Abstract
Therapeutic interventions to treat acute lung injury (ALI) remain largely limited to lung-protective strategies, as a real molecular pathophysiologically driven therapeutic intervention has yet to become available. While we have previously documented the expression of HVEM on leukocytes of septic mice and critically ill patients, its functional role in shock/sepsis-induced ALI has not yet been studied. Inasmuch; a murine model of indirect ALI (iALI) was induced by hemorrhagic shock (HEM) followed by cecal ligation and puncture (CLP), septic challenge and HVEM-siRNA or PBS was administrated by intra-tracheal instillation 2 hours after hemorrhage to determine the role of HVEM in the development of experimental iALI. Indices of lung injury were measured. HVEM expression was significantly elevated in iALI mice. Compared to PBS treated iALI mice, HVEM knock-down by siRNA caused a reduction of cytokine/chemokine levels, MPO activity, BAL cell count, and BALF protein concentration. HVEM-siRNA treatment reduced inflammation and attenuated pulmonary architecture destruction as well as provided an early (60 hours post HEM-CLP) survival benefit in iALI mice. This ability of anti-HVEM treatment to prevent the development of iALI and provide a transient survival benefit implies that mitigating signaling through HVEM may be a novel target worth further investigation.
Keywords: co-inhibitory receptor/ligand, siRNA, indirect lung injury, mouse
Introduction
Shock/sepsis-induced acute lung injury (indirect-acute lung injury, iALI) and its more severe form, acute respiratory distress syndrome (ARDS) have high morbidity and mortality [1]. Current interventions are limited to supportive therapy, including low tidal volume ventilation, as no real molecularly-based therapeutic approach has yet become available [2]. The pathophysiologic process of iALI includes immune cell accumulation, cytokine release, epithelial/endothelial cell injury, and inhibited PMN apoptosis [2]. However, the detailed pathogenic mechanism is not yet clear.
Herpes virus entry mediator (HVEM), also known as tumor necrosis factor receptor superfamily-14 (TNFRSF14), is a transmembrane molecule expressed in nearly all internal organs. The highest expression of HVEM is in the lung, kidney and liver. The cellular distribution of HVEM is relatively wide; it is found on T cells, B cells, dendritic cells, myeloid cells, epithelial cells, and endothelial cells [3]. HVEM can engage up to five different ligands (CD160, B and T lymphocyte attenuator [BTLA], LIGHT [tumor necrosis factor superfamily member 14, TNFSF14], lymphotoxin α, and herpes simplex virus glycoprotein D) to deliver inhibitory or stimulatory signals [4]. Studies have found that HVEM signaling activates intestinal epithelial cells to produce inflammatory cytokines that assist to provide host defense against pathogenic enteric bacteria [5, 6]. However, the role of HVEM in the development of shock/sepsis-induced ALI in mice is not well studied. We hypothesize that HVEM signaling may activate pulmonary cells to promote inflammation in iALI. To test this hypothesis, we utilized a mouse model of combined shock (hemorrhage, HEM) and sepsis (cecal ligation and puncture, CLP) induced iALI, to investigate the relationship of pulmonary HVEM expression with local inflammation and lung injury.
Materials and Methods
Mice
C57BL/6 male mice, 20–25g body weight, ages 8–12 weeks, were obtained from the Jackson Laboratory (Bar Harbor, ME) and were housed in RI Hospital’s Central Research Facilities for 7–10 days before experiments commenced. All protocols were carried out in the morning (8–11AM) according to the NIH guide for animal use and care, and were approved by the Lifespan-Rhode Island Hospital Institutional animal care and use committee (approval number: 0040-16). Animals were housed in ventilated racks, with water and fed standard mouse chow ad libitum under standard environmental conditions (12-hours: 12-hours light/dark cycle, 68–72°F, 30–70% humidity).
Shock/sepsis-induced iALI
A mouse model of iALI was induced by hemorrhagic shock (HEM) followed 24 hours later by cecal ligation and puncture septic challenge (CLP), as previously described by our laboratory [7, 8]. After being anesthetized with isoflurane, mice were bled from one femoral artery to a mean blood pressure of 30–40 mm Hg, kept stable for 90 minutes, and resuscitated with Ringers lactate (4 times the shed volume). Sham mice incurred bilateral femoral artery ligated, but no blood was drawn. 24 hours post-HEM or post-sham HEM, mice were subjected to a septic insult by CLP. The cecum was isolated and ligated at a point approximately 1 cm from the cecal tip, punctured twice with a 22-gauge needle, then gently squeezed to extrude a small amount of fecal material from the perforation sites. The midline wound was closed in two layers and mice were resuscitated with 1 mL of Ringers lactate subcutaneously. In the sham CLP group mice, the cecum was exposed but neither ligated nor punctured.
