Abstract
The incidence of alcohol use disorder (AUD) is higher among people living with HIV (PLWH). The advent and continued development of antiretroviral therapy (ART) has significantly reduced mortality, shifting the course of HIV infection to a chronic illness. However, this is associated with an increased incidence of comorbid conditions, including type 2 diabetes mellitus, insulin resistance, and cardiovascular complications. Using a nonhuman primate model of simian immunodeficiency virus (SIV) infection, previous studies have demonstrated that chronic binge alcohol (CBA) administration decreases whole body insulin responsiveness, irrespective of ART administration. The objective of the current study was to determine the effects of CBA and ART on insulin-sensitive peripheral tissues before the development of overt clinical symptoms of SIV disease. Our results show that CBA reduced omental adipocyte cell size, increased collagen expression, and decreased the in vitro differentiation potential of adipose-derived stem cells. In contrast, it did not alter skeletal muscle or omental or hepatic expression of insulin signaling proteins. However, ART significantly decreased skeletal muscle expression of phosphatase and tensin homolog, total mechanistic target of rapamycin, and ribosomal protein S6. In addition, ART increased hepatic phosphorylation of AMP-activated protein kinase α and increased gene expression of key enzymes required for gluconeogenesis and fatty acid synthesis. These findings suggest that CBA and ART differentially promote adverse metabolic effects in an organ-specific manner that may underlie insulin resistance associated with alcohol, SIV, and ART. Whether this is translated in PLWH with AUD remains to be determined.
Keywords: alcohol, antiretroviral therapy, insulin signaling, metabolism, SIV disease
INTRODUCTION
People living with HIV (PLWH) frequently engage in hazardous alcohol use (22, 34). The advent and continued development of new antiretroviral therapy (ART) has significantly reduced HIV/AIDS mortality and altered the course of HIV infection to a chronic illness with a near-normal life span for ART-compliant PLWH (40). However, this is associated with an increased incidence of comorbid conditions, including type 2 diabetes mellitus, insulin resistance (IR), metabolic syndrome, and cardiovascular complications. Moreover, the direct effects of ART (38) and lifestyle choices, such as alcohol, tobacco, and illicit drug use (23, 30), contribute significantly to exacerbate these comorbid conditions.
Our previous studies in simian immunodeficiency virus (SIV)-infected rhesus macaques demonstrated that chronic binge alcohol (CBA) administration significantly reduced disposition index (DI), acute insulin response to glucose (AIRg), plasma insulin, and c-peptide values in asymptomatic ART-treated macaques. In addition, CBA reduced serum adiponectin, an insulin-sensitizing adipokine, and glucose effectiveness (SG) (20), providing evidence for CBA-mediated dysregulation of glucose insulin dynamics in SIV infection.
Chronic alcohol (32, 53, 63, 66, 72), HIV (8, 48), and ART (25, 27) adversely affect insulin signaling in peripheral insulin-sensitive tissues. The complexity and continued development and changes in the use of an ART regimen have made the identification of direct ART effects on metabolic parameters in PLWH difficult, yet metabolic dysregulation in PLWH is partly attributed to ART effects (13, 28, 39). However, few studies have determined the effects of chronic alcohol and ART on insulin-sensitive peripheral tissues in SIV/HIV infection.
Chronic alcohol consumption decreases adipose tissue mass and adipocyte size in mice (72, 73) and decreases the expression of key adipogenic transcription factors, peroxisome proliferator-activated receptor (PPAR) and CCAAT/enhancer-binding protein alpha (CEBP-a) (14, 73). Chronic alcohol also decreases insulin-stimulated glucose uptake in adipose tissue, reviewed in Ref. 67. However, alcohol does not appear to affect phosphoinositide-3-kinase (PI3K)/AKT signaling or mRNA expression of insulin receptor, insulin receptor substrate-1, or PP1 (50). A recent comprehensive review highlights alcohol-mediated adipose tissue alterations that potentially contribute to metabolic dysregulation (67). Adipose tissue from lipodystrophic PLWH has an increased percentage of small adipocytes (4, 47), a decreased expression of major adipogenic transcription factors [PPAR, CEBPa, and sterol regulatory element-binding protein 1c (SREBP-1c)], an impaired expression of adipocyte insulin sensitivity markers [insulin receptor b-subunit and AKT/PKB (4, 24)], and increased fibrosis (4, 36). Moreover, the incidence of IR in PLWH correlates with a decreased expression and secretion of adiponectin by adipose tissue (29, 65). A newer generation ART regimen seems to have less adverse effects, and the incidence of IR because of drug toxicity among PLWH is potentially decreasing (31, 51, 55).
Our previous work demonstrated that CBA administration accentuates a dysfunctional skeletal muscle phenotype, which was associated with an accelerated time to end-stage disease in non-ART-treated SIV-infected macaques (42, 43). This was characterized by increased skeletal muscle proteasomal activity, attenuation of anabolic pathways, alterations in insulin signaling (35), a profibrotic milieu (17), dysregulation of gene networks that regulate muscle homeostasis (59), and altered mitochondrial function (19). Our recent work also provides evidence that CBA decreases myoblast differentiation (58) and dysregulates mitochondrial function (18) in ART-treated macaques. These findings suggest that CBA potentially affects skeletal muscle insulin responsiveness in SIV-infected animals.
Chronic alcohol exposure decreases hepatic insulin signaling by increasing hepatic phosphatase and tensin homolog (PTEN) expression (16). Expression of TNFα, an anti-insulin cytokine, increases following alcoholic liver injury, HIV infection, and ART (3, 45, 68) and potentially alters hepatic glucose metabolism. Adiponectin inhibits gluconeogenesis (5, 70) and enhances fatty acid β oxidation, and these effects are mediated by activation of AMP-activated protein kinase (AMPK) (69, 70), implicating a central role for adiponectin to regulate hepatic metabolic processes and suggest that impaired adiponectin signaling may lead to IR. The activation of fatty acid synthesis pathways, including SREBP-1c, and key regulatory/rate-limiting step enzymes acetyl coA carboxylase (ACC) and fatty acid synthase increase hepatic triglyceride synthesis and deposition, which have been characterized in alcoholic fatty liver disease (52, 54). In addition to chronic alcohol exposure, prolonged ART regimens also contribute to abnormal hepatic fat deposition with possible progression to the development of a fatty liver (62).
This study aimed to identify CBA- and ART-mediated mechanisms of metabolic dysregulation in peripheral insulin-sensitive tissues of SIV-infected male macaques. We found that CBA reduced adipocyte cell size, increased collagen expression, and decreased differentiation of adipose-derived stem cells, whereas ART increased hepatic gluconeogenic and fatty acid synthesis enzyme mRNA expression and decreased skeletal muscle expression of insulin-signaling proteins. These findings suggest that CBA and ART differentially contribute to metabolic dysregulation in SIV infection but that their effects may not be synergistic.
METHODS
Animal Study Design
All experiments reported in this manuscript were approved by the Institutional Animal Care and Use Committee at both Tulane National Primate Research Center (Covington, LA) and Louisiana State University Health Sciences Center (New Orleans, LA) and adhered to the National Institutes of Health guidelines for the care and use of experimental animals. The detailed experimental design, virological, immune, and metabolic data collected from the parent longitudinal study have been previously published (20, 41, 58, 61). Briefly, 4- to 6-yr-old male rhesus macaques (Macaca mulatta) were used for the study. Macaques were administered alcohol daily through an intragastric catheter (13–14 g of ethanol·kg body wt−1·week−1; 30% wt/vol water) or isocaloric sucrose (SUC) for 3 mo before SIV infection and continued for the duration of the study (2, 41). Following 3 mo of daily alcohol or SUC administration, animals were inoculated intrarectally with SIVmac251. After 2.5 mo of SIV infection, animals were randomized to receive ART (provided by Gilead Sciences, Inc.) consisting of daily subcutaneous injections of 20 mg/kg of tenofovir (TFV) (9-[R-2-(phosphonomethoxy) propyl] adenine, PMPA) and 30 mg/kg of emtricitabine (FTC) for a total of 4 treatment groups: SUC/SIV/ART− (n = 4), SUC/SIV/ART+ (n = 7), CBA/SIV/ART− (n = 6), and CBA/SIV/ART+ (n = 7). This protocol has been previously shown to effectively suppress viral load in both SUC and CBA animals and to have no renal or hepatic toxicity (41). Skeletal muscle biopsies were collected during the fed state 1 wk before necropsy. Skeletal muscle, omental adipose tissue (OmAT), and liver samples were collected in the fasted state at the time of necropsy.
OmAT Analysis
OmAT samples were collected from fasted animals at necropsy, and fresh omental tissue was used to isolate adipose-derived stem cells (ADSCs). Tissue was also flash-frozen for gene and protein expression analysis. To determine adipocyte size, collagen expression, and immune cell infiltration by immunohistochemistry, a portion of the sample was fixed in zinc formalin and processed for standard paraffin embedding.
Isolation of OmAT-derived stem cells.
