Abstract
Background
After being eliminated during the 1950s, dengue reemerged in Brazil in the 1980s. Since then, incidence of the disease has increased, as serotypes move within and between cities. The co-circulation of multiple serotypes contributes to cycles of epidemic and interepidemic years, and a seasonal pattern of transmission is observed annually. Little is known regarding possible differences in the epidemiology of dengue under epidemic and interepidemic scenarios. This study addresses this gap and aims to assess the epidemiological characteristics and determinants of epidemic and interepidemic dengue transmission, utilizing data from the 5th largest city in Brazil (Fortaleza), at fine spatial and temporal scales.
Methods/Principal findings
Longitudinal models of monthly rates of confirmed dengue cases were used to estimate the differential contribution of contextual factors to dengue transmission in Fortaleza between 2011 and 2015. Models were stratified by annual climatological schedules and periods of interepidemic and epidemic transmission, controlling for social, economic, structural, entomological, and environmental factors. Results revealed distinct seasonal patterns between interepidemic and epidemic years, with persistent transmission after June in interepidemic years. Dengue was strongly associated with violence across strata, and with poverty and irregular garbage collection during periods of low transmission, but not with other indicators of public service provision or structural deprivation. Scrapyards and sites associated with tire storage were linked to incidence differentially between seasons, with the strongest associations during transitional precipitation periods. Hierarchical clustering analysis suggests that the dengue burden concentrates in the southern periphery of the city, particularly during periods of minimal transmission.
Conclusions/Significance
Our findings have direct programmatic implications. Vector control operations must be sustained after June even in non-epidemic years. More specifically, scrapyards and sites associated with tires (strongly associated with incidence during periods of minimal transmission), require sustained entomological surveillance, particularly during interepidemic intervals and in the urban periphery. Intersectoral collaborations that address urban violence are critical for facilitating the regular activities of vector control agents.
Author summary
Almost half of the world population is at risk of a dengue infection. Although dengue often presents annual transmission seasonality (peaking during the summer) and features epidemics occuring between multi-year interepidemic intervals, little is known about how the epidemiology and determinants of dengue vary seasonally and during different stages of the epidemic cycle. As a result, control efforts likely neglect factors that sustain transmission between epidemics, or that set epidemics in motion. Using five years of monthly data at fine spatial scale from the 5th largest city in Brazil, we observed that dengue epidemiology in interepidemic years deviates from the common pattern, showing a much longer and sustained transmission season. Occurrence of violence was a strong and consistent predictor of high dengue incidence, while specific areas in the city (e.g. with a high concentration of scrapyards and locations used for tire storage) were critical during transitional precipitation periods. Indicators of social deprivation were only correlated with dengue incidence during periods of low transmission, suggesting that practices typifying these areas–such as water storage–may extend viremic circulation during dry seasons. These findings can be translated into revised and better strategies of control, but also call for the need to better understand how variations in the epidemiology of dengue may affect the success of current control recommendations.
Introduction
Dengue virus (DENV, genus Flavivirus, family Flaviviridae) is an arbovirus with at least four distinct serotypes (DENV1, DENV2, DENV3, and DENV4) that confer homologous immunity [1]. Manifestations of DENV infection range from completely asymptomatic in as many as three quarters of cases, to dengue hemorrhagic fever (DHF) and dengue shock-syndrome (DSS), with an intervening continuum of febrile symptoms and nonspecific signs of infection such as headache and rash [2]. Dengue occurs primarily in tropical and sub-tropical latitudes with an estimated burden of 390 million cases annually, of which 96 million cases manifest symptomatically [3]. These figures represent a thirty-fold increase in disease globally over the last fifty years that exposes nearly three billion people to the risk of infection [4]. The heaviest burden is in Asia, with an estimated 67 million symptomatic cases annually, followed by the Americas, with 13 million, of which more than half occur in Brazil and Mexico [3].
Dengue is transmitted primarily by Aedes aegypti and secondarily by Ae. albopictus [5], in predominantly urban human transmission cycles [6]. Ae. aegypti prefers human blood [7–9]; is more likely to oviposit in water storage containers than natural depressions [10, 11]; and its eggs withstand desiccation for more than four months, on average, in high humidity settings [12]. Common breeding habitats include containers for water storage; domestic and cookware receptacles; flower pots; and discarded objects, including tires and trash [13]. As a result, Ae. aegypti flourishes in crowded human settlements lacking access to piped water, waste collection, and adequate health and vector control systems. Such areas typify expansive and unplanned urban peripheries in tropical and subtropical latitudes [14]. In those settings, even when access to piped water is prevalent, intermittent and unreliable provision may compel populations to store water in containers, introducing Aedes oviposition sites [15]. High population density (and thus easy availability of a blood meal or breeding site) and anthropogenic constraints on urban low-level flight (such as highways, railroads, and buildings), likely limit mean Ae. aegypti flight range to < 200m in urban settings [10, 16, 17]. Notwithstanding average and maximum flight distances, adult Ae. aegypti remain in close proximity to the site of their larval/pupal development [18], predominantly concentrating and transmitting DENV indoors [19], such that a substantial share of viral circulation is believed attributable to the socially structured movement of human hosts through areas where they are exposed to DENV-infected mosquitoes [20, 21].
A variety of vector control strategies are available, targeting different stages of the mosquito [22], as well as educational campaigns aimed at promoting behaviors that minimize the proliferation of breeding habitats [23–25]. The World Health Organization (WHO) endorses an integrated approach to vector control [26], including an ecological, biological, social (‘Eco-bio-social’) dengue transmission model emphasizing community participation, as well as identification of local sources of dengue exposure [23, 27]. A primary challenge to interventions, however, is implementation at scale in a manner that can be sustained across periods of epidemic and interepidemic transmission [28, 29]. Insecticide treated bednets are inadequate to rebuff diurnal, endophilic Aedes mosquitoes, and large-scale adulticide fogging has been characterized as a cosmetic measure [30]. There is evidence that treatment of curtains and screens with insecticide reduces Aedes density [31, 32] with communal spillover effects [33], and that indoor residual [17, 34] and space [35] spraying reduce both vector density and dengue incidence.
Determining the time, place, and combination of control interventions requires proper knowledge of dengue epidemiology, which is influenced not only by the characteristics of the vector, but also by many biological (e.g., serotype), social (e.g., education), behavioral (e.g., storage of water), economic (e.g., income), and environmental (e.g., climate) factors [15, 36–43]. Temporal trends in dengue incidence within an endemic area are commonly characterized by typical inter-annual and seasonal variability. First, a cyclical inter-annual pattern of epidemic and lower-level interepidemic transmission exists, with as much as a tenfold difference in observed cases year-to-year [35, 44]. Second, regardless of the degree of endemicity, within each year dengue incidence exhibits seasonality, with peaks usually observed during warmer and wetter months [37, 45]. The appearance of DENV genetic variants with greater epidemic potential is linked to sustained urban interepidemic transmission [44, 46], increasing the importance of identifying factors contributing to transmission during these intervals.
This cyclical, seasonal, and context-dependent nature of urban dengue transmission present challenges for targeting control, and a better understanding of possible differences in drivers of transmission within and between interepidemic and epidemic years is needed. This study addresses this need, and aims to assess the role of structural, environmental, and human factors in the spatial and temporal distribution of reported dengue cases in Fortaleza, the 5th largest city in Brazil. The analysis considers five consecutive years (2011 to 2015), capturing both epidemic and interepidemic transmission. Observing the ubiquity of reported dengue infections in Fortaleza during epidemic months of peak transmission, we hypothesize that ecological correlates of infection differ within and between years according to the scale of transmission (epidemic vs. interepidemic) and the season (low vs. high precipitation). If factors associated with dengue incidence in Fortaleza during periods of low transmission are different than those that characterize incidence during epidemic peaks, vector control efforts may inadvertently neglect issues that sustain local viremic circulation between epidemics. Since suppression of interepidemic transmission may be a means to forestall or avert epidemics, identifying conditions that are associated with incidence during each stage of the epidemic cycle represents much needed evidence to inform dengue prevention and control.
Materials and methods
Study area
Located in the Northeast (NE) region of Brazil, Fortaleza is the capital of Ceará state and the 5th most populous city in Brazil (estimated at 2.6 million inhabitants in 2018) [47]. Fortaleza is divided into six regionais (administrative districts) and 119 bairros (administrative sub-districts, or neighborhoods) (Fig 1). The United Nations income-based urban inequality index classifies Fortaleza as the 9th most unequal city in the world [48]. The city has the highest homicide rate in Brazil, and the 12th highest homicide rate in the world, with approximately 61 homicides per 100,000 people in 2015 [49].
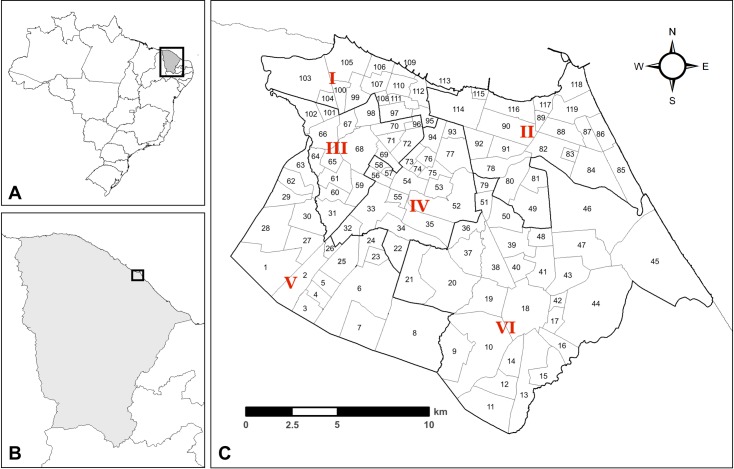
Fig 1. Administrative districts of Fortaleza, Ceará, Brazil.
A: Brazil state boundaries; B: Ceará state; C: Fortaleza municipality. Red numerals (I-VI) refer to Fortaleza regionais (districts), numbers refer to bairros (neighborhoods). A bairro name key is included in supplementary material (S1 Table).
The Köppen climate classification describes Ceará as equatorial savannah, with dry winters and humid summers [50]. The seasonality of annual precipitation cycles in Ceará is modified by the Atlantic Multidecadal Oscillation (AMO) climate cycle, which causes anomalous wet-dry phases lasting for multiple decades [51].
Dengue transmission in Fortaleza
After the elimination of Ae. aegypti from most of the Americas in the 1950s –except for the United States, Suriname, Venezuela, and several Caribbean states [52]–the mosquito was reintroduced in Brazil in the late 1970s, at a time when entomological surveillance activities and vector control teams were, for the most part, non-existent [53]. Dengue reemerged in Brazil during the early 1980s, with epidemics of DENV1 in Northern Brazil and Rio de Janeiro state in 1981 [54]. These outbreaks were followed by a series of epidemics in coastal cities of the Southeast (SE) and NE regions, and nearly 300,000 cases were reported nationally between 1986 and 1993 [55]. Between 2000 and 2010, national incidence varied widely by year, ranging from 74,000 cases in 2004 to more than 1 million in 2010 [56].
Following an absence of over fifty years, cases of DENV1 were first recorded in Fortaleza in 1986. DENV1 was the only serotype identified in the city until 1994, when DENV2 was associated with an epidemic of nearly 30,000 cases. DENV1 and DENV2 predominated until 2002, when DENV3 was first observed in the city. While reported annual incidence between 1994 and 2008 did not exceed 20,000 cases, recent epidemics of DENV2 (2008), DENV1 (2011) and allochthonous DENV4 (2012) all exceeded 34,000 reported cases [57].
Dengue control in Fortaleza
Within the Fortaleza Municipal Health Secretariat, the Vector Control Program coordinates activities to prevent and respond to dengue epidemics in accordance with national protocols. The municipality employs approximately 1,260 individuals to conduct Ae. aegypti monitoring and control activities [58]. Epidemiological surveillance and vector control departments carry out three to four sampled entomological surveys annually to estimate property infestation, and visit targeted surveillance sites (called strategic points) every fifteen days. In parallel with epidemiological surveillance, Vector Control Program agents conduct community canvassing operations throughout the year to identify and destroy breeding sites, chemically treat potential breeding sites that cannot be destroyed (e.g. elevated water tanks and cisterns), and implement public information campaigns to promote community-based vector control [59].
Data sources
This study assembled data from multiple sources. Dengue cases recorded in Fortaleza between 2011 and 2015 were acquired from Brazil’s Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação, SINAN) [60]. By law, suspected dengue cases are reported by clinicians and health professionals to SINAN within 24 hours of initial diagnosis [61]. Suspected dengue cases include all episodes of fever lasting two to seven days which are accompanied by at least two symptoms including nausea, vomiting, positive tourniquet test (i.e. capillary fragility test), petechiae, leukopenia, headache, retro-orbital pain, myalgia, or rash; and exposure to areas with active dengue transmission or presence of Ae. aegypti during the prior fourteen days [62]. Within 60 days of reporting, suspected cases are coded by the Municipal Health Secretariat as discarded/inconclusive (when not all criteria for a suspect case, as described above, was met), or coded as confirmed dengue according to: (i) laboratory analysis–based on results of IgM serology (ELISA), NS1, viral isolation, RT-PCR, or postmortem immunohistochemistry; or (ii) clinical/epidemiological criteria–when a laboratory test was not performed, but all criteria were met [63]. The MoH recommends that as many cases as possible should be confirmed by laboratory analysis, except in epidemic periods, when about 10% of laboratory confirmation is consired to be sufficient to characterize the epidemiological situation [63]. In this study we considered only confirmed dengue cases.