HVEM siRNA treatment
To confirm the efficacy of anti-HVEM siRNA (SMART pool siGENOME Mouse Tnfrsf14 siRNA [M-058214-00-0050]) (GE Dharmacon, Lafayette, CO), in vitro cell culture of mouse lung epithelial cell line-LA4, obtained from ATCC (Manassas, VA), were cultured with DMEM/F-12 media containing 10% FBS according to manufacturer’s directions. Cells were seeded in tissue culture plates until they reached 60–80% confluent. The culture media were replaced by the Opti-MEM media with Lipofestamine containing anti-HVEM siRNA (100 nM) or PBS and cells were cultured for another 24–48 hours. Cells were collected to determine HVEM expression by flow cytometry.
For in vivo mouse studies, animals were randomized to receive either anti-HVEM siRNA, at a dose of 50μg in 100μl PBS or 100μl PBS per mouse, by intra-tracheal (I.T.) instillation 2 hours after HEM [9]. Of note, we have shown in prior studies that nonsense control siRNA, at the concentration used here, has little effect on indices of experimental iALI as produced by this model when compared to the PBS vehicle treatment [10–12]. Therefore, we opted to compare these animals to a PBS alone control for these studies.
Sample collection
At 24 hours post-CLP or sham CLP (48 hours after Hem+CLP), mice were euthanized with an isoflurane overdose. Broncho-alveolar lavage fluid (BALF) was collected. Briefly, the trachea was exposed via a midline incision and cannulated with a sterile polypropylene 21-gauge catheter. The lungs were lavaged twice with 0.6 mL of normal saline. The lavage fluid was collected for cell count and protein concentration measurement. The whole lung tissue was harvested and homogenized for Western blot, cytokine and MPO analyses as previously outlined [13] or in some cases the lungs were perfused with PBS, then enzymatically dissociated to produce a single cell suspension for flow cytometric staining/analysis as described in detail by our laboratory before [14, 15].
Western blot
Investigated proteins include: HVEM (D-5) (Santa Cruz Biotechnology, Inc., Dallas, TX), Phospho-Stat3 (Tyr705), Stat3 (124H6), and Phospho-p65 (Ser536) antibodies (Cell Signaling Technology, Danvers, MA). Corresponding secondary antibodies were used according to manufacturer protocol. Image densitometry was collected and analyzed with ImageJ software. GAPDH was used as a loading control (Life Technologies) [16].
Flow cytometry
HVEM expression in the dissociated lung tissue cell populations or the LA-4 cell line was determined with PE-labeled anti-HVEM (clone HMHV-1B18) (Biolegend, San Diego, CA), combined with antibodies to denote cell type: APC-labeled anti-Epcam (clone G8.8), anti-CD11c (clone N418), anti-B220 (clone RA3-6B2), anti-CD31 (clone 390), anti-Gr1 (clone 1A8-Ly6g), anti-F4/80 (clone BM8) and anti-CD3 (clone SK7) antibodies (eBioscience, San Diego, CA).
Measurement of cytokines
Concentrations of keratinocyte chemoattractant (KC), macrophage inflammatory protein-2 (MIP-2), monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-10 from lung lysates were assessed via ELISA according to manufacturer’s directions (BD Bioscience, San Jose, CA).
Lung myeloperoxidase (MPO) activity
Lung tissue MPO activity was measured using an assay kit (ab105136) according to manufacturer’s directions (Abcam, Cambridge, MA).
Assessment of lung morphology
The left lung was inflated at 15 mm Hg and fixed with 10% formalin. The specimens were processed, stained with hematoxylin and eosin, and examined by light microscopy. The images were collected from the microscope (Nikon Eclipse 80i) using a 20× objective and a Spot RT3 camera [13].
Survival study
Both HVEM siRNA and PBS treated mice (n=15/group) were subjected to the combined insults of Hem/CLP, and were observed for survival over a ten-day period.
Statistics
Results for all studies other than survival are expressed as Mean ± SEM; n=4–6 mice per group. Statistical significance of the results presented were determined by one-way ANOVA with Bonferroni post-hoc multiple comparisons test. Log-rank test was used for the survival study. Statistical software used was Graph Pad Prism 5 (La Jolla, CA, USA). P<0.05 was used as a cutoff for establishing evidence of a significant difference between a given group.
