Abstract
Lactobacillus reuteri is a gut symbiont inhabiting the gastrointestinal tract of numerous vertebrates. The surface-exposed serine-rich repeat protein (SRRP) is a major adhesin in Gram-positive bacteria. Using lectin and sugar nucleotide profiling of wild-type or L. reuteri isogenic mutants, MALDI-ToF-MS, LC–MS and GC–MS analyses of SRRPs, we showed that L. reuteri strains 100-23C (from rodent) and ATCC 53608 (from pig) can perform protein O-glycosylation and modify SRRP100-23 and SRRP53608 with Hex-Glc-GlcNAc and di-GlcNAc moieties, respectively. Furthermore, in vivo glycoengineering in E. coli led to glycosylation of SRRP53608 variants with α-GlcNAc and GlcNAcβ(1→6)GlcNAcα moieties. The glycosyltransferases involved in the modification of these adhesins were identified within the SecA2/Y2 accessory secretion system and their sugar nucleotide preference determined by saturation transfer difference NMR spectroscopy and differential scanning fluorimetry. Together, these findings provide novel insights into the cellular O-protein glycosylation pathways of gut commensal bacteria and potential routes for glycoengineering applications.
Keywords: accessory secretion system, glycosyltransferase, gut commensal bacteria, O-linked glycosylation, sugar nucleotides
Introduction
Although originally believed to be restricted to eukaryotes, protein glycosylation, i.e., the covalent attachment of a carbohydrate moiety to specific protein targets, is emerging as an important feature in bacteria and archaea, revealing an important diversity of glycan structures and pathways within and between microbial species (Schäffer and Messner 2017). To date, protein glycosylation has been widely studied in pathogenic bacteria, where glycoproteins are often essential for virulence and pathogenicity (Eichler and Koomey 2017). However, the nature and function of protein glycosylation in gut commensal bacteria remains largely unexplored (Latousakis and Juge 2018).
Lactobacillus reuteri is a Gram-positive bacterial symbiont inhabiting the gastrointestinal (GI) tract of a range of vertebrates (including humans) that displays a remarkable degree of host specialization (Oh et al. 2010; Frese et al. 2011; Frese et al. 2013; Wegmann et al. 2015; Duar et al. 2017). One of the mechanisms mediating specific interaction of L. reuteri strains with the host is provided by cell surface proteins that facilitate adherence to epithelial or mucosal surface along the GI tract, depending on the niche colonized by the bacteria (Mackenzie et al. 2010; Etzold et al. 2014; Sequeira et al. 2018). Previous analyses of the rodent strain L. reuteri 100-23C identified a gene encoding a predicted surface-exposed serine-rich repeat protein (SRRP100-23) that was essential for L. reuteri biofilm formation in the forestomach of mice (Frese et al. 2013). Inactivation of SRRP100-23 completely abrogated epithelial association, indicating that initial adhesion represented the most significant step in biofilm formation, likely conferring host specificity (Frese et al. 2013).
SRRPs are a family of adhesins found in many Gram-positive bacteria (Lizcano et al. 2012). These proteins were originally identified in pathogenic bacteria, such as streptococci and staphylococci (Wu et al. 1998; Bensing and Sullam 2002; Zhou and Wu 2009; Seo et al. 2013; Li et al. 2014), where their expression has been linked to virulence (Shivshankar et al. 2009; Sanchez et al. 2010). SRRPs are composed of distinct subdomains: a cleavable and unusually long signal peptide which, in some cases, is followed by an alanine-serine-threonine rich (AST) motif, a short serine rich repeat region (SRR1), a binding region (BR), a second and much larger SRR2, and an LPXTG cell wall anchoring motif (Rigel and Braunstein 2008). Previous studies on SRRPs from pathogenic organisms have shown that these proteins are O-glycosylated on serine or threonine residues and exported via an accessory secretion (SecA2/Y2) system (Bensing and Sullam 2002; Bensing et al. 2004; Takamatsu et al. 2004; Siboo et al. 2008; Chaze et al. 2014; Li et al. 2014). This specialized secretion system is encoded by genes that are normally co-located within a gene cluster and is composed of the motor protein SecA2, the translocon channel SecY2 and three to five accessory Sec proteins (Asp1-5). In addition, this gene cluster also contains genes encoding a variable number of glycosyltransferases (GTs), ranging between two and ten (Bensing et al. 2014). The best studied examples of SecA2/SecY2-mediated glycosylation systems are from pathogenic Streptococcus parasanguinis, Streptococcus pneumoniae, Streptococcus gordonii, Streptococcus agalactiae and Staphylococcus aureus (Takamatsu et al. 2004; Zhu et al. 2016; Jiang et al. 2017). In all cases, the glycosylation process is initiated by a two-protein glycosyltransferase complex, consisting of GtfA and GtfB, that mediate the addition of N-acetylglucosamine (GlcNAc) to serine and threonine residues within the SRR domains of the adhesins. This is sometimes followed by the extension of the core glycan via the action of additional GTs whose number and type vary between species, resulting in a range of glycan structures (Zhu et al. 2016; Jiang et al. 2017; Chen et al. 2018). Recently, a SecA2/Y2 cluster encoding three SRRPs has been identified in the commensal species Streptococcus salivarius JIM8777; unusually the first glycosylation step was carried out by two genetically linked GTs outside of the cluster (Couvigny et al. 2017).
To date, SecA2/Y2 clusters have been identified in the genomes of various Lactobacillus species (Tytgat and de Vos 2016; Latousakis and Juge 2018; Sequeira et al. 2018). In L. reuteri, the intact cluster has mostly been found in strains of murine or porcine origin, and it appears to be absent from strains of human origin (Frese et al. 2011; Frese et al. 2013; Wegmann et al. 2015; Sequeira et al. 2018). The SecA2/Y2 cluster in the L. reuteri rodent strain 100-23C is crucial for ecological fitness and adhesion of the bacteria to the forestomach epithelium of the murine GI tract (Frese et al. 2013). Using proteomics, we showed that SRRP100-23 is the primary cell wall-associated protein of L. reuteri 100-23C strain that is secreted through the accessory SecA2/Y2 system (Frese et al. 2013). In addition, our analysis of the completed genome of the pig isolate L. reuteri ATCC 53608 revealed the presence of a SecA2/Y2 system with an associated SRRP sharing the same domain organization as SRRP100-23 (Wegmann et al. 2015). Further analysis of the pangenome of L. reuteri pig isolates also revealed the presence of a SecA2/Y2 system with an associated SRRP in these strains (Wegmann et al. 2015), suggesting a conserved role of SecA2/Y2 among L. reuteri strains that possess the cluster. We confirmed that the SRRPs from L. reuteri pig strains were secreted during growth in vitro (Sequeira et al. 2018), as previously shown for SRRP100-23 (Frese et al. 2013). However, despite the central importance of the SecA2/Y2 cluster and SRRPs in specific L. reuteri strains, how SRRPs are glycosylated in lactobacilli has not yet been determined.
Here we provide a comprehensive analysis of the glycosylation of L. reuteri SRRPs (LrSRRPs) from L. reuteri ATCC 53608 (pig) and 100-23C (rodent) strains. Using a combination of bioinformatics analysis, lectin screening, LC–MS-based sugar nucleotide profiling, MALDI-ToF and GC–MS analyses, we showed that the L. reuteri ATCC 53608 and 100-23C strains are capable of performing protein glycosylation and that SRRP100-23 and SRRP53608 are glycosylated with hexose (Hex)2-N-acetylhexosamine (HexNAc) and di-HexNAc moieties, respectively. Following in vivo glycoengineering in E. coli, NMR analysis and enzymatic treatment showed that SRRP53608 is glycosylated with GlcNAcβ(1→6)GlcNAcα moieties. In addition, using Differential Scanning Fluorimetry (DSF) and Saturation Transfer Difference (STD) NMR, we provide biochemical insights into the specificity of the glycosyltransferases involved in the SecA2/Y2 accessory pathway leading to the protein glycosylation of these adhesins in gut symbionts.
Results
SRRPs from L. reuteri strains 100-23C and ATCC 53608 are glycosylated
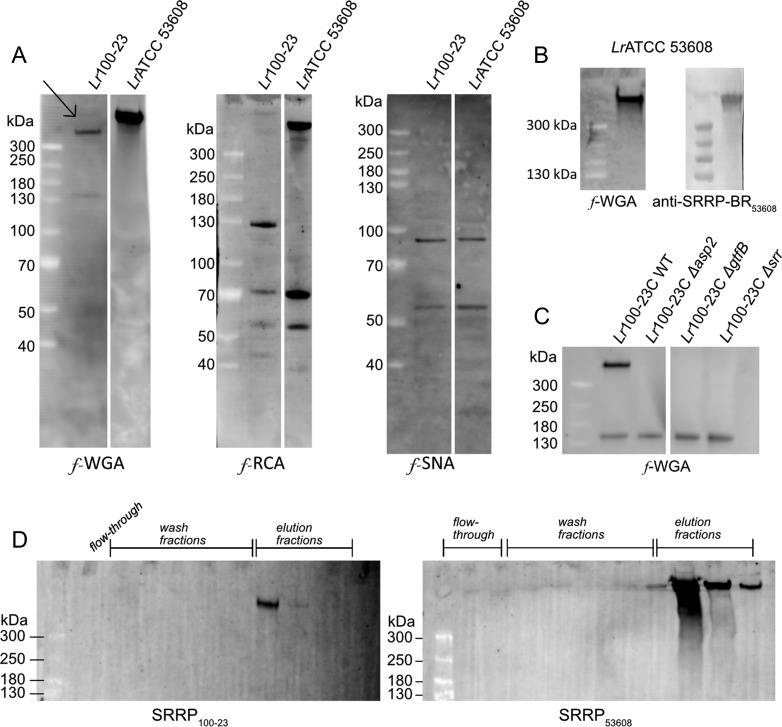
To determine whether L. reuteri strains 100-23C and ATCC 53608 are capable of performing protein glycosylation of LrSRRPs, the proteins from the spent media (SM) were separated by SDS-PAGE and analyzed by western blot using a range of fluorescein (f)-labeled lectins. A similar lectin recognition profile was observed between proteins from both L. reuteri strains with binding to f-WGA, f-RCA and f-SNA (Figure 1A) while no binding was observed with f-ConA, f-LTL, f-PNA or f-UEA (data not shown). This suggests the presence of glycoproteins carrying GlcNAc, sialic acid or galactose (Gal) residues. A large protein with an apparent molecular weight (MW) > 300 kDa was detected in both L. reuteri strains by f-WGA but not with any of the other lectins tested. This protein was also recognized by anti-SRRP-BR53608 antibodies in L. reuteri ATCC 53608 SM, suggesting that it corresponds to SRRP53608 (Figure 1B). It is of note that Coomassie-staining cannot efficiently detect LrSRRPs, probably due to their unusual amino acid composition and glycosylation. The anti-SRRP-BR53608 does not cross-react with SRRP100-23 which may be due to the low amino acid similarity (48%) between the two binding regions of the two adhesins (Sequeira et al. 2018). Previous reports have also shown that lectins can detect SRRPs with greater sensitivity than antibodies, since the high degree of glycosylation masks the underlying amino acid and protein antigens (Siboo et al. 2008). Therefore, to confirm the identity of the putative SRRP glycoprotein secreted by L. reuteri 100-23C, the lectin binding profile of L. reuteri 100-23C Δsrr mutant (lacking SRRP100-23 expression, see (Frese et al. 2013)) was determined as above following western blot analysis with f-labeled lectins. The protein band >300 kDa recognized by f-WGA in the L. reuteri 100-23C wild-type strain was missing in the Δsrr mutant (Figure 1C) while no other difference in the lectin recognition pattern was observed with f-WGA or when the SM proteins were probed with f-RCA or f-SNA (data not shown), confirming that this protein is SRRP100-23 (marked with an arrow in Figure 1A). It is interesting to note that the theoretical MW of SRRP53608 and SRRP100-23 is 116 kDa and 224 kDa, respectively, therefore the high apparent MW of LrSRRPs is in line with the potential glycosylation of these adhesins. The lectin recognition pattern of LrSRRPs suggests that these adhesins are glycosylated with glycans carrying GlcNAc residues.
