Abstract
The clinical significance of a single Mycobacterium kansasii (MK) isolation in multiple sputum samples remains unknown. We conducted this study to evaluate the outcome and predictors of developing MK-pulmonary disease (PD) within 1 year among these patients. Patients with a single MK isolation from ≥3 sputum samples collected within 3 months and ≥2 follow-up sputum samples and chest radiography in the subsequent 9 months between 2008 and 2016 were included. The primary outcome was development of MK-PD within 1 year, with its predictors explored using multivariate logistic regression analysis. A total of 83 cases of a single MK isolation were identified. The mean age was 68.9 ± 17.9, with a male/female ratio of 1.96. Within 1 year, 16 (19%) cases progressed to MK-PD; risk factors included high acid-fast smear (AFS) grade (≥3), elementary occupation workers, and initial radiographic score >6, whereas coexistence with other nontuberculous mycobacterium species was protective. Among patients who developed MK-PD, all experienced radiographic progression, and 44% died within 1 year. Although a single MK isolation does not fulfil the diagnostic criteria of MK-PD, this disease may develop if having above-mentioned risk factors. Early anti-MK treatment should be considered for high-risk patients.
Introduction
The burden of nontuberculous mycobacteria (NTM) pulmonary disease (PD) has been increasing worldwide in recent decades1–3. Mycobacterium kansasii (MK) is one of the most virulent species among all NTM species4, with similar clinical manifestation and radiographic characteristics of pulmonary tuberculosis (TB)5,6. Current reports reveal that both the number of respiratory isolates of MK and the incidence of MK-PD have increased worldwide5–8, including Taiwan9. The predisposing factors of MK-PD include structural lung disease and immunocompromised status of the host7,10,11. Water pollution exposure and occupational history have also been reported to be associated with the development of MK-PD5,12.
The presence of NTM in respiratory specimens does not indicate true infection, and the diagnosis of NTM-PD depends on composite microbiological and clinical criteria1,13; at least two positive sputum cultures of the same NTM species is required to fulfil the microbiological criteria from current guideline1. Therefore, a single NTM isolation from multiple respiratory specimens is usually considered colonization or contamination rather than indicative of a true pathogen. In a study conducted in South Korea, 26 (14%) of 190 patients with a single sputum isolation of pathogenic NTM met the diagnostic criteria for NTM-PD within a median follow-up period of 16 months, and none of the 8 patients with a single MK isolation had a subsequent positive culture14. Under this clinical entity, follow-up sputum sampling and clinical monitoring are necessary to diagnose NTM-PD; yet, the clinical outcome and optimal duration of follow-up remain uncertain14,15. Furthermore, patients may have different NTM species in the respiratory tract presenting as transitional, alternating, or simultaneous pattern in chronological order1,16,17. Little is known of the clinical significance of coexistence of different NTM species.
In Taiwan, MK is the third most common NTM causing PD, and the numbers of MK isolates increased 4.7-fold from 2010 to 20149. Being familiar with the outcome of different clinical entities of MK is crucial in clinical practice. We therefore conducted this retrospective, longitudinal cohort study to investigate the predictors of developing MK-PD within 1 year among patients with single MK isolation from multiple sputum samples, with a special emphasis on coexistence of NTM species other than MK.
Results
Study population
Figure 1 shows the flowchart of patient selection and the enrolment criteria. Between 2008 and 2016, a total of 1,852 respiratory MK isolates in 1,183 patients were identified from six hospitals. By applying the selection criteria, a total of 83 (7.0%) cases of single MK isolation were finally selected for further analysis.
Figure 1.
Flowchart of selection of new cases of Mycobacterium kansasii (MK) pulmonary disease (PD) in six hospitals.
Among the 83 patients, the mean age of the patients was 68.9 ± 17.9 years, with a male/female ratio of 1.96. Among the 83 patients, 52% were ex-smokers and 54% had received education for <6 years. 20 (24%) cases were employed in elementary occupations. Of them, 18 were in industrialized areas, including 14 construction laborers and 4 iron-steel manufacturing laborers. Of the remaining two patients, one was a truck driver and the other was a cleaner. The most common pulmonary comorbidity was chronic obstructive pulmonary disease (COPD: 35%), and the most common systemic comorbidity was chronic kidney disease stages 3–5 (23%). Higher (but nonsignificantly) prevalence rates of COPD and pneumoconiosis (respectively, 50% vs. 28%, p = 0.086; 11% vs. 0%, p = 0.060) were noted in patients with elementary occupations from industrialized areas than in others. Fifteen (18%) patients had coexistence of NTM species other than MK, including Mycobacterium avium complex (MAC) in nine, M. fortuitum in two, one each for M. abscessus and M. scrofulaceum, and unidentified NTM species in two.