ADSCs were isolated according to previously published protocols (12, 21). OmAT was washed in PBS, minced, and enzymatically digested in collagenase buffer [0.25% collagenase type I (Sigma-Aldrich, St. Louis, MO), 2% penicillin streptomycin (P/S), 0.25 ug/ml Fungizone in HBSS] by incubating at 37°C, 5% CO2 for 45–60 min. The enzyme reaction was neutralized with adipose stem cell media (20% FBS + 2% P/S + 1% glutamine + 0.25 μg/ml Fungizone in α-MEM) and the tissue homogenate filtered through a sterile gauze. The filtrate was centrifuged at 186 g for 7 min, and the pellet was resuspended in red blood cell lysis buffer (Qiagen, Hilden, Germany) and incubated at room temperature for 5 min. The pellet was washed by centrifugation at 500 g for 5 min, passed through a cell strainer (70 μm), and plated in a 10-cm tissue culture dish. The cells were incubated at 37°C and 5% CO2. Half of the media was replaced 24 h later; thereafter, media was changed every 3 days, and cells were passaged when 70% confluent. Three primary cell lines from each treatment group were used for the study. All experiments were run in triplicate and repeated three times with each cell line.
Adipogenic differentiation of ADSCs.
ADSCs were plated at a density of 1 × 105 cells/well of a 6-well plate in 2 ml of stromal media (α-MEM, 10% FBS, 1% l-glutamine, 2% P/S, and 0.25 ug/ml Fungizone). At 90%–100% confluency, the cell layer was rinsed with PBS, and 2 ml of adipogenic differentiation medium (0.5 ug/ml dexamethasone, 0.5 mM isobutylmethylxanthine, and 50 μM indomethacin in stromal media) added. The media were replaced every 3 days, and differentiated adipocytes are generally observed approximately at 14 days. After 14 days of differentiation, cells were incubated with boron-dipyrromethene (BODIPY, 2 μg/ml, final concentration; Thermo Fisher Scientific, Waltham, MA) at 37°C for 30 min. The cells were then fixed with 4% paraformaldehyde for 10 min and mounted with fluorescent mounting media (Vectashield Antifade, Vector Laboratories, Burlingame, CA). Slides were imaged in a blinded manner using an Olympus DP72 Digital Camera System mounted to an Olympus BX51 TRF Microscope (Olympus, Center Valley, PA). RNA was isolated during the predifferentiation and differentiation phases to determine changes in gene expression.
Measurement of adipocyte diameter and collagen.
Adipose tissue slides were stained for collagen expression and for adipocyte diameter measurements using picrosirius red staining, as published previously (17). Five μm sections of OmAT were deparaffinized and hydrated. The slides were washed in PBS and stained in Bouins reagent (Sigma-Aldrich) for 30 min. The sections were rinsed in water, stained in picrosirius red (Sigma Direct Red 80, Sigma-Aldrich) for 1 h, and rinsed in acidic water (5 ml glacial acetic acid in 1 liter of water) and picric alcohol (10% picric acid, 20% ethanol) rinse. The slides were then cleared in xylene and mounted with permount medium (Sigma-Aldrich). Slides were imaged in a blinded manner using an Olympus DP72 Digital Camera System mounted to an Olympus BX51 TRF Microscope (Olympus) and the diameter determined using Image J.
Immunohistochemical analysis to detect inflammatory cell infiltration.
OmAT sections were stained with toluidine blue (Sigma-Aldrich) for histological analysis of mast cells (49, 64) and HAM56 for macrophages (15), and control experiments were performed by omitting the primary antibody. Representative color micrographs were obtained from tissue sections using ×20 objectives for mast cell analyses. Slides were imaged in a blinded manner using an Olympus DP72 Digital Camera System mounted to an Olympus BX51 TRF Microscope (Olympus) and number of cells counted using Image J.
Western blot for expression of insulin signaling cascade proteins in quadricep muscle, OmAT, and liver.
Fasted and fed-state quadricep muscle biopsies, fasted OmAT, and fasted liver samples were homogenized in Tissue Protein Extraction Reagent (Thermo Fisher Scientific) buffer with added protease inhibitor cocktail and phosphatase inhibitor cocktails II and III (Sigma-Aldrich). Concentration of protein samples was determined using a bicinchoninic acid assay (Pierce BCA Assay, Thermo Fisher Scientific), according to manufacturer’s instructions. Protein (20 µg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad Mini-PROTEAN TGX Stain-Free Precast Gels and Criterion TGX Precast Gels) and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA) for immunoblot analysis. Phosphorylated and total protein expression was assessed using specific antibodies (detailed in Table 1). Polyvinylidene fluoride membranes were incubated briefly with chemiluminescent substrate (Luminata Crescendo Western HRP Substrate) (EMD Millipore), and autoradiography films (HyBlot CL Autoradiography Film; Denville Scientific, Inc., Holliston, MA) were utilized to capture images. Quantification of protein band density was performed using Image J software (National Institutes of Health, Bethesda, MD). Phosphorylated and total protein expression were normalized to the loading control, growth factor receptor-bound protein 2 (GRB2).
Table 1.
List of antibodies used for Western blot analysis
| Antibody | Catalog | Dilution |
|---|---|---|
| P-4E-BP1 (Thr37/46) | 2855S | 1:1,000 |
| 4E-BP1 | 9644S | 1:1,000 |
| P-AKT (Ser473) | 4060S | 1:2,000 |
| P-AKT (Thr308) | 13038S | 1:1,000 |
| AKT | 9272S | 1:1,000 |
| P-AMPKα | 2535S | 1:1,000 |
| AMPKα | 5831S | 1:1,000 |
| P-eEF2 (Thr56) | 2331S | 1:1,000 |
| eEF2 | 2332S | 1:1,000 |
| GRB2 | 3972S | 1:1,000 |
| P-GSK3B (Ser9) | 9323S | 1:1,000 |
| GSK3B (3D10) | 9832S | 1:1,000 |
| PP2A catalytic subunit | 2038 | 1:500 |
| P-p70S6 kinase (Thr421/Ser424) | 9204S | 1:1,000 |
| p70S6 kinase | 2708S | 1:1,000 |
| PTEN | 9188S | 1:1,000 |
| PTP1B | 5311S | 1:1,000 |
| P-S6 Ribosomal protein (Ser235/236) | 4858S | 1:2,000 |
| P-S6 Ribosomal protein (Ser240/244) | 5364S | 1:2,000 |
| S6 Ribosomal protein | 2317S | 1:1,000 |
| Anti-mouse IgG, HRP-linked | 7076S | 1:25,000 |
| Anti-rabbit IgG, HRP-linked | 7074S | 1:25,000 |
All antibodies are from Cell Signaling Technologies. AMPKα, AMP-activated protein kinase alpha; GRB2, growth factor receptor-bound protein 2; HRP, horseradish peroxidase; PTEN, phosphatase and tensin homolog; Thr, threonine.
Quantitative PCR for gene expression.
RNA was extracted from liver, OmAT, and ADSCs using RNeasy Mini Kits (Qiagen, cat. no. 74106). cDNA was synthesized using the reverse transcriptase kit (Qiagen) and quantitative PCR was performed using RT2 SYBR Green Fluorescent qPCR Master Mix (Qiagen) and specific primers (Integrated DNA Technologies, Coralville, IA). RPS13 was used as the endogenous control, and values are expressed as the relative fold-change according to the ∆∆Ct method. All primer sequences are included in Table 2.
Table 2.
List of primers used for quantitative PCR analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACC | CACGGATATCATCGGGAAAG | GTGACGATCTCTTCGTAAGC |
| AdipoR1 | CTGGACTATTCAGGGATTGC | CAGACGATGGAGAGGTAGAT |
| AdipoR2 | CCACAACCTTGCTTCATCTA | GAAACACTCCTGCTCTTACTC |
| EGR2 | CATGACTGGAGAGAAGAGGT | GGAGAACTTGCCCATGTAAG |
| FAS | CAACCTGATAGTGAGTGGGAA | CAGACGCAGCTCCTTGTAAA |
| F16BP | CTCTCCAACGACCTGGTTATG | TCCACTATGATGGCGTGTTTAT |
| GCK | GCATCCTTCTCAACTGGAC | TGCCACCACATCCATTTC |
| GLU6p | CTGGGAGGGTGTATGTGATA | GCCTGGCTTATTCCTATGTG |
| IL1B | TGGCATCCAGCTACAAATCTCCCA | AAGGGAATCAAGGTGCTCAGGTCA |
| IL-6 | AGCCACTGACCTCTTCAGAACGAA | AGTGCCTCTTTGCTGCTTTCACAC |
| PC | CGGCAGAAAGCAGATGAA | CCTTGATGATGTCTGGGATG |
| PCK2 | GAGAATACTGCCACACTGAC | CACCGTCTTGCTCTCTATTC |
| PCK1 | CCATCCAGAAGAACACCATC | CTGGGCTCCATTCCTTATTC |
| PPARγ | TGAAGGATGCAAGGGTTTCTTCCG | CGCCCAAACCTGATGGCATTATGA |
| SREBP1C | GTCCTCTCACAGCAAAGAAG | CCCTTCACAGAACAGGAAAC |
| TNFα | TTTCAGCTGGAGAAGGGT | GGGCAATGATCCCAAAGTAG |
Primers are from IDT Technologies.