Results
HVEM expression is elevated in response to iALI
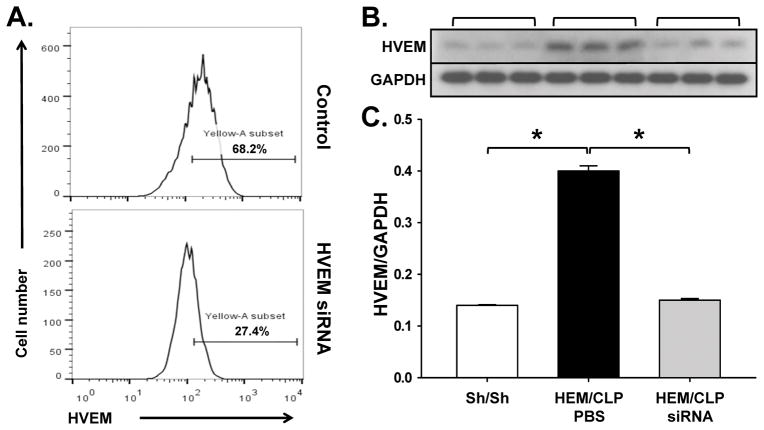
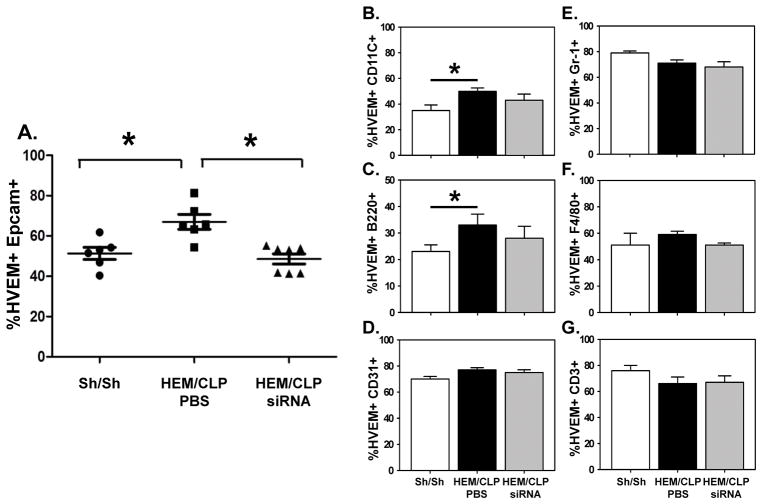
Following the establishment of our anti-HVEM siRNA SMART constructs capacity to selectively in the expression of HVEM protein by cultured of mouse lung epithelial cell line-LA4 (Fig 1A), we first sought to determine whether HVEM was localized in pulmonary cells during ALI. As shown in Fig. 1B–C, HVEM is detected in lung tissue, and the expression is significantly elevated in iALI mice compared to sham mice. This suggests the possibility that HVEM expression may play a role in the development of iALI. By flow cytometry, we subsequently found that HVEM expression was upregulated significantly on Epcam+ (epithelial cell, Fig. 2A), CD11c+ (dendritic cell, Fig. 2B) and B220+ (B cell, Fig. 2C) cell populations. There was no statistically significant change of HVEM expression on CD31+ (endothelial cell, Fig. 2D), Gr1+ (neutrophil, Fig. 2E), F4/80+ (macrophage, Fig. 2F) and CD3+ (T cell, Fig. 2G) cells during iALI.
Figure 1. HEM/CLP enhances HVEM expression.
Initially, the efficacy of anti-HVEM siRNA SMART pool construct was confirmed, vs. Control, by documenting its’ capacity to suppress transfected LA-4 cells ability to express cell surface HVEM (the number of cells and intensity of HVEM cell surface expression [% HVEM (Yellow-A-subset)+ ] by flow cytometry) over a 24 hours in cell culture (representative histograms of one of 3 repeat experiments are provided) (A). Subsequently, at 24 hours after iALI, HVEM expression in lung tissue increased significantly compared to sham surgery mice by Western blot (B) and densitometry analysis (C). HVEM siRNA treatment by I.T. injection down-regulated HVEM expression. Mean ± SEM, n=4–6 mice per group; *P<0.05 vs. PBS treated iALI group, ANOVA.
Figure 2. HEM/CLP enhances HVEM expression on Epcam+, CD11c+ and B220+ cells in the lung.
At 24 hours after iALI, HVEM expression in lung cells increased significantly compared to sham surgery mice. HVEM siRNA treatment by I.T. injection down-regulated HVEM expression only in Epcam+ (A), CD11c+ (B) and B220+ (C) cells, but no changes in other cell types (D–G). Mean ± SEM, n=4–6 mice per group; *P<0.05 vs. PBS treated iALI (Hem/CLP) group as compared to the Sham/Sham (Sh/Sh) group, ANOVA.