We used data from years 2011 to 2015 to capture both the cyclical and seasonal patterns of dengue transmission: 2011, 2012, and 2015 were considered epidemic years, and 2013, and 2014 were interepidemic years. Case home addresses were geocoded and then aggregated to the bairro-level. Sufficient information to geocode cases to bairros was available for 97.5% of confirmed cases in 2011, 77.5% in 2012, 98.1% in 2013, 95.4% in 2014, and 77.7% in 2015. Temporally, cases had the exact date of first symptoms, which allowed aggregation to epidemiological weeks and to months. All data analyzed were anonymized.
We obtained annual population data by bairro from the 2010 Demographic Census [64] and estimated bairro population growth from 2011–2015 using estimates of population growth for the city of Fortaleza from the Brazilian Institute of Geography and Statistics (IBGE). Using dengue cases and population, we calculated incidence rates per 100,000 people for each bairro, and created a binary variable to indicate low and high transmission months in each year: (i) high corresponds to months with more than 9% of annual transmission (or more than one would expect if cases were uniformly distributed over the year), or more than 1,000 total reported cases citywide, and (ii) low corresponds to the remaining months.
We obtained bairro-level socio-ecological data from the 2010 Demographic Census [64]. These include: mean number of inhabitants per household; percent of households connected to electricity, piped water, sewage, and regular garbage collection; population density; average income per household in Reais (R$); and literacy rates for men and women separately. Two new bairros were created after 2010 (both originated from larger bairros split into two). In those cases, socio-ecological data for the original bairro in 2010 was extended to the new bairros.
We estimated the degree of structural deprivation in a bairro using two data sources. First, we obtained information for subnormal agglomerations (AS, aglomerados subnormais) by census tract from the 2010 Demographic Census. AS is a habitation class characterized by tenuous legal claim and poor structural development. An AS is constituted by, at minimum, 51 inhabited units on illegally occupied land or land obtained within the prior ten years; constructed haphazardly or outside preexisting structural standards; and lacking access to essential public services such as electricity, garbage collection, water grids, and sewage systems [65]. Second, from the Planning Institute of Fortaleza (IPLANFOR, Instituto de Planejamento de Fortaleza) [66], we obtained data on precarious settlements (AP, assentamentos precários). AP is an exclusively urban classification introduced by the National Housing Secretariat (Secretaria Nacional de Habitação) that extends the AS definition to include settlement types that are structurally insecure, neglected, or unplanned, even if legally occupied [67]. The spatial boundaries of AP and AS were merged, then overlaid on the bairro boundaries to estimate the intersecting areal proportion for each bairro. We refer to this aggregate class as subnormal settlements (SS).
Annual homicide counts by bairro were collected from the Mortality Information System (Sistema de Informações de Mortalidade, SIM), and used to calculate a homicide rate per 10,000 people. It is established that interpersonal violence can deter and disrupt provision of human services by introducing risk for individuals providing and receiving health-related services [68]. As such, we hypothesized that bairro-level homicide rates are associated with spatial heterogeneity in access to health and vector control services, and with higher dengue transmission.
Entomological surveillance data were acquired from Ae. aegypti infestation surveys, conducted by Brazilian Municipal Health Secretariats at a local level. The Aedes aegypti Infestation Rapid Survey (Levantamento Rápido de Índice para Aedes aegypti, LIRAa) is a sample survey that randomly selects properties for inspection for immature forms (mosquito larvae and pupae). Municipalities are divided into areal groups of between 8,100–12,000 properties, from which a sample of 450 properties is drawn. LIRAa was introduced in Fortaleza in October 2011 to replace the Ae. aegypti Infestation Survey (Levantamento de índice de infestação amostral, LIA), which covered a much larger sample of properties in the municipality (about 10%), and required considerably more time to complete. LIRAa is repeated three to four times per year, with a national survey conducted every October. The Brazilian Ministry of Health uses three categories of infestation intensity to classify risk: (i) satisfactory, <1% infestation; (ii) alert, 1–3.9%; and (iii) risk of outbreak, >3.9% [62]. We obtained data on all surveys available for LIA in 2011 and for LIRAa from 2011 to 2015. For the purposes of this analysis we used data from the January surveillance of both programs–immediately preceding the early-spring rainy season characteristic of NE Brazil–that is considered the most valuable for forecasting regional epidemic risk. In 2014, the January LIRAa survey was delayed until February, resulting in elevated bairro infestation indexes relative to the other years; as a result, we designated 2014 LIRAa results as missing, and used a missingness indicator variable [69]. Under the assumption of representative sampling, we used total houses sampled by bairro as a denominator to create a bairro-level household infestation variable for each year.
We also used data from surveillance of strategic points (SP)–locations identified by the municipality as higher risk for harboring Aedes breeding sites–to assess the epidemiological relevance of Aedes infestation at targeted non-residential locations. In Brazil, SP are a central component of national directives for vector control to prevent dengue. Municipal vector control operations agents are tasked with creating a roster of sites likely to be susceptible to mosquito oviposition and inspecting those sites every 15 days. These operations are coordinated locally, such that each municipality is responsible for its own roster of strategic points [62]. Vector control teams inspect SPs in 15-day cycles and code the visit as positive or negative for the presence of Aedes larvae or pupae. Between 2011 and 2015, surveillance agents identified 82 types of SPs in Fortaleza; we aggregated those into six categories: construction, industrial fabrication, recyclable processing, scrapyards, sites associated with tire storage–such as tire repair shops and garages, and an “other” category, which included sites associated with vacant lots, public and private facilities (e.g. hospitals and schools), husbandry (e.g. cattle pens, vacarias), private residences, outdoor locations (e.g. cemeteries), and general commercial properties.
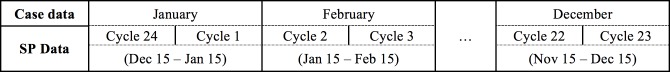
We created two bairro-level variables for each SP category: (i) positivity, defined as the proportion of successfully visited sites that were registered as positive for larvae/pupae; and (ii) proximity, defined as the areal proportion of the bairro that falls within a 150-meter buffer area around a positive strategic point. We selected the buffer size using two criteria: (i) expected Aedes mosquito flight distance in an urban setting [10, 16, 17]; and (ii) buffer size used by the Brazilian Ministry of Health to define the transmission block area around identified vector oviposition sites [62]. For the positivity variable, bairros without an SP visit during an inspection cycle were coded as missing with a missingness indicator variable. The proximity variable was calculated in ArcGIS. To properly associate the presence of mosquito larvae/pupae with monthly dengue virus transmission, SP-related covariates were lagged by a 15-day cycle, as shown in Fig 2.
Fig 2. Lag time scheme to relate monthly dengue cases and 15-day inspection cycle of strategic points.
Cycles are numbered sequentially, starting with Cycle 1 for the first 15 days of the year.
We acquired rainfall data from the Climate Hazards Infrared Precipitation with Stations (CHIRPS) dataset [70]. CHIRPS has a spatial resolution of 0.05°, thus Fortaleza was covered by 15 pixels. Daily precipitation estimates were aggregated to weeks, and spatially joined to bairros. Efforts to associate precipitation volume with dengue incidence must incorporate a time lag to adjust for vector development. While lags of zero [45], one [71, 72], and two months [37, 73] were reported, the mechanistic role of precipitation in dengue transmission can be confounded by water storage behavior [43], and with the potential for heavy rains to wash out exposed oviposition sites [74]. For the purposes of our analysis, precipitation was included as a continuous variable (monthly sum in millimeters), and lagged by two weeks, consistent with the lag used with the SP data. To assess potential effects of rainfall anomalies [75] during the period, we calculated the average monthly rainfall by bairro over the five years of study and computed the deviation of each bairro-month from its five year average. This variable was included in all models, except those stratified by monthly precipitation volume.
Lastly, daily temperature values (high, low, and daily average) recorded at Fortaleza’s Pinto Martins International Airport were obtained from the Weather Underground data archive [76] and used to generate two measures of temperature variability over the course of a month [77]: monthly average of daily temperature ranges (high minus low daily temperature), and monthly standard deviation of daily temperature averages.
Analytical approach
We defined the outcome variable as the monthly case incidence rate (dengue cases per 100,000 people) by bairro, observed from January 2011 to December 2015, and used a zero-inflated negative binomial longitudinal regression model (hereafter, longitudinal model). The zero-inflated negative binomial model accounts for extra-variation (overdispersion) in the data [78, 79], and was considered as an alternative to the negative binomial hurdle model given the likelihood that seasonally inflated zeros are attributable to both true changes in ecological dynamics that depress transmission, as well as reduced surveillance or misclassification during low seasons and interepidemic years [80–82]. The final model was selected based on comparison of Akaike Information Criterion (AIC) values for alternative distributions, including negative binomial, poisson, and zero-inflated poisson. We chose longitudinal models because our data are longitudinal at the bairro level, and used Huber-White standard errors [83]. Climatological and ecological independent variables–selected based on previously identified associations with Aedes-borne disease transmission and summarized by bairro-month–included: mean household size; percentage of households with access to electricity; percentage of households with access to piped water; percentage of households with access to regular garbage collection; percentage of households connected to sewage network; male literacy rate; female literacy rate; household income; population density; proportion of a bairro classified as a subnormal settlement; homicide rate; separate lagged strategic point proximity variables for each of the following types: construction, material fabrication, recyclables, scrapyard, tires, and others; lagged total infestation index for all SP categories combined; categories of infestation (LIA and LIRAa); lagged total precipitation (mm) and deviation from average precipitation (mm); and standard deviation of daily temperature averages (degrees Celsius). The AIC was used to select the final temperature covariate, and likelihood ratio test statistics (chi-square) were used to compare the full model with naïve intercept-only models, indicating that the full model was a better fit (p<0.001) for all analyses.
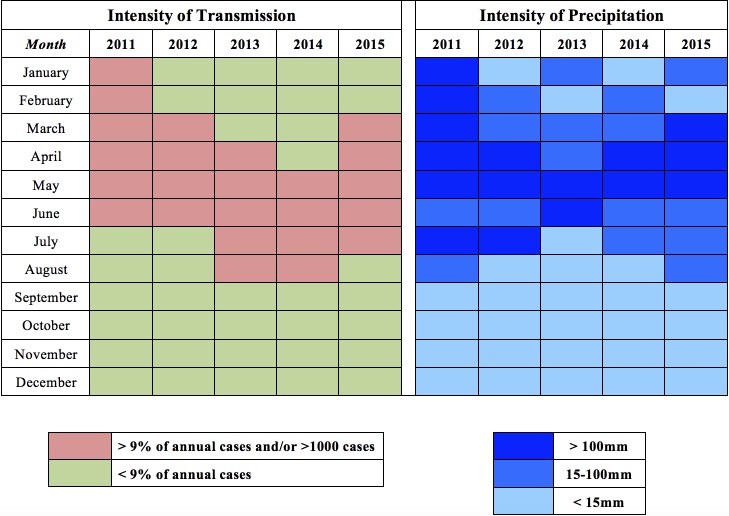
Considering the cyclical and seasonal patterns of dengue transmission, we run eight temporally stratified models. Model 1 contains all 7,140 bairro-months for which data were collected. Models 2 and 3 distinguish between epidemic (2011, 2012, and 2015) and interepidemic (2013, 2014) years. Models 4 and 5 stratify the analysis by transmission intensity, while Models 6, 7, and 8 are stratified by the intensity of precipitation (Fig 3). We addressed the family-wise error rate in all models using the false discovery rate (FDR) [84]. All data preparation and regression analysis was done in Stata v.14.2 (Stata Corp., College Station, TX, USA).
Fig 3. Classification of months according to the intensity of dengue transmission and of precipitation, Fortaleza, 2011–15.
Months with high intensity of transmission (more than 9% of total annual dengue cases or more than 1,000 total cases citywide) are represented in pink, and those with low intensity in green. Months with high intensity of rainfall (more than 100 millimeters), medium (15-100mm), and low (less than 15mm) are represented in shades of blue.
Lastly, to characterize the spatial and temporal patterns of epidemic intensity between years we used average linkage hierarchical clustering with an Euclidean distance dissimilarity measure, based on eight metrics, standardized as z-scores, reflecting the duration and burden of dengue incidence in a bairro. The number of clusters selected for each year was determined with reference to the gap statistic [85], reflecting the difference of within-cluster variability (as the within-cluster sum of squares around cluster means) at each number of k clusters in the observed data from its expectation in a null reference distribution computed from repeated sampling using the package factoextra [86]. Values of the gap statistic indicate the strength of clustering at each quantity of clusters considered (i.e. sequential values k). Maxima of the gap statistic may be local (in comparison to their immediate neighboring values k) or global (in comparison to all possible or considered levels k), and a plateau in the statistic indicates the negligible added value of additional partitions between clustering groups [85]. We considered between 3–7 clusters for each year to preserve interpretative value, prioritizing global maxima of the gap statistic (for years 2012, 2013, 2015 –S1 Fig), or local maxima that distinguish between well-separated groups, where further divisions between clusters in the considered range would exclusively partition single-bairro clusters (2011). Though the gap statistic first plateaued at three clusters in 2014, we partitioned the middle cluster–with high within-cluster variability along the first principal component of our clustering parameters–to distinguish two clusters, each with low internal variability along that axis (see S2 Fig, clusters II and III). In addition to plots of the first two principal components of clustering parameters (S2 Fig), grouping decisions considered potential informational value to surveillance operations. After grouping, clusters were ordered by the average full-year case rate of clustered units. Clustering analysis and mapping were conducted with R version 3.3.2 [87]. Data used in this study are available in S1 Dataset.
