Abstract
There exists little human neuroscience research to explain why some individuals lose their appetite when they become depressed, while others eat more. Answering this question may reveal much about the various pathophysiologies underlying depression. The present study combined neuroimaging, salivary cortisol, and blood markers of inflammation and metabolism collected prior to scanning. We compared the relationships between peripheral endocrine, metabolic, and immune signaling and brain activity to food cues between depressed participants experiencing increased (N=23) or decreased (N=31) appetite and weight in their current depressive episode and healthy control participants (N=42). The two depression subgroups were unmedicated and did not differ in depression severity, anxiety, anhedonia, or body mass index. Depressed participants experiencing decreased appetite had higher cortisol levels than subjects in the other two groups, and their cortisol values correlated inversely with the ventral striatal response to food cues. In contrast, depressed participants experiencing increased appetite exhibited marked immunometabolic dysregulation, with higher insulin, insulin resistance, leptin, CRP, IL-1RA, and IL-6, and lower ghrelin than subjects in other groups, and the magnitude of their insulin resistance correlated positively with the insula response to food cues. These findings provide novel evidence linking aberrations in homeostatic signaling pathways within depression subtypes to the activity of neural systems that respond to food cues and select when, what, and how much to eat. In conjunction with prior work, the present findings strongly support the existence of pathophysiologically distinct depression subtypes for which the direction of appetite change may be an easily measured behavioral marker.
Keywords: major depressive disorder, fMRI, cortisol, leptin, ghrelin, inflammation
INTRODUCTION
Although changes in appetite and weight have long been recognized as important features of depression1 and are codified in the DSM-5 as diagnostic markers of major depressive disorder (MDD), remarkably little attention has been paid to what the direction of appetite changes per se reveal about the dissociable pathophysiologies that underlie the clinical diagnosis of MDD. This paucity of research is surprising in light of evidence that appetite and weight changes are often the most discriminating symptoms in latent class analyses of depression subtypes 2–5. Nearly half of MDD patients exhibit depression-related decreases in appetite and nearly a third experience depression-related weight-loss 6. By contrast, approximately a third of MDD patients exhibit depression-related increases in appetite with a fifth experiencing depression-related weight-gain. These changes in appetite and weight are stable across depressive episodes7 and within a two-year time window 8, suggesting that they may be enduring markers of how depression manifests within an individual.
In a recent functional magnetic resonance imaging (fMRI) study, Simmons and colleagues demonstrated that unmedicated depressed adults grouped solely based on self-reported appetite changes in their current depressive episode exhibit marked differences in brain activity to food cues 9. Depressed participants with increased appetite exhibited exaggerated food cue responses within regions previously implicated in the mesocorticolimbic dopamine reward system, including the medial OFC, the ventral striatum, and the putamen. In contrast, depressed subjects with decreased appetite exhibited decreased food cue responses in regions of the posterior/mid-insula, relative to depressed subjects with increased appetite and healthy comparison subjects.
The locations of the findings in the insula and reward circuitry are particularly significant because (A) the posterior/mid-insula is considered part of the brain’s primary interoceptive cortex, where afferents carrying signals about the body’s visceral, metabolic, and immune status first reach the cortex 10–13, and (B) endocrine, metabolic, and immune signals modulate the activity of reward regions in humans 14–20. For example, cortisol can regulate reward motivation through glucocorticoid receptor activation in the hypothalamus, striatum, and amygdala 16, 21, leptin is known to regulate the reward value of food through leptin receptor activation in both the ventral tegmental area and hypothalamus 22, and inflammatory cytokines can alter striatal dopamine uptake thereby leading to reduced sensitivity to rewards (e.g., 23). Indeed, in addition to influences on food reward and appetite, hypothalamic-pituitary-adrenal (HPA), immune, and metabolic signaling pathway aberrations have also been implicated in depression 24–27. Importantly, using data-driven approaches to subtype depressed participants, multi-site naturalistic cohort studies have found that depression with atypical features (of which increased appetite is a prominent marker) is associated with lower cortisol, higher leptin, and higher inflammatory cytokines than depression with melancholic features (of which appetite loss is a prominent marker)28–30.
Although much is now known about how HPA, inflammatory, and metabolic signaling pathways influence eating and mood, key questions remain unanswered. First, do subgroups of unmedicated depressed patients identified solely by the direction of their appetite and weight changes exhibit HPA, inflammatory, or metabolic differences? Second, and more significantly, how do endocrine, immune, and metabolic differences between those with depression-related increases versus decreases in appetite relate to group differences in brain activity in response to food cues? This question is critical, as it directly relates to how aberrations in homeostatic signaling pathways in specific depression subtypes might ultimately alter activity of neural systems that select when, what, and how much to eat. Answering this question would begin to close the loop between the subgroup differences in brain activity observed with functional neuroimaging 9 and the endocrine, metabolic, and immune differences among depression subgroups that are intimated by large multi-site naturalistic cohort studies 28–30.
To address these questions, we recruited three groups of unmedicated adults: depressed participants experiencing increased appetite and weight gain within their current depressive episode (MDD appetite↑), depressed participants experiencing decreased appetite and weight loss within their current depressive episode (MDD appetite↓), and healthy non-depressed comparison (HC) participants. Cortisol, inflammation, and metabolic markers were assessed in all three groups prior to undergoing fMRI scanning, during which subjects performed a task in which they viewed food pictures.
MATERIALS AND METHODS
Participants
Ninety-six volunteers with a body mass index (BMI) ≥18.5 participated in the study: 23 participants with MDD reporting increased appetite in the current depressive episode (MDD-appetite↑; female N=19; age 18–51 years), 31 participants with MDD reporting decreased appetite in the current depressive episode (MDD-appetite↓; female N=19; age 18–49 years), and 42 healthy control subjects (HC; female N=27; age 18–47 years).
All depressed subjects met DSM-IV-TR criteria for current MDD. None of the depressed participants had received any psychotropic medication within the preceding 3 weeks (6 weeks for fluoxetine). Depressed subjects were assigned to either the MDD-appetite↑ or MDD-appetite↓ groups based on responses to the appetite change questions in the mood disorders module of the Structured Clinical Interview for DSM-IV, Axis I Disorders and confirmed in an interview with a psychiatrist. Additionally, all depressed participants included in the study reported either weight gain or loss in the current depressive episode (see Supplemental Methods for additional sample information). Study procedures conformed to IRB oversight through Western IRB, and all participants provided written informed consent.
