Because of advances in health care and an aging population, the number of cancer survivors is increasing. How to best identify and address the needs of cancer survivors remains undefined. This article explores distinct subgroups of patients with similar needs and presents a cluster analysis based on self‐reported care needs among cancer survivors.
Keywords: Survivors, Health services research, Needs assessment, Health services needs and demand, Patient care planning
Abstract
Background.
In efforts to inform clinical screening and development of survivorship care services, we sought to characterize patterns of health care needs among cancer survivors by (a) identifying and characterizing subgroups based on self‐reported health care needs and (b) assessing sociodemographic, clinical, and psychosocial factors associated with these subgroups.
Methods.
We conducted a cross‐sectional self‐administered survey among patients presenting for routine follow‐up care for early‐stage cancer at our academic medical center. Latent class cluster analysis was used to identify clusters of survivors based on survivorship care needs within seven domains. Multiple logistic regression analyses were used to assess factors associated with these clusters.
Results.
Among 292 respondents, the highest unmet needs were related to the domains of side effects (53%), self‐care (51%), and emotional coping (43%). Our analysis identified four clusters of survivors: (a) low needs (n = 123, 42%), (b) mainly physical needs (n = 46, 16%), (c) mainly psychological needs (n = 57, 20%), and (d) both physical and psychological needs (n = 66, 23%). Compared with cluster 1, those in clusters 2, 3, and 4 were younger (p < .03), those in clusters 3 and 4 had higher levels of psychological distress (p < .05), and those in clusters 2 and 4 reported higher levels of fatigue (p < .05).
Conclusion.
Unmet needs among cancer survivors are prevalent; however, a substantial group of survivors report low or no health care needs. The wide variation in health care needs among cancer survivors suggests a need to screen all patients, followed by tailored interventions in clinical care delivery and research.
Implications for Practice.
The characterization of patients as having few needs, predominantly physical needs, predominantly psychological needs, or substantial needs that are both physical and psychological provides a productive framework for clinical care of cancer survivors and to guide further research in this field. Further research is needed to define the tailored information and services appropriate for each group of patients and to define optimal screening tools to efficiently identify the needs of individuals in oncology practice.
Introduction
Because of advances in health care and aging of the population, the number of cancer survivors is increasing. In the U.S., there were approximately 15.5 million cancer survivors in 2016, and this number is expected to exceed 20.3 million by 2026 [1]. This growing group of cancer survivors often faces physical and psychosocial issues as a result of cancer and cancer treatment [2]. Previous work has found that survivors have unmet needs regarding management of persistent complications from cancer therapy, practical assistance in daily living, and help with psychosocial problems such as anxiety and fear of recurrence [3], [4], [5]. However, the level and type of needs differ across survivor populations and settings [6]. Identifying needs clusters, as opposed to focusing only on distinct individual symptoms and problems, may provide insight into the challenges faced by cancer survivors and the best way to address them.
Over the past decades, the need to identify and address health concerns for patients who are expected to live years beyond initial therapy for cancer (defined as cancer survivors, for purposes of this article) has been well described [7], [8]. In the American Institute of Medicine's report, From Cancer Patient to Cancer Survivor: Lost in Translation, Survivorship Care Plans (SCPs) containing a treatment summary and follow‐up care plan were advocated as an available and efficient means to meet the needs of cancer survivors [7]. Although the face validity of SCPs seemed acceptable, several randomized controlled trials have failed to identify beneficial effects of SCPs in various study populations [9], [10], [11], [12]. In addition, the provision of SCPs has shown to be more resource intensive than anticipated, resulting in low implementation and dissemination of SCPs in clinical practice [13], [14], [15]. However, SCPs may be beneficial for subgroups of patients, particularly underserved populations [16]. Consequently, debates on how best to address the needs of cancer survivors persist [6], [17], [18].
The optimal method to effectively and efficiently identify and address the needs of cancer survivors remains undefined. Because cancer survivors constitute a heterogeneous population with different cancer types, treatment plans, ongoing and maintenance therapy, long‐term health risks, and individual health care needs and preferences, we sought to determine if distinct subgroups of patients could be identified with similar needs that might be addressed through select group interventions and services. In this study, we present a cluster analysis based on individual self‐reported care needs among cancer survivors that aims to (a) characterize subgroups of patients with similar patterns of health care needs and (b) assess the sociodemographic, clinical, and psychosocial factors associated with these subgroups.
