Abstract
The female ventromedial hypothalamic nucleus (VMN) is a focal substrate for estradiol (E) regulation of energy balance, feeding, and body weight, but how E shapes VMN gluco-regulatory signaling in each sex is unclear. This study investigated the hypothesis that estrogen receptor-alpha (ERα) and/or -beta (ERβ) control VMN signals that inhibit [γ-aminobutyric acid] or stimulate [nitric oxide, steroidogenic factor-1 (SF-1)] counter-regulation in a sex-dependent manner. VMN nitrergic neurons monitor astrocyte fuel provision; here, we examined how these ER regulate astrocyte glycogen metabolic enzyme, monocarboxylate transporter, and adrenoreceptor protein responses to insulin-induced hypoglycemia (IIH) in each sex. Testes-intact male and E-replaced ovariectomized female rats were pretreated by intracerebroventricular ERα antagonist (MPP) or ERβ antagonist (PHTPP) administration before IIH. Data implicate both ER in hypoglycemic inhibition of neuronal nitric oxide synthase protein in each sex and up-regulation of glutamate decarboxylase65/67 and SF-1 expression in females. ERα and -β enhance astrocyte AMPK and glycogen synthase expression and inhibit glycogen phosphorylase in hypoglycemic females, while ERβ suppresses the same proteins in males. Differential VMN astrocyte protein responses to IIH may partially reflect ERα and -β augmentation of ERβ and down-regulation of alpha1, alpha2, and beta1 adrenoreceptor proteins in females, versus ERβ repression of GPER and alpha2 adrenoreceptor profiles in males. MPP or PHTPP pretreatment blunted counter-regulatory hormone secretion in hypoglycemic males only, suggesting that in males one or more VMN neurotransmitters exhibiting sensitivity to forebrain ER may passively regulate this endocrine outflow, whereas female forebrain ERα and -β are apparently uninvolved in these contra-regulatory responses.
Keywords: Ventromedial hypothalamic nucleus, estrogen receptor, neuronal nitric oxide synthase, glycogen synthase, 5-adenosine monophosphate-activated protein kinase, glucagon
Introduction:
The brain consumes a disproportionate amount of bodily energy in order to execute vital nerve cell functions. Iatrogenic insulin-induced hypoglycemia (IIH) is an unremitting complication of requisite rigorous therapeutic management of insulin-dependent diabetes mellitus [Cryer et al., 2003; Cryer, 2010]. By depriving the brain of an adequate energy fuel supply, IIH poses a significant risk for neurological dysfunction and injury. The central neural network that maintains glucostasis is continuously appraised of cellular energy paucity by specialized metabolic sensors positioned in the brain and periphery, and responds to those cues by activating coordinated contra-regulatory autonomic, neuroendocrine, and behavioral functions. The ventromedial hypothalamic nucleus (VMN) integrates nutrient, endocrine, and neurochemical signals of metabolic state to shape contra-regulatory responses to IIH [Watts and Donovan, 2010, Donovan and Watts, 2014]. VMN metabolic-sensory neurons increase (‘fuel-inhibited’) or decrease (‘fuel-excited’) synaptic firing when ambient substrate fuel levels decline [Oomura et al., 1969; Ashford et al., 1990; Silver and Erecinska, 1998]. VMN detection of neuro-energetic shortage is obligatory for optimal counter-regulatory hormone and gluconeogenic responses to IIH [Borg et al., 1997; 2003]. In male rats, VMN neurotransmitter effectors of local and extrinsic metabolic-sensory readout likely include γ-aminobutyric acid (GABA), which inhibits pancreatic glucagon and adrenomedullary catecholamine release during IIH [Chan et al., 2006], and signals that intensity counter-regulatory hormone secretion, e.g. nitric oxide (NO) and steroidogenic factor-1 (SF-1) [Fioramonti et al., 2011; Routh et al., 2014]. SF-1 is also implicated in neural regulation of energy expenditure in both sexes [Choi et al., 2013; Kinyua et al., 2016]. The role of these neurochemicals in counter-regulatory outflow in the female remains unclear.
Estradiol (E) regulation of energy balance involves multiple systemic mechanisms, such as governance of energy intake (including meal size and frequency), fuel storage, and energy expenditure [Wade and Schneider, 1992; Richard D, 1986; Dagnault et al., 1996; Asarian and Geary, 2002; Laudenslager et al., 1980; Wade and Gray, 1979]. E influences glucostasis by controlling carbohydrate intake and metabolism, glucose tolerance, and hepatic gluconeogenesis and glycogenesis [Ahmed-Sorour and Bailey, 1980; Bailey and Ahmed-Sorour, 1980; Wurtman and Baum, 1980; Ahmed-Sorour and Bailey, 1981; Lenzen and Bailey, 1984]. Insulin and counter-regulatory hormone, e.g. glucagon, epinephrine, and corticosterone secretion is regulated by E [Ahmed-Sorour and Bailey, 1980; Faure et al., 1988; Komesaroff et al., 1988; Adams et al., 2005; Briski and Nedungadi, 2009]. The classical estrogen nuclear receptors estrogen receptor-alpha (ERα) and -beta (ERβ) occur in both common and distinctive sites in the hypothalamus. The VMN and paraventricular hypothalamic nucleus reportedly contain only ERα or -β mRNA, respectively, whereas both receptor variant transcripts are expressed in arcuate and dorsomedial nuclei and lateral hypothalamic area [Shughrue et al., 1997]. This project utilized pharmacological tools alongside high-resolution microdissection/high-sensitivity molecular analytical techniques to address the premise that ERα and/or -β control VMN gluco-regulatory neuron reactivity to IIH in a sex-dependent manner. Circulating E levels vary significantly over the rat estrous cycle [Butcher et al., 1974]. Here, female rats were ovariectomized (OVX) and implanted with E-releasing capsules to achieve uniformity of plasma E levels among subjects at metestrus-like levels. E-treated OVX female and testes-intact male rats were pretreated by lateral ventricular administration of the selective ERα antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), the ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), or vehicle prior to subcutaneous insulin injection. VMN tissue obtained by micropunch dissection was analyzed by Western blot for protein markers of GABAergic [glutamate decarboxylase65/67 (GAD65/67)], nitrergic [neuronal nitric oxide synthase (nNOS)], SF-1, and brain-derived neurotrophic factor (BDNF) neuron function. Sex-specific patterns of VMN BDNF responses to IIH were evaluated here as this neurochemical coordinates metabolic interactions between neurons and astrocytes [Ishii et al., 2018]. The current rationale for delivery of ER antagonists into the cerebral ventricular system as opposed to the VMN was to achieve drug access to ER expressed in the VMN as well as forebrain metabolic structures that innervate the VMN [Bouret, 2017].
In the brain, conversion of glucose to energy occurs within astrocyte and nerve cell compartments. Glucose is taken up from the circulation into the astrocyte cell compartment, where it is catabolized to the oxidizable fuel L-lactate for transport to support neuronal aerobic respiration [Pellerin et al., 2007; Pellerin and Magistretti, 2012]. Glial (MCT1)-and neuron (MCT2)-specific monocarboxylate transporters (MCT) transfer lactate between these cell types [Broer et al., 1997]. Astrocytes maintain an energy reserve in the form of the complex glucose polymer glycogen, involving incorporation or release of glycosyl residues by action of glycogen synthase (GS) and glycogen phosphorylase (GP) activity, respectively [Stobart and Anderson, 2013]. A significant fraction of glucose in the astrocyte compartment is actively cycled through the glycogen store, e.g. glycogen shunting, prior to breakdown by glycolysis [Walls et al., 2009; Schousboe et al., 1020]. Ventromedial hypothalamic lactoprivation is a stimulus for counter-regulatory outflow as local lactate infusion reduces glucagon and catecholamine secretion during IIH [Borg et al., 2003], by mechanisms involving intensified GABAergic transmission [Chan et al., 2006]. Recent studies show that pharmacological inhibition of VMN GP activity increases activity of the ultra-sensitive energy sensor 5’ AMP-activated protein kinase (AMPK) and nNOS expression in the male rat VMN, implying that astrocyte glycogen-derived fuel stream may affect neuro-metabolic stability in this critical gluco-regulatory structure [Alhamami et al., 2018a]. The current study examined the premise that forebrain ERα and/or -β exert sex-specific effects on astrocyte glycogen metabolic enzyme and astrocyte-and nerve cell-specific MCT protein expression during IIH.