There was no significant difference in clinical characteristics between the 15 patients with coexistence of other NTM species and the other 68 without such coexistence (Table 1), except that the coexistence group had a significantly higher prevalence of congestive heart failure (33% vs. 7%, p = 0.018). The initial symptoms, laboratory data, lung function, radiographic findings, and sputum mycobacteriologic results were similar between the two groups.
Table 1.
Clinical characteristics of patients with a single Mycobacterium kansasii isolation, stratified by coexistence of other nontuberculous mycobacteria species.
| Coexistence of other NTM (N = 15) | No coexistence of other NTM (N = 68) | p-value | |
|---|---|---|---|
| Age (year) | 69.5 ± 17.2 | 68.7 ± 18.2 | 0.877 |
| Male sex | 12 (80%) | 43 (63%) | 0.214 |
| Body-mass index (kg/m2) | 20.2 ± 4.2 | 19.8 ± 4.2 | 0.586 |
| <18.5 | 5 (33%) | 30 (44%) | 0.476 |
| Smoking status | |||
| Never smoker | 6 (40%) | 25 (37%) | 0.815 |
| Ex-smoker | 8 (53%) | 35 (52%) | 0.896 |
| Current smoker | 1 (7%) | 8 (12%) | 0.908 |
| Alcoholism | 5 (33%) | 26 (38%) | 0.722 |
| Education period (n = 13, 65) | |||
| <6 years | 7 (54%) | 35 (53%) | >0.999 |
| 6 ~ 9 years | 2 (15%) | 7 (11%) | 0.582 |
| 9 ~ 12 years | 1 (8%) | 14 (22%) | 0.271 |
| ≥12 years | 3 (23%) | 9 (14%) | 0.405 |
| Occupation historya | |||
| Elementary occupations | 1 (7%) | 19 (28%) | 0.103 |
| Professionals | 1 (7%) | 1 (2%) | 0.331 |
| Technicians and associate professionals | 0 | 1 (2%) | >0.999 |
| Service and sales workers | 3 (20%) | 12 (17%) | 0.830 |
| Craft and related trades workers | 0 | 3 (4%) | >0.999 |
| Plant/machine operators and assemblers | 1 (7%) | 2 (3%) | 0.455 |
| Retired or unemployed | 9 (60%) | 29 (43%) | 0.222 |
| Pulmonary comorbidity | |||
| Chronic obstructive pulmonary disease | 4 (27%) | 24 (35%) | 0.735 |
| Bronchiectasis | 4 (27%) | 17 (25%) | 0.893 |
| History of pulmonary tuberculosis | 5 (33%) | 18 (27%) | 0.591 |
| Lung cancer | 2 (13%) | 7 (10%) | 0.663 |
| Other pulmonary diseases | 1 (7%)b | 9 (13%)c | 0.665 |
| Systemic comorbidity | |||
| Chronic kidney disease, stage 3–5 | 3 (20%) | 16 (24%) | 0.768 |
| Congestive heart failure | 5 (33%) | 5 (7%) | 0.018 |
| Diabetes mellitus | 7 (47%) | 20 (29%) | 0.197 |
| Extra-pulmonary cancer | 1 (7%)d | 13 (19%)e | 0.468 |
| Steroid userf | 0 | 8 (12%) | 0.361 |
| HIV infection | 0 | 1 (2%) | >0.999 |
| Other systemic diseases | 0 | 6 (9%)g | 0.563 |
| Initial symptoms | |||
| Sputum | 10 (67%) | 58 (85%) | 0.090 |
| Cough | 11 (73%) | 42 (62%) | 0.399 |
| Hemoptysis | 1 (7%) | 15 (22%) | 0.281 |
| Dyspnea | 5 (33%) | 21 (31%) | 0.853 |
| Initial laboratory data | |||
| Leukocyte > 9000/uL | 2 (13%) | 24 (36%) | 0.126 |
| Segment > 70% (n = 13, 50) | 3 (23%) | 25 (50%) | 0.119 |
