Abstract
Background:
Several studies, including a randomized controlled trial by our group, support applying anodal tDCS (A-tDCS) to the left hemisphere during behavioral aphasia treatment to improve outcomes. A clear mechanism explaining A-tDCS’s efficacy has not been established, but modulation of neuroplasticity may be involved.
Objective/hypothesis:
The brain-derived neurotrophic factor (BDNF) gene influences neuroplasticity and may modulate the effects of tDCS. Utilizing data from our recently completed trial, we conducted a planned test of whether aphasia treatment outcome is influenced by interaction between A-tDCS and a single-nucleotide polymorphism of the BDNF gene, rs6265.
Methods:
Seventy-four individuals with chronic stroke-induced aphasia completed 15 language therapy sessions and were randomized to receive 1 mA A-tDCS or sham tDCS (S-tDCS) to the intact left temporoparietal region for the first 20 min of each session. BDNF genotype was available for 67 participants: 37 participants had the typical val/val genotype. The remaining 30 participants had atypical BDNF genotype (Met allele carriers). The primary outcome factor was improvement in object naming at 1 week after treatment completion. Maintenance of treatment effects was evaluated at 4 and 24 weeks.
Results:
An interaction was revealed between tDCS condition and genotype for treatment-related naming improvement (F = 4.97, p = 0.03). Participants with val/val genotype who received A-tDCS showed greater response to aphasia treatment than val/val participants who received S-tDCS, as well as the Met allele carriers, regardless of tDCS condition.
Conclusion:
Individuals with the val/val BDNF genotype are more likely to benefit from A-tDCS during aphasia treatment.
Keywords: Aphasia, Stroke, tDCS, Electrical brain stimulation, Rehabilitation, Aphasia treatment
Aphasia is a language problem typically caused by damage to the cortical language network. Even for individuals with relatively mild aphasia, the effects on communication abilities are often profound, and, by extension, affect employment opportunities and overall quality of life. Aphasia is present in approximately 30% of all cases of acute stroke [1], and it is estimated that over two million longterm stroke survivors are living with chronic aphasia in the United States [2]. Most people who persist with chronic aphasia never fully recover, especially those with moderate or severe impairment. Although the incidence of stroke is declining among older adults, the overall prevalence of stroke is not decreasing, as stroke incidence is increasing among younger adults, and life expectancy is increasing [3]. Consequently, the societal effects of aphasia are increasing as younger individuals are likely to live longer with aphasia compared to those who acquire aphasia at a later age.
The standard of care for aphasia is behavioral speech-language treatment (aphasia treatment), which is usually administered by a speech-language pathologist in a one-on-one setting. The effectiveness of aphasia treatment is supported by randomized controlled trials (RCT) and meta-analyses of treatment studies [1]. A recent RCT showed that three weeks of speech-language treatment compared to deferred treatment improves verbal communication (Cohen’s d = 0.58) and communication quality of life (Cohen’s d = 0.21) in chronic aphasia [4]. Although these data are encouraging, the effect sizes are somewhat small, which may result in only modest improvements in many cases. Therefore, it is crucial to find ways to improve the effectiveness of aphasia treatment.
One method that has shown promising results in enhancing the effects of aphasia treatment is transcranial direct current stimulation (tDCS). The first study to combine aphasia treatment and A-tDCS found that 1 mA stimulation targeting the left frontal lobe improved the effect of naming treatment over sham tDCS (S-tDCS) immediately at the conclusion of treatment and at one week-posttreatment [5]. A follow-up study using A-tDCS applied to posterior regions of the left hemisphere during aphasia treatment resulted in improved naming reaction times compared to S-tDCS [6]. A-tDCS applied to the left motor cortex during aphasia treatment was also shown to result in greater improvement in naming of trained and untrained items at 6 months after treatment completion [7]. Notably, the treatment effect generalized to improvements in functional communication.
Most of the early studies on A-tDCS and aphasia included only a small number of participants [5,6,8–12]. This was the motivation for the much larger and more definitive double-blinded RCT comparing the outcome of three weeks of aphasia treatment (5 days/week) in a group of chronic post-stroke participants with aphasia who were randomized to receive A-tDCS (N = 34) or S-tDCS (N = 40) [13]. This study utilized a futility design: the null hypothesis stated that A-tDCS results in better aphasia treatment outcome than S-tDCS, whereas the alternative hypothesis stated that there is no evidence that A-tDCS is superior to S-tDCS. The primary analysis was adjusted for aphasia severity using the Aphasia Quotient (AQ) from the Western Aphasia Battery, Revised (WAB-R [14]). The results of this RCT were: participants who received A-tDCS, compared to those who received S-tDCS, had large improvements in naming of trained and untrained items at oneweek (70% higher), 4 weeks- (79% higher), and 24 weeks- (110% higher) post treatment. Crucially, the futility hypothesis was not supported by the data, suggesting that further study of A-tDCS to enhance aphasia treatment is warranted.
