophy (MSA) [18], hepatopulmonary syndrome (HPS) [19], cardiac fibroblasts (CFs) [20], HBV-associated chronic hepatitis [21], Alzheimer [22], pulmonary fibrosis [23], acute kidney injury (AKI) [24], gestational diabetes mellitus (GDM) [25] and so on. Evidence had been proven that miR-101 played a pivotal role in the initiation and development of multifarious diseases, especially malignant neoplasms.
In this review, we will focus on the function of miR-101 in cancer-related biological processes, including proliferation, apoptosis, angiogenesis, drug resistance, invasion and metastasis, and explore the potential role of miR-101 as a biomarker in various neoplasms, which might offer a novel guidance in molecular targeting cancer treatment.
MiR-101 in proliferation
Cell proliferation is crucial in cellular processes and miR-101 has been recognized to suppress tumor proliferation by regulating several target genes. It was demonstrated that miR-101 inhibited cell proliferation directly by decreasing the expression of enhancer of zeste homolog 2 (EZH2) in lung cancer [17], BTCC [11] and embryonal rhabdomyosarcoma (eRMS) [26]. EZH2, a member of the polycomb group (PcG) protein family, functioned as a histone methyltransferase by catalyzing histone H3 lysine 27 (H3-K27) trimethylation, which played an important role in maintaining gene silence and was greatly participated in the process of proliferation in various neoplasms [27]. Besides, in mesenchymal stem cell of Wilms tumor, miR-101, cooperating with miR-26a and Wilm’s tumor suppressor gene1 (WT1), remarkably suppressed cell proliferation by targeting EZH2 [28]. Moreover, in SiO2 particle-induced lung cancer, SiO2 particle stimulated the accumulation of IL-1b, which negatively modulated miR-101. Then miR-101, suppressed by IL-1b, inhibited the EZH2 expression and thus restrained cell proliferation [29]. Interestingly, it was also announced that miR-101 reduced the expression of Lin28B in NSCLC and thereby inhibited cell proliferation, which could be restrained by IL-1b via the COX2-HIF1α pathway [30]. In addition, miR-101 refrained cell proliferation of HCC directly by targeting SOX9 [31]. What’s more, miR-101 was also discovered to be diminished by HBV in HCC and possessed a great potential in decreasing cell proliferation via targeting ras-associated protein-1b (Rap1b) [32]. Rap1b was one of the isoforms of Rap1. Rap1, a member of Ras superfamily of G proteins, was a little GTPase located in cellular membranes and had been reported to be associated with cell proliferation [33]. Furthermore, miR-101 was elucidated to dilute the level of DNA methyltransferase 3A (DNMT3A) in breast cancer, accordingly suppressing cell proliferation [34]. Meanwhile, it was also discovered that DNMT3A accumulated in NSCLC and accelerated tumor cell proliferation via activating the PTEN/AKT pathway, which could be repressed by miR-101 [35]. Additionally, in breast cancer, miR-101 worked as a suppressor in cell proliferation through targeting proteasome maturation protein (POMP) and Stathmin 1 (Stmn1) [14,36]. Notably, enhancing the expression of miR-101 could dilute the level of Ras-related C3 botulinum toxin substrate 1 (Rac1), thereby leading to inhibited cell proliferation of papillary thyroid carcinoma (PTC) [37]. Also, it was claimed that the miR-101/c-FOS signaling pathway contributed to the weakened proliferation in bladder cancer [38]. In addition, the suppressed cell proliferation of cervical cancer was proven to be related with the high-expressed miR-101, via negatively regulating COX-2 [12]. What’s more, miR-101 was also affirmed to weaken prostate cancer (PCa) cell proliferation via down-regulating COX-2 and SUB1 [39,40]. Besides, in endometrial cancer, miR-101 restrained the level of MCL-1, thereby contributing to the decreased tumor proliferation [41] (Figure 1 and Table 1).
Figure 1.
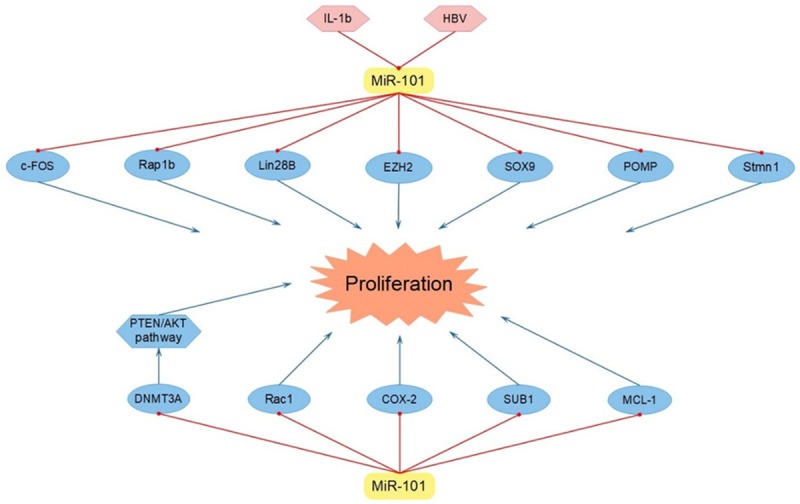
MiR-101 inhibited tumor cell proliferation via targeting c-FOS, Rap1b, Lin28B, EZH2, SOX9, POMP, Stmn1, DNMT3A, Rac1, COX-2, SUB1 and MCL-1.
Table 1.