In previous studies [12, 17], we have shown that intra-tracheal (I.T.) delivery of naked siRNA reduced target molecule expression primarily in pulmonary epithelial cells, but did not appear to markedly affect other cells. Inasmuch; we initially chose to examine the impact the I.T. administration of naked anti-HVEM siRNA on the iALI-induced elevation of HVEM protein expression in the lung, as assessed by Western immunoblot analysis. We found that the I.T. administration of anti-HVEM siRNA markedly attenuated the rise in HVEM expression seen in the iALI (PBS) group (Fig. 1A–B). With respect to the impact of I.T. anti-HVEM siRNA delivery on lung cell sub-population level, we further observed that HVEM was significantly decreased, specifically on Epcam+ cells (Fig. 2A). This effect was not found among other cell sub-populations, including CD11c+, B220+, CD31+, Gr1+, F4/80+ and CD3+ cells (Fig. 2B–G).
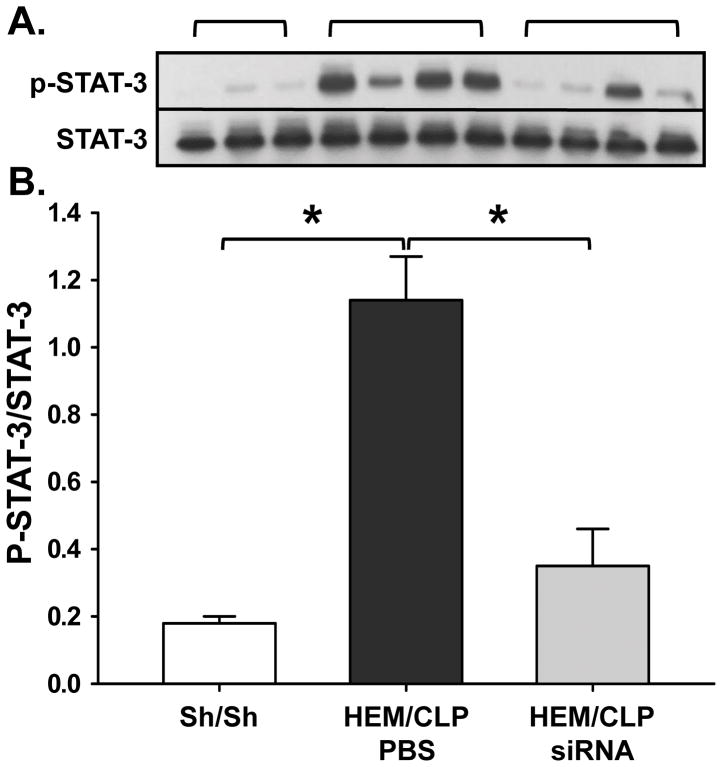
To investigate which signaling pathways may be involved in HVEM expression, we measured phosphorylated STAT-3 in lung tissue lysates by Western blot. Phosphorylated STAT-3 was increased in iALI mice compared to sham mice, and decreased in HVEM-siRNA treated mice compared to the PBS treated iALI group (Fig. 3A–B). There was no relationship between HVEM expression and p65 phosphorylation of the NFκB cascade (Data not shown).
Fig. 3. HEM/CLP enhances HVEM expression on lung cells and is associated with the activation of pSTAT3.
At 24 hours after iALI, HVEM expression in lung tissue increased significantly compared to sham surgery mice, which is associated with an activation of STAT-3 phosphorylation detected in lung tissue lysates after iALI (I). Mean ± SEM, n=4–6 mice per group; *P<0.05 vs. PBS treated iALI group, ANOVA.