Fig. 1.
Lectin screening of L. reuteri SM proteins. (A) Western blot analysis of L. reuteri 100-23C and ATCC 53608 SM proteins, using f-WGA, f-RCA and f-SNA. The arrow indicates SRRP in L. reuteri 100-23C. (B) Western blot analysis of L. reuteri ATCC 53608 SM proteins with f-WGA and anti-SRRP-BR53608 antibody. (C) Western blot analysis of L. reuteri 100-23C WT, Δasp2, ΔgtfB and Δsrr mutant SM proteins with f-WGA. (D) Purification of LrSRRPs by affinity chromatography, using agWGA. LrSRRPs were eluted with 0.5 M GlcNAc.
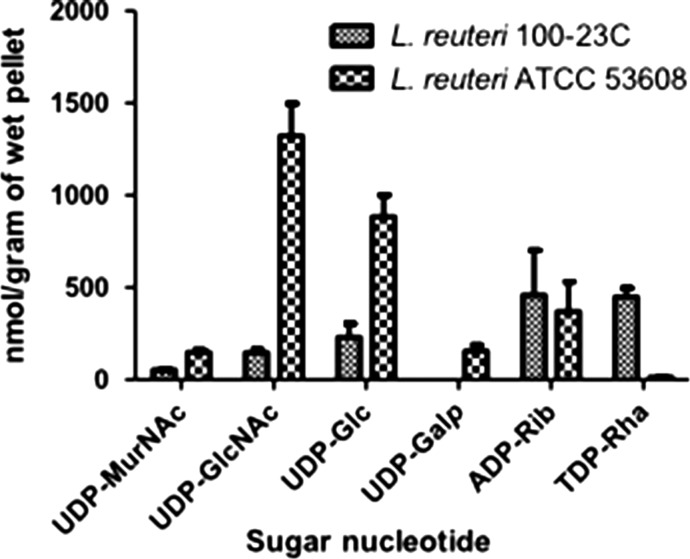
In support of this analysis, the profile of intracellular sugar nucleotides produced by L. reuteri strains was determined as described in Rejzek et al. (2017) with some modifications specific for the cell lysis of Gram-positive bacteria. The LC–MS/MS based analysis revealed the presence of six abundant nucleotide 5’-diphosphosugar (NDP-sugar) species in L. reuteri 100-23C and ATCC 53608 (Figure 2) at concentrations ranging from low nmol to low μmol per gram of wet cell pellet (Table S1). UDP-GlcNAc and UDP-Glc were detected in both L. reuteri strains at high levels (Figure 2). UDP-Gal was also found in both strains but at significantly lower levels in L. reuteri 100-23C, under the conditions tested. These results are in line with the bioinformatics analyses showing the genetic requirement for the synthesis of UDP-GlcNAc, UDP-Glc, UDP-Gal (data not shown) which are commonly used as sugar donors by GTs in protein glycosylation (Freeze et al. 2017) and in agreement with the presence of GlcNAc moieties onto LrSRRPs, as suggested by the lectin screening.
Fig. 2.
LC–MS sugar nucleotide profiling of L. reuteri 100-23C and ATCC 53608 strains. The bars represent the standard error of three biological replicates. See also Table S1 for MRM transitions, retention times and quantity of the sugar nucleotides.
SRRP100-23 and SRRP53608 are glycosylated with Hex2GlcNAc and di-GlcNAc moieties, respectively
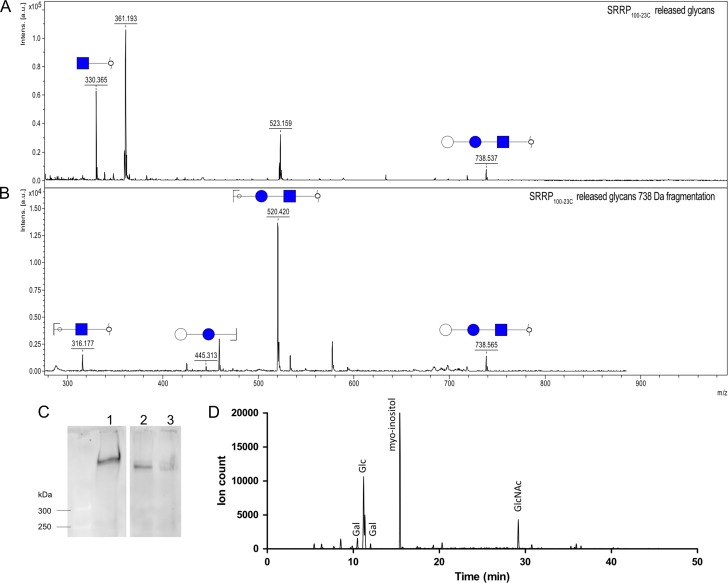
To identify the glycans decorating LrSRRPs, SRRP100-23 and SRRP53608 were purified from L. reuteri culture supernatant by affinity chromatography using an agarose-bound WGA (agWGA) column. The purified proteins migrated at a MW > 300 kDa on SDS-PAGE and were recognized by f-WGA (Figure 1D) on western blot. The purified LrSRRPs were then subjected to reductive β-elimination, and the chemically released glycans permethylated and analyzed by MALDI-ToF. The spectra of SRRP100-23 showed a peak at 738 Da, corresponding to Hex2HexNAc (Figure 3A) and fragmentation of this ion species suggested a linear glycan structure (Figure 3B). The peak at 330 Da corresponds to reduced, permethylated HexNAc, suggesting some degree of heterogeneity in the glycosylation of SRRP100-23 which may also explain the recognition of SRRP100-23 by WGA. Interestingly, the Hex-HexNAc intermediate could not be identified in the sample. As further support of SRRP100-23 glycosylation, SM proteins from L. reuteri 100-23C asp2 and gtfB mutants (Frese et al. 2013) were analyzed by western blot using f-WGA. The WGA-band corresponding to SRRP100-23 was missing in both mutants (Figure 1C) and glycomics analysis of SM proteins from the gtfB mutant showed a loss of the peak at 738 Da compared to the wild-type strain (Supplementary data, Figure S1), further confirming that this modification was due to SecA2/Y2-mediated protein glycosylation. To identify the nature of the monosaccharides constituting SRRP100-23 glycans, the adhesin was treated with α- or β-glucosidase, or α-, or β-galactosidase and the reaction product was analyzed by western blot, using f-WGA. The results showed that treatment with either α-glucosidase or α-galactosidase led to reduction of the apparent MW of the adhesin after SDS-PAGE (Figure 3C), suggesting that the terminal hexoses could be either Glc or Gal. Further analysis of the monosaccharides in the elution fraction of the agWGA affinity chromatography by GC–MS, following methanolysis, N-acetylation and TMS-derivatization of the released methyl-glycosides, showed that Glc and Gal were the only hexoses present, supporting the enzymatic deglycosylation data (Figure 3D). The analysis also showed that GlcNAc was the only HexNAc present. Together these results suggest that SRRP100-23 is modified with GlcNAc and Glc or Gal moieties with GlcNAc being at the reducing end of the glycans.
Fig. 3.
Structural analysis of SRRP100-23 glycosylation. (A) MALDI-ToF analysis of SRRP100-23 released glycans found in the 35% ACN elution fraction. (B) Fragmentation of the 738 Da peak. (C) Western blot analysis of enzymatically deglycosylated SRRP100-23. 1. SRRP100-23 (1), treated with α- and β-glucosidase (2), or α- and β- galactosidase (3). (D) Monosaccharide composition analysis of SRRP100-23 glycans. Extracted ion chromatogram for ions at 204 and 173 Da, characteristic for monosaccharides. See also Figure S1 for comparison of MALDI-ToF spectra of the fraction containing the released glycans of L. reuteri 100-23 WT and ΔgtfB mutant.