Target genes and dysregulation of miR-101 in proliferation, apoptosis, angiogenesis, drug resistance
| MiR-101 | Disease | Target genes | Reference | Participation |
|---|---|---|---|---|
| Downregulated | NSCLC | DNMT3A | Liu J, et al. | Proliferation |
| Lin28B | Wang L, et al. | Proliferation | ||
| EZH2 | Zhang JG, et al. | Proliferation | ||
| Caspase 3 | Yin J, et al. | Apoptosis | ||
| HCC | Rap1b | Sheng Y, et al. | Proliferation | |
| SOX9 | Zhang Y, et al. | Proliferation | ||
| EZH2 | Xu L, et al. | Drug resistance | ||
| MCL-1 | He H, et al. | Drug resistance | ||
| Breast Cancer | POMP | Zhang X, et al. | Proliferation | |
| Stmn1 | Wang R, et al. | Proliferation | ||
| DNMT3A | Liu J, et al. | Proliferation | ||
| EYA1 | Guan H, et al. | Apoptosis | ||
| VHL | Liu N, et al. | Apoptosis | ||
| SOX2 | Wang J, et al. | Apoptosis | ||
| Jak2 | Wang L, et al. | Apoptosis | ||
| MCL-1 | Liu X, et al. | Drug resistance | ||
| Bladder cancer | EZH2 | Friedman JM, et al. | Proliferation | |
| c-FOS | Long Y, et al. | Proliferation | ||
| PCa | SUB1 | Chakravarthi BV, et al. | Proliferation | |
| COX-2 | Hao Y, et al. | Proliferation | ||
| RLIP76 | Yang J, et al. | Apoptosis | ||
| Wilms Tumor | EZH2 | Akpa MM, et al. | Proliferation | |
| Cervical Cancer | COX-2 | Lin C, et al. | Proliferation | |
| GC | VEGF-C | Li G, et al. | Apoptosis | |
| Liu HT, et al. | Angiogenesis | |||
| PDA | RRM1 | Fan P, et al. | Apoptosis | |
| CRC | Sphk1 | Chen MB, et al. | Angiogenesis | |
| PTC | Rac1 | Lin X, et al. | Proliferation | |
| OS | DNA-PKcs | Zhen YF, et al. | Apoptosis | |
| mTOR | Lin S, et al. | Apoptosis | ||
| T-ALL | Notch 1 | Qian L, et al. | Drug resistance | |
| NPC | ITGA3 | Tang XR, et al. | Angiogenesis | |
| GBM | GSK3β | Tian T, et al. | Drug resistance | |
| ACC | Pim-1 | Liu XY, et al. | Drug resistance | |
| eRMS | EZH2 | Vella S, et al. | Proliferation | |
| Upregulated | - | - | - | - |
MiR-101 in apoptosis
Tumor cell apoptosis is an indispensable step in cellular processes and miR-101 has been clarified to serve as a promoter in apoptosis of diverse cancers. It was confirmed that miR-101 increased cell apoptosis of breast cancer directly through decreasing the expression of Janus Kinase 2 (Jak2), eyes absent homolog1 (EYA1) and sex-determing region Y-box2 (SOX2) [42-44]. What’s more, the capacity of breast cancer cell apoptosis was regulated by more complex mechanisms. In detail, miR-101 reduced the level of von Hippel-Lindau (VHL), which could negatively regulate hypoxia inducible factor-1α (HIF1α), thus leading to enhancing HIF1α-dependent cell apoptosis in breast cancer [45]. In addition, miR-101 served as a promotor in PCa cell apoptosis via inhibiting the level of Ral binding protein 1 (RLIP76), accordingly down-regulating the activation of PI3K/Akt/Bcl-2 signaling pathway [46]. Besides, miR-101 enhanced NSCLC cell apoptosis directly via stimulating the augmentation of caspase 3 [47]. What’s more, in pancreatic ductal adenocarcinoma (PDA), miR-101 impaired the expression of ribonucleotide reductase M1 (RRM1), thus leading to the induced progression of cell apoptosis [48]. Meanwhile, it was proclaimed that miR-101 contributed to the increased GC cell apoptosis via diluting the VEGF-C expression level [49]. Furthermore, in OS, the action of miR-101 in promoting apoptosis attributed to its negative regulation of mTOR and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) [50,51]. Based on available researches, we found that some classical apoptosis-related genes, like p53, Fas, ATM, had not been mentioned. So it is still urgent for us to find out whether miR-101 can modulate other apoptosis-related genes in diverse neoplasms in future (Figure 2 and Table 1).
Figure 2.
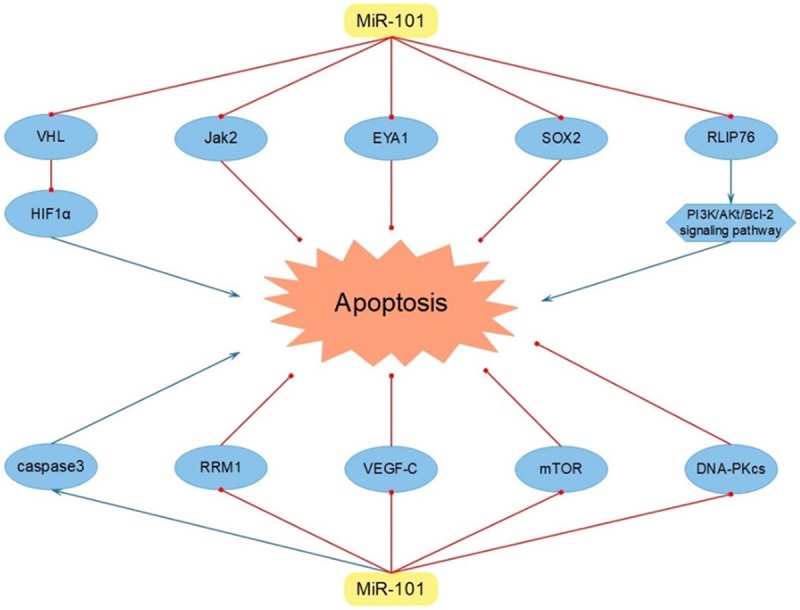
MiR-101 promoted tumor cell apoptosis via targeting VHL, Jak2, EYA1, SOX2, RLIP76, caspase3, RRM1, VEGF-C, mTOR and DNA-PKcs.
MiR-101 in angiogenesis
Tumor angiogenesis is based on the interaction between neoplasm cells and endothelial cells, which plays a significant role in the initiation and development of cancers. It was indicated that miR-101 acted as an inhibitor of tube formation in human umbilical vascular endothelial cells (HUVECs) in GC via targeting VEGF-C [52]. VEGF-C, a member of the VEGF family, was reported to be related with higher lymphatic density [53]. However, other VEGF family members, like VEGF-A, VEGF-B, VEGF-D and PGF, are also involved in the regulation of hemangiogenesis or lymphangiogenisis. So more efforts should be put to find out whether they could also serve as target genes of miR-101. In addition, the repression of angiogenesis caused by miR-101 was clarified in colorectal cancer (CRC), by the way of targeting sphingosine kinase 1 (SphK1) [54]. SphK1 mediated the phosphorylation of sphingosine to S1P, which had been found to be connected with angiogenesis [55]. What’s more, it was disclosed that, in nasopharyngeal carcinoma (NPC), increasing the level of miR-101 could negatively modulate the expression of integrin subunit alpha 3 (ITGA3), accordingly leading to the weakened capacity of angiogenesis [56] (Figure 3 and Table 1).