Knocking down HVEM in pulmonary epithelial cells with HVEM-specific siRNA attenuates lung inflammation and reduces early mortality after iALI
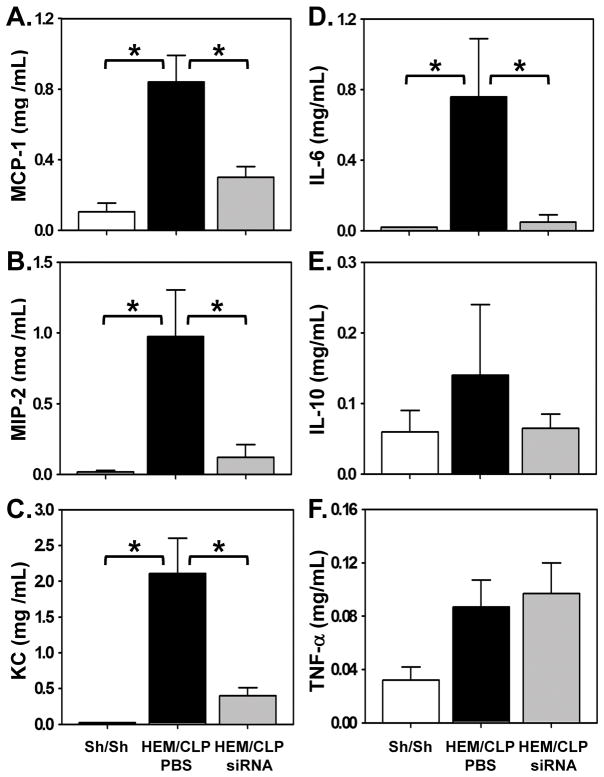
Increased levels in inflammatory cytokines are thought to be indices of local pulmonary inflammation and injury [18]. In the assessment of whether local pulmonary inflammation was affected by I.T. instillation of HVEM-siRNA into the lungs, especially relative to epithelial cells, we found that chemokines MCP-1 (Fig. 4A), MIP-2 (Fig. 4B) and KC (Fig. 4C) in lung lysates were increased significantly in mice subjected to iALI, and decreased in HVEM-siRNA treated iALI mice. This indicates that HVEM expression may affect immune cell recruitment to the lungs. In addition, IL-6 (Fig. 4D) levels were increased significantly in PBS treated iALI mice when compared with sham mice, and were decreased to sham levels with HVEM-siRNA treatment in iALI mice. However, there were no changes in IL-10 or TNF-α levels in lung tissue lysates between groups (Fig. 4E–F).
Figure 4. Effects of HVEM gene knockdown on lung inflammation after iALI.
At 24 hours after iALI, lung tissue lysates are collected for chemokine and cytokine level analysis. MCP-1 (A), MIP-2 (B) and KC (C) are increased significantly in mice subjected to iALI, and decreased in mice with HVEM-siRNA treatment. IL-6 (2D) level is increased significantly in iALI mice and is decreased to sham levels with HVEM-siRNA treatment. There was no change in TNF-α or IL-10 level in lung tissue lysates between all groups (Fig. 2E–F). Mean ± SEM, n=4–6 mice per group; *P<0.05 vs. PBS treated iALI group, ANOVA.
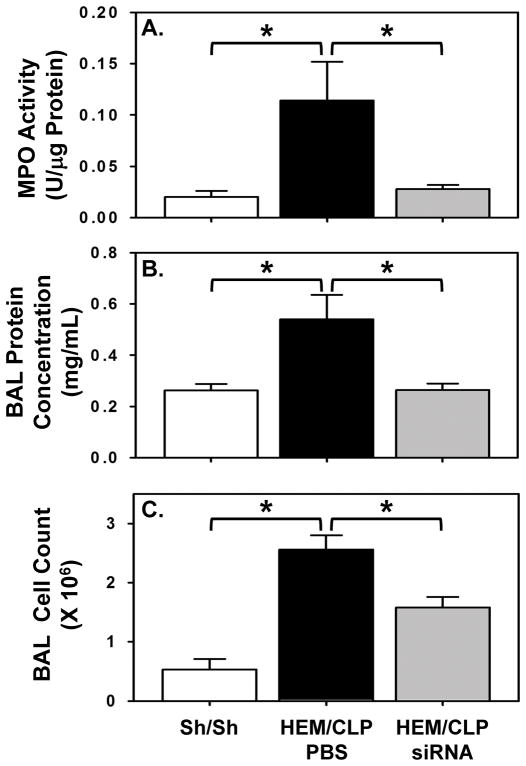
MPO activity, which is used as a measure of neutrophil presence in lung tissue, was elevated in iALI mice compared to sham controls, and was decreased significantly with local silencing of HVEM (Fig. 5A).
Figure 5. Effects of HVEM gene knockdown on neutrophil recruitment and pulmonary permeability in mice with iALI.
At 24 hours after iALI, lung tissue lysates are collected for MPO activity analysis and BALF is collected for protein concentration determination and total cell count. MPO activity (A), BALF protein concentration (B) and BALF cell count (C) are increased in PBS treated mice after iALI, while these parameters are decreased in HVEM-siRNA treated iALI mice. Mean ± SEM, n=4–6 mice per group; *P<0.05 vs. PBS treated iALI group, ANOVA.
Increased protein concentration in BALF is commonly used as a marker of vascular pulmonary permeability/lung injury [19]. In this study, BALF protein concentration was significantly elevated in iALI mice when compared to sham controls, while it was decreased significantly in iALI mice treated with HVEM-siRNA compared to PBS treatment (Fig. 5B). Moreover, the increased total cell count of BALF seen in iALI mice declined in the HVEM-siRNA treated group (Fig. 5C).