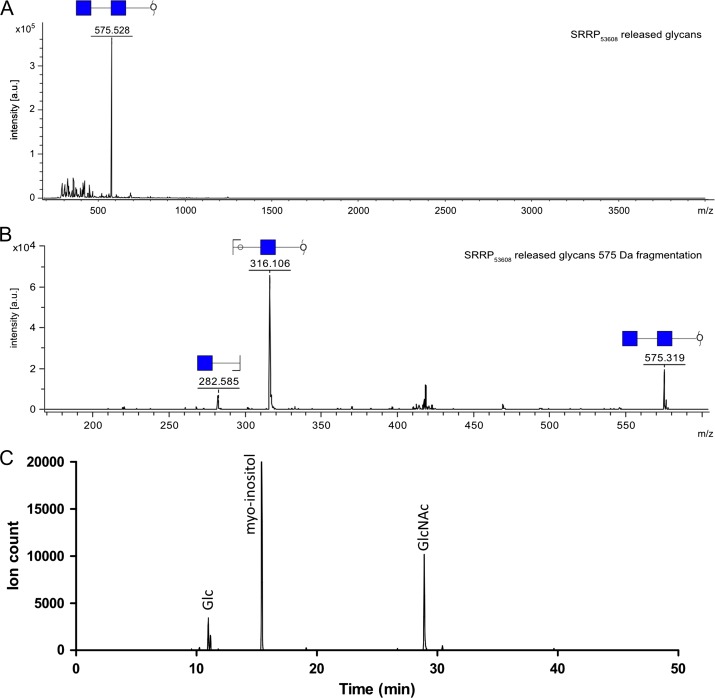
MALDI-ToF analysis of SRPP53608 glycans revealed a single peak at 575 Da, which corresponds to the mass of a reduced, permethylated sodiated di-HexNAc (Figure 4A). Further fragmentation of this species confirmed the nature of the glycan, as it produced two main peaks at 282 Da and 316 Da, corresponding to a non-reducing and a reducing terminal HexNAc, respectively (Figure 4B). To determine the nature of the glycan residues, the carbohydrate content of purified SRRP53608 was further analyzed by GC–MS. The chromatogram showed a single HexNAc peak with a retention time (~29 min) corresponding to that of GlcNAc (Figure 4C).
Fig. 4.
Structural analysis of SRRP53608 glycosylation (A) MALDI-ToF analysis of SRRP53608 released glycans. (B) Fragmentation of the 575 Da peak. (C) Monosaccharide composition analysis of SRRP53608 glycans. Extracted ion chromatogram for ions at 204 and 173 Da, characteristic for monosaccharides.
Taken together, these data suggest that SRRP100-23 is mainly glycosylated with Hex-Hex-GlcNAc and SRRP53608 with di-GlcNAc moieties. These results are in agreement with the lectin and sugar nucleotide profiling of L. reuteri strains 100-23C and ATCC 53608.
SRRP100-23 and SRRP53608 display different glycosylation pathways
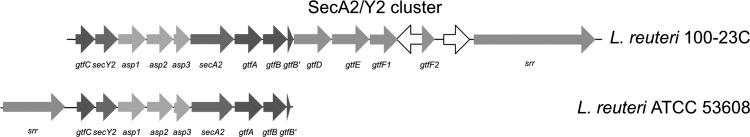
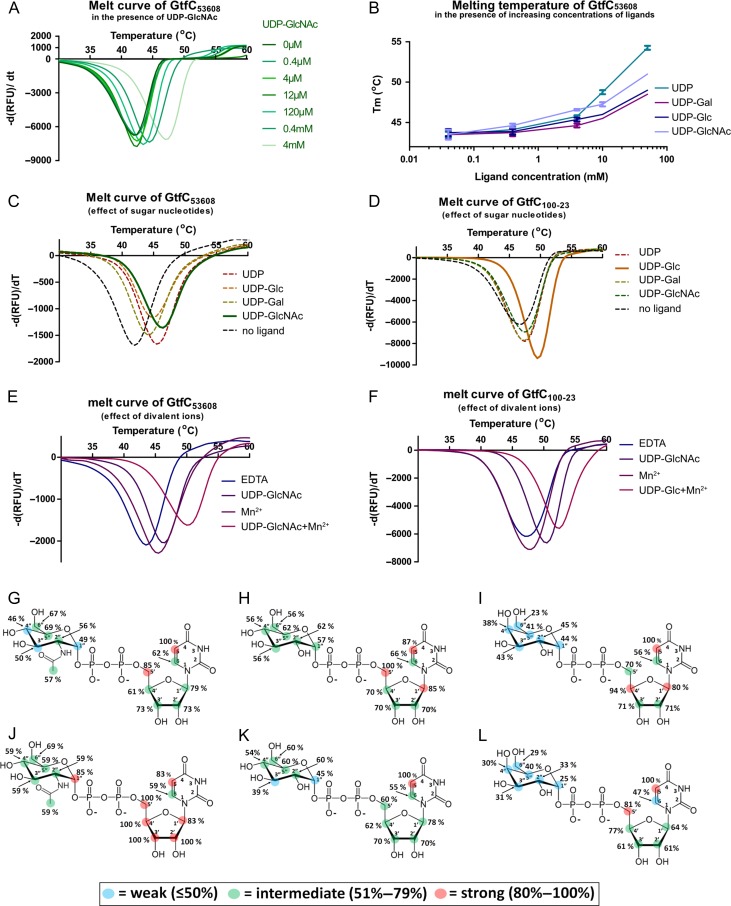
In addition to the SecA2 and SecY2 translocases and the accessory secretion associated proteins Asp1-3, the L. reuteri ATCC 53608 SecA2/Y2 glycosylation system contains genes encoding the priming GtfA53608 and GtfB53608, and a gene encoding GtfC53608 (Figure 5) whereas, in L. reuteri 100-23C, the SecA2/Y2 cluster includes eight genes encoding predicted GTs, including GtfA100-23, GtfB100-23 and GtfC100-23 (Figure 5). Based on homologous SecA2/Y2 clusters in streptococcal and staphylococcal systems, GtfA and GtfB are predicted to act together to initiate glycosylation of SRRPs by the addition of a GlcNAc residue, whereas GtfC is predicted to mediate the second glycosylation step (Zhu et al. 2016; Couvigny et al. 2017; Jiang et al. 2017). Based on the SRRP100-23 and SRRP53608 glycosylation profiles determined above, GtfC53608 and GtfC100-23 are predicted to add a GlcNAc residue or a Hex residue, respectively, to the GlcNAc core, while sharing 97% identity in amino acid sequence (Supplementary data, Figure S2). To confirm the ligand specificity of these enzymes, GtfC53608 and GtfC100-23 were heterologously expressed in E. coli and the recombinant enzymes first analyzed by differential scanning fluorimetry (DSF). Interactions of proteins with their ligands often lead to increased stabilization of the protein, and this is reflected by an increased melting temperature (Tm) (D’Urzo et al. 2012). GtfC53608 showed a UDP-GlcNAc concentration-dependent increase in Tm, from 42°C in the absence of the ligand to 47°C in the presence of 4 mM UDP-GlcNAc (Figure 6A). The specificity of GtfC53608 interaction was further tested against UDP, UDP-Gal and UDP-Glc, showing a concentration-dependent increase in Tm for all ligands tested (Figure 6B) but lower than the interaction with UDP-GlcNAc (Figure 6B, C), indicating a preference of GtfC53608 towards UDP-GlcNAc. GtfC100-23 showed an increase in Tm of up to 3°C in the presence of UDP-Glc, whereas other ligands had a reduced effect at concentrations up to 4 mM (Figure 6D), indicating a preference of GtfC100-23 for UDP-Glc. DSF was also used to investigate the dependency of GtfC53608 and GtfC100-23 to metal ions. The Tm of GtfC53608 was increased by 2.5°C in the presence of 5 mM of the divalent ions (Mg2+, Mn2+, Ca2+) and by 7°C when both the sugar ligand UDP-GlcNAc and metal ions were present (Figure 6E). A smaller shift in Tm (<1°C) was detected when the ions were added to GtfC100-23 in the absence or presence of UDP-Glc (Figure 6F). These results suggest that GtfC53608 and GtfC100-23 have different requirements for divalent ions for optimum binding.
Fig. 5.
Schematic representation of the accessory SecA2/Y2 clusters from L. reuteri 100-23C and ATCC 53608.
Fig. 6.
Analysis of GtfC100-23 and GtfC53608 ligand specificity. (A–F) Differential scanning fluorimetry (DSF) analysis. (A) Melt curve of GtfC53608 in the presence of increasing concentrations of UDP-GlcNAc. (B) Tm of GtfC53608 in the presence of increasing concentrations of UDP, UDP-Gal, UDP-Glc and UDP-GlcNAc. Error bars represent the standard error of the mean of four technical replicates. (C) Melt curve of GtfC53608 in the presence of 4 mM UDP-GlcNAc, UDP-Glc, UDP-Gal and UDP. (D) Melt curve of GtfC100-23C in the presence of 4 mM UDP-GlcNAc, UDP-Glc, UDP-Gal and UDP. (E) Melt curves of GtfC53608 in the presence of 5 mM Mn2+ (left), or 5 mM Mn2+ and 4 mM UDP-GlcNAc. (F) Melt curves of GtfC100-23C in the presence of 5 mM Mn2+ (left), or 5 mM Mn2+ and 4 mM UDP-Glc. Since no significant difference was observed between the different divalent ions, only Mn2+ is shown. (G–L) Saturation Transfer Difference (STD) NMR analysis. (G), (H), (I) Binding epitope maps for the complexes of GtfC100-23 with UDP-GlcNAc, UDP-Glc and UDP-Gal, respectively. Bottom row, (J), (K), (L) binding epitope maps for the complexes of GtfC53608 with UDP-GlcNAc, UDP-Glc and UDP-Gal, respectively. See also Table I and Figure S2 for the competition assays of the sugar nucleotides against GtfC100-23 and GtfC53608.