Figure 3.
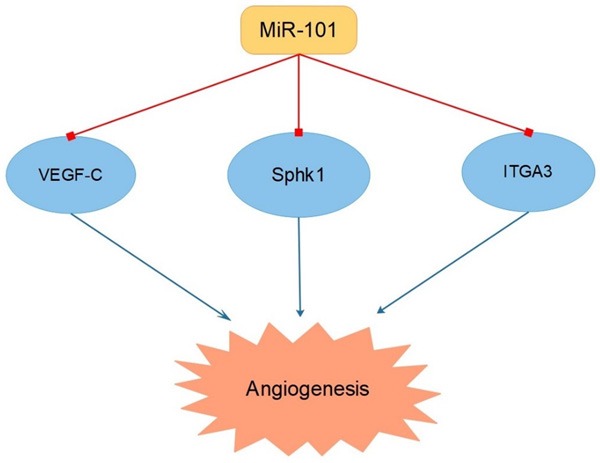
MiR-101 suppressed tumor cell angiogenesis via targeting VEGF-C, Sphk1 and ITGA3.
MiR-101 in invasion and metastasis
Tumor invasion and metastasis is frequently known as the main cause of cancer-related deaths and miR-101 shows a great potential in inhibiting tumor cell invasion and metastasis in multifarious neoplasms. It was found that miR-101 decreased cell metastasis in OS through directly reducing the expression of v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) [8]. Meanwhile, miR-101 was discovered to repress cell metastasis of HCC via regulating the miR-101-ERK2/c-FOS-AP-1 feedback loop [7,57]. In detail, miR-101 inhibited the expression of ERK2 and c-FOS, which were two critical components of activator protein-1 (AP-1), thus suppressing AP-1-dependent cell metastasis. AP-1, as a transcription factor (TF), could promote miR-101 transcription, which made the pathway a feedback loop. In addition, EZH2 was participated in the process of invasion and metastasis as well. It was elucidated that miR-101 restrained cell metastasis by down-regulating the level of EZH2 in lung cancer and OS [58,16]. Moreover, in epithelial ovarian cancer (EOC), miR-101 decreased the expression of EZH2, which mediated the transcriptional repression of p21 wafl/cipl, thus leading to the inhibition of cell metastasis [59]. Meanwhile, miR-101 was demonstrated to down-regulate the EZH2 level in GC and thereby enhanced the restoration of E-cadherin (CDH1), which weakened the capacity of cell metastasis [60]. Besides, it was recognized that miR-101 was negatively regulated by androgen and HIF-1α/HIF-1β in PCa and miR-101 functioned as a suppressor via diluting EZH2 in the process of invasion and metastasis [61]. What’s more, a variety of signaling pathways were explored to play a critical role in the process of tumor invasion and metastasis. For instance, miR-101 inhibited tumor metastasis in OS by impairing the activation of PI3K/AKT and JAK/STAT signaling pathways via targeting ROCK1 [62]. Moreover, the inhibition of the Wnt/β-catenin signaling pathway was proven in laryngeal squamous cell carcinoma (LSCC), which was caused by miR-101 through targeting CDK8 [63]. Apart from the above-mentioned complex molecular mechanisms, there were still a number of target genes which were mediated by miR-101 and directly produced effects on the process of invasion and metastasis in multifarious cancers. For example, in NSCLC, miR-101 inhibited cell metastasis through targeting ceramide synthase 6 (CERS6) [9]. CERS6 played a key role in ceramide synthesis in cellular membrane, which was connected with the formation of lamellipodia and metastasis [64]. Besides, the reduced capacity of metastasis was reported to be regulated by miR-101 through targeting Ubiquitin-like with PHD and ring finger domains 1 (UHRF1) and VEGF-C [6,65,66]. In addition, it was recognized that miR-101 decreased cell metastasis via inhibiting the expression of zinc finger E-box binding homeobox 1 (ZEB1) in HCC and OSCC [67,10]. ZEB1 was confirmed to possess a great potential in organ fibrosis and cancer metastasis via reducing E-cadherin and triggering epithelial-to-mesenchymal transition (EMT) [68]. Moreover, in cervical cancer, the miR-101/COX2 regulatory axis was indicated to be negatively correlated with tumor metastasis [69]. Interestingly, miR-101, cooperating with miR-217, down-regulated the level of onco-lncRNA MALAT1 and refrained cell metastasis in esophageal squamous cell carcinoma (ESCC) [70]. It was clarified that miR-101 reduced the expression of EP4 and inhibited CRC cell metastasis [71]. Subsequently, the suppressed ability to invade caused by miR-101 was explored in PTC, as it negatively modulated Ubiquitin-specific protease 22 (USP22) and Rac1 [72,73]. It was affirmed that miR-101 was also participated in the miR-101/c-Met pathway in bladder cancer to prevent cell metastasis [74]. Notably, in OSCC, the function of miR-101 in decreasing metastasis resulted from targeting CX chemokine receptor 7 (CXCR7) [75]. Furthermore, the accumulation of miR-101 was illustrated to suppress the level of sex-determining region Y-box9 protein (SOX9), thereby controlling invasion and metastasis in glioblastoma multiforme (GBM) [76] (Figure 4 and Table 2).
Figure 4.
