Abstract
Background:
Short Bowel Syndrome (SBS) results from extensive bowel resection. Patients with SBS require Total Parenteral Nutrition (TPN) for survival. Understanding mechanisms contributing to TPN-associated liver injury and gut atrophy are critical in developing SBS therapies. Existing SBS models using tethered animals have significant limitations and are unlike ambulatory human SBS patients. We hypothesized that we could induce SBS in piglets and develop an ambulatory TPN-SBS model.
Material and Methods:
18 neonatal pigs received duodenal and jugular catheters. They were fitted with a jacket holding TPN and a miniaturized pump. 6 piglets had 90% small bowel resection and catheter placement (SBS group). Non-SBS piglets were randomized into enteral nutrition (EN) or TPN.
Results:
Bowel resection was successfully accomplished in SBS animals. Weight gain was similar in all groups. SBS animals had increased serum bilirubin compared to EN. Mean conjugated bilirubin ± SD was 0.045 ± 0.01 for EN, (p=0.03 EN vs TPN and p=0.03 SBS vs EN) and 1.09 ± 1.25 for TPN, (p=0.62 TPN vs SBS). Gut density was reduced in the TPN group compared to EN and SBS groups. Mean gut density ± SD was 0.11 ± 0.04 for TPN (p=0.0004 TPN vs SBS and p=0.00007 TPN vs EN) and not statistically different for EN vs SBS (p=0.32).
Conclusion:
We created a novel, ambulatory TPN-SBS model using piglets, mimicking longterm TPN delivery in human SBS patients. Our model demonstrated TPN-related conjugated hyperbilirubinemia and compensatory gut hypertrophy, as noted in humans with SBS. This model holds great potential for future research.
Keywords: Model, Animal, TPN, Short Bowel Syndrome, Ambulatory, Cholestasis, Gut atrophy
INTRODUCTION:
Short Bowel Syndrome (SBS) is an often debilitating condition that results from bowel resection usually secondary to necrotizing enterocolitis, gastroschisis, inflammatory bowel disease, ischemic injury or trauma1. Patients with SBS require Total Parenteral Nutrition (TPN) for survival2,3.
TPN is known to cause liver injury as well as significant morbidity and mortality in adult and pediatric populations4,5, the mechanisms of which remain elusive and are the major focus of ongoing research6,7.
Efforts to understand mechanistic pathways contributing to TPN associated injury as well as the development of ameliorative and preventative strategies are critical in the ongoing care for such patients8,9. A key component of such endeavors is the availability of model systems replicative of human SBS.
Testing systems using a tethered animal with indwelling catheters are not ideal due to short duration TPN therapy secondary to animal stress from a lack of free mobility10,11. Animal stress has been shown to cause alterations in hepatobiliary receptors as well as intestinal motility, ion secretion and intestinal permeability, 12. Additional concern is the viability of long term indwelling catheters, which are prone to dislodgement in traditional fixed tethered systems. Conversely, in human SBS patients, TPN can be delivered long term in an unobstructed, ambulatory manner13-15
Hypothesis:
Given this predicament, we hypothesized that we could develop an ambulatory model of short bowel syndrome utilizing neonatal pigs that undergo 90% surgical bowel resection. Such animals would be maintained on TPN, infused via secured catheters using miniaturized infusion pumps carried by the animal thus permitting completely untethered animal mobility and nutrition delivery. This would be a significant step forward in establishing a robust model to test SBS and a platform for future mechanistic studies.
METHODS AND MATERIALS:
Animal procurement:
Saint Louis University (SLU) is a registered research facility with the United States Department of Agriculture. The study was initiated upon approval by the Institutional Animal Care and Use Committee of SLU (SLU No. 2657, US Department of Agriculture registration 43-R-011) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Seven to ten day old, term neonatal pigs were used for this study. Animals were procured from an approved Class A vendor. Animals were identified by ear tags. Upon arrival they were immediately placed in heated cages.
Acclimatization and Housing:
In accordance with University guidelines, animals were acclimatized for three days upon arrival. Piglets were fed ad lib, swine milk replacer formula (LitterLife; Merrick's Inc, Middleton, WI, USA) with close monitoring of their daily intake. All animals were kept in a thermally controlled environment for the duration of the study at temperatures mandated by the facility’s veterinarian.
