Abstract
Acute kidney injury (AKI) is a major clinical problem in native and transplanted kidneys. Bidirectional interaction between gut microbiota and kidney tissue or the ‘colo-renal’ system is being recognized as an important modulating factor in AKI. Gut microbes appear to have a complex but yet poorly understood communication with renal cellular and molecular processes that affect normal kidney function and response to injury. There have been major recent advances in the study of the microbiome that provide an opportunity to apply this knowledge to improve our understanding and treatment of patients with AKI. This mini-review aims to focus on select general concepts about the microbiome, mechanisms by which the microbiome can modify kidney function, and data on microbiome and AKI. We have briefly touched on a few topics rather than comprehensively reviewing the role of microbiome in kidney diseases. We also propose future gut microbiota-AKI studies based on advances in gut microbiota studies in other human diseases and experimental models.
Keywords: Intestinal microbiota, acute kidney injury, SCFA, olfactory receptor, colo-renal
Microbiome overview
An estimated 3.8 × 1013 microbes inhabit the human body, and consequently the human body has nearly equal numbers of microbial cells and human cells [1]. The bidirectional evolutionary relationship between intestinal microbes and humans has resulted in a unique and dynamic interdependence between the two species [2]. Recent advancements in metagenomic analysis has markedly improved our understanding of microbiota-related effects in health and disease. Investigations on intestinal microbiota have been the focus of multiple studies in recent years, and the relationship of intestinal microbiota with the kidney is being referred to as colo-renal axis. Alterations in intestinal microbiota dynamics (dysbiosis) has been linked to multiple human diseases, including that of the kidneys [3,4]. Similarly, kidney diseases appear to results in dysbiosis that could affect complications and progression of kidney diseases.
Microbiome and AKI
Acute kidney injury (AKI) as a result of ischemia reperfusion (IR) or nephrotoxins is a significant clinical problem in both native and transplanted kidneys. There has been a large body of work on immune cells in the kidney and AKI, but it is unclear why immune cells inhabit the kidney, a traditional non-immune organ. Work on kidney-distant organ cross talk in AKI generated the possibility that gut bacterial load could alter kidney inflammation [5]. The first mechanistic work on microbiome and AKI initially hypothesized that a low bacterial burden would decrease kidney lymphocytes. To test this, germ free (GF) mice kidneys were analyzed for lymphocyte content [6]. Contrary to expectations, GF mice were found to have an increased kidney natural killer T (NKT) cells and ample numbers of other T cells. The increased NKT cells in GF mice was confirmed by a later study demonstrating increased invariant NKT (iNKT) cell numbers and activity in GF mice predisposed to inflammatory bowel disease and asthma [7]. GF mice subjected to kidney IR injury (IRI) had worse kidney function. When these GF mice were conventionalized with bacteria from wild type (WT) mice, the kidney lymphocyte content normalized, and after IR both kidney function and tissue injury became similar to WT mice [6].
In another study using a kidney IRI model as well as a cisplatin-induced AKI model in normal mice, 16s RNA metagenomics sequencing of stool post AKI demonstrated unique patterns of fecal microbiota that were distinct in AKI compared to normal, and different in ischemic AKI compared to cisplatin-induced AKI [8]. Both Erysipelotrichia and Lactobacillus were found to increase in AKI. Colonization of mixed AKI-specific bacteroides sp. to GF mice led to decrease in kidney NKT and B cell frequency, whereas the frequency of CD8 T cells increased.
The existing data supports the hypothesis that gut microbiota affects kidney function and kidney resident immune cells through short chain fatty acids (SCFAs), mainly acetate and propionate. SCFAs are produced as the end products of the fermentation of dietary fibers by gut microbiota, and are released into the systemic circulation. Administration of SCFAs (acetate, propionate, and butyrate) was found to significantly improve renal dysfunction in a model of IR-induced AKI [9]. Although all three SCFAs protected from AKI, both functionally and histologically, the protection was most pronounced in the group that received acetate. However, short- and long-term effects of SCFA treatment on kidney need to be investigated more carefully, especially given a recent study that showed T cell mediated ureteritis and hydroneophrosis following chronic SCFA treatment [10]. Parallel investigation demonstrated that an olfactory receptor 78 (Olfr78) present in the juxtaglomerular apparatus interacts with SCFAs (acetate and propionate) to regulate kidney function [11]. Furthermore, G protein-coupled receptor 41 (Gpr41) expressed in endothelial cells of blood vessels is also responsive towards SCFAs [12]. Although, both Olfr78 and Gpr41 respond to SCFAs to modulate blood pressure regulation and/or kidney function, their roles in AKI development and progression have not been determined. In addition to producing hemodynamic effects, SCFAs have also been shown to induce immunologic responses by regulating immune cell function and differentiation. In immunocompetent mice, intestinal colonization stimulated the production of secretory IgA, the differentiation of effector T helper 1 (TH1), TH2 and TH17 cells, and the development of regulatory T (Treg) cells [13].