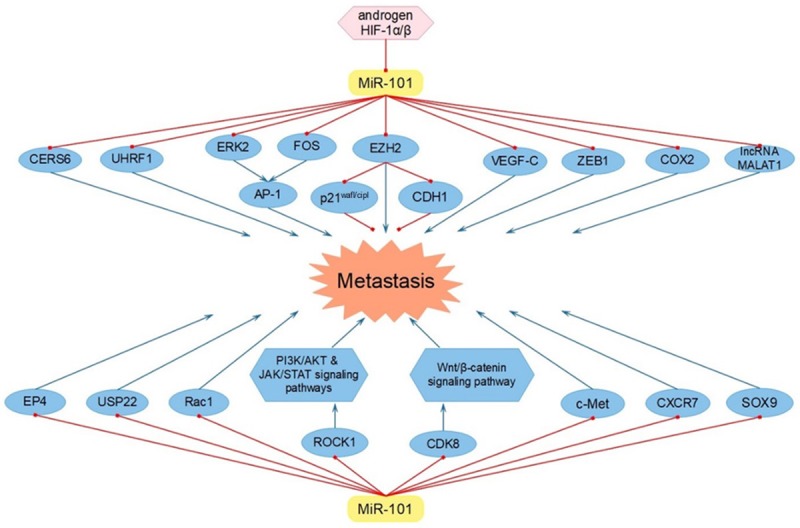
MiR-101 refrained tumor cell metastasis via targeting CERS6, UHRF1, ERK2, FOS, EZH2, VEGF-C, ZEB1, COX2, lncRNA MALAT1, EP4, USP22, Rac1, ROCK1, CDK8, c-Met, CXCR7 and SOX9.
Table 2.
Target genes and dysregulation of miR-101 in metastasis
| MiR-101 | Disease | Target genes | Reference |
|---|---|---|---|
| Downregulated | NSCLC | CERS6 | Suzuki M, et al. |
| EZH2 | Cho HM, et al. | ||
| HCC | VEGF-C | Liu Z, et al. | |
| ERK2 | Liu JJ, et al. | ||
| c-FOS | Liu JJ, et al. | ||
| FOS | Li S, et al. | ||
| ZEB1 | Zhao S, et al. | ||
| Bladder Cancer | c-Met | Hu Z, et al. | |
| PCa | EZH2 | Cao P, et al. | |
| RCC | UHRF1 | Goto Y, et al. | |
| Cervical Cancer | COX-2 | Huang F, et al. | |
| EOC | EZH2 | Semaan A, et al. | |
| GC | EZH2 | Carvalho J, et al. | |
| ESCC | Onco-IncRNA MALAT1 | Li RQ, et al. | |
| CRC | EP4 | Chandramouli A, et al. | |
| PTC | Rac1 | Wang C, et al. | |
| USP22 | Zhao H, et al. | ||
| OS | EZH2 | Zhang K, et al. | |
| ROCK1 | Jiang R, et al. | ||
| c-FOS | Wang Z, et al. | ||
| GBM | SOX9 | Liu N, et al. | |
| LSCC | CDK8 | Li M, et al. | |
| OSCC | CXCR7 | Hui Y, et al. | |
| ZEB1 | Wu B, et al. | ||
| Upregulated | - | - | - |
MiR-101 in drug resistance
Drug resistance is a major obstacle in chemotherapy and increasing evidences had indicated that miR-101 took part in the regulation of drug resistance in sorts of cancers. It was disclosed that, in triple-negative breast cancer, high-expressed miR-101 enhanced tumor cell chemotherapeutic sensitivity to paclitaxel via decreasing the MCL-1 expression level [15]. Moreover, the chemotherapeutic sensitivity to fluorouracil in HCC cell was indicated to be promoted by the up-regulated miR-101, via targeting EZH2 [77]. Interestingly, based on previous researches, miR-197 was illustrated to promote paclitaxel resistance while inhibiting fluorouracil resistance in cancers [78,79]. This phenomenon contributed to the synergistic effect or the antagonistic effect in drug resistance remained to be obscure and urged for more investigation. In addition, miR-101 was clarified to induce chemotherapeutic sensitivity to adriamycin in T-cell acute lymphoblastic leukemia (T-ALL) and HCC via targeting Notch 1 and MCL-1 respectively [80,81]. Besides, the chemotherapeutic sensitivity to cisplatin in salivary gland adenoid cystic carcinoma (ACC) cell was indicated to be promoted by miR-101, via targeting provirus integration site for moloney murine leukemia virus 1 (Pim-1) [82]. Furthermore, the augmentation of miR-101 in GBM suppressed the level of glycogen synthase kinase 3β (GSK3β), accordingly leading to the increased ability to sensitize resistant GBM cells to temozolomide [83]. The above-mentioned researches almost focused on single drug resistance. However, an increasing number of cancer patients are suffering from multi-drug resistance, whose molecular mechanism is still unclear and worthy of further investigation (Figure 5 and Table 1).
Figure 5.
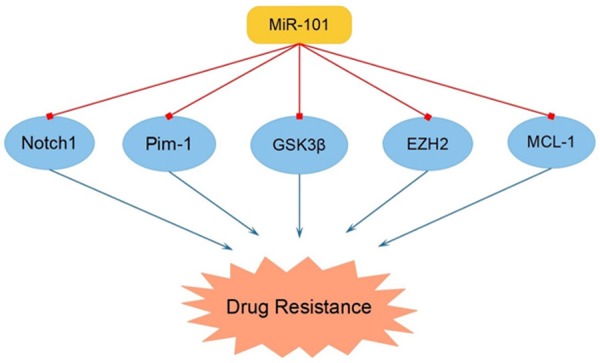
MiR-101 modulated tumor cell drug resistance via targeting Notch1, Pim-1, GSK3β, EZH2 and MCL-1.
MiR-101 in clinical application
Based on mounts of researches, miR-101 was speculated to have the potential of serving as a biomarker in multifarious neoplasms, which might bring guidance for the molecular targeting treatment. It was claimed that down-regulated miR-101 was remarkably associated with increased tumor size, advanced TNM classification and poor survival in GC cell [52]. Moreover, an underlying connection between the miR-101 expression, T3-4 tumor grade and worse prognosis was announced in LSCC [63]. Besides, the poor prognosis of cancer patients caused by the low level of miR-101 was demonstrated in OSCC [10]. Additionally, it was also reported that the level of miR-101 played a critical role in the pathological grade and TNM classification in breast cancer cell [42]. With ample evidences in cancer, we considered miR-101 as a considerable biomarker in multifarious cancers, which might offer a novel sight for clinical application.