Along with previous studies of this kind, our RCT suggests AtDCS may enhance the effects of aphasia treatment. Although we believe these findings are promising, very little is known about why adjunctive A-tDCS boosts the effect of the behavioral treatment. A considerable body of evidence suggests aphasia treatment leads to both functional and structural changes in the cortex [15–20]. One mechanism by which A-tDCS may work is by modulating brain plasticity; for example, at the level of synaptic activity [21,22]. Relying on a mouse model, a study by Fritsch et al. [21] showed that anodal direct current stimulation (DCS) administered for 15 min paired with simultaneous synaptic activation lead to an increase in postsynaptic potentials in M1 slices. This increase lasted at least 2 h beyond the termination of the DCS. Crucially, Fritsch et al.’s study showed that activity-dependent secretion of brain derived neurotrophic factor (BDNF) is necessary for long-term synaptic plasticity induced by DCS.
BDNF is encoded by the BDNF gene, and a common singlenucleotide polymorphism (SNP), rs6265 (also called val66met or val/met), is associated with an 18e30% reduction in secretion of BDNF in humans [23]. Among Caucasians, the ratio of typical BDNF genotype (val/val) to atypical BDNF genotype (val/met or met/met; i.e., “Met allele carriers”) is approximately 65/35 (see Petryshen et al. [24] on proportion of rs6265 in different ethnic and racial groups). The presence of the Met allele is associated with poorer memory performance [25,26] and decreased plasticity of the motor cortex [27,28]. In stroke patients, the Met allele has been associated with poorer motor rehabilitation outcome in patients with moderate-mild impairment [29], poorer learning of a novel motor task [30], as well as lower ipsilateral cortical brain activation [31]. If A-tDCS enhances synaptic plasticity during aphasia treatment, it is straightforward to suggest that individuals with typical BDNF genotype on SNP rs6265 (val/val) would benefit more from adjuvant A-tDCS during aphasia treatment compared to those with atypical BDNF genotype.
The purpose of the current study was to test whether response to A-tDCS to preserved left hemisphere temporo-parietal regions during aphasia treatment is BDNF genotype specific. The data presented here were collected as part of the aforementioned RCT trial on A-tDCS and aphasia treatment [13]. We hypothesized that an interaction would exist between tDCS condition (A-tDCS vs. StDCS) and BDNF genotype (typical BDNF genotype [val/val] vs. atypical BDNF genotype [val/met or met/met]). Specifically, we expected that participants with typical BDNF genotype receiving AtDCS would respond better to aphasia treatment (demonstrate greater improvements in object naming post-therapy) compared to those with i) typical BDNF genotype who received S-tDCS, and ii) participants with atypical genotype (Met allele carriers), regardless of tDCS condition. In addition, based on studies that suggest BDNF genotype influences overall learning [25,28,29,32], we expected a main effect of BDNF genotype on aphasia treatment outcome.
1. Methods
Participants:
Seventy-four participants were included in the RCT, which was performed at the University of South Carolina (USC) and at the Medical University of South Carolina (MUSC). BDNF genotype was available for 67 participants due to lost or contaminated sample (6 participants) or decline in blood draw (1 participant). One participant with typical BDNF genotype (val/val) discontinued the study before completing the treatment phase. Therefore, BDNF genotype and post-treatment assessment data were available for 66 participants. For greater detail on the background, methods, and results of the primary outcome of the RCT, readers are referred to Fridriksson et al. [13].
BDNF genotyping:
During each participant’s first session for neuroimaging and behavioral testing, 2 mL of whole blood were collected. The blood sample was labeled with each participant’s deidentified study number and frozen. All samples were sent in one batch to DNA Genotek (https://www.dnagenotek.com/), where DNA extraction and SNP genotyping was completed on rs6265. Blood samples were extracted using Qiagen PureGene reagents and a validated extraction protocol. Genotyping for the SNP was accomplished using a TaqMan® single tube genotyping assay. The TaqMan assay is an allele discrimination assay using PCR amplification and a pair of fluorescent dye detectors that target the SNP. One fluorescent dye is attached to the detector that is a perfect match to the first allele (e.g. an “A” nucleotide) and a different fluorescent dye is attached to the detector that is a perfect match to the second allele (e.g. a “C” nucleotide). During PCR, the polymerase releases the fluorescent probe into solution where it is detected using endpoint analysis in a Life Technologies, Inc. (Foster City, CA) 7900HT Real-Time instrument. Primers and probes were obtained through Life Technologies design and manufacturing. Genotypes were determined using Life Technologies’ Taqman Genotyper v1.0.1 software. Participants with a val/val (e.g. “C/C00) expression were considered typical BDNF genotype and those with val/met (e.g. “C/A”) or met/met (e.g. “A/A”) were considered to have atypical BDNF genotype. Samples were then destroyed as per protocol.