A correlated objective of the present project was to characterize expression profiles of classical and membrane ER in VMN astrocytes in each sex in vivo, and to determine how IIH may impact these receptor proteins. VMN astrocytes were identified in situ by glial fibrillary acid protein immuno-labeling for individual collection by laser-catapult microdissection from the VMN of male and female rats pretreated with MPP, PHTPP, or vehicle prior to IIH, and cell lysates were analyzed by Western blot for ERα, ERβ, and G protein-coupled estrogen receptor 1 (GPER) [Micevych and Kelly, 2012] protein expression. This technological means to procure pure astrocyte cell samples also enabled investigation here of sex-specific reactivity of astrocyte AMPK, as opposed to whole-VMN AMPK, to IIH [Tamrakar and Briski, 2015]. Lastly, glycogen metabolism in cortical astrocytes is regulated in vitro by norepinephrine (NE). Those cells respond directly to NE via alpha1 (α1), alpha2 (α2) and beta1 (β1) adrenoreceptors (AR) [Hertz et al., 2010], and undergo glycogenolysis upon beta AR stimulation [Fillenz et al., 1999; Dong et al., 2012]. In this study, VMN astrocyte lysates were evaluated by Western blot to ascertain if α1AR, α2AR, and β1AR proteins are expressed in each sex, and determine if one or more receptor profiles are modified in a sex-specific manner during IIH.
Materials and Methods:
Experimental Design:
Adult male and female Sprague Dawley rats (3–4 months of age) were housed in individual shoe-box cages, containing Aspen Sani chip bedding (Envigo, Houston, TX), under a 14 hr light/10 hr dark cycle (lights on at 05.00 h). Animals were provided standard laboratory chow and tap water ad-libitum, and acclimated to daily handling prior to experimentation. All surgical and experimental protocols were conducted in accordance with NIH guidelines for care and use of laboratory animals, and approved by the ULM Institutional Animal Care and Use Committee. On day 1, animals of each sex were anesthetized with ketamine/xylazine (0.1 mL/100 g bw; 90 mg ketamine:10 mg xylazine/mL; Henry Schein Inc., Melville, NY), and implanted with an PE-20 cannula aimed at the the left lateral ventricle (LV) [Singh and Briski, 2005] at the following coordinates: 0.0 mm posterior to bregma; 1.5 mm lateral to bregma; 5.0 mm ventral to skull surface. While under anesthesia, females were also bilaterally OVX. After surgery, animals were injected subcutaneously (sc) with ketoprofen (1 mg/kg body weight) and intramuscularly with enrofloxacin (10 mg/0.1 mL), treated by topical 0.25% bupivacaine to closed incisions, and transferred to individual cages. On day 7, female rats were anesthetized with isoflurane prior to sc implantation of a silastic capsule (i.d. 0.062/in. o.d. 0.125 in.; 10 mm/100 g bw) containing 30 ug 17β estradiol-3-benzoate/mL safflower oil. This steroid replacement regimen yields approximate plasma E concentrations of 22 pg/ml [Briski et al., 2001], replicating circulating hormone levels characteristic of metestrus in 4-day cycling animals [Butcher et al., 1974]. At 08.45 hr on day 10, male rats were divided into four treatment groups, and injected to the LV with the vehicle dimethyl sulfoxide (V) (groups 1 and 2; n=6/group), the ERα antagonist MPP (10 µM/200 nL [Briski and Shrestha, 2016]; Tocris/Bio-Techne Corp., Minneapolis, MN) (group 3; n=6), or the ERβ antagonist PHTPP (10 µM/200 nL [Briski and Shrestha, 2016]; Tocris) (group 4; n=6) [Table 1]. At 9:00 hr on day 10, animals in group 1 were injected sc with sterile insulin diluent (V; Eli Lilly & Co., Indianapolis, IN); at the same time, groups 2–4 were treated by injection of neutral protamine Hagedorn insulin (INS; 10.0 U/kg bw; Butler Schein Animal Health, Dublin, OH). Rats were sacrificed at 10:00 hr for trunk blood and brain collection. On day 10, groups of E-implanted OVX female rats (n=6/ group) were similarly pretreated at 8.45 hr by LV administration of V (groups 1 and 2), MPP (group 3), or PHTPP (group 4), injected at 09.00 hr with V (group 1) or 10 U INS/kg bw (groups 2–4), and then sacrificed at 10.00 hr [Table 1]. Brains were individually snap-frozen in liquid nitrogen-cooled isopentane for storage at −80°C. Plasma was stored at −20°C.
Table 1.
Experimental design.
| Male | Female | ||||
|---|---|---|---|---|---|
| Icv Pretreatment | Sc Injection | Icv Pretreatment | Sc Injection | ||
| Group 1 | V-Icv (n = 6) | V-Sc (n = 6) | Group 1 | V-Icv (n = 6) | V-Sc (n = 6) |
| Group 2 | V-Icv (n = 6) | INS-Sc (n = 6) | Group 2 | V-Icv (n = 6) | INS-Sc (n = 6) |
| Group 3 | MPP-Icv (n = 6) | INS-Sc (n = 6) | Group 3 | MPP-Icv (n = 6) | INS-Sc (n = 6) |
| Group 4 | PHTPP-Icv (n = 6) | INS-Sc (n = 6) | Group 4 | PHTPP-Icv (n = 6) | INS-Sc (n = 6) |
Icv: intracerebroventricular, INS: neutral protamine Hagedorn insulin, 10.0 U/kg bw, MPP (ERα antagonist): 11,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride; 10 μM/200 nL, PHTPP (ERβ antagonist): 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl] phenol; 10 μM/200 nL.
VMN Tissue Microdissection and Analysis:
Forebrains were cut into alternating series of 100 µm-or 10 µm-thick frozen sections over the length of the VMN, over alternating distances of 200 µm (2 × 100 μm sections) and 120 μm (12 × 10 μm thin sections), respectively. For each animal, VMN tissue was bilaterally micropunch-dissected from thick sections using calibrated hollow needle tools (Stoelting Co., Wood Dale, IL) and collected into lysis buffer (2% sodium dodecyl sulfate [SDS], 0.05 M dithiothreitol, 10% glycerol, 1 mM EDTA, 60 mM Tris-HCl, pH 7.2) for heat denaturation. For each treatment group, tissue aliquots from individual subjects were combined for protein separation in Bio-Rad Stain-Free 10–12% gradient acrylamide gels (Hercules, CA); proteins were subsequently transblotted to 0.45-µm PVDF-Plus membranes (Osmonics, Gloucester, MA) [Shakya et al., 2018]. Membranes were blocked with Tris-buffered saline, pH 7.4, containing 0.1 % Tween-20 and 2% bovine serum albumin prior to 24–48 hour incubation with primary antisera. Proteins of interest were probed with polyclonal antibodies raised in rabbit against nNOS (1:1,000; prod. mo. sc-648; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), GAD65/67 (1:2,000; prod. no. AB1511; EMD Millipore, Billerica, MA), BDNF (1:2,000; prod. no. NBP1–46750; Novus Biologicals, Littleton, CO), SF1 (1:2,000; prod. no. PA5–41967l; ThermoFisherScientific, Rockford, IL MCT1 (1;1,000; prod. no. AB3540P; EMD Millipore), or GS (1:2,000; prod. no. 3893S; Cell Signaling Technology, Danvers, MA), or in goat against GP (1:1,000; prod. no. sc 46347; Santa Cruz Biotechnol.) or MCT2 (1:1,000; prod. no. sc-14926; Santa Cruz Biotechnol.). After primary incubation, membranes were sequentially exposed to horseradish peroxidase-labeled goat anti-rabbit (1:5,000; prod. no. NEF812001EA; PerkinElmer, Waltham, MA) or rabbit anti-goat (1:5,000; prod. no. AP106P; EMD Millipore, Billerica, MA) secondary antisera, and SuperSignal West Femto maximum sensitivity chemiluminescent substrate (prod. no. 34096; ThermoFisherScientific). Signals were visualized in a ChemiDoc MP Imaging System (Bio-Rad). Total in-lane protein and chemiluminescence band optical density (O.D.) values were determined densitometrically using BioRad Image Lab 6.0.0 software. Protein bands were normalized to total protein content of their respective lane. Immunoblots were performed in triplicate at minimum for each protein of interest. Bio-Rad precision plus protein dual color standards (prod. no. 161–0374) were included in each Western blot analysis.
