Abstract
Injury to the central nervous system (CNS) can leave patients with devastating neurological deficits that may permanently impair independence and diminish quality of life. Recent insights into how the CNS responds to injury and reacts to critically timed interventions are being translated into clinical applications that have the capacity to drastically improve outcomes for patients suffering from permanent neurological deficits due to spinal cord injury, stroke, or other CNS disorders. The translation of such knowledge into practical and impactful treatments involves the strategic collaboration between neurosurgeons, clinicians, therapists, scientists, and industry. Therefore, a common understanding of key neuroscientific principles is crucial. Conceptually, current approaches to CNS revitalization can be divided by scale into macroscopic (systems-circuitry) and microscopic (cellular-molecular). Here we review both emerging and well-established tenets that are being utilized to enhance CNS recovery on both levels, and we explore the role of neurosurgeons in developing therapies moving forward. Key principles include plasticity-driven functional recovery, cellular signaling mechanisms in axonal sprouting, critical timing for recovery after injury, and mechanisms of action underlying cellular replacement strategies. We then discuss integrative approaches aimed at synergizing interventions across scales, and we make recommendations for the basis of future clinical trial design. Ultimately, we argue that strategic modulation of microscopic cellular behavior within a macroscopic framework of functional circuitry re-establishment should provide the foundation for most neural restoration strategies, and the early involvement of neurosurgeons in the process will be crucial to successful clinical translation.
Keywords: Stroke, Spinal cord injury, Neurorehabilitation, Neural repair, Axonal regrowth, Neuroregeneration, Brain-machine interface (BMI), Electrical stimulation
ABBREVIATIONS
- BMI
brain-machine interface
- CNS
central nervous system
- CST
corticospinal tract
- ECoG
electrocorticography
- EES
epidural electrical stimulation
- ESS
European Stroke Scale
- FES
functional electrical stimulation
- GAP43
growth-associated protein 43
- MAP
myelin-associated protein
- PTEN
phosphatase and tensin
- SCI
spinal cord injury
- STDP
spike timing-dependent plasticity
- TMS
transcranial magnetic stimulation
Injury to the central nervous system (CNS) can leave patients with devastating functional deficits that may permanently impair independence and diminish quality of life. From a societal perspective, this remains a huge and costly burden.1 Although the CNS has some capacity for recovery during the first year after injury, chronic deficits tend to be static and display minimal improvement over time.2,3 Recent neuroscientific advances, however, have led to new hope for conditions previously considered untreatable.4 For example, for the first time in history, novel interventions have allowed patients with chronic and clinically complete spinal cord injuries (SCI) to regain some degree of voluntary motor control of the legs5–9 and arms.10 Furthermore, through combined immunotherapy and task-based rehabilitation protocols, functional corticospinal tract (CST) regeneration11 and functional synapse formation12 have been produced in animal models of SCI, while full functional recovery of forelimb activity has been demonstrated in animal models of stroke.13 Collectively, these advances are based on a set of emerging neuroscience principles that are being translated from the laboratory to the clinic, providing the first tangible evidence of meaningful recovery in such patients.
Conceptually, approaches to repairing the CNS can be classified by scale into systems-circuitry level approaches (ie, macroscopic) and cellular-molecular interventions (ie, microscopic). Macroscopic approaches currently under investigation include rehabilitation paradigms14,15 with or without neural interfaces5,16 and electrical stimulation strategies6–10,17–20 aimed at increasing the excitability of intact neural elements and inducing circuit plasticity across lesions. Contemporary microscopic strategies, most of which will require surgical administration, include cellular replacement (ie, stem or embryonic cell) therapy,21–26 induction of axonal growth via molecular mechanisms,27–33 optogenetic modulation,34 immunotherapy,13,35–37 and/or enhancement of neurotrophic guidance.38 Emerging evidence suggests that strategically combining approaches on both scales and utilizing conscious intent to re-engage damaged circuitry will be essential to achieving full neurological recovery.4,39 In this paper, we review key scientific principles, discuss integrative approaches, and examine the role of neurosurgeons in translating such techniques into clinical realities.
SYSTEMS-CIRCUITRY PRINCIPLES
Plasticity Drives Functional Recovery
In the acute-to-subacute period after a CNS injury (ie, several days to several weeks), some level of spontaneous clinical improvement often occurs due to reduction in edema, resolution of diaschisis, and optimization of residual dormant (or recovering) but intact functional elements.40,41 Further recovery is achieved through intrinsic plasticity mechanisms such as collateral sprouting from nearby intact neurons and/or dynamic alterations in existing synapses through changes in neurotransmitters, ionic gradients, gap junctions, and glial cells.42–49 During this period, axonal and synaptic plasticity are enabled because the extracellular neural environment has relatively loose extracellular space, more neurotrophic factors, additional open synaptic sites, and probing axonal growth cones.33,50,51 Neurogenesis, on the other hand, does not significantly contribute to recovery of function.52–54 After 6 to 12 mo, further clinical progress wanes55,56 as the environment stabilizes by forming a glial scar with inhibitory mechanical properties57,58 and re-expresses inhibitory molecules such as myelin-associated proteins (MAPs) and proteoglycans.59–62 Interventions aiming to maximize neural recovery, therefore, have tended to focus on the critical time period before 1 yr when functional plasticity mechanisms remain active.63 Efforts after this period usually emphasize strengthening existing circuits, building endurance, and treating the deleterious effects of inappropriate plasticity (eg, spasticity and seizures).
More recently, however, emerging evidence suggests that even patients with chronic and complete SCIs may retain some capacity for functional improvements through previously untapped plasticity mechanisms. In 2016, Donati et al5 demonstrated that some recovery could be achieved in patients with chronic and complete thoracic SCIs by implementing extensive training with a brain-controlled exoskeleton. In doing so, this group provided the first report of a therapeutic strategy that enabled the reclassification of patients from chronic-complete to incomplete SCIs. A similar result was recently published in 2017 by Rejc et al,9 showing that extensive training combined with epidural electrical stimulation (EES) of spinal elements distal to a lesion could achieve similar long-term results.