Results
Between 2011 and 2015, 130,430 dengue cases were reported in Fortaleza. In total, 98,339 cases were confirmed by clinical/epidemiological or lab criteria and had sufficient information to be geocoded to bairros and included in this analysis. Annual citywide case rates per 100,000 varied from a low of 192 in 2014, to a high of 1,360 in 2011. Monthly rates within a single bairro exceeded 3,500 cases per 100,000 people in January 2011, April 2011, and June 2012. In total 26.5% (1,891 of 7,140) of bairro-months did not register a case; 75.2% of bairro-months with zero cases occurred between August and January, reflecting dengue seasonality. However, the pattern differed between epidemic and interepidemic years, as shown by the percent of annual reported cases that occurred between July and December each year (Table 1 and Fig 4). Incidence during epidemic years was higher, but more concentrated seasonally, while in interepidemic years transmission continued into seasons that were less climatologically hospitable to mosquitoes.
Table 1. Number of dengue cases by year, and percent of cases reported between July and December–Fortaleza, 2011–2015.
| Year | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Reported Cases | 37,021 | 34,134 | 16,381 | 10,178 | 32,716 |
| % of total 2011–15 | 28.4 | 26.2 | 12.6 | 7.8 | 25.1 |
| % cases in Jul-Dec | 9.4 | 6.0 | 38.1 | 39.3 | 14.4 |
| Confirmed Cases | 33,642 | 30,272 | 8,611 | 4,935 | 20,879 |
| % of total 2011–15 | 34.2 | 30.8 | 8.8 | 5.0 | 21.2 |
| % cases in Jul-Dec | 8.8 | 5.1 | 41.8 | 39.0 | 13.8 |
Note: Epidemic years were 2011, 2012, and 2015; interepidemic years were 2013 and 2014.
Fig 4. Dengue epidemiological curves and weekly precipitation, Fortaleza, 2011–2015.

Average precipitation by week is presented in blue. Line of weekly dengue case count citywide is presented in black. Scale is adjusted for dengue cases (left y-axis) between interepidemic (2013, 2014) and epidemic years (2011, 2012, 2015), and constant for precipitation (right y-axis).
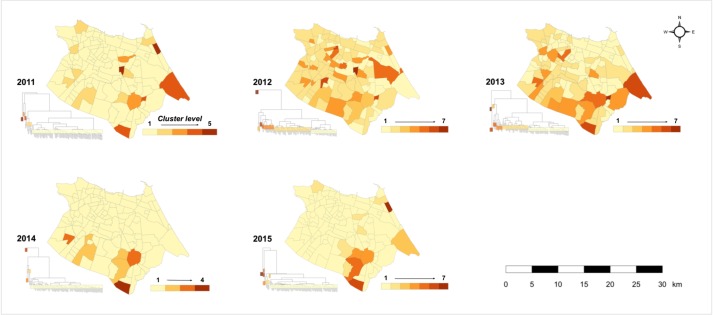
Hierarchical cluster analysis grouped bairros into ascending patterns according to the scale and duration of their dengue burden during annual transmission cycles (Fig 5, S2 Table). Spatially, results suggested that the epidemics of 2011 and 2012 were distinguishable by the increased prevalence of cases in northern, coastal bairros–such as the northern coast and northeastern peninsula near Mucuripe–after which incidence concentrated farther from the coastal urban core. Small, outlying clusters (typically composed of only one or two bairros) with elevated incidence rates and shorter periods of transmission were present in all years, such that the distance between observations in patterns 1 and 2 was smaller than between other clustering levels, and clustering was strongest during years when a large majority of bairros reported minimal dengue transmission (such as 2014 and 2015) (S2 Fig and S3 Table).
Fig 5. Hierarchical cluster maps of annual dengue transmission intensity in Fortaleza.
Averages and ranges of parameter values by year and pattern are presented in S2 Table. Patterns were ordered low-high by annual case rates. Highest level patterns, particularly during lower-transmission years (patterns 6 and 7 in 2013 and 2015; pattern 4 in 2014), classify bairros with outlying case rates but shorter transmission intervals. Middle ranked patterns (patterns 2 and 3 in 2011; pattern 3 in 2012; pattern 5 in 2013 and 2015) were characterized by more populous bairros with longer intervals with continuous transmission, typically exceeding 40 weeks annually, and higher total case counts.
In 2011, clustering isolated 15 bairros (patterns 2–5), spatially dispersed throughout the city, with substantially elevated or protracted transmission (Fig 5); five of these bairros (patterns 4 and 5) exhibited outlying incidence rates (greater than 6,000 per 100,000) over shorter intervals (S2 Table). During the 2011 epidemic, transmission was present or elevated in all regionais: two bairros in the lowest clustering level experienced more than 3,000 cases per 100,000, over 44 and 48 weeks with confirmed incidence.
The 2012 epidemic and 2013 interepidemic years featured lower levels of transmission overall and fewer outlying bairros, decreasing clustering distances between high and low patterns. As a result, clustering distinguished additional patterns at both high and low scales of transmission. Similar to the 2011 epidemic, transmission during the 2012 epidemic was spatially diffuse, with coastal, affluent bairros grouped amongst peripheral communities in pattern 2. In contrast, in 2013 northeastern coastal bairros were almost exclusively grouped in pattern 1 (Fig 5).
This trend continued in subsequent years, when outlying transmission was either largely isolated (2014) or concentrated (2015) in bairros of the southern periphery. Confirmed incidence was negligible in 112 bairros of pattern 1 in 2014, yet low level residual dengue circulation is evident in six bairros of patterns 2 and 3 (Messejana, Jangurussu, Barroso, Maraponga, Bom Jardim, and Mondubim), where cases were confirmed for 38.5 and 44.5/52 weeks, on average. The scale of annual transmission in these six bairros is comparable to pattern 1 bairros in 2011, 2012, and 2015, but occurred over much longer intervals (S2 Table). Confirmed dengue incidence in three of these bairros (Messejana, Jangurussu, and Barroso) increased to epidemic levels in 2015 (identifiable in 2015 patterns 4 and 5), inflating case rates for the city as a whole.
Table 2 presents descriptive statistics. Mean household size in Fortaleza was 3.42 persons. Access to infrastructural and municipal services was high (except for sewage), though unequally distributed. Reported literacy exceeded 86% for both men and women in all bairros. Minimum reported average annual household income by bairro was R$ 800 reais (approximately US$450, 2010 exchange rate). Population density exceeded 100 persons per km2 in all bairros ranging from 148 in Sabiaguaba to more than 34,000 persons per km2 in Pirambu. Ten bairros included less than 1% SS in their area, and 13 bairros had more than 50% SS (highest values of 74%, 87%, and 92% observed in Genibau, Pirambu, and Curio). Homicide rates by bairro ranged from zero to 585.6 per 100,000 persons per year; rates for the whole city ranged from a low of 48.33 per 100,000 in 2011 to 76.93 per 100,000 in 2014, exceeding regional averages.
Table 2. Descriptive statistics of the bairro-level variables used in the analysis.
| Variable | Mean | SD | Range | ||
|---|---|---|---|---|---|
| Reported dengue cases by bairro-month (outcome) | |||||
| Count (number of cases) |
2011 | 23.56 | 48.52 | (0–531) | |
| 2012 | 21.20 | 51.11 | (0–568) | ||
| 2013 | 6.03 | 10.29 | (0–108) | ||
| 2014 | 3.46 | 7.68 | (0–119) | ||
| 2015 | 14.62 | 38.17 | (0–548) | ||
| Rate (cases per 100,000) |
2011 | 127.33 | 291.55 | (0–4,628.3) | |
| 2012 | 105.43 | 255.89 | (0–3,660.9) | ||
| 2013 | 28.76 | 48.81 | (0–666.9) | ||
| 2014 | 15.87 | 29.44 | (0–355.4) | ||
| 2015 | 71.33 | 184.12 | (0–3,197.3) | ||
| Demographic (exposure) | |||||
| Mean Household size (persons) | 3.42 | 0.19 | (2.9–4.0) | ||
| Access to electricity (%) | 99.65 | 0.55 | (95–100) | ||
| Access to piped water (%) | 93.02 | 6.32 | (55.8–99.5) | ||
| Access to regular garbage collection (%) | 98.42 | 3.33 | (78.2–100) | ||
| Access to sewage (%) | 57.99 | 33.47 | (0.5–99.9) | ||
| Literacy (%) | Male | 93.61 | 3.39 | (86.4–99.1) | |
| Female | 93.69 | 2.94 | (87.5–98.5) | ||
| Household income (R$) | 2,532.13 | 1,970.8 | (803.4–10,504.7) | ||
| Population density (persons/km2) | 10,929.1 | 5,817.8 | (148–34,007) | ||
| Homicide rate (per 100,000) | 2011 | 48.33 | 48.25 | (0–369.0) | |
| 2012 | 67.06 | 60.37 | (0–340.2) | ||
| 2013 | 76.91 | 59.60 | (0–262.1) | ||
| 2014 | 76.93 | 78.11 | (0–585.6) | ||
| 2015 | 59.82 | 51.64 | (0–423.1) | ||
| Subnormal Settlement (% of bairro) | 18.28 | 21.03 | (0–91.8) | ||
| Climate (exposure) | |||||
| Precipitation (sum in mm) |
2011 | 138.27 | 139.19 | (0–385.4) | |
| 2012 | 53.48 | 63.89 | (0–209.6) | ||
| 2013 | 48.67 | 53.86 | (0–171.6) | ||
| 2014 | 64.23 | 77.50 | (0–269.2) | ||
| 2015 | 70.65 | 82.12 | (0–248.3) | ||
| Temperature (°C) | Daily average | 2011 | 26.19 | 0.53 | (25.4–27.1) |
| 2012 | 26.92 | 0.51 | (26.1–27.9) | ||
| 2013 | 27.38 | 0.61 | (26.3–28.3) | ||
| 2014 | 27.07 | 0.39 | (26.4–27.7) | ||
| 2015 | 27.07 | 0.60 | (26.1–28.2) | ||
| SD of daily averages (by month) | 2011 | 0.86 | 0.34 | (0.51–1.57) | |
| 2012 | 0.70 | 0.24 | (0.48–1.35) | ||
| 2013 | 0.66 | 0.22 | (0.41–1.11) | ||
| 2014 | 0.64 | 0.19 | (0.35–0.96) | ||
| 2015 | 0.68 | 0.26 | (0.40–1.28) | ||
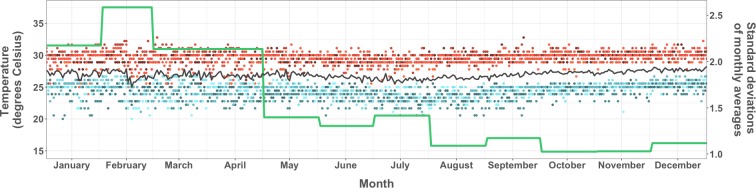
Temperature in Fortaleza did not vary substantially over the course of our five year study period, but daily temperature averages were marginally higher earlier in the year; the lowest daily temperature average was 22.2 degrees on January 10, 2011 and the highest daily average was 29.4, on March 3, 2013 (Fig 6). In contrast, precipitation differed seasonally, annually, and between bairros. At least one bairro registered no precipitation during 76% of our study period (197 weeks), and in 37% (96 weeks) no precipitation occurred anywhere in the city. The highest weekly sum precipitation (226mm) was recorded in the second week of March 2015, and precipitation exceeding 83mm was recorded during 10% of all bairro-weeks. The largest bairro differential in weekly precipitation was 124.2mm, with an average weekly differential of 16.2mm. Average deviation from monthly averages, measured at the bairro level, was highest in February 2011, when average bairro precipitation exceeded five-year averages for that month by nearly 240mm. This trend continued for the following three months (with positive deviations exceeding 100mm in March, April, and May), amidst the 2011 epidemic. In comparison, citywide averages during spring months of interepidemic years 2013 and 2014 were starkly lower than five-year averages (April 2013 = -137mm, and March 2014 = -101mm).
Fig 6. Daily temperature highs and lows, daily average temperature for the 5-year interval, and standard deviation of daily averages by month, Fortaleza, 2011–2015.
Values of daily high, low, and average daily temperatures were obtained from each year. High values are represented by dots colored in shades of red, and low values by dots colored in shades of blue. The black line summarizes the daily average for the 5-year period, and the green line shows the standard deviation of daily averages by month.
Entomological surveillance data is summarized in Table 3. On average, bairro-level infestation was higher during the LIA (2011) survey than for LIRAa surveys (2012, 2013, and 2015), though the maximum bairro-level infestation value (6.45% of visited sites positive for vector immature forms) was recorded in Vila Ellery in January, 2015. The highest average January bairro infestation over the course of the four-years (excluding 2014) was in Cambeba, 3.4%.