| Hemoglobin < 12 g/dL | 9 (60%) | 30 (46%) | 0.309 |
| Platelet count < 140 K/uL | 2 (13%) | 11 (17%) | 0.751 |
| C-reactive protein > 10 mg/L (n = 5, 40) | 3 (60%) | 27 (68%) | 0.737 |
| Aspartate transaminase > 40 U/L (n = 13,66) | 1 (7%) | 12 (19%) | 0.444 |
| Alanine transaminase > 40 U/L (n = 14, 66) | 2 (13%) | 12 (19%) | 0.638 |
| Creatinine > 1.4 mg/dL (n = 15, 67) | 2 (13%) | 13 (19%) | 0.726 |
| Albumin < 3.5 g/dL (n = 3, 20) | 2 (67%) | 14 (70%) | >0.999 |
| Lung function (n = 8, 32) | |||
| FEV1 (% of predicted) | 74.2 ± 37.0 | 71.7 ± 36.9 | 0.584 |
| FEV1/FVC | 72.9 ± 10.0 | 72.2 ± 10.8 | 0.871 |
| Obstructive type | 3 (38%) | 14 (44%) | 0.959 |
| Restrictive type | 1 (13%) | 8 (25%) | 0.571 |
| Radiographic finding | |||
| Predominant pattern | |||
| Fibrocavitory | 1 (7%) | 11 (16%) | 0.360 |
| Nodular bronchiectasis | 0 | 9 (13%) | 0.279 |
| Multifocal involvement | 12 (80%) | 56 (81%) | >0.999 |
| Initial radiographic score | 5.5 ± 2.2 | 5.7 ± 3.3 | 0.818 |
| Follow-up radiographic score | 6.1 ± 3.6 | 6.8 ± 4.3 | 0.552 |
| Initial sputum study | |||
| Number of sputum samples | 4.1 ± 1.3 | 3.8 ± 1.4 | 0.552 |
| Acid-fast smear | |||
| Negative | 9 (60%) | 53 (78%) | 0.148 |
| Low-grade positive (Gr. 1, 2) | 6 (40%) | 12 (18%) | 0.065 |
| High-grade positive (Gr. 3, 4) | 0 (0%) | 3 (4%) | >0.999 |
Abbreviations: FEV1: forced expiratory volume in first second; FVC: forced vital capacity; NTM: nontuberculous mycobacteria.
Data are number (percentage) or mean ± standard deviation.
aThe occupational category was noted according to the International Standard Classification of Occupations, 2008 (ISCO-08)28.
bOne had asthma.
cThree had interstitial lung disease, three had asthma, two had pneumoconiosis, and the remaining one had both interstitial lung disease and asthma.
dOne had oesophageal cancer.
eFive had leukaemia and eight had solid organ tumours, including thymic cancer in two; prostate cancer in two; and one each for gastric cancer, papilla of Vater cancer, colon cancer, and basal cell carcinoma.
fSteroid user was defined as receiving at least 1 week with a dose of ≥30 mg/day of oral prednisone (or equivalent).
gFour had autoimmune disease, and two had liver cirrhosis.
Outcome and risk factors of MK-PD
Within 1 year, MK-PD developed in 16 (19%) patients, including 6 (38%) in the second quarter and 8 (50%) in the third quarter (Fig. 2). All of these patients experienced radiographic progression during follow-up. The radiographic findings during MK-PD development were FC pattern in 10 (63%) patients and NB pattern in 6 (38%), and all showed multifocal involvement.
Figure 2.
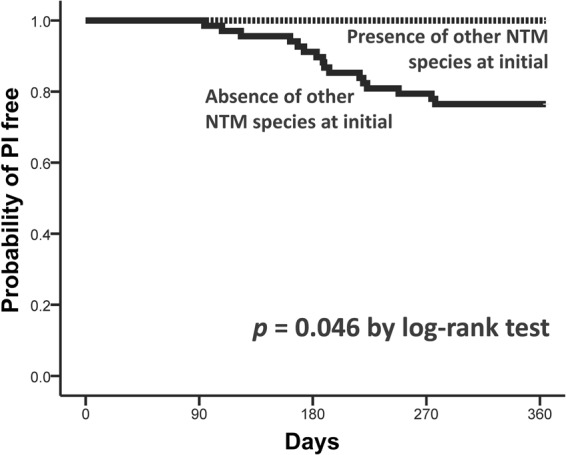
Kaplan–Meier curves for time to development of Mycobacterium kansasii (MK) pulmonary disease (PD), stratified by coexistence of nontuberculous mycobacteria (NTM) species other than MK.