Materials and Methods
Study Design
We conducted a cross‐sectional, self‐administered, comprehensive needs assessment survey. All English‐speaking adult patients with a history of cancer presenting for follow‐up care to the Massachusetts General Hospital Cancer Center between February and July of 2016 were eligible to participate. Only English questionnaires were used because of our pragmatic method of survey distribution. Surveys were provided by clinicians in clinic, research assistants in the waiting rooms, and clinic staff at the check‐in desk across multiple disease‐specific clinics and two affiliated community oncology practices. Because of the pragmatic method of survey distribution, the number of patients offered or eligible for the survey cannot be assessed. The Dana‐Farber/Harvard Cancer Center institutional review board approved the study.
Study Population
A total of 636 patients participated in the survey. For the purposes of this analysis, we included only patients participating in the cancer‐center‐wide survey who self‐identified as having no evidence of distant metastases and who were (a) on therapy with curative intent within 2 years of cancer diagnosis, (b) on adjuvant endocrine therapy, or (c) off therapy in long‐term follow‐up. Patients reporting incurable cancer, or reporting curable cancer but currently receiving radiation therapy or chemotherapy more than 2 years from the time of diagnosis (indicating potential stage IV disease), were excluded (n = 298). In addition, patients with early‐stage disease who did not complete the survivorship care needs assessment (n = 46) were excluded from the current analysis.
Measures
Sociodemographic variables included age, gender, race, marital status, employment, internet access, educational level, and income. Clinical variables included cancer type category, years since diagnosis, treatment (both current and previous), and number of listed comorbidities.
Survivorship Care Needs
The comprehensive needs assessment survey was developed by a multidisciplinary team of health services researchers, oncologists, psychologists, nurses, and primary care physicians with input from patients and family members of patients. The survey included 66 study‐specific items on information to help survivors cope with their cancer or treatments, subdivided into seven domains: side effects, lifestyle and self‐care, emotional coping, social support, sexual health, complementary services, and practical support. Participants were asked to report the concern (“I do have this problem”) and to indicate their interest in receiving information to help them cope with this concern (“I have enough information,” “Not at all interested,” “A little interested,” and “Very interested”). All items were dichotomized into needs (“A little interested” and “Very interested) versus no needs (no concern indicated, “I have enough information,” and “Not at all interested”). For each of the seven domains, a dichotomous scale was computed based on needs in domain (needs on at least one item in domain) versus no needs in domain. Internal consistency of the domain scales in our sample was good (Cronbach's alpha: side effects, 0.89; emotional coping, 0.88; social support, 0.88; lifestyle and self‐care, 0.77; sexual health, 0.75; complementary services, 0.91; practical support, 0.74).
Emotional and Physical Symptoms
Fear of recurrence was measured using the revised five‐item Assessment of Survivor Concerns [19]. Internal consistency of the scale in our sample was good (Cronbach's alpha, 0.88). Depression, anxiety, insomnia, pain, and fatigue were single‐item scales based on a modified version of the FACT‐G [20]. Items were measured on a five‐point Likert frequency scale (“never,” “rarely,” “sometimes,” “very often,” and “always”) and included depression (“I feel sad or depressed”), anxiety (“I feel nervous or worried”), insomnia (“I have problems falling or staying asleep”), pain (“I have pain”), and fatigue (“my fatigue interferes with my usual activities”). Higher scores indicated greater problems.
Statistical Analysis
Latent class cluster analysis was conducted to identify clusters of cancer survivors based on survivorship care needs across seven domains. Latent class modeling is a data‐driven approach that aims to classify similar objects, with respect to a set of variables, into mutually exclusive groups [21]. Variables used to define the need clusters were seven dichotomous scales indicating needs in domain. The optimal number of clusters was derived from goodness‐of‐fit statistics. Bivariate residuals were assessed to check if the local independency assumption was met (values <3) [21]. Cluster analyses were conducted with Latent GOLD version 5.2.0 (Statistical Innovations Inc., Belmont, MA).
Further statistical analyses were conducted using SAS version 9.4. (SAS Institute, Cary, NC). Differences in baseline characteristics between need clusters were assessed using analysis of variance (ANOVA) for continuous variables and chi‐square tests for categorical variables. Characteristics that differed across clusters (p ≤ .1) were entered into a multiple logistic regression analysis. Both univariate and multivariate models were built to assess the odds that a cancer survivor was in a need cluster as indicated by the conditional sociodemographic or clinical characteristic, compared with cluster 1. Odds ratios (ORs) and 95% confidence intervals are shown.
For further exploratory purposes, emotional and physical symptoms that were assessed separately from the survivorship care needs survey were compared between need clusters. Bonferroni‐corrected ANOVA pairwise comparisons were made to statistically assess the differences between clusters. Means, standard deviations, and statistical differences between the clusters compared with cluster 1 are shown. P values smaller than .05 were considered to be statistically significant.