Table 3. Infestation indices, Fortaleza, 2011–2015.
| Parameter | Mean | SD | Range | |||||
| a. Sample-based Entomological Surveys (January cycle) | ||||||||
| LIA (%) | 2011 | 1.80 | 1.04 | (0–5.29) | ||||
| LIRAa (%) | 2012 | 0.80 | 0.76 | (0–3.45) | ||||
| 2013 | 1.18 | 0.94 | (0–4.62) | |||||
| 2014 (excluded from analysis) | 2.11 | 1.76 | (0–12.5) | |||||
| 2015 | 1.42 | 1.19 | (0–6.45) | |||||
| b. Strategic Points (targeted surveillance) | ||||||||
| Class | Year | Number of sites | Positivity (% infested) | Proximity (% areal proportion) | ||||
| Mean | SD | Range | Mean | SD | Range | |||
| Construction | 2011 | 658 | 10.89 | 20.26 | (0–100) | 1.75 | 4.20 | (0–43.6) |
| 2012 | 838 | 6.87 | 14.60 | (0–100) | 1.35 | 3.28 | (0–27.3) | |
| 2013 | 1,115 | 6.96 | 14.17 | (0–100) | 1.99 | 4.19 | (0–31.4) | |
| 2014 | 1,225 | 7.79 | 16.03 | (0–100) | 1.13 | 3.06 | (0–27.8) | |
| 2015 | 1,089 | 9.64 | 18.82 | (0–100) | 1.70 | 4.11 | (0–37.0) | |
| Average | 985 | 8.43 | 16.78 | — | 1.58 | 3.81 | — | |
| Fabrication | 2011 | 128 | 9.60 | 20.79 | (0–100) | 0.48 | 1.62 | (0–16.0) |
| 2012 | 138 | 8.37 | 20.03 | (0–100) | 0.45 | 1.43 | (0–16.0) | |
| 2013 | 162 | 9.88 | 20.33 | (0–100) | 0.56 | 1.56 | (0–16.2) | |
| 2014 | 155 | 10.18 | 22.54 | (0–100) | 0.38 | 1.37 | (0–16.2) | |
| 2015 | 143 | 9.79 | 21.23 | (0–100) | 0.38 | 1.26 | (0–9.2) | |
| Average | 145.2 | 9.56 | 20.98 | — | 0.45 | 1.46 | — | |
| Recyclables | 2011 | 277 | 8.05 | 18.46 | (0–100) | 0.76 | 2.21 | (0–23.3) |
| 2012 | 292 | 4.43 | 13.64 | (0–100) | 0.46 | 1.55 | (0–18.4) | |
| 2013 | 325 | 4.38 | 11.16 | (0–100) | 0.56 | 1.77 | (0–18.4) | |
| 2014 | 323 | 5.96 | 14.75 | (0–100) | 0.47 | 1.49 | (0–14.5) | |
| 2015 | 284 | 5.18 | 15.41 | (0–100) | 0.41 | 1.52 | (0–18.4) | |
| Average | 300.2 | 5.60 | 14.68 | — | 0.53 | 1.73 | — | |
| Scrapyard | 2011 | 333 | 9.74 | 21.61 | (0–100) | 0.86 | 2.45 | (0–37.7) |
| 2012 | 352 | 7.43 | 18.32 | (0–100) | 0.67 | 1.87 | (0–18.0) | |
| 2013 | 397 | 7.40 | 16.81 | (0–100) | 0.78 | 2.06 | (0–23.3) | |
| 2014 | 406 | 7.94 | 18.30 | (0–100) | 0.51 | 1.49 | (0–13.8) | |
| 2015 | 364 | 9.33 | 21.26 | (0–100) | 0.62 | 1.63 | (0–10.8) | |
| Average | 370.4 | 8.37 | 19.26 | — | 0.69 | 1.93 | — | |
| Tires | 2011 | 975 | 5.62 | 12.71 | (0–100) | 1.84 | 3.66 | (0–30.2) |
| 2012 | 1,042 | 3.46 | 8.41 | (0–100) | 1.34 | 2.92 | (0–22.4) | |
| 2013 | 1,200 | 3.65 | 9.24 | (0–100) | 1.44 | 2.98 | (0–19.5) | |
| 2014 | 1,110 | 5.87 | 14.20 | (0–100) | 0.85 | 2.14 | (0–19.8) | |
| 2015 | 829 | 7.61 | 16.78 | (0–100) | 0.88 | 2.03 | (0–18.7) | |
| Average | 1,031.2 | 5.24 | 12.27 | — | 1.27 | 2.83 | — | |
| Others | 2011 | 236 | 15.29 | 24.36 | (0–100) | 1.33 | 3.22 | (0–40.6) |
| 2012 | 226 | 10.73 | 22.10 | (0–100) | 0.87 | 2.49 | (0–32.3) | |
| 2013 | 284 | 11.29 | 21.55 | (0–100) | 1.04 | 2.88 | (0–42.5) | |
| 2014 | 303 | 11.22 | 21.88 | (0–100) | 0.64 | 1.79 | (0–22.3) | |
| 2015 | 262 | 11.84 | 22.29 | (0–100) | 0.79 | 2.24 | (0–19.4) | |
| Average | 262.2 | 12.07 | 22.44 | — | 0.93 | 2.58 | — | |
| Total | 2011 | 2,607 | 8.38 | 12.02 | (0–100) | 6.59 | 8.48 | (0–70.15) |
| 2012 | 2,888 | 5.38 | 9.36 | (0–100) | 4.88 | 6.89 | (0–54.77) | |
| 2013 | 3,483 | 5.84 | 9.09 | (0–100) | 5.99 | 7.50 | (0–42.46) | |
| 2014 | 3,522 | 6.78 | 12.46 | (0–100) | 3.80 | 5.55 | (0–35.08) | |
| 2015 | 2,971 | 8.91 | 14.47 | (0–100) | 4.58 | 5.92 | (0–38.78) | |
| Average | 3,094.2 | 7.06 | 11.48 | — | 5.17 | 6.87 | — | |
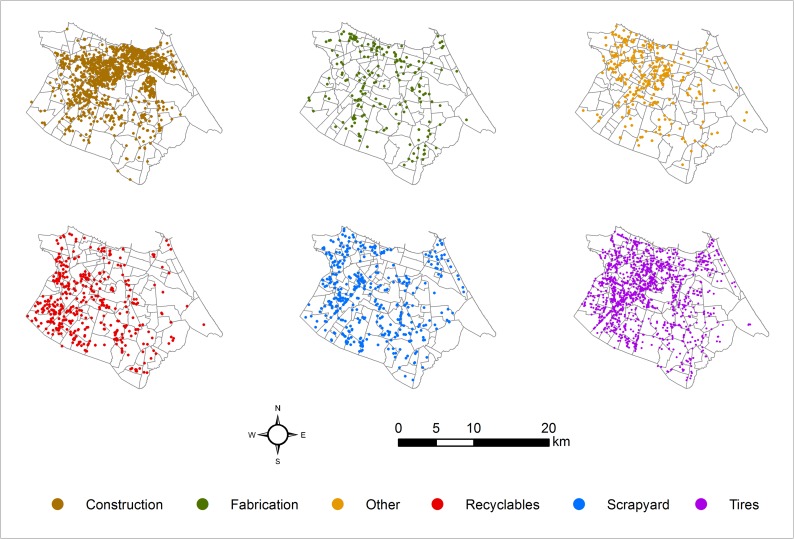
The spatial distribution of SP types varied across the city (Fig 7 shows their location in 2015), and around 3,000 SP sites were inspected annually, on average, during the study period. The largest number of SP inspected by surveillance agents was 3,522 in 2014, the majority being tire repair shops and garages (Tires category in Table 3). Considering all types of SPs, the highest infestation indices were registered in 2011 (8.38%) and 2015 (8.91%) (Table 3). There were large differences between infestation by site types: on average, sites in the “Others” category registered the highest monthly infestation indices, while those under the tire category had the lowest (Table 3). SP infestation exceeded household infestation (LIRAa/LIA), on average, and was substantially more variable at the bairro level. The monthly areal proportion of a bairro within 150 meters of a positive SP varied by type of site, reflecting differences in SP prevalence and infestation rates by class. On average, about 5% of bairro area was within 150-meter radius of a positive SP citywide, and more than half of this exposure was attributable to sites in the construction and tire categories.
Fig 7. Location of strategic points by type in Fortaleza, 2015.
In the analysis of all 7,140 bairro-months (Model 1, Table 4), amongst socio-ecological variables only bairro homicide rates was a statistically significant correlate of dengue incidence rates: on average, increases in annual household income by R$1,000 were accompanied by an 8% decrease in dengue incidence rates, while an additional ten homicides per 100,000 people was associated with a 6% increase. Over the entire study period, a 1% increase in the areal proportion of a bairro within 150 meters of a SP in the tires class was associated with 3% increases in bairro-month dengue incidence rates. Indicator variables of epidemic year, peak transmission season, and of precipitation intensity were statistically significant, supporting stratified analyses.
Table 4. Longitudinal model considering all bairro-months (Model 1).
| Parameter | Model 1 (n = 7,140) | ||||
|---|---|---|---|---|---|
| IRR | 95% CI | z | FDR adjusted p-value | ||
| Social & structural factors | Household size | 0.764 | [0.419–1.392] | -0.88 | 0.54 |
| Electricity | 0.956 | [0.790–1.158] | -0.46 | 0.74 | |
| Water | 0.995 | [0.983–1.007] | -0.87 | 0.54 | |
| Garbage | 0.978 | [0.948–1.010] | -1.34 | 0.33 | |
| Sewage | 0.999 | [0.995–1.002] | -0.83 | 0.55 | |
| Literacy (male) | 1.100 | [0.979–1.236] | 1.60 | 0.26 | |
| Literacy (female) | 0.936 | [0.828–1.059] | -1.05 | 0.47 | |
| Income | 0.924 | [0.865–0.987] | -2.35 | 0.07 | |
| Pop. Density | 0.981 | [0.961–1.002] | -1.81 | 0.19 | |
| Subnormal (%) | 1.000 | [0.992–1.008] | -0.05 | 0.97 | |
| Homicides | 1.060*** | [1.047–1.073] | 9.44 | <0.01 | |
| Strategic Points | Construction | 1.006 | [0.991–1.022] | 0.81 | 0.56 |
| Fabrication | 1.011 | [0.986–1.036] | 0.83 | 0.55 | |
| Recyclables | 1.013 | [0.995–1.031] | 1.44 | 0.30 | |
| Scrapyard | 1.019 | [1.000–1.038] | 1.97 | 0.14 | |
| Tires | 1.032*** | [1.018–1.046] | 4.49 | <0.01 | |
| Other | 0.981 | [0.961–1.002] | -1.80 | 0.19 | |
| Total Infestation | 0.998 | [0.995–1.002] | -0.90 | 0.53 | |
| LIA/LIRAa | Alert | 1.065 | [0.946–1.200] | 1.05 | 0.47 |
| Risk | 1.100 | [0.792–1.528] | 0.57 | 0.68 | |
| Climate | Rainfall (med) | 1.240*** | [1.145–1.342] | 5.31 | <0.01 |
| Rainfall (high) | 2.035*** | [1.816–2.280] | 12.25 | <0.01 | |
| Rainfall (deviation) | 1.000 | [0.999–1.000] | -1.82 | 0.19 | |
| Temperature | 1.424*** | [1.238–1.638] | 4.95 | <0.01 | |
| Scale | Epidemic Year | 2.603*** | [2.372–2.856] | 20.16 | <0.01 |
| High Month | 3.936*** | [3.629–4.271] | 32.97 | <0.01 | |
Results expressed as incidence rate ratios (IRR) associated with a unit change in covariate value. Model adjusted for multiple testing using the false discovery rate (FDR). Significance is represented as:
*** = 0.01.
Tables 5 and 6 show the models stratified by transmission intensity inter-annually (Models 2 and 3) and seasonally (Models 4 and 5). Results indicate that some covariates were strongly associated with dengue incidence rates during periods of low (Models 3 and 5), but not high (Models 2 and 4) transmission. Though income was not a statistically significant correlate of dengue incidence during epidemic years (Model 2), during years of interepidemic transmission a R$1,000 increase in average annual bairro household income is associated with reduced dengue incidence by more than 10% (Model 3). Further, while exhibiting a negative, protective effect in all strata, the percentage of properties with regular garbage collection was only statistically significant during interepidemic years (Model 3).