For each animal, thin (10 µm-thick) sections were mounted on PEN membrane-coated slides (Carl Zeiss Microscopy, LLC, Thornwood, NY) for immunocytochemical labeling of the astrocyte marker protein glial fibrillary acid protein (GFAP) [Tamrakar et al., 2014, 2015; Tamarkar and Briski, 2015]. Briefly, tissues were fixed with acetone, blocked with 1.5% normal horse serum (prod. no. S-2000, Vector Laboratories, Burlingame, CA), then incubated with a mouse monoclonal antiserum against the astrocyte marker protein glial fibrillary acidic protein (1:500; prod. no. 3670S; Cell Signaling Technol.). Sections were next exposed, in the following order, to Vectastain Elite ABC-HRP mouse IgG kit reagents, e.g. biotinylated horse anti-mouse IgG secondary antibody and ABC reagent (prod. no. PK-6101; Vector Lab.). Labeled cells were visualized using Vector DAB peroxidase substrate kit reagents (prod. no. SK-4100; Vector Lab.). A Zeiss P.A.L.M. UV-A microlaser IV was used to sequentially circumdissect and eject individual GFAP-ir-positive astrocytes from tissue sections into microcentrifuge tubes containing lysis buffer, as described in the above referenced work. Individual target proteins were probed in triplicate astrocyte pools (n=50 astrocytes per treatment group) at minimum using Stain-Free technology. Primary antisera were raised in rabbit against AMPKα1,2 (1:2,000; prod. no. 2532S; Cell Signaling Technol.), ERβ (1:2,000; prod. no. NB120–3577; Novus Biol.), α1AR (1:2,000; prod. no. NB100–78585; Novus Biol.), α2AR (1:2,000; prod. no. NBP2–22452; Novus Biol.), or GPER (1:2,000; prod. no. NLS 4271; Novus Biol.), in mouse against pAMPKα1/2 (Thr 172; 1:2,000; prod. no. 2535S; Cell Signaling Technol.) or ERα (1:2,000; prod. no. NB300560; Novus Biol.), or in goat against β1AR (1:2,000; prod. no. NB600–978; Novus Biol.). Protein molecular weight markers were included in each Western blot analysis.
Blood Glucose and Plasma Hormone Measurements:
Blood glucose was measured with an ACCU-CHECK Aviva Plus glucometer (Roche Diagnostics USA, Indianapolis, IN), as described [Kale et al., 2006]. Plasma glucagon and corticosterone concentrations were determined using ELISA kit reagents (EZGLU-30K, EMD Millipore; ADI-900–097; Enzo Life Sciences, Inc., Farmingdale, NY).
Statistics:
Mean circulating glucose, glucagon, and corticosterone levels, and normalized protein O.D. data were analyzed between groups in each sex by a one-way ANOVA and Duncan’s multiple range test, using Graph pad prism 5.0 and IBM SPSS Statistics 22.0. Differences of p <0.05 were considered significant. Graphical representation was constructed using Sigma plot 10.0.3.
Results:
A foremost objective of the present study was to investigate whether ERα-and/or -β exert sex-dimorphic effects on VMN metabolic effector nerve cell function during IIH. Figure 1 depicts effects of LV administration of MPP or PHTPP on hypoglycemic-associated patterns of VMN GAD65/67, nNOS, SF-1, and BDNF protein expression in E-implanted OVX female versus testes-intact male rats. IIH caused significant augmentation of GAD65/67in the female (Panel A left [F(3,8)=9.31; p=0.0005]), but not male (Panel A right [F(3,8)=3.77; p=0.032]) rat VMN relative to V-injected controls [V/INS versus V/V]; this sex-specific response was prevented by ERα or -β antagonism [MPP/INS or PHTPP/INS versus V/INS]. VMN nNOS content was diminished in female (Panel B left [F(3,8)=16.49; p<0.0001]) and male (Panel B right [F(3,8)=9.98; p=0.0006]) hypoglycemic rats as a consequence of ERα-and -β activity. SF-1 protein profiles were increased in hypoglycemic females (Panel C left [F(3,8)=10.10; p=0.0002], but not males (Panel C right [F(3,8)=4.68; p=0.01]), and normalized in INS-injected females by pretreatment with either MPP or PHTPP. IIH stimulated BDNF by ER-independent mechanisms in female rats (Panel D left [F(3,8)=8.42; p<0.003); this profile was refractory to hypoglycemia in males (Panel D right [F(3,8)=3.62; p=0.036]). ERα, but not ERβ antagonism amplified hypoglycemia-associated levels of SF-1 and BDNF expression in male rats [MPP/INS versus V/INS].
Figure 1. Effects of Lateral Ventricular (LV) Pretreatment of the ERα Antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP) or ERβ Antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) on Ventromedial Hypothalamic Nucleus (VMN) Metabolic Neurotransmitter Marker Protein Responses to Insulin-Induced Hypoglycemia (IIH) in Female Versus Male Rats.
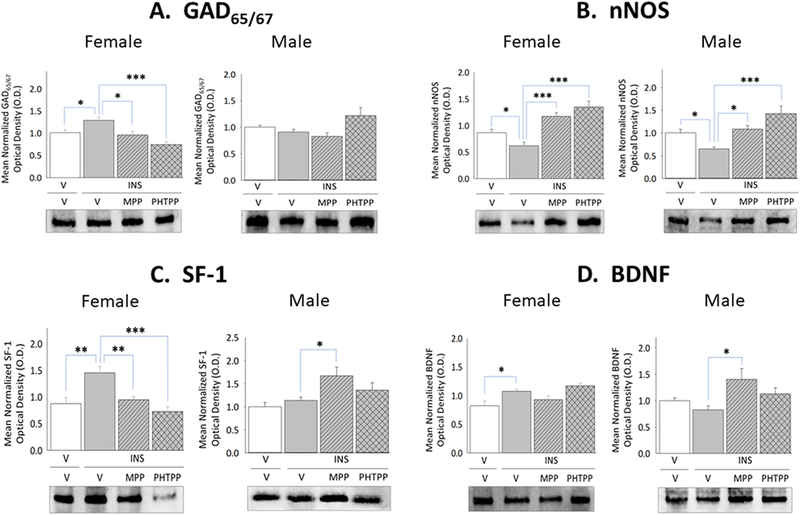
Micropunch-dissected VMN tissue was obtained from groups of estradiol (E) – implanted ovariectomized (OVX) female and testes-intact male rats pretreated by LV administration of MPP, PHTPP, or vehicle prior to sc insulin (INS) injection for Western blot analysis of glutamate decarboxylase65/67 (GAD65/67) [Panel A; female data at left, male data at right], neuronal nitric oxide synthase (nNOS) [Panel B; female data at left, male data at right], steroidogenic factor-1 (SF-1) [Panel C; female data at left, male data at right], and brain-derived neurotrophic factor (BDNF) [Panel D; female data at left, male data at right] expression. Data depict for each sex mean normalized protein optical density (O.D.) values ± S.E.M. for vehicle-pretreated animals injected sc with vehicle-(solid white bars; n=6) or INS (solid gray bars; n=6) or INS-injected rats pretreated with MPP (diagonal-striped gray bars; n=6) or PHTPP (cross-hatched gray bars; n=6). *p<0.05; **p<0.01; **p<0.001.
Current research also examined whether forebrain ERα and/or -β exert differential effects on astrocyte glycogen metabolic enzyme and astrocyte-and nerve cell-specific MCT protein profiles during IIH. Data in Figure 2 illustrate effects of IIH, initiated after ER antagonist versus vehicle pretreatment, on VMN GS, GP, MCT1, and MCT2 protein profiles in female and male rats. Females exhibited up-regulated GS (Panel A left [F(3,8)=6.44; p<0.003]) and inhibition of GP (Panel B left [F(3,8)=23.1; p=0.003]) protein expression during hypoglycemia, responses that were each prevented by MPP or PHTPP pretreatment. In hypoglycemic male rats, GS (Panel A right [F(3,8)=22.60; p<0.0001]) and GP (Panel B right) [F(3,8)=16.139; p<0.0001] proteins were down-regulated owing to ERβ activity. IIH caused PHTPP-reversible augmentation of VMN MCT1 protein levels in hypoglycemic females (Panel C left [F(3,8)=10.95; p<0.003]), but inhibited MCT2 profiles in males (Panel D right [F(3,8)=44.8; p<0.0001]) via ERα-dependent mechanisms. Figure 3 depicts levels of VMN astrocyte AMPK and Pampk protein expression in male and female rats during hypoglycemia. Total AMPK protein and pAMPK levels were both reduced in laser-microdissected astrocytes obtained from INS-injected male rats (Panel A right [F(3,8)=5.21; p=0.008]) (Panel B right [F(3,8)=11.11; p=0.0003]); however, both profiles were amplified in cells from hypoglycemic females (Panel A left [F(3,8)=17.60; p<0.0001]) (Panel B left [F(3,8)=53.22; p<0.0001]).