Aphasia treatment and tDCS:
The aphasia treatment consisted of a computerized picture-word matching task, administered 5 times/ week for three weeks [33]. For each trial, participants viewed a picture of an everyday object, followed by an audiovisual model of a speaker saying a word (only the mouth of the speaker was displayed [33]). The participant then indicated, via button press, whether the audiovisual input matched the picture displayed (i.e., pressing a green button for correct matches and a red button for incorrect matches). The treatment paradigm included 160 trials, composed of low-, medium-, and high-frequency words. Immediate feedback was provided following each trial so participants could self-monitor their performance. Treatment session duration was approximately 45 min. Two tests of naming were selected as the primary outcome measure: 1. A picture naming test consisting of 80 items selected from the aphasia treatment paradigm; for the sake of brevity, we refer to this test as ‘Naming 80; ‘ and 2. The Philadelphia Naming Test (PNT), which is also a picture naming test and is widely used in the literature [34]. The PNT includes 175 ‘untreated’ items and potential participants were excluded from study enrollment if they correctly named more than 140 pictures at baseline (to allow room for treatment-related improvement). Both the Naming 80 and PNT were administered twice at baseline, on two separate days, and then twice at each of the following post-treatment time points: 1 week, 4 weeks, and 24 weeks (also on two separate days). Here, we report results combined for trained items (Naming 80) and untrained items (PNT) for a total of 255 items. The proportional improvement in naming was calculated as pre-to-post treatment changes in naming divided by the potential improvement at baseline. Specifically, the number of correctly named items at baseline was subtracted from the number of correctly named items after treatment. Then, this difference was divided by the difference between the number of items named correctly at baseline and possible maximum score on the tests, 255 For example, if a participant named, on average, 55 items at baseline and then named 105 items at 1 week post-treatment, her proportional improvement was calculated as: (105–55)/(255–55) = 0.25.
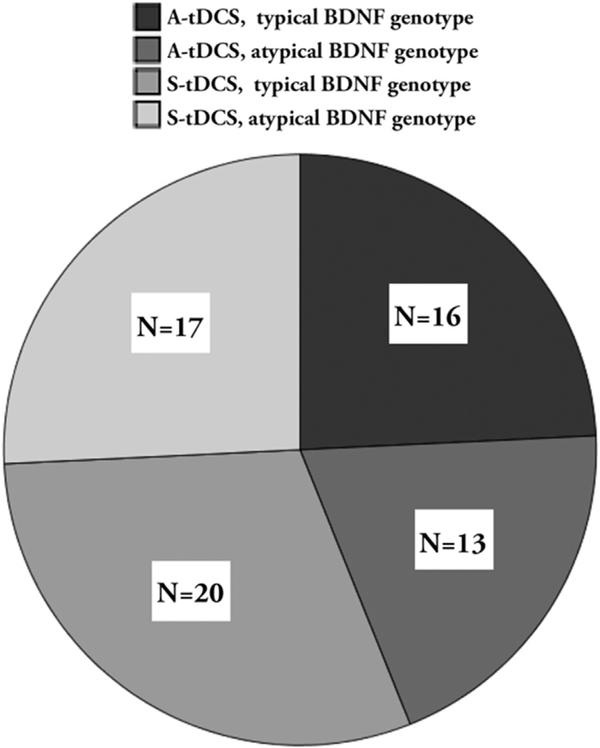
At the beginning of the behavioral task, participants received 20 min of A-tDCS or S-tDCS, depending on their group assignment at randomization. Participants were randomized to tDCS condition using a randomization algorithm that utilized minimal sufficient balancing to ensure that participants at both testing sites (USC and MUSC) were balanced by baseline age, as well as aphasia type and severity. Among the 66 participants whose genotype was analyzed, 29 were randomized to receive A-tDCS and 37 were randomized to receive S-tDCS (Fig. 1). A relationship was not revealed between the distributions of genotype and race, X2=3.2, p = 0.20. This was a double-blind study - all investigators, clinicians (including those who scored the outcome measures), study coordinators, and participants were blind to tDCS condition assignment.
Fig. 1.
Distribution of participants across genotype and tDCS conditions. Frequency for each sub-group is shown in the box within each quadrant of the pie chart.