Surgery and Catheter Placement:
Following three days of acclimatization, the piglets underwent surgery for creation of iatrogenic short bowel syndrome and placement of both jugular and duodenal catheters. Each piglet was taken to the veterinary operating room and placed in an individual chamber containing 3% to 5% isoflurane for anesthetic induction. Subsequently the animal was transferred to a heated surgery table. Using a cone mask, appropriate anesthesia was maintained (2%-4% isoflurane). Oxygen saturation, body temperature, heart rate and the respiratory rate were continuously monitored throughout the surgical procedure. Once deeply anesthetized, the neck and abdomen were surgically prepared and draped for aseptic surgery.
Jugular Catheter Placement:
Jugular catheters were placed in the right and left jugular vein by a vascular cut-down technique. The catheters were secured to the vessel via a purse string suture. Patency was confirmed by injecting 3mL heparinized saline. The catheters were subsequently tunneled subcutaneously to exit the skin just caudal to the scapulae. The catheter was then sutured to the skin via a catheter flange. In order to limit catheter slide, medical grade silicone glue was applied to additionally secure the catheter.
Duodenal Catheterization:
A midline 5 inch abdominal incision was made cranial to the umbilicus. Placement of a catheter into the duodenal lumen was performed as previously published10. Once the catheter was secured and tunneled, we proceed with 90% mid-small bowel resection.
Creation of Short Bowel:
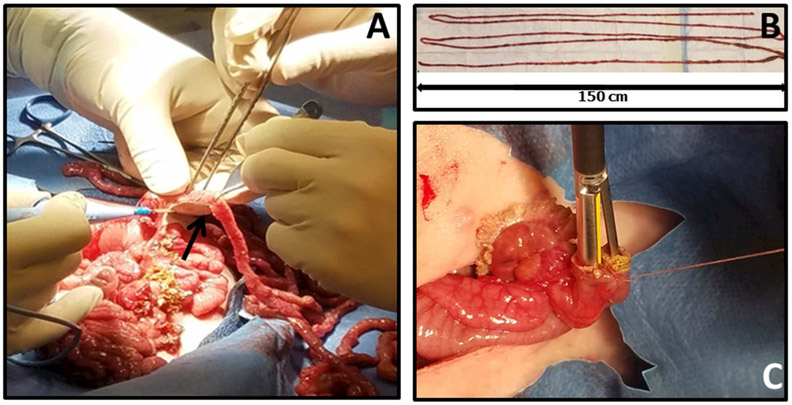
A 50 cm segment of the bowel proximal to the ileocecal valve as well as a 50cm segment distal to the ligament of Treitz was identified. We measured the bowel using sterile silk ribbon placed along the anti-mesenteric border of the gently stretched small intestine. Using doyen-clamps the proximal and distal ends were clamped to prevent gross contamination. The small bowel in between these two segments was resected using electrocautery and measured outside of the piglet (Figure 1A).
Figure 1:
(A) Electrocautery is used to obliterate the mesentery to accomplish the resection. (B) Resected bowel is measured to confirm 90% resection. (C) Jejuno-ileal side to side stapled anastomosis (Just Right® 5 mm Stapler)
Once the resected segment was freed from the mesentery, the doyen-clamped ends of each remnant bowel section (jejunum and ileum) were brought together. Stay sutures at the mesenteric and anti-mesenteric borders were used to ensure correct apposition. To ensure 90% resection we measured the entire length of resected bowel and if necessary, we made adjustments by resecting further bowel (Figure 1B). Luminal continuity of the two remnant sections was restored by jejunoileal side to side (functional end to end) stapled (Just Right® 5 mm Stapler) anastomosis (Figure 1C).
The anastomosis was copiously washed in a bowl of warm saline before being returned to the abdomen. The abdominal incision was closed in two layers with 2.0 Vicryl (absorbable; all layers of abdominal wall except skin) and staples. Piglets were administered buprenorphine (0.1 mg/kg, subcutaneously) for postoperative pain relief and monitored until full recovery.