Saturation Transfer Difference (STD) NMR was used to obtain structural insights into the interaction between GtfC53608 or GtfC100-23 and these sugar nucleotides. We obtained binding epitope maps (maps of distribution of STD0(%) factors along the molecule) for each ligand tested (UDP, UDP-Gal, UDP-Glc and UDP-GlcNAc), reflecting the main contacts with the surface of the protein in the bound state. For each ligand, the highest STD0(%) factors were observed for the uracil and ribose moieties whereas the hexopyranose moieties (Glc, GlcNAc and Gal) showed lower STD0(%) factors (Figure 6G–L). In addition, there were differences between the ligand binding epitopes in complex with GtfC53608 or GtfC100-23. UDP-GlcNAc showed higher STD0(%) factors on average in the presence of GtfC53608 (Figure 6J), supporting a preference of this protein for UDP-GlcNAc whereas GtfC100-23 showed a binding preference for UDP-Glc (Figure 6H). UDP-Gal showed only weak interactions with GtfC100-23 or GtfC53608 (Figure 6I, L). STD NMR titrations were carried out to determine the ligand affinity of GtfC53608 and GtfC100-23. Since the stability of the protein samples imposed time constraints on the NMR measurements precluding an STD initial slope titration approach to get thermodynamic values (Angulo et al. 2010), the KD values were considered as apparent. All apparent KD values, were in excellent agreement with the binding epitope data, except for the KD of the complex GtfC100-23/UDP-Gal which was lower than GtfC100-23/UDP-Glc. In order to explore this further, a competitive STD NMR study was performed where the STD factors for the complexes GtfC100-23/UDP-Glc, GtfC100-23/UDP-GlcNAc, GtfC53608/UDP-GlcNAc and GtfC53608/UDP-Glc were determined in the absence or presence of UDP-Gal. The results (Table I, Supplementary data, Figure S3) were in excellent agreement with the epitope mappings of the sugar nucleotides, supporting the preference of GtfC100-23 towards UDP-Glc, despite the lower apparent KD obtained for UDP-Gal. The difference in apparent KD may be due to a conformational rearrangement of GtfC100-23 in the presence of UDP-Glc, reducing the kinetics rate of the association process (on-rate, kON), leading to an underestimation of affinity due to ligand rebinding (Angulo et al. 2010), as was previously reported for the complex of the human blood group B galactosyltransferase and its donor substrate UDP-Gal (Angulo et al. 2006).
Table I.
Affinity ranking of UDP, UDP-GlcNAc, UDP-Glc and UDP-Gal for GtfC53608 and GtfC100-23 from different 1H STD NMR approaches
| STD-NMR determination of the ligand affinity of GtfC100-23 and GtfC53608 | ||||
|---|---|---|---|---|
| Ligands | GtfC53608 | GtfC100-23 | ||
| KD (mM) | Affinity from competition | KD (mM) | Affinity from competition | |
| UDP-Glc | 1.8 | + | 0.99 | ++++ |
| UDP-GlcNAc | 0.43 | ++++ | 2.4 | + |
| UDP-Gal | 1.66 | + | 0.31 | + |
Taken together, these results suggest that GtfA/B are involved in GlcNAc attachment to SRRP100-23 and SRRP53608 while GtfC53608 extends the chain with a GlcNAc residue and GtfC100-23 with Glc.
In vivo glycoengineering of SRR1 domain
To gain further insights into the glycosylation of SRRP53608, a sequence encoding a His-tagged SRR1 region covering aa 81-236 of SRRP53608 was co-expressed in E. coli together with an operon encoding GtfA53608, GtfB53608 and GtfC53608. MS analysis after trypsin digest of protein bands at 60, 50 and 40 kDa (Supplementary data, Figure S4A), confirmed that these correspond to the successfully expressed GtfA53608, GtfB53608 and GtfC53608, respectively (data not shown). The protein extract was further analyzed by western blot, using f-WGA. A protein migrating between 45 and 60 kDa was detected by f-WGA when GtfA/B/C53608 and SRR1, were co-expressed, but not in the control experiment expressing SRR1 only (Supplementary data, Figure S4B), suggesting that this protein corresponds to glycosylated SRR1 (gSRR1). The his-tagged gSRR1 was purified by IMAC and subjected to reductive β-elimination. Analysis of the permethylated glycans by MALDI-ToF MS showed a peak at 575 Da (Supplementary data, Figure S5A), consistent with the presence of di-HexNAc species, as seen for the glycans from the native SRRP53608. The assignment of this peak as a di-HexNAc-ol was also supported by fragmentation of the species at 575 Da that showed dominant peaks at 316 and 282 Da (Supplementary data, Figure S5A). Two weak signals at 330 Da and at 534 Da, corresponding to the mass of a permethylated, sodiated HexNAc and Hex-HexNAc-ol, respectively, were also observed (Supplementary data, Figure S5A).
The released, underivatized glycans were analyzed using 2D NMR and DEPT experiments in order to characterize the conformation and linkage of the disaccharide. NMR spectra of α/ β-GlcNAc and GlcNAc-ol standards were recorded for comparison with the experimental samples. The NMR analysis of the gSRR1 glycans confirmed the presence of a di-GlcNAc disaccharide (Table II), in agreement with the MS analysis of gSRR1 and the glycosylation of native SRRP53608. The disaccharide was determined to be β-GlcNAc-(1→6)-GlcNAc-ol (Supplementary data, Figure S5B-C). In addition, the released glycan fraction also revealed the presence of free GlcNAc-ol and the two mixture components were present in the proportions GlcNAc-ol (60%): disaccharide (40%) (Supplementary data, Figure S5B), suggesting that the glycosylation of gSRR1 in E. coli consists of a combination of mono- and di-GlcNAc side chains. A minor doublet was detected at 4.50 ppm, suggesting the presence of a second disaccharide on gSRR1, in agreement with the MALDI-ToF analysis that showed the presence of a Hex-HexNAc-ol. The β-conformation of the non-reducing GlcNAc was further confirmed by treatment of recombinant gSRR1 with a commercially available β-N-acetylhexosaminidasef. The enzymatically-treated gSRR1 showed reduced apparent size on western blot following detection by f-WGA as compared to non-treated gSRR1 (Supplementary data, Figure S5C).
Table II.
1H and 13C chemical shifts of reference standards, glycan released from gSRR1 and glycan units present in intact gSRR1
| NMR characterization of the sSRR1 released glycans | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | CH3 | C=O | ||
| Reference Standards | |||||||||
| α-GlcNAc | H | 5.21 | 3.88 | 3.78 | 3.50 | 3.86 | 3.86,3.80 | 2.06 | – |
| C | 93.70 | 56.96 | 73.52 | 72.91 | 74.44 | 63.42 | 24.77 | 177.40 | |
| β-GlcNAc | H | 4.72 | 3.68 | 3.55 | 3.47 | 3.47 | 3.92,3.76 | 2.06 | – |
| C | 97.79 | 59.54 | 76.73 | 72.69 | 78.81 | 63.58 | 25.05 | 177.65 | |
| GlcNAc-ol (R) | H | 3.64,3.74 | 4.08 | 3.97 | 3.60 | 3.76 | 3.66,3.83 | 2.06 | – |
| C | 63.68 | 56.58 | 71.14 | 73.79 | 73.93 | 65.62 | 24.96 | 177.35 | |
| Glycan released from gSRR1, β-GlcNAc-(1→6)-GlcNAc-ol | |||||||||
| β-GlcNAc(1→(B) | H | 4.55 | 3.75 | 3.57 | 3.46 | 3.47 | 3.95,3.76 | 2.07 | – |
| C | 104.45 | 58.44 | 76.65 | 72.81 | 78.68 | 63.58 | 25.09 | 177.65 | |
| →6)GlcNAc-ol (G) | H | 3.64,3.74 | 4.08 | 3.97 | 3.60 | 3.84 | 4.09 | 2.05 | – |
| C | 63.73 | 56.55 | 70.95 | 73.65 | 72.49 | 73.75 | 24.94 | 177.35 | |
| GlcNAc units present in gSRR1, M = monosaccharide, D = disaccharide side-chain | |||||||||
| t-α-GlcNAc→Ser (αM) | H | 4.87 | 3.92 | 3.72 | 3.47 | 3.62 | 3.84,3.78 | ~2.05 | – |
| C | 100.61 | 56.35 | 73.86 | 72.68 | 75.15 | 63.44 | ~25.0 | ~177.0 | |
| →6)-α-GlcNAc→Ser (αD) | H | 4.88 | n.d. | n.d | n.d. | n.d. | 4.13,3.80 | n.d. | n.d. |
| C | 100.61 | n.d. | 73.87 | 72.54 | n.d. | 71.13 | n.d. | – | |
| t-β-GlcNAc(1→(βD) | H | 4.54 | 3.75 | 3.58 | 3.47 | 3.47 | 3.94,3.77 | ~2.07 | – |
| C | 104.51 | 58.41 | 76.54 | 72.67 | 78.74 | 63.68 | ~25.2 | ~177.3 | |
See also Supplementary data, Figure S5 and Table S3 for information on the expression of GtfA, GtfB and GtfC, and glycosylation of gSRR1 and Supplementary data, Figures S5 and S6 for information on the structural characterization of the gSRR1 released and native glycans by NMR.
n.d. = not determined.
To determine the configuration of GlcNAc linked to the protein, NMR experiments were carried out on the intact gSRR-1 protein. NMR assignments of the sugar residues in gSRR1 are reported in Table II and details of how the assignments were made are provided in the Supplementary data, Figure S5 captions (Supplementary data, Figure S6). The analysis revealed that GlcNAc was α-linked to gSRR1 and confirmed that both single α-GlcNAc and GlcNAcβ-(1→6)-GlcNAcα disaccharide side chains were present. In the 1H spectrum of gSRR1, the anomeric signal of β-GlcNAc appeared as a simple doublet, J1,2 = 8.6 Hz, at δ 4.54, but the anomeric signal of α-GlcNAc appeared as a broad feature centered at δ 4.87. This broad feature consisted of a superposed series of doublets, all with J1,2 = 3.9 Hz, but with displaced δH1 chemical shifts in the range 4.91–4.85 ppm (Supplementary data, Figure S6C). The displacement arises because the sugars are linked to serine or threonine residues that occupy slightly different environments as a result of the protein secondary structure. By integrating the α- and β- 1H anomeric signals (Supplementary data, Figure S6D) it was possible to estimate the proportions of mono- to disaccharide side chains as 64%:36%, in agreement with the result obtained from the released glycans mixture.