Univariate logistical regression (Table 2, left panel) revealed that MK-PD was more likely to develop in patients with BMI < 18.5 kg/m2 (31% vs. 11%, p = 0.033), elementary occupations (60% vs. 6%, p < 0.001), COPD (56% vs. 12%, p = 0.010), steroid use (50% vs. 16%, p = 0.032), haemoptysis (38% vs. 15%, p = 0.047), leucocytosis (white blood cells >9000/μL; 42% vs. 9%, p = 0.001), haemoglobin <12 g/dL (28% vs. 12%, p = 0.034), high-grade (grade 3 or 4) positivity of sputum AFS (100% vs. 16%, p = 0.007), and initial radiographic score >6 (33% vs. 9%, p = 0.008). By contrast, coexistence with NTM species other than MK was associated with a lower risk of developing MK-PD (0% vs. 25%, p = 0.037).
Table 2.
Univariate and multivariate logistic regression analysis for predictors of progression to Mycobacterium kansasii pulmonary disease.
| Variables | Univariate (n = 83) | Multivariate (n = 65)a | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age > 65 | 2.27 (0.59–8.76) | 0.236 | ||
| Male sex | 0.72 (0.26–2.53) | 0.723 | ||
| Body-mass index < 18.5 | 3.58 (1.11–11.55) | 0.033 | 1.65 (0.05–7.54) | 0.696 |
| Elementary occupationsb | 22.12 (5.73–85.45) | <0.001 | 10.77 (1.65–70.51) | 0.013 |
| Chronic obstructive pulmonary disease | 4.54 (1.44–14.29) | 0.010 | 2.48 (0.24–25.74) | 0.447 |
| History of pulmonary tuberculosis | 0.50 (0.13–1.95) | 0.314 | ||
| Bronchiectasis | 0.63 (0.16–2.46) | 0.505 | ||
| Diabetes mellitus | 0.24 (0.05–1.15) | 0.073 | 0.27 (0.03–2.91) | 0.280 |
| Congestive heart failure | 1.05 (0.20–5.52) | 0.951 | ||
| Steroid use | 5.25 (1.15–23.94) | 0.032 | 3.58 (0.20–63.18) | 0.383 |
| Hemoptysis | 3.42 (1.02–11.53) | 0.047 | 1.67 (0.13–22.26) | 0.698 |
| Cough | 2.93 (0.76–11.25) | 0.118 | ||
| Sputum | 1.69 (0.34–8.35) | 0.523 | ||
| Dyspnea | 1.97 (0.64–6.03) | 0.238 | ||
| Leukocyte > 9000/uL | 6.36 (2.02–20.05) | 0.001 | 1.94 (0.27–13.87) | 0.509 |
| Hemoglobin < 12 g/dL | 3.34 (1.06–10.57) | 0.034 | 3.55 (0.48–26.09) | 0.213 |
| Initial radiographic scores > 6 | 5.38 (1.56–18.52) | 0.008 | 10.25 (1.24–84.59) | 0.031 |
| Anti-MK treatment: intent to treatc | 2.69 (0.76–9.44) | 0.114 | ||
| Anti-MK treatment: per-protocold | 4.33 (0.56–33.29) | 0.129 | ||
| High-grade (Gr. 3 or 4) positive for AFSa | 32.59 (1.60–665.77) | 0.007 | ||
| Co-existence of NTM species other than MKa | 0.78 (0.68–0.88) | 0.037 | ||
Abbreviation: AFS, acid-fast smear; NTM, nontuberculous mycobacteria.
aAmong the 15 episodes with coexistence of other NTM species, none progressed to MK-PD during 1-year follow-up, whereas 16 (24%) of the 68 without concomitant NTM species did (p = 0.037). Of the 68 episodes, 3 had high-grade positivity (grades 3 and 4) in sputum AFS, and all developed MK-PD, whereas 13 (20%) of the 65 episodes without high AFS grade did (p = 0.001). Thus, we conclude that coexistence with other NTM was a significant protector against MK-PD and that high AFS grade was a significant predictor for MK-PD. We excluded 18 episodes from the data in fitting the multivariate logistic model.
bThe category of occupation was noted according to the International Standard Classification of Occupations, 2008 (ISCO-08)28.
cPatients who had ever received any drugs against MK, regardless of duration.
dPatients who had ever received combination chemotherapy against MK for more than 2 months in the first 3 months.