A constant current stimulator (Phoresor® II PM850; Iomed® Inc., Salt Lake City, Utah) was used for A-tDCS application. In-house hardware was used to mask treatment type so A-tDCS and S-tDCS setups did not differ in appearance. All participants were fitted with 5 5 cm saline soaked sponges (electrodes) at two sites: i) the anode site was the location of the left scalp that was coregistered to the brain region with the highest naming-related activation from a baseline fMRI (for more details on the fMRI setup see Ref. [35]), and ii) the cathode was always placed on the right supraorbital forehead region. The mean location of stimulation was in the temporoparietal junction. Mean coordinate locations (MNI coordinates, X,Y,Z in mm) for each stimulation group were as follows: A-tDCS: −49, −50, 15; SD = 10, 19, 19; S-tDCS: −50, 50, 11; SD = 10, 19, 19; and mean coordinate location across all participants was 40, 50, 12; SD = 9, 18, 18. Participants in the active condition received AtDCS for the first 20 min of the 45-min language treatment session. To blind participants who received S-tDCS to condition, a brief scalp sensation (30 s, with a gradual decrease over 15 s) was induced for all participants at the beginning of the language treatment session. tDCS setup and treatment procedures were identical for all participants, regardless of A-tDCS or S-tDCS. Complete details are provided in Fridriksson et al. [13].
Statistical analyses:
Descriptive statistics and two-sample t-tests were used to compare BDNF genotype status for participant characteristics at baseline. A mixed effects linear model of the repeated measures of proportional change in correct naming (calculated as described above) was fit with the following independent variables: tDCS condition (A-tDCS or S-tDCS), BDNF genotype status (typical or atypical), tDCS x BDNF, time of testing (1 week post-, 4 weeks post-, 24 weeks post-treatment), tDCS x time, BDNF Genotype x tDCS x time, baseline WAB-AQ, and lesion size (time was considered a classification variable using SAS v 9.4 PROC MIXED repeated time/AR(1)). As revealed in Fridriksson et al. [13], WAB-AQ was associated with treatment outcome, in that participants with milder aphasia tended to improve more on the naming outcome tests (p = 0.01). Therefore, we included WAB-AQ as a cofactor in the mixed models.
2. Results
Among the 66 participants included in this analysis, 36 (55%) had typical BDNF genotype and 30 (45%) had atypical BDNF genotype, carrying the met allele. Racial distribution was as follows: 57 Caucasian, 7 African-American, and 2 Asian. Twenty participants were women and the mean age was 59.8 (SD: 10.2; range: 30–77). The mean education level was 14.8 years (SD: 2.46; range: 10e20) and the mean time post-stroke was 43.3 months (SD: 41.1; range: 6.1e204.9). Baseline neuropsychological testing included the following: WAB-R [14] to determine aphasia type and severity (AQ mean: 58.9; SD: 19.9; range: 20.1–93.7); National Institutes of Health Stroke Scale to assess overall stroke severity (NIHSS; mean: 5; SD: 3; range: 1–14); Pyramids and Palm Trees test [36] to assess semantic processing (PPTT; mean: 46.3/52; SD: 3.94; range: 34–52); and matrix sub-test on the Wechsler Adult Intelligence Scale-3rd Edition to test executive functioning [37](WAIS III; mean: 11.9; SD: 5.4; range: 3–23).
Atypical BDNF genotype was associated with more severe language impairment at baseline (Table 1). Aphasia severity was considerably milder in the participants with typical BDNF as demonstrated by a mean WAB-AQ that was more than 12 points higher than their counterparts who had atypical BDNF. The atypical BDNF participants also scored significantly lower on the Naming 80 and PNT, indicating that they had more severe anomia than typical BDNF participants. In contrast, no performance differences were found between the groups on the PPTT, the matrix subtest of the WAIS III, or in stroke severity as indexed by NIHSS score. Similarly, no group differences were found for other factors such as age, lesion size, education, or time post stroke.
Table 1.
Baseline data for participants with typical or atypical BDNF genotype. A two-sided t-test was used to compare the two groups on each baseline behavioral test, age, years of education, and lesion size. Significant group differences are highlighted by bolded text for the p-values in the last column.