Together these data showed that GtfA, GtfB and GtfC can glycosylate gSRR1 in E. coli. Detailed NMR analysis of the intact glycoprotein, as well as the released glycans, showed that gSRR1 is modified with α-linked GlcNAc residues and GlcNAcβ(1→6)GlcNAcα moieties at a ~4:6 ratio with a small fraction of a Hex-GlcNAc species further identified by MS and NMR.
Discussion
Protein glycosylation is emerging as an important feature in bacteria. Protein glycosylation systems have been reported and studied in many pathogenic bacteria, revealing an important diversity of glycan structures and pathways within and between bacterial species. Studies focused on SRRPs from streptococci and staphylococci have demonstrated that these adhesins are O-glycosylated. In these closely related bacteria, glycosylation of SRRPs is initiated by a complex between GtfA and GtfB that adds GlcNAc to the SRR domains of the adhesins while additional GTs, including GtfC, may further modify SRR glycosylation by sequentially adding other glycan moieties onto the GlcNAc core (Takamatsu et al. 2004; Shi et al. 2014; Zhu et al. 2016; Jiang et al. 2017). Here we showed that the gut symbiont L. reuteri is capable of performing O-glycosylation on proteins, and that L. reuteri strains differentially modify SRRPs. SRRP100-23 is glycosylated with GlcNAc and Hex-Glc-GlcNAc whereas SRRP53608 is glycosylated with GlcNAc and di-GlcNAc moieties. L. reuteri GtfAB are expected to be involved in the addition of the core GlcNAc to serine or threonine, in agreement with the glycan structure of SRRP100-23 and SRRP53608 and with their high sequence homology with other functionally characterized GtfAs (e.g., ~46% identity with GtfA from S. pneumoniae TIGR4 (Jiang et al. 2017), E-value < 10–150). In addition to the SecA2/SecY2 export system dedicated to the glycosylation of SRRPs, a general O-glycosylation system has been reported in L. plantarum WCFS1 where homologs of L. reuteri SecA2/Y2 GtfA and GtfB have been shown to be involved in the addition of a single HexNAc molecule onto the glycosylation site of the acceptor proteins (Lee et al. 2014). These two enzymes contain a DUF1975 in the N-terminus which probably mediates the interaction between the two GTs and the target proteins and a GT domain in the C-terminus, as demonstrated for GtfA and GtfB from S. parasanguinis FW213 (Wu and Wu 2011), suggesting a similar mode of action to the SecA2/Y2-specific GtfA and GtfB.
The glycosylation of SRRP100-23 with Hex-Glc-GlcNAc is in line with the specificity of GtfC100-23 to UDP-Glc by DSF and STD NMR. The second Hex (either Glc or Gal) may be the result of another GT present in the L. reuteri 100-23C SecA2/Y2 cluster (see Figure 5). The number of GTs in the L. reuteri 100-23C SecA2/Y2 cluster exceeds the number of sugars on SRRP100-23, as also reported for the pneumonococcal SecA2/Y2 system (Jiang et al. 2017). Here the putative GtfD100-23 and GtfE100-23 encoded genes share a similar organization with a GT4 in the N-terminus and a DUF1792 in the C-terminus. In addition, GtfF1100-23 and GtfF2100-23 may be part of the same gene separated by a gene encoding a putative transposase, with GtfF1100-23 encoding a GT4 domain in the N-terminus and part of a DUF1792 domain in the C-terminus and GtfF2100-23 encoding the remaining part of the DUF1792 domain. Glycosyltransferases possessing a DUF1792 has been shown to be involved in the third glycosylation step of the SRRPs, Fap1 and PsrP, from S. parasanguinis FW213 and S. pneumoniae TIGR4, respectively (Zhang et al. 2014; Jiang et al. 2017). While DUF1792 has been shown to expand the Fap1 glycan with Glc moieties in S. parasanguinis (Zhang et al. 2014), DUF1792 from S. pneumoniae showed a relaxed specificity transferring either Glc or Gal to SRR1 in E. coli (Jiang et al. 2017). As all additional GTs in the L. reuteri 100-23C SecA2/Y2 cluster contain such a domain, it is possible that only one of these enzymes is active or that there is redundancy in their function. Taken together with the SRRP100-23 enzymatic deglycosylation data, it is likely that SRRP100-23 is modified by Glc-Glc-GlcNAc or Gal-Glc-GlcNAc. Interestingly, the Glc-GlcNAc intermediate could not be identified by MALDI-ToF analysis, suggesting that the addition of the third monosaccharide onto the expanding glycan is a rapid reaction, as observed for Fap1 in S. parasanguinis FW213 (Zhang et al. 2014).
To date, all characterized GtfCs have been shown to add a Glc residue onto the GlcNAc core, therefore the glycosylation of SRRP53608 by di-GlcNAc was unexpected. The specificity of L. reuteri GtfC53608 was further supported by DSF and STD NMR analyses, showing a preference for UDP-GlcNAc, in line with the MS/GC–MS analyses. This is therefore the first report of a GtfC from the SecA2/Y2 system showing ligand specificity to UDP-GlcNAc. In addition, we showed that GtfC53608 (and GtfC100-23 to a lesser extent) bound to divalent ions, suggesting that they may contribute to optimum enzyme activity. Although these enzymes do not possess the DxD motif, commonly involved in ion binding, they harbor a DxE motif that could have a similar role. Such dependency for divalent ions is well established in Leloir GTs, and some examples have recently been reported in prokaryotic systems such as the dGT1-mediated glycosylation of Fap1 in S. parasanguinis (Zhang et al. 2014). However, no divalent ions have been identified so far in GtfCs from other microorganisms (Zhu et al. 2011).
SRRP53608 glycosylation was further confirmed by the introduction of GtfA/B/C53608 into E. coli, resulting in glycosylation of a co-expressed SRR1 domain by mono- and di-GlcNAc, as shown by MS and NMR. Heterogeneity in the glycosylation of SRRPs has been reported in SRR glycoproteins from Streptococcus species (Chaze et al. 2014; Zhang et al. 2014; Couvigny et al. 2017; Jiang et al. 2017), where deposition of GlcNAc moieties is not followed by further elongation of the glycan, suggesting this is a common feature among SRRPs. This heterogeneity was also observed in the glycosylation of SRRP100-23 (see Results section) and could explain the recognition of SRRP100-23 by WGA.
The NMR analysis also indicated that SRRP53608 is glycosylated with GlcNAcβ(1→6)-GlcNAcα moieties, providing a unique example of SRRP glycans extended with GlcNAc residues in the second position. Although only so far reported for GlcNAc residues that are directly attached onto the protein backbone, it is possible that SRRP53608 contains additional O-acetyl group moieties as previously identified in SRRPs from S. gordonii M99 (Seepersaud et al. 2017), S. agalactiae H36b (Chaze et al. 2014) and S. salivarius JIM8777 (Couvigny et al. 2017). In these Streptoccocus SRRPs, Asp2 was found to be responsible for this modification, probably on the O-6 position (Seepersaud et al. 2017). Since L. reuteri SecA2/Y2 clusters harbor a gene encoding a predicted Asp2 with conserved catalytic residues, Asp2 may also carry out this function in L. reuteri. However, since the O-AcGlcNAc modification is lost under the conditions used in our MALDI-ToF or GC–MS analyses (the high pH used for the release of the glycans leads to base-catalyzed ester hydrolysis and thus loss of the modification), more work is required to establish whether Asp2 functions as an acetyltransferase that modifies GlcNAc moieties of SRRP53608. The α-linked configuration we demonstrated here for the first time for an SRRP is in agreement with the retaining mechanism reported for GtfA from S. gordonii (Chen et al. 2016) and S. pneumoniae (Shi et al. 2014).
Interestingly, a small fraction of the gSRR1 glycans consisted of Hex-HexNAc moieties, a modification that was not found on the native protein. This suggests that GtfC could mediate the transfer of either Glc or GlcNAc in the E. coli glycosylation model, while showing a preference for GlcNAc in L. reuteri ATCC 53608, in agreement with the enzyme donor specificity and the increased levels of UDP-GlcNAc in L. reuteri ATCC 53608.
In L. reuteri 100-23C, the Δasp2 and ΔgtfB mutants lost the WGA band corresponding to SRRP100-23, indicating that, in this strain, Asp2 and GtfB are essential for glycosylation and/or export of SRRP100-23. In S. gordonii, Asp2 is involved in both the post-translational modification and transport of SRR glycoproteins during their biogenesis (Yen et al. 2011; Seepersaud et al. 2012; Seepersaud et al. 2017). This requirement for the coupling of glycosylation and secretion has been proposed as a mechanism underpinning the co-evolution of SRR glycoproteins with their dedicated accessory SecA2/Y2 system such that the adhesin is optimally modified for binding (Seepersaud et al. 2012).
In conclusion, we showed that LrSRRP adhesins are differentially glycosylated in L. reuteri strains 100-23C and ATCC 53608, reflecting differences in the organization of the SecA2/Y2 accessory cluster of these strains. In addition, LrSRRPs from pig and rodent strains differ with respect to the number of repeat motifs and their sequences of their SRR regions (Sequeira et al. 2018). The glycosylation of SRRPs in Lactobacillus species, as demonstrated for the first time in this study, is likely to impact on the adhesion capacity of these strains. A recent analysis of all available genomes of L. reuteri strains showed that homologs of functional SRRPs (and the corresponding linked SecA2/Y2 gene cluster) were exclusively found in rodent and pig isolates, with the exception of one chicken isolate (Sequeira et al. 2018). Differences in LrSRRP glycosylation profile may therefore contribute to the mechanisms underpinning L. reuteri adaptation to these hosts. In addition, bioinformatics analyses revealed the presence of complete SecA2/Y2 clusters with an intact SRRP in the genomes of other Lactobacillus species including strains from Lactobacillus oris, Lactobacillus salivarius, Lactobacillus johnsonii and Lactobacillus fructivorans (Latousakis and Juge 2018; Sequeira et al. 2018), suggesting a common role of SRR glycoproteins in adhesion to host epithelia, which may be related to the ecological context of these strains (see Duar et al. (2017) for a review). This aspect can be particularly important in the selection of probiotics targeting different vertebrate hosts. Furthermore, knowledge of the cellular pathways of glycosylation in gut symbionts expands the range of glycoengineering applications for the recombinant production of glycoprotein conjugates in different cell types.