Multivariate logistic regression analysis was performed to investigate the independent predictors for developing MK-PD. We found a statistical separation phenomenon existed in two variables, coexistence with other NTM species and high-grade positivity in sputum AFS. Among the 15 episodes with coexistence of other NTM species, none progressed to MK-PD within the 1-year follow-up, whereas 16 (24%) of the 68 without concomitant other NTM species did (p = 0.037). Of the remaining 68 episodes, 3 had high-grade positivity (grades 3 and 4) in sputum AFS, with all developing into MK-PD; by contrast, 13 (20%) of the other 65 episodes without high AFS grade developed into MK-PD (p = 0.001). Therefore, we excluded above 18 episodes from the data in fitting the multivariate logistic model. Subsequent analysis of the remaining 65 episodes revealed that independent predictors of developing MK-PD within 1 year were an elementary occupation (odds ratio [95% confidence interval] = 10.77 [1.65–70.51], p = 0.013) and initial radiographic score >6 (10.25 [1.24–84.59], p = 0.031) (Table 2, right panel).
One-year outcome
Treatment courses and 1-year mortality are summarized in Table 3. Treatment for MK was more frequently, though not significantly, prescribed in the 16 cases developing into MK-PD according to either intention-to-treat analysis (29% vs. 13%, p = 0.087) or per-protocol analysis (13% vs. 3%, p = 0.166). Of the 16 patients who developed MK-PD within 1 year, 7 (44%) died, 4 of whom died of MK-PD. By contrast, 6 (9%) of the 67 without development of MK-PD died (p = 0.002). Neither of the two patients with MK-PD who received standard anti-MK treatment died, whereas two patients who received transient anti-MK treatment died.
Table 3.
Treatment and 1-year outcome of patients, stratified by progression to Mycobacterium kansasii pulmonary disease (MK-PD).
| Progressed to MK-PD (N = 16) | Not progress to MK-PD (N = 67) | p-value | |
|---|---|---|---|
| Treatment against MK | |||
| Intent to treat analysisa | 5 (29%) | 9 (13%) | 0.087 |
| Per-protocol analysisb | 2 (13%) | 2 (3%) | 0.166 |
| Mortality in one year | 7 (44%) | 6 (9%) | 0.002 |
| Time to mortality | 245 (192–299) | 286 (190–302) | 0.681c |
| Cause of Death | |||
| Sepsis with bacterial pathogen | 3 (19%) | 4 (6%) | |
| Mycobacterium kansasii | 4 (25%) | 0 (0%) | |
| Others | 0 | 2 (3%)d | |
Data are number (percentage) or median ± interquartile range.
p value was calculated using the chi-squared test unless otherwise mentioned. aPatients who had ever received any drugs against MK, regardless of duration.
bPatients who had ever received combination chemotherapy against MK for more than 2 months in the first 3 months.
cp value was calculated by log-rank test.
dThe cause of death was lung cancer in one and acute myocardial infarction in the other.
Discussion
To our knowledge, this is the first longitudinal, multicentre study investigating the incidence and predictors of developing MK-PD within 1 year among patients with single MK isolation from multiple sputum samples. There were two major findings in this study. First, MK-PD developed in 19% of cases, with 88% of the MK-PD occurring in the second and third quarters after the index date. All patients who progressed to MK-PD had typical radiographic patterns with multifocal involvement, and radiographic progression occurred thereafter. The 1-year mortality rate was 44% in MK-PD patients with MK-PD, which was 4.9 times higher than in those without MK-PD. Second, high-grade sputum AFS at initial presentation, elementary occupations, and higher initial radiographic scores (>6) were independent risk factors for developing MK-PD within 1 year, whereas coexistence with other NTM species was protective.