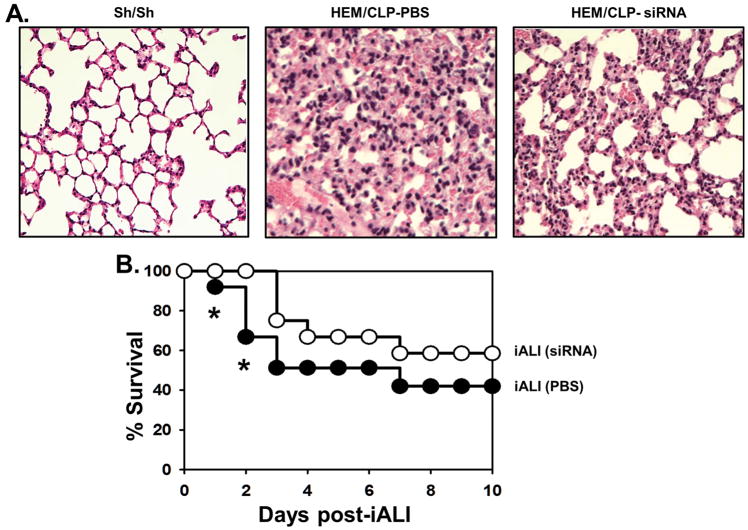
Morphologically, paraffin embedded sections of the lung stained with hematoxylin and eosin showed evidence of increased congestion and neutrophil influx in iALI mice when compared to sham controls. However, the HVEM-siRNA treated animals subjected to iALI appeared to have reduced lung inflammatory infiltrates and improved alveolar architecture, which was more similar to sham controls (Fig. 6A). Finally, the survival rate was elevated significantly in HVEM-siRNA treated group compared to PBS treated iALI mice during the first 60 hours after CLP; however, this survival advantage was lost by 10 days (Fig. 6B).
Figure 6. Effects of HVEM gene knockdown on lung histology and survival in mice with iALI.
At 24 hours after iALI, lung tissue are collected for histology. Representative sections of hematoxylin and eosin stain of the lung from Sham mice, PBS treated or HVEM siRNA treated iALI mice (A) (magnification, ×200). An increased lung cellularity, septal thickening, and alveolar congestion are observed in PBS treated iALI mice that such changes are reduced in HVEM siRNA treated iALI mice. For survival study (B), HVEM siRNA treatment shows a reduced mortality during the first 60 hours compared to PBS treated iALI mice (n=15/group). However, the survival rates of the groups are not different by 10 days. Kaplan-Meier LogRank test.
Discussion
The pathogenic mechanism of iALI is not yet clear [2]. The function of HVEM in this disease-state is to promote inflammation and to stimulate the clearance of bacteria as previously described [5, 20]. Our study reveals that HVEM may play an important role in the development of iALI.
We observed that HVEM expression was elevated on multiple cell types in the lungs during iALI, including epithelial cells, dendritic cells and B cells. Beyond the antigen presenting cells, we also found an increased trend of HVEM expression on endothelial cells and decreased HVEM expression on neutrophils and T cells, a finding consistent with the presence of severe inflammation coexisting with dampened bacterial clearance in iALI. The treatment with naked HVEM-specific siRNA delivered by I.T. injection has previously been documented to primarily affect epithelial cells [10, 12, 17, 21]; thus, the following results could be partially taken as identifying a pathological role of HVEM signaling in pulmonary epithelial cells.
In the past, the quantity of PMNs and macrophages in the lungs has been correlated with an ALI prognosis [22, 23]. Here, we found that HVEM knock-down reduced pulmonary inflammation in our experimental model of iALI. As epithelial cells are reported to be one of the main sources of chemokines [24] and as I.T. injection favors lung epithelial cells as a target [10, 12, 17], we chose to look at changes in lung chemokine levels. Down-regulation of epithelial HVEM expression brought about a reduction of chemokine (MCP-1, KC and MIP-2) levels. This in turn was associated with local decline in lung neutrophil numbers as well as BAL inflammatory cell numbers. This indicates that HVEM signaling through pulmonary epithelial cells, potentially via augmented chemokine release, etc., influences immune cell recruitment -- one of the central links in iALI development [2, 21, 25].