Statistical Analyses
All data are presented as means ± SE, where n = 4–7 for each treatment group at study end-point [SUC/SIV/ART− (n = 4), SUC/SIV/ART+ (n = 7), CBA/SIV/ART− (n = 6), and CBA/SIV/ART+ (N = 7)]. The discrepancy in total number of animals between the two time points is due to three non-ART-treated SIV-infected macaques that met criteria for euthanasia before reaching study end-point (11 mo post-SIV infection). Two-way analysis of variance, using Prism Graph Pad Version 5 (La Jolla, CA), was used to compare the measures at 11 mo post-SIV infection. Statistical significance was established at P < 0.05.
RESULTS
CBA dysregulates OmAT phenotype in SIV-infected macaques.
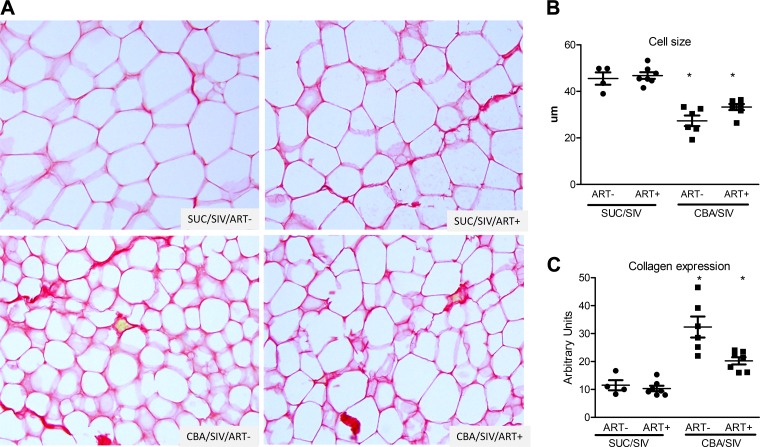
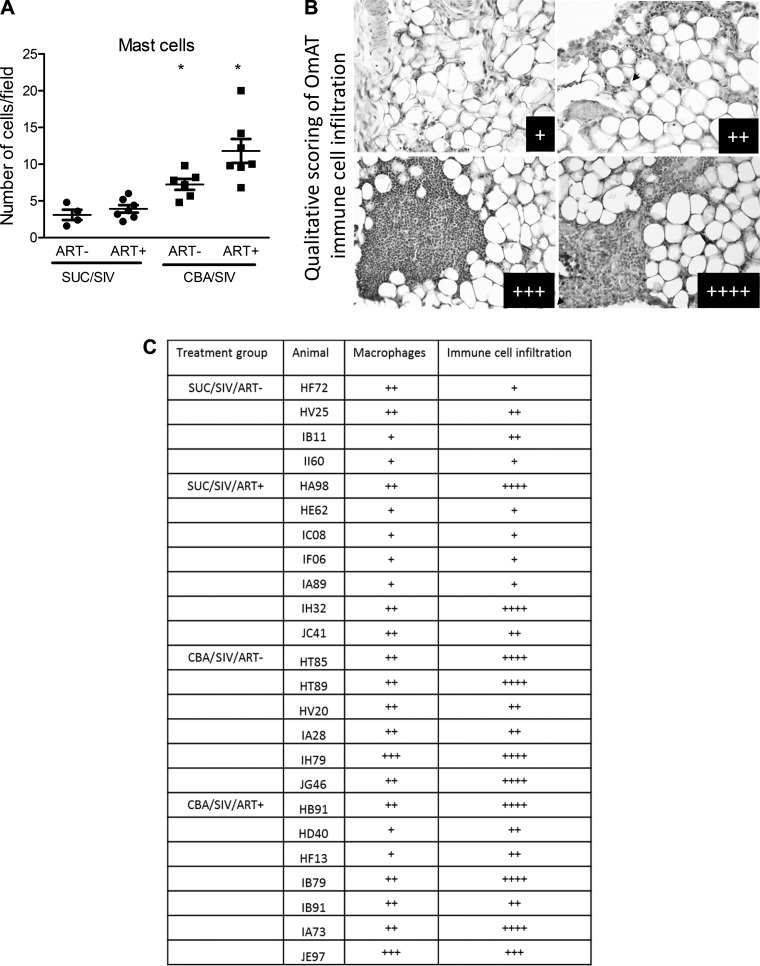
CBA significantly decreased adipocyte cell size (Fig. 1, A and B) and increased collagen expression (Fig. 1, A and C) in OmAT, irrespective of ART status. CBA significantly increased the number of mast cells relative to SUC-administered macaques, irrespective of ART status (Fig. 2A). Although there were no significant differences in the number of HAM56+ cells (macrophages) (Fig. 2C), histology revealed significant immune cell infiltration in OmAT of CBA-administered macaques compared with SUC-administered macaques (Fig. 2, B and C). There were no significant effects of CBA and ART to alter the protein expression of TNF-α, Il-6, or MCP-1 (Table 3).
Fig. 1.
Omental adipose tissue (OmAT) histology. OmAT isolated from sucrose (SUC)- or chronic binge alcohol (CBA)-administered macaques with and without antiretroviral therapy (ART) was stained for picrosirius red (PSR). A: representative micrographs of PSR staining in the OmAT. B: there was a main effect of CBA to decrease cell size in OmAT. C: there was a main effect of CBA to increase PSR staining (collagen expression) in OmAT. SUC/SIV/ART− and SUC/SIV/ART+ (black closed circles) and CBA/SIV/ART− and CBA/SIV/ART+ (black closed squares). *P < 0.05. Data are expressed as means ± SE. n = 4–7/group. SIV, simian immunodeficiency virus.
Fig. 2.
Omental adipose tissue (OmAT) inflammatory cell infiltration. OmAT isolated from sucrose (SUC)- or chronic binge alcohol (CBA)-administered macaques with and without antiretroviral therapy (ART) was stained for toluidine blue for determining mast cell numbers and for HAM56 for macrophages. A: there was a main effect of CBA to increase number of mast cells in OmAT. B: representative micrographs of HAM56 staining in OmAT showing the scoring for immune cell infiltration: +, ++, +++, ++++. C: qualitative scoring of macrophage and immune cells in the OmAT. SUC/SIV/ART− and SUC/SIV/ART+ (black closed circles) and CBA/SIV/ART− and CBA/SIV/ART+ (black closed squares). *P < 0.05. Data are expressed as means ± SE. n = 4–7/group. SIV, simian immunodeficiency virus.
Table 3.
Cytokine and adiponectin expression in the omental adipose tissue
| SUC/SIV/ART− | SUC/SIV/ART+ | CBA/SIV/ART− | CBA/SIV/ART+ | |
|---|---|---|---|---|
| TNF-α, pg/mg | 674.27 ± 138.58 | 1,230.17 ± 684.05 | 2,224.09 ± 1,621.98 | 707.36 ± 107.31 |
| IL-6, U/mg | 70.3 ± 13.74 | 54.7 ± 6.71 | 44.62 ± 7.36 | 79.55 ± 21.26 |
| MCP-1, pg/mg | 1,448.38 ± 144.62 | 1,179.89 ± 63 | 1,128.9 ± 167.25 | 1,661.51 ± 314.01 |
| Adiponectin, ng/mg | 2,798 ± 548.9 | 2,666 ± 394.3 | 2,253 ± 411.6 | 3,372 ± 624.1 |
MCP, monocyte chemoattractant protein.
CBA decreases differentiation of ADSCs in SIV-infected macaques.
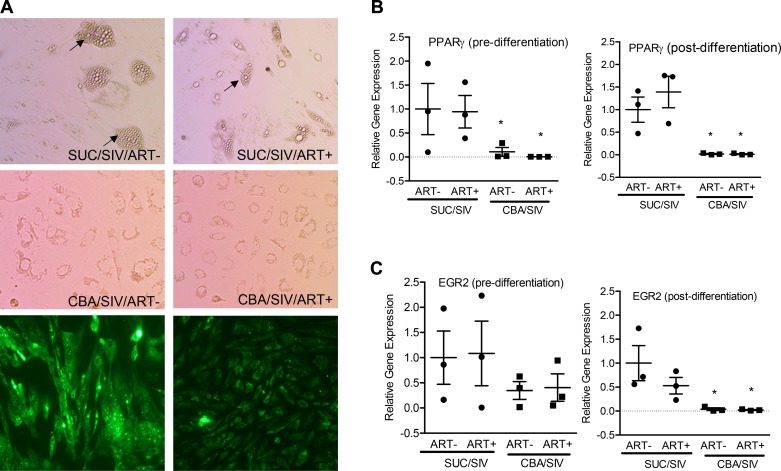
Under conditions that promote adipogenic differentiation, adipose cell differentiation was significantly reduced in cultures of ADSCs isolated from CBA/SIV/ART−/+ compared with those isolated from SUC/SIV/ART−/+ macaques (Fig. 3A). Expression of genes that are essential for adipogenic differentiation was determined at day 0 and 14 of differentiation. CBA significantly decreased the mRNA expression of PPARγ both at day 0 and 14 (Fig. 3B) and early growth response 2 (EGR2) at day 14 of differentiation (Fig. 3C).
Fig. 3.