Table 5. Longitudinal model stratified by epidemic years and interepidemic years.
| Parameter | Annual Transmission Intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 2 (Epidemic) n = 4,284 | Model 3 (Interepidemic) n = 2,856 | ||||||||
| IRR | 95% CI | z | FDR p-value | IRR | 95% CI | z | FDR p-value | ||
| Social & structural factors | Household size | 0.750 | [0.382–1.472] | -0.84 | 0.55 | 0.807 | [0.448–1.451] | -0.72 | 0.61 |
| Electricity | 0.903 | [0.734–1.110] | -0.97 | 0.50 | 1.111 | [0.897–1.377] | 0.96 | 0.50 | |
| Water | 0.985 | [0.971–0.999] | -2.15 | 0.10 | 1.009 | [0.996–1.022] | 1.35 | 0.33 | |
| Garbage | 0.991 | [0.953–1.032] | -0.43 | 0.75 | 0.954** | [0.921–0.988] | -2.65 | 0.03 | |
| Sewage | 1.000 | [0.996–1.003] | -0.14 | 0.92 | 0.996 | [0.992–1.000] | -2.16 | 0.10 | |
| Literacy (m) | 1.066 | [0.941–1.208] | 1.00 | 0.49 | 1.119 | [0.972–1.287] | 1.56 | 0.26 | |
| Literacy (f) | 0.955 | [0.834–1.092] | -0.68 | 0.63 | 0.930 | [0.800–1.080] | -0.95 | 0.50 | |
| Income | 0.959 | [0.883–1.042] | -0.99 | 0.50 | 0.893*** | [0.844–0.946] | -3.89 | <0.01 | |
| Pop. Density | 0.978 | [0.955–1.002] | -1.79 | 0.19 | 0.987 | [0.968–1.007] | -1.23 | 0.39 | |
| Subnormal (%) | 1.001 | [0.993–1.010] | 0.26 | 0.85 | 0.997 | [0.988–1.005] | -0.81 | 0.56 | |
| Homicides | 1.076*** | [1.057–1.094] | 8.24 | <0.01 | 1.044*** | [1.030–1.057] | 6.45 | <0.01 | |
| Strategic Points | Construction | 0.995 | [0.980–1.011] | -0.59 | 0.67 | 1.019 | [0.998–1.040] | 1.81 | 0.19 |
| Fabrication | 1.008 | [0.983–1.033] | 0.61 | 0.66 | 1.006 | [0.976–1.038] | 0.40 | 0.76 | |
| Recyclables | 1.019 | [0.996–1.042] | 1.58 | 0.26 | 1.019 | [0.996–1.043] | 1.59 | 0.26 | |
| Scrapyard | 1.022 | [1.002–1.042] | 2.14 | 0.10 | 1.028 | [1.003–1.053] | 2.22 | 0.09 | |
| Tires | 1.033*** | [1.016–1.051] | 3.73 | <0.01 | 1.017 | [0.995–1.040] | 1.51 | 0.28 | |
| Other | 0.983 | [0.960–1.006] | -1.45 | 0.30 | 0.979 | [0.959–0.999] | -2.01 | 0.13 | |
| Total | 0.996 | [0.991–1.000] | -1.76 | 0.20 | 1.001 | [0.996–1.006] | 0.29 | 0.83 | |
|
LIA/ LIRAa |
Alert | 1.194** | [1.042–1.368] | 2.55 | 0.04 | 0.944 | [0.791–1.128] | -0.63 | 0.65 |
| Risk | 1.344 | [0.978–1.849] | 1.82 | 0.19 | 1.541 | [0.727–3.263] | 1.13 | 0.43 | |
| Climate | Rainfall (sum) | 1.006*** | [1.005–1.007] | 11.71 | <0.01 | 0.999 | [0.999–1.000] | -1.57 | 0.26 |
| Rainfall (deviation) | 0.994*** | [0.993–0.995] | -10.48 | <0.01 | 1.003*** | [1.002–1.004] | 6.30 | <0.01 | |
| Temperature | 1.641*** | [1.368–1.970] | 5.32 | <0.01 | 2.245*** | [1.779–2.833] | 6.81 | <0.01 | |
| Scale | High Month | 3.672*** | [3.219–4.189] | 19.36 | <0.01 | 2.944*** | [2.692–3.219] | 23.70 | <0.01 |
Results expressed as incidence rate ratios (IRR) associated with a unit change in covariate value. Models adjusted for multiple testing using the false discovery rate (FDR). Significance is represented as:
** = 0.05
*** = 0.01
Table 6. Longitudinal model stratified by high and low transmission months.
| Parameter | Monthly Transmission Intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 4 (High, peak) n = 4,284 | Model 5 (Low, residual) n = 2,856 | ||||||||
| IRR | 95% CI | z | FDR p-value | IRR | 95% CI | z | FDR p-value | ||
| Social & structural factors | Household size | 0.847 | [0.432–1.660] | -0.48 | 0.73 | 0.705 | [0.402–1.235] | -1.22 | 0.39 |
| Electricity | 1.119 | [0.943–1.328] | 1.29 | 0.35 | 0.830 | [0.670–1.027] | -1.71 | 0.22 | |
| Water | 0.990 | [0.977–1.004] | -1.42 | 0.31 | 1.002 | [0.991–1.014] | 0.42 | 0.75 | |
| Garbage | 0.985 | [0.950–1.022] | -0.80 | 0.56 | 0.964 | [0.934–0.995] | -2.24 | 0.08 | |
| Sewage | 0.997 | [0.994–1.001] | -1.43 | 0.31 | 0.999 | [0.996–1.003] | -0.41 | 0.76 | |
| Literacy (m) | 1.043 | [0.898–1.210] | 0.55 | 0.69 | 1.156** | [1.040–1.284] | 2.69 | 0.03 | |
| Literacy (f) | 0.994 | [0.845–1.168] | -0.07 | 0.96 | 0.891 | [0.800–0.992] | -2.09 | 0.11 | |
| Income | 0.915** | [0.860–0.973] | -2.84 | 0.02 | 0.908** | [0.849–0.971] | -2.83 | 0.02 | |
| Pop. Density | 0.975 | [0.954–0.997] | -2.27 | 0.08 | 0.990 | [0.970–1.010] | -1.01 | 0.49 | |
| Subnormal (%) | 1.001 | [0.992–1.010] | 0.14 | 0.92 | 0.999 | [0.992–1.007] | -0.13 | 0.92 | |
| Homicides | 1.073*** | [1.056–1.090] | 8.56 | <0.01 | 1.046*** | [1.035–1.057] | 8.23 | <0.01 | |
| Strategic Points | Construction | 1.007 | [0.992–1.022] | 0.91 | 0.53 | 1.009 | [0.988–1.031] | 0.87 | 0.54 |
| Fabrication | 1.017 | [0.989–1.046] | 1.18 | 0.41 | 1.009 | [0.970–1.049] | 0.44 | 0.74 | |
| Recyclables | 1.017 | [0.993–1.042] | 1.40 | 0.32 | 1.016 | [0.987–1.047] | 1.07 | 0.46 | |
| Scrapyard | 1.015 | [0.996–1.035] | 1.56 | 0.26 | 1.030 | [0.996–1.064] | 1.74 | 0.21 | |
| Tires | 1.029*** | [1.013–1.046] | 3.46 | <0.01 | 1.030 | [1.004–1.056] | 2.27 | 0.08 | |
| Other | 0.983 | [0.962–1.004] | -1.59 | 0.26 | 1.000 | [0.971–1.030] | 0.01 | 0.99 | |
| Total | 0.997 | [0.993–1.001] | -1.50 | 0.28 | 0.996 | [0.990–1.002] | -1.39 | 0.32 | |
|
LIA/ LIRAa |
Alert | 1.121 | [0.963–1.304] | 1.48 | 0.29 | 1.062 | [0.945–1.194] | 1.01 | 0.49 |
| Risk | 1.198 | [0.809–1.773] | 0.90 | 0.53 | 0.974 | [0.768–1.236] | -0.22 | 0.88 | |
| Climate | Rainfall (sum) | 1.005*** | [1.004–1.006] | 9.28 | <0.01 | 1.003*** | [1.002–1.004] | 5.84 | <0.01 |
| Rainfall (deviation) | 0.997*** | [0.996–0.998] | -7.09 | <0.01 | 0.999 | [0.998–1.000] | -1.40 | 0.32 | |
| Temperature | 0.644*** | [0.513–0.810] | -3.77 | <0.01 | 1.556*** | [1.295–1.870] | 4.72 | <0.01 | |
| Scale | Epidemic | 3.379*** | [2.902–3.934] | 15.69 | <0.01 | 1.467*** | [1.335–1.613] | 7.95 | <0.01 |
Results expressed as incidence rate ratios (IRR) associated with a unit change in covariate value. Models adjusted for multiple testing using the false discovery rate (FDR). Significance is represented as:
** = 0.05
*** = 0.01
With respect to SPs, a 1% increase in areal exposure to tires SP sites was associated with 3.3% increased dengue incidence during epidemic years (Model 2). Conversely, though unit increases in exposure to construction, recycling, scrapyard, and tire sites were all positively correlated with dengue rates during interepidemic years, none were statistically significant when corrected for multiple testing (Model 3). Unlike every other model, the continuous variable for monthly sum precipitation (mm) was not positively associated with incidence over the course of interepidemic years in our sample, reflecting the absence of typical dengue seasonality during those years.
Models 4 and 5 (Table 6) present results stratified by monthly transmission intensity (Fig 3). Though homicides were associated with dengue rates in all models, an additional 10 homicides per 100,000 was associated with a 7.3% higher incidence rate during high transmission months (Model 4), and 7.6% higher rate during epidemic years (Model 2), exceeding increases of 4.6% and 4.4% estimated for periods of lower transmission (Models 3 and 5). Income was a statistically significant protective correlate during both high and low transmission months (Models 4 and 5), and–though not statistically significant when corrected for multiple testing–models estimated stronger associations between regular garbage collection and dengue incidence during late-season months of residual transmission (Model 5). Finally, literacy was a statistically significant bairro-level correlate of dengue incidence rates during low months (Model 5), but with divergent effects: male literacy was associated with increased dengue incidence rates, while female literacy was correlated with lower rates. The strongest associations between proximity to infested SPs and dengue incidence related to scrapyards and tire sites within both seasonal strata (Models 4 and 5); while the estimated effects were larger during residual months (Model 5), the corrected associations were not statistically significant. Movement from satisfactory (<1% property infestation) to alert (1–3.9%) levels of larval infestation, as measured by cross-sectional LIRA and LIA surveys conducted in January, was a statistically significant correlate of incidence in bairros across epidemic years (Model 2), but not when the sample was isolated to peak months (Model 4). Temperatures were slightly lower and more variable, on average, during epidemic years (Table 2); nevertheless, a negative correlation between variability and incidence during months of seasonal transmission (Model 4) suggests lower temperature variance during peak months of epidemic years relative to interepidemic years.
Models 6, 7, and 8 are stratified by precipitation intensity and presented in Table 7. Consistent with results for low-transmission months (Model 5), literacy and garbage collection were correlates of dengue incidence only during months with minimal rainfall (Model 6). Correlation between dengue incidence and income peaked during “transition” precipitation strata (Model 7)–where R$ 1,000 increases in income were associated with an 11% decrease in dengue incidence rates–but the effect was nearly halved during periods of high precipitation (Model 8). Relative to dry months (Model 6), during wetter months (Models 7 & 8) the magnitude of the association with homicides doubled and a negative association with population density increased in magnitude. Though a relationship between tires SP sites and dengue incidence was observed during low precipitation months (Model 6), associations between dengue incidence and SP proximity were strongest amidst intermediate precipitation (Model 7), when marginal increases in the areal proportion for tire and scrapyard sites were associated with over 4% increased incidence rates. The estimated effect for these SP types declined to 2.9% and 1.1%, respectively, during high precipitation periods (Model 8). While temperature variability was a strong predictor of monthly dengue incidence rates during periods with minimal precipitation (Model 6), it registered a null association during wetter months (Model 8).
Table 7. Longitudinal model considering data stratified by precipitation volume (mm).