Relative to pulmonary inflammation, levels of the pro-inflammatory cytokine IL-6 were reduced after HVEM gene knock-down. Consistent with the pro-inflammatory profile of the TNFR superfamily, HVEM has previously been shown to induce IL-6 secretion [5, 26]. Additionally, the decreased local inflammatory cell influx (lower BALF cell count) into the lungs likely contributed to the suppressed levels of IL-6 in the anti-HVEM siRNA treated iALI mouse group. Another facet of this diminished IL-6 may have been the muted inflammatory response leading to a milder epithelial injury, which in turn incited a less vehement IL-6 production. This is not the case for TNF-α and IL-10, however, as these cytokines did not correlate with HVEM expression, implying that their regulation is independent of HVEM expression. It may also be that as delivery of naked siRNA construct I.T. appears to be taken up largely by pulmonary epithelial cells and not macrophage or endothelial cells (which are reported to be significant sources of TNF-α and/or IL-10 in this model) [12, 17]. Therefore, the production of these cytokines may not have been affected much here. Increased BALF protein concentration is indicative of pulmonary vascular permeability/lung injury [27]. We further observed that with anti-HVEM siRNA intervention, BALF protein levels were markedly reduced, suggesting the alleviation of pulmonary epithelial/endothelial barrier injury, consistent with our findings on histologic analysis.
The marked, although transient, improvement of survival rate seen here is likely the combined consequence of the above factors. We think that the nature of the transient survival benefit may be related to the inability of the single time-point administration of siRNA’s capacity to sustain suppression of HVEM gene expression in high turn-over cells like pulmonary epithelial cells, which we have shown to be a target of I.T. siRNA delivery [17]. It is possible that if the delivery frequency were increased, the duration of the survival benefit might be improved further. With this stated, it must be appreciated that semi-selective epithelial targeting of I.T. delivered siRNA may preclude targeting of other more distal cell/tissues/organs that are known to be affected by shock/sepsis (e.g. liver, kidney, intestine). The modulation of these organ systems following shock/sepsis contributes to overall mortality and warrants further exploration. We aim to examine this in future studies by assessing the impact of not only alternative routes of delivery, but also alternative modes of siRNA encapsulation.
HVEM has been reported to activate the NF-κβ and STAT3 pathways [5, 28–30]. In our study, the increase in iALI-induced HVEM expression correlated with STAT3 phosphorylation, but not NFκB p65. However, as iALI-induced STAT3 activation/phosphorylation was suppressed by I.T. delivery of anti-HVEM siRNA, this would support the epithelial cell-HVEM-STAT3 mediated regulation of mucosal cell inflammation in this model, in line with what has been reported by others [5, 31]. Yet, which specific pathway(s) HVEM utilizes to recruit/activate STAT3 within the lung epithelial cells remains to be established. Beyond this, HVEM has the ability to promote cell-survival [32]. The decreased neutrophil apoptosis, together with the increased epithelial/endothelial cells apoptosis, are thought to be important pathologic events in iALI [33, 34], and HVEM is expressed on all of these cell types. The type of influence that HVEM exerts on cellular apoptosis in this iALI model remains to be elucidated.
Finally, a number of questions remain unresolved that were outside the scope of our initial study design. What are the HVEM ligands that are expressed in response to insults associated with experimental iALI? Since HVEM has been reported to either enhance or prevent inflammation depending on its interaction with the potential ligands or receptors in different configurations [35], which ones might be pathologically dominant in the iALI lung? Do these ligands/receptors provide stimulatory or inhibitory signals, or both? Alternatively, as many of these HVEM ligands and receptors (e.g., LIGHT and BTLA) also transduce intra-cellular signals themselves when engaged by HVEM, what might this mean when translated into the murine Hem/CLP model? The initial findings here provide foundation for further investigation.
In conclusion, our study shows, for the first time, that HVEM plays an important role in experimental iALI. Further, anti-HVEM siRNA, when delivered intra-tracheally, can reduce inflammatory cell recruitment, mitigate local pulmonary inflammatory response, alleviate lung injury, and transiently reduce mortality induced by experimental iALI. The fact that HVEM is poised at the interconnection of several signaling pathways, many of which act bidirectionally, render this molecule an interesting and potentially unique therapeutic target in iALI.
Acknowledgments
The authors would like to thank Mr. Paul Monfils and Ms. Virginia Hovanesian, Core Research Laboratories, Rhode Island Hospital, for assistance with histology and microscopy/imagine analysis of these specimens.
This work was supported by the following grants: R35 GM118097 (A.A.) from the National Institute of Health/National Institute of General Medical Sciences as well as “Armand D. Versaci” fellowship (E.A.F.)
Footnotes
Conflict of Interest Disclosure: The authors declare no conflicts of interests.