Jacket Placement:
Animals were fitted into a jacket with pockets on both sides. An ambulatory, miniaturized battery-operated infusion pump (Orchestra 500) was placed in the right pocket. Nutrition bag tubes were placed in the left pocket. Both of the jugular catheters were sieved through the left pocket fabric. While one was heparin locked, the other was connected to the pump via a PEGA tube mechanism.
Subsequently, the TPN solution (Clinimax E and Intralipid) in EVA bags (EVA, code 66050; Medtec Medical, IL, USA) was placed in the left pocket. This was connected via cannula clamps to the tubing supplying the pump. All piglets were closely monitored by veterinary staff until fully recovered.
Animal Monitoring:
Animals were weighed each morning and examined by a veterinary doctor. Additionally, they were monitored with scheduled visits by the research personnel in accordance with the Institutional Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals.16
Group allocation and Statistical Analysis:
After 48-hour post-surgery recovery, 18 animals were randomly assigned to three experimental groups (Table 1). The EN animal group (n=6) received enteral nutrition via the duodenal catheter and no TPN. The TPN animal group (n=6) received no enteral nutrition but were provided TPN via the jugular catheter. The SBS animal group (n=6) underwent 90% bowel resection and received TPN via the jugular catheter but no enteral nutrition. Mean and standard deviation were calculated for each group for the weight gain, serum bilirubin and the gut density. The student’s t test was used for statistical analysis. All tests were 2 sided using a statistical significance level of 0.05.
Table 1:
Group Allocation
| Group | Assigned Treatment |
|---|---|
| EN Group | Animal with catheter placements and abdominal incision but no bowel resection. Animals received regular enteral nutrition but no TPN. |
| TPN Group | Animal with catheter placements and abdominal incision but no bowel resection. Animals received TPN but no enteral nutrition. |
| SBS Group | Animal with catheter placements and abdominal incision with 90% bowel resection. Animals received TPN but no enteral nutrition. |
Nutrition Intake:
Isocaloric and isonitrogenous nutrition was provided to all animals for a period of approximately 3 weeks. The EN group received the swine replacement formula LitterLife (Merrick's Inc). Enteral nutrition was delivered via the duodenal catheter at a rate of 260 mL kg–1 d–1 with 25 g/kg lactose, 12.4 g/kg protein, and 5 g/kg fat along with electrolytes, trace minerals, and vitamins, for a total of 187 kcal kg–1 d–1.
Total parenteral nutrition piglets received the commercially available parenteral nutrition preparation (Clinimix E; Baxter, IL, USA;) via the jugular catheter. This provided fluids at 260 mL kg–1 d–1 with 26 g/kg dextrose, 11.05 g/kg protein, and 5 g/kg fat along with electrolytes, trace minerals, and vitamins, for a total of 182 kcal kg–1 d–1 via the jugular catheter.
The TPN or EN was placed in nutrition bags (EVA, product code 66050; Medtec Medical) with nutritional constituents as seen in table 2. All nutrition bags were replaced every 12 hours using aseptic precautions.
Table 2:
Nutritional Constituents
| Total Parenteral Nutrition | Enteral Nutrition |
|---|---|
| Ingredients: Leucine, Isoleucine, Valine, Lysine, Phenylalamine, Histidine, Threonine, Methionine, tryptophan, Alanine, Arginine, Glycine, Proline, Serine, Tyrosine, Sodium, Potassium, Magnesium, Calcium, Acetate, Chloride, Phosphate, Dextrose) and Intralipid (Fresenius Kabi, Germany, Ingredients: Soybean Oil, Egg Yolk Phospholipids, Glycerin and Water. | Ingredients: Dried Whey Protein Concentrate, Animal Plasma, Animal and Vegetable Fat preserved with BHA, Dried Lactose, Lecithin, Dicalcium Phosphate, Magnesium Sulfate, Manganese Sulfate, Ferrous Sulfate, Zinc Sulfate, Cobalt Sulfate, Copper Sulfate, Calcium Iodate, Sodium Selenite, Vitamin A Acetate, d-Activated Animal Sterol, Vitamin E Supplement, Menadione Dimethylpyrimidinol Bisulfite, Choline Chloride, Riboflavin Supplement, Calcium Pantothenate, Niacin Supplement, Vitamin B12 Supplement, Biotin, Ascorbic Acid, Yucca Schidigera Extract, Natural and Artificial Flavors. |
Animal Euthanasia and Sample Collection:
Blood samples were obtained for conjugated bilirubin prior to administration of euthanasia. Piglets were euthanized using a pentobarbital sodium (100 mg/kg)–based euthanasia solution (Beuthanasia-D; Schering-Plough Animal Health, Kenilworth, NJ, USA) delivered intravenously. Immediately after euthanasia, the abdomen was opened and the liver was procured in its entirety. The proximal and distal segments were identified based on suture location and removed in their entirety. Removed bowel from all groups was immediately flushed with cold saline and weighed.