Discussions and conclusions
In this review, on account of available researches, miR-101 was reported to be down-regulated in almost carcinoma tissues, which disclosed that miR-101 could function as a tumor suppressor in diverse malignant neoplasms. Nonetheless, no one had claimed the up-regulation of miR-101 in any carcinoma tissue. This phenomenon of tissue specificity was different from that of other microRNAs. For instance, the majority of miR-29a was down-regulated in human cancers while a few was illustrated to be overexpressed in some cancers, like cholangiocarcinoma and CRC [84]. Consequently, it is controversial that miR-101 could be up-regulated in some neoplasms and possess the possibility to serve as oncogene or tumor suppressed gene. The distinct tissue specificity of miR-101 still deserves for further exploration.
Besides, we discovered that miR-101 could modulate various target genes while one single target gene could be regulated by multifarious miRNAs, which added the difficulty of identifying specific downstream targets of miR-101 in clinical application. Fortunately, an increasing number of new materials have entered into public view, like extracellular vesicles (EVs), long non-coding RNAs (lncRNAs) and so on, which possess the potential of carrying functional miRNAs and delivering them to specific targets. However, the connection between miR-101 and new materials is still mysterious and more attention should be paid for further research.
In conclusion, miR-101 greatly contributed to the incidence and development of a variety of neoplasms. Kinds of physiological and pathological cell processes were discovered to be under the regulation of miR-101, such as cell proliferation, apoptosis, angiogenesis, drug resistance, invasion, metastasis and so on. In addition, miR-101 was also detected to possess the potential to be a biomarker for diverse neoplasms. However, the connection between miR-101 and its target genes was complex and there were still a lot of mysterious mechanisms remaining unknown, which urged us for further exploration before the research findings turning to clinical application. We suggest that miR-101 might provide a novel, secure and effective insight in the future molecular targeting treatment and could bring dawn for people suffering from cancers.
Acknowledgements
We thank Dan-Dan Wang for useful discussions and help in revision of the present paper. This research was supported by the National Key Research and Development Program of China (No. 2016YFC0905900), the “333” Talent Project of Jiangsu Province [No. 4(2016)], the National Key Clinical Specialist Construction Programs of China [No. 544(2013)] and Natural Science Foundation of Jiangsu Province (No. BK20151579).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wardle J, Robb K, Vernon S, Waller J. Screening for prevention and early diagnosis of cancer. Am Psychol. 2015;70:119–133. doi: 10.1037/a0037357. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT, Xia YJ, Ye ZY, Tao HQ. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Deng G, Teng Y, Huang F, Nie W, Zhu L, Huang W, Xu H. MicroRNA-101 inhibits the migration and invasion of intrahepatic cholangiocarcinoma cells via direct suppression of vascular endothelial growth factor-C. Mol Med Rep. 2015;12:7079–7085. doi: 10.3892/mmr.2015.4239. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, He R, Xia H, Wei YU, Wu S. MicroRNA-101 has a suppressive role in osteosarcoma cells through the targeting of c-FOS. Exp Ther Med. 2016;11:1293–1299. doi: 10.3892/etm.2016.3085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J, Sun Z, Wei L, Zheng X. MicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene in human hepatocellular carcinoma. Hepatology. 2009;49:1194–1202. doi: 10.1002/hep.22757. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Cao K, Kato S, Komizu Y, Mizutani N, Tanaka K, Arima C, Tai MC, Yanagisawa K, Togawa N, Shiraishi T, Usami N, Taniguchi T, Fukui T, Yokoi K, Wakahara K, Hasegawa Y, Mizutani Y, Igarashi Y, Inokuchi J, Iwaki S, Fujii S, Satou A, Matsumoto Y, Ueoka R, Tamiya-Koizumi K, Murate T, Nakamura M, Kyogashima M, Takahashi T. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J Clin Invest. 2016;126:254–265. doi: 10.1172/JCI79775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Wu B, Lei D, Wang L, Yang X, Jia S, Yang Z, Shan C, Yang X, Zhang C, Lu B. MiRNA-101 inhibits oral squamous-cell carcinoma growth and metastasis by targeting zinc finger E-box binding homeobox 1. Am J Cancer Res. 2016;6:1396–1407. [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 12.Lin C, Huang F, Shen G, Yiming A. MicroRNA-101 regulates the viability and invasion of cervical cancer cells. Int J Clin Exp Pathol. 2015;8:10148–10155. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakahara O, Takamori H, Iwatsuki M, Baba Y, Sakamoto Y, Tanaka H, Chikamoto A, Horino K, Beppu T, Kanemitsu K, Honda Y, Iyama K, Baba H. Carcinogenesis of intraductal papillary mucinous neoplasm of the pancreas: loss of microRNA-101 promotes overexpression of histone methyltransferase EZH2. Ann Surg Oncol. 2012;19(Suppl 3):S565–571. doi: 10.1245/s10434-011-2068-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Schulz R, Edmunds S, Kruger E, Markert E, Gaedcke J, Cormet-Boyaka E, Ghadimi M, Beissbarth T, Levine AJ, Moll UM, Dobbelstein M. MicroRNA-101 suppresses tumor cell proliferation by acting as an endogenous proteasome inhibitor via targeting the proteasome assembly factor POMP. Mol Cell. 2015;59:243–257. doi: 10.1016/j.molcel.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Tang H, Chen J, Song C, Yang L, Liu P, Wang N, Xie X, Lin X, Xie X. MicroRNA-101 inhibits cell progression and increases paclitaxel sensitivity by suppressing MCL-1 expression in human triple-negative breast cancer. Oncotarget. 