References
- 1.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 2.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 5.Shui J-W, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. HVEM signaling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C, Wickham ME, Guttman JA, Deng W, Walker J, Madsen KL, Jacobson K, Vogl WA, Finlay BB, Vallance BA. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map) Cell Microbiol. 2006;8:1669–1686. doi: 10.1111/j.1462-5822.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 7.Bai J, Tang L, Lomas-Neira J, Chen Y, McLeish KR, Uriarte SM, Chung CS, Ayala A. TAT-SNAP-23 treatment inhibits the priming of neutrophil functions contributing to shock and/or sepsis-induced extra-pulmonary acute lung injury. Innate Immun. 2015;21:42–54. doi: 10.1177/1753425913516524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A. Programmed cell death receptor ligand 1 modulates the regulatory T cells’ capacity to repress shock/sepsis-induced indirect acute lung injury by recruiting phosphatase SRC homology region 2 domain-containing phosphatase 1. Shock. 2015;43:47–54. doi: 10.1097/SHK.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomas-Neira J, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 10.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8 in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkar RK, Chung CS, Chen Y, Monaghan SF, Lomas-Neira J, Cioffi WG, Ayala A. Local tissue expression of the cell death ligand, FasL, plays a central role in the development of extra-pulmonary acute lung injury. Shock. 2011;36:138–143. doi: 10.1097/SHK.0b013e31821c236d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomas-Neira J, Perl M, Venet F, Chung CS, Ayala A. The role and source of tumor necrosis factor-a in hemorrhage-induced priming for septic lung injury. Shock. 2012;37:611–620. doi: 10.1097/SHK.0b013e318254fa6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomas-Neira J, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 14.Venet F, Chung CS, Huang X, Lomas-Neira J, Chen Y, Ayala A. Indirect lung injury: a role for regulatory T lymphocytes in the development of pulmonary inflammation. J Immunology. 2009;183:3472–80. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monaghan SF, Thakkar RK, Heffernan DS, Tran ML, Huang X, Chung CS, Chen Y, Lomas-Neira J, Cioffi WG, Ayala A. Mechanisms of indirect acute lung injury: a novel role for the co-inhibitory receptor, programmed death-1 (PD-1) Ann Surg. 2012;255:158–64. doi: 10.1097/SLA.0b013e31823433ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Chung CS, Chen Y, Monaghan SF, Patel S, Huang X, Heffernan DS, Ayala A. A novel role for programmed cell death receptor ligand-1 (PD-L1) in sepsis-induced intestinal dysfunction. Mol Med. 2016;22:830–840. doi: 10.2119/molmed.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messer MP, Kellermann P, Weber SJ, Hohmann C, Denk S, Klohs B, Schultze A, Braumüller S, Huber-Lang MS, Perl M. Silencing of fas, fas-associated via death domain, or caspase 3 differentially affects lung inflammation, apoptosis, and development of trauma-induced septic acute lung injury. Shock. 2013;39:19–27. doi: 10.1097/SHK.0b013e318277d856. [DOI] [PubMed] [Google Scholar]
- 18.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 19.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A. Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol. 2014;96:809–820. doi: 10.1189/jlb.4MA1213-647RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shui JW, Kronenberg M. HVEM: An unusual TNF receptor family member important for mucosal innate immune responses to microbes. Gut Microbes. 2013;4:146–151. doi: 10.4161/gmic.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perl M, Chung CS, Perl U, Lomas-Neira JL, De Paepe M, Cioffi WG, Ayala A. Fas induced pulmonary apoptosis and inflammation during extrapulmonary acute lung injury. Amer J Resp Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomas-Neira J, Venet F, Chung CS, Thakkar RK, Heffernan DS, Ayala A. Neutrophil-endothelial interactions mediate angiopoietin-2 associated pulmonary cell dysfunction in indirect ALI in mice. Am J Respir Cell Mol Biol. 2014;50:193–200. doi: 10.1165/rcmb.2013-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 24.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–L15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 26.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Bio. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holter JF, Weiland JE, Pacht ER, Gadek JE, Davis WB. Protein permeability in the adult respiratory-distress syndrome - loss of size selectivity of the alveolar epithelium. J Clin Invest. 1986;78:1513–1522. doi: 10.1172/JCI112743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So T, Croft M. Regulation of PI-3-kinase and Akt signaling in T lymphocytes and other cells by TNFR family molecules. Front Immunol. 2013;4:139. doi: 10.3389/fimmu.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2012;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 33.Fialkow L, Filho LF, Bozzetti MC, Milani AR, Filho EMR, Ladniuk RM, Pierozan P, Moura RM, Prolla JC, Vachon E, Gregory P, Downey GP. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care. 2006;10:R155. doi: 10.1186/cc5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 35.Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186–201. doi: 10.1111/j.1600-065X.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]