Though a single respiratory isolation of pathogenic NTM, such as MAC, M. abscessus, and MK, is insufficient to establish the diagnosis of NTM-PD1, the results of previous studies suggest that it is clinically significant in appropriate clinical settings, especially in cavitary lung disease5,17–19. Some experts also suggest a lower diagnostic threshold for patients with positive respiratory cultures of MK, particularly in people living with human immunodeficiency virus18,20,21. Another study obtained the opposite result, showing that 14% of patients with a single sputum isolation of NTM eventually developed NTM-PD within a median follow-up period of 16 months14. Another retrospective observational study had a similar finding, showing that none of 18 patients with a single MK isolation developed MK-PD within a median of 12 months22. However, it’s difficult to draw a definite conclusion due to the limitation of power (small sample size). Therefore, an appropriate follow-up period is proposed to be essential to determine the clinical relevance of a single MK respiratory isolation14,15. The present study demonstrated that 19% patients with a single respiratory isolation of MK would progress to MK-PD, and they should be followed for at least 9 months to determine its clinical relevance. Given the high probability of adverse drug reactions, regular monitoring is recommended, rather than immediate anti-MK treatment.
Among patients with a single MK respiratory isolation who developed MK-PD within 1 year, it is striking that all experienced radiographic progression and 44% of them died. The mortality rate in this study is much higher than that in other reports, ranging from 9% to 15.8%3,23, probably because the present study was conducted in medical centres where patients tended to have high disease severity. In addition, the diagnosis of MK-PD in the present study strictly followed the American Thoracic Society/Infectious Diseases Society of America guidelines1, whereas previous reports exclusively employed the microbiological component of the current guidelines, which may underestimate the severity of disease9. Given the poor outcome, predicting subsequent progression to MK-PD in patients with single MK isolation in sputum is crucial for early and accurate initiation of anti-MK treatment.
Different NTM species can coexist in 8% to 25% of patients with NTM infection16,19. This highlights the dynamic nature of NTM and puzzles clinicians. However, the clinical significance of this phenomenon is unclear. In patients receiving treatment for MAC-PD, single respiratory isolation of M. abscessus probably requires no therapy17. However, the change from MAC to M. abscessus is usually accompanied by symptomatic and radiographic worsening24. Unlike other studies, the present analysis revealed that coexistence of MK and another NTM may be associated with a lower risk of MK-PD and initial high-grade sputum AFS is a risk factor for MK-PD, supporting the hypothesis that quantitative organism load might determine the clinical relevance of NTM-PD8,11,25. Coexistence with other NTM species may therefore reflect that MK is not the dominant microorganism.
The epidemiology of MK is predominantly urban and has been associated with high-density and low-income communities5,7. The major reservoir has been postulated to include water systems associated with habitation or industry, and infection probably occurs via an aerosol route1,11,20. These factors may explain why the incidence of MK-PD was higher among the patients employed in elementary occupations in the present study; such workers extensively use aerosolized water for dust control5,20,26.
There are several limitations of the present study. First, the lack of subtyping for MK precluded us from distinguishing its pathogenic (such as MK subtype 1) and nonpathogenic subtypes27. Second, we excluded patients providing less than three sputum samples even if single MK isolation was noted, which may have resulted in underestimation of the incidence of subsequent MK-PD. Third, no standardized microbiological or radiographic follow-up protocols were used in this retrospective study. Fourth, the data were retrieved from medical centres in Taiwan and may not be generalizable to all populations.
In conclusion, approximately one-fifth of the patients with single MK isolation from multiple sputum samples progress to MK-PD within 1 year; the majority of progressions occur in the second and third quarters thereafter. However, once MK-PD develops, radiographic progression is inevitable, with 1-year mortality of >40%. Early treatment for MK should be considered for vulnerable populations. Having an elementary occupation, high-grade sputum AFS positivity, and high initial radiographic score (>6 points) are risk factors for MK-PD, whereas coexistence with NTM other than MK is protective.
Methods
Study population
This retrospective study was conducted in two medical centres, the National Taiwan University Hospital (NTUH) and Kaohsiung Medical University Hospital (KMUH), and their four branch hospitals. This multicentre study was approved by the medical centres’ institutional review boards (NTUH REC 201508017RIND and KMUH IRB-SV[I]-2015200266) and the need for informed consent was waived because data utilized in this retrospective study have been de-identified.