Differentiation potential of omental adipose derived stem cells (ADSCs). Four top panels (A) show representative light micrographs obtained at day 14 of differentiation of ADSCs isolated from SUC/SIV/ART−, SUC/SIV/ART+, CBA/SIV/ART−, and CBA/SIV/ART+ macaques. Two bottom panels (A) show micrographs of boron-dipyrromethene (BODIPY) staining with and without lipid droplet accumulation. Relative expression of peroxisome proliferator-activated receptor gamma (PPARγ) (B) and early growth factor 2 (EGR-2) (C) pre- (left) and post- (right) differentiation of ADSCs isolated from SUC- (closed circles) or CBA- (closed squares) administered macaques with and without ART. There was a main effect of CBA to decrease the mRNA expression of PPARγ pre- and 14 days postdifferentiation and to decrease the mRNA expression of EGR2 at day 14 of differentiation. *P < 0.05. Data are expressed as means ± SE. n = 3 primary lines/group. ART, antiretroviral therapy; CBA, chronic binge alcohol; SIV, simian immunodeficiency virus; SUC, sucrose.
Effects of CBA and/or ART on phosphorylation and total expression of insulin signaling cascade proteins in the OmAT.
At study end-point, there were no differences among treatment groups in the phosphorylated/total protein ratio or the total protein expression normalized to loading control for proteins in the insulin signaling cascade, including p-mTOR (Ser2448)/total mTOR, p-AKT (Ser473)/total AKT, GSK3β (Ser9)/total GSK3β, PTEN, PTP1B, and PP2A, in the OmAT (Table 4).
Table 4.
OmAT expression of proteins in the insulin signaling cascade
| Protein | SUC/SIV/ART- | SUC/SIV/ART+ | CBA/SIV/ART- | CBA/SIV/ART+ |
|---|---|---|---|---|
| P-AKT/total AKT (Ser473) | 1 ± 0.23 | 0.57 ± 0.07 | 0.7 ± 0.07 | 0.96 ± 0.11 |
| P-mTOR/total mTOR | 1 ± 0.19 | 0.7 ± 0.22 | 1.48 ± 0.15 | 1.22 ± 0.17 |
| P-GSK3B/total GSK3B | 1 ± 0.43 | 1.2 ± 0.21 | 0.72 ± 0.32 | 1.3 ± 0.14 |
| PTP1B | 1 ± 0.11 | 0.93 ± 0.14 | 1.00 ± 0.17 | 0.97 ± 0.24 |
| PP2A | 1 ± 0.13 | 1.02 ± 0.07 | 1.07 ± 0.05 | 1.12 ± 0.06 |
| PTEN | 1 ± 0.26 | 1.19 ± 0.28 | 1.4 ± 0.21 | 1.6 ± 0.19 |
mTOR, mammalian target of rapamycin; OmAT, omental adipose tissue; PTEN, phosphatase and tensin homolog.
ART alters skeletal muscle protein expression of insulin signaling cascade in SIV-infected macaques.
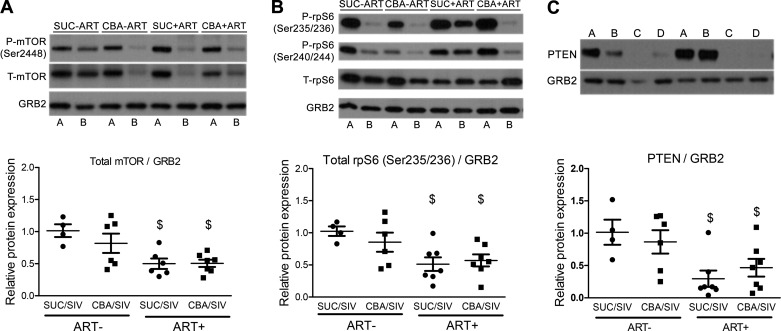
Skeletal muscle biopsies were obtained in the fed state and after 18 h of fasting, and the phosphorylated and total expression of proteins in the insulin signaling cascade was determined. CBA had no effect on the expression of any of the proteins measured. ART significantly decreased the relative total mTOR protein expression (0.50 ± 0.08 arbitrary units for SUC/SIV/ART+ and 0.51 ± 0.06 for CBA/SIV/ART+) relative to ART-naïve macaques (1.00 ± 0.23 for SUC/SIV/ART− and 0.82 ± 0.15 for CBA/SIV/ART−) in the fed-state muscle biopsy sample (Fig. 4A). However, neither CBA nor ART altered the ratio of fed or fasted p-mTOR (Ser2448)/total mTOR protein expression. Total rpS6 protein expression in the fed-state muscle biopsy sample in ART-treated (0.54 ± 0.12 arbitrary units for SUC/SIV/ART+ and 0.57 ± 0.09 for CBA/SIV/ART+) macaques was significantly reduced relative to ART-naïve macaques (1.00 ± 0.17 for SUC/SIV/ART− and 0.85 ± 0.15 arbitrary units for CBA/SIV/ART−) (Fig. 4B). Neither CBA nor ART altered the fed- or fasted-state ratio of p-AKT (Ser473 or Thr309) or total expression of AKT, p-4E-BP1 (Thr37/46)/total 4E-BP1 or total 4E-BP1, or p-eEF2 (Thr56)/total eEF2 or total eEF2 expression (Table 5). ART significantly decreased the relative PTEN protein expression in the fed-state muscle biopsy sample (Fig. 4C). Neither CBA nor ART altered fed-state protein expression of PTP1B or PP2A (Table 5).
Fig. 4.
Chronic binge alcohol (CBA) and antiretroviral therapy (ART) effects on proteins in the insulin-signaling cascade in skeletal muscle. Representative Western blots for mammalian target of rapamycin (mTOR) (A) and ribosomal protein S6 (rpS6) (B) determined in skeletal muscle. Alternating fasted [A] and fed [B] state samples from SUC/SIV/ART−, CBA/SIV/ART−, SUC/SIV/ART+, and CBA/SIV/ART+ macaques. Phosphatase and tensin homolog (PTEN) expression in fed-state biopsy samples (C) relative to growth factor receptor-bound protein 2 (GRB2) [A: SUC/SIV/ART−; B: CBA/SIV/ART−; C: SUC/SIV/ART+; D: CBA/SIV/ART+]. There was a main effect of ART to decrease total mTOR/GRB2 (A), total rpS6/GRB2 (B), and PTEN expression (C). SUC/SIV/ART− and SUC/SIV/ART+ (black closed circles) and CBA/SIV/ART− and CBA/SIV/ART+ (black closed squares) in the fed-state muscle biopsy samples. There were no differences in the phosphorylated mTOR (p-mTOR)/total mTOR (t-mTOR) or p-rpS6/T-rpS6 expression. Data are expressed as means ± SE. $P < 0.05. n = 4–7/group. SIV, simian immunodeficiency virus; SUC, sucrose.
Table 5.
Skeletal muscle expression of proteins in the insulin signaling cascade
| Protein | SUC/SIV/ART− | SUC/SIV/ART+ | CBA/SIV/ART− | CBA/SIV/ART+ |
|---|---|---|---|---|
| P-AKT/total AKT (Ser473) | 1 ± 0.8 | 0.5 ± 0.14 | 0.3 ± 0.05 | 0.7 ± 0.2 |
| P-AKT/total AKT (Thr308) | 1 ± 0.2 | 0.89 ± 0.09 | 1.05 ± 0.2 | 1.03 ± 0.12 |
| P-4EBP1/total 4EBP1 | 1 ± 0.2 | 1.08 ± 0.06 | 1.27 ± 0.14 | 0.97 ± 0.12 |
| P-eEF2/total eEF2 | 1 ± 0.4 | 0.93 ± 0.09 | 0.67 ± 0.13 | 1.11 ± 0.16 |
| PTP1B | 1 ± 0.42 | 0.9 ± 0.11 | 1.16 ± 0.12 | 1.04 ± 0.16 |
| PP2A | 1 ± 0.4 | 0.8 ± 0.2 | 1.12 ± 0.2 | 0.78 ± 0.15 |
ART alters hepatic gene expression of enzymes of gluconeogenesis, glycolysis, and fatty acid synthesis.