RESULTS:
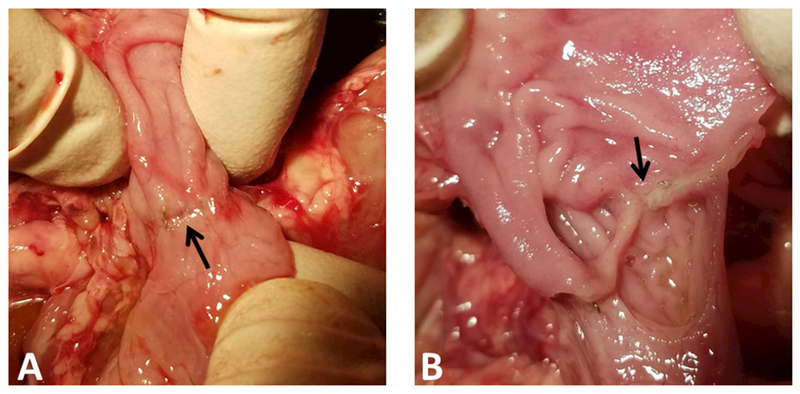
A total of 18 piglets were implanted with duodenal and jugular vein catheters. At the time of euthanasia we inspected all bowel anastomosis in SBS animals (Figure 2A), which remained intact (Figure 2B).
Figure 2:
(A) Intact anastomosis on serosal surface (arrow marks staples). (B) Anastomosis has been divided, intact mucosa is visualized.
Weight gain:
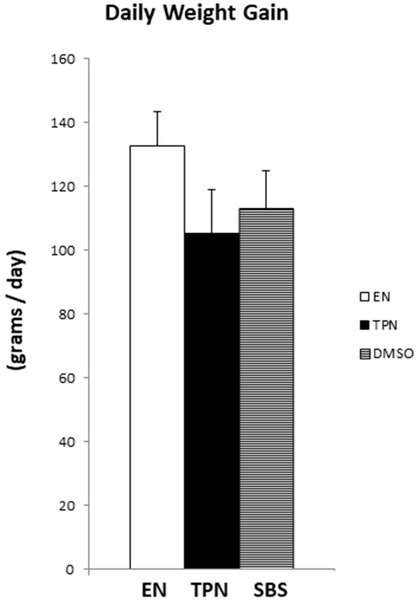
An indicator of adequate caloric intake is weight gain. We evaluated the weight of each animal daily. The daily weight gain was similar for all groups with no statistically significant differences, verifying adequate caloric support using ambulatory pump for nutrition (Figure 3). The mean daily weight gain ± SD was 132.89 ± 29.73 for EN, (p=0.06 EN vs TPN); 105.26 ± 13.72 for TPN, (p=0.31 TPN vs SBS) and 113.15 ± 11.88 for SBS, (p=0.15 SBS vs EN).
Figure 3:
Daily weight gain in each group. Each column reflects the mean for that group. Error bars represent standard error. Differences between groups are based on the T test. All test were 2 sided using a significance level of 0.05. Note: No difference in daily weight gain between the groups.