Research Design Overview
For two nights prior to scan day, participants collected saliva at the beginning of each hour from 19:00–22:00. On the night prior to scan day, subjects were instructed to fast from eating beginning at midnight. They arrived at Laureate Institute for Brain Research at 8:00 on the morning of the scan, at which point blood was collected for fasting glucose and insulin measurements. Next, participants were given a breakfast standardized for micro-, and macronutrient content (see Online Supplemental Methods). At 12:00 participants were positioned in the scanner and blood was sampled for the metabolic and immune assays. Immediately after the blood draw participants began the food/non-food picture task while undergoing fMRI. Structural scans were collected at the end of the scan session.
fMRI Food/non-food picture task
During fMRI the participants performed a food/nonfood picture task. Participants saw 180 food photographs and 45 nonfood photographs. Foods included high fat/sugar appetizing items, as well as fruits and vegetables. Nonfood photographs depicted manipulable household/office implements. See online Supplemental Methods for details of the task, stimuli, and stimulus presentation.
Cortisol assays
Saliva samples were collected from each participant using salivettes (Sarstedt, Numbrecht, Germany) and kept cold until they could be centrifuged at 1000g for 2 minutes at room temperature and stored at −30 °C until analysis. Cortisol concentrations were measured in duplicates using an enzyme immunoassay (Salimetrics, PA). For group comparisons and imaging analyses, the average value of eight samples across four nighttime measurements was used for each subject (See online supplemental methods).
Metabolic and immune assays
Plasma and serum samples were collected at approximately 8:00. Fasting glucose and insulin were measured within one hour by a CLIA-certified lab. Prior to the fMRI scan (i.e., the post-prandial blood draw occurring approximately 3.25 hours after the breakfast; see Supplemental Methods), venous blood was collected in two BD Vacutainer EDTA tubes and plasma was used for inflammatory and metabolic assays. Inflammatory markers obtained were a high sensitivity measure of c-reactive protein (CRP), interleukin 1 receptor antagonist (IL-1ra), and interleukin 6. Metabolic markers consisted of acylated ghrelin, leptin, and fasting insulin and glucose which were used for estimation of insulin resistance (HOMA-IR). See the online supplemental methods for details of the assay analyses, variations in the number of participant samples available for each analyte, and Supplemental Table S3 for coefficients of variation for each assay.
MRI data acquisition and preprocessing
The online Supplemental Methods contains details of the imaging parameters, data pre-processing, and subject-level regression models applied to the task-based data.
Group analyses of bioassays
The online Supplemental Methods contains details of the group analyses of bioassay analytes. In brief, for each analyte, Shapiro-Wilkes tests of distribution normality were performed, and those with non-Gaussian distributions were either log-transformed or analyzed using non-parametric tests.
fMRI group analyses
Using random-effects multivariate modeling we performed whole-brain voxel-wise analyses on all subjects’ beta coefficients derived from the subject-level regression analyses for food and nonfood stimuli. The goal of this analysis was to identify brain regions exhibiting a difference in activity between any two of the groups. To ensure that we identified voxels where there were differences between the two depressed groups, or between one of depressed groups and the healthy control participants, three statistical images were computed (MDD-appetite↑ vs MDD-appetite↓, MDD-appetite↑ vs HC, and MDD-appetite↓ vs HC) with each contrast map separately corrected for multiple comparisons (see online Supplemental Methods). This analysis identified eight brain regions exhibiting group mean activity differences: the right putamen, right parahippocampal gyrus, right occipital lobe, right dorsal mid-insula, right posterior insula, the left ventral striatum, and the ventral tegmental area (see Table S1 for information on these regions, and Table S2 for group comparisons). Next, robust regression using an M estimator in R was used to test a model of the slope differences among the three groups, and thereby identify if the subgroups differed in the slope of the relationship between the humoral markers and activity in any of these eight regions to food pictures. False Discovery Rate (FDR) correction for multiple comparisons31 was used across the resulting 64 tests.
RESULTS
Behavioral Results
The two depressed groups did not differ on ratings of depression, anhedonia, or state/trait anxiety (Table 1).
Table 1.
Sample Demographic and Clinical Characteristics
| Healthy | MDD Appetite↑ | MDD Appetite↓ | p-value | |
|---|---|---|---|---|
|
|
||||
| Mean(sd) | Mean(sd) | Mean(sd) | ||
| N | 42 | 23 | 31 | |
| Age in years | 31.33 (8.56) | 33.57 (10.28) | 29.90 (8.58) | 0.339a |
| Body Mass Index (kg/m2) | 28.50 (4.94) | 31.68 (5.73) | 29.18 (5.61) | 0.073a |
| Gender = M (%) | 15 (35.7) | 4 (17.4) | 12 (38.7) | 0.208b |
| Modified Hamilton Depression Rating Scale | 2.62 (2.43) | 22.64 (5.46) | 21.55 (6.03) | 0.497c |
| Modified Snaith-Hamilton Pleasure Scale | 16.78 (4.87) | 25.70 (5.44) | 27.57 (6.11) | 0.264c |
| State-Trait Anxiety Inventory - State | 24.62 (4.22) | 41.59 (11.20) | 44.19 (10.90) | 0.404c |
| State-Trait Anxiety Inventory - Trait | 26.32 (4.53) | 56.86 (9.22) | 55.19 (10.17) | 0.537c |
| Hamilton Anxiety Rating Scale | 2.41 (3.01) | 17.91 (5.71) | 19.10 (6.89) | 0.497c |
Depressed subjects were assigned to either the appetite increase or decrease groups based on consistent responses to all three of the following: (1) their responses to the SCID-I Mood Disorders Module appetite change questions and confirmed in an interview with a research psychiatrist AND (2) reported increased/decrease appetite the day of data collection AND (3) reported an increase/decrease in weight. One subject from Depressed-appetite increase group and five subjects from healthy group did not complete clinical ratings. Modified Hamilton Depression Rating Scale: Appetite and food related questions were excluded from the scoring. Modified Snaith-Hamilton Pleasure Scale: Food and drink related questions were excluded from the scoring.