The implication of these pilot human experiments is that there are likely surviving but dormant, or subclinical, white matter tracts that are recruitable for strengthening and plasticity induction in select cases. In most SCIs which are usually due to blunt traumatic compression, hemorrhage tends to occur preferentially in the central gray matter due to its softer consistency and relatively increased vascularity (Figure 1).64 Thus, there is potential for some more mechanically resilient peripheral white matter tracts to remain intact (ie, central cord contusions). Importantly, the volume of residual white matter tracts has been shown to directly relate to postinjury locomotive ability in rat models of SCI.65 In parallel, human SCIs that are clinically complete may also demonstrate residual subclinical supraspinal connections. Such injuries are now being called “discomplete,” indicating potential for a clinical response to the aforementioned interventions.66,67 As such, exoskeleton and spinal cord stimulation strategies are now being combined to help further facilitate rehabilitation in motor complete, or discomplete, paraplegics.8
FIGURE 1.
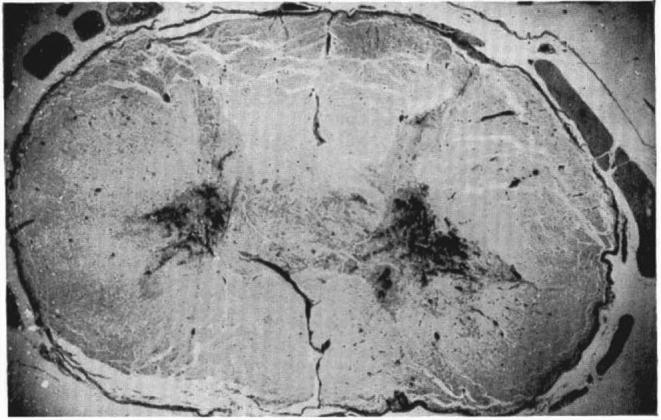
Transverse section of a central spinal cord contusion at C7. Hemorrhage is seen preferentially in the central gray matter with largely intact surrounding white matter. Reprinted by permission from SpringerNature: Paraplegia. The disturbance of circulation in traumatic paraplegia in acute and late stages: A pathological study. Wolman L.64 Copyright 1965.
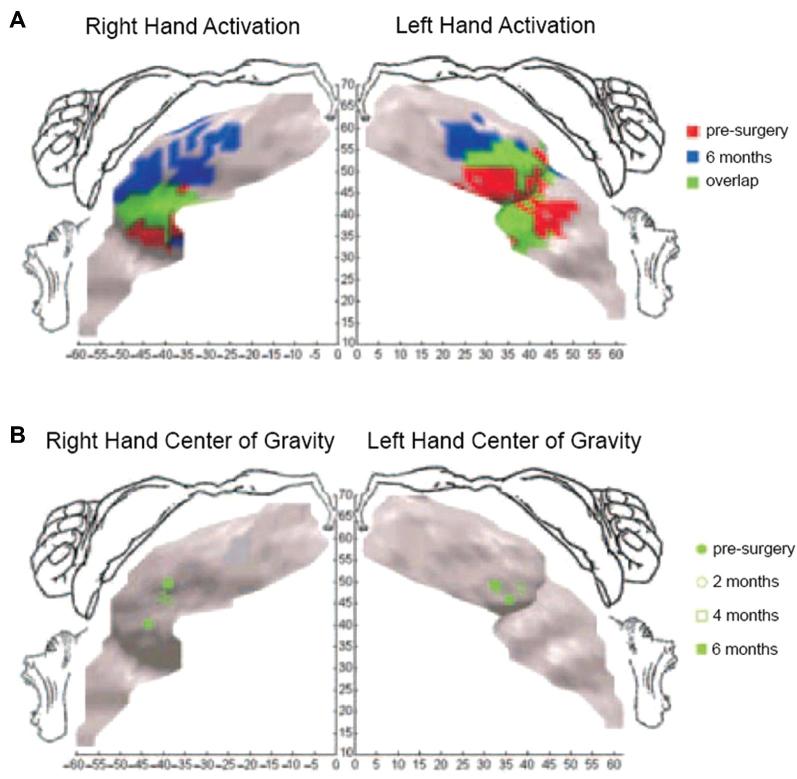
In addition to intrinsic spinal plasticity, the relative role of cortical plasticity in facilitating such recovery is yet undefined. Significant cortical reorganization is known to occur after chronic nonuse or amputation of a limb68–71 as well as in response to directly injured motor cortex,72 and such reorganization tends to include the expansion of somatotopically neighboring functions into newly dormant or damaged areas in a behavior-dependent fashion.72 Furthermore, there is some evidence that this plasticity is reversible.73,74 Therefore, it is also likely that re-engagement of previously lost functions can help maintain or induce cortical plasticity to re-establish and/or re-grow critical somatotopy and connectivity for improved performance (Figure 2).74,75
FIGURE 2.
Cortical plasticity induced by bilateral hand transplantation in an amputee. A, Hand activation in M1 before surgery and 6 mo after grafting. After receiving the graft, hand representation expanded medially to reoccupy the entire hand region. B, Center of gravity shifts over time. The right-hand representation shifted 10 mm, and the left-hand representation shifted 6 mm from the lateral to the central part of the M1 hand region. Reprinted by permission from SpringerNature: Nature Neuroscience. Cortical reorganization in motor cortex after graft of both hands. Giraux P et al.74 Copyright 2001.
Experimental approaches aimed at enhancing cortical plasticity after stroke have included electrical stimulation of the cortex,18,76 vagus nerve stimulation,77 paired associative stimulation (ie, paired peripheral nerve and transcranial magnetic stimulation [TMS]),78 and brain-state-dependent stimulation (ie, paired TMS and neural interfacing).79 While cortical stimulation paradigms have shown promise in animal models,17,80,81 a recent phase III clinical trial was negative at its primary endpoints.19 However, future studies may incorporate a variety of novel stimulation protocols and/or combine stimulation with a host of microscopic interventions discussed later in this review. The lack of an FDA-approved device for this type of stimulation remains a major barrier. However, a fully implantable electrocorticography (ECoG) device with wireless transmission capabilities is now undergoing clinical trials in Europe for brain-machine interface (BMI) applications, but it is currently being used for recording only.82
Neurons that Fire Together Wire Together
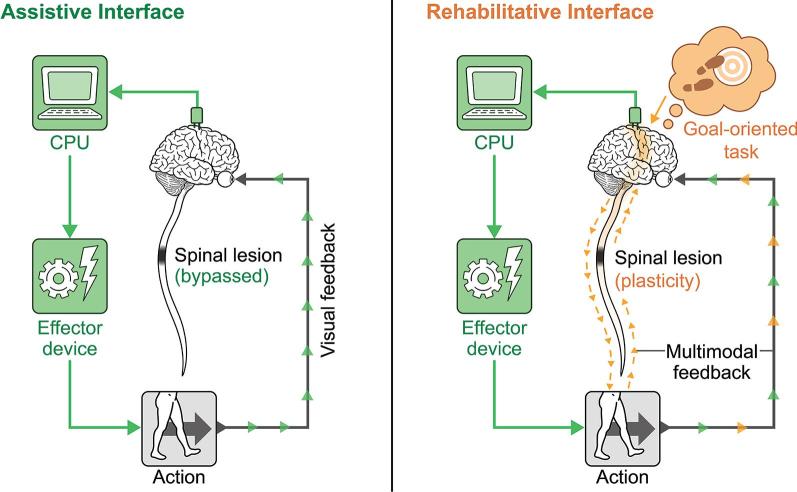
Also known as Hebbian plasticity, the principle of spike timing-dependent plasticity (STDP) states that synaptic strength is redistributed to favor functionally relevant pathways that are coincidently active.83–85 This tenet underlies several new rehabilitation paradigms that utilize neural interfaces and invasive stimulation strategies to pair goal-oriented intention with critically timed feedback to encourage positive plasticity. The paradigm shift from assistive to rehabilitative interfaces has been explored in several recent reviews,4,86,87 and a schematic diagram outlining the conceptual evolution in this approach is reproduced in Figure 3. The experiments of Donati et al5 and Rejc et al9 explored in the previous section also likely owe much of their success to their utilization of STDP principles, as these systems pair conscious intention with exoskeleton and EES-aided movements, respectively. Overlapping principles are also explored in the section “Conscious Engagement is Key for Long-term Functional Improvement,” wherein the importance of conscious engagement is discussed.