Figure 2. Effects of MPP or PHTPP Pretreatment on VMN Glycogen Metabolic Enzyme and Cell Type-Specific Monocarboxlyate Transporter Protein Expression in Hypoglycemic Female and Male Rats.
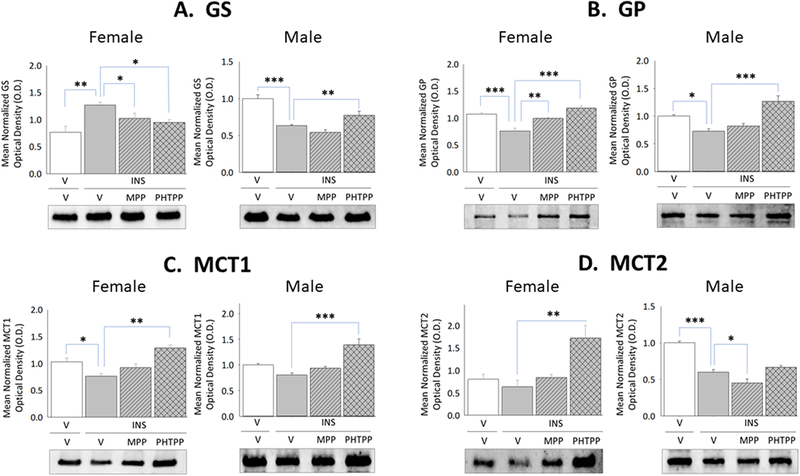
Data show for OVX + E female and testes-intact male rats mean normalized VMN glycogen synthase (GS) [Panel A; female data at left, male data at right], glycogen phosphorylase (GP) [Panel B; female data at left, male data at right], astrocytic monocarboxylate transporter-1 (MCT1) [Panel C; female data at left, male data at right], and neuronal monocarboxylate transporter-2 (MCT2) [Panel D, female data at left, male data at right] protein O.D. measures ± S.E.M. for treatment groups consisting of vehicle-pretreated eu-and hypoglycemic rats and ER antagonist-pretreated hypoglycemic animals. *p<0.05; **p<0.01; **p<0.001.
Figure 3. Effects of MPP or PHTPP Pretreatment on VMN Astrocyte 5’-AMP-Activated Protein Kinase (AMPK) and Phospho-AMPK (pAMPK) Protein Content in Hypoglycemic Female and Male Rats.
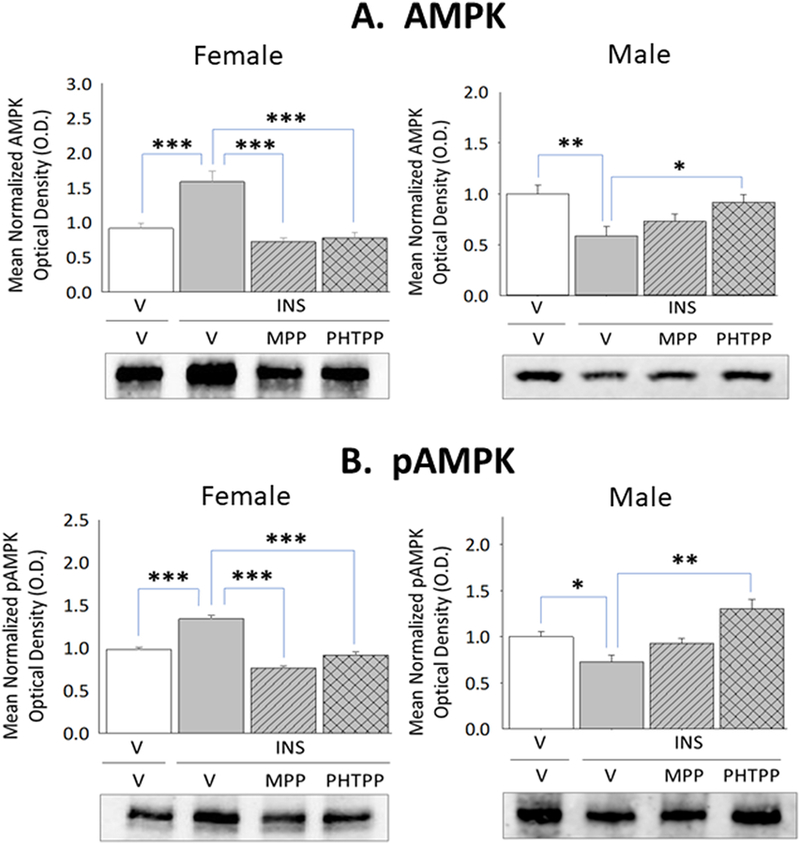
Glial fibrillary acidic protein (GFAP)-immunopositive astrocytes were laser-microdissected from the VMN of vehicle-pretreated eu-(solid white bars) and hypoglycemic (solid gray bars) animals and MPP-(diagonal-striped bars) or PHTPP (cross-hatched gray bars)-pretreated INS-injected rats for Western blot analysis of AMPK [Panel A; female data at left, male data at right] and pAMPK [Panel B; female data at left, male data at right] protein expression. Data illustrate for each sex mean normalized astrocyte protein O.D. measures ± S.E.M. for each treatment group. *p<0.05; **p<0.01; **p<0.001.
The current study sought to characterize baseline and hypoglycemia-associated expression of VMN astrocyte classical and membrane ER in each sex in vivo. Figure 4 shows hypoglycemia-associated modifications in ERα, ERβ, and GPER protein expression in astrocytes collected from vehicle-versus ER antagonist-pretreated hypoglycemic female and male rats. Data show that astrocyte ERβ protein expression was up-regulated by ERα and ERβ – dependent mechanisms (Panel B left [[F(3,8)=9.68; p=0.002]), whereas ERα (Panel A left [F(3,8)=8.62; p=0.025]) and GPER (Panel C left [F(3,8)=3.81; p=0.04]) protein profiles were insensitive to hypoglycemia in females. Astrocytes from hypoglycemic male rats exhibited ERβ-mediated inhibition of GPER content (Panel C right [F(3,8)=50.33; p<0.0001]), alongside stabilized levels of ERα (Panel A right [F(3,8)=21.60; p<0.0001]) and ERβ (Panel B right [F(3,8)=7.59; p=0.003]) expression.
Figure 4. Effects of MPP versus PHTPP on VMN Astrocyte Estrogen Receptor (ER)-Alpha (ERα), ER-Beta (ERβ), and G Protein-Coupled Estrogen Receptor (GPER) Protein Expression in Hypoglycemic Female and Male Rats.
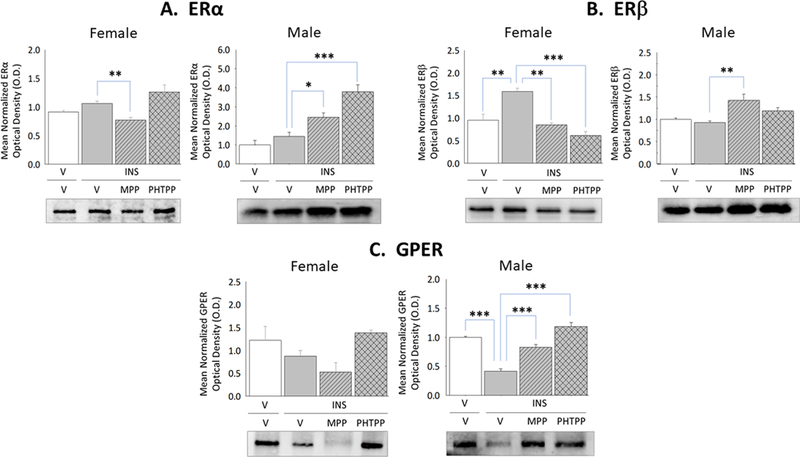
VMN astrocyte lysates were evaluated by Western blot for ERα [Panel A; female data at left, male data at right], ERβ [Panel B; female data at left, male data at right], and GPER [Panel C; female data at left, male data at right] protein expression in groups of vehicle-pretreated eu-(solid white bars) and hypoglycemic (solid gray bars) animals and MPP-(diagonal-striped bars) or PHTPP (cross-hatched gray bars)-pretreated INS-injected rats. Data depict for each sex mean normalized astrocyte protein O.D. measures ± S.E.M. for each treatment group. *p<0.05; **p<0.01; **p<0.001.