A more recent study showed that depletion of gut microbiota using broad spectrum antibiotics (1% solution containing ampicillin, metronidazole, neomycin and vancomycin) protected from IR-induced AKI, and that transplantation of fecal material from untreated mice abolished the protective effect of antibiotics [14]. Microbiota depletion following antibiotic treatment was accompanied by a reduction in F4/80 and chemokine receptors (CX3CR1 and CCR2) expression in the F4/80+ kidney resident and bone marrow macrophages. The reasons for the seemingly opposing effects of intestinal microbiota on AKI outcome in this study compared to that of Jang et al [6] are not clear. We speculate that in contrast to the study using GF mice that lacked all bacteria, antibiotic treatment in normal mice resulted in selective depletion of deleterious gut microbiota but not AKI protective bacteria. The effect of microbiota on the development of the immune system in GF mice may have also contributed to the differences. Nonetheless, the findings of this study in conjunction with the other direct studies on experimental AKI, suggests a complex role for microbiota in AKI and the importance of future very carefully designed experiments to establish the exact pathophysiological role of gut microbiota during AKI.
At the time of the writing of this manuscript, PubMed searches on ‘human AKI and microbiome’, ‘human AKI and bacteria’, and similar terms found no published original research on the effects of human AKI on gut microbiome, or vice versa. However, a recent retrospective study in cirrhotic patients undergoing rifaximin treatment showed reduced incidence of AKI. The protection observed in this study was thought to be due to an altered gut microbiota following rifaximin treatment rather than a complete depletion of gut microflora; however, no microbiota related assessment was carried out [15]. Furthermore, metagenomic analysis of renal transplant patients found that maintenance immunosuppressive therapy impacts their fecal microbiome [4]. Similarly, alterations in fecal microbiota were also observed in adults undergoing liver transplantation. In studies on AKI to CKD transition and ESRD, there are also changes in intestinal microbiota. Recently, alterations in intestinal microbiota in ESRD patients were found following uremia [3]. There were marked differences in the abundance of a large number of bacterial operational taxonomic units (OTUs, a DNA sequence that represents a species or group of similar species) between ESRD and control patients. Many uremic toxins (e.g. indoxyl sulfate, p-cresyl sulfate, phenyl sulfate, cholate, hippurate, dimethylglycine, γ-guanidinobutyrate, glutarate etc.) are known to be microbial in nature [16]. It is speculated that uremia directly or indirectly affects the composition of gut microbiota, and translocation of bacterial products across the leaky intestinal barrier activates the immune system, resulting in systemic inflammation associated with ESRD and CKD. Similar metabolic changes due to an altered colo-renal relationship are postulated in AKI, but remain relatively unexplored.
Microbiome targeted therapy for AKI treatment?
Despite limited published literature, increasing data provides evidence that the gut microbiota can modulate kidney function in health and disease. Harnessing the recent developments in microbiome science is a promising direction to develop ways to improve the outcomes of patients with AKI. In addition to directly decreasing incidence and improving renal recovery, patients may be stratified on the basis of the composition of their intestinal microbiomes and their metabolites. These microbiota and metabolomics assessment may allow personalized treatment options for patients. The public may also be more receptive to therapies based on “natural” approaches targeting the gut microbiota compared to pharmacologic and recombinant DNA based therapeutics felt to be less holistic. These “natural’ approaches using prebiotic, probiotics and synbiotics could potentially treat dysbiosis during AKI and increase disposal of harmful metabolites. Furthermore, microbiome interventions could have a major impact on the high incidence of serious infection during AKI as well as cases of sepsis leading to AKI.