Conjugated Hyperbilirubinemia:
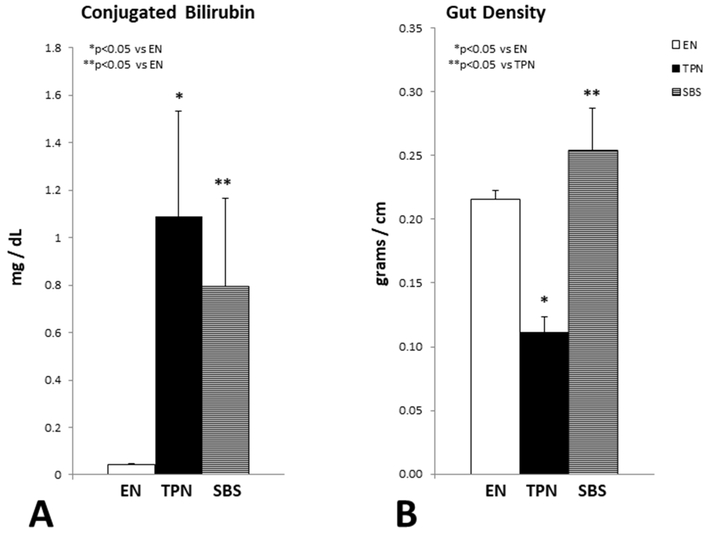
Increased conjugated bilirubin level is characteristically noted with TPN therapy. We measured the direct bilirubin fraction prior to animal euthanasia at the completion of the study. As expected, animals on TPN had significant conjugated hyperbilirubinemia in comparison to the EN animal group. The SBS animal group also had significant elevation of serum conjugated bilirubin (Figure 4A).
Figure 4.
(A): Serum conjugated bilirubin. Each column reflects the mean for that group. Error bars represent standard error. Differences between groups are based on the T test. All test were 2 sided using a significance level of 0.05. Note: Significant elevation of bilirubin in the TPN and SBS group vs EN. No statistical differences in serum bilirubin were noted between TPN and SBS. (B) Gut density as measured in grams per centimeter. Each column reflects the mean for that group. Error bars represent standard error. Differences between groups are based on the T test. All test were 2 sided using a significance level of 0.05. Note: Significant reduction of gut density with TPN vs EN. SBS had the highest gut density, likely secondary to gut adaptation.
The mean conjugated bilirubin level ± SD was 0.045 ± 0.01 mg/dL for EN, (p=0.03 EN vs TPN); 1.09 ± 1.25 mg/dL for TPN, (p=0.62 TPN vs SBS) and 0.79 ± 0.09 mg/dL for SBS, (p=0.03 SBS vs EN). We also measured hepatic cholestatic deposits. While the cholestatic deposits were higher in animals on TPN, this did not reach statistical significance (p=0.87).
Gut Density:
We have previously published gut atrophic changes with TPN2. To further quantify the atrophic changes we determined the density of the gut as a measure of weight in grams per centimeter of bowel length. We noted a significant reduction in gut density in TPN animals compared to those on EN. Paradoxically, animals with SBS, despite being on TPN had higher gut density compared to their TPN counterparts with intact bowel. Additionally, there was no statistical difference in the gut density between EN and SBS animals (Figure 4B). The mean gut density ± SD was 0.22 ± 0.02 gm/cm for EN, (p=0.00007 EN vs TPN); 0.11 ± 0.04 gm/cm for TPN, (p=0.0004 TPN vs SBS) and 0.25 ± 0.08 gm/cm for SBS, (p=0.32 SBS vs EN).
DISCUSSION:
Worldwide, every year a large number of patients undergo bowel resection for several disorders, including necrotizing enterocolitis, volvulus, inflammatory bowel disease (IBD), malignancy, mesenteric ischemia, gastroschisis, trauma and others17,18. Significant bowel resection compromises digestive and absorptive processes impairing proper nutritional status. These patients thus need nutritional support via the intravenous route – a modality called total parenteral nutrition (TPN)19,20.
Since TPN associated liver injury21 can lead to significant morbidity and mortality, weaning from TPN remains a major goal for such patients22,23. While significant research is focused in finding preventative or ameliorative strategies to manage the complications associated with chronic TPN, a major limitation in moving the field forward is the lack of a robust SBS animal model replicating human ambulatory TPN infusion. This is a critical step for translational studies.