From January 2008 to December 2016, respiratory specimens were retrieved from the mycobacteriology databases. Mycobacteriologic examinations were performed as described previously9. Only patients with new episodes of MK since 2008 were selected9. Patients who provided ≥3 sputum samples within 3 months with only one MK isolation, and who had ≥2 follow-up sputum samples and chest radiography in the subsequent 9 months were selected into the study. Only the first episode of single MK isolation for each patient was selected for analysis. We excluded patients lacking demographic data and having TB concomitantly. The index date was defined as the date when the index culture of MK isolation was plated. The patients were followed up until diagnosis of MK-PD, death, or 1 year after the index date.
Data collection
Patient characteristics, including age, sex, body-mass index (BMI), smoking status, education level, occupation, comorbidities (pulmonary and systemic), symptoms, laboratory data, serial radiographic and microbiological reports, treatment course, and outcome were recorded. The categorical classification of occupation was based on the International Standard Classification of Occupation, 200828, in which elementary occupations included (1) sales and services elementary occupations; (2) agricultural, fishery and related labourers; and (3) labourers in mining, construction, manufacturing and transport.
Chest radiographs and computed tomographic scans were interpreted independently by two pulmonologists. We categorized the patterns as fibrocavitary (FC) and nodular bronchiectatic (NB) and the extent as focal and multifocal. Radiographic scores for severity assessment were recorded as previously described29.
The MK treatment administered was analysed in two ways: (1) intention-to-treat analysis for patients who had ever received any drugs against MK, regardless of duration; and (2) per-protocol analysis for patients who had received combination chemotherapy against MK for >2 months in the first 3 months after the index date.
Outcome assessment
The primary outcome was development of MK-PD within 1 year of the index date. The diagnosis of MK-PD was according to current guidelines1. The secondary outcome was radiographic progression and mortality within 1 year for those developing MK-PD. MK was considered the cause of death if no pathogens other than MK were identified and radiographic progression of MK-PD was noted.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with interquartile range and were compared using independent samples t tests. Categorical variables are expressed as percentages and were compared using the chi-squared test or Fisher’s exact test, as appropriate. The independent factors associated with development of MK-PD were determined using multivariate logistic regression analysis. Time-to-event curves for development of MK-PD were generated and compared using the log-rank test. Statistical significance was set at p < 0.05 (two-sided). All statistical analyses were performed using IBM SPSS version 22.0 (IBM, Armonk, NY, USA).
Data Sharing Statement
All data were deposited in the Information Technology Office of National Taiwan University Hospital and Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital. The data were not available for sharing without permission.
Acknowledgements
The authors thank the Information Technology Office of National Taiwan University Hospital and the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital, for providing patient data. This study was supported by the Taiwan Ministry of Science and Technology (NSC101-3114-Y-002-003, MOST104-2321-B-002-058, MOST 107-2314-B-037 -106 -MY3) and Kaohsiung Medical University Hospital Research Program (KMUH105-5M09). The funders had no role in the study design, data analysis, or manuscript writing.
Author Contributions
H.L.H., J.Y.W. and I.W.C. designed the study. M.H.C., C.J.L. and P.L.L. performed the database analysis. H.L.H. and C.J.L. contributed to the statistical analysis. H.L.H., J.Y.W. and I.W.C. contributed to data interpretation and prepared the first draft of the manuscript. M.H.C. and P.L.L. critically revised the draft manuscript. J.Y.W. and I.W.C. were responsible for the coordination. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing Interests
Some data of this study was presented in the annual meeting of the 49th Union World Conference on Lung Health as a poster presentation at October 27th, 2018. The poster number is PS38-817-27. All authors report no relationships that could be construed as a conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Hoefsloot W, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 3.Marras TK, et al. Pulmonary Nontuberculous Mycobacteria–Associated Deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis. 2017;23:468. doi: 10.3201/eid2303.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philley JV, et al. Treatment of Non-Tuberculous Mycobacterial Lung Disease. Curr Treat Options Infect Dis. 2016;8:275–296. doi: 10.1007/s40506-016-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett E, et al. Mycobacterium kansasii and M. scrofulaceum isolates from HIV-negative South African gold miners: incidence, clinical significance and radiology. Int J Tuberc Lung Dis. 1999;3:501–507. [PubMed] [Google Scholar]