| Baseline Characteristic | Typical BDNF genotype (val/val) N = 36 |
Atypical BDNF (val/met, met/met) N = 30 |
Two sided p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Lower 95% CI | Upper 95% CI | Mean | SD | Lower 95% CI | Upper 95% CI | ||
| WAB-R AQ | 63.5 | 18.6 | 57.2 | 69.8 | 52.2 | 19.3 | 45 | 59.4 | 0.01 |
| Naming 80 (treated items) | 23.0 | 18.0 | 16.9 | 29.1 | 14.4 | 15 | 8.8 | 20 | 0.03 |
| Philadelphia Naming Test | 69.3 | 45.0 | 54.1 | 84.5 | 48.6 | 38 | 34.4 | 62.8 | 0.04 |
| PNT þ Naming 80 | 92.3 | 62.4 | 71.2 | 113.4 | 63.0 | 52.4 | 43.4 | 82.5 | 0.04 |
| PPTT Total Score | 46.6 | 3.4 | 45.4 | 47.7 | 46.1 | 4.6 | 44.4 | 47.8 | 0.65 |
| WAIS III Matrix Score | 12.4 | 5.8 | 10.5 | 14.4 | 11.6 | 4.9 | 9.7 | 13.4 | 0.63 |
| NIH Stroke Scale | 4.8 | 2.9 | 3.8 | 5.8 | 5.2 | 3.4 | 4 | 6.5 | 0.55 |
| Age | 61.9 | 9.2 | 58.8 | 65.0 | 57.3 | 11.1 | 53.2 | 61.5 | 0.07 |
| Years of Education | 14.8 | 2.1 | 14.1 | 15.5 | 14.9 | 2.8 | 13.9 | 16 | 0.74 |
| Time post stroke (months) | 50.3 | 42.8 | 35.9 | 64.8 | 35.5 | 38.9 | 21.0 | 50.0 | 0.16 |
| Lesion Size | 118.5 | 62.8 | 97.3 | 139.8 | 141.6 | 84 | 119 | 172 | 0.17 |
When adjusting for time post stroke and lesion size the main effect of BDNF genotype on WAB-R AQ and PNT+Naming 80 is no longer statistically significant (p = 0.11, p = 0.17, respectively).
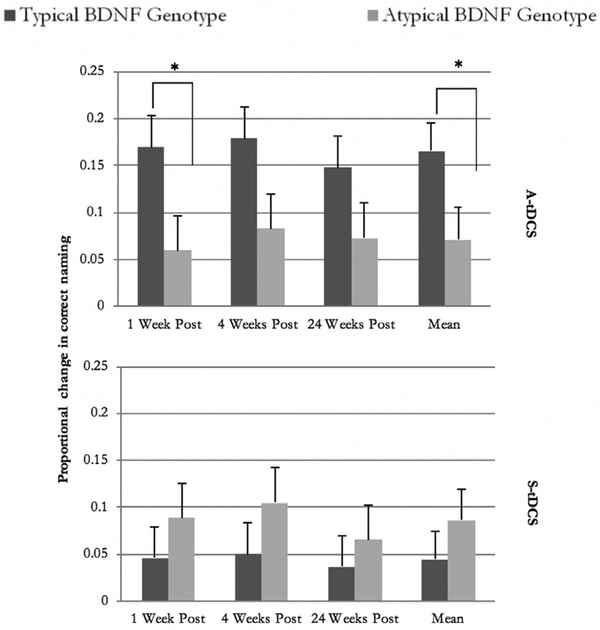
Based on the repeated measures mixed model, an interaction between BDNF genotype and tDCS condition was revealed for naming improvement (Table 2). Participants with typical BDNF genotype who received A-tDCS during the aphasia treatment improved more than those with typical BDNF genotype but received S-tDCS (Fig. 2). The participants with typical BDNF genotype who received A-tDCS also improved more than participants with atypical genotype, regardless of tDCS condition.
Table 2.
Repeated Measures Model of Mean proportional improvement: to address the primary study question regarding interaction between BDNF genotype and tDCS condition when adjusting for baseline aphasia severity (WAB-R AQ) and lesion size.
| Tests of Fixed Effects from Repeated Measures Mixed Effect model | ||||
|---|---|---|---|---|
| Effect | Numerator DF | Denominator DF | F Value | Two-sided p-value |
| Genotype | 1 | 60 | 0.71 | 0.40 |
| tDCS condition | 1 | 60 | 3.1 | 0.08 |
| Genotype × tDCS | 1 | 60 | 4.97 | 0.03 |
| Time of testing | 2 | 120 | 2.5 | 0.08 |
| tDCS × Time of testing | 2 | 120 | 0.08 | 0.92 |
| Genotype × tDCS × Time of testing | 4 | 120 | 0.36 | 0.84 |
| Baseline WAB-R AQ | 1 | 60 | 15.09 | 0.0003 |
| Lesion Size | 1 | 60 | 0.78 | 0.3803 |
Fig. 2.
Mean proportional improvement (y-axis) at 1 week, 4 weeks, and 24 weeks (x-axis) after treatment completion for subgroups who had typical or atypical BDNF genotype and received either A-tDCS or S-tDCS. Means are adjusted for WAB-R AQ at baseline, lesion size, time, BDNF, tDCS, BDNF x time, tDCS x time and BDNF x time x tDCS. The error bars denote standard error of the adjusted means. Asterisks indicate statistically significant difference (p < 0.05).