We hypothesized that we could create an ambulatory SBS piglet model with miniaturized pumps carried by the animal. We developed surgical techniques to resect 90% bowel in neonatal pigls and achieve end to end anastomosis. We were also able to introduce intra-duodenal as well as jugular catheters sieved subcutaneously to exit at the animals dorsal with minimal complications. Subsequently, using miniaturized ambulatory pumps we were able to successfully deliver TPN to these animals and such placement facilitated animal mobility.
We demonstrated equal weight gain in short bowel animals compared to animals without bowel resection on TPN as well as those on enteral nutrition indicating adequate nutrition delivery with our new model.
A key component of liver injury that occurs in patients with SBS on TPN is jaundice which is a clinical manifestation of hyperbilirubinemia24. Validating this aspect of hepatocellular injury, we noted significantly higher conjugated bilirubin level in animals on TPN.
Recent evidence also points to atrophic changes in the gut while on TPN. This is believed to occur due to a lack of trophic signals secondary to a lack of luminal nutrients2,5. As expected animals on TPN had gut atrophic changes as documented by a reduction in the gut density. Interestingly, the gut density in animals with SBS was higher. We speculate that this response was due to gut adaptation secondary to the extensive bowel resection and mechanisms driving such gut growth would be an important research area.
Our model demonstrated that the establishment of an ambulatory SBS model with TPN is feasible and replicates the physiopathology of SBS in humans. This model is promising in many ways since it may allow experimental evaluation of the gut and liver alterations with SBS, as well as could prove critical in the development of novel therapies for SBS.
Emerging data supports the use of bile acids25 to ameliorate TPN associated liver injury via activation of gut derived signals which modulate the gut-liver crosstalk2,26,27. An important use of our model would be therapeutic drug testing, as well as to further define mechanistic links mediating such crosstalk. Given its ambulatory nature, our SBS model poses minimal animal stress in comparison to the tethered animal models and thus long term effects of TPN infusion could be ascertained and tested.
CONCLUSION:
We created a successful short bowel syndrome animal model using neonatal pigs and delivered TPN using miniaturized pumps in an ambulatory fashion. We believe this model recapitulates long-term ambulatory TPN delivery in human SBS patients. Our TPN-SBS model, with significant bowel resection shows the classical hyperbilirubinemia as well as compensatory gut hypertrophy noted with significant bowel resection and thus validates key injury elements noted in human short bowel syndrome. This model holds great potential for translational research supporting drug development and in our understanding of SBS as well as complications of TPN.
Acknowledgments
FINANCIAL SUPPORT FOR MANUSCRIPT PREPARATION:
None
GRANT FUNDING:
AKJ received funding from the National Institute of Health (NIH) under grant number K08DK098623-01. Additional funding was provided to GV and AKJ via the Saint Louis University internal grants.
ABBREVIATIONS:
- TPN
Total Parenteral Nutrition
- JV
Jugular Vein
- DC
Duodenal Catheters
- USDA
United States Department of Agriculture
Footnotes
Conflict of Interest:
AKJ serves as a consultant and speaker for Alexion Pharmaceuticals; however this association is not relevantto the current manuscript.