One way test
χ2 test
Two Sample t-test between MDD Appetite↑ and MDD Appetite↓ group.
Bio-assay Results
Correlations between bio-assay values and brain activity across all participants
We first examined the correlation matrix showing the relationships between all the participants’ bio-assay values (Table 2) and fMRI activity within the eight brain regions exhibiting group mean activity differences. After correcting for the 64 comparisons, only one significant relationship was observed: activity in the right posterior insula was negatively correlated with subjects’ ghrelin levels (Table 3). The failure to observe many reliable correlations across all subjects might be due however to (A) heterogeneity among the groups both in mean bio-assay levels, and/or (B) differences in the groups’ relationships between brain activity and the humoral markers. We thus used ANOVA and post-hoc t-tests (and corrections for multiple comparisons) to assess group differences in mean bioassay levels, and robust regression to test the slope differences among the three groups in the humoral markers and activity in any of the eight brain regions of interest. Again, FDR correction for multiple comparisons was used across the resulting 64 tests.
Table 2.
Mean Biomarker Values for Each Group
| Healthy | MDD Appetite↑ |
MDD Appetite↓ |
p- value |
HC vs. MDD Appetite↑ |
HC vs. MDD Appetite↓ |
MDD Appetite↑vs. MDD Appetite↓ |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mean(sd) | Mean(sd) | Mean(sd) | p- value |
Cohen’s dc |
p- value |
Cohen’s dc |
p- value |
Cohen’s dc |
||
| Cortisol(nmol/L)a | 1.24 (0.51) | 1.03 (0.38) | 1.58 (0.74) | 0.005 | 0.360 | 0.440 | 0.050 | −0.562 | 0.004 | −0.912 |
| Metabolic | ||||||||||
| Insulin (uIU/mL)b | 6.82(3.52) | 20.39(18.62) | 9.74(5.44) | 0.012 | 0.008 | −1.170 | 0.058 | −0.649 | 0.117 | 0.848 |
| 1 HOMA_IRa | 0.49(0.62) | 1.21(0.88) | 0.33(0.72) | 0.000 | 0.002 | −1.163 | 0.677 | 0.177 | 0.0004 | 1.120 |
| 1 Leptin (ng/mL)a | 2.63 (0.91) | 3.31 (0.55) | 2.48 (0.99) | 0.004 | 0.017 | −0.710 | 0.777 | 0.177 | 0.005 | 0.976 |
| 1 Ghrelin (pg/mL)a | 3.89(0.63) | 3.47(0.71) | 4.13(0.66) | 0.007 | 0.070 | 0.622 | 0.381 | −0.399 | 0.005 | −0.969 |
| Immune | ||||||||||
| CRP (mg/L)b | 2.49 (2.48) | 4.39 (3.35) | 1.71 (1.62) | 0.024 | 0.036 | −0.686 | 0.349 | 0.351 | 0.009 | 1.064 |
| IL-1ra (ng/mL)b | 0.35 (0.28) | 0.44 (0.24) | 0.26 (0.08) | 0.02 | 0.027 | −0.336 | 0.842 | 0.405 | 0.003 | 1.083 |
| 1 IL6 (pg/L)a | 6.36(0.67) | 6.84(0.5) | 6.74(0.57) | 0.029 | 0.049 | −0.772 | 0.115 | −0.579 | 0.886 | 0.111 |
Log-transformed.
One way ANOVA followed by Tukey’s ‘Honest Significant Difference’ method;
p-values are from nonparametric result using Kruskal test (Wilcox test for the two-group case).
Cohen’s d effect sizes computed with the effsize package in R.
HOMA-IR: The homeostasis model assessment of insulin resistance. CRP: C-reactive protein, IL: interleukin, IL-1ra: IL-1 receptor antagonist.
HC: Healthy
Table 3.
Correlations between bio-assay values and brain activity across all participants
| R. Putamen | R. Parahippocampal Gyrus |
R. Occipital Lobe |
R. Dorsal Mid-Insula |
R. Posterior Insula |
R. Mid- Insula |
Ventral Tegmental Area |
L. Ventral Striatum |
|
|---|---|---|---|---|---|---|---|---|
| Cortisol(nmol/L) | −0.1 | −0.06 | −0.04 | −0.1 | −0.31 | −0.15 | 0.21b | −0.13 |
| Insulin (uIU/mL) | 0.04 | −0.02 | 0.05 | 0.09 | 0.17 | −0.04 | −0.35 | 0.04 |
| 1 HOMA_IR | 0.19 | −0.07c | 0.09 | 0.2c | 0.26c | 0.06 | −0.25 | 0.17 |
| 1 Leptin (ng/mL) | 0 | −0.08 | −0.02 | 0.08 | 0.13 | −0.14 | −0.27 | −0.02 |
| 1 Ghrelin (pg/mL) | −0.22 | −0.22 | −0.22 | −0.31 | −0.39a | −0.27 | 0.09 | −0.15 |
| CRP (mg/L) | 0.14 | 0.08 | 0.02 | 0.11 | −0.03 | −0.05 | −0.24 | 0.07 |
| IL-1ra (ng/mL) | 0.1 | 0.07 | 0.08 | 0.18 | 0.14 | 0.17 | −0.2 | 0.02 |
| 1 IL6 (pg/L) | −0.14 | −0.34 | −0.27 | −0.23c | −0.18c | −0.14 | −0.25 | −0.16 |
Log-transformed.
p < .04 after FDR correction for multiple comparisons
Subsequent analyses reveal group interaction for the MDD appetite↓ group
Subsequent analyses reveal group interaction for the MDD appetite↑ group.
HOMA-IR: The homeostasis model assessment of insulin resistance. CRP: C-reactive protein, IL: interleukin, IL-1ra: IL-1 receptor antagonist.