Moreover, a main effect was not found for BDNF genotype or tDCS condition (Table 2). In contrast, a main effect of time of followup testing was observed. The proportional improvement was fairly stable over time. Interaction effects were not revealed for tDCS condition vs. time of testing or a three-way interaction between BDNF genotype, tDCS condition, and time of testing.
3. Discussion
The analyses revealed two significant effects. First, there was an unexpected baseline difference, such that individuals with typical BDNF tended to have milder symptoms. Second, we found the predicted interaction, where individuals who had both typical BDNF as well as A-tDCS demonstrated better improvement.
A baseline difference in language performance between carriers of typical or atypical BDNF gene was an unexpected finding as others have not revealed similar results. A study by de Boer et al. [38] found neither baseline nor overall recovery differences in a group of 53 patients with stroke induced aphasia. However, there was one crucial difference between their research and the current study: Baseline examination of the patients in the de Boers et al. study occurred within one-month post-stroke and aphasia treatment commenced immediately thereafter. If BDNF genotype influences recovery in aphasia, it is possible that its effects are too subtle to be detected so soon after stroke. Specifically, had de Boers et al. [38] followed their patients several years post stroke, it is possible that the effects of BDNF genotype may have been detected. In the current study, the average time post-stroke at enrollment was greater than 3.5 years. The difference in activity dependent BDNF secretion by BDNF genotype may be too subtle (18e30%) to be detected except on a relatively long timescale. This point is supported by the current data where a main effect was not detected for BDNF genotype on the outcome of aphasia treatment dispensed over three weeks.
Although baseline differences in aphasia severity were revealed between the two BDNF genotype groups, no differences were noted on the NIHSS, a measure of overall stroke severity. In this context, it is important to note that among the participants included here, aphasia was the most pronounced stroke impairment measured on the NIHSS, in most cases. In fact, the primary inclusion criterion was presence of aphasia and potential participants were excluded if their naming abilities were too mild in order to avoid ceiling effects on the dependent measures. Language impairment is only assessed on one of the 11 items that comprise the NIHSS. Therefore, the NIHSS may not have been sensitive enough to detect group differences among participants whose most common behavioral impairment was aphasia. If we assume the two genotype groups were similar with regard to aphasia severity at the time of stroke, a difference in recovery of more than 12 AQ points constitutes a fairly pronounced effect of BDNF genotype. In most cases, experienced clinicians would probably notice an AQ difference of 12 points between patients and, we speculate, that its impact would probably surpass a ‘minimum clinically significant difference.’ However, because the participants included here were not followed since the time of their stroke, we cannot definitively state that those aphasia severity differences reflect genotype differences. Although not statistically significant, the group with atypical BDNF genotype had numerically larger cortical damage and was not as far post-stroke, which are factors that may relate to long-term outcome [39]. However, those same participants also tended to be younger than those with typical BDNF genotype, which indicates a more favorable recovery from stroke and aphasia [40–42]. It should be noted that the baseline difference on language measures was not statistically significant once group means were adjusted for time post-stroke and lesion size, which suggests these findings may reflect other potential differences besides BDNF genotype. However, the non-significance for adjusted means does not discount the potential role of BDNF genotype in long-term language recovery and suggests that further investigation of this issue is warranted in a more targeted study. It is also important to note that we did not compare levels of depression between participants with typical and atypical BDNF genotype. In a rat model of stroke, differences in levels of BDNF protein secretion have been reported between individuals with and without depression [43]. Accordingly, future studies may need to consider assessing depression as a potential factor relating to group differences in participants with typical and atypical BDNF genotype.
Among participants who were randomized to receive A-tDCS during the aphasia treatment, those with typical BDNF genotype benefitted more from the language treatment than those with atypical BDNF genotype. As was stated earlier, atypical BDNF genotype is associated with lower levels of activity dependent BDNF secretion. Therefore, it is possible that A-tDCS boosts the effect of aphasia treatment by modulating long-term synaptic plasticity, a process that relies on BDNF secretion. Accordingly, participants who received A-tDCS but had atypical BDNF genotype did not respond as well to aphasia treatment because of lower production of BDNF. A comparison between the two genotypes among participants who received S-tDCS did not reveal a difference in treatment outcome. In fact, among the participants who received S-tDCS, those with typical BDNF genotype actually improved less than participants with atypical BDNF genotype, although this difference was not statistically significant. These findings are in agreement with what Fritsch et al. [21] found in a mouse model: A-tDCS has a neuroplastic effect that is BDNF dependent.