Adrenoreceptor protein expression has characterized in cortical but not hypothalamic astrocytes. In this study, VMN astrocyte lysates were evaluated by Western blot to ascertain if α1AR, α2AR, and β1AR proteins are expressed in each sex, and determine if one or more receptor profiles are modified in a sex-specific manner during IIH. Results presented in Figure 5 illustrate effects of forebrain ERα versus ERβ activity on patterns of astrocyte α1AR, α2AR, and β1AR protein expression in INS-injected female and male rats. MPP and PHTPP each blunted hypoglycemic suppression of α1AR (Panel A left [F(3,8)=43.86; p<0.0001]), α2AR (Panel B left [F(3,8)=17.79; p<0.0001]), and β1AR (Panel C left [F(3,8)=143.90; p<0.0001]) protein levels in astrocytes harvested from the female VMN. In contrast, ERβ (Panel B right [F(3,8)=7.59; p=0.002] is implicated in down-regulation of astrocyte α2AR protein levels in hypoglycemic male animals.
Figure 5. Effects of MPP versus PHTPP on VMN Astrocyte Alpha1-Adrenergic Receptor (α1AR), Alpha2-AR (α2AR), and Beta1-AR (β1AR) Protein Expression in Hypoglycemic Female and Male Rats.
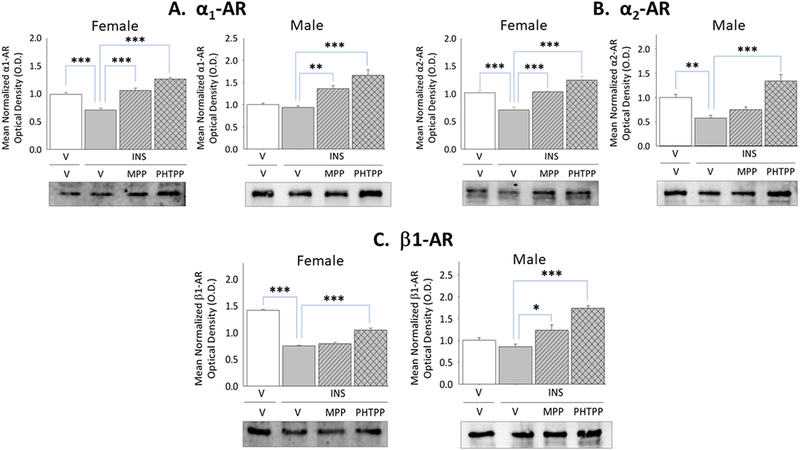
VMN astrocyte lysates were evaluated by Western blot for α1AR [Panel A; female data at left, male data at right], α2AR [Panel B; female data at left, male data at right], and β1AR [Panel C; female data at left, male data at right] protein expression in groups of vehicle-pretreated eu-(solid white bars) and hypoglycemic (solid gray bars) animals and MPP-(diagonal-striped bars) or PHTPP (cross-hatched gray bars)-pretreated INS-injected rats. Data depict for each sex mean normalized astrocyte protein O.D. measures ± S.E.M. for each treatment group. *p<0.05; **p<0.01; **p<0.001.
Current research evaluated the role of forebrain ERα and -β in glycemic and counter-regulatory hormone responses to insulin administration. As shown in Figure 6, Panel A, circulating glucose levels were significantly decreased after INS injection to female (left [F(3,20)=150.80; p<0.0001] and male (right [F(3,20)=108.50; p<0.0001] rats, respectively. Data show that neither MPP nor PHTPP pretreatment altered the magnitude of glucose decline measured at 1 hour after induction of hypoglycemia. Hypoglycemic animals of each sex exhibited elevated glucagon secretion at this time point (Panel B). In male rats (right [F(3,20)=15.12; p=0.0002]), this stimulatory response was completely or partially reversed by corresponding ERα or ERβ blockade; in females, however, neither antagonist caused significant change in this hormone profile (left [F(3,20)=12.24; p<0.0006]). Plasma corticosterone levels were elevated in male rats at +1 hr after INS treatment, but were decreased at the same time point in female rats (Panel C). In INS-injected males (right [F(3,20)=22.36; p=0.001]), PHTPP prevented hypercorticosteronemia, whereas MPP attenuated output of this hormone. Conversely, patterns of corticosterone secretion in hypoglycemic female rats were refractory to ERα or ERβ antagonism (left [F(3,20)=7.51; p=0.01] ).
Figure 6. Impact of MPP or PHTPP Pretreatment on Insulin-Induced Hypoglycemia and Counter-Regulatory Hormone Secretion in Female versus Male Rats.
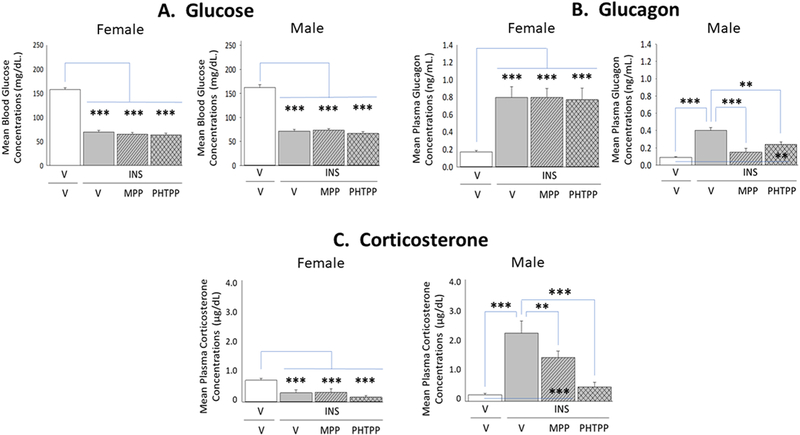
Data show circulating glucose [Panel A; female data at left, male data at right], glucagon [Panel B; female data at left, male data at right], and corticosterone [Panel C; female data at left, male data at right] levels in groups of groups of vehicle-pretreated eu-(solid white bars) and hypoglycemic (solid gray bars) animals and MPP-(diagonal-striped bars) or PHTPP (cross-hatched gray bars)-pretreated INS-injected rats. Bars depict for each sex mean concentrations ± S.E.M. for each treatment group.
Discussion:
The present study utilized selective ERα and -β antagonists as pharmacological tools to explore how these forebrain ER variants regulate, in each sex, hypoglycemic patterns of VMN transmitter signaling purported to inhibit or enhanced glucose counter-regulation. Within one hour after INS injection, both ERα and -β act to suppress nNOS expression in males and females, while ERβ up-regulates GAD65/67 and SF-1 profiles in the female VMN. These receptors thus likely promote, at least over the acute hypoglycemic time frame investigated here, a degree of metabolic stability within the VMN that may vary according to sex. There remains a critical need to determine if post-INS injection patterns of gluco-regulatory signaling observed here indeed reflect a positive gain in energy state, and if so, to characterize the molecular mechanisms that mediate that outcome. Recent studies show that VMN gluco-stimulatory nitrergic neurons respond astrocyte glycogen-derived fuel supply [Alhamami et al., 2018a]. Evidence here for sex-specific adjustments in GS (females: increased; males: decreased) and sex-unrelated down-regulation GP profiles in hypoglycemic animals suggests that ERα and -β may together stimulate astrocyte fuel storage in females, whereas ERβ acts to inhibit glycogen shunt activity and spare glycogen breakdown soon after induction of this metabolic stress. Further research is needed to determine if these sex-distinctive ER-driven glial protein responses to hypoglycemia are controlled by astrocyte AMPK and involve ER regulation of astrocyte receptivity to NE. Evidence for blunted glucagon and corticosterone secretion in hypoglycemic male rats pretreated with MPP or PHTPP establishes forebrain ERα and -β stimulation of this hormone outflow, but VMN GABA-and nitrergic neurons likely do not mediate that positive action. Surprisingly, outcomes show that forebrain ER antagonism does not modify either counter-regulatory hormone profile in females, suggesting that observed receptor actions on VMN substrates exert a local, rather than systemic impact.