HC: Healthy
Nighttime salivary cortisol
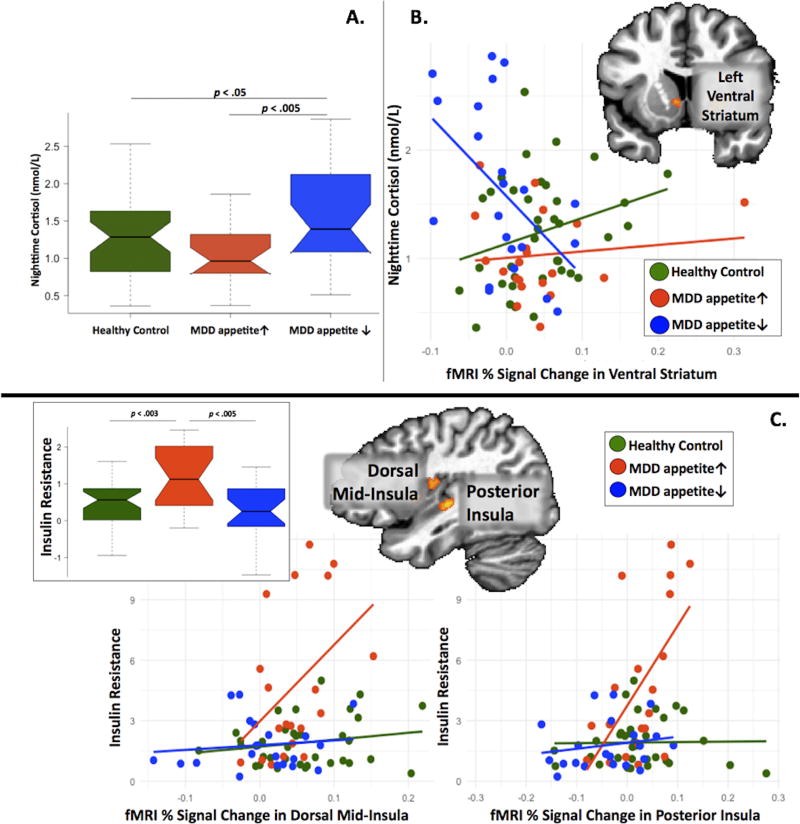
The three groups differed in cortisol concentrations on the two nights prior to scanning (p<0.005), with post-hoc tests confirming higher cortisol in the MDD appetite↓ group versus either the MDD appetite↑ group (pcorrected=0.004) or HC groups (pcorrected=0.05) (Figure 1A, Table 2, see online Supplemental Results for detailed statistical test values).
Figure 1. Group differences in the relationships between brain activity to food pictures, cortisol, and insulin resistance.
Panel A: Boxplots show that nighttime cortisol was higher in the MDD appetite↓ group compared to both the MDD appetite↑ and HC groups. The boxes show the interquartile range, with plot “whiskers” indicating the range. The line inside the boxes indicates the median of the group’s distribution. Panel B: Scatterplots show that in the MDD appetite↓ group, higher nighttime salivary cortisol was associated with lower activity in the left ventral striatum region of interest (ROI). Panel C: The inset boxplots show that insulin resistance (HOMA-IR) was higher in the MDD appetite↑ group compared to both the MDD appetite↓ and HC groups. The scatterplots show that in the MDD appetite↑ group, higher insulin resistance was associated with greater activity in the dorsal mid-insula, and posterior insula regions of interest (ROI). In panels B and C, the inset brain images show the ROIs defined in the analysis of group differences in the response to food pictures (see Supplemental Table S1).
Plasma metabolic markers
We observed higher insulin levels in the MDD-appetite↑ group than the HC group (pcorrected=0.008), and a trend toward higher insulin in the MDD appetite↓ group compared to the HC group (pcorrected=0.058). Relatedly, the MDD appetite↑ group exhibited higher insulin resistance than either the MDD appetite↓ (pcorrected=0.0004) or HC participants (pcorrected=0.002), with the latter two groups not differing from each other (pcorrected=0.68) (Figure 1C).
Group differences were observed in plasma (log)leptin levels, with the MDD appetite↑ subjects exhibiting higher (log)leptin than the MDD appetite↓ (pcorrected=0.005) and HC participants (pcorrected=0.017).
Finally, group differences were observed in log-transformed plasma acylated ghrelin levels. The MDD appetite↑ subjects exhibited lower (log)ghrelin than the MDD appetite↓ subjects (pcorrected=0.005). The difference between the MDD appetite↑ and HC participants showed a nonsignificant trend (pcorrected=0.07).
Plasma immune markers
MDD appetite↑ subjects exhibited higher CRP than either the MDD appetite↓ (p<.009) or HC participants (p = .036) (Supplemental Figure S1). Similar group differences were observed for IL-1RA, where MDD appetite↑ subjects exhibited significantly higher IL-1RA than either the MDD appetite↓ participants (Wilcoxon p=0.003) or HC participants (Wilcoxon p < 0.03). Group differences were also observed in log-transformed IL-6, with the MDD appetite↑ subjects exhibiting higher IL-6 than the HC participants (pcorrected=0.049) but not the MDD appetite↓ subjects (pcorrected=0.89).
Neuroimaging Results
Associations between endocrine, metabolic, and immune factors and brain activity to food cues
Of the eight brain regions exhibiting group mean activity differences to food stimuli (see Methods and Table S1), four regions exhibited differences between the MDD subtypes in the relationships between brain activity and cortisol, metabolic, or inflammatory factors. First, group differences were observed in the slope of the relationship between nighttime salivary cortisol and ventral striatum response to food cues (pcorrected=0.037). This effect was driven by the MDD appetite↓ group, which exhibited a strong negative correlation between cortisol and ventral striatum activity (r = −0.50, pcorrected=0.02; Figure 1B).
Second, both the dorsal mid-insula and posterior insula exhibited group differences in the slope of the relationship between insulin resistance and response to food cues (dorsal mid-insula pcorrected=0.045; posterior insula pcorrected=0.001). This effect was driven by the MDD appetite↑ group which exhibited positive associations between insulin resistance and insula activity (dorsal mid-insula r = 0.42, pcorrected=0.06; posterior insula r = 0.59, pcorrected=0.006; Figure 1C). Additionally, at p-levels uncorrected for multiple comparisons, both insula regions also exhibited group differences in the relationship between activity to food pictures and IL-6 (dorsal mid-insula p=0.04; posterior insula p=0.009). In both insula regions, the MDD appetite↓ participants who exhibited the highest IL-6 levels exhibited the weakest responses to food cues, while the MDD appetite↑ participants who exhibited the highest IL-6 levels exhibited the highest responses to food cues (Supplemental Figure S2, see Figure S3–Figure S9 for results in all ROIs).