| Parameter | Bairro Precipitation Intensity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 6 (Low, <15mm) n = 3,054 | Model 7 (Mid, 15-100mm) n = 1,969 | Model 8 (High, >100mm) n = 2,117 | |||||||||||
| IRR | 95% CI | z | FDR p-val |
IRR | 95% CI | z | FDR p-val |
IRR | 95% CI | z | FDR p-val |
||
| Social & structural factors | Household size | 0.680 | [0.389–1.191] | -1.35 | 0.33 | 0.920 | [0.452–1.871] | - 0.23 | 0.87 | 0.703 | [0.345–1.431] | - 0.97 | 0.50 |
| Electricity | 0.879 | [0.680–1.138] | - 0.98 | 0.50 | 0.935 | [0.737–1.187] | - 0.55 | 0.69 | 1.046 | [0.865–1.266] | 0.47 | 0.74 | |
| Water | 1.001 | [0.988–1.014] | 0.10 | 0.94 | 0.997 | [0.984–1.011] | - 0.38 | 0.77 | 0.994 | [0.977–1.011] | - 0.72 | 0.61 | |
| Garbage | 0.952** | [0.921–0.984] | - 2.89 | 0.02 | 0.989 | [0.958–1.020] | - 0.71 | 0.61 | 0.981 | [0.939–1.024] | - 0.89 | 0.53 | |
| Sewage | 0.998 | [0.995–1.001] | - 1.17 | 0.41 | 1.000 | [0.996–1.004] | - 0.09 | 0.94 | 0.998 | [0.994–1.002] | - 1.13 | 0.43 | |
| Literacy (m) | 1.126 | [1.019–1.244] | 2.32 | 0.07 | 1.079 | [0.946–1.230] | 1.13 | 0.43 | 1.080 | [0.917–1.271] | 0.92 | 0.52 | |
| Literacy (f) | 0.908 | [0.813–1.015] | - 1.70 | 0.22 | 0.964 | [0.846–1.100] | - 0.54 | 0.69 | 0.951 | [0.792–1.143] | - 0.53 | 0.70 | |
| Income | 0.918 | [0.847–0.995] | - 2.09 | 0.11 | 0.890*** | [0.836–0.947] | - 3.68 | <0.01 | 0.938 | [0.869–1.012] | - 1.65 | 0.24 | |
| Pop. Density | 1.002 | [0.981–1.024] | 0.17 | 0.91 | 0.976 | [0.954–0.998] | - 2.12 | 0.10 | 0.970** | [0.947–0.994] | - 2.48 | 0.05 | |
| Subnormal (%) | 0.997 | [0.990–1.005] | - 0.70 | 0.61 | 1.001 | [0.992–1.009] | 0.19 | 0.89 | 1.000 | [0.990–1.010] | - 0.03 | 0.98 | |
| Homicides | 1.036*** | [1.025–1.047] | 6.61 | <0.01 | 1.072*** | [1.056–1.088] | 9.26 | <0.01 | 1.073*** | [1.054–1.093] | 7.53 | <0.01 | |
| Strategic Points | Construction | 1.008 | [0.984–1.032] | 0.61 | 0.66 | 1.018 | [0.997–1.039] | 1.67 | 0.23 | 1.006 | [0.989–1.023] | 0.70 | 0.61 |
| Fabrication | 1.039 | [0.984–1.096] | 1.39 | 0.32 | 1.007 | [0.976–1.040] | 0.45 | 0.74 | 1.013 | [0.983–1.044] | 0.87 | 0.54 | |
| Recyclables | 1.025 | [0.995–1.056] | 1.64 | 0.23 | 1.013 | [0.990–1.037] | 1.10 | 0.45 | 1.008 | [0.983–1.035] | 0.64 | 0.65 | |
| Scrapyard | 1.032 | [0.981–1.086] | 1.21 | 0.39 | 1.041*** | [1.017–1.066] | 3.36 | <0.01 | 1.011 | [0.992–1.031] | 1.15 | 0.43 | |
| Tires | 1.040 | [1.005–1.076] | 2.25 | 0.08 | 1.041*** | [1.024–1.058] | 4.85 | <0.01 | 1.029** | [1.008–1.051] | 2.71 | 0.03 | |
| Other | 0.972 | [0.945–0.999] | - 2.05 | 0.12 | 0.987 | [0.971–1.004] | - 1.46 | 0.30 | 0.980 | [0.952–1.009] | - 1.34 | 0.33 | |
| Total Infestation | 0.999 | [0.991–1.006] | - 0.37 | 0.77 | 0.993** | [0.988–0.998] | - 2.86 | 0.02 | 0.999 | [0.994–1.003] | - 0.66 | 0.63 | |
| LIA/LIRAa | Alert | 1.101 | [0.952–1.273] | 1.30 | 0.35 | 0.971 | [0.849–1.111] | - 0.43 | 0.75 | 1.114 | [0.943–1.318] | 1.27 | 0.37 |
| Risk | 0.914 | [0.636–1.314] | - 0.48 | 0.73 | 1.131 | [0.833–1.535] | 0.79 | 0.57 | 1.186 | [0.768–1.832] | 0.77 | 0.58 | |
| Climate | Temperature | 2.114*** | [1.663–2.688] | 6.11 | <0.01 | 1.306** | [1.078–1.582] | 2.72 | 0.02 | 1.162 | [0.956–1.412] | 1.50 | 0.28 |
| Scale | Epidemic Year | 1.194** | [1.056–1.351] | 2.82 | 0.02 | 3.395*** | [2.909–3.962] | 15.50 | <0.01 | 4.471*** | [3.796–5.266] | 17.93 | <0.01 |
| High Month | 2.706*** | [2.404–3.045] | 16.50 | <0.01 | 4.075*** | [3.658–4.540] | 25.48 | <0.01 | 3.562*** | [3.069–4.134] | 16.71 | <0.01 | |
Results expressed as incidence rate ratios (IRR) associated with a unit change in covariate value. Models adjusted for multiple testing using the false discovery rate (FDR). Significance is represented as:
** = 0.05
*** = 0.01.
Discussion
This study analyzed five years of dengue incidence at fine spatial and temporal scales. One of the most important findings was the distinct seasonal pattern between interepidemic and epidemic years. Our results indicate that dengue epidemiological curves differed according to the intensity of annual transmission: while the pattern of transmission during epidemic years conforms to widely documented seasonality, during non-epidemic years low-level transmission persisted into climatologically inhospitable conditions. Sustained transmission after June could result from relaxed control activity following a high season with minimal transmission. Such lapses during interepidemic periods are likely to compound entomological and epidemiological challenges for the next annual cycle, and call for sustained vector control activities regardless of transmission intensity. Bairros in the southern periphery of the city experienced a larger dengue burden than the coastal city center, particularly during interepidemic years, suggesting that sustained, non-seasonal transmission is linked to conditions in those peripheral areas. Socio-ecological indicators of poverty and deprivation were correlated with higher bairro-level dengue incidence rates during seasonal and non-seasonal months–highest during non-epidemic years–but not across epidemics years. The most pronounced associations between Ae. aegypti surveillance at targeted sites and dengue incidence occurred during transitional precipitation seasons, suggesting that they may be one factor linking stages of residual and epidemic transmission. In contrast to the SP class covariates (which quantify the proportion of a bairro spatially proximate to an infested site in bi-monthly intervals), measures of the SP infestation index and entomological cross-sectional surveys (LIA and LIRAa)–ostensibly conducted to identify areas with heightened vulnerability to dengue transmission–did not reliably capture the variability in risk between seasons or bairros, corroborating other studies [88, 89]. These results are not surprising, given that the relationship between larval indices and adult densities is diminished by adult flight [90], and variable survival rates of immature forms and productivity by container type [91]. In fact, the association between cross-sectional entomological surveys (LIRAa/LIA) and incidence during epidemic years was dependent upon the inclusion of the precipitation deviation covariate in the model, reinforcing the need for closer scrutiny of those surveys. These findings have direct programmatic implications, identifing places and stages of the epidemic cycle when targeted community interventions can be prioritized by the Fortaleza Municipal Health Secretariat.
While the contribution of non-residential sites (such as the SPs) to vector propagation has been discussed [92], minimal attention is given to their epidemiological relevance. In some cases, non-residential surveillance has been limited to natural sites and niches uncharacteristic of Ae. aegypti oviposition [93] or a restricted class of non-residential sites, such as schools [94, 95]. Consideration of non-residential structures and spaces that sustain oviposition during dry seasons–such as vacant lots [96], septic tanks [97], and drains [98, 99]–is sparse. Although special surveillance of SPs is mandatory in Brazil, the absence of rigorous examination of the importance of SP infestation for dengue virus transmission limits the capacity of municipal actors to take evidence-based steps to improve their routine control activities. To the best of our knowledge, this is the first study to use fine scale information on SP inspection.
Infestation of SPs was spatially and temporally associated with dengue incidence in Fortaleza, and the magnitude of the associations differed by SP type and according to the scale of transmission and precipitation. Specifically, scrapyards and sites associated with tire collection and storage–such as repair shops and garages–showed associations with dengue incidence during periods of both interepidemic and epidemic transmission. The proportion of a bairro proximate to sites known for storing tires was a strong and reliable correlate of dengue incidence within nearly all strata, but the magnitude of the coefficient was largest during transitional precipitation regimes (when rainfall was neither sparse nor extreme) and dry seasons. Further, after adjustment for FDR, scrapyards registered statistically significant correlations with dengue incidence rates during the same intermediate rainfall strata. These results, combined with the new findings regarding the seasonal pattern of dengue transmission in interepidemic years, strongly suggest that enhanced surveillance should be sustained during low transmission periods, in both epidemic and interepidemic years. The Municipal Health Secretariat could characterize scrapyards and tire repair shops as “high risk” strategic points; such a classification could entail more frequent and thorough surveillance proportional to the physical size of the site, and to the number of potential oviposition habitats. An enhanced program would also impose strict and transparent consequences for sustained infestation at these locations (such as revocation of licenses or more effective fine enforcement), and where operators exhibit disregard for vector control protocol during successive visits.
Further, many of the socioeconomic, structural, and environmental factors expected to be associated with dengue transmission showed varied significance according to transmission and precipitation intensity. Among the factors related to access to public services, regular garbage collection was the most consistent correlate of dengue incidence, consistent with previous studies [100, 101]. However, we also show that the link is most pronounced during low transmission and low precipitation periods. Empty lots filled with abandoned trash–as well as discarded materials such as cans, plastic bottles, and debris commonly observed in yards of houses and along sidewalks [15]–could preserve desiccation-resistant eggs, which hatch following intermittent late-season rains. Thus, closer property surveillance and scrutiny of empty lots may be an important component of targeted vector control during interepidemic periods to prevent the escalation to epidemic-scale transmission.
With regard to income, negative [102] and null [100] associations between poverty and dengue incidence have been reported for Fortaleza. In contrast, with the exception of the epidemic year (Model 2) and high precipitation (Model 8) models, when incidence was shown to be more widely dispersed throughout the city, our results indicate a persistent concentration of dengue cases in lower income bairros. The association is strongest during periods of intermediate precipitation (Model 7), where a unit increase in bairro average household income (equal to one half standard deviation) is associated with 11% decrease in dengue incidence. The associations with income were highly statistically significant during months of high and low transmission (Models 4 & 5), but not in the combined dataset (Model 1), demonstrating the importance of stratifying models by transmission scale. Nonetheless, these results need to be interpreted with caution: health care provided by the private sector is largely underreported in SINAN, despite the fact that notification is mandatory [103]. In Fortaleza, approximately 30% of the population uses private health services, and in more affluent bairros this proportion reaches 85%. While the results observed may reflect underreporting of care provided to higher income populations, when stratified annually (Models 2 & 3) correlations with poverty were isolated to non-epidemic years, suggesting that changing dengue transmission dynamics at different stages of the interannual epidemic cycle also underly this association.
Consistent and large correlations between dengue incidence and interpersonal violence, which was also observed for tuberculosis [104], exemplify the difficulty of implementing effective population health measures in expansive cities such as Fortaleza, as well as the importance of involving different governmental sectors to address these challenges. In contrast to many other exposures, homicides were more strongly associated with dengue rates during epidemics and high transmission months. When violence deters actors tasked with providing municipal services it limits access to health services and implementation of responsive vector control [105]. Vector control agents–who are often tasked with canvassing bairros and entering properties–may neglect areas that are rife with violence, replicating challenges to effective control of yellow fever that were encountered by Oswaldo Cruz in the early 20th century [106]. In conjunction, wary property owners may refuse entry to vector control and health service officers out of fear for their personal safety [107, 108]. In the context of Aedes control, while achieving total coverage is rare, the larger the areas of the city that remain uninspected (and thus untreated), the higher the threat to effective vector control [109].
With regard to bairro structural factors, subnormal settlements were not associated with dengue transmission. This may reflect data limitations. Classification of a census tract as AS does not characterize the degree of subnormality, such as the proportion of houses that are subnormal, or the nature of the AS that predominates in a tract. Persistent negative correlations between population density and dengue incidence may reflect the dengue burden in Fortaleza’s peripheral bairros–where large expanses without dense development and disconnected from proper urban planning are common (e.g. Prefeito José Walter)–and lower incidence rates in densely populated coastal bairros, whether affluent (e.g. Meireles) or not (e.g. Pirambu).
Though we did not analyze serological data, the Fortaleza Municipal Health Secretariat conducts limited sampling to monitor the introduction of allochthonous serotypes. All four serotypes circulated in Fortaleza between 2011–2015, but DENV1 and DENV4 predominated. DENV1 was reintroduced in 2011 after 10 years, and was the primary serotype in 2011, 2014 and 2015. DENV4 was first introduced in 2012, and was responsible for the majority of cases in 2012 and 2013. In all years, the predominant serotype was present in more than 90% of serological samples, indicating that spatial transmission dynamics may be driven by the degree of population susceptibility throughout Fortaleza’s neighborhoods. As a result of diminished population susceptibility, it is unlikely that DENV1 (which caused an epidemic in 2011) was solely responsible for the estimated disease burden in 2015. The Fortaleza Health Secretariat has found that a significant number of Zika virus (ZIKV) cases were misclassified as dengue in 2015, and that DENV1 and ZIKV co-circulated. Though imported cases of chikungunya (CHIKV) were detected by the surveillance of the City of Fortaleza in 2014, autochthonous cases were not confirmed until December 2015. Therefore, the circulation of ZIKV in 2015 offers one possible explanation for the epidemiological curve observed for that year. The epidemic peak in 2015 was lower than in 2011 and 2012, and incidence in 2015 extended into non-seasonal months at a scale that is more characteristic of interepidemic years (Table 1). It is possible that the peak of 2015 transmission is attributable to seasonal misdiagnosis of ZIKV and CHIKV cases, in which protracted incidence would be characteristic of interepidemic dengue virus transmission in Fortaleza. Alternatively, it is possible that 2015 did have seasonal epidemic dengue transmission, and that protracted non-seasonal transmission is attributable to Zika misdiagnosis.
This study has many strengths. First, data are drawn from multiple years, permitting analysis of interepidemic and epidemic transmission patterns, with and without typical patterns of dengue seasonality. Second, by using temperature and rainfall information, seasonality by year could be properly characterized, accounting for possible weather anomalies. Third, the analysis combined entomological surveys with socio-ecological and climatological data to provide a comprehensive assessment of transmission patterns and correlates. Lastly, the study incorporates routine cross-sectional surveillance surveys of immature forms (considered to be a proxy of the concentration of dengue cases in an urban landscape).
This study has some limitations. First, as any analysis that uses administrative records, data refer to passive surveillance, and do not include asymptomatic infections and/or mild cases that do not trigger search for care [110]. This issue may be qualitatively dependent on the intensity of transmission [56], and communities with access to private healthcare providers may underreport at greater rates than low-income bairros [111]. Second, given the circulation of ZIKV in Fortaleza in 2015, the fact that about 20% of cases were lab confirmed in 2015, and the lack of serological tests with high specificity, we expect that some dengue cases reported that year were, in fact, ZIKV infections. Yet, since both diseases are Aedes-borne, they are subject to the same socio-ecological factors, and thus their co-circulation should not detract from our conclusions. Third, some variables may not properly capture access and behavior. For example, while we might have expected associations between dengue and access to piped water during dry seasons (as a result of increases in water storage behavior), an ethnographic study in Fortaleza suggested that potable water storage is pervasive [15], such that bairros-scale statistics on access to piped water may not be adequate to capture the phenomenon. Similarly, including socio-demographic covariates such as income, access to municipal services, interpersonal violence, and prevalence of subnormal agglomerations at the bairro level may not satisfactorily control for socio-economic factors expected to be associated with dengue incidence. As a result, in addition to their role as Aedes breeding sites, the prevalence of strategic points–such as scrapyards and tire shops–in a bairro may be a proxy for unobserved differences that are relevant to the transmission cycle. Fourth, we only have access to one weather station for temperature data; yet, considering the low variability in temperature observed in Fortaleza, we do not expect this to be a major limitation. Lastly, the entomological surveillance data may only be a partial depiction of the scale of infestation as a result of barriers that vector surveillance agents face when conducting their inspection. Since we are using data from a targeted surveillance program, we assume that some areas may be prioritized because of their known high risk for mosquito breeding.