So far, one other study has examined BDNF and tDCS in aphasia treatment outcome [44]. Marangolo and colleagues [44] tested whether tDCS, both anodal and cathodal, administrated during language treatment, modulated serum BDNF levels in seven participants with chronic aphasia. Although a positive effect of tDCS was found for aphasia treatment outcome, no changes in serum levels of BDNF were detected. If these findings are confirmed in larger studies, it could mean that A-tDCS does not actually alter the secretion of BDNF. Rather, it could be the case that the neuroplastic effect of A-tDCS is dependent on baseline levels of BDNF secretion; accordingly, participants in the A-tDCS group who have typical BDNF genotype and higher levels of BDNF secretion benefit more from the aphasia treatment than their counterparts who have atypical BDNF genotype and lower levels of BDNF secretion. This would also explain why participants in the S-tDCS group benefitted less from the aphasia treatment. However, Marongolo et al. tested serum levels of BDNF but did not verify BDNF genotype in their participants. Given that this study only included seven participants, it is possible that their null result was driven by including a significant proportion of participants with atypical BDNF genotype. Therefore, we cannot adjudicate whether the beneficial effect of AtDCS seen in the current study is driven by baseline levels of BDNF or whether A-tDCS actually modulates the levels of BDNF. At the very least, our study provides motivation to examine this issue in greater detail in a targeted experiment.
Studies that have applied A-tDCS during aphasia treatment have targeted several different cortical regions, including the left inferior frontal gyrus [10,45], motor cortex [7], and posterior language cortex [6,11,46]. If, as our data may suggest, A-tDCS promotes BDNF driven neuroplasticity, our study provides strong evidence that modulation of residual language cortex promotes aphasia recovery. Compared to the right hemisphere or left hemisphere regions outside of the language network, we believe that plastic changes in residual language cortex promote the best aphasia treatment outcome. A relatively large body of research has focused on changes in cortical activity and structure associated with aphasia treatment outcome [15–20]. Most of these studies have focused on single cases or relatively small group of participants. However, the largest studies in this area [16,47] suggest that preservation and modulation of residual language cortex is crucial for better aphasia treatment outcome. Along with the current study, these findings represent emerging evidence that improved lexical-semantic processing associated with aphasia treatment is driven by changes in residual language cortex in the left hemisphere. Moreover, the current results provide a clear cortical candidate to target in future studies and, if supported by further data, in clinical practice.
Acknowledgemtnts
This work was supported by: National Institute on Deafness and Other Communication Disorders (PI Fridriksson: U01 DC011739). Co-author AB supported by NIH T32 DC014435 (trainee).
Footnotes
Conflicts of interest
The authors report no conflicts of interest with this work.
References
- [1].Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Libr 2016;6 https://doi.org/10.1002/14651858.CD000425.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simmons-Mackie N Aphasia in North America. Moorestown NJ: Aphasia Access; 2018. [Google Scholar]
- [3].Ramirez L, Kim-Tenser MA, Sanossian N, Cen S, Wen G, He S, et al. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc 2016;5(5), e003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Breitenstein C, Grewe T, Flöel A, Ziegler W, Springer L, Martus P, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017;389(10078):1528e38. [DOI] [PubMed] [Google Scholar]
- [5].Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 2010;41(6):1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke 2011;42(3):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meinzer M, Darkow R, Lindenberg R, Flöel A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016;139(4):1152–63. [DOI] [PubMed] [Google Scholar]
- [8].Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, et al. Speech facilitation by left inferior frontal cortex stimulation. Curr Biol 2011;21(16): 1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Flöel A, Meinzer M, Kirstein R, Nijhof S, Deppe M, Knecht S, et al. Short-term anomia training and electrical brain stimulation. Stroke 2011;42(7):2065–7. [DOI] [PubMed] [Google Scholar]
- [10].Marangolo P, Marinelli C, Bonifazi S, Fiori V, Ceravolo M, Provinciali L, et al. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res 2011;225(2):498–504. [DOI] [PubMed] [Google Scholar]
- [11].Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J Cognit Neurosci 2011;23(9):2309–23. [DOI] [PubMed] [Google Scholar]
- [12].Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- [13].Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after strokeA randomized clinical trial. JAMA Neurol 2018. https://doi.org/10.1001/jamaneurol.2018.2287. Published online August 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kertesz A Western aphasia battery-revised. San Antionio, TX: Pearson; 2007. [Google Scholar]