Materials and Methods
Materials, strains and culture conditions
Uridine diphosphate (UDP), UDP-glucuronic acid (UDP-GlcA), UDP-N-acetylglucosamine (UDP-GlcNAc), UDP-N-acetylgalactosamine (UDP-GalNAc), UDP-glucose (UDP-Glc), UDP-galactopyranose (UDP-Gal), thymidine diphosphate (TDP)-Glc and all chemical reagents were from Merck (Gottingen, Germany), unless stated otherwise. TDP-rhamnose (TDP-Rha) was prepared as described (Wagstaff et al., 2018). Polyclonal antiserum against immobilized metal affinity chromatography (IMAC)-purified His6-SRRP53608-BR was raised in rabbits by BioGenes GmbH (Berlin, Germany) and provided at a titer of >1:200,000, as previously reported (Sequeira et al. 2018). The lectins used in this study were purchased from Vector Laboratories (Peterborough, UK) and are listed in Table S1.
The bacterial strains and plasmids used in this study are described in Table S2. The deMan-Rogosa-Sharpe (MRS; Oxoid, Loughborough, UK) or lactobacillus defined medium-II (LDM-II (Kotarski and Savage 1979)) medium was used for growth of L. reuteri strains at 37°C, and the media were supplemented with erythromycin (10 μg/mL) for L. reuteri 100-23C mutants. The Luria-Bertani (LB) or terrific broth-based auto induction media supplemented with trace elements (AIM; Formedium, Hunstanton, UK) were used for E. coli growth at 37°C, 250 rpm. The media were supplemented with the relevant antibiotics as described in Table S2.
Lectin screening by western blot
L. reuteri strains were grown in LDM-II overnight at 37°C under static conditions. This culture was used to inoculate fresh LDM-II at 0.2% vol/vol. Following incubation under static conditions at 37°C overnight, the cultures were centrifuged at 4000 × g for 5 min and the spent media (SM) concentrated 10-fold by spin filtration using 10 kDa MWCO spin filters. The SM proteins were analyzed by SDS-PAGE, using Bis-Tris 4–12% or Tris-Acetate 3–8% NuPAGE gels (ThermoFisher Scientific, Loughborough, UK) in 3-Morpholinopropane-1-sulfonic acid (MOPS) or Tris-Acetate NOVEX buffer for 50 min at 200 V. The gels were then stained with InstantBlue protein stain (Expedeon, Over, UK). Alternatively, proteins were transferred onto PVDF membranes in NuPAGE transfer buffer, using an X-cell II blot module (ThermoFischer Scientific, Loughborough, UK) at 30 V for 2 h. The membrane was then blocked for 1 h at RT and probed with either fluorescein (f)-labeled lectins at 5 μg/mL or with anti-SRRP-BR53608 primary antibody (1000-fold dilution). Alkaline phosphatase-conjugated anti-rabbit IgG antibody Merck (Gottingen, Germany) was used as secondary antibody. Three washes with PBS supplemented with 0.1% vol/vol Tween-20 were included between antibody incubations. Bound antibody was detected using alkaline phosphatase substrate (nitroblue tetrazolium 0.1 mM, 5-bromo-4-chloro-indolyl phosphate p-toluidine 1 mM, in Tris-HCl 0.1 M containing 4 mM MgCl2) at pH 9.6 and scanned in a GS-800 calibrated densitometer (Bio-Rad, UK).
LrSRRP purification
L. reuteri 100-23C and ATCC 53608 strains were grown in LDM-II for 24 h at 37°C. The bacteria were removed following centrifugation at 10,000×g for 10 min. Ammonium sulfate was added to the SM at a final concentration of 60% (w/v) to precipitate the proteins. The suspension was stirred overnight. The precipitated proteins were recovered by centrifugation at 10,000 × g for 20 min. The proteins were resuspended in HEPES buffer (HEPES 10 mM, NaCl 150 mM, pH 7.5) and LrSRRP purified by gravity flow affinity chromatography, using agarose-bound wheat germ agglutinin (agWGA). Loosely bound proteins were removed with 10 column vol of HEPES buffer and the bound proteins were eluted with six column vol of HEPES buffer containing 0.5 mM GlcNAc. The proteins were extensively dialyzed in 50 mM ammonium bicarbonate to remove free GlcNAc.
Proteomics
Protein bands of interest were excised from SDS-NuPAGE gels and cut up to small cubic pieces. After two washes with 200 μL of ABC buffer (200 mM aqueous ammonium bicarbonate in 50% acetonitrile; ACN) for 15 min and then ACN for 10 min, the gel plugs were air-dried for 15 min. Proteins were reduced in a DL-dithiothreitol solution (200 μL, 10 mM in 50 mM ammonium bicarbonate) at 60°C for 30 min and carboxymethylated with iodoacetamide (10 mM in 50 mM ammonium bicarbonate) in the dark for an additional 30 min. The iodoacetamide solution was removed and the washing and drying steps were repeated. Trypsin Gold (10 μL; 10 ng/μL; Promega, UK) was added to the gel plugs along with equal amount of 10 mM ammonium bicarbonate. After incubation at 37°C for 3 h, 20 μL of 1% formic acid was added and the samples were further incubated at room temperature for 10 min. The solution was then transferred to a clean tube and tryptic peptides were further extracted from the gel plugs by addition of 40 μL of 50% ACN and incubation for 10 min at room temperature. The samples were pooled together and dried on a centrifugal evaporator. The peptide mixtures were analyzed by nano-scale liquid chromatographic tandem mass spectrometry (nLC MS/MS), using an Orbitrap Fusion trihybrid mass spectrometer coupled with a nano flow ultra-high performance liquid chromatography (UHPLC) system (ThermoFischer Scientific, UK). The peptides were separated on a C18 pre-column, using a gradient of 3–40% ACN in 0.1% formic acid (vol/vol) over 50 min at a flow rate of 300 nL/min at 40°C. The peptides were fragmented in the linear ion trap by a data-dependent acquisition method, selecting the 40 most intense ions. Mascot (Matrix Science, UK) was used to analyze the raw data against an in-house maintained database of the L. reuteri and/or E. coli proteome. The tolerance on parent ions was 5 ppm and on fragments was 0.5 Da. Carboxymethylation of cysteine was selected as fixed modification and oxidation of methionine as variable modification. One miscleavage was allowed.
Enzymatic treatment of SRRPs
LrSRRP was treated with α-glucosidase from Saccharomyces cerevisiae, α-galactosidase from green coffee beans, β-glucosidase from almonds or β-galactosidase from Αspergillus oryzae (0.5 U/μL; Merck Gottingen, Germany) in 50 mM sodium acetate, 5 mM CaCl2, pH 6 for 16 h. The reaction products were analyzed by SDS-PAGE and western blot, as described above.
Glycan analysis by Matrix Assisted Laser Desorption/ionization Time of Flight Mass Spectrometry (MALDI-ToF)
LrSRRP glycans were released by β-elimination, after treatment of the purified proteins with 1 M NaBH4 in 50 mM NaOH for 16 h at 45°C. Excess of NaBH4 was neutralized by the addition of acetic acid, before sodium ions were removed by ion-exchange chromatography, using a DOWEX 50Wx8 H+ column. Glycans were collected in the flow-through and wash fractions using 5% acetic acid. These fractions were pooled and freeze-dried, prior to permethylation of the glycans with 300 μL NaOH – anhydrous dimethylsulfoxide (DMSO) slurry and 400 μL iodomethane. The reaction was incubated at room temperature for 60 min under vigorous shaking and quenched by the dropwise addition of H2O, until fizzing stopped. The permethylated glycans were extracted in 2 mL chloroform, washed three times with 2 mL H2O. After drying the organic phase under nitrogen, glycans were dissolved in 50 μL aqueous methanol 50% vol/vol and loaded onto a pre-washed with methanol, acetonitrile and water Empore™ C18-SD cartridge (7 mm; Merck, Germany). Hydrophilic contaminants were washed with 500 μL H2O and 400 μL 15% vol/vol aqueous acetonitrile. Permethylated carbohydrates were eluted with 400 μL of 35%, 50% and 75% vol/vol aqueous ACN. The eluants were dried under a gentle stream of nitrogen, dissolved in 10 μL of TA30 [30% (vol/vol) ACN, 0.1% (vol/vol) trifluoroacetic acid] and mixed with equal amount of 2,5-dihydroxybenzoic acid (DHB; Sigma-Aldrich, UK; 20 mg/mL in TA30), before being spotted onto an MTP 384 polished steel target plate (Bruker, UK). The samples were analyzed by MALDI-ToF, using the Bruker Autoflex™ analyzer mass spectrometer (Bruker, UK) in the positive-ion and reflectron mode.
Monosaccharide analysis by gas chromatography (GC)–MS
LrSRRPs were treated with methanolic HCl (1 M) for 16 h and 5 μg of myo-inositol added as internal standard. Silver carbonate (~50 mg) was added to the solution, followed by 100 μL acetic anhydride and the reactions were incubated at room temperature for 16 h in the dark. Lipids were removed by three washes with heptane and the remaining methanolic phase was dried under a gentle nitrogen flow. Tri-Sil HTP reagent (200 μL) (ThermoFischer Scientific, Loughborough, UK) was added to the dried sample and the reaction was incubated at 80°C for 30 min. The solution was dried under nitrogen and 1 mL of hexane was used to extract sugars by sonication for 15 min. The samples were transferred to clean vials, dried and dissolved in dichloromethane (100 μL) before injection onto the GC–MS. The samples were analyzed on an Agilent 7890B GC–MS system paired with an Agilent 5977 A mass spectrometry detector (Agilent, UK), using a BPX70 column (SGE Analytical Science, Australia). Helium was used as the carrier gas. The inlet was maintained at 220°C, 12.9 psi, and 23 mL/min flow. The injection volume was 1 μL in split mode (1:20). The oven temperature increased initially from 100°C to 120°C over 5 min, followed by a second increase from 120°C to 230°C over 40 min.