Finally, within the right parahippocampal gyrus, a group difference was evident in the relationship between insulin resistance and hemodynamic response to food cues (p=0.036). This reflected a negative association within the MDD appetite↑ group between insulin resistance and parahippocampal gyrus response to food pictures, while the parahippocampal response and insulin resistance were not correlated in the MDD appetite↓ group.
DISCUSSION
In the present study, depression with decreased appetite was associated with higher nighttime cortisol, while depression with increased appetite was associated with greater insulin resistance, higher leptin, lower ghrelin, and higher CRP, IL-1RA, and IL-6 levels. Additionally, there were three key imaging findings. First, the ventral striatal activity to food cues was lowest in the depressed subjects with decreased appetite who had the highest cortisol levels. No such relationship was observed for the other groups. Second, dorsal mid-insula, posterior insula, and parahippocampal activity to food cues was highest in the depressed subjects with increased appetite who were the most insulin resistant. Third, dorsal mid- and posterior insula activity to food cues was highest in the depressed subjects with increased appetite who had the highest IL-6 levels, but lowest in the depressed subjects with decreased appetite who had the highest IL-6 levels.
Cortisol, depression, and the ventral striatum
Although meta-analyses have consistently confirmed the association between elevated cortisol and depression 32–36, effect sizes across studies have varied with clinical heterogeneity identified as a significant contributor to the variance 37. There are strong indications that higher cortisol is associated with depression with melancholic features, for which appetite loss is a prominent symptom, and may be a key indicator of differential pathophysiology among depression subtypes 27, 29, 38–42 (See online Supplement for discussion of our MDD subgroups and the atypical/melancholic distinction). The negative association between cortisol and ventral striatum response to food cues in our decreased appetite subgroup provides an important clue regarding how previous cortisol findings in melancholic depression may translate into decreased appetite specifically, or reduced reward processing more broadly. The ventral striatum plays a critical role in both the hedonic representation and motivational salience for food stimuli 43, 44 and previous studies in non-depressed samples have demonstrated associations between circulating cortisol (which crosses the blood brain barrier) and reward responses in the striatum (which contains neurons that possess glucocorticoid receptors) 16, 45, 46.
Immunometabolic dysfunction in depression with increased appetite
The present study is the first to report differences in insulin resistance, leptin, CRP, IL-1RA, and IL-6 between depression subgroups defined strictly based on self-reported appetite and weight change. These findings are consistent with earlier work by Penninx and colleagues demonstrating that depression with atypical features, which is prominently linked with elevated appetite, is also associated with immunometabolic dysregulation 28–30.
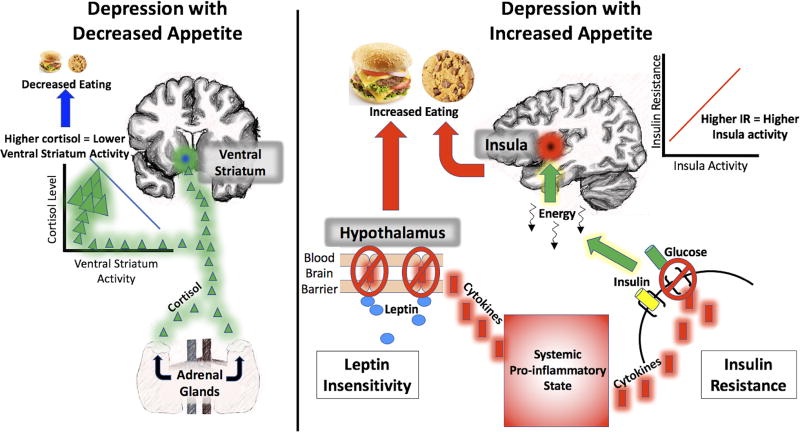
The leptin finding in the present study is particularly striking as the two MDD subgroups did not differ significantly on BMI (although there was a numerical difference of 2.7 points in BMI). Leptin is a hormone released by adipose tissue into the circulatory system that serves as a signal about energy stored in fat reserves. Once in the brain, leptin is an appetite inhibitor and mood regulator 15, 47–50, that has been implicated in depression 51–55. High levels of leptin in obesity can be associated with leptin insensitivity, resulting in a decrease in leptin’s capacity to regulate appetite 56–58. There are generally two explanations for leptin insensitivity in obesity: leptin transport failures across the blood brain barrier, and failure of leptin-mediated intracellular signaling in hypothalamic neurons. Importantly, elevated concentrations of IL-1, IL-6, and CRP are central to the mechanisms underlying both types of failure 57–60, which is noteworthy since the MDD appetite↑ group manifested elevated leptin as well as elevated IL-1RA, IL-6, and CRP levels. Interestingly, systemic inflammation can also promote insulin resistance 61, 62, another prominent feature in the metabolic disturbance of the MDD-appetite↑ group. IL-1B, for which IL-1RA is a counter-regulatory analog, impairs insulin signaling in macrophages and peripheral body tissues 63, 64, and IL-6 reduces the expression of GLUT-4 and IRS-1 which are initiated by binding of insulin to the insulin receptor and transport glucose into cells 65, 66. Taken together, these findings suggest the possibility that some individuals may manifest chronic low-grade inflammation that brings about (1) reduced leptin sensitivity, thereby releasing their appetite from the anorexigenic signals emitted by their adipose tissue, and (2) increased insulin resistance, which impairs glucose utilization and thus presumably signals to the CNS that the body is in a state of energy need, thereby promoting eating (Figure 2).
Figure 2. Conceptual Models of Appetite Change in Depression.