Results
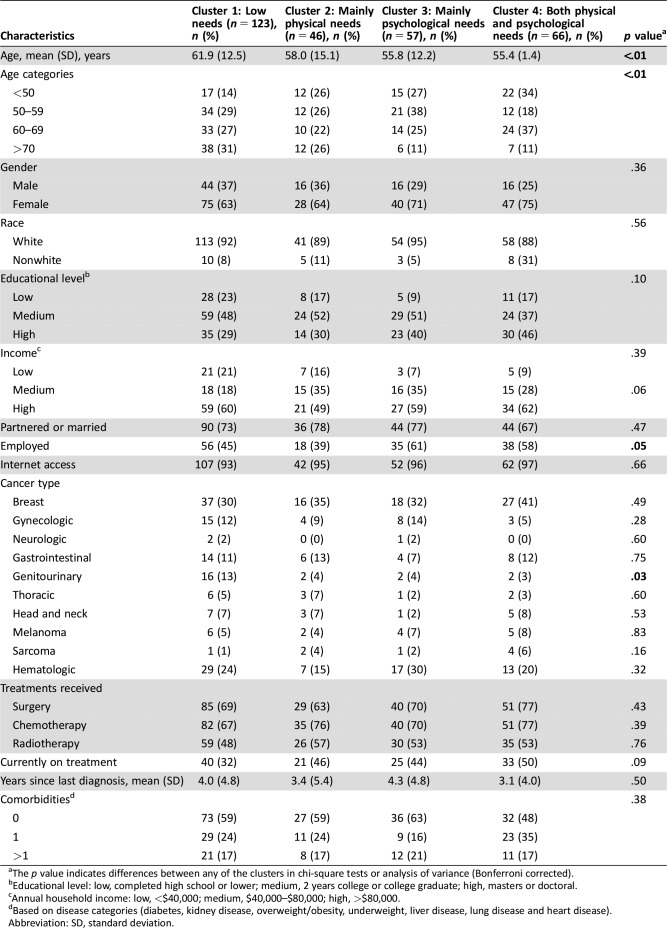
The 292 patients included in the current study had an average age of 58.6 years. The majority was female (67%), of white race (91%), of high income (59%), and not currently on treatment (59%). The most common cancer types were breast (34%), hematologic (23%), and gastrointestinal (11%). Participants completed the survey on average 3.7 years after the most recent cancer diagnosis (Table 1).
Table 1. Survivor characteristics.
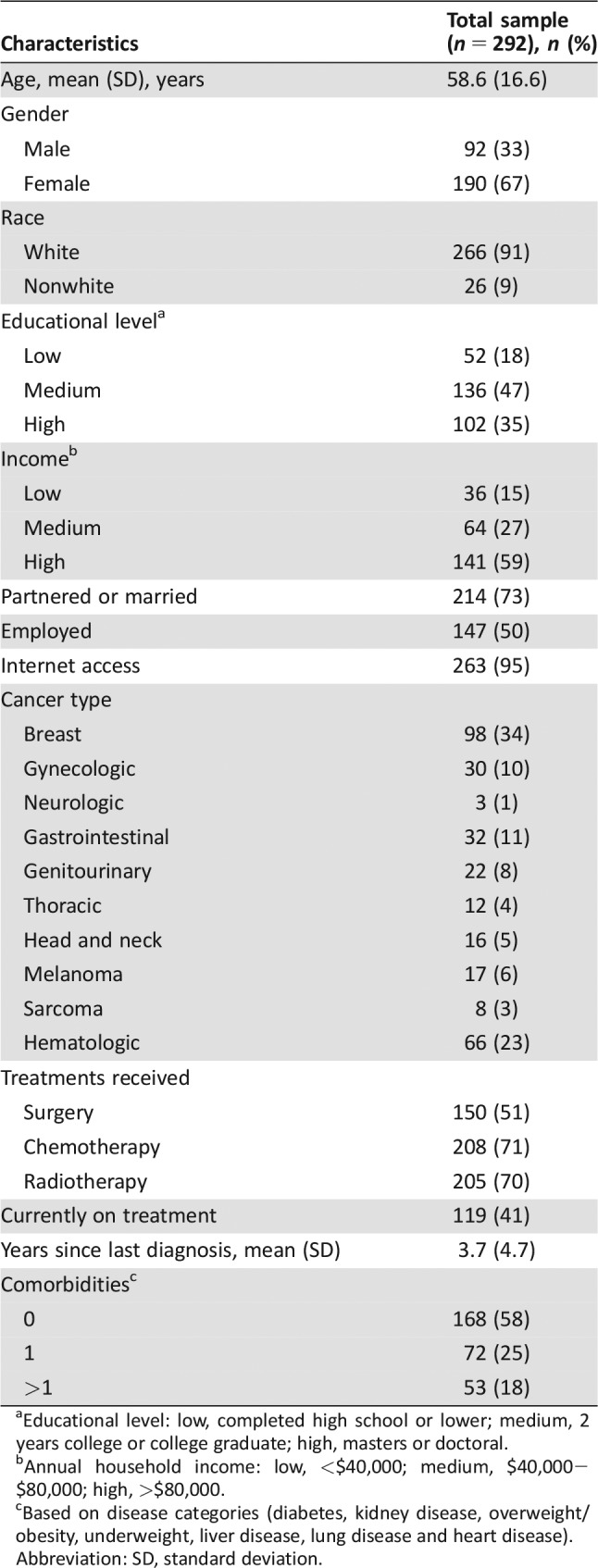
aEducational level: low, completed high school or lower; medium, 2 years college or college graduate; high, masters or doctoral.