FIGURE 3.
Assistive vs rehabilitative interfaces. The assistive interface bypasses a lesion to produce an action, whereas the rehabilitative interface times a goal-oriented intention with positive feedback to induce plasticity. The effector device can be anything that produces a desired output, including cursor on a screen, an exoskeleton, FES, EES, EMG, or a typing device, for example. Reproduced from Krucoff et al, 20164 under license CC BY-ND 4.0.
Intact Neuromuscular Elements Distal to a CNS Lesion can be Recruited for Function
SCI leads to a “disconnection syndrome” where cognitive intent can no longer communicate with distal neuromuscular anatomy. Therefore, indirectly reconnecting motor plans to execution of its intended action could theoretically restore function. This concept has led to the development of bypass (ie, assistive) BMI, or ways to circumvent lesions to restore critical functions.88 Such strategies89,90 are not necessarily designed to engender plasticity; however, it turns out that improved performance during long-term training with such BMIs is likely due in part to significant neuroplasticity.73
Approaches to reanimating paralyzed extremities include functional electrical stimulation (FES) of distal musculature10,91,92 and EES of distal spinal elements.6–8,88 FES involves the stimulation of electrodes in target muscles directed by signals decoded from a neural implant. So far, FES systems have enabled brain-controlled joint-specific movements of paralyzed limbs in 3 dimensions, and have assisted quadriplegic patients in feeding themselves.10 Continuous EES, on the other hand, lowers the excitation threshold of intact distal neuronal circuitry such that any subclinical supraspinal connections can re-exert their influence and enable volitional control of the distal anatomy.66,67,93 Demonstrations of such techniques have enabled both volitional and nonvolitional stepping movements, task-specific single-joint movements, and standing in patients with complete and chronic SCI6–8 that in 1 case persisted after stimulation was ceased.9 To help further develop control techniques for EES, FES, and proprioceptive stimulation modalities, ethical animal models for reversible paraplegia are also being developed.94
CELLULAR-MOLECULAR PRINCIPLES
Cellular Signaling can Alter Axonal Sprouting
In the mature CNS, neurons do not spontaneously regenerate, and attempts at axonal regrowth generally fail due to a lack of appropriate extracellular guidance.95–97 Therefore, altering intrinsic transcription factors and regeneration-associated genes may provide pharmacological solutions to enhance regrowth, guidance, and reinnervation.50,98–100 To date, several important targets have been identified, such as phosphatase and tensin homolog (PTEN) 101,102 and Socs3.103 Additionally, the proto-oncogene bcl-2 is known to play a key role in preventing cell death after injury.104,105 Also, growth and differentiation factor 10 and growth-associated protein 43 (GAP43) are known to promote axonal growth and are released in the subacute period after stroke in rat models.31,32 Moreover, use of the purine nucleoside inosine in animal models of SCI and stroke have been shown to restore GAP43 levels and improve behavioral outcomes.27–29,101,106,107
In addition, extrinsic factors like MAPs and proteoglycans can also prevent axonal regeneration, especially in glial scars formed after injury.59–62 However, recent evidence suggests that the glial scar itself may provide a necessary scaffolding for successful iatrogenically induced regeneration.108 Removal or blockage of extracellular inhibitory factors alone has generally failed to achieve effective axonal regeneration.109,110 One exception has been neutralization of Nogo, a negative regulator of growth.111 Anti-Nogo immunotherapies have successfully demonstrated increased sprouting associated with functional recovery in both rat35,112,113 and primate36 models of SCI and stroke.13 Surgical intervention may be required to iatrogenically deliver therapies such as these to critical targets.
Inflammation is Complex and Important
While some components of inflammation cause tissue damage and apoptosis/necrosis, others promote phagocytosis, debris removal, cell survival, and axonal sprouting depending on timing after injury.101,114–118 Both oncomodulin, a macrophage-derived growth factor, and injury-induced cytokine release appear to play a role in inflammation-induced axonal regeneration.116,119,120 Traditional anti-inflammatory therapies (eg, nonsteroidal anti-inflammatory drugs) may stifle both helpful and harmful components of the immune response.59,115,121 For example, when combined with PTEN deletion and elevation of cyclic adenosine monophosphate, intraocular inflammation has been shown to enable some retinal ganglion cells to regenerate injured axons from the eye to the brain and restore simple visual responses.61 Therefore, therapeutic approaches might aim at balancing cellular phenotypes in the injury microenvironment, as microglia, macrophages, and astrocytes exhibit a spectrum of states that are under active investigation.122,123
Cellular Replacement may Work Through a Variety of Mechanisms
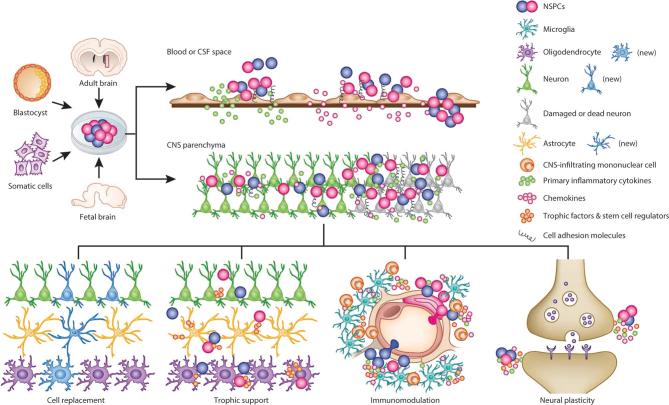
Rates and extent of recovery across patients with CNS injuries can vary widely. It is now recognized that some of this variability may be due to a host of cellular processes, such as (1) number and neuroplasticity of surviving neurons, synapses, and circuits; (2) extent of reorganization and neural innervation; (3) degree of dendritic arborization, synaptogenesis, and remyelination; (4) release of trophic factors; (5) activity of immune cells; and (6) generation of new neurons, glial, and endothelial cells from endogenous stem cells that integrate into injured neuronal networks.124 Therefore, while traditional neural grafting has emphasized the role of neurons in reconstituting neural circuitry through synaptic connectivity,125,126 many new approaches emphasize a much broader range of cell sources and actions once grafted (Figure 4).127–130
FIGURE 4.