2015;6:20070–20083. doi: 10.18632/oncotarget.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B. MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol Rep. 2014;32:2143–2149. doi: 10.3892/or.2014.3459. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JG, Guo JF, Liu DL, Liu Q, Wang JJ. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thorac Oncol. 2011;6:671–678. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 18.Valera E, Spencer B, Mott J, Trejo M, Adame A, Mante M, Rockenstein E, Troncoso JC, Beach TG, Masliah E, Desplats P. MicroRNA-101 modulates autophagy and oligodendroglial alpha-synuclein accumulation in multiple system atrophy. Front Mol Neurosci. 2017;10:329. doi: 10.3389/fnmol.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhuang L, Rong H, Guo Y, Ling X, Wang R, Yu X, Zhang W. MicroRNA-101 inhibits proliferation of pulmonary microvascular endothelial cells in a rat model of hepatopulmonary syndrome by targeting the JAK2/STAT3 signaling pathway. Mol Med Rep. 2015;12:8261–8267. doi: 10.3892/mmr.2015.4471. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Wang K, Hu F, Qian C, Guan H, Feng K, Zhou Y, Chen Z. MicroRNA-101 protects cardiac fibroblasts from hypoxia-induced apoptosis via inhibition of the TGF-beta signaling pathway. Int J Biochem Cell Biol. 2015;65:155–164. doi: 10.1016/j.biocel.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Yao Q, Butt AM, Guo J, Tian Z, Bao X, Li H, Meng Q, Lu J. Expression profiling of serum microRNA-101 in HBV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cancer Biol Ther. 2014;15:1248–1255. doi: 10.4161/cbt.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long JM, Lahiri DK. MicroRNA-101 downregulates Alzheimer’s amyloid-beta precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun. 2011;404:889–895. doi: 10.1016/j.bbrc.2010.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Xiao X, Yang Y, Mishra A, Liang Y, Zeng X, Yang X, Xu D, Blackburn MR, Henke CA, Liu L. MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast proliferation and activation. J Biol Chem. 2017;292:16420–16439. doi: 10.1074/jbc.M117.805747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Hua R, Gong Z, Shang B, Huang Y, Guo L, Liu T, Xue J. Human amniotic epithelial cells inhibit CD4+ T cell activation in acute kidney injury patients by influencing the miR-101-c-Rel-IL-2 pathway. Mol Immunol. 2017;81:76–84. doi: 10.1016/j.molimm.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Floris I, Descamps B, Vardeu A, Mitic T, Posadino AM, Shantikumar S, Sala-Newby G, Capobianco G, Mangialardi G, Howard L, Dessole S, Urrutia R, Pintus G, Emanueli C. Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microRNA-101 and histone methyltransferase enhancer of zester homolog-2. Arterioscler Thromb Vasc Biol. 2015;35:664–674. doi: 10.1161/ATVBAHA.114.304730. [DOI] [PubMed] [Google Scholar]
- 26.Vella S, Pomella S, Leoncini PP, Colletti M, Conti B, Marquez VE, Strillacci A, Roma J, Gallego S, Milano GM, Capogrossi MC, Bertaina A, Ciarapica R, Rota R. MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin Epigenetics. 2015;7:82. doi: 10.1186/s13148-015-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 28.Akpa MM, Iglesias D, Chu L, Thiebaut A, Jentoft I, Hammond L, Torban E, Goodyer PR. Wilms tumor suppressor, WT1, cooperates with MicroRNA-26a and MicroRNA-101 to suppress translation of the polycomb protein, EZH2, in mesenchymal stem cells. J Biol Chem. 2016;291:3785–3795. doi: 10.1074/jbc.M115.678029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei YM, Zu YF, Wang J, Bai S, Shi YF, Shi R, Duan J, Cui D, Chen J, Xiang Y, Dong J. Interleukin-1beta-mediated suppression of microRNA-101 and upregulation of enhancer of zeste homolog 2 is involved in particle-induced lung cancer. Med Oncol. 2015;32:387. doi: 10.1007/s12032-014-0387-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, Lou JT, Liu MF. IL-1beta-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. 2014;74:4720–4730. doi: 10.1158/0008-5472.CAN-14-0960. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Guo X, Xiong L, Kong X, Xu Y, Liu C, Zou L, Li Z, Zhao J, Lin N. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362–4370. doi: 10.1016/j.febslet.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 32.Sheng Y, Ding S, Chen K, Chen J, Wang S, Zou C, Zhang J, Cao Y, Huang A, Tang H. Functional analysis of miR-101-3p and Rap1b involved in hepatitis B virus-related hepatocellular carcinoma pathogenesis. Biochem Cell Biol. 2014;92:152–162. doi: 10.1139/bcb-2013-0128. [DOI] [PubMed] [Google Scholar]
- 33.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Pang Y, Wang H, Li Y, Sun X, Xu F, Ren H, Liu D. miR-101 inhibits the proliferation and migration of breast cancer cells via downregulating the expression of DNA methyltransferase 3a. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:299–303. [PubMed] [Google Scholar]
- 35.Wang L, Yao J, Sun H, He K, Tong D, Song T, Huang C. MicroRNA-101 suppresses progression of lung cancer through the PTEN/AKT signaling pathway by targeting DNA methyltransferase 3A. Oncol Lett. 2017;13:329–338. doi: 10.3892/ol.2016.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Wang HB, Hao CJ, Cui Y, Han XC, Hu Y, Li FF, Xia HF, Ma X. MiR-101 is involved in human breast carcinogenesis by targeting Stathmin1. PLoS One. 2012;7:e46173. doi: 10.1371/journal.pone.0046173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin X, Guan H, Li H, Liu L, Liu J, Wei G, Huang Z, Liao Z, Li Y. miR-101 inhibits cell proliferation by targeting Rac1 in papillary thyroid carcinoma. Biomed Rep. 2014;2:122–126. doi: 10.3892/br.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Y, Wu Z, Yang X, Chen L, Han Z, Zhang Y, Liu J, Liu W, Liu X. MicroRNA-101 inhibits the proliferation and invasion of bladder cancer cells via targeting c-FOS. Mol Med Rep. 2016;14:2651–2656. doi: 10.3892/mmr.2016.5534. [DOI] [PubMed] [Google Scholar]