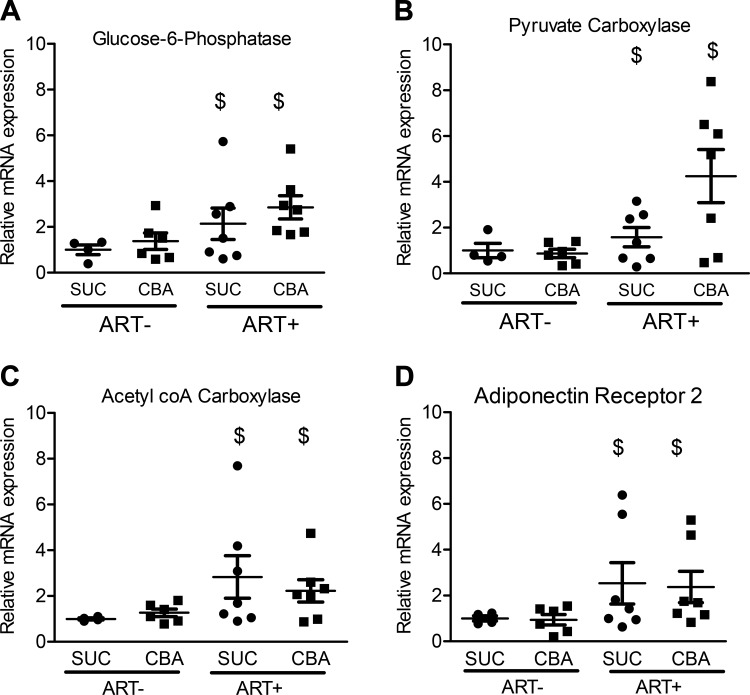
Our previously published work demonstrated that CBA decreased circulating adiponectin levels and potentially mediated an increase in glucose-independent glucose stimulation (20). Hence, we determined the hepatic expression of enzymes involved in gluconeogenesis, fatty acid synthesis, and glycolysis. CBA did not alter the expression of any of the genes determined. ART (2.13 ± 0.69 for SUC/SIV/ART+ and 2.85 ± 0.51 for CBA/SIV/ART+) significantly increased glucose-6-phosphatase mRNA expression (1.00 ± 0.22 for SUC/SIV/ART− and 1.38 ± 0.37 for CBA/SIV/ART+) (Fig. 5A). ART (1.58 ± 0.42 for SUC/SIV/ART+ and 4.25 ± 1.16 for CBA/SIV/ART+) significantly increased pyruvate carboxylase mRNA (1.00 ± 0.31 for SUC/SIV/ART− and 0.87 ± 0.18 for CBA/SIV/ART+) (Fig. 5B). ART significantly increased ACC mRNA expression (2.83 ± 0.93, SUC/SIV/ART+ and 2.23 ± 0.49, CBA/SIV/ART+) compared with macaques that were not administered ART (1.00 ± 0.04, n = 4 for SUC/SIV/ART− and 1.27 ± 0.16, n = 6 for CBA/SIV/ART−) (Fig. 5C). ART (2.53 ± 0.90 for SUC/SIV/ART− and 2.37 ± 0.69 for CBA/SIV/ART+) significantly increased AdipoR2 mRNA expression (1.00 ± 0.12 for SUC/SIV/ART− and 0.94 ± 0.23 for CBA/SIV/ART−) (Fig. 5D). Neither CBA nor ART altered the mRNA expression of F-1, 6-BPase, PCK-1, PCK-2, fatty acid synthase, SREBP-1c, GCK, PFK-1, or AdipoR1 (Table 6).
Fig. 5.
Chronic binge alcohol (CBA) and antiretroviral therapy (ART) effects on expression of hepatic gene expression. There was a main effect of ART to increase the hepatic mRNA expression of glucose-6-phosphatase (A), pyruvate carboxylase (B), acetyl coA carboxylase (C), and adiponectin receptor 2 (D). SUC/SIV/ART− and SUC/SIV/ART+ (black closed circles) and CBA/SIV/ART− and CBA/SIV/ART+ (black closed squares). Data are expressed as means ± SE. $P < 0.05. n = 4–7/group. SIV, simian immunodeficiency virus; SUC, sucrose.
Table 6.
Hepatic expression of genes involved in metabolic processes
| Genes | SUC/SIV/ART− | SUC/SIV/ART+ | CBA/SIV/ART− | CBA/SIV/ART+ |
|---|---|---|---|---|
| F-1,6-BPase | 1 ± 0.3 | 0.97 ± 0.23 | 0.54 ± 0.17 | 1.2 ± 0.31 |
| PCK-1 | 1 ± 0.2 | 1.12 ± 0.38 | 1.79 ± 0.8 | 2.18 ± 0.34 |
| PCK-2 | 1 ± 0.14 | 1.87 ± 0.69 | 3.54 ± 1.3 | 3.2 ± 1.04 |
| AdipoR1 | 1 ± 0.15 | 1.42 ± 0.46 | 1.28 ± 0.36 | 1.93 ± 0.37 |
| GCK | 1 ± 0.28 | 1.1 ± 0.32 | 0.98 ± 0.4 | 0.61 ± 0.17 |
| PFK1 | 1 ± 0.62 | 0.52 ± 0.19 | 0.34 ± 0.05 | 0.29 ± 0.06 |
| FAS | 1 ± 0.08 | 1.02 ± 0.36 | 1.15 ± 0.22 | 0.76 ± 0.13 |
| SREBP-1C | 1 ± 0.15 | 0.67 ± 0.16 | 0.79 ± 0.16 | 0.57 ± 0.09 |
| TNF | 1 ± 0.26 | 0.65 ± 0.2 | 0.4 ± 0.13 | 1.85 ± 0.58 |
| IL-1B | 1 ± 0.2 | 1.07 ± 0.35 | 1.52 ± 0.66 | 1.86 ± 0.44 |
| IL-6 | 1 ± 0.25 | 0.33 ± 0.08 | 1.08 ± 0.39 | 3.76 ± 1.9 |
Effects of CBA and/or ART on phosphorylation and total expression of insulin signaling cascade proteins in the liver.
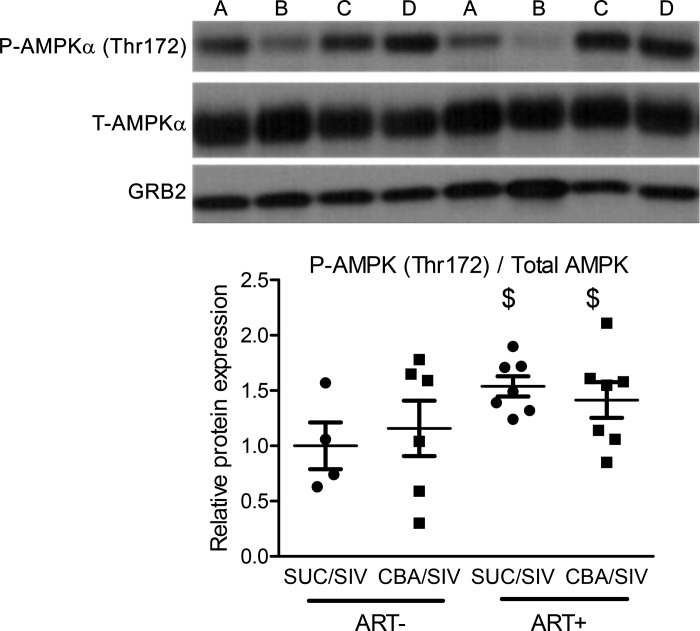
At study end-point, there were no differences in the phosphorylated/total protein ratio or the total protein expression normalized to loading control for proteins in the insulin signaling cascade, including p-mTOR (Ser2448)/total mTOR, p-AKT (Ser473)/total AKT, GSK3β (Ser9)/total GSK3β, PTEN, PTP1B, and PP2A (Table 7). ART (1.54 ± 0.09 for SUC/SIV/ART+ and 1.41 ± 0.16 for CBA/SIV/ART+) significantly increased the ratio of p-AMPKα (Thr172)/total AMPKα (1.00 ± 0.21 for SUC/SIV/ART− and 1.16 ± 0.25 for CBA/SIV/ART−) in the liver (Fig. 6).
Table 7.
Hepatic expression of proteins in the insulin signaling cascade
| Protein | SUC/SIV/ART− | SUC/SIV/ART+ | CBA/SIV/ART− | CBA/SIV/ART+ |
|---|---|---|---|---|
| P-AKT/total AKT (Ser473) | 1 ± 0.02 | 0.91 ± 0.05 | 1.08 ± 0.15 | 0.9 ± 0.17 |
| P-mTOR/total mTOR | 1 ± 0.09 | 0.91 ± 0.07 | 0.97 ± 0.06 | 0.99 ± 0.08 |
| P-GSK3B/total GSK3B | 1 ± 0.42 | 0.71 ± 0.09 | 0.69 ± 0.12 | 0.63 ± 0.14 |
| PTP1B | 1 ± 0.11 | 0.93 ± 0.14 | 1.00 ± 0.17 | 0.97 ± 0.24 |
| PP2A | 1 ± 0.16 | 1.1 ± 0.06 | 1.12 ± 0.25 | 1.12 ± 0.15 |
| PTEN | 1 ± 0.16 | 1.29 ± 0.18 | 1.21 ± 0.2 | 1.5 ± 0.28 |
mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homolog.
Fig. 6.
Chronic binge alcohol (CBA) and antiretroviral therapy (ART) effects on AMP-activated protein kinase alpha (AMPKα) expression in the liver. Representative Western blots for phosphorylated AMPKα (p-AMPKα), total AMPKα (t-AMPKα), and growth factor receptor-bound protein 2 (GRB2) in liver samples from [A: SUC/SIV/ART−; B: CBA/SIV/ART−; C: SUC/SIV/ART+; and D: CBA/SIV/ART+] macaques. There was a main effect of ART to increase hepatic expression of p-AMPKα [threonine 172 (Thr172)]/t-AMPK. SUC/SIV/ART− and SUC/SIV/ART+ (black closed circles) and CBA/SIV/ART− and CBA/SIV/ART+ (black closed squares). Data are expressed as means ± SE. $P < 0.05. n = 4–7/group. SIV, simian immunodeficiency virus; SUC, sucrose.
Effects of CBA and/or ART on hepatic proinflammatory cytokine mRNA expression.