Possible mechanisms of action of cellular replacement therapy. After transplantation, stem cells can promote CNS repair through several mechanisms, such as cellular replacement, neurotrophic support, immunomodulation, and/or induction of plasticity at existing synapses. These mechanisms are not mutually exclusive. Reprinted by permission from SpringerNature: Nature Neuroscience. Kokai Z et al. Cross-talk between neural stem cells and immune cells: the key to better brain repair?127 Copyright 2012.
Though cellular transplantation has shown promise in animal models,12,131,132 translation to neurological improvement in human studies has proven difficult as the underlying mechanisms of action remain poorly understood and unexpected toxicity has occurred.133 In animal models of Huntington's disease131 and ischemic stroke, direct injection of embryonic cells has been shown to improve deficits,132 and a phase 1 clinical trial of human fetal brain-derived immortalized neural stem cells for stroke demonstrated safety with some suggestion of neurological improvement.134 Recently, Kodoya et al12 demonstrated robust CST regeneration and synapse formation caudal to an SCI after grafting homologous multipotent neural progenitor cells to the site of injury in rats. Similarly, cultured human neurons derived from an embryonal carcinoma cell line135 were studied in an open-label phase 1 trial that showed improvement on the European Stroke Scale (ESS) and metabolism by fluorodeoxyglucose-positron emission tomography.136 However, a subsequent phase 2 randomized study demonstrated no statistically significant difference in ESS or overall motor outcome, although improvement was seen on Fugl-Meyer Assessments and in cognitive function.137
In addition to embryonic or carcinoma-derived stem cells, it is possible to genetically reprogram differentiated mature somatic cells, such as fibroblasts, into pluripotent stem cells that exhibit the morphology and growth properties of embryonic stem cells.138 Use of autologous induced pluripotent stem cells has the potential to avoid immunosuppression and ethical issues associated with the use of human embryonic cells. An open-label phase 1/2a study of stereotactic injection of these cells into the area of a previous ischemic stroke demonstrated significant improvement in stroke scale and motor scores, leading to randomized controlled trials.25 Thus, critical questions for future studies in addition to efficacy include defining viable cell sources, understanding safety concerns, delineating effects on endogenous cell populations, and understanding mechanisms of action for different cell lines.
INTEGRATIVE PRINCIPLES
Regeneration ≠ Functional Restoration
Robust axonal regeneration and development of appropriate connectivity alone does not necessarily ensure restoration of function.139 In 1 example, Bei et al140 were able to induce adult mouse retinal axons to regrow and synapse in the superior colliculus; however, these connections did not restore visual function on their own—addition of a voltage-gated potassium channel blocker was required to enable the proper conduction of action potentials, as the newly regenerated axons were not properly myelinated. For more complex functions, targeted behavioral training will almost certainly need to accompany anatomic realignment to ensure establishment of the proper functional connectivity,72 as not all plasticity is known to be beneficial (eg, spasticity, postinjury seizures, and pathological pain). Research groups remain heavily focused on both understanding the precise roles of different neuronal populations in repair, as well as crossing the bridge from structural to functional restoration.
Micro- and Macroscopic Interventions Might be Synergistic or Antagonistic, and Timing is Critical
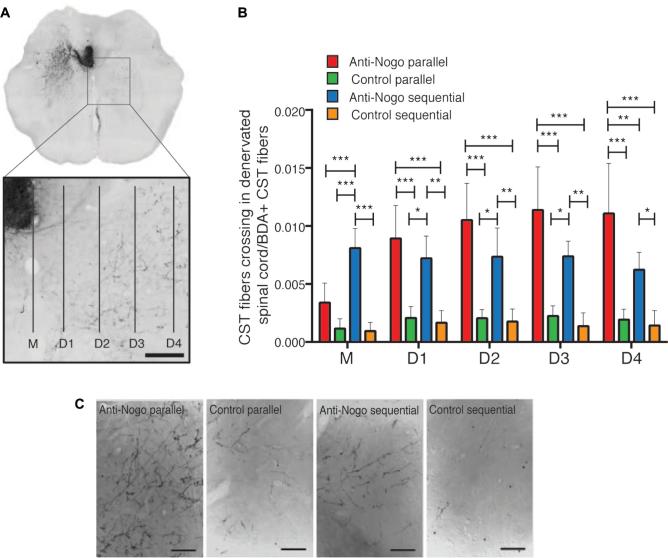
While microscopic interventions can alter cell populations, improve cellular signaling, and induce axonal sprouting and synapse formation, macroscopic paradigms may strengthen and stabilize functional circuits to enhance performance. Therapy on each scale can affect the other in crucial ways. Wahl et al13 perhaps most clearly demonstrated this principle when they injected an anti-NogoA antibody intrathecally into rats with large strokes, and then followed the injection with intensive task-specific training (Figure 5). When immunotherapy and training were combined simultaneously, greater axonal sprouting was seen, but fiber branching was chaotic and functional outcome was worse compared to no treatment (also seen by Maier et al35). This demonstration illustrates both the distinction between simple regrowth and functional restoration, as well as the importance of timing between interventions on different scales to stabilize essential circuitry. In general, the principle of micro- and macroscopic interaction is only recently being rigorously explored. Such experiments can be difficult to design, execute, and interpret, as they require multidisciplinary expertise. However, achieving a better understanding and appreciation of such interplay appears to be critical for developing therapeutic strategies that hope to realize a positive clinical impact.