At the outset of this work, we predicted that IIH would reduce VMN GAD65/67, while enhancing nNOS protein expression, and presumed that the magnitude of those adjustments might differ in male versus female rats. Yet, current outcomes reveal that nNOS levels are decreased in hypoglycemic animals of each sex, alongside GAD up-regulation in INS-injected females. This unexpected evidence for likely suppressed production of the counter-regulatory stimulus NO implies that a state of metabolic sufficiency exists within the VMN for at least one hour after hypoglycemia induction, despite blood glucose decline. Prior findings of elevated VMN nNOS profiles in males 2 hr after administration of a similar INS dosage [Alhamami et al., 2018a] imply that such stability is transient, at least in males. Decrements in VMN NO signaling owing to decreased nNOS enzyme activity may likely reflect, in part, a reduction in AMPK activation state, which is governed by adjustments in cellular AMP/ATP ratio; however, the possibility that NO signaling may provide a readout of additional metabolic parameters cannot be overlooked. Further research is justified to examine whether down-regulated nNOS expression here signifies a positive gain in energy state, and to characterize the molecular mechanisms that bolster metabolic stability within the current acute time frame. The premise of enhanced VMN positive energy balance in females at +1 hr post-INS injection is bolstered by evidence for elevated GAD65/57 expression. Lactate abundance is correlated with heightened GABAergic signaling in recurrent hypoglycemic male rats [Chan et al., 2013]. Observations here of suppressed MCT1 expression in hypoglycemic females imply that astrocyte lactate release may be similarly diminished, suggesting that up-regulated GAD expression in these animals may occur independently of lactate provision. Additional work will be needed to establish whether metabolic parameters that regulate GABAergic signaling vary by sex and over time after onset of hypoglycemia. In male rats, ventromedial hypothalamic GABA transmission is obligatory for optimal counter-regulatory outflow during acute IIH [Chan et al., 2006], and mal-adaptive augmentation of this neurochemical stimulus is implicated in diminished counter-regulatory responses to recurring IIH [Chan et al., 2013]. Present data infer that in males, GABA signaling during acute IIH may be a passive requirement for ideal counter-regulatory function. It remains unclear if ERα and -β are co-expressed in VMN nitrergic neurons in each sex or if instead these neurons are regulated by ER-sensitive upstream neurons, and to establish, similarly, if GABAergic nerve cells in the female VMN are direct or indirect targets for ERβ control during hypoglycemia.
INS-injected female rats exhibited ERβ-dependent augmentation of VMN SF-1 profiles and ER-independent amplification of BDNF expression, whereas both transmitters profiles were refractory to IIH in males. Indeed, we previously reported that SF-1 expression does not differ between eu-and hypoglycemia male rats [Alhamami et al., 2018b]. It remains unclear if SF-1 neurons in the female VMN express ERβ, or respond to ERβ regulation of upstream neurons. SF-1 is implicated in neural control of counter-regulatory hormone secretion and energy expenditure. As present data show that forebrain ERβ are not involved in hypoglycemic patterns of glucagon or corticosterone release in female subjects, SF-1 may be principally involved in the latter metabolic function under current circumstances. Present efforts seek to elucidate how sex-specific patterns of hypoglycemic VMN BDNF signaling may contribute to counter-regulatory function in males versus females, and to characterize the ER-independent mechanisms that up-regulate this neurotransmitter in hypoglycemic females.
IIH produced opposite adjustments in VMN GS expression in hypoglycemic males (down-regulated) versus females (up-regulated) and inhibited GP profiles in each sex; these modifications were promoted by ERα and -β activity in females, but ERβ action alone in males. Current proof that VMN astrocytes express both classical ER implies that these cells are direct substrates for combinatory or singular receptor effects on astrocyte protein responses to hypoglycemia. Sex-dimorphic adjustments in GS profiles, together with down-regulated GP in each sex, support the likelihood of enhanced glycogen amassment (and diversion of glucose away from glycolysis) in female astrocytes over the first hour after IIH onset, versus suppression of glycogen shunt activity in males. As VMN tissue glycogen content was not measured here, this assumption remains speculative. This premise is supported by current evidence for decreased MCT1 or MCT2 protein expression in the hypoglycemic female or male VMN, respectively, outcomes that correlate with corresponding diminution of astrocyte lactate export or uptake. Current data collectively bolster the unique notion that VMN glycogen may be spared over the acute post-hypoglycemia time frame, e.g. +1 hr, examined here. Brain tissue glycogen levels are similarly stable for several hours after initiation of vigorous exercise [Matui et al., 2011]. Contemporary studies show that non-glucose substrates, e.g. glutamate, glutamine, and aspartate via the pyruvate recycling pathway, can be oxidized within distinctive nerve cell populations to delay ATP depletion during glucoprivation [Amaral, 2013]. For example, in vitro studies show that glutamine is a critical energy source to cerebellar granular neurons immediately after glucose removal [Peng et al., 2007]. At present, contributions of the minor energy substrates referenced above to expression patterns of VMN counter-regulatory inhibitor (GABA) and/or enhancer (NO; SF-1) neurotransmitter/biosynthetic enzyme proteins within an acute time frame after induction of hypoglycemia, and the role of NE in potential adjustments in their oxidation rate during hypoglycemia, have yet to be investigated.
Astrocyte total AMPK protein and pAMPK expression were altered in opposite directions in hypoglycemic male (both down-regulated by ERβ) and female (both up-regulated by ERα and -β) rats. Regarding males, a plausible interpretation of decrements in total astrocyte AMPK enzyme protein levels is a commensurate increase in cellular pAMPK/AMPK ratios, i.e. percentage of activated enzyme, which would in turn facilitate kinase inhibition of acetyl Co-A carboxylase (ACC) enzyme conversion of acetyl Co-A to malonyl Co-A. Alternatively, a reduction in total AMPK protein could, depending upon magnitude of decline, eventually limit enzyme mass available for activation by phosphorylation. Phosphorylation is a rapid post-translational modification that produces an appropriate acute response to hypoglycemia, whereas altered total AMPK protein expression may possibly serve as a more protracted adaptive response. It remains unclear how augmentation (in females) versus reductions (in males) in total AMPK expression and phosphorylation described here impacts AMPK regulation of ACC function in each sex during acute IIH. It also remains to be determined if and how sex-specific patterns of astrocyte phosphorylation AMPK may impact glycogen metabolism and lactate export.
Current data confirm that VMN astrocytes in each sex express α1AR, α2AR, and β1AR proteins in vivo, and document sex-dimorphic changes in the former and latter receptor profiles during hypoglycemia. Astrocytes from hypoglycemic females showed a decline in each A-R variant protein, in response to combinatory ERα and -β action, whereas only α2AR was reduced in INS-injected males owing to ERβ signaling. Hertz et al. [2010] note that α2AR up-regulate glycogen synthesis, whereas βAR and α2AR stimulate astrocyte glycogenolysis. Findings here imply that IIH-associated GP protein down-regulation in each sex may reflect, in part, diminished astrocyte α2AR signaling in each sex alongside decreased β1AR action in females, and that suppression of GS profiles in males may be correlated with a reduction in α2AR expression. On the other hand, proof for enhanced GS expression in females despite decreased α2AR signaling suggests that this protein may be up-regulated by non-noradrenergic mechanisms.