bAnnual household income: low, <$40,000; medium, $40,000−$80,000; high, >$80,000.
cBased on disease categories (diabetes, kidney disease, overweight/obesity, underweight, liver disease, lung disease and heart disease).
Abbreviation: SD, standard deviation.
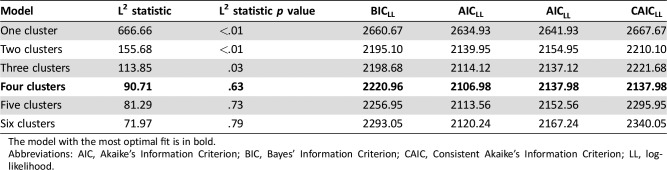
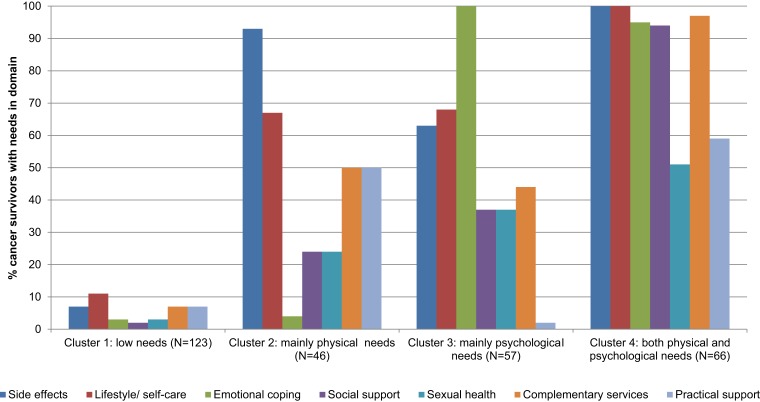
In our latent class cluster analysis, the four‐cluster model was shown to have the best model fit, based on goodness‐of‐fit statistics (Akaike's Information Criterion; Consistent Akaike's Information Criterion; Table 2). Although the more conventional Bayes’ Information Criterion showed that the two‐cluster model had the best model fit, we felt that the more liberal four‐cluster model best described variation in survivorship care needs in our sample. This model identified four clusters of survivors, including (a) low needs (n = 123, 42%), (b) mainly physical needs (n = 46, 16%), (c) mainly psychological needs (n = 57, 20%), and (d) both physical and psychological needs (n = 66, 23%; Fig. 1). Among those with low needs (cluster 1), 77 survivors (26% of the total) reported no needs on any domain.
Table 2. Goodness‐of‐fit indexes for cluster models.
The model with the most optimal fit is in bold.
Abbreviations: AIC, Akaike's Information Criterion; BIC, Bayes’ Information Criterion; CAIC, Consistent Akaike's Information Criterion; LL, log‐likelihood.
Figure 1.
Distribution of survivorship care needs across domains, per need cluster.
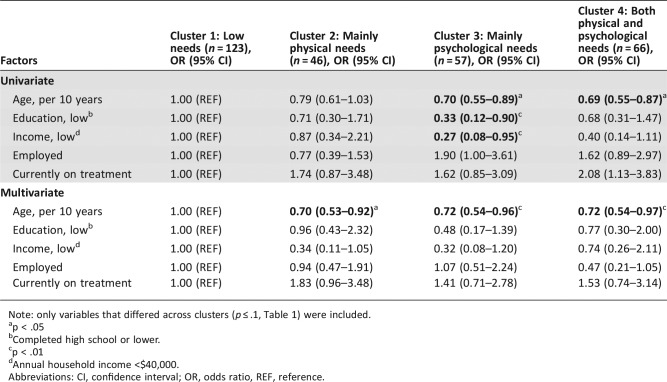
Baseline characteristics significantly differed across clusters with respect to age (continuous and categorical; p < .01), employment (p = .05), and genitourinary cancer type (p = .03; Table 3). Multiple logistic regression analysis showed that survivors with mainly physical needs (cluster 2), mainly psychological needs (cluster 3), and both physical and psychological needs (cluster 4) were younger compared with survivors with low needs (cluster 1), independent of income, education, employment, or being currently on treatment (continuous age per 10 years, ORs 0.70, 0.72, 0.72; p < .03; Table 4).