FIGURE 5.
Functional CST sprouting depends on relative timing of anti-NogoA injection and rehabilitation training in rat models of stroke. Four rehabilitation schedules (anti-Nogo-A/parallel, control/parallel, anti-Nogo-A/sequential, and control/sequential) were tested and differently influenced CST fiber sprouting from the intact hemicord (left) across midline. Abhorrent growth (anti-Nogo-A/parallel group) displayed worse functional outcomes compared to control groups, whereas organized growth (anti-Nogo-A/sequential) demonstrated improved functionality. A, Micrographs of CST fibers in the intact spinal hemicord (left) growing into the stroke-denervated hemicord (right) at C4. B, Fibers crossing the midline (M) and branching in the gray matter at distances D1 to D4 were counted and normalized to the number CST fibers in the main tract. C, Micrographs showing different sprouting patterns of corticospinal fibers from the ipsilateral cortex in the denervated cervical spinal cord (C4) in lamina 7. Scale bar—200 mm; M—midline; BDA—biotinylated dextran amine. *P < .05, **P < .01, ***P < .001. From Wahl AS, Omlor W, Rubio JC, et al. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344(6189):1250-1255. doi:10.1126/science.1253050. Reprinted with permission from AAAS.
Conscious Engagement is Key for Long-Term Functional Improvement
One of the first studies to demonstrate the restoration of supraspinal control of gait in a rat model of SCI also demonstrated the importance of conscious engagement in long tract regrowth.11 In this study, all rats were trained with EES. However, the rats that were trained with passive treadmill rehabilitation achieved no restoration of volitional motor control, whereas the rats that were trained with goal-oriented tasks both regained volitional control of ambulation and showed evidence of functional long-tract regrowth. Additionally, in a human clinical trial of recovery after hemiparetic stroke, passively assisted robotic arm movements showed less improvement than unassisted, patient-direct movements.141 Exactly how conscious agency relates to neuroanatomical principles of circuitry and guidance remains a mystery, but they are intimately connected.142–144 From a clinical perspective, this implies that patients who suffer from disorders of consciousness (eg, comatose or vegetative patients) may need completely different therapeutic strategies which are much farther from being realized. It also suggests that experiments that have failed in vitro may still be viable therapies when integrated into a framework that includes conscious intent and goal-directed therapy, and therefore should not be explicitly excluded from clinical trial design.4
THE NEUROSURGEON'S ROLE IN CNS RESTORATION
Neurosurgeons have a unique opportunity to play a critical role in the advancement of therapeutic modalities aimed at functional CNS restoration. While most neurosurgeons interact with patients with neurological deficits from brain tumors, strokes, traumatic brain injury, or SCIs daily, they have limited tools to help such patients regain function after their condition has been stabilized. At this point in treatment, neurosurgeons generally take a back seat to other providers such as physical, occupational, and speech therapists. To advance the utility of interventions such as BMI, FES, EES, stem cell therapy, immunotherapy, pharmacotherapy, optogenetics, and gene therapy, collaborations between neurosurgeons, clinicians, therapists, basic scientists, funding agencies, and industry will be essential. Neurosurgeons have already played prominent roles in the BrainGate10,145 and the Northstar Neuroscience (Everest) trials,19 and the progression of such techniques into the realm of CNS repair may provide opportunities for neurosurgeons to expand their capabilities in the care of these patients beyond just the implantation-stabilization phase. Furthermore, such BMI and cortical stimulation strategies will likely continue to play a large role in future macroscopic frameworks within which microscopic advancements will be tested.
The engagement of neurosurgeons early in the process of developing CNS repair strategies is essential not only because neurosurgeons maintain critical access to patients with CNS injuries and the ability to perform invasive CNS procedures, but neurosurgeons also have intimate clinical experience with the relationship between structural and functional CNS anatomy and its response to injury and intervention. Additionally, as any currently proposed therapy will need to navigate the tortuous pathway to FDA approval before realizing any large-scale implementation, neurosurgeons should be involved early to help stave off potential pitfalls of human translation and clinical trial design. Therefore, a basic understanding of the translational principles outlined in this manuscript and a sense for where therapeutic advances may be heading are necessary.
CONCLUSION
Despite numerous scientific advances, many patients continue to experience persistent functional deficits following SCI, stroke, and other CNS disorders. As explored in this review, new interventions are starting to provide hope for better outcomes, and strategic approaches that utilize both micro- and macroscopic interventions will be the most likely to have a broad clinical impact. While macroscopic (ie, systems-circuitry) techniques such as neural interfaces, FES, and EES have begun to demonstrate positive results in human patients, most microscopic (ie, cellular-molecular) therapies such as cellular treatments, immunotherapies, molecular interventions, and optogenetics remain in the in vitro or animal model stage and have encountered significant hurdles to clinically relevant translation. To make the leap, 3 strategic and harmonious integrative principles are important for future translational clinical trial design: (1) axonal regeneration does not by itself ensure functional restoration, (2) timing between cellular and systems-level (ie, behavioral) interventions is critical, and (3) conscious engagement plays a vital role in neurological restoration. Neurosurgeons will have the opportunity to play a variety of roles in the adaptation of such therapies into mainstream clinical practice, which can range from passive observers to expert technicians or intellectual leaders. Which role is played will largely depend on active and early engagement, an understanding of important translational neuroscience principles, and a willingness to collaborate and help facilitate clinical trial design.
Disclosures
Dr Krucoff's time is supported by NINDS grant 5R25NS065731-08. Dr Turner's time is supported by VA grants I21 BX003023-01 and I21 RX002223, as well the NIH grant R21 AG051103-01. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
In light of recent developments positing improved functional recovery from central nervous system injuries, this timely review seeks to lay down microscopic and macroscopic principles that guide CNS restoration based on a review of recent data. The target audience includes neurosurgeons interested in helping push this field forward. The authors summarize the impact of different “cellular-molecular principles”, meaning transcription factors, factors in the extra-cellular matrix, and stem cells as well as the influence of different “system-circuitry principles” (macroscopic) like neuronal plasticity and brain-machine interfaces on CNS restoration. They then argue that the best and necessary approach to achieve functional recovery is to synergistically combine these 2 approaches of different scales. This point is demonstrated by studies that effectively elicited post-injury neuronal connectivity but not a return of proper function. They describe some recent data connecting immunotherapeutic and behavioral interventions.