CBA and ART increased TNFα mRNA expression. Neither CBA nor ART altered the expression of IL-1 or IL-6. While expression of all three proinflammatory cytokines trended to have the highest hepatic gene expression in CBA/SIV/ART+ macaques (Table 6), this difference did not reach statistical significance.
DISCUSSION
In this study, we examined the effects of CBA and ART on aspects of metabolic regulation in peripheral insulin sensitive tissues (skeletal muscle, adipose tissue, and liver) during the asymptomatic phase of SIV infection. CBA decreased adipose-derived stem cell differentiation and adipocyte cell size and increased collagen expression in the OmAT. ART decreased the expression of proteins in the insulin-mediated protein synthesis pathway in the skeletal muscle and increased expression of gluconeogenic and fatty acid synthesis enzymes, adiponectin receptor, and proinflammatory cytokine TNF-α in the liver. Taken together, these results provide evidence for a differential contribution of CBA and ART to metabolic dysregulation in SIV-infection.
Previously, we demonstrated CBA-mediated reductions in plasma insulin and C-peptide response to a glucose challenge, indicating decreased pancreatic insulin secretion. CBA also decreased the kinematic measures of glucose-insulin dynamics, namely DI and AIRg, in response to a glucose challenge (20). DI is a derived value determined as the product of the minimal model kinematic values for both insulin secretion and insulin responsiveness of peripheral tissues, and it serves as a marker for the dynamic regulation of glucose (6, 46). Hence, the objective of the study was to determine specific alterations in peripheral insulin-sensitive tissues that may account for the decreased insulin responsiveness.
Adipose tissue contributes to whole body metabolic regulation, where homeostasis is dictated by the balance between lipogenesis and lipolysis. Our results show that CBA decreased adipocyte cell size and ADSC differentiation in the OmAT in SIV-infected animals. Our results also indicate that CBA increased collagen expression in OmAT. These findings are in agreement with those reported in chronic alcohol-fed rodents (72, 73) or HIV (4, 47) and suggest that alcohol use could exacerbate the decrease in adipocyte cell size and could contribute to lipoatrophy in PLWH. Increased extracellular matrix impairs adipose tissue growth and adipocyte differentiation (1) that is potentially translated to impaired triglyceride storage and IR. Collagens are secreted by preadipocytes, adipocytes, and macrophages (11). Whether the CBA-mediated decreased adipogenic differentiation of ADSCs indicates a profibrogenic differentiation lineage remains to be determined. The mechanisms of CBA-mediated increased adipose tissue collagen expression are currently being investigated. We have previously demonstrated that skeletal muscle myoblasts isolated from CBA-administered macaques have decreased differentiation potential (58, 60). These studies were performed in cultured myoblasts that were not exposed to alcohol (ethanol) or the SIV virus in vitro, suggesting that these myoblasts had a “memory” that preserved gene expression changes and in which epigenetic mechanisms are involved (58). Adipogenic differentiation is principally regulated by the transcription factor PPARγ, and epigenomic alterations of PPARγ have been demonstrated in obesity (33) and in ADSCs isolated from low birth weight individuals (9, 10). Our studies indicate that PPARγ expression is decreased in ADSCs isolated from CBA-administered macaques, and together with evidence from the literature and our published and current data, we speculate that epigenetic mechanisms contribute to the decreased differentiation ability of ADSCs.
We have previously published that there were no significant differences in body composition, including changes in fat mass, waist circumference, and waist-to-hip ratio in these animals, indicating that at this stage of disease, there are no overt signs of lipodystrophy (20). However, our previous studies indicate that CBA decreases body weight and body mass index at end-stage disease in non-ART-treated SIV-infected macaques (42). Moreover, the observed CBA-mediated decrease in serum adiponectin (20) provides evidence that adipocyte function is dysregulated and contributes to metabolic dysregulation.
ART, but not CBA, reduced the total protein expression of mTOR and rpS6 in the skeletal muscle, suggesting ART’s adverse effect to reduce protein synthesis. Decreases in protein synthesis can be due to perturbation in the signaling cascade (reduced phosphorylation of target downstream molecules) or decreased expression of signaling cascade molecules. There was an ART-mediated reduction in the total PTEN protein expression in the skeletal muscle. PTEN is a phosphatase enzyme that dephosphorylates phosphoinositide-3-kinase and effectively attenuates downstream insulin signaling. The ART-mediated downregulation of PTEN did not translate to a statistically significant alteration in AKT phosphorylation. It is possible that other proteins are involved in the regulation of AKT. The rictor-mTOR complex positively regulates AKT phosphorylation at Ser473 (57). The observed upregulation of PTEN, in parallel to an ART-mediated downregulation of mTOR, is a possible explanation for the changes in AKT phosphorylation. There were no CBA-mediated impairments in the ratios of phosphorylated/total protein expression of molecules in the insulin signaling cascade, including canonical insulin signaling protein synthesis and glycogen synthesis, in either fasted-state or fed-state skeletal muscle biopsies.
Based on our previous findings of CBA-mediated reductions in serum adiponectin and SG, it was hypothesized that hepatic glucose and fatty acid metabolism would primarily be driven by a CBA-mediated reduction in gluconeogenesis and increased fatty acid β oxidation (70). Despite the CBA-mediated reductions in adiponectin and SG (20), and contrary to our prediction, hepatic changes were primarily associated with the ART regimen, with minimal CBA-associated effects. Hepatic gene expression of enzymes of gluconeogenesis was significantly greater in the livers of ART-treated macaques. Interestingly, the expression observed in hepatic samples from CBA/SIV/ART+ animals was greater than that observed in SUC/SIV/ART+ animals for both enzymes, which suggests that CBA administration may have also contributed to the observed increase in expression. However, there was no effect of CBA or ART to alter gene expression of key glycolytic enzymes, GCK and PFK-1. These findings suggest that ART alters the balance of glucose metabolism, favoring greater glucose production over glucose utilization. In addition, ART also increased the expression of ACC, the rate-limiting enzyme of fatty acid synthesis.
There were no CBA- or ART-mediated alterations in the ratios of phosphorylated/total protein expression in the liver for any of the insulin signaling molecules examined. However, there was an ART-associated increase in the ratio of p-AMPKα (Thr172)/total AMPKα. AMPK acts as a cellular energy sensor, responsive to increased cellular AMP and ADP and/or reduced cellular ATP (26). The addition of efavirenz, a nonnucleoside reverse transcriptase inhibitor, increases the ratio of p-AMPKα (Thr172)/total AMPKα protein expression in hepatocytes and increased accumulation of cytoplasmic lipid droplets. This is suggested to be a compensatory response to reductions in mitochondrial oxygen consumption and ATP production caused by efavirenz-mediated inhibition of mitochondrial complex I (7). Whereas mechanistic studies are lacking for an ART-mediated compromise in mitochondrial function, numerous studies have reported mitochondrial toxicity of ART medications (37, 44, 56). The ART regimen used in the current study consists of TFV and FTC, drugs that are safe and with minimal toxicities (55). However, our tightly controlled studies allow the outcome measures to reflect alterations because of the manipulated variables and suggest an ART (nucleoside reverse transcriptase inhibitor, NRTI)-mediated metabolic dysregulation. Though the current study did not focus on mitochondrial function in the liver and adipose tissue, we recently published that alcohol and SIV mediate mitochondrial dysfunction in skeletal muscle myoblasts, even in ART-treated, virally suppressed SIV-infected macaques (18). It is suggested that cells with mitochondrial dysfunction promote cell survival by activating the AMPK pathway and autophagy (71). Whether the observed ART-induced AMPK activation in the liver is a compensatory mechanism for mitochondrial dysfunction and metabolic dysregulation warrants further investigation.
CBA and ART also increased the expression of TNFα, and its anti-insulin properties could contribute to development of hepatic IR. Our results indicate an ART-mediated impairment in gluconeogenic and fatty acid synthesis gene expression, fatty acid/triglyceride fat deposition, and adiponectin receptor gene expression, supporting an ART-mediated impairment of glucose-insulin dynamics in the liver. It is worth noting that CBA administration decreased systemic adiponectin levels and impaired insulin secretion, and there was a trend for increased hepatic TNFα expression in the CBA/SIV/ART group, suggesting these two insults may interact to impair metabolic regulation in an organ-specific manner.
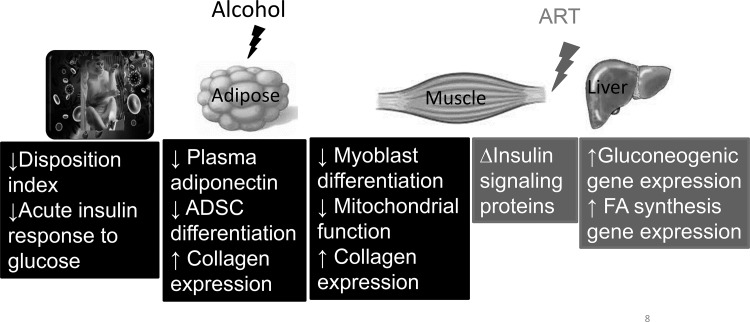
Our results show differential effects of chronic alcohol and the ART regimen, TFV and FTC, on metabolic dysregulation in SIV-infected macaques. CBA decreased in vitro differentiation potential of adipose-derived stem cells, adipocyte cell size, and increased collagen expression in OmAT. There was an ART-mediated increase in hepatic gene expression of key gluconeogenic and fatty acid synthesis enzymes. This adds to our previous reports of CBA-mediated decreases in AIRg, DI, circulating adiponectin levels (20), in vitro differentiation potential of skeletal muscle myoblasts (58), and mitochondrial function in skeletal muscle and myoblasts (18) (Fig. 7). The tissue specific changes seen are in the absence of overt dysglycemia or dyslipidemia, despite the consumption of a nutritionally balanced diet and lack of significant alterations in body weights. Therefore, whether a high-fat diet, age, and sex produce differential responses warrants investigation. Moreover, whether similar alterations are seen in nonobese, nondiabetic PLWH with alcohol use disorder remains to be determined.