The schematic models illustrate plausible accounts for the relationship between cortisol, inflammatory, and metabolic disturbance and appetite changes in the two depression subtypes based on the findings in the present study and in prior published research. These are not intended to reflect comprehensive models of how cortisol, inflammation, and metabolic factors relate to appetite changes in the two depression subtypes, and the ideas suggested here will benefit from additional research and replication. On the left, elevated levels of cortisol secretion by the adrenal glands in MDD with decreased appetite appear to bring about lower ventral striatum responses to food cues. As the ventral striatum plays important roles in food hedonics and incentive salience, this may lead to decreased appetite and eating. In contrast, on the right side of the figure, MDD with increased appetite appears to be associated with elevated systemic inflammation (as indexed here by CRP, IL-1RA, and IL-6). Systemic inflammation is known to promote leptin resistance by interrupting leptin trafficking across the blood brain barrier, and disrupting intracellular signaling in hypothalamic arcuate neurons sensitive to leptin. This has the effect of abrogating one of the body’s primary anorexogenic signaling pathways in response to increased adiposity. At the same time, systemic inflammation impairs insulin signaling in macrophages and peripheral body tissues and reduces the expression of factors that transport glucose into cells upon the binding of insulin to insulin receptors. The primary interoceptive cortex of the mid- and posterior insula, which is sensitive to homeostatic energy signals from the body, may detect the lack of energy in the viscera (likely via vagal afferents) and send signals to the wider brain that food intake is necessary.
One would expect these signals of altered energy utilization to be detected in the brain’s primary interoceptive cortex, located in the mid- and posterior insula, which is known to exhibit altered responses to food cues based on circulating markers of energy availability, including insulin and glucose 18, 67, 68. Indeed, in the present study the MDD-appetite↑ subjects who were most insulin resistant exhibited the highest insular response to food pictures. This neuroimaging finding thus closes the loop between the immunometabolic dysfunction observed in depressed subjects with increased appetite (or atypical features in prior studies), and one of the neural pathways by which the brain interocepts the body’s homeostatic state and initiates behaviors to address the body’s energy needs. This pattern was mirrored in the association (observed at an uncorrected p-level) between activity in these regions and circulating IL-6, which foundational research has shown is related to MDD69, 70. In both the dorsal mid- insula and posterior insula, those MDD-appetite↑ subjects who had the highest IL-6 showed the highest hemodynamic response to food pictures. In contrast, the MDD-appetite↓ group exhibited the opposite relationship between IL-6 and activity in these regions. Interestingly, recent evidence suggests that in response to stress, peripherally circulating IL-6 may leak into the brain, causing neural inflammation and promoting depression 71.
Finally, the ghrelin findings contribute additional evidence that depression with increased appetite is associated with metabolic dysfunction. Ghrelin is secreted primarily by cells in the upper part of the stomach and binds to receptors located in organ systems throughout the body, and particularly the hypothalamus where it promotes appetite 72. Given that ghrelin is an orexigenic hormone, it might appear counter-intuitive to find lower levels of ghrelin in participants selected on the basis of increased appetite (also, see Supplemental Table S4 for evidence that the group differences remain after covarying for BMI). Again, the heightened inflammation in the MDD-appetite↑ group may provide an explanation. There is a growing literature demonstrating complex relationships between ghrelin and inflammation, with both rodent and human studies demonstrating that systemic inflammation is associated with decreased circulating ghrelin 73. Researchers have examined whether ghrelin might be implicated in depression 74, 75 as well as eating disorders 76, with mixed results. Although some studies obtained no evidence of ghrelin differences between depressed and healthy samples 77, others have found higher ghrelin associated with depression 78, 79. One possible explanation for these mixed findings is the heterogeneity of the MDD populations. To date, this is the first study to compare ghrelin levels among depression subtypes with increased and decreased appetite.
Limitations
In the present dataset, we were not able to reliably assess age of depression onset in our MDD sample, nor do we have reliable measurements of the number of previous independent depressive episodes in the participants. Future prospective studies should seek to understand whether MDD subgroups defined solely by appetite change differ in these variables.
All participants ate the same standardized meal on the morning of the scan. We were unable however to provide meals that were matched to individual subjects’ metabolic rate/activity. Additionally, recent research in female participants has demonstrated menstrual cycle modulation of hemodynamic responses to both food 80 and non-food 81 reward stimuli. We considered scanning female participants in only one phase (e.g., follicular) but decided against this restriction as the MDD subjects were all unmedicated and doing so would have required some women to either delay enrolling in treatment for up to two weeks in order to participate in the study. Nevertheless, the influence of gonadal hormones on the effects reported in the present study is an important topic that warrants investigation in future studies. Additionally, as shown in Supplemental Table S6, data were not available for all analytes from all subjects. This was particularly the case for IL-6, and may contribute to why the IL-6 findings did not survive strict correction for multiple comparisons. As a result, future studies should be undertaken to replicate the findings reported here.
Conclusion
Accumulating evidence suggests that the direction of appetite change during depressive episodes provides the most discriminative marker of depression subtypes 2–5, and that increased versus decreased appetite MDD phenotypes reflect dissociable genetic influences 82. The present study has demonstrated that cortisol, inflammation, and metabolic differences exist between MDD subtypes defined solely on the direction of self-reported appetite and weight changes. Moreover, this study showed for the first time that these MDD subtypes exhibit differential relationships between markers of peripheral endocrine, immune, and metabolic signaling and the activity of brain regions underlying appetitive responses to food, which may thereby translate into the behaviors of increased and decreased eating.
Here we argue that the metabolic dysregulation that attends depression with increased appetite arises from these individuals’ systemic pro-inflammatory state. This, however, leaves two critical questions unanswered: Why do these individuals have higher inflammation in the first place, and is their depression a cause or consequence of the chain of immunometabolic processes described above? Although inflammation likely precedes metabolic disturbance in these patients, we acknowledge that virtually all of the hormones and cytokines studied here have multi-directional effects, making it challenging to infer causality.
More generally, it is difficult to infer the causality among the various endocrine, inflammatory, metabolic, and neural findings in the depression subtypes. This is because the activity in reward and interoceptive brain regions can be both consequence and cause of abnormal peripheral endocrine, immune, and metabolic dysregulation. Addressing the causality of the relationships reported here will require subsequent studies in which mood and appetite are measured in the presence of interventions that alter activity in basic signaling pathways underlying responses to stress, inflammation, and cellular energy regulation.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Mental Health (K01MH096175-01) grant to WKS, and NARSAD Young Investigator Award to WKS. WKS also receives funding from a National Institute of General Medical Sciences Center Grant (1P20GM121312). We also wish to thank the University of Oklahoma Integrative Immunology Center for assistance with running immunoassays.