Table 3. Survivor characteristics per need cluster.
The p value indicates differences between any of the clusters in chi‐square tests or analysis of variance (Bonferroni corrected).
Educational level: low, completed high school or lower; medium, 2 years college or college graduate; high, masters or doctoral.
Annual household income: low, <$40,000; medium, $40,000–$80,000; high, >$80,000.
Based on disease categories (diabetes, kidney disease, overweight/obesity, underweight, liver disease, lung disease and heart disease).
Abbreviation: SD, standard deviation.
Table 4. Multiple logistic regression analysis of sociodemographic and clinical factors associated with clusters.
Note: only variables that differed across clusters (p ≤ .1, Table 1) were included.
p < .05
Completed high school or lower.
p < .01
Annual household income <$40,000.
Abbreviations: CI, confidence interval; OR, odds ratio, REF, reference.
Emotional and physical issues, which were assessed in a distinct section of the survey from the needs assessment questions, were associated with the needs clusters. Emotional issues, including fear of recurrence, depression, anxiety, and insomnia, were more prevalent among survivors with mainly psychological needs (cluster 3) and with both physical and psychological needs (cluster 4) compared with low needs (cluster 1; p < .05). Fatigue was more prevalent among survivors in clusters with mainly physical needs (cluster 2) and with both physical and psychological needs (cluster 4) compared with low needs (cluster 1; p < .05; supplemental online Appendix I).
Overall, survivors reported the highest unmet needs in the domains of side effects (53%), self‐care (51%), and emotional coping (43%). Needs related to side effects were highest for fatigue (30%), memory problems (20%), and weight gain (20%); needs related to self‐care were highest for diet and nutrition counselling (34%), physical activity (30%), and meditation and relaxation (30%); and needs related to emotional coping were highest for fear of recurrence (34%), anxiety or worry (28%), and managing stress (22%; supplemental online Appendix II).
Discussion
We demonstrate that the unmet needs of cancer survivors who participated in this study are indeed heterogeneous. Interestingly, our analysis suggests that over 40% of survivors report no or low unmet needs for survivorship care. For others who reported needs, we show that they may be broken down into separate subgroups: those with (a) primarily physical needs related to chronic or late effects of cancer or cancer therapy, (b) primarily psychological needs, and (c) both physical and psychological unmet needs.
The identification of these subgroups and sociodemographic and clinical predictors of each subgroup does not diminish the need to screen each individual patient for survivorship care needs. It does, however, provide clinical insight into how programs to address the needs of diverse survivors might be structured, and it underscores the importance of both tailored information provision and accounting for differences in needs when testing interventions in survivorship care research.
Overall, unmet survivorship care needs were highest regarding side effects, followed by the self‐care and emotional coping domains. This is in line with earlier studies using other surveys, including the Supportive Care Needs Survey [22] and the Cancer Survivors Unmet Needs measure [23], that reported the importance of psychological issues such as anxiety and fear of recurrence, information on things to do to get well, and physical issues such as side effects [3], [4], [5]. Our study demonstrates that needs within these domains do not always occur together and that high needs in all areas simultaneously are uncommon. Our findings are consistent with a similar cluster analysis in a population of breast cancer survivors that characterized an even greater 63% of patients within a “few‐needs” cluster [24]. This suggests that one of the goals for cancer survivorship programs could be to screen for patients’ needs (or lack thereof) and then connect individuals to services focused on management of their specific issues, rather than trying to develop a single program to address all needs.
In line with previous research [25], [26], [27], older age was associated with lower unmet survivorship care needs compared with younger survivors, suggesting that more effort is needed to identify and address the needs of younger cancer survivors. Previous work suggests that younger patients experience a greater impact from cancer and treatments on functioning and psychological well‐being [28], [29] and have higher expectations of services and people around them than older patients [30], [31], whereas older patients show better adjustment to cancer [32]. Resilience among older patients has been explained by more experience with coping with challenging events throughout life [33]. We did not find substantial differences in cancer type across clusters, except that survivors with a genitourinary cancer more often had low or no needs compared with the other cancer types, probably because of older average age (68 years). Other clinical factors, such as treatment type, being currently on treatment, and time since diagnosis, were not associated with need patterns in our study. Possibly the statistical power was too low to detect these associations because of the relatively small cluster sizes.