- [15].Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci 2010;30(35): 11558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage 2012;60(2):854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McKinnon ET, Fridriksson J, Glenn GR, Jensen JH, Helpern JA, Basilakos A, et al. Structural plasticity of the ventral stream and aphasia recovery. Ann Neurol 2017;82(1):147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nenert R, Allendorfer JB, Martin AM, Banks C, Ball A, Vannest J, et al. Neuroimaging correlates of post-stroke aphasia rehabilitation in a pilot randomized trial of constraint-induced aphasia therapy. Med Sci Mon Int Med J Exp Clin Res: Int Med J Exp Clin Res 2017;23:3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang 2014;136:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mohr B, MacGregor LJ, Difrancesco S, Harrington K, Pulvermüller F, Shtyrov Y. Hemispheric contributions to language reorganisation: an MEG study of neuroplasticity in chronic post stroke aphasia. Neuropsychologia 2016;93: 413–24. [DOI] [PubMed] [Google Scholar]
- [21].Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010;66(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci 2014;37(12): 742–53. [DOI] [PubMed] [Google Scholar]
- [23].Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006;314(5796):140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatr 2010;15(8):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 2003;23(17):6690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ho B-C, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brainderived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatr 2006;63(7): 731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci 2006;9(6):735. [DOI] [PubMed] [Google Scholar]
- [28].McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Crespo LM, Procaccio V, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cerebr Cortex 2009;20(5):1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shiner CT, Pierce KD, Thompson-Butel AG, Trinh T, Schofield PR, McNulty PA. BDNF genotype interacts with motor function to influence rehabilitation responsiveness poststroke. Front Neurol 2016;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Helm EE, Tyrell CM, Pohlig RT, Brady LD, Reisman DS. The presence of a singlenucleotide polymorphism in the BDNF gene affects the rate of locomotor adaptation after stroke. Exp Brain Res 2016;234(2):341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim DY, Quinlan EB, Gramer R, Cramer SC. BDNF Val66Met polymorphism is related to motor system function after stroke. Phys Ther 2016;96(4):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lamb YN, McKay NS, Thompson CS, Hamm JP, Waldie KE, Kirk IJ. Brainderived neurotrophic factor Val66Met polymorphism, human memory, and synaptic neuroplasticity. Wiley Interdiscipl Rev: Cognit Sci 2015;6(2):97e108. [DOI] [PubMed] [Google Scholar]
- [33].Fridriksson J, Baker JM, Whiteside J, Eoute D Jr, Moser D, Vesselinov R, et al. Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke 2009;40(3):853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: scoring and rationale. Clin Aphasiology 1996;24:121–34. [Google Scholar]
- [35].Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebr Cortex 2010;20(5):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Howard D, Patterson K. The Pyramids and Palm Trees Test: a test of semantic access from words and pictures. Cambridge: Thames Valley Test Company; 1992. [Google Scholar]
- [37].Wechsler D Wechsler adult intelligence scaleeThird Edition (WAISeIII). San Antonio, TX: NCS Pearson; 1997. [Google Scholar]
- [38].de Boer RG, Spielmann K, Heijenbrok-Kal MH, van der Vliet R, Ribbers GM, van de Sandt-Koenderman WME. The role of the BDNF Val66Met polymorphism in recovery of aphasia after stroke. Neurorehabilitation Neural Repair 2017;31(9):851–7. [DOI] [PubMed] [Google Scholar]
- [39].Ellis C, Dismuke C, Edwards KK. Longitudinal trends in aphasia in the United States. NeuroRehabilitation 2010;27(4):327–33. [DOI] [PubMed] [Google Scholar]
- [40].Johnson L, Basilakos A, Yourganov G, Cai B, Bonilha L, Rorden C, et al. Natural history of aphasia in the chronic stages after a stroke. [submitted for publication].
- [41].Laska A, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med 2001;249(5):413–22. [DOI] [PubMed] [Google Scholar]
- [42].Holland AL, Greenhouse JB, Fromm D, Swindell CS. Predictors of language restitution following stroke: a multivariate analysis. J Speech Lang Hear Res 1989;32(2):232–8. [DOI] [PubMed] [Google Scholar]
- [43].Ifergane G, Boyko M, Frank D, Shiyntum HN, Grinshpun J, Kuts R, et al. Biological and behavioral patterns of post-stroke depression in rats. Can J Neurol Sci 2018:1–11. [DOI] [PubMed] [Google Scholar]
- [44].Marangolo P, Fiori V, Gelfo F, Shofany J, Razzano C, Caltagirone C, et al. Bihemispheric tDCS enhances language recovery but does not alter BDNF levels in chronic aphasic patients. Restor Neurol Neurosci 2014;32(2):367–79. [DOI] [PubMed] [Google Scholar]
- [45].Marangolo P, Fiori V, Calpagnano M, Campana S, Razzano C, Caltagirone C, et al. tDCS over the left inferior frontal cortex improves speech production in aphasia. Front Hum Neurosci 2013;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Richardson J, Datta A, Dmochowski J, Parra LC, Fridriksson J. Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. NeuroRehabilitation 2015;36(1):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Griffis JC, Nenert R, Allendorfer JB, Szaflarski JP. Linking left hemispheric tissue preservation to fMRI language task activation in chronic stroke patients. Cortex 2017;96:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]