Fig. 7.
Schematic summary of alcohol- and antiretroviral therapy (ART)-mediated metabolic dysregulation in simian immunodeficiency virus (SIV) disease. ADSC, adipose-derived stem cell; FA, fatty acid.
GRANTS
Research reported in this publication was supported by the NIH under award numbers P60-AA-009803, T32-AA-07577, F30-AA-024030, P51-RR-000164, and K01-AA-024494.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.F. Jr, L.S.P., J.D., G.B., S.N., and P.E.M. conceived and designed research; S.M.F. Jr, L.S.P., P.B., G.C., C.V.S., and J.D. performed experiments; S.M.F. Jr, L.S.P., P.B., G.C., and C.V.S. analyzed data; S.M.F. Jr, L.S.P., G.C., and P.E.M. interpreted results of experiments; S.M.F. Jr and L.S.P. prepared figures; S.M.F. Jr and L.S.P. drafted manuscript; S.M.F. Jr, L.S.P., P.B., G.C., C.V.S., J.D., G.B., S.N., and P.E.M. edited and revised manuscript; S.M.F. Jr, L.S.P., P.B., C.V.S., J.D., G.B., S.N., and P.E.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for the excellent technical assistance from Larissa Devlin, Wayne A. Cyprian, and Nancy Dillman at the Tulane National Primate Research Center Pathology Laboratory. From the Louisiana State University Health Sciences Center New Orleans, we are grateful for the technical support of Kejing Song, Jean Carnal, Jane Schexnayder, Amy B. Weinberg, and Rhonda R. Martinez.
REFERENCES
- 1.Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K, Winlove CP. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab 305: E1427–E1435, 2013. doi: 10.1152/ajpendo.00111.2013. [DOI] [PubMed] [Google Scholar]
- 2.Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res 30: 1781–1790, 2006. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 3.Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, Joshi-Barve S, McClain CJ. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health 33: 229–236, 2010. [PMC free article] [PubMed] [Google Scholar]
- 4.Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, Luboinski J, Laville M, Maachi M, Girard PM, Rozenbaum W, Levan P, Capeau J. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 359: 1026–1031, 2002. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 5.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 6.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blas-García A, Apostolova N, Ballesteros D, Monleón D, Morales JM, Rocha M, Victor VM, Esplugues JV. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology 52: 115–125, 2010. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]
- 8.Broholm C, Mathur N, Hvid T, Grøndahl TS, Frøsig C, Pedersen BK, Lindegaard B. Insulin signaling in skeletal muscle of HIV-infected patients in response to endurance and strength training. Physiol Rep 1: e00060, 2013. doi: 10.1002/phy2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broholm C, Olsson AH, Perfilyev A, Gillberg L, Hansen NS, Ali A, Mortensen B, Ling C, Vaag A. Human adipogenesis is associated with genome-wide DNA methylation and gene-expression changes. Epigenomics 8: 1601–1617, 2016. doi: 10.2217/epi-2016-0077. [DOI] [PubMed] [Google Scholar]
- 10.Broholm C, Olsson AH, Perfilyev A, Hansen NS, Schrölkamp M, Strasko KS, Scheele C, Ribel-Madsen R, Mortensen B, Jørgensen SW, Ling C, Vaag A. Epigenetic programming of adipose-derived stem cells in low birthweight individuals. Diabetologia 59: 2664–2673, 2016. doi: 10.1007/s00125-016-4099-9. [DOI] [PubMed] [Google Scholar]
- 11.Buechler C, Krautbauer S, Eisinger K. Adipose tissue fibrosis. World J Diabetes 6: 548–553, 2015. doi: 10.4239/wjd.v6.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45: 115–120, 2008. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 353: 2093–2099, 1999. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 14.Crowell KT, Steiner JL, Coleman CS, Lang CH. Decreased whole-body fat mass produced by chronic alcohol consumption is associated with activation of S6K1-mediated protein synthesis and increased autophagy in epididymal white adipose tissue. Alcohol Clin Exp Res 40: 1832–1845, 2016. doi: 10.1111/acer.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings TJ, Hulette CM, Bigner SH, Riggins GJ, McLendon RE. Ham56-immunoreactive macrophages in untreated infiltrating gliomas. Arch Pathol Lab Med 125: 637–641, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 16.de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. J Gastroenterol Hepatol 27, Suppl 2: 33–41, 2012. doi: 10.1111/j.1440-1746.2011.07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodd T, Simon L, LeCapitaine NJ, Zabaleta J, Mussell J, Berner P, Ford S, Dufour J, Bagby GJ, Nelson S, Molina PE. Chronic binge alcohol administration accentuates expression of pro-fibrotic and inflammatory genes in the skeletal muscle of simian immunodeficiency virus-infected macaques. Alcohol Clin Exp Res 38: 2697–2706, 2014. doi: 10.1111/acer.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duplanty AA, Siggins RW, Allerton T, Simon L, Molina PE. Myoblast mitochondrial respiration is decreased in chronic binge alcohol administered simian immunodeficiency virus-infected antiretroviral-treated rhesus macaques. Physiol Rep 6: e13625, 2018. doi: 10.14814/phy2.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duplanty AA, Simon L, Molina PE. Chronic binge alcohol-induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus-infected rhesus macaques at end-stage disease. Alcohol Alcohol 52: 298–304, 2017. doi: 10.1093/alcalc/agw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford SM Jr, Simon L, Vande Stouwe C, Allerton T, Mercante DE, Byerley LO, Dufour JP, Bagby GJ, Nelson S, Molina PE. Chronic binge alcohol administration impairs glucose-insulin dynamics and decreases adiponectin in asymptomatic simian immunodeficiency virus-infected macaques. Am J Physiol Regul Integr Comp Physiol 311: R888–R897, 2016. doi: 10.1152/ajpregu.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliardi C, Bunnell BA. Isolation and culture of rhesus adipose-derived stem cells. Methods Mol Biol 702: 3–16, 2011. doi: 10.1007/978-1-61737-960-4_1. [DOI] [PubMed] [Google Scholar]
- 22.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol 63: 179–186, 2002. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 23.Gamarel KE, Brown L, Kahler CW, Fernandez MI, Bruce D, Nichols S; Adolescent Medicine Trials Network for HIV/AIDS Intervention . Prevalence and correlates of substance use among youth living with HIV in clinical settings. Drug Alcohol Depend 169: 11–18, 2016. doi: 10.1016/j.drugalcdep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gougeon ML, Pénicaud L, Fromenty B, Leclercq P, Viard JP, Capeau J. Adipocytes targets and actors in the pathogenesis of HIV-associated lipodystrophy and metabolic alterations. Antivir Ther 9: 161–177, 2004. [PubMed] [Google Scholar]
- 25.Hadigan C. Insulin resistance among HIV-infected patients: unraveling the mechanism. Clin Infect Dis 41: 1341–1342, 2005. doi: 10.1086/496990. [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong-Brown LQ, Pruznak AM, Frost RA, Vary TC, Lang CH. Indinavir alters regulators of protein anabolism and catabolism in skeletal muscle. Am J Physiol Endocrinol Metab 289: E382–E390, 2005. doi: 10.1152/ajpendo.00591.2004. [DOI] [PubMed] [Google Scholar]
- 28.Hruz PW. HIV protease inhibitors and insulin resistance: lessons from in-vitro, rodent and healthy human volunteer models. Curr Opin HIV AIDS 3: 660–665, 2008. doi: 10.1097/COH.0b013e3283139134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jan V, Cervera P, Maachi M, Baudrimont M, Kim M, Vidal H, Girard PM, Levan P, Rozenbaum W, Lombès A, Capeau J, Bastard JP. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther 9: 555–564, 2004. [PubMed] [Google Scholar]
- 30.Justice A, Sullivan L, Fiellin D; Veterans Aging Cohort Study Project Team . HIV/AIDS, comorbidity, and alcohol: can we make a difference? Alcohol Res Health 33: 258–266, 2010. [PMC free article] [PubMed] [Google Scholar]
- 31.Koethe JR. Adipose tissue in HIV infection. Compr Physiol 7: 1339–1357, 2017. doi: 10.1002/cphy.c160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang CH, Derdak Z, Wands JR. Strain-dependent differences for suppression of insulin-stimulated glucose uptake in skeletal and cardiac muscle by ethanol. Alcohol Clin Exp Res 38: 897–910, 2014. doi: 10.1111/acer.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavebratt C, Almgren M, Ekström TJ. Epigenetic regulation in obesity. Int J Obes 36: 757–765, 2012. doi: 10.1038/ijo.2011.178. [DOI] [PubMed] [Google Scholar]
- 34.Lazo M, Gange SJ, Wilson TE, Anastos K, Ostrow DG, Witt MD, Jacobson LP. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis 45: 1377–1385, 2007. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 35.LeCapitaine NJ, Wang ZQ, Dufour JP, Potter BJ, Bagby GJ, Nelson S, Cefalu WT, Molina PE. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis 204: 1246–1255, 2011. doi: 10.1093/infdis/jir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloreta J, Domingo P, Pujol RM, Arroyo JA, Baixeras N, Matias-Guiu X, Gilaberte M, Sambeat MA, Serrano S. Ultrastructural features of highly active antiretroviral therapy-associated partial lipodystrophy. Virchows Arch 441: 599–604, 2002. doi: 10.1007/s00428-002-0667-0. [DOI] [PubMed] [Google Scholar]