Consistent with previous literature, physical and psychological problems were associated with higher unmet needs [34]. Specifically, patients reporting current psychological issues, including fear of recurrence, depression, and anxiety, and, interestingly, those with insomnia, were more likely to identify needs for care and information related specifically to coping or across all care domains. Patients reporting current physical issues, such as fatigue, were more likely to report needs for care and information related to management of chronic and late effects of disease and treatment, or needs across all care domains. These associations are not surprising, but it is important to note that these were distinct questions in different sections of our survey, the former focused on current symptoms or problems and the latter on patients’ reported needs for assistance in care. Although we feel that further research is needed to confirm associations with need clusters using validated questionnaires measuring physical and psychological symptoms, our findings may suggest that screening for current symptoms and problems, as advocated by the National Comprehensive Cancer Network survivorship guideline panel, can be an effective way of identifying patients’ needs for survivorship care [35]. This is also consistent with recent efforts to integrate patient‐reported outcome measures into survivorship care pathways [36].
Limitations
This is a single institution study, conducted primarily at an urban academic medical center serving a population with relatively high socioeconomic status. Because of the pragmatic nature of offering this survey across the cancer center, we cannot determine response rates, making it more difficult to assess selection bias. Although this study includes a broad range of patients with regard to age and cancer type, it is possible that patients with higher symptom burden or care needs may have been more motivated to complete the survey and may be overrepresented in our sample [37]. However, alternatively, patients with higher symptom burden may have been too stressed or fatigued to complete the questionnaire and may therefore be underrepresented in our sample. Furthermore, our resources precluded translation of the survey into Arabic, Cantonese, French, Mandarin, Spanish, and Russian, which are spoken by a minority of patients at our center. Needs may differ among non‐English speaking patients, and in particular there may be greater barriers related to access to care and practical assistance faced by these patients who for pragmatic reasons were not included in our sample. Nevertheless, even in a selective sample of cancer survivors, there were variations in survivorship care needs and meaningful clusters of needs among this population. These patterns may be more pronounced in a more diverse sample. Furthermore, a larger sample size could increase the number of clusters to be identified, allowing for assessment of need patterns in more detail.
Our study is cross‐sectional and did not account for changes in survivorship care needs over time. Although time from diagnosis was not associated with need clusters, individual patterns of survivorship care needs may not be static. Repeated evaluations of survivorship care needs could elucidate the degree to which individual survivors have changing needs over time. In addition, we included all patients with curable disease, including those who were on intravenous therapy, consistent with the survivorship definition from the National Coalition for Cancer Survivors and endorsed by the American Society of Clinical Oncology, which begins at diagnosis [38]. This definition mirrors the work of Dr. Mullan, who recognized different phases of survivorship [39]. Needs are expected to vary based on specific regimens, time of assessment within a given regimen or from completion of therapy, and use of any long‐term or maintenance therapies. A growing number of extended oral, intramuscular, and intravenous therapies that can extend for years now blurs the lines of completion of initial therapy and precludes a simple definition of survivorship. We chose to use a broad definition and to be inclusive of curable patients in our analysis to mirror the pragmatic challenges of clinical practice, when needs must be assessed and addressed across this continuum. Needs for individual patients clearly do change over time depending on changes in therapy and many other factors.
We used primarily study‐specific survey questions. Although scales of need within domains showed good internal consistency, we cannot assume that constructs of domains were fully captured using our questionnaire. This survey relied on patient‐reported clinical data, not chart review, and we did not feel that determination of detailed staging information was possible.
Future Directions
Because of increasing health care costs and limited resources for survivorship care, there is a need for efficient provision of information, interventions, and care to cancer survivors. The classification of survivors into clusters based on survivorship care needs is a novel way to assess patterns of needs across survivors, in contrast to a body of literature focusing on associations with the number of needs or individual domains [3]. To our knowledge, this is the first study that assessed clusters of needs on multiple domains among patients with multiple types of cancer.