Footnotes
Financial Disclosures
WCD is an employee of Janssen Research & Development, LLC., of Johnson & Johnson, and holds equity in Johnson & Johnson. WCD and WKS are co-inventors on a patent regarding appetite change in depression. All other authors have no financial interests to disclose.
References
- 1.Kraepelin E. Lectures on Clinical Psychiatry. Wood; New York, NY: 1904. [Google Scholar]
- 2.Li Y, Aggen S, Shi S, Gao J, Li Y, Tao M, et al. Subtypes of major depression: latent class analysis in depressed Han Chinese women. Psychological medicine. 2014;44(15):3275–3288. doi: 10.1017/S0033291714000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PF, Kessler RC, Kendler KS. Latent class analysis of lifetime depressive symptoms in the national comorbidity survey. The American journal of psychiatry. 1998;155(10):1398–1406. doi: 10.1176/ajp.155.10.1398. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PF, Prescott CA, Kendler KS. The subtypes of major depression in a twin registry. Journal of affective disorders. 2002;68(2–3):273–284. doi: 10.1016/s0165-0327(00)00364-5. [DOI] [PubMed] [Google Scholar]
- 5.Ten Have M, Lamers F, Wardenaar K, Beekman A, de Jonge P, van Dorsselaer S, et al. The identification of symptom-based subtypes of depression: A nationally representative cohort study. Journal of affective disorders. 2016;190:395–406. doi: 10.1016/j.jad.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell MA, Cole DA. Weight change and appetite disturbance as symptoms of adolescent depression: toward an integrative biopsychosocial model. Clinical psychology review. 2009;29(3):260–273. doi: 10.1016/j.cpr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Nierenberg AA, Pava JA, Clancy K, Rosenbaum JF, Fava M. Are neurovegetative symptoms stable in relapsing or recurrent atypical depressive episodes? Biological psychiatry. 1996;40(8):691–696. doi: 10.1016/0006-3223(96)00029-7. [DOI] [PubMed] [Google Scholar]
- 8.Lamers F, Rhebergen D, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Stability and transitions of depressive subtypes over a 2-year follow-up. Psychological medicine. 2012;42(10):2083–2093. doi: 10.1017/S0033291712000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, et al. Depression-Related Increases and Decreases in Appetite: Dissociable Patterns of Aberrant Activity in Reward and Interoceptive Neurocircuitry. The American journal of psychiatry. 2016;173(4):418–428. doi: 10.1176/appi.ajp.2015.15020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;33:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 11.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature reviews Neuroscience. 2015;16(7):419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human brain mapping. 2013;34(11):2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jastreboff AM, Lacadie C, Seo D, Kubat J, Van Name MA, Giannini C, et al. Leptin Is Associated With Exaggerated Brain Reward and Emotion Responses to Food Images in Adolescent Obesity. Diabetes care. 2014;37:3061–3068. doi: 10.2337/dc14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J. Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biological psychiatry. 2009;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. The Journal of clinical investigation. 2011;121(10):4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell metabolism. 2008;7(5):400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. The American journal of clinical nutrition. 2014;99(6):1319–1330. doi: 10.3945/ajcn.113.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology & behavior. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 22.Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, et al. Leptin regulates the reward value of nutrient. Nature neuroscience. 2011;14(12):1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of general psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain, behavior, and immunity. 2007;21(2):147–152. doi: 10.1016/j.bbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC medicine. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Molecular psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 28.van Reedt Dortland AK, Giltay EJ, van Veen T, van Pelt J, Zitman FG, Penninx BW. Associations between serum lipids and major depressive disorder: results from the Netherlands Study of Depression and Anxiety (NESDA) The Journal of clinical psychiatry. 2010;71(6):729–736. doi: 10.4088/JCP.08m04865blu. [DOI] [PubMed] [Google Scholar]
- 29.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular psychiatry. 2013;18(6):692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 30.Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW. Leptin Dysregulation Is Specifically Associated With Major Depression With Atypical Features: Evidence for a Mechanism Connecting Obesity and Depression. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini A, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 32.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21(2):76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- 34.Herbert J. Cortisol and depression: three questions for psychiatry. Psychological medicine. 2013;43(3):449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 35.Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, et al. Pathophysiology of hypercortisolism in depression. Acta psychiatrica Scandinavica Supplementum. 2007;(433):90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 36.Carroll BJ, Iranmanesh A, Keenan DM, Cassidy F, Wilson WH, Veldhuis JD. Pathophysiology of hypercortisolism in depression: pituitary and adrenal responses to low glucocorticoid feedback. Acta psychiatrica Scandinavica. 2012;125(6):478–491. doi: 10.1111/j.1600-0447.2011.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35(9):1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, et al. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. Journal of affective disorders. 2005;87(2–3):305–311. doi: 10.1016/j.jad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 39.O’Keane V, Frodl T, Dinan TG. A review of Atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology. 2012;37(10):1589–1599. doi: 10.1016/j.psyneuen.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Karlovic D, Serretti A, Vrkic N, Martinac M, Marcinko D. Serum concentrations of CRP, IL-6, TNF-alpha and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry research. 2012;198(1):74–80. doi: 10.1016/j.psychres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Casper RC, Kocsis J, Dysken M, Stokes P, Croughan J, Maas J. Cortisol measures in primary major depressive disorder with hypersomnia or appetite increase. Journal of affective disorders. 1988;15(2):131–140. doi: 10.1016/0165-0327(88)90081-x. [DOI] [PubMed] [Google Scholar]
- 42.Gold PW, Chrousos GP. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18(6):632–634. doi: 10.1038/mp.2013.5. [DOI] [PubMed] [Google Scholar]