- 6.Park HK, Koh W-J, Shim TS, Kwon OJ. Clinical characteristics and treatment outcomes of Mycobacterium kansasii lung disease in Korea. Yonsei Medical J. 2010;51:552–556. doi: 10.3349/ymj.2010.51.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch KC, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Internal Med. 1998;129:698–704. doi: 10.7326/0003-4819-129-9-199811010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H-L, et al. Epidemiology and Predictors of NTM Pulmonary Infection in Taiwan-a Retrospective, Five-Year Multicenter Study. Sci. Rep. 2017;7:16300. doi: 10.1038/s41598-017-16559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johanson WG, Jr, Nicholson DP. Pulmonary disease due to Mycobacterium kansasii: an analysis of some factors affecting prognosis. Am Rev Respir Dis. 1969;99:73–85. doi: 10.1164/arrd.1969.99.1.73. [DOI] [PubMed] [Google Scholar]
- 11.Stout JE, Koh W-J, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis. 2014;14:279. doi: 10.1186/1471-2334-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haworth, C.S. & Floto, R.A. Introducing the new BTS Guideline: Management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). (BMJ Publishing Group Ltd, 2017). [DOI] [PubMed]
- 14.Koh W-J, et al. Clinical significance of a single isolation of pathogenic nontuberculous mycobacteria from sputum specimens. Diagn Microbiol Infect Dis. 2013;75:225–226. doi: 10.1016/j.diagmicrobio.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Lee M-R, et al. Factors associated with subsequent nontuberculous mycobacterial lung disease in patients with a single sputum isolate on initial examination. Clin Microbiol Infect. 2015;21:250. e251–250. e257. doi: 10.1016/j.cmi.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Lim H-J, et al. Isolation of multiple nontuberculous mycobacteria species in the same patients. Int J Infet Dis. 2011;15:e795–e798. doi: 10.1016/j.ijid.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Griffith DE, et al. The significance of Mycobacterium abscessus subspecies abscessus isolation during Mycobacterium avium complex lung disease therapy. CHEST. 2015;147:1369–1375. doi: 10.1378/chest.14-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett EL, et al. The impact of HIV infection on Mycobacterium kansasii disease in South African gold miners. Am J Respir Crit Care Med. 1999;160:10–14. doi: 10.1164/ajrccm.160.1.9808052. [DOI] [PubMed] [Google Scholar]
- 19.Griffith DE. Management of disease due to Mycobacterium kansasii. Clin Chest Med. 2002;23:613–621. doi: 10.1016/S0272-5231(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, J. C., Chiang, L. & Elwood, K. Mycobacterium kansasii. Microbiol Spectr. 5 (2017). [DOI] [PMC free article] [PubMed]
- 21.Witzig RS, et al. Clinical manifestations and implications of coinfection with Mycobacterium kansasii and human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:77–85. doi: 10.1093/clinids/21.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Moon SM, et al. Clinical significance of Mycobacterium kansasii isolates from respiratory specimens. PLoS One. 2015;10:e0139621. doi: 10.1371/journal.pone.0139621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gommans E, et al. Risk factors for mortality in patients with pulmonary infections with non-tuberculous mycobacteria: a retrospective cohort study. Respir Med. 2015;109:137–145. doi: 10.1016/j.rmed.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, et al. Implication of species change of Nontuberculous Mycobacteria during or after treatment. BMC Pul Med. 2017;17:213. doi: 10.1186/s12890-017-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan S-W, et al. Microbiological persistence in patients with Mycobacterium avium complex lung disease: the predictors and the impact on radiographic progression. Clin Infect Dis. 2017;65:927–934. doi: 10.1093/cid/cix479. [DOI] [PubMed] [Google Scholar]
- 26.Etxebarrieta M, et al. Clinical importance of Mycobacterium kansasii and evaluation of the need to identify nontuberculous mycobacteria. Emferm Infecc Microbiol Clin. 2002;20:113–116. doi: 10.1016/S0213-005X(02)72760-2. [DOI] [PubMed] [Google Scholar]
- 27.Taillard C, et al. Clinical implications of Mycobacterium kansasii species heterogeneity: Swiss National Survey. J Clin Microbiol. 2003;41:1240–1244. doi: 10.1128/JCM.41.3.1240-1244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ILO, I. International Standard Classification of Occupations ISCO-08. (vol, 2012).
- 29.Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971;103:625–640. doi: 10.1164/arrd.1971.103.5.625. [DOI] [PubMed] [Google Scholar]