Lateral ventricular administration of MPP or PHTPP caused significant attenuation of hypoglycemic patterns of glucagon and corticosterone secretion in male, but not female rats. These results signify that forebrain ERα and -β function is vital for maximal counter-regulatory hormone release in male rats. Present data rule out involvement of VMN GABA-and nitrergic neurons in ER stimulation of these endocrine profiles as neither antagonist modified GAD65/67 protein expression, and both drugs amplified the gluco-stimulatory transmitter NO. VMN SF-1 and BDNF expression in hypoglycemic male rats was elevated by ERα antagonism. It remains to be clarified if one or both neurochemicals function as an ERα-inhibited counter-regulatory suppressor in this sex under current experimental circumstances. As the current intraventricular route of drug administration was intended to antagonize ERα and -β expressed in the VMN as well as forebrain metabolic structures that innervate that structure, it is highly plausible that extra-VMN ERα-and/or ERβ – sensitive neurotransmitters may mediate intensifying effects of these receptors on counter-regulatory hormone responses to hypoglycemia in male rats. Intriguingly, current outcomes argue against forebrain ERα or ERβ participation in counter-regulatory glucagon and corticosterone outflow in female rats. It will be necessary to characterize in future work the neuroanatomical location(s) in the female brain and resident gluco-regulatory neuron populations that are targets for estrogenic control of endocrine counter-regulation. Current work thus illuminates important sex distinctions for VMN neurotransmitters implicated in hypoglycemic hyperglucagonemia and hypercorticosteronemia by studies performed in males. E is reported to act within the VMN to control peripheral and hepatic insulin sensitivity [Liu et al., 2013]. The possibility that in the female, ERα and -β regulation of VMN GABA, NO, and SF-1 signaling may have systemic consequences via effects on insulin resistance and hepatic gluconeogenesis, or alternatively, have fulfill a local function by mediating or reporting local non-gluco-regulatory cell adaptations to the metabolic stress of hypoglycemia.
As hormone data were not directly compared between sexes in the present study, definitive assertion of sex differences in plasma corticosterone levels between euglycemic males and females cannot be made here. However, sex-dimorphic patterns of basal corticosterone release in the rat have been described [Kitay, 1961; Critchlow et al., 1963; LeMevel et al., 1979]. Further research is needed to elucidate the mechanism(s) that underlie opposite effects of hypoglycemia on corticosterone secretion in male versus female rats at the early time point examined here. As our prior work showed that corticosterone release did not differ between eu- and hypoglycemic females at +2 hr after insulin injection, but was elevated in the latter at later time points [Briski and Nedungadi, J. Neuroendocrinol. 2009; 21: 578–585], the possibility that hypoglycemia elicits a biphasic pattern of response in this sex cannot be overlooked. Estradiol elicits acute compensatory up-regulation of substrate catabolism and coincident maintenance of energy stability in hindbrain metabolic-sensory A2 noradrenergic neurons in hypoglycemic female rats [Tamrakar et al., 2015]. In light of reports that hindbrain catecholaminergic signaling is required for glucoprivic augmentation of corticosterone secretion [Ritter et al., 2003], we speculate that sex-specific estrogen-mediated neuroprotective effects on hindbrain nerve cell energy state may result in discrepant output of corticosterone within an early time frame after induction of this metabolic stress.
It should be noted that stabilized, unvarying plasma estradiol levels, achieved here by sc capsule implantation, deviate from the dynamic pattern of estradiol secretion that occurs over the estrous cycle in ovary-intact adult female rats. Thus, consideration must be made that in the presence of static estradiol levels, low or high, VMN glucoregulatory neuron and astrocyte responses to IIH, may not mimic those transpiring in the presence of fluctuating endogenous hormone release. The continuum of growth and development over the female lifespan involves transition between distinct reproductive states, including juvenile quiescence, fecundity, and reproductive senescence, that exhibit unique patterns of ovarian estradiol secretion. Long-term goals of our research include clarification of distinguishing features of diverse physiological patterns of estradiol release, including prepubertal hormone output, changeable secretion over the estrous cycle, elevated ovarian hormone release during pregnancy, and significant decline in hormone output during old age on brain responses to glucose dyshomeostasis.
In summary, this research addressed the hypothesis that forebrain ERα and ERβ exert sex-specific effects on hypoglycemic patterns of VMN gluco-regulatory signaling, VMN astrocyte glycogen metabolic enzyme and receptor protein expression, and counter-regulatory hormone secretion. Results associate both ER with hypoglycemic inhibition of VMN nNOS profiles in each sex, indicative of suppression of the gluco-stimulatory transmitter NO, and amplified expression SF-1 and GAD65/67, a marker enzyme for the counter-regulatory inhibitor GABA, in females. ERα and -β together enhance astrocyte AMPK total protein and activity and glycogen synthase profiles, while inhibiting glycogen phosphorylase expression at the same time in hypoglycemic females, whereas ERβ alone suppresses these proteins in males. Down-regulated GP expression supports the possibility of early sparing of glycogen stores in this hypothalamic structure in each sex. Differential VMN astrocyte protein responses to IIH may reflect in part ERα and -β augmentation of ERβ and down-regulation of α1AR, α2AR, and β1AR in females, versus ERβ inhibition of GPER30 and α2AR in males. Data show that MPP or PHTPP pretreatment blunted counter-regulatory hormone secretion in hypoglycemic male, but not female rats. Results infer that in males, signaling by one or more VMN neurotransmitters characterized here as sensitive to forebrain ER influence may have a passive role in this endocrine outflow, whereas ERα and -β expressed in the female forebrain apparently do not participate in neural regulation of these contra-regulatory responses.
Highlights:
Estrogen (E) control of ventromedial hypothalamic nucleus (VMN) gluco-regulation is unclear.
E receptor (ER) antagonists were given icv to male or female rats before insulin-induced hypoglycemia.
ER-alpha and -beta cause hypoglycemic inhibition of neuronal nitric oxide synthase protein in each sex.
ER up- or down-regulate VMN astrocyte AMPK expression and activity in females and males, respectively.
ER stimulate glucogen synthase expression in females only, but inhibit glycogen phosphorylase in both sexes.
ER antagonism blunted counter-regulatory hormone secretion in hypoglycemic males, but not females.
Acknowledgements:
This research was funded by NIH DK 109382.
Abbreviations:
- α1AR
alpha1 adrenergic receptor
- α2AR
alpha2 adrenergic receptor
- β1AR
beta1 adrenergic receptor
- BDNF
brain-derived neurotrophic factor
- E
estradiol
- ERα
estrogen receptor-alpha
- ERβ
estrogen receptor-beta
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase65/67
- GFAP
glial fibrillary acidic protein
- GP
glycogen phosphorylase
- GPER
G protein-coupled estrogen receptor 1
- GS
glycogen synthase
- IIH
insulin-induced hypoglycemia
- INS
insulin
- MCT1
monocarboxylate transporter-1
- MCT2
monocarboxylate transporter-2
- MPP
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- NE
norepinephrine
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- OVX
ovariectomy
- PHTPP
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- SF-1
steroidogenic factor-1
- VMN
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Adams JM, Legan SJ, Ott CE, Jackson BA. Modulation of hypoglycemia-induced increases in plasma epinephrine by estrogen in the female rat. J. Neurosci. Res 2005; 79: 360–367. [DOI] [PubMed] [Google Scholar]
- Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation, and gluconeogenesis. Ann. Nutr. Metab 1981; 25: 208–212. [DOI] [PubMed] [Google Scholar]
- Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis. Interaction with insulin, glucagon, and epinephrine. Horm. Res 1980; 13: 396–403. [DOI] [PubMed] [Google Scholar]
- Alhamami HN, Alshamrani A, Briski KP. Effects of the glycogen phosphorylase inhibitor 1,4-dideoxy-1,4-imino-D-arabinitol on ventromedial hypothalamic nucleus 5’-adenosine monophosphate-activated protein kinase activity and metabolic neurotransmitter biosynthetic enzyme protein expression in eu-versus hypoglycemic male rats. Physiological Reports 2018a; 103: 236–249. [Google Scholar]
- Alhamami HN, Uddin MM, Mahmood ASMH, Briski KP. Lateral but not Medial Hypothalamic AMPK Activation Occurs at the Hypoglycemic Nadir in Insulin-injected Male Rats: Impact of Caudal Dorsomedial Hindbrain Catecholamine Signaling. Neuroscience 2018b; 379:103–114. [DOI] [PubMed] [Google Scholar]
- Amaral AI. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. J. Inherit. Metab. Dis; 36: 621–634. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 2002; 42: 461–471. [DOI] [PubMed] [Google Scholar]