The authors conclude by sharing a few insights to bring neurosurgeons who may become involved in clinical trial design in this field up to speed with current thinking. Namely, that cellular regeneration does not necessitate a return of proper circuit-level function, and that both the proper combination and timing of microscopic and macroscopic interventions and the conscious engagement of patients in their rehabilitative efforts is central to their recovery.
Bradley Lega
Dallas, Texas
REFERENCES
- 1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. 2015. https://www.nscisc.uab.edu/Public/Facts2015.pdf.
- 2. Waters RL, Yakura JS, Adkins RH, Sie I. Recovery following complete paraplegia. Arch Phys Med Rehabil. 1992;73(9):784–789. [PubMed] [Google Scholar]
- 3. El Tecle NE, Dahdaleh NS, Bydon M, Ray WZ, Torner JC, Hitchon PW. The natural history of complete spinal cord injury: a pooled analysis of 1162 patients and a meta-analysis of modern data. J Neurosurg Spine. 2018:1–8. doi: 10.3171/2017.7.SPINE17107. [DOI] [PubMed] [Google Scholar]
- 4. Krucoff MO, Rahimpour S, Slutzky MW, Edgerton VR, Turner DA. Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Front Neurosci. 2016;10(December):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donati ARC, Shokur S, Morya E et al. . Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep. 2016;6(1):30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(5):1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harkema SJ, Gerasimenko Y, Hodes J et al. . Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grahn PJ, Lavrov IA, Sayenko DG et al. . Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544–554. [DOI] [PubMed] [Google Scholar]
- 9. Rejc E, Angeli CA, Atkinson D, Harkema SJ. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep. 2017;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ajiboye AB, Willett FR, Young DR et al. . Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389(10081):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Brand R, Heutschi J, Barraud Q et al. . Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336(6085):1182–1185. [DOI] [PubMed] [Google Scholar]
- 12. Kadoya K, Lu P, Nguyen K et al. . Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22(5):479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahl AS, Omlor W, Rubio JC et al. . Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344(6189):1250–1255. [DOI] [PubMed] [Google Scholar]
- 14. Wolf SL, Winstein CJ, Miller JP et al. . Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 2006;296(17):2095–2104. [DOI] [PubMed] [Google Scholar]
- 15. Lo AC, Guarino PD, Richards LG et al. . Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloch J, Lacour SP, Courtine G. Electronic dura mater meddling in the central nervous system. JAMA Neurol. 2017;74(4):470–475. [DOI] [PubMed] [Google Scholar]
- 17. Plautz EJ, Barbay S, Frost SB et al. . Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–810. [DOI] [PubMed] [Google Scholar]
- 18. Levy RM, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108(4):707–714. [DOI] [PubMed] [Google Scholar]
- 19. Levy RM, Harvey RL, Kissela BM et al. . Epidural electrical stimulation for stroke rehabilitation. Neurorehabil Neural Repair. 2016;30(2):107–119. [DOI] [PubMed] [Google Scholar]
- 20. Edwardson MA, Lucas TH, Carey JR, Fetz EE. New modalities of brain stimulation for stroke rehabilitation. Exp Brain Res. 2013;224(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cote DJ, Bredenoord AL, Smith TR et al. . Ethical clinical translation of stem cell interventions for neurologic disease. Neurology. 2017;88(3):322–328. [DOI] [PubMed] [Google Scholar]
- 22. Smith EJ, Stroemer RP, Gorenkova N et al. . Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30(4):785–796. [DOI] [PubMed] [Google Scholar]
- 23. Wang Q, Duan F, Wang MX, Wang XD, Liu P, Ma LZ. Effect of stem cell-based therapy for ischemic stroke treatment: a meta-analysis. Clin Neurol Neurosurg. 2016;146(69):1–11. [DOI] [PubMed] [Google Scholar]
- 24. Moniche F, Escudero I, Zapata-Arriaza E et al. . Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): Protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015;10(7):1149–1152. [DOI] [PubMed] [Google Scholar]
- 25. Steinberg GK, Kondziolka D, Wechsler LR et al. . Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke. Stroke. 2016;47(7):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tornero D, Tsupykov O, Granmo M et al. . Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140(3):692–706. [DOI] [PubMed] [Google Scholar]
- 27. Zai L, Ferrari C, Subbaiah S et al. . Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29(25):8187–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D, Zai L, Liang P, Schaffling C, Ahlborn D, Benowitz LI. Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS One. 2013;8(12):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen P, Goldberg DE, Kolb B et al. . Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci. 2002;99(13):9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carmichael ST, Chesselet M-F. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22(14):6062–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li S, Nie EH, Yin Y et al. . GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18(12):1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135–2144. [DOI] [PubMed] [Google Scholar]
- 33. Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J Comp Neurol. 1996;373(4):484–497. [DOI] [PubMed] [Google Scholar]
- 34. Jarvis S, Schultz SR. Prospects for optogenetic augmentation of brain function. Front Syst Neurosci. 2015;9(November):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maier IC, Ichiyama RM, Courtine G et al. . Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132(6):1426–1440. [DOI] [PubMed] [Google Scholar]
- 36. Freund P, Schmidlin E, Wannier T et al. . Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12(7):790–792. [DOI] [PubMed] [Google Scholar]
- 37. Lee J-K, Kim J-E, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24(27):6209–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31(10):3766–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krucoff MO, Harward SC, Rahimpour S et al. . Integrating molecular, cellular, and systems approaches to repairing the brain after stroke. In: Lapchak PA, Zhang JH, eds, Stroke: Cellular and Molecular Approaches to Regeneration & Repair. Cham, Switzerland: Springer; 2018: 365–382. [Google Scholar]
- 40. Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75(5):2144–2149. [DOI] [PubMed] [Google Scholar]
- 41. Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17(5):817–830. [DOI] [PubMed] [Google Scholar]
- 42. DeFina P, Fellus J, Polito MZ, Thompson JWG, Moser RS, DeLuca J. The new neuroscience frontier: promoting neuroplasticity and brain repair in traumatic brain injury. Clin Neuropsychol. 2009;23(8):1391–1399. [DOI] [PubMed] [Google Scholar]
- 43. Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2012;27(4):274–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nahmani M, Turrigiano GG. Adult cortical plasticity following injury: Recapitulation of critical period mechanisms? Neuroscience. 2014;283(12):4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villamar MF, Santos Portilla A, Fregni F, Zafonte R. Noninvasive brain stimulation to modulate neuroplasticity in traumatic brain injury. Neuromodulation. 2012;15(4):326–338. [DOI] [PubMed] [Google Scholar]