Supporting information
(PDF)
(PDF)
(PDF)
Range is bounded at seven clusters. Selected number of clusters identified by vertical dotted line.
(PDF)
Outlying single- and multi-bairro clusters are labeled.
(PDF)
(ZIP)
Acknowledgments
We thank the Fortaleza Municipal Health Secretariat for providing access to the data used in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was partially funded by Fundação de Ciência, Tecnologia e Inovação (CITINOVA), and by the Harvard University Lemann Brazil Research Fund (MCC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci U S A. 1994;91(7):2395–400. Epub 1994/03/29. ; PubMed Central PMCID: PMCPMC43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–96. Epub 1998/07/17. ; PubMed Central PMCID: PMCPMC88892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. Epub 2013/04/09. 10.1038/nature12060 ; PubMed Central PMCID: PMCPMC3651993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Dengue Fact Sheet 2017 [March 16, 2017]. Available from: http://www.searo.who.int/entity/vector_borne_tropical_diseases/data/data_factsheet/en/
- 5.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4(5):e646 Epub 2010/06/04. 10.1371/journal.pntd.0000646 ; PubMed Central PMCID: PMCPMC2876112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9(7):532–41. Epub 2011/06/15. 10.1038/nrmicro2595 ; PubMed Central PMCID: PMCPMC3321645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg. 1997;57(2):235–9. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 8.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38(3):411–22. Epub 2001/05/25. . [DOI] [PubMed] [Google Scholar]
- 9.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42(5):844–9. Epub 2005/12/21. . [DOI] [PubMed] [Google Scholar]
- 10.Hemme RR, Thomas CL, Chadee DD, Severson DW. Influence of urban landscapes on population dynamics in a short-distance migrant mosquito: evidence for the dengue vector Aedes aegypti. PLoS Negl Trop Dis. 2010;4(3):e634 Epub 2010/03/20. 10.1371/journal.pntd.0000634 ; PubMed Central PMCID: PMCPMC2838782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie S. Dengue Vector Bionomics: Why Aedes aegypti is such a good vector In: Gubler DJ, Ooi E. E., Vasudevan S., & Farrar J., editor. DENV and DENV hemorrhagic Fever. 2 ed Boston, MA: CAB International; 2014. p. 455–80. [Google Scholar]
- 12.Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90(3):353–8. Epub 1992/06/01. 10.1007/BF00317691 . [DOI] [PubMed] [Google Scholar]
- 13.Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, et al. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41(6):1123–42. Epub 2004/12/21. . [DOI] [PubMed] [Google Scholar]
- 14.Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop Med Health. 2011;39(4 Suppl):3–11. Epub 2012/04/14. 10.2149/tmh.2011-S05 ; PubMed Central PMCID: PMCPMC3317603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caprara A, Lima JW, Marinho AC, Calvasina PG, Landim LP, Sommerfeld J. Irregular water supply, household usage and dengue: a bio-social study in the Brazilian Northeast. Cad Saude Publica. 2009;25 Suppl 1:S125–36. Epub 2009/03/17. . [DOI] [PubMed] [Google Scholar]
- 16.Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58(3):277–82. Epub 1998/04/18. . [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4(12):e920 Epub 2011/01/05. 10.1371/journal.pntd.0000920 ; PubMed Central PMCID: PMCPMC3006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Rejon J, Lorono-Pino MA, Farfan-Ale JA, Flores-Flores L, Del Pilar Rosado-Paredes E, Rivero-Cardenas N, et al. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79(6):940–50. Epub 2008/12/05. . [PubMed] [Google Scholar]
- 19.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69(5):494–505. Epub 2003/12/26. . [PubMed] [Google Scholar]
- 20.Reiner RC Jr., Stoddard ST, Scott TW. Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics. 2014;6:30–6. Epub 2014/03/07. 10.1016/j.epidem.2013.12.003 ; PubMed Central PMCID: PMCPMC3971836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110(3):994–9. Epub 2013/01/02. 10.1073/pnas.1213349110 ; PubMed Central PMCID: PMCPMC3549073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achee NL, Gould F, Perkins TA, Reiner RC Jr., Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9(5):e0003655 Epub 2015/05/08. 10.1371/journal.pntd.0003655 ; PubMed Central PMCID: PMCPMC4423954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, et al. Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ. 2010;88(3):173–84. Epub 2010/04/30. 10.2471/BLT.09.067892 ; PubMed Central PMCID: PMCPMC2828788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caprara A, Lima JW, Peixoto AC, Motta CM, Nobre JM, Sommerfeld J, et al. Entomological impact and social participation in dengue control: a cluster randomized trial in Fortaleza, Brazil. Trans R Soc Trop Med Hyg. 2015;109(2):99–105. Epub 2015/01/22. 10.1093/trstmh/tru187 ; PubMed Central PMCID: PMCPMC4299523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell-Foster K, Ayala EB, Breilh J, Spiegel J, Wilches AA, Leon TO, et al. Integrating participatory community mobilization processes to improve dengue prevention: an eco-bio-social scaling up of local success in Machala, Ecuador. Trans R Soc Trop Med Hyg. 2015;109(6):419 Epub 2015/04/22. 10.1093/trstmh/trv028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. Handbook for integrated vector management. Geneva 2012.
- 27.Sommerfeld J, Kroeger A. Eco-bio-social research on dengue in Asia: a multicountry study on ecosystem and community-based approaches for the control of dengue vectors in urban and peri-urban Asia. Pathog Glob Health. 2012;106(8):428–35. Epub 2013/01/16. 10.1179/2047773212Y.0000000055 ; PubMed Central PMCID: PMCPMC3541880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5(3):e68 Epub 2008/03/21. 10.1371/journal.pmed.0050068 ; PubMed Central PMCID: PMCPMC2267811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiner RC Jr., Achee N, Barrera R, Burkot TR, Chadee DD, Devine GJ, et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl Trop Dis. 2016;10(5):e0004588 Epub 2016/05/27. 10.1371/journal.pntd.0004588 ; PubMed Central PMCID: PMCPMC4881945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiter P. Surveillance and Control of Urban Dengue Vectors In: Gubler DJ, Ooi E. E., Vasudevan S., & Farrar J., editor. DENV and DENV hemorrhagic Fever. 2 ed Boston, MA: CAB International; 2014. p. 481–518. [Google Scholar]
- 31.Manrique-Saide P, Che-Mendoza A, Barrera-Perez M, Guillermo-May G, Herrera-Bojorquez J, Dzul-Manzanilla F, et al. Use of insecticide-treated house screens to reduce infestations of dengue virus vectors, Mexico. Emerg Infect Dis. 2015;21(2):308–11. Epub 2015/01/28. 10.3201/eid2102.140533 ; PubMed Central PMCID: PMCPMC4313634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson AL, Dhiman RC, Kitron U, Scott TW, van den Berg H, Lindsay SW. Benefit of insecticide-treated nets, curtains and screening on vector borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(10):e3228 Epub 2014/10/10. 10.1371/journal.pntd.0003228 ; PubMed Central PMCID: PMCPMC4191944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, et al. Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ. 2006;332(7552):1247–52. Epub 2006/06/01. 10.1136/bmj.332.7552.1247 ; PubMed Central PMCID: PMCPMC1471956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie SA, Long S, Smith G, Pyke A, Knox TB. Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J Med Entomol. 2004;41(1):1–4. Epub 2004/03/03. . [DOI] [PubMed] [Google Scholar]
- 35.Stoddard ST, Wearing HJ, Reiner RC Jr., Morrison AC, Astete H, Vilcarromero S, et al. Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8(7):e3003 Epub 2014/07/18. 10.1371/journal.pntd.0003003 ; PubMed Central PMCID: PMCPMC4102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braga C, Luna CF, Martelli CM, de Souza WV, Cordeiro MT, Alexander N, et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 2010;113(3):234–40. Epub 2009/11/10. 10.1016/j.actatropica.2009.10.021 ; PubMed Central PMCID: PMCPMC3847853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson MA, Dominici F, Glass GE. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl Trop Dis. 2009;3(2):e382 Epub 2009/02/18. 10.1371/journal.pntd.0000382 ; PubMed Central PMCID: PMCPMC2637540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madeira NG, Macharelli CA, Pedras JF, Delfino MC. Education in primary school as a strategy to control dengue. Rev Soc Bras Med Trop. 2002;35(3):221–6. Epub 2002/06/05. . [DOI] [PubMed] [Google Scholar]
- 39.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. Epub 2013/08/31. 10.2147/CLEP.S34440 ; PubMed Central PMCID: PMCPMC3753061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–93. Epub 2006/05/19. 10.3201/10.3201/eid1206.051210 ; PubMed Central PMCID: PMCPMC3373041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9(1):86–9. Epub 2003/01/21. 10.3201/eid0901.020220 ; PubMed Central PMCID: PMCPMC2873752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci U S A. 2006;103(31):11802–7. Epub 2006/07/27. 10.1073/pnas.0602960103 ; PubMed Central PMCID: PMCPMC1544250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady OJ, Messina JP, Scott TW, Hay SI. Mapping the Epidemiology of Dengue In: Gubler DJ, Ooi E. E., Vasudevan S., & Farrar J., editor. DENV and DENV hemorrhagic Fever. 2 ed Boston, MA: CAB International; 2014. p. 30–49. [Google Scholar]
- 44.Cummings DA, Irizarry RA, Huang NE, Endy TP, Nisalak A, Ungchusak K, et al. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature. 2004;427(6972):344–7. Epub 2004/01/23. 10.1038/nature02225 . [DOI] [PubMed] [Google Scholar]
- 45.Descloux E, Mangeas M, Menkes CE, Lengaigne M, Leroy A, Tehei T, et al. Climate-based models for understanding and forecasting dengue epidemics. PLoS Negl Trop Dis. 2012;6(2):e1470 Epub 2012/02/22. 10.1371/journal.pntd.0001470 ; PubMed Central PMCID: PMCPMC3279338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gubler DJ. Cities spawn epidemic dengue viruses. Nat Med. 2004;10(2):129–30. Epub 2004/02/05. 10.1038/nm0204-129 . [DOI] [PubMed] [Google Scholar]
- 47.IBGE, Diretoria de Pesquisas—DPE, Coordenação de População e Indicadores Sociais—COPIS. Estimativas da população residente nos municípios brasileiros com data de referência em 1 de julho de 2018. https://www.ibge.gov.br/estatisticas-novoportal/sociais/populacao/9103-estimativas-de-populacao.html?=&t=resultados. 2018.
- 48.UN-HABITAT. State of the world’s cities 2010/2011: bridging the urban divide UN-HABITAT; 2010. [Google Scholar]
- 49.Ortega J. Listado de las 50 ciudades más violentas del mundo en 2015. Consejo Ciudadano para la Seguridad Pública y Justicia Penal A.C., 2016. [Google Scholar]
- 50.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15(3):259–63. [Google Scholar]
- 51.Knight JR, Folland CK, Scaife AA. Climate impacts of the Atlantic multidecadal oscillation. Geophysical Research Letters. 2006;33(L17706). [Google Scholar]
- 52.Braga IA, Valle D. Aedes aegypti: histórico do controle no Brasil. Epidemiologia e serviços de saúde. 2007;16(2):113–8. [Google Scholar]
- 53.Teixeira MG, Costa MCN, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cadernos de Saúde Pública. 2009;25(supl.1):S7–S18. [DOI] [PubMed] [Google Scholar]
- 54.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica. 2006;20(6):407–15. Epub 2007/03/08. . [DOI] [PubMed] [Google Scholar]
- 55.Siqueira JB Jr., Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11(1):48–53. Epub 2005/02/12. 10.3201/eid1101.031091 ; PubMed Central PMCID: PMCPMC3294356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira MG, Siqueira JB Jr., Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7(12):e2520 Epub 2014/01/05. 10.1371/journal.pntd.0002520 ; PubMed Central PMCID: PMCPMC3871634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prefeitura de Fortaleza. Boletim Semanal da Dengue: Ano 2016 – 12a Semana Epidemiológica. Secretaria Municipal de Saúde, Célula de Vigilância Epidemiológica, 2016.
- 58.Prefeitura de Fortaleza. Plano de Contingência do Município de Fortaleza para Epidemias de Dengue Fortaleza, Brazil: Secretaria Municipal da Saúde, 2015. [Google Scholar]
- 59.Prefeitura de Fortaleza. Prefeitura de Fortaleza realiza nova etapa de operação de combate ao mosquito da Dengue no Jangurussu: Secretaria Municipal de Saúde; 2017 [April 23, 2017]. Available from: https://www.fortaleza.ce.gov.br/noticias/prefeitura-de-fortaleza-realiza-nova-etapa-da-operacao-inverno-no-jangurussu-2.