“The authors declare that there is no conflict of interest regarding the publication of this paper.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Sigalet DL. Short bowel syndrome in infants and children: an overview. Seminars in pediatric surgery. 2001;10(2):49–55. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. American journal of physiology Gastrointestinal and liver physiology. 2012;302(2):G218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharadwaj S, Gohel T, Deen OJ, DeChicco R, Shatnawei A. Fish oil-based lipid emulsion: current updates on a promising novel therapy for the management of parenteral nutrition-associated liver disease. Gastroenterology report. 2015;3(2):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tazuke Y, Wildhaber BE, Yang H, Washburn J, Teitelbaum DH. Total parenteral nutrition leads to alteration of hepatocyte cell cycle gene expression and proliferation in the mouse. Digestive diseases and sciences. 2007;52(4):920–930. [DOI] [PubMed] [Google Scholar]
- 5.Kumar JA, Teckman JH. Controversies in the Mechanism of Total Parenteral Nutrition Induced Pathology. Children (Basel). 2015;2(3):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlaardingerbroek H, Ng K, Stoll B, et al. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. Journal of lipid research. 2014;55(3):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nature clinical practice Gastroenterology & hepatology. 2007;4(5):277–287. [DOI] [PubMed] [Google Scholar]
- 8.Wales PW, Allen N, Worthington P, et al. A.S.P.E.N. Clinical Guidelines: Support of Pediatric Patients With Intestinal Failure at Risk of Parenteral Nutrition-Associated Liver Disease. JPEN Journal of parenteral and enteral nutrition. 2014;38(5):538–557. [DOI] [PubMed] [Google Scholar]
- 9.Platell CF, Coster J, McCauley RD, Hall JC. The management of patients with the short bowel syndrome. World journal of gastroenterology : WJG. 2002;8(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain AK, Wen JX, Arora S, et al. Validating hyperbilirubinemia and gut mucosal atrophy with a novel ultramobile ambulatory total parenteral nutrition piglet model. Nutrition research. 2015;35(2):169–174. [DOI] [PubMed] [Google Scholar]
- 11.van der Staay FJ, Schuurman T, Hulst M, et al. Effects of chronic stress: a comparison between tethered and loose sows. Physiol Behav 2010;100(2):154–164. [DOI] [PubMed] [Google Scholar]
- 12.Caso JR, Leza JC, Menchen L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Curr Mol Med 2008;8(4):299–312. [DOI] [PubMed] [Google Scholar]
- 13.Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol 2011;17(4):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofstetter S, Stern L, Willet J. Key issues in addressing the clinical and humanistic burden of short bowel syndrome in the US. Curr Med Res Opin 2013;29(5):495–504. [DOI] [PubMed] [Google Scholar]
- 15.Matarese LE. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN Journal of parenteral and enteral nutrition. 2013;37(2):161–170. [DOI] [PubMed] [Google Scholar]
- 16.Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC)2011. [Google Scholar]
- 17.Choi SJ, Lee KJ, Choi JS, et al. Poor Prognostic Factors in Patients with Parenteral Nutrition-Dependent Pediatric Intestinal Failure. Pediatr Gastroenterol Hepatol Nutr 2016;19(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganousse-Mazeron S, Lacaille F, Colomb-Jung V, et al. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clinical nutrition. 2015;34(3):428–435. [DOI] [PubMed] [Google Scholar]
- 19.Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr 2009;61(3):283–291. [PubMed] [Google Scholar]
- 20.Dudrick SJ, Palesty JA. Historical highlights of the development of total parenteral nutrition. Surg Clin North Am 2011;91(3):693–717. [DOI] [PubMed] [Google Scholar]
- 21.Kelly DA. Preventing parenteral nutrition liver disease. Early human development. 2010;86(11):683–687. [DOI] [PubMed] [Google Scholar]
- 22.Cowles RA, Ventura KA, Martinez M, et al. Reversal of intestinal failure-associated liver disease in infants and children on parenteral nutrition: experience with 93 patients at a referral center for intestinal rehabilitation. Journal of pediatric surgery. 2010;45(1):84–87; discussion 87–88. [DOI] [PubMed] [Google Scholar]
- 23.Nucci A, Burns RC, Armah T, et al. Interdisciplinary management of pediatric intestinal failure: a 10-year review of rehabilitation and transplantation. J Gastrointest Surg 2008;12(3):429–435; discussion 435–426. [DOI] [PubMed] [Google Scholar]
- 24.Alkharfy TM, Ba-Abbad R, Hadi A, Sobaih BH, AlFaleh KM. Total parenteral nutrition-associated cholestasis and risk factors in preterm infants. Saudi J Gastroenterol 2014;20(5):293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliewer SA, Mangelsdorf DJ. Bile Acids as Hormones: The FXR-FGF15/19 Pathway. Digestive diseases. 2015;33(3):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boesjes M, Brufau G. Metabolic effects of bile acids in the gut in health and disease. Current medicinal chemistry. 2014;21(24):2822–2829. [DOI] [PubMed] [Google Scholar]
- 27.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46(4):302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]