- Ashford MLJ, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurons is mediated by ATP-sensitive K+ channels. Pfugers Arch 415: 479–483, 1990. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia 1980; 19: 475–481. [DOI] [PubMed] [Google Scholar]
- Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 2003; 52: 663–666. [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J. Clin. Invest 1997; 99: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG. Development of Hypothalamic Circuits That Control Food Intake and Energy Balance. In: Harris RBS, editor. Appetite and Food Intake: Central Control 2nd edition Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 7. [PubMed] [Google Scholar]
- Briski KP, Koshy Cherian A, Genabai NK, Vavaiya KV. In situ coexpression of glucose and monocarboxylate transporter mRNAs in metabolic-sensitive dorsal vagal complex catecholaminergic neurons: transcriptional reactivity to insulin-induced hypoglycemia and caudal hindbrain glucose or lactate repletion during insulin-induced hypoglycemia. Neuroscience 2009; 164: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Briski KP, Marshall ES, Sylvester PW. Effects of estradiol on glucoprivic transactivation of catecholaminergic neurons in the female rat caudal brainstem. Neuroendocrinology 2001; 73: 369–377. [DOI] [PubMed] [Google Scholar]
- Briski KP, Nedungadi TP. Adaptation of feeding and counter-regulatory hormone responses to intermediate insulin-induced hypoglycaemia in the ovariectomised female rat: effects of oestradiol. J. Neuroendocrinol 2009; 21: 578–585. [DOI] [PubMed] [Google Scholar]
- Briski KP, Shrestha PK. Hindbrain estrogen receptor-beta antagonism normalizes reproductive and counter-regulatory hormone secretion in hypoglycemic steroid-primed ovariectomized female rats. Neuroscience 2016; 331: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem 1997; 272: 30096–30102. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentrations of LH, FSH, progesterone, and estradiol-17beta throughout the 4-day estrous cycle of the rat . Endocrinology 1974; 94: 1704–1708. [DOI] [PubMed] [Google Scholar]
- Chan O, Paranjape SA, Horblitt A, Zhu W, Sherwin RS. Lactate-induced release of GABA in the ventromedial hypothalamus contributes to counterregulatory failure in recurrent hypoglycemia and diabetes. Diabetes 2013; 62: 4239–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 2006; 55: 1080–1087. [DOI] [PubMed] [Google Scholar]
- Choi YH, Fujikawa T, Lee J, Reuter A, Kim KW. Revisiting the ventral medial nucleus of the hypothalamus: The roles of SF-1 neurons in energy homeostasis. Front Neurosci 2013; 7:71. doi: 10.3389/fnins.2013.00071. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003; 26: 1902–1912. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt A, Bar-sela M, Mountcastle W, Lipscomb H. Sex differences in resting pituitary-adrenal function in the rat. Amer. J. Physiol 1963; 205: 807–815. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. North Am 2010; 39: 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnault A, Deshaies Y, Richard D. Involvement of type I corticosteroid receptor in the effects of ovariectomy on energy balance. Amer. J. Physiol. Regul. Integr. Comp. Physiol 1996; 270:R199–R206. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Watts AG. Peripheral and central glucose sensing in hypoglycemic detection. Physiology 2014; 29: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JH, Chen X, Cui M, Yu X, Pang Q, Sun JP. Β2-adrenergic receptor and astrocyte glucose metabolism. J. Mol. Neurosci 2012; 48: 456–463. [DOI] [PubMed] [Google Scholar]
- Faure A, Haouari M, Sutter BC. Short term and direct influence of oestradiol on glucagon secretion stimulated by arginine. Diabetes Metab 1988; 14: 452–454. [PubMed] [Google Scholar]
- Fillenz M, Lowry JP, Boutelle MG, Fray AE. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol. Scand 1999; 167: 275–284. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes 2010; 59: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoreceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]. Neurochem. Intl 2010; 57: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Warabi E, Mann GE. Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c-Rel to reset energy metabolism in astrocytes via brain-derived neurotrophic factor. Free Radic. Biol. Med 2018; 119: 34–44. [DOI] [PubMed] [Google Scholar]
- Kale AY, Vavaiya KV, Briski KP. Effects of acute and chronic insulin-induced hypoglycemia on type II glucocorticoid receptor (GR) gene expression in characterized CNS metabolic loci in male rat brain. Brain Res. Bull 2006; 70: 240–244. [DOI] [PubMed] [Google Scholar]
- Kinyua AW, Yang DJ, Chang I, Kim KW. Steroidogenic factor 1 in the ventromedial nucleus of the hypothalamus regulates age-dependent obesity. PLoS One 2016; 11(9):e0162352. doi: 10.1371/journal.pone.0162352. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology 1961; 68: 818–824. [DOI] [PubMed] [Google Scholar]
- Komesaroff PA, Esler M, Clarke IJ, Fullerton MJ, Funder JW. Effects of estrogen and estrous cycle on glucocorticoid and catecholamine responses to stress in sheep. Amer. J. Physiol. Endocrinol. Metab 1988; 275: E671–E678. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estradiol. Amer.. J Physiol. Regul. Integr. Comp. Physiol 1980; 238:R400–R405. [DOI] [PubMed] [Google Scholar]
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology 1979; 105: 812–817. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Bailey CJ. Thyroid hormones, gonadal and adrenocortical steroids and the function of the islets of Langerhans. Endocr. Rev 1984; 5: 411–434. [DOI] [PubMed] [Google Scholar]
- Liu J, Bisschop PH, Eggels L, Foppen E, Ackermans MT, Zhou JN, Fliers E, Kalsbeek A. Intrahypothalamic estradiol regulates glucose metabolism via the sympathetic nervous system in female rats. Diabetes 2013; 62: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Soya S, Okamoto M, Ichitani Y, Kawanaka K, Soya H. Brain glycogen decreases during prolonged exercise. J. Physiol 2011; 589: 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology 2012; 96: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Ono H, Ooyama H, Wayner MJ. Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222: 282–284, 1969. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J. Cereb. Blood Flow. Metab 2012; 32: 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 2007; 55: 1251–1262. [DOI] [PubMed] [Google Scholar]
- Richard D, Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Amer. J. Physiol 1986; 250: R245–R249. [DOI] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 2003; 144: 1357–1367. [DOI] [PubMed] [Google Scholar]
- Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front. Syst. Neurosci 2014; 8:236. doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Sickmann HM, Walls AB, Bak LK, Waagepetersen HS. Functional importance of the astrocytic glycogen-shunt and glycolysis for maintenance of an intact intra/extracellular glutamate gradient. Neurotox. Res 2010; 18: 94–99. [DOI] [PubMed] [Google Scholar]
- Shakya M, Shrestha PK, Briski KP. Hindbrain 5’-adenosine monophosphate-activated protein kinase mediates short-term food deprivation inhibition of the gonadotropin-releasing hormone-luteinizing hormone axis: role of nitric oxide. Neuroscience 2018; 383: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 1997. December 1;388(4):507–25. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecińska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J. Neurophysiol 79: 1733–1745, 1998. [DOI] [PubMed] [Google Scholar]
- Singh SR, Briski KP. Septopreoptic mu opioid receptor mediation of hindbrain gluco-privic inhibition of reproductive neuroendocrine function in the female rat. Endocrinology 2004; 145: 5322–5331. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Cell. Neurosci 7: 1–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamrakar P, Briski KP. Estradiol regulates hypothalamic astrocyte adenosine 5’-monophosphate-activated protein kinase activation by hypoglycemia: role of hindbrain catecholamine signaling. Brain Res. Bull 2015; 110: 47–53. [DOI] [PubMed] [Google Scholar]
- Tamrakar P, Ibrahim BA, Gujar AK, Briski KP. Estrogen regulates energy metabolic pathway and upstream AMPK kinase and phosphatase enzyme expression in dorsal vagal complex metabolo-sensory neurons during glucostasis and hypoglycemia. J. Neurosci. Res 2015; 93: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamrakar P, Shrestha P, Briski KP. Sex-specific basal and hypoglycemic patterns of in vivo caudal dorsal vagal complex astrocyte glycogen metabolic enzyme protein expression. Brain Res 2014; 1586:90–8. [DOI] [PubMed] [Google Scholar]
- Tamrakar P, Shrestha P, Briski KP. Dorsomedial hindbrain catecholamine regulation of hypothalamic astrocyte glycogen metabolic enzyme protein expression: Impact of estradiol. Neuroscience 2015; 292: 34–45. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev 1992; 16: 235–272. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav 1979; 22: 583–593. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimbürger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience 158: 284–292, 2009. [DOI] [PubMed] [Google Scholar]
- Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front. Neuroendocrinol 2010; 31: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman JJ, Baum MJ. Estrogen reduces total food and carbohydrate intake, but not protein intake, in female rats. Physiol. Behav 1980; 24: 823–827 [DOI] [PubMed] [Google Scholar]