- 37.Lo Re V, Zeldow B, Kallan MJ, Tate JP, Carbonari DM, Hennessy S, Kostman JR, Lim JK, Goetz MB, Gross R, Justice AC, Roy JA. Risk of liver decompensation with cumulative use of mitochondrial toxic nucleoside analogues in HIV/hepatitis C virus coinfection. Pharmacoepidemiol Drug Saf 26: 1172–1181, 2017. doi: 10.1002/pds.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenc A, Ananthavarathan P, Lorigan J, Jowata M, Brook G, Banarsee R. The prevalence of comorbidities among people living with HIV in Brent: a diverse London borough. London J Prim Care (Abingdon) 6: 84–90, 2014. doi: 10.1080/17571472.2014.11493422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallewa JE, Wilkins E, Vilar J, Mallewa M, Doran D, Back D, Pirmohamed M. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother 62: 648–660, 2008. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 40.May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, Chadwick D, Martin F, Hill T, Walsh J, Post F, Fisher M, Ainsworth J, Jose S, Leen C, Nelson M, Anderson J, Sabin C; UK Collaborative HIV Cohort (UK CHIC) Study . Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 28: 1193–1202, 2014. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molina PE, Amedee AM, Veazey R, Dufour J, Volaufova J, Bagby GJ, Nelson S. Chronic binge alcohol consumption does not diminish effectiveness of continuous antiretroviral suppression of viral load in simian immunodeficiency virus-infected macaques. Alcohol Clin Exp Res 38: 2335–2344, 2014. doi: 10.1111/acer.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32: 138–147, 2008. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res 30: 2065–2078, 2006. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 44.Morén C, Bañó M, González-Casacuberta I, Catalán-Garcia M, Guitart-Mampel M, Tobías E, Cardellach F, Pedrol E, Peraire J, Vidal F, Domingo P, Miró Ò, Gatell JM, Martínez E, Garrabou G. Mitochondrial and apoptotic in vitro modelling of differential HIV-1 progression and antiretroviral toxicity. J Antimicrob Chemother 70: 2330–2336, 2015. doi: 10.1093/jac/dkv101. [DOI] [PubMed] [Google Scholar]
- 45.Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology 30: 934–943, 1999. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- 46.Nittala A, Ghosh S, Stefanovski D, Bergman R, Wang X. Dimensional analysis of MINMOD leads to definition of the disposition index of glucose regulation and improved simulation algorithm. Biomed Eng Online 5: 44, 2006. doi: 10.1186/1475-925X-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolan D, Hammond E, Martin A, Taylor L, Herrmann S, McKinnon E, Metcalf C, Latham B, Mallal S. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17: 1329–1338, 2003. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 48.Parfieniuk-Kowerda A, Czaban SL, Grzeszczuk A, Jaroszewicz J, Flisiak R. Assessment of serum IGF-1 and adipokines related to metabolic dysfunction in HIV-infected adults. Cytokine 64: 97–102, 2013. doi: 10.1016/j.cyto.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells 28: 2065–2072, 2010. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 50.Poirier LA, Rachdaoui N, Nagy LE. GLUT4 vesicle trafficking in rat adipocytes after ethanol feeding: regulation by heterotrimeric G-proteins. Biochem J 354: 323–330, 2001. doi: 10.1042/bj3540323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price J, Hoy J, Ridley E, Nyulasi I, Paul E, Woolley I. Changes in the prevalence of lipodystrophy, metabolic syndrome and cardiovascular disease risk in HIV-infected men. Sex Health 12: 240–248, 2015. doi: 10.1071/SH14084. [DOI] [PubMed] [Google Scholar]
- 52.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res 33: 191–205, 2009. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramirez T, Tong M, Chen WC, Nguyen QG, Wands JR, de la Monte SM. Chronic alcohol-induced hepatic insulin resistance and endoplasmic reticulum stress ameliorated by peroxisome-proliferator activated receptor-δ agonist treatment. J Gastroenterol Hepatol 28: 179–187, 2013. doi: 10.1111/j.1440-1746.2012.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasineni K, Casey CA. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol 44: 299–303, 2012. doi: 10.4103/0253-7613.96297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribera E, Paradiñeiro JC, Curran A, Sauleda S, García-Arumí E, Castella E, Puiggròs C, Crespo M, Feijoo M, Diaz M, Del Saz SV, Planas M, Sureda D, Falcó V, Ocaña I, Pahissa A. Improvements in subcutaneous fat, lipid profile, and parameters of mitochondrial toxicity in patients with peripheral lipoatrophy when stavudine is switched to tenofovir (LIPOTEST study). HIV Clin Trials 9: 407–417, 2008. doi: 10.1310/hct0906-407. [DOI] [PubMed] [Google Scholar]
- 56.Samuels R, Bayerri CR, Sayer JA, Price DA, Payne BAI. Tenofovir disoproxil fumarate-associated renal tubular dysfunction: noninvasive assessment of mitochondrial injury. AIDS 31: 1297–1301, 2017. doi: 10.1097/QAD.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 58.Simon L, Ford SM Jr, Song K, Berner P, Vande Stouwe C, Nelson S, Bagby GJ, Molina PE. Decreased myoblast differentiation in chronic binge alcohol-administered simian immunodeficiency virus-infected male macaques: role of decreased miR-206. Am J Physiol Regul Integr Comp Physiol 313: R240–R250, 2017. doi: 10.1152/ajpregu.00146.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon L, Hollenbach AD, Zabaleta J, Molina PE. Chronic binge alcohol administration dysregulates global regulatory gene networks associated with skeletal muscle wasting in simian immunodeficiency virus-infected macaques. BMC Genomics 16: 1097, 2015. doi: 10.1186/s12864-015-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon L, LeCapitaine N, Berner P, Vande Stouwe C, Mussell JC, Allerton T, Primeaux SD, Dufour J, Nelson S, Bagby GJ, Cefalu W, Molina PE. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am J Physiol Regul Integr Comp Physiol 306: R837–R844, 2014. doi: 10.1152/ajpregu.00502.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon L, Siggins R, Winsauer P, Brashear M, Ferguson T, Mercante D, Song K, Vande Stouwe C, Nelson S, Bagby G, Amedee A, Molina PE. Simian immunodeficiency virus infection increases blood ethanol concentration duration after both acute and chronic administration. AIDS Res Hum Retroviruses 34: 178–184, 2018. doi: 10.1089/aid.2017.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonderup MW, Wainwright HC. Human immunodeficiency virus infection, antiretroviral therapy, and liver pathology. Gastroenterol Clin North Am 46: 327–343, 2017. doi: 10.1016/j.gtc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Souza-Smith FM, Ford SM Jr, Simon L, Molina PE. Repeated binge-like alcohol intoxication: depot-specific adipose tissue immuno-metabolic dysregulation. Shock 48: 243–250, 2017. doi: 10.1097/SHK.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 64.Souza-Smith FM, Siggins RW, Molina PE. Mesenteric lymphatic-perilymphatic adipose crosstalk: role in alcohol-induced perilymphatic adipose tissue inflammation. Alcohol Clin Exp Res 39: 1380–1387, 2015. doi: 10.1111/acer.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srdic D, Khawla AM, Soldatovic I, Nikolic J, Jevtovic D, Nair D, Dragovic G. Correlation of leptin, adiponectin, and resistin levels in different types of lipodystrophy in HIV/AIDS patients. Metab Syndr Relat Disord 15: 153–159, 2017. doi: 10.1089/met.2016.0068. [DOI] [PubMed] [Google Scholar]
- 66.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985) 117: 1170–1179, 2014. doi: 10.1152/japplphysiol.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steiner JL, Lang CH. Alcohol, adipose tissue and lipid dysregulation. Biomolecules 7: E16, 2017. doi: 10.3390/biom7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabo G, Zakhari S. Mechanisms of alcohol-mediated hepatotoxicity in human-immunodeficiency-virus-infected patients. World J Gastroenterol 17: 2500–2506, 2011. doi: 10.3748/wjg.v17.i20.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99: 16309–16313, 2002. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 71.Zhao B, Qiang L, Joseph J, Kalyanaraman B, Viollet B, He YY. Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis 3: 82–87, 2016. doi: 10.1016/j.gendis.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, McClain CJ, Li X, Feng W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J Lipid Res 56: 1481–1491, 2015. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 180: 998–1007, 2012. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]