- 43.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology & behavior. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudenga KJ, Sinha R, Small DM. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. International journal of obesity. 2013;37(2):309–316. doi: 10.1038/ijo.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Sescousse G, Dreher JC. Endogenous cortisol levels are associated with an imbalanced striatal sensitivity to monetary versus non-monetary cues in pathological gamblers. Frontiers in behavioral neuroscience. 2014;8:83. doi: 10.3389/fnbeh.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Licinio J, Negrao AB, Wong ML. Plasma leptin concentrations are highly correlated to emotional states throughout the day. Translational psychiatry. 2014;4:e475. doi: 10.1038/tp.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo M, Huang TY, Garza JC, Chua SC, Lu XY. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16(4):857–867. doi: 10.1017/S1461145712000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farr OM, Fiorenza C, Papageorgiou P, Brinkoetter M, Ziemke F, Koo BB, et al. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. The Journal of clinical endocrinology and metabolism. 2014;99(12):E2529–2538. doi: 10.1210/jc.2014-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stieg MR, Sievers C, Farr O, Stalla GK, Mantzoros CS. Leptin: A hormone linking activation of neuroendocrine axes with neuropathology. Psychoneuroendocrinology. 2015;51:47–57. doi: 10.1016/j.psyneuen.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Current opinion in pharmacology. 2007;7(6):648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zupancic ML, Mahajan A. Leptin as a neuroactive agent. Psychosomatic medicine. 2011;73(5):407–414. doi: 10.1097/PSY.0b013e31821a196f. [DOI] [PubMed] [Google Scholar]
- 53.Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW. Leptin Dysregulation Is Specifically Associated With Major Depression With Atypical Features: Evidence for a Mechanism Connecting Obesity and Depression. Biological psychiatry. 2017;81:807–814. doi: 10.1016/j.biopsych.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 54.Milaneschi Y, Simonsick EM, Vogelzangs N, Strotmeyer ES, Yaffe K, Harris TB, et al. Leptin, abdominal obesity, and onset of depression in older men and women. The Journal of clinical psychiatry. 2012;73(9):1205–1211. doi: 10.4088/JCP.11m07552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milaneschi Y, Sutin AR, Terracciano A, Canepa M, Gravenstein KS, Egan JM, et al. The association between leptin and depressive symptoms is modulated by abdominal adiposity. Psychoneuroendocrinology. 2014;42:1–10. doi: 10.1016/j.psyneuen.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nature neuroscience. 2005;8(5):566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 57.Jung CH, Kim MS. Molecular mechanisms of central leptin resistance in obesity. Arch Pharm Res. 2013;36(2):201–207. doi: 10.1007/s12272-013-0020-y. [DOI] [PubMed] [Google Scholar]
- 58.Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol. 2017 doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hribal ML, Fiorentino TV, Sesti G. Role of C reactive protein (CRP) in leptin resistance. Curr Pharm Des. 2014;20(4):609–615. doi: 10.2174/13816128113199990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Chen R, Wang H, Liang F. Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol. 2015;2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su D, Coudriet GM, Hyun Kim D, Lu Y, Perdomo G, Qu S, et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes. 2009;58(11):2624–2633. doi: 10.2337/db09-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boni-Schnetzler M, Donath MY. How biologics targeting the IL-1 system are being considered for the treatment of type 2 diabetes. Br J Clin Pharmacol. 2013;76(2):263–268. doi: 10.1111/j.1365-2125.2012.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serrano-Marco L, Barroso E, El Kochairi I, Palomer X, Michalik L, Wahli W, et al. The peroxisome proliferator-activated receptor (PPAR) beta/delta agonist GW501516 inhibits IL-6-induced signal transducer and activator of transcription 3 (STAT3) activation and insulin resistance in human liver cells. Diabetologia. 2012;55(3):743–751. doi: 10.1007/s00125-011-2401-4. [DOI] [PubMed] [Google Scholar]
- 66.Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T, et al. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. International journal of environmental research and public health. 2014;11(4):3586–3598. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, et al. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nature neuroscience. 2013;16(11):1551–1552. doi: 10.1038/nn.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstadt-Klein S, Wolfensteller U, et al. (Still) longing for food: insulin reactivity modulates response to food pictures. Human brain mapping. 2013;34(10):2367–2380. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher Than Normal Plasma Interleukin-6 Concentrations in Cancer Patients With depression: Preliminary Findings. American Journal of Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 70.Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain, behavior, and immunity. 2015;48:8–18. doi: 10.1016/j.bbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nature neuroscience. 2017;20(12):1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otero M, Nogueiras R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Chronic inflammation modulates ghrelin levels in humans and rats. Rheumatology (Oxford) 2004;43(3):306–310. doi: 10.1093/rheumatology/keh055. [DOI] [PubMed] [Google Scholar]
- 74.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature neuroscience. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wittekind DA, Kluge M. Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology. 2015;52:176–194. doi: 10.1016/j.psyneuen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Holsen LM, Lawson EA, Christensen K, Klibanski A, Goldstein JM. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry research. 2014;223(2):94–103. doi: 10.1016/j.pscychresns.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kluge M, Schussler P, Schmid D, Uhr M, Kleyer S, Yassouridis A, et al. Ghrelin plasma levels are not altered in major depression. Neuropsychobiology. 2009;59(4):199–204. doi: 10.1159/000223731. [DOI] [PubMed] [Google Scholar]
- 78.Akter S, Pham NM, Nanri A, Kurotani K, Kuwahara K, Jacka FN, et al. Association of serum leptin and ghrelin with depressive symptoms in a Japanese working population: a cross-sectional study. BMC Psychiatry. 2014;14:203. doi: 10.1186/1471-244X-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozsoy S, Besirli A, Abdulrezzak U, Basturk M. Serum ghrelin and leptin levels in patients with depression and the effects of treatment. Psychiatry Investig. 2014;11(2):167–172. doi: 10.4306/pi.2014.11.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, et al. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. The American journal of clinical nutrition. 2011;94(2):377–384. doi: 10.3945/ajcn.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, et al. 17beta-Estradiol Differentially Regulates Stress Circuitry Activity in Healthy and Depressed Women. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milaneschi Y, Lamers F, Peyrot WJ, Abdellaoui A, Willemsen G, Hottenga JJ, et al. Polygenic dissection of major depression clinical heterogeneity. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.