Figure. Colo-renal interactions during AKI.
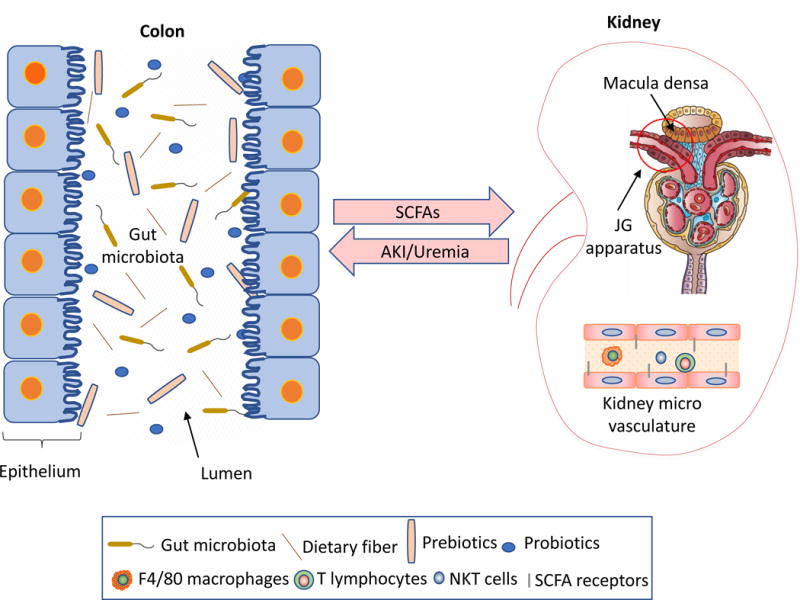
Bacteria in the gut can communicate with kidney, and among the identified factors are short chain fatty acids (SCFAs). During experimental AKI, gut bacteria can modify immune cells and other pathophysiologic mediators to alter the course of AKI. AKI can in turn modify gut bacteria. Therapeutic strategies for AKI include modification of gut bacteria with pre-and pro-biotics, as well as targeting SCFAs and their receptors.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noel S, Martina-Lingua MN, Bandapalle S, Pluznick J, Hamad AR, Peterson DA, Rabb H. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract. 2014;127:139–143. doi: 10.1159/000363209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 4.Zaza G, Dalla Gassa A, Felis G, Granata S, Torriani S, Lupo A. Impact of maintenance immunosuppressive therapy on the fecal microbiome of renal transplant recipients: Comparison between an everolimus- and a standard tacrolimus-based regimen. PLoS One. 2017;12:e0178228. doi: 10.1371/journal.pone.0178228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–851. doi: 10.1038/ki.2008.390. [DOI] [PubMed] [Google Scholar]
- 6.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1457–65. doi: 10.1152/ajprenal.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson D, Chen Y, Noel S, Bandapalle S, Martina-Lingua M, White J, Hamad A, Rabb H. Acute kidney injury in mice induces specific changes in gut microbiota. J Am Soc Nephrol. 2013;24:638. [Google Scholar]
- 9.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Camara NO. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26:1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J, Craig J, Goergen C, HogenEsch H, Kim C. Chronically elevated levels of short-chain fatty acids induce T cell–mediated ureteritis and hydronephrosis. J Immunol (Baltimore, Md: 1950) 2016;196:2388. doi: 10.4049/jimmunol.1502046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 14.Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, Florquin S, Leemans JC, Dessing MC. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2017;28:1450–1461. doi: 10.1681/ASN.2016030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong T, Aronsohn A, Gautham Reddy K, Te HS. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci. 2016;61:3621–3626. doi: 10.1007/s10620-016-4313-0. [DOI] [PubMed] [Google Scholar]
- 16.Mishima E, Fukuda S, Mukawa C, Yuri A, Kanemitsu Y, Matsumoto Y, Akiyama Y, Fukuda NN, Tsukamoto H, Asaji K, Shima H, Kikuchi K, Suzuki C, Suzuki T, Tomioka Y, Soga T, Ito S, Abe T. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 2017;92:634–645. doi: 10.1016/j.kint.2017.02.011. [DOI] [PubMed] [Google Scholar]