Cloning, expression and purification of glycosyltransferases
For the production of recombinant GtfC53608, the coding region of gtfC53608 was amplified by PCR from the genomic DNA of L. reuteri ATCC 53608 using 0907-F and 0907-R primers (Table S2) and cloned into a pOPINF vector linearized with KpnI-HF and HindIII-HF, using the In-Fusion HD kit (Clonetech, California, USA), following the manufacturer’s instructions. The recombinant vector was used to transform E. coli BL21 (DE3). AIM medium was inoculated with an overnight culture of the recombinant clone at 1%. The fresh culture was incubated at 37°C for 3 h and then 16°C for 48 h. The cells were harvested by centrifugation at 10,000×g, resuspended in Tris buffer (Tris-HCl 50 mM, NaCl 150 mM, pH 7.5). The bacteria were lysed by 10 cycles of sonication and soluble, His6-tagged proteins were purified by immobilized metal ion affinity chromatography (IMAC). Bound proteins were eluted with Tris buffer containing 100 mM EDTA, concentrated by spin filtration, using a 10 kDa MWCO Vivaspin® Turbo 15 spin filter (Sartorious, Gottingen, Germany) and buffer-exchanged in Tris buffer using PD10 desalting columns (GE Healthcare Lifesciences, Little Chalfont, UK), following the manufacturer’s instructions. Purified recombinant GtfC100-23 produced in E. coli was a kind gift from Carl Young (Prozomix, UK).
Glycosylation of SRR1
For the glycosylation of SRR acceptor in E. coli, an artificial gtfCAB53608 operon was cloned into pETcoco™-1 (Merck, Gottingen, Germany). Briefly, primer pairs nss_F and nss_R or gtfA_F and gtfB_R (Table S2) were used together with ATCC 53608 template DNA to generate two PCR products of 1055 bp or 2905 bp, respectively. Next, equimolar amounts of these products were mixed and used as template together with the primers nss_F and gtf_R (Table S2) to generate the final 3915 bp splice PCR product. Subsequently, the NotI restricted product was cloned into pETcoco™-1 that had been restricted with SphI, treated with T4-polymerase (New England Biolabs) and subsequently cut with NotI, resulting in pETcoco_gtfCAB53608. Partial srr gene was cloned into pET-15b. Briefly, a primer pair dsrr_F and dsrr_R (Table S2) was used to amplify a 487 bp product encoding the 81-236 aa region of SRRP53608 that corresponds to the first serine-rich repeat region (SRR1) of SRRP53608. Restriction sites incorporated into the primers (Table S2) enabled the restriction with NdeI and BamHI and the subsequent ligation into pET-15b that had been restricted in the same way resulting in pET-15b_srr1. Both pETcoco_gtfCAB53608 and pET-15b_srr1 were then used to transform E. coli BL21 (DE3). Induction of the expression and purification of the His-tagged SRR1 were performed as described above for GtfC53608.
Differential scanning fluorimetry (DSF)
DSF was used to assess glycosyltransferase – sugar donor interactions by measuring changes in the melting temperature (Tm) of the protein upon interaction with sugar nucleotides. The reactions were set up at a final volume of 20 μL in Tris-HCl 50 mM, pH 7.5. Proteins were used at a final concentration of 10 μM and SYPRO Orange (ThermoFischer Scientific, UK), the fluorescent dye used in the assay was used at 5× final concentration. Ligand and ion concentration ranged from 0 to 50 mM. To measure the effect of divalent ions on the protein–ligand interaction, sugar donors were used at 4 mM and divalent ions at 5 mM. The reactions were initially kept at 10°C for 10 min and then the temperature increased in a step-wise manner, with increments of 0.5°C every 15 s, up to 90°C. Measurement of the fluorescence was taken every 15 s on a Real-Time PCR Detection System (Bio-Rad CFX96 Touch™). The results were analyzed using CFX Manager 3.5 (Bio-Rad, UK).
Saturation Transfer Difference (STD) NMR experiments
Proteins were exchanged using an Amicon centrifuge filter unit with a 3 kDa MW cutoff in 20 mM d19-2,2-bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol pH 7.4 (uncorrected for the deuterium isotope effect on the pH glass electrode) and 50 mM NaCl. Ligands (UDP, UDP-GlcNAc, UDP-Glc, UDP-Gal) were dissolved in 20 mM d19-2,2-bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol pH 7.4, 50 mM NaCl. The final ligand concentration was measured using 4,4-dimethyl-4-silapentane-1-sulfonic acid as an internal standard of known concentration. The protein concentration in the NMR tube (volume 500 μL) was 28 μM for GtfC100-23 and 21 μM for GtfC53608. Ligands were used in concentrations ranging from 0.3 to 3.5 mM. The STD NMR spectra were performed on a Bruker Avance 500 MHz at 298 K following published methodology (Mayer and Meyer 1999). The on- and off-resonance spectra were acquired using a train of 50 ms Gaussian selective saturation pulses at a fixed saturation time of 2 s (for KD determination) or variable saturation time from 0.5 s to 4 s (for binding epitope mapping determination). The water signal was suppressed by using the WATERGATE technique as described in Piotto et al. (1992) while the remaining protein resonances were filtered using a T2 filter of 40 ms. The selective on-resonance irradiation was performed at 0.7 ppm while the off-resonance irradiation was performed at 40 ppm. The spectra were performed with a spectral width of 5 KHz and 32768 data points. For determination of apparent KD, the spectra were collected with either 32 or 64 scans and 8 dummy scans at 2 s saturation time, while for the binding epitope mapping the spectra were collected with 512 scans, 8 dummy scans and a 4 s relaxation delay for all the spectra. For each ligand interacting with GtfC100-23 or GtfC53608, the STD build up curve was obtained and the STD0 parameter (STD factor at time 0) was used to derive the binding epitope. STD0 was obtained by fitting the build-up curve data to the equation STD(tsat) = STDmax * (1–exp(–ksat * tsat)) where the STD0 factor is calculated by STDmax * ksat = STD0. For each proton STD0 factors were normalized to the highest STD0 within each ligand, and expressed as relative STD0(%) so that the binding epitope mappings could be derived.
Sugar nucleotide profiling by liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS)
L. reuteri strains 100-23C and ATCC 53608 were grown in 1 l MRS until OD600 reached ~1.0, harvested by centrifugation at 10,000×g for 10 min, washed three times in ice-cold PBS, and resuspended in 70% ethanol. UDP-GlcA (1.6 nmol/g wet pellet) was added to the suspension as an internal standard. Cells were then lysed for five cycles of 50 s each using 100 μm long glass beads on a FastPrep®-24 homogenizer (MP Biomedicals, UK). Cells were kept on ice for 2 min between cycles. After centrifugation at 10,000 ×g for 20 min, the supernatant was recovered and ethanol was evaporated under a stream of nitrogen. The aqueous residue was freeze-dried and contaminating lipids were extracted with butan-1-ol as previously described (Turnock and Ferguson 2007). Sugar nucleotides were dissolved in ammonium bicarbonate 5 mM and extracted using ENVI-Carb cartridges as described in Rabina et al. (2001). The samples were dissolved in 50 μL formic acid (80 mM) brought to pH 9.0 with ammonia (mobile phase A) and analyzed on a surface-conditioned porous graphitic carbon (PGC) column (Hypercarb™, 100 × 1 mm, 5 μm; ThermoFischer. Loughborough, UK) with detection by tandem quadrupole mass spectrometer in electrospray ionization mode (ESI-MS/MS) (Pabst et al. 2010), using Xevo TQ-S coupled to an Acquity UPLC (Waters, Elstree, UK), as described previously (Rejzek et al. 2017). Available sugar nucleotide standards (10 μM) were injected (5 μL) to determine retention times. The mass spectrometer was operated in multiple reaction monitoring (MRM) mode. MRM transitions for sugar nucleotide standards were generated using IntelliStart software as described in Rejzek et al. (2017). For generic groups (e.g., UDP-N-acetylhexosamines, UDP-HexNAc) or where authentic standard was not available (UDP-N-acetylmuramic acid, UDP-MurNAc) predicted MRM functions were generated (Turnock and Ferguson 2007) (Supplementary Table S1). MassLynx software (Waters) was used to collect, to analyze and to process data. When needed, co-injection of samples with standards was used to further confirm analyte identification. Analysis of three biological replicates was performed. To ensure reproducible retention times, the Hypercarb PGC column was freshly regenerated before the analysis, as described in Supplemental methods.
Supplementary Material
Acknowledgements
We would like to acknowledge Carl Young (Prozomix) for providing GtfC100-23 and Dr. Gerhard Saalbach for assistance with proteomics.
Abbreviations
- AST, alanine-serine-threonine; BR, binding region; DSF, differential scanning fluorimetry; GT, glycosyltransferase; IMAC, immobilized metal affinity chromatography; SM, spent media; SRRP, serine-rich repeat protein; STD, saturation transfer difference; UDP, uridine diphosphate.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programmes for The Gut Health and Food Safety (BB/J004529/1), Gut Microbes and Health (BB/R012490/1) and Understanding and Exploiting Metabolism (BB/j004561/1), the BBSRC grant BB/P010660/1 and the John Innes Foundation. D.L. acknowledges a PhD studentship with financial support from the Institute of Food Research (IFR)/Quadram Institute Bioscience (QIB) Extra.
Conflict of interest statement
None declared.