Our study demonstrates that the majority of patients (58%) do have substantial needs, even years after diagnosis, and these patients need to be identified through careful screening in follow‐up care. However, we also found that a large group of cancer survivors (42%) report relatively few symptoms or problems after cancer diagnosis and identify few care and informational needs. These patients still need quality cancer follow‐up care, screening for late effects and recurrence, and ongoing assessment of needs, but they do not appear to need intensive survivorship care resources. Consequently, our study may provide an explanation for the failure of some survivorship interventions, such as SCPs, to improve outcomes in randomized trials [9], [10], [11], [12]. The effect of these interventions may be diluted by a substantial subgroup of survivors with few or no needs. In future research, interventional studies should consider identifying patients with documented needs and seeking to improve outcomes, rather than delivering an intervention to an unselected population of cancer survivors. In this respect, survivorship care interventions may need to be tested with the same rigor applied to precision cancer therapy, with the right intervention for the right patient at the right time.
Conclusion
We feel that the needs clusters identified in our study merit validation using other needs assessment tools and across more diverse populations of patients with cancer. However, the characterization of patients as having few needs, predominantly physical needs, predominantly psychological needs, or substantial needs that are both physical and psychological provides a productive framework for clinical care of cancer survivors and to guide further research in this field. Further research is needed to define the tailored information and services appropriate for each group of patients and to define optimal screening tools to efficiently identify the needs of individuals in oncology practice.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by institutional funds from the Massachusetts General Hospital Cancer Survivorship Program.
Author Contributions
Conception/design: Belle H. de Rooij, Jeffrey Peppercorn
Provision of study material or patients: Don S. Dizon, Kathryn E. Post, Allison L. McDonough, Jeffrey Peppercorn
Collection and/or assembly of data: Giselle K. Perez, Julia Rabin, Katharine M. Quain, Jeffrey Peppercorn
Data analysis and interpretation: Belle H. de Rooij, Elyse R. Park, Jeffrey Peppercorn
Manuscript writing: Belle H. de Rooij, Elyse R. Park, Giselle K. Perez, Julia T. Rabin, Katharine M. Quain, Don S. Dizon, Kathryn E. Post, Garrett M. Chinn, Allison L. McDonough, Rachel B. Jimenez, Lonneke V. van de Poll‐Franse, Jeffrey Peppercorn
Final approval of manuscript: Belle H. de Rooij, Elyse R. Park, Giselle K. Perez, Julia T. Rabin, Katharine M. Quain, Don S. Dizon, Kathryn E. Post, Garrett M. Chinn, Allison L. McDonough, Rachel B. Jimenez, Lonneke V. van de Poll‐Franse, Jeffrey Peppercorn
Disclosures
Allison L. McDonough: Swedish Orphan Biovitrium AB (E—spouse); Jeffrey Peppercorn: Pfizer (RF), GlaxoSmithKline (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 2. Aaronson NK, Mattioli V, Minton O et al. Beyond treatment ‐ psychosocial and behavioural issues in cancer survivorship research and practice. EJC Suppl 2014;12:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison JD, Young JM, Price MA et al. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009;17:1117–1128. [DOI] [PubMed] [Google Scholar]
- 4. Geller BM, Vacek PM, Flynn BS et al. What are cancer survivors’ needs and how well are they being met? J Fam Pract 2014;63:E7–E16. [PubMed] [Google Scholar]
- 5. Kent EE, Arora NK, Rowland JH et al. Health information needs and health‐related quality of life in a diverse population of long‐term cancer survivors. Patient Educ Couns 2012;89:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol 2017;18:e11–e18. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine; National Research Council ; Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Translation. Washington, D.C: National Academies Press; 2006. [Google Scholar]
- 8.Institute of Medicine ; Adler NE, Page AE, eds. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, D.C: National Academies Press; 2008. [PubMed] [Google Scholar]
- 9. Brothers BM, Easley A, Salani R et al. Do survivorship care plans impact patients’ evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol Oncol 2013;129:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grunfeld E, Julian JA, Pond G et al. Evaluating survivorship care plans: Results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol 2011;29:4755–4762. [DOI] [PubMed] [Google Scholar]
- 11. Hershman DL, Greenlee H, Awad D et al. Randomized controlled trial of a clinic‐based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat 2013;138:795–806. [DOI] [PubMed] [Google Scholar]