- 46. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22(3-5):281–299. [PubMed] [Google Scholar]
- 47. Alia C, Spalletti C, Lai S et al. . Neuroplastic Changes Following Brain Ischemia and their Contribution to Stroke Recovery: Novel Approaches in Neurorehabilitation. Front Cell Neurosci. 2017;11(March):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hermann DM, Chopp M. Promoting neurological recovery in the post-acute stroke phase: benefits and challenges. Eur Neurol. 2014;72(5-6):317–325. [DOI] [PubMed] [Google Scholar]
- 49. Corbett D, Nguemeni C, Gomez-Smith M. How can you mend a broken brain? - Neurorestorative approaches to stroke recovery. Cerebrovasc Dis. 2014;38(4):233–239. [DOI] [PubMed] [Google Scholar]
- 50. Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193(2):291–311. [DOI] [PubMed] [Google Scholar]
- 51. Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci. 2013;7(December):887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105(1):33–41. [DOI] [PubMed] [Google Scholar]
- 53. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. [DOI] [PubMed] [Google Scholar]
- 54. Ohab JJ, Carmichael ST. Poststroke Neurogenesis: emerging principles of migration and localization of immature neurons. Neurosci. 2008;14(4):369–380. [DOI] [PubMed] [Google Scholar]
- 55. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- 56. Teasell RW, Murie Fernandez M, McIntyre A, Mehta S. Rethinking the continuum of stroke rehabilitation. Arch Phys Med Rehabil. 2014;95(4):595–596. [DOI] [PubMed] [Google Scholar]
- 57. Moeendarbary E, Weber IP, Sheridan GK et al. . The soft mechanical signature of glial scars in the central nervous system. Nat Commun. 2017;8:14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saxena T, Gilbert J, Stelzner D, Hasenwinkel J. Mechanical characterization of the injured spinal cord after lateral spinal hemisection injury in the rat. J Neurotrauma. 2012;29(9):1747–1757. [DOI] [PubMed] [Google Scholar]
- 59. Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: Strategies for overcoming inhibitory signals and activating neurons' intrinsic growth state. Devel Neurobio. 2007;67(9):1148–1165. [DOI] [PubMed] [Google Scholar]
- 61. de Lima S, Koriyama Y, Kurimoto T et al. . Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci. 2012;109(23):9149–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Omura T, Omura K, Tedeschi A et al. . Robust axonal regeneration occurs in the injured CAST/Ei Mouse CNS. Neuron. 2015;86(5):1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Teasell R, Mehta S, Pereira S et al. . Time to rethink long-term rehabilitation management of stroke patients. Top Stroke Rehabil. 2012;19(6):457–462. [DOI] [PubMed] [Google Scholar]
- 64. Wolman L. The disturbance of circulation in traumatic paraplegia in acute and late stages: a pathological study. Paraplegia. 1965;2(4):213–226. [DOI] [PubMed] [Google Scholar]
- 65. Metz GAS, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17(1):1–17. [DOI] [PubMed] [Google Scholar]
- 66. Dimitrijevic MR. Neurophysiology in spinal cord injury. Paraplegia. 1987;25(3):205–208. [DOI] [PubMed] [Google Scholar]
- 67. McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil Neural Repair. 2004;18(3):144–153. [DOI] [PubMed] [Google Scholar]
- 68. Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19(17):7679–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qi H-X, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque monkeys with long-standing amputations. J Neurophysiol. 2000;84(4):2133–2147. [DOI] [PubMed] [Google Scholar]
- 70. Ojemann JG, Silbergeld DL. Cortical stimulation mapping of phantom limb rolandic cortex. J Neurosurg. 1995;82(4):641–644. [DOI] [PubMed] [Google Scholar]
- 71. Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. Brain. 1991;114(1):615–627. [DOI] [PubMed] [Google Scholar]
- 72. Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–1019. [DOI] [PubMed] [Google Scholar]
- 73. Balasubramanian K, Vaidya M, Southerland J et al. . Changes in cortical network connectivity with long-term brain-machine interface exposure after chronic amputation. Nat Commun. 2017;8(1):1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giraux P, Sirigu A, Schneider F, Dubernard JM. Cortical reorganization in motor cortex after graft of both hands. Nat Neurosci. 2001;4(7):691–692. [DOI] [PubMed] [Google Scholar]
- 75. Zheng M-X, Hua X-Y, Feng J-T et al. . Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378(1):22–34. [DOI] [PubMed] [Google Scholar]
- 76. Levy RM, Harvey RL, Kissela BM et al. . Epidural electrical stimulation for stroke rehabilitation. Neurorehabil Neural Repair. 2016;30(2):107–119. [DOI] [PubMed] [Google Scholar]
- 77. Khodaparast N, Hays SA, Sloan AM et al. . Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60(12):80–88. [DOI] [PubMed] [Google Scholar]
- 78. Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123 Pt 3(3):572–584. [DOI] [PubMed] [Google Scholar]
- 79. Gharabaghi A, Kraus D, Leão MT et al. . Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci. 2014;8(March):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25(8):789–793. [DOI] [PubMed] [Google Scholar]
- 81. Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25(8):780–788. [DOI] [PubMed] [Google Scholar]
- 82. Mestais CS, Charvet G, Sauter-starace F, Foerster M, Ratel D. WIMAGINE: wireless 64-channel ECOG recording implant for long term clinical applications. IEEE Trans Neural Syst Rehabil Eng. 2015;23(1):10–21. [DOI] [PubMed] [Google Scholar]
- 83. Cooper SJ. Donald O. Hebb's synapse and learning rule: a history and commentary. Neurosci Biobehav Rev. 2005;28(8):851–874. [DOI] [PubMed] [Google Scholar]
- 84. Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 85. Rebesco JM, Miller LE. Enhanced detection threshold for in vivo cortical stimulation produced by Hebbian conditioning. J Neural Eng. 2011;8(1):16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gharabaghi A, Naros G, Walter A et al. . From assistance towards restoration with epidural brain-computer interfacing. Restor Neurol Neurosci. 2014;32(4):517–525. [DOI] [PubMed] [Google Scholar]
- 87. Ethier C, Gallego JA, Miller L. Brain-controlled neuromuscular stimulation to drive neural plasticity and functional recovery. Curr Opin Neurobiol. 2015;33(8):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Capogrosso M, Milekovic T, Borton D et al. . A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539(7628):284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lobel DA, Lee KH. Brain machine interface and limb reanimation technologies: Restoring function after spinal cord injury through development of a bypass system. Mayo Clin Proc. 2014;89(5):708–714. [DOI] [PubMed] [Google Scholar]
- 90. Soekadar SR, Birbaumer N, Slutzky MW, Cohen LG. Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol Dis. 2015;83(11):172–179. [DOI] [PubMed] [Google Scholar]