References
- Angulo J, Enriquez-Navas PM, Nieto PM. 2010. Ligand-receptor binding affinities from saturation transfer difference (STD) NMR spectroscopy: The binding isotherm of STD initial growth rates. Chem-Eur J. 16:7803–7812. [DOI] [PubMed] [Google Scholar]
- Angulo J, Langpap B, Blume A, Biet T, Meyer B, Krishna NR, Peters H, Palcic MM, Peters T. 2006. Blood group B galactosyltransferase: Insights into substrate binding from NMR experiments. J Am Chem Soc. 128:13529–13538. [DOI] [PubMed] [Google Scholar]
- Bensing BA, Gibson BW, Sullam PM. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J Bacteriol. 186:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensing BA, Seepersaud R, Yen YT, Sullam PM. 2014. Selective transport by SecA2: An expanding family of customized motor proteins. Biochim Biophys Acta. 1843:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensing BA, Sullam PM. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Molecular Microbiol. 44:1081–1094. [DOI] [PubMed] [Google Scholar]
- Chaze T, Guillot A, Valot B, Langella O, Chamot-Rooke J, Di Guilmi A-M, Trieu-Cuot P, Dramsi S, Mistou M-Y. 2014. O-glycosylation of the N-terminal region of the serine-rich adhesin Srr1 of Streptococcus agalactiae explored by mass spectrometry. Mol Cell Proteomics. 13:2168–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bensing BA, Seepersaud R, Mi W, Liao M, Jeffrey PD, Shajahan A, Sonon RN, Azadi P, Sullam PM et al. 2018. Unraveling the sequence of cytosolic reactions in the export of GspB adhesin from Streptococcus gordonii. J Biol Chem. 293:5360–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Seepersaud R, Bensing BA, Sullam PM, Rapoport TA. 2016. Mechanism of a cytosolic O-glycosyltransferase essential for the synthesis of a bacterial adhesion protein. Proc Natl Acad Sci USA. 113:E1190–E1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvigny B, Lapaque N, Rigottier-Gois L, Guillot A, Chat S, Meylheuc T, Kulakauskas S, Rohde M, Mistou M-Y, Renault P et al. 2017. Three glycosylated serine-rich repeat proteins play a pivotal role in adhesion and colonization of the pioneer commensal bacterium, Streptococcus salivarius. Environ Microbiol 19:3579–3594. [DOI] [PubMed] [Google Scholar]
- Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, Pérez-Muñoz ME, Leulier F, Gänzle M, Walter J. 2017. Lifestyles in transition: Evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. 41:S27–S48. [DOI] [PubMed] [Google Scholar]
- D’Urzo N, Malito E, Biancucci M, Bottomley MJ, Maione D, Scarselli M, Martinelli M. 2012. The structure of Clostridium difficile toxin A glucosyltransferase domain bound to Mn2+ and UDP provides insights into glucosyltransferase activity and product release. FEBS J. 279:3085–3097. [DOI] [PubMed] [Google Scholar]
- Eichler J, Koomey M. 2017. Sweet new roles for protein glycosylation in prokaryotes. Trends Microbiol. 25:662–672. [DOI] [PubMed] [Google Scholar]
- Etzold S, Kober OI, Mackenzie DA, Tailford LE, Gunning AP, Walshaw J, Hemmings AM, Juge N. 2014. Structural basis for adaptation of lactobacilli to gastrointestinal mucus. Environ Microbiol. 16:888–903. [DOI] [PubMed] [Google Scholar]
- Freeze HH, Hart GW, Schnaar RL. 2017. Glycosylation precursors In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH et al., editors. Essentials of Glycobiology. New York: Cold Spring Harbor; p. 51–63. [Google Scholar]
- Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, Oh PL, Heng NCK, Patil PB, Juge N et al. 2011. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 7:e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese SA, Mackenzie DA, Peterson DA, Schmaltz R, Fangman T, Zhou Y, Zhang C, Benson AK, Cody LA, Mulholland F et al. 2013. Molecular characterization of host-specific biofilm formation in a vertebrate gut symbiont. PLoS Genet. 9:e1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y-L, Jin H, Yang H-B, Zhao R-L, Wang S, Chen Y, Zhou C-Z. 2017. Defining the enzymatic pathway for polymorphic O-glycosylation of the pneumococcal serine-rich repeat protein PsrP. J Biol Chem 292:6213–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski SF, Savage DC. 1979. Models for study of the specificity by which indigenous lactobacilli adhere to murine gastric epithelia. Infect Immun. 26:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latousakis D, Juge N. 2018. How sweet are our gut beneficial bacteria? A focus on protein glycosylation in Lactobacillus. International Journal of Molecular Sciences. 19:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, van Swam II, Tomita S, Morsomme P, Rolain T, Hols P, Kleerebezem M, Bron PA. 2014. GtfA and GtfB are both required for protein O-glycosylation in Lactobacillus plantarum. J Bacteriol. 196:1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang X, Li J, Zeng J, Zhu F, Fan W, Hu L. 2014. Both GtfA and GtfB are required for SraP glycosylation in Staphylococcus aureus. Curr Microbiol. 69:121–126. [DOI] [PubMed] [Google Scholar]
- Lizcano A, Sanchez CJ, Orihuela CJ. 2012. A role for glycosylated serine-rich repeat proteins in gram-positive bacterial pathogenesis. Mol Oral Microbiol. 27:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, Roos S, Walter J, Juge N. 2010. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology (Reading, Engl). 156:3368–3378. [DOI] [PubMed] [Google Scholar]
- Mayer M, Meyer B. 1999. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew Chem Int Edit. 38:1784–1788. [DOI] [PubMed] [Google Scholar]
- Oh PL, Benson AK, Peterson DA, Patil PB, Moriyama EN, Roos S, Walter J. 2010. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. ISME J. 4:377–387. [DOI] [PubMed] [Google Scholar]
- Pabst M, Grass J, Fischl R, Leonard R, Jin CS, Hinterkorner G, Borth N, Altmann F. 2010. Nucleotide and nucleotide sugar analysis by liquid chromatography-electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal Chem. 82:9782–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. 1992. Gradient-tailored excitation for single-quantum NMR-spectroscopy of aqueous solutions. J Biomol NMR. 2:661–665. [DOI] [PubMed] [Google Scholar]
- Rabina J, Maki M, Savilahti EM, Jarvinen N, Penttila L, Renkonen R. 2001. Analysis of nucleotide sugars from cell lysates by ion-pair solid-phase extraction and reversed-phase high-performance liquid chromatography. Glycoconj J. 18:799–805. [DOI] [PubMed] [Google Scholar]
- Rejzek M, Hill L, Hems ES, Kuhaudomlarp S, Wagstaff BA, Field RA. 2017. Profiling of sugar nucleotides. Method Enzymol. 597:209–238. [DOI] [PubMed] [Google Scholar]
- Rigel NW, Braunstein M. 2008. A new twist on an old pathway – accessory secretion systems. Mol Microbiol. 69:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PWM, Orihuela CJ. 2010. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog. 6:e1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer C, Messner P. 2017. Emerging facets of prokaryotic glycosylation. FEMS Microbiol Rev. 41:49–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seepersaud R, Bensing BA, Yen YT, Sullam PM. 2012. The accessory Sec protein Asp2 modulates GlcNAc deposition onto the serine-rich repeat glycoprotein GspB. J Bacteriol. 194:5564–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seepersaud R, Sychantha D, Bensing BA, Clarke AJ, Sullam PM. 2017. O-acetylation of the serine-rich repeat glycoprotein GspB is coordinated with accessory Sec transport. PLoS Pathog. 13:e1006558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Xiong YQ, Sullam PM. 2013. Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis. PLoS One. 8:e64204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira S, Kavanaugh D, MacKenzie DA, Šuligoj T, Walpole S, Leclaire C, Gunning AP, Latousakis D, Willats WGT, Angulo J et al. 2018. Structural basis for the role of serine-rich repeat proteins from Lactobacillus reuteri in gut microbe–host interactions. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed]
- Shi W-W, Jiang Y-L, Zhu F, Yang Y-H, Shao Q-Y, Yang H-B, Ren Y-M, Wu H, Chen Y, Zhou C-Z. 2014. Structure of a novel O-linked N-acetyl-D-glucosamine (O-GlcNAc) transferase, GtfA, reveals insights into the glycosylation of pneumococcal serine-rich repeat adhesins. J Biol Chem. 289:20898–20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. 2009. The Streptococcus pneumoniae adhesin PsrP binds to Keratin 10 on lung cells. Mol Microbiol. 73:663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siboo IR, Chaffin DO, Rubens CE, Sullam PM. 2008. Characterization of the accessory Sec system of Staphylococcus aureus. J Bacteriol. 190:6188–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu D, Bensing BA, Sullam PM. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J Bacteriol. 186:7100–7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock DC, Ferguson MAJ. 2007. Sugar nucleotide pools of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Eukaryot Cell. 6:1450–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat HLP, de Vos WM. 2016. Sugar coating the envelope: Glycoconjugates for microbe-host crosstalk. Trends Microbiol. 24:853–861. [DOI] [PubMed] [Google Scholar]
- Wagstaff BA, Rejzek M, Kuhaudomlarp S, Hill L, Mascia I, Nepogodiev SA, Field RA. 2018. Discovery of an RmlC/D fusion protein in the microalga, Prymnesium parvum, and implications for NDP-β-L-rhamnose biosynthesis among microalgae. J Biol Chem, submitted. [DOI] [PMC free article] [PubMed]
- Wegmann U, MacKenzie DA, Zheng J, Goesmann A, Roos S, Swarbreck D, Walter J, Crossman LC, Juge N. 2015. The pan-genome of Lactobacillus reuteri strains originating from the pig gastrointestinal tract. BMC Genomics. 16:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Mintz KP, Ladha M, Fives-Taylor PM. 1998. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol. 28:487–500. [DOI] [PubMed] [Google Scholar]
- Wu R, Wu H. 2011. A molecular chaperone mediates a two-protein enzyme complex and glycosylation of serine-rich streptococcal adhesins. J Biol Chem. 286:34923–34931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen YT, Seepersaud R, Bensing BA, Sullam PM. 2011. Asp2 and Asp3 interact directly with GspB, the export substrate of the Streptococcus gordonii accessory Sec System. J Bacteriol. 193:3165–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu F, Yang T, Ding L, Zhou M, Li J, Haslam SM, Dell A, Erlandsen H, Wu H. 2014. The highly conserved domain of unknown function 1792 has a distinct glycosyltransferase fold. Nat Commun. 5:4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology (Reading, Engl). 155:317–327. [DOI] [PubMed] [Google Scholar]
- Zhu F, Erlandsen H, Ding L, Li J, Huang Y, Zhou M, Liang X, Ma J, Wu H. 2011. Structural and functional analysis of a new subfamily of glycosyltransferases required for glycosylation of serine-rich streptococcal adhesins. J Biol Chem. 286:27048–27057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang H, Yang T, Haslam SM, Dell A, Wu H. 2016. Engineering and dissecting the glycosylation pathway of a streptococcal serine-rich repeat adhesin. J Biol Chem. 291:27354–27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.