- 12. Nicolaije KA, Ezendam NP, Vos MC et al. Impact of an automatically generated cancer survivorship care plan on patient‐reported outcomes in routine clinical practice: Longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol 2015;33:3550–3559. [DOI] [PubMed] [Google Scholar]
- 13. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: Prevalence and barriers to use. J Cancer Educ 2013;28:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stricker CT, Jacobs LA, Risendal B et al. Survivorship care planning after the Institute of Medicine recommendations: How are we faring? J Cancer Surviv 2011;5:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salz T, McCabe MS, Onstad EE et al. Survivorship care plans: Is there buy‐in from community oncology providers? Cancer 2014;120:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maly RC, Liang LJ, Liu Y et al. Randomized controlled trial of survivorship care plans among low‐income, predominantly Latina breast cancer survivors. J Clin Oncol 2017;35:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nekhlyudov L, Ganz PA, Arora NK et al. Going beyond being lost in transition: A decade of progress in cancer survivorship. J Clin Oncol 2017;35:1978–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parry C, Kent EE, Forsythe LP et al. Can't see the forest for the care plan: A call to revisit the context of care planning. J Clin Oncol 2013;31:2651–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gotay CC, Pagano IS. Assessment of Survivor Concerns (ASC): A newly proposed brief questionnaire. Health Qual Life Outcomes 2007;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 21. Vermunt JK, Magidson J. Latent GOLD 4.0 User's Guide. Belmont, Massachusetts: Statistical Innovations Inc, 2005. [Google Scholar]
- 22. Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients’ perceived needs: Development and validation of the 34‐item Supportive Care Needs Survey (SCNS‐SF34). J Eval Clin Pract 2009;15:602–606. [DOI] [PubMed] [Google Scholar]
- 23. Hodgkinson K, Butow P, Hunt GE et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: The CaSUN (Cancer Survivors’ Unmet Needs measure). Psychooncology 2007;16:796–804. [DOI] [PubMed] [Google Scholar]
- 24. Aranda S, Schofield P, Weih L et al. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. Eur J Cancer Care (Engl) 2005;14:211–222. [DOI] [PubMed] [Google Scholar]
- 25. Barg FK, Cronholm PF, Straton JB et al. Unmet psychosocial needs of Pennsylvanians with cancer: 1986–2005. Cancer 2007;110:631–639. [DOI] [PubMed] [Google Scholar]
- 26. Sanson‐Fisher R, Girgis A, Boyes A et al. The unmet supportive care needs of patients with cancer. Cancer 2000;88:226–237. [DOI] [PubMed] [Google Scholar]
- 27. Soothill K, Morris SM, Harman J et al. The significant unmet needs of cancer patients: Probing psychosocial concerns. Support Care Cancer 2001;9:597–605. [DOI] [PubMed] [Google Scholar]
- 28. Ganz PA, Schag CC, Heinrich RL. The psychosocial impact of cancer on the elderly: A comparison with younger patients. J Am Geriatr Soc 1985;33:429–435. [DOI] [PubMed] [Google Scholar]
- 29. Kroenke CH, Rosner B, Chen WY et al. Functional impact of breast cancer by age at diagnosis. J Clin Oncol 2004;22:1849–1856. [DOI] [PubMed] [Google Scholar]
- 30. Sitzia J, Wood N. Patient satisfaction: A review of issues and concepts. Soc Sci Med 1997;45:1829–1843. [DOI] [PubMed] [Google Scholar]
- 31. Thompson AG, Suñol R. Expectations as determinants of patient satisfaction: Concepts, theory and evidence. Int J Qual Health Care 1995;7:127–141. [DOI] [PubMed] [Google Scholar]
- 32. Cohen M, Baziliansky S, Beny A. The association of resilience and age in individuals with colorectal cancer: An exploratory cross‐sectional study. J Geriatr Oncol 2014;5:33–39. [DOI] [PubMed] [Google Scholar]
- 33. Brandtstädter J. Sources of resilience in the aging self: Toward integrating perspectives In: Hess TM, Blanchard‐Fields F, eds., Social Cognition and Aging. San Diego, CA: Academic Press; 1999:123–141. [Google Scholar]
- 34. Hodgkinson K, Butow P, Hunt GE et al. Breast cancer survivors’ supportive care needs 2–10 years after diagnosis. Support Care Cancer 2007;15:515–523. [DOI] [PubMed] [Google Scholar]
- 35. Denlinger CS, Ligibel JA, Are M et al. Survivorship: Screening for cancer and treatment effects, version 2.2014. J Natl Compr Cancer Netw 2014;12:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors ‐ a role for patient‐reported outcome measures and health informatics. Acta Oncol 2015;54:600–608. [DOI] [PubMed] [Google Scholar]
- 37. Paganini‐Hill A, Hsu G, Chao A et al. Comparison of early and late respondents to a postal health survey questionnaire. Epidemiology 1993:375–379. [DOI] [PubMed] [Google Scholar]
- 38. McCabe MS, Bhatia S, Oeffinger KC et al. American Society of Clinical Oncology statement: Achieving high‐quality cancer survivorship care. J Clin Oncol 2013;31:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mullan F. Seasons of survival: Reflections of a physician with cancer: N Engl J Med 1985;313:270–273. [DOI] [PubMed] [Google Scholar]