- 91. Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485(7398):368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Memberg WD, Polasek KH, Hart RL et al. . Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch Phys Med Rehabil. 2014;95(6):1201–1211.e1e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sherwood AM, Dimitrijevic MR, Barry McKay W. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci. 1992;110(1-2):90–98. [DOI] [PubMed] [Google Scholar]
- 94. Krucoff MO, Zhuang K, Macleod DB et al. . A novel paraplegia model in awake behaving macaques. J Neurophysiol. 2017;118(3):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bernstein DR, Stelzner DJ. Plasticity of the corticospinal tract following midthoracic spinal injury in the postnatal rat. J Comp Neurol. 1983;221(4):382–400. [DOI] [PubMed] [Google Scholar]
- 96. Bulinski JC, Ohm T, Roder H, Spruston N, Turner DA, Wheal HV. Changes in Dendritic structure and function following Hippocampal Lesions: correlations with developmental events? Prog Neurobiol. 1998;55(6):641–650. [DOI] [PubMed] [Google Scholar]
- 97. Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. [DOI] [PubMed] [Google Scholar]
- 98. Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2004;58(1):60–69. [DOI] [PubMed] [Google Scholar]
- 99. Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol. 2010;20(4):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tedeschi A. Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci. 2011;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de Lima S, Habboub G, Benowitz LI. Combinatorial therapy stimulates long-distance regeneration, target reinnervation, and partial recovery of vision after optic nerve injury in mice. Int Rev Neurobiol. 2012;106:153–172. [DOI] [PubMed] [Google Scholar]
- 102. Park KK, Liu K, Hu Y et al. . Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR Pathway. Science. 2008;322(5903):963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith PD, Sun F, Park KK et al. . SOCS3 Deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64(5):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen D, Schneider G, Martinou J, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385(6615):434–439. [DOI] [PubMed] [Google Scholar]
- 105. Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default. Neuron. 2002;33(5):689–702. [DOI] [PubMed] [Google Scholar]
- 106. Zai L, Ferrari C, Dice C et al. . Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci. 2011;31(16):5977–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555(3):78–88. [DOI] [PubMed] [Google Scholar]
- 108. Anderson MA, Burda JE, Ren Y et al. . Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alilain W, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475(7355):196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34(1):131–152. [DOI] [PubMed] [Google Scholar]
- 111. Chew DJ, Fawcett JW, Andrews MR. The challenges of long-distance axon regeneration in the injured CNS. Prog Brain Res. 2012;201:253–294. [DOI] [PubMed] [Google Scholar]
- 112. Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378(6556):498–501. [DOI] [PubMed] [Google Scholar]
- 113. Liebscher T, Schnell L, Schnell D et al. . Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58(5):706–719. [DOI] [PubMed] [Google Scholar]
- 114. Baldwin KT, Carbajal KS, Segal BM, Giger RJ. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc Natl Acad Sci USA. 2015;112(8):2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24(6):577–583. [DOI] [PubMed] [Google Scholar]
- 116. Kurimoto T, Yin Y, Habboub G et al. . Neutrophils express oncomodulin and promote optic nerve regeneration. J Neurosci. 2013;33(37):14816–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yin Y, Cui Q, Li Y et al. . Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23(6):2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137(3):707–723. [DOI] [PubMed] [Google Scholar]
- 119. Yin Y, Henzl MT, Lorber B et al. . Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9(6):843–852. [DOI] [PubMed] [Google Scholar]
- 120. Yin Y, Cui Q, Gilbert H-Y et al. . Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci. 2009;106(46):19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Benowitz L, Yin Y. Rewiring the injured CNS: lessons from the optic nerve. Exp Neurol. 2008;209(2):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hu X, Leak RK, Shi Y et al. . Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67(2):181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shetty AK, Turner DA. Development of fetal hippocampal grafts in intact and lesioned hippocampus. Prog Neurobiol. 1996;50(5-6):597–653. [DOI] [PubMed] [Google Scholar]
- 126. George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci. 2012;15(8):1078–1087. [DOI] [PubMed] [Google Scholar]
- 128. Dunnett SB, Björklund A. Mechanisms and use of neural transplants for brain repair. Prog Brain Res. 2017;230:1–51. [DOI] [PubMed] [Google Scholar]
- 129. Dihné M, Hartung HP, Seitz RJ. Restoring neuronal function after stroke by cell replacement: anatomic and functional considerations. Stroke. 2011;42(8):2342–2350. [DOI] [PubMed] [Google Scholar]
- 130. Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158(11):94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Isacson O, Deacon T, Pakzaban P, Galpern W, Dinsmore J, Burns L. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat Med. 1995;1(11):1189–1194. [DOI] [PubMed] [Google Scholar]
- 132. Dinsmore J, Martin J, Siegan J et al. . CNS Grafts for Treatment of Neurologic Disorders. In: Methods of Tissue Engineering. 1st ed San Diego: Academic Press; 2002. [Google Scholar]
- 133. Stoker TB, Barker RA. Cell therapies for Parkinson's disease: how far have we come? Regen Med. 2016;11(8):777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kalladka D, Sinden J, Pollock K et al. . Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. [DOI] [PubMed] [Google Scholar]
- 135. Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149(2):310–321. [DOI] [PubMed] [Google Scholar]
- 136. Kondziolka D, Wechsler L, Goldstein S et al. . Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565–569. [DOI] [PubMed] [Google Scholar]
- 137. Kondziolka D, Steinberg GK, Wechsler L et al. . Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103(1):38–45. [DOI] [PubMed] [Google Scholar]
- 138. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 139. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(05):637–647. [DOI] [PubMed] [Google Scholar]
- 140. Bei F, Lee HHC, Liu X et al. . Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164(1-2):219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study. J Neuroengineering Rehabil. 2006;3(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Breakspear M, Stam CJ. Dynamics of a neural system with a multiscale architecture. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1051–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Womelsdorf T, Schoffelen J-M, Oostenveld R et al. . Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316(5831):1609–1612. [DOI] [PubMed] [Google Scholar]
- 144. Brogaard B, Gatzia DE. What can neuroscience tell us about the hard problem of consciousness? Front Neurosci. 2016;10(September):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hochberg LR, Bacher D, Jarosiewicz B et al. . Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]