- 39.Chakravarthi BV, Goswami MT, Pathi SS, Robinson AD, Cieslik M, Chandrashekar DS, Agarwal S, Siddiqui J, Daignault S, Carskadon SL, Jing X, Chinnaiyan AM, Kunju LP, Palanisamy N, Varambally S. MicroRNA-101 regulated transcriptional modulator SUB1 plays a role in prostate cancer. Oncogene. 2016;35:6330–6340. doi: 10.1038/onc.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao Y, Gu X, Zhao Y, Greene S, Sha W, Smoot DT, Califano J, Wu TC, Pang X. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev Res (Phila) 2011;4:1073–1083. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konno Y, Dong P, Xiong Y, Suzuki F, Lu J, Cai M, Watari H, Mitamura T, Hosaka M, Hanley SJ, Kudo M, Sakuragi N. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5:6049–6062. doi: 10.18632/oncotarget.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Zeng H, Li H, Chen T, Wang L, Zhang K, Chen J, Wang R, Li Q, Wang S. MicroRNA-101 inhibits growth, proliferation and migration and induces apoptosis of breast cancer cells by targeting sex-determining region Y-Box 2. Cell Physiol Biochem. 2017;43:717–732. doi: 10.1159/000481445. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Li L, Guo R, Li X, Lu Y, Guan X, Gitau SC, Wang L, Xu C, Yang B, Shan H. miR-101 promotes breast cancer cell apoptosis by targeting Janus kinase 2. Cell Physiol Biochem. 2014;34:413–422. doi: 10.1159/000363010. [DOI] [PubMed] [Google Scholar]
- 44.Guan H, Dai Z, Ma Y, Wang Z, Liu X, Wang X. MicroRNA-101 inhibits cell proliferation and induces apoptosis by targeting EYA1 in breast cancer. Int J Mol Med. 2016;37:1643–1651. doi: 10.3892/ijmm.2016.2557. [DOI] [PubMed] [Google Scholar]
- 45.Liu N, Xia WY, Liu SS, Chen HY, Sun L, Liu MY, Li LF, Lu HM, Fu YJ, Wang P, Wu H, Gao JX. MicroRNA-101 targets von Hippel-Lindau tumor suppressor (VHL) to induce HIF1alpha mediated apoptosis and cell cycle arrest in normoxia condition. Sci Rep. 2016;6:20489. doi: 10.1038/srep20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Song Q, Cai Y, Wang P, Wang M, Zhang D. RLIP76-dependent suppression of PI3K/AKT/Bcl-2 pathway by miR-101 induces apoptosis in prostate cancer. Biochem Biophys Res Commun. 2015;463:900–906. doi: 10.1016/j.bbrc.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Wang M, Jin C, Qi Q. miR-101 sensitizes A549 NSCLC cell line to CDDP by activating caspase 3-dependent apoptosis. Oncol Lett. 2014;7:461–465. doi: 10.3892/ol.2013.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan P, Liu L, Yin Y, Zhao Z, Zhang Y, Amponsah PS, Xiao X, Bauer N, Abukiwan A, Nwaeburu CC, Gladkich J, Gao C, Schemmer P, Gross W, Herr I. MicroRNA-101-3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Lett. 2016;373:130–137. doi: 10.1016/j.canlet.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Yang F, Gu S, Li Z, Xue M. MicroRNA-101 induces apoptosis in cisplatin-resistant gastric cancer cells by targeting VEGF-C. Mol Med Rep. 2016;13:572–578. doi: 10.3892/mmr.2015.4560. [DOI] [PubMed] [Google Scholar]
- 50.Lin S, Shao NN, Fan L, Ma XC, Pu FF, Shao ZW. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technolog Med Sci. 2014;34:889–895. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- 51.Zhen YF, Li ST, Zhu YR, Wang XD, Zhou XZ, Zhu LQ. Identification of DNA-PKcs as a primary resistance factor of salinomycin in osteosarcoma cells. Oncotarget. 2016;7:79417–79427. doi: 10.18632/oncotarget.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu HT, Xing AY, Chen X, Ma RR, Wang YW, Shi DB, Zhang H, Li P, Chen HF, Li YH, Gao P. MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget. 2015;6:37458–37470. doi: 10.18632/oncotarget.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen MB, Yang L, Lu PH, Fu XL, Zhang Y, Zhu YQ, Tian Y. MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem Biophys Res Commun. 2015;463:954–960. doi: 10.1016/j.bbrc.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 55.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang XR, Wen X, He QM, Li YQ, Ren XY, Yang XJ, Zhang J, Wang YQ, Ma J, Liu N. MicroRNA-101 inhibits invasion and angiogenesis through targeting ITGA3 and its systemic delivery inhibits lung metastasis in nasopharyngeal carcinoma. Cell Death Dis. 2017;8:e2566. doi: 10.1038/cddis.2016.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu JJ, Lin XJ, Yang XJ, Zhou L, He S, Zhuang SM, Yang J. A novel AP-1/miR-101 regulatory feedback loop and its implication in the migration and invasion of hepatoma cells. Nucleic Acids Res. 2014;42:12041–12051. doi: 10.1093/nar/gku872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho HM, Jeon HS, Lee SY, Jeong KJ, Park SY, Lee HY, Lee JU, Kim JH, Kwon SJ, Choi E, Na MJ, Kang J, Son JW. MicroRNA-101 inhibits lung cancer invasion through the regulation of enhancer of zeste homolog 2. Exp Ther Med. 2011;2:963–967. doi: 10.3892/etm.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semaan A, Qazi AM, Seward S, Chamala S, Bryant CS, Kumar S, Morris R, Steffes CP, Bouwman DL, Munkarah AR, Weaver DW, Gruber SA, Batchu RB. MicroRNA-101 inhibits growth of epithelial ovarian cancer by relieving chromatin-mediated transcriptional repression of p21(waf(1)/cip(1)) Pharm Res. 2011;28:3079–3090. doi: 10.1007/s11095-011-0547-x. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho J, van Grieken NC, Pereira PM, Sousa S, Tijssen M, Buffart TE, Diosdado B, Grabsch H, Santos MA, Meijer G, Seruca R, Carvalho B, Oliveira C. Lack of microRNA-101 causes E-cadherin functional deregulation through EZH2 up-regulation in intestinal gastric cancer. J Pathol. 2012;228:31–44. doi: 10.1002/path.4032. [DOI] [PubMed] [Google Scholar]