- 60.Ministério da Saúde. Sistema de Informação de Agravos de Notificação (SINAN). In: Ministério da Saúde, editor. Brasília 2017.
- 61.Ministério da Saúde. Sistema de Informação de Agravos de Notificação–SINAN: normas e rotinas. In: Ministério da Saúde, editor. Brasília Ministério da Saúde; 2006.
- 62.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Diretrizes Nacionais para a Prevenção e Controle de Epidemias de DENV. Brasília: Ministério da Saúde, 2009.
- 63.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde. Guia de Vigilância em Saúde. Brasília, DF: Ministério da Saúde, Secretaria de Vigilância em Saúde; http://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_unificado.pdf, 2014. [Google Scholar]
- 64.IBGE. Censo Demográfico Instituto Brasileiro de Geografia e Estatística (IBGE), 2011.
- 65.IBGE. Censo Demográfico 2010: Aglomerados subnormais, informações territoriais In: Ministério do Planejamento OaeGo, editor. Rio de Janeiro, Brazil: Instituto Brasileiro de Geografia e Estatística (IBGE); 2011. [Google Scholar]
- 66.IPLANFOR. Assentamentos Precários Institute of Planning of Fortaleza (IPLANFOR); 2015.
- 67.Ministério das Cidades. Política Habitacional e a Integração Urbana de Assentamentos Precários Parâmetros conceituais, técnicos e metodológicos Secretaria Nacional de Habitação SNH, Ministério das Cidades; 2008. [Google Scholar]
- 68.Krug EG, Mercy JA, Dahlberg LL, Zwi AB. The world report on violence and health. Lancet. 2002;360(9339):1083–8. Epub 2002/10/18. 10.1016/S0140-6736(02)11133-0 . [DOI] [PubMed] [Google Scholar]
- 69.Groenwold RH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–9. Epub 2012/03/01. 10.1503/cmaj.110977 ; PubMed Central PMCID: PMCPMC3414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Funk C, Peterson P, Landsfeld M, Pedreros D, Verdin J, Shukla S, et al. The climate hazards infrared precipitation with stations—a new environmental record for monitoring extremes. Sci Data. 2015;2:150066 Epub 2015/12/10. 10.1038/sdata.2015.66 ; PubMed Central PMCID: PMCPMC4672685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes AF, Nobre AA, Cruz OG. Temporal analysis of the relationship between dengue and meteorological variables in the city of Rio de Janeiro, Brazil, 2001–2009. Cad Saude Publica. 2012;28(11):2189–97. Epub 2012/11/14. . [DOI] [PubMed] [Google Scholar]
- 72.Luz PM, Mendes BV, Codeco CT, Struchiner CJ, Galvani AP. Time series analysis of dengue incidence in Rio de Janeiro, Brazil. Am J Trop Med Hyg. 2008;79(6):933–9. Epub 2008/12/05. . [PubMed] [Google Scholar]
- 73.Wu PC, Guo HR, Lung SC, Lin CY, Su HJ. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007;103(1):50–7. Epub 2007/07/07. 10.1016/j.actatropica.2007.05.014 . [DOI] [PubMed] [Google Scholar]
- 74.Hii YL, Rocklov J, Ng N, Tang CS, Pang FY, Sauerborn R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Glob Health Action. 2009;2 Epub 2010/01/07. 10.3402/gha.v2i0.2036 ; PubMed Central PMCID: PMCPMC2799326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anyamba A, Linthicum KJ, Small JL, Collins KM, Tucker CJ, Pak EW, et al. Climate Teleconnections and Recent Patterns of Human and Animal Disease Outbreaks. PLOS Neglected Tropical Diseases. 2012;6(1):e1465 10.1371/journal.pntd.0001465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weather History and Data Archive [Internet]. The Weather Company. 2017. Available from: https://www.wunderground.com/history/.
- 77.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A. 2011;108(18):7460–5. Epub 2011/04/20. 10.1073/pnas.1101377108 ; PubMed Central PMCID: PMCPMC3088608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breslow NE. Extra-Poisson Variation in Log-Linear Models. Journal of the Royal Statistical Society Series C (Applied Statistics). 1984;33(1):38–44. [Google Scholar]
- 79.Lawless JF. Negative binomial and mixed Poisson regression. Canadian Journal of Statistics. 1987;15(3):209–25. [Google Scholar]
- 80.Rose CE, Martin SW, Wannemuehler KA, Plikaytis BD. On the use of zero-inflated and hurdle models for modeling vaccine adverse event count data. Journal of biopharmaceutical statistics. 2006;16(4):463–81. Epub 2006/08/09. 10.1080/10543400600719384 . [DOI] [PubMed] [Google Scholar]
- 81.Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, et al. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecology letters. 2005;8(11):1235–46. Epub 2005/11/01. 10.1111/j.1461-0248.2005.00826.x . [DOI] [PubMed] [Google Scholar]
- 82.Brito RN, Gorla DE, Diotaiuti L, Gomes ACF, Souza RCM, Abad-Franch F. Drivers of house invasion by sylvatic Chagas disease vectors in the Amazon-Cerrado transition: A multi-year, state-wide assessment of municipality-aggregated surveillance data. PLoS Negl Trop Dis. 2017;11(11):e0006035 Epub 2017/11/18. 10.1371/journal.pntd.0006035 ; PubMed Central PMCID: PMCPMC5689836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–6. Epub 2000/07/06. . [DOI] [PubMed] [Google Scholar]
- 84.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 85.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2001;63(2):411–23. 10.1111/1467-9868.00293 [Google Scholar]
- 86.Kassambara A, Mundt F. factoextra. R package version 1.0.5. https://cran.r-project.org/web/packages/factoextra/index.html. 2017.
- 87.RStudio. RStudio: Integrated Development for R Boston, MA: RStudio, Inc; 2016. [Google Scholar]
- 88.Bowman LR, Runge-Ranzinger S, McCall PJ. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis. 2014;8(5):e2848 Epub 2014/05/09. 10.1371/journal.pntd.0002848 ; PubMed Central PMCID: PMCPMC4014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cromwell EA, Stoddard ST, Barker CM, Van Rie A, Messer WB, Meshnick SR, et al. The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infection. PLoS Negl Trop Dis. 2017;11(3):e0005429 Epub 2017/03/24. 10.1371/journal.pntd.0005429 ; PubMed Central PMCID: PMCPMC5363802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honorio NA, Silva Wda C, Leite PJ, Goncalves JM, Lounibos LP, Lourenco-de-Oliveira R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2003;98(2):191–8. Epub 2003/05/24. . [DOI] [PubMed] [Google Scholar]
- 91.Focks DA, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases A review of entomological sampling methods and indicators for dengue vectors. Geneva: World Health Organization. http://www.who.int/iris/handle/10665/68575; 2004. [Google Scholar]
- 92.Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, et al. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100 Suppl 1:S73–S86. Epub 2006/04/25. 10.1179/136485906X105534 . [DOI] [PubMed] [Google Scholar]
- 93.Gurtler RE, Garelli FM, Coto HD. Effects of a five-year citywide intervention program to control Aedes aegypti and prevent dengue outbreaks in northern Argentina. PLoS Negl Trop Dis. 2009;3(4):e427 Epub 2009/04/29. 10.1371/journal.pntd.0000427 ; PubMed Central PMCID: PMCPMC2669131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Rejon JE, Lorono-Pino MA, Farfan-Ale JA, Flores-Flores LF, Lopez-Uribe MP, Najera-Vazquez Mdel R, et al. Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Merida, Mexico. Am J Trop Med Hyg. 2011;84(3):489–96. Epub 2011/03/03. 10.4269/ajtmh.2011.10-0654 ; PubMed Central PMCID: PMCPMC3042828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olano VA, Matiz MI, Lenhart A, Cabezas L, Vargas SL, Jaramillo JF, et al. Schools as Potential Risk Sites for Vector-Borne Disease Transmission: Mosquito Vectors in Rural Schools in Two Municipalities in Colombia. J Am Mosq Control Assoc. 2015;31(3):212–22. Epub 2015/09/17. 10.2987/moco-31-03-212-222.1 . [DOI] [PubMed] [Google Scholar]
- 96.Baak-Baak CM, Arana-Guardia R, Cigarroa-Toledo N, Lorono-Pino MA, Reyes-Solis G, Machain-Williams C, et al. Vacant lots: productive sites for Aedes (Stegomyia) aegypti (Diptera: Culicidae) in Merida City, Mexico. J Med Entomol. 2014;51(2):475–83. Epub 2014/04/15. ; PubMed Central PMCID: PMCPMC4064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackay AJ, Amador M, Diaz A, Smith J, Barrera R. Dynamics of Aedes aegypti and Culex quinquefasciatus in septic tanks. J Am Mosq Control Assoc. 2009;25(4):409–16. Epub 2010/01/27. 10.2987/09-5888.1 . [DOI] [PubMed] [Google Scholar]
- 98.Arana-Guardia R, Baak-Baak CM, Lorono-Pino MA, Machain-Williams C, Beaty BJ, Eisen L, et al. Stormwater drains and catch basins as sources for production of Aedes aegypti and Culex quinquefasciatus. Acta Trop. 2014;134:33–42. Epub 2014/03/04. 10.1016/j.actatropica.2014.01.011 ; PubMed Central PMCID: PMCPMC4070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Souza RL, Mugabe VA, Paploski IAD, Rodrigues MS, Moreira P, Nascimento LCJ, et al. Effect of an intervention in storm drains to prevent Aedes aegypti reproduction in Salvador, Brazil. Parasit Vectors. 2017;10(1):328 Epub 2017/07/13. 10.1186/s13071-017-2266-6 ; PubMed Central PMCID: PMCPMC5505146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heukelbach J, de Oliveira FA, Kerr-Pontes LR, Feldmeier H. Risk factors associated with an outbreak of dengue fever in a favela in Fortaleza, north-east Brazil. Trop Med Int Health. 2001;6(8):635–42. Epub 2001/09/14. . [DOI] [PubMed] [Google Scholar]
- 101.Cordeiro R, Donalisio MR, Andrade VR, Mafra AC, Nucci LB, Brown JC, et al. Spatial distribution of the risk of dengue fever in southeast Brazil, 2006–2007. BMC Public Health. 2011;11:355 Epub 2011/05/24. 10.1186/1471-2458-11-355 ; PubMed Central PMCID: PMCPMC3128013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vasconcelos PF, Lima JWO, da Rosa APT, Timbó MJ, da Rosa EST, Lima HR, et al. Epidemia de dengue em Fortaleza, Ceará: inquérito soro-epidemiológico aleatório. Revista de Saúde Pública. 1998;32(5):447–54. [DOI] [PubMed] [Google Scholar]
- 103.Duarte HHP, Franca EB. Qualidade dos dados da vigilância epidemioógica da dengue em Belo Horizonte, MG. Revista de Saude Publica 2006;40:134–42. [DOI] [PubMed] [Google Scholar]
- 104.Harling G, Lima Neto AS, Sousa GS, Machado MMT, Castro MC. Determinants of tuberculosis transmission and treatment abandonment in Fortaleza, Brazil. BMC Public Health. 2017;17(1):508 Epub 2017/05/27. 10.1186/s12889-017-4435-0 ; PubMed Central PMCID: PMCPMC5445312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loewenberg S. Tackling the causes of ill health in Rio's slums. Lancet. 2005;365(9463):925–6. Epub 2005/03/18. 10.1016/S0140-6736(05)71062-X . [DOI] [PubMed] [Google Scholar]
- 106.Lourenco-de-Oliveira R. Rio de Janeiro against Aedes aegypti: yellow fever in 1908 and dengue in 2008—editorial. Mem Inst Oswaldo Cruz. 2008;103(7):627–8. Epub 2008/12/06. . [DOI] [PubMed] [Google Scholar]
- 107.Jones CH, Benitez-Valladares D, Guillermo-May G, Dzul-Manzanilla F, Che-Mendoza A, Barrera-Perez M, et al. Use and acceptance of long lasting insecticidal net screens for dengue prevention in Acapulco, Guerrero, Mexico. BMC Public Health. 2014;14:846 Epub 2014/08/16. 10.1186/1471-2458-14-846 ; PubMed Central PMCID: PMCPMC4152567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maciel de Freitas R, Marques WA, Peres RC, Cunha SP, Lourenço-de-Oliveira R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Memórias do Instituto Oswaldo Cruz. 2007;102(4):489–96. [DOI] [PubMed] [Google Scholar]
- 109.World Health Organization (WHO), Special Programme for Research and Training in Tropical Diseases (TDR). Dengue. Guidelines for Diagnosis, Treatment, Prevention and Control New Edition ed. Geneva: World Health Organization; http://www.who.int/rpc/guidelines/9789241547871/en/; 2009. [Google Scholar]
- 110.Ooi EE, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saude Publica. 2009;25 Suppl 1:S115–24. Epub 2009/03/17. . [DOI] [PubMed] [Google Scholar]
- 111.Shepard DS, Undurraga EA, Betancourt-Cravioto M, Guzman MG, Halstead SB, Harris E, et al. Approaches to refining estimates of global burden and economics of dengue. PLoS Negl Trop Dis. 2014;8(11):e3306 Epub 2014/11/21. 10.1371/journal.pntd.0003306 ; PubMed Central PMCID: PMCPMC4238988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Range is bounded at seven clusters. Selected number of clusters identified by vertical dotted line.
(PDF)
Outlying single- and multi-bairro clusters are labeled.
(PDF)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.