- 61.Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 2017;7:88–97. [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Tian L, Ren H, Chen X, Wang Y, Ge J, Wu S, Sun Y, Liu M, Xiao H. MicroRNA-101 is a potential prognostic indicator of laryngeal squamous cell carcinoma and modulates CDK8. J Transl Med. 2015;13:271. doi: 10.1186/s12967-015-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z, Wang J, Mao Y, Zou B, Fan X. MicroRNA-101 suppresses migration and invasion via targeting vascular endothelial growth factor-C in hepatocellular carcinoma cells. Oncol Lett. 2016;11:433–438. doi: 10.3892/ol.2015.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto Y, Kurozumi A, Nohata N, Kojima S, Matsushita R, Yoshino H, Yamazaki K, Ishida Y, Ichikawa T, Naya Y, Seki N. The microRNA signature of patients with sunitinib failure: regulation of UHRF1 pathways by microRNA-101 in renal cell carcinoma. Oncotarget. 2016;7:59070–59086. doi: 10.18632/oncotarget.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao S, Zhang Y, Zheng X, Tu X, Li H, Chen J, Zang Y, Zhang J. Loss of MicroRNA-101 promotes epithelial to mesenchymal transition in hepatocytes. J Cell Physiol. 2015;230:2706–2717. doi: 10.1002/jcp.24995. [DOI] [PubMed] [Google Scholar]
- 68.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 69.Huang F, Lin C, Shi YH, Kuerban G. MicroRNA-101 inhibits cell proliferation, invasion, and promotes apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma cells. Asian Pac J Cancer Prev. 2013;14:5915–5920. doi: 10.7314/apjcp.2013.14.10.5915. [DOI] [PubMed] [Google Scholar]
- 70.Li RQ, Ren Y, Liu W, Pan W, Xu FJ, Yang M. MicroRNA-mediated silence of onco-lncRNA MALAT1 in different ESCC cells via ligand-functionalized hydroxyl-rich nanovectors. Nanoscale. 2017;9:2521–2530. doi: 10.1039/c6nr09668a. [DOI] [PubMed] [Google Scholar]
- 71.Chandramouli A, Onyeagucha BC, Mercado-Pimentel ME, Stankova L, Shahin NA, LaFleur BJ, Heimark RL, Bhattacharyya AK, Nelson MA. MicroRNA-101 (miR-101) post-transcriptionally regulates the expression of EP4 receptor in colon cancers. Cancer Biol Ther. 2012;13:175–183. doi: 10.4161/cbt.13.3.18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao H, Tang H, Huang Q, Qiu B, Liu X, Fan D, Gong L, Guo H, Chen C, Lei S, Yang L, Lu J, Bao G. MiR-101 targets USP22 to inhibit the tumorigenesis of papillary thyroid carcinoma. Am J Cancer Res. 2016;6:2575–2586. [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C, Lu S, Jiang J, Jia X, Dong X, Bu P. Hsa-microRNA-101 suppresses migration and invasion by targeting Rac1 in thyroid cancer cells. Oncol Lett. 2014;8:1815–1821. doi: 10.3892/ol.2014.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Z, Lin Y, Chen H, Mao Y, Wu J, Zhu Y, Xu X, Xu X, Li S, Zheng X, Xie L. MicroRNA-101 suppresses motility of bladder cancer cells by targeting c-Met. Biochem Biophys Res Commun. 2013;435:82–87. doi: 10.1016/j.bbrc.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 75.Hui Y, Li Y, Jing Y, Feng JQ, Ding Y. miRNA-101 acts as a tumor suppressor in oral squamous cell carcinoma by targeting CX chemokine receptor 7. Am J Transl Res. 2016;8:4902–4911. [PMC free article] [PubMed] [Google Scholar]
- 76.Liu N, Zhang L, Wang Z, Cheng Y, Zhang P, Wang X, Wen W, Yang H, Liu H, Jin W, Zhang Y, Tu Y. MicroRNA-101 inhibits proliferation, migration and invasion of human glioblastoma by targeting SOX9. Oncotarget. 2017;8:19244–19254. doi: 10.18632/oncotarget.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu L, Beckebaum S, Iacob S, Wu G, Kaiser GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, Lu M, Cicinnati VR. MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity. J Hepatol. 2014;60:590–598. doi: 10.1016/j.jhep.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 78.Sun Z, Zhou N, Han Q, Zhao L, Bai C, Chen Y, Zhou J, Zhao RC. MicroRNA-197 influences 5-fluorouracil resistance via thymidylate synthase in colorectal cancer. Clin Transl Oncol. 2015;17:876–883. doi: 10.1007/s12094-015-1318-7. [DOI] [PubMed] [Google Scholar]
- 79.Zou D, Wang D, Li R, Tang Y, Yuan L, Long X, Zhou Q. MiR-197 induces Taxol resistance in human ovarian cancer cells by regulating NLK. Tumour Biol. 2015;36:6725–6732. doi: 10.1007/s13277-015-3365-7. [DOI] [PubMed] [Google Scholar]
- 80.Qian L, Zhang W, Lei B, He A, Ye L, Li X, Dong X. MicroRNA-101 regulates T-cell acute lymphoblastic leukemia progression and chemotherapeutic sensitivity by targeting Notch1. Oncol Rep. 2016;36:2511–2516. doi: 10.3892/or.2016.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He H, Tian W, Chen H, Deng Y. MicroRNA-101 sensitizes hepatocellular carcinoma cells to doxorubicin-induced apoptosis via targeting Mcl-1. Mol Med Rep. 2016;13:1923–1929. doi: 10.3892/mmr.2015.4727. [DOI] [PubMed] [Google Scholar]
- 82.Liu XY, Liu ZJ, He H, Zhang C, Wang YL. MicroRNA-101-3p suppresses cell proliferation, invasion and enhances chemotherapeutic sensitivity in salivary gland adenoid cystic carcinoma by targeting Pim-1. Am J Cancer Res. 2015;5:3015–3029. [PMC free article] [PubMed] [Google Scholar]
- 83.Tian T, Mingyi M, Qiu X, Qiu Y. MicroRNA-101 reverses temozolomide resistance by inhibition of GSK3beta in glioblastoma. Oncotarget. 2016;7:79584–79595. doi: 10.18632/oncotarget.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang JY, Zhang Q, Wang DD, Yan W, Sha HH, Zhao JH, Yang SJ, Zhang HD, Hou JC, Xu HZ, He YJ, Hu JH, Zhong SL, Tang JH. MiR-29a: a potential therapeutic target and promising biomarker in tumors. Biosci Rep. 2018:38. doi: 10.1042/BSR20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]


