Summary
Background
Overproduction of free radicals accompanied with their insufficient removal/neutralization by antioxidative defense system impairs redox hemostasis in living organisms. Oxidative stress has been shown to be involved in all the stages of carcinogenesis and malignant melanocyte transformation. The aim of this study was to examine association between oxidative stress development and different stages of melanoma.
Methods
The measured oxidative stress parameters included: superoxide anion radical, total and manganese superoxide dismutase, catalase and malondialdehyde. Oxidative stress parameters were measured spectrophotometrically in serum samples from melanoma patients (n=72) and healthy control subjects (n=30). Patients were classified according to AJCC clinical stage.
Results
Average superoxide anion and malondialdehyde concentrations were significantly higher in melanoma patients than in control group, with the highest value of superoxide anion in stage III, while malondialdehyde highest value was in stage IV. The activity of total and manganese superoxide dismutase was insignificantly higher in melanoma patients than in control group, while catalase activity was significantly higher. The highest activity of total activity of manganese superoxide dismutase was in stage IV. Catalase activity was increasing with the disease progression achieving the maximum in stage III.
Conclusion
Results of our study suggest that melanoma is oxidative stress associated disease, as well as deteriorated cell functioning at mitochondrial level.
Keywords: antioxidants, free radicals, melanoma, oxidative stress
Kratak sadržaj
Uvod
Prekomerna produkcija slobodnih radikala i njihova nedovoljna eliminacija/neutralizacija putem sistema antioksidativne odbrane, narušava redoks homeostazu u organizmu. Oksidativni stres je uključen u sve faze razvoja karcinoma i maligne transformacije melanocita. Cilj ove studije bio je da se ispita razvoj oksidativnog stresa u razlićitim stadijumima melanoma.
Metode
Parametri za procenu oksidativnog stresa su: superoksil anjon radikal, ukupna i mangan superoksidna dizmutaza, katalaza i malondialdehid. Parametri oksidativnog stresa su mereni metodom spektrofotometrije u serumu bo lesnika sa melanomom (n=72) i zdravih kontrolnih oso ba (n=30). Bolesnici su klasifikovani prema AJCC kriterijumu.
Rezultati
Prosečne koncentracije superoksil anjon radikala i malonaldehida bile su značajno veće kod bolesnika sa melanomom u odnosu na kontrolnu grupu, najveća vrednost superoksil anjon radikala bila je u III stadijumu, a malondialdehida u IV stadijumu. Aktivnost ukupne i mangan superoksidne dizmutaze bila je neznačajno povećana kod obolelih od melanoma u odnosu na kontrolnu grupu, dok je aktivnost katalaze bila statistički značajno veća. Najveća aktivnost ukupne superoksidne dizmutaze bila je u III stadijumu, a mangan superoksidne dizmutaze u IV stadijumu.Zaključak Aktivnost katalaze je rasla sa napredovanjem bolesti i dostigla maksimum u III stadijumu.
Zaključak
Rezultati našem studije ukazuju na povezanost melanoma i oksidativnog stresa, kao i na pogoršanu funkciju ćelija na nivou mitohondrija.
Ključne reči: antioksidansi, melanom, oksidativni stres, slobodni radikali
Introduction
World Health Organization classified melanoma into four common types: superficial spreading, nodular, lentigo maligna and acral lentiginous; and six less frequent (1). Although melanoma accounts for only 4% of all skin cancers, it causes the greatest number of skin cancer related deaths worldwide (2). It also affects other extra-cutaneous pigment-containing sites including eyes, meninges, esophagus and mucous membranes. Cutaneous melanoma is the most common and aggressive subtypes of melanoma, arising from malignant transformation of epidermal melano cytes (3), while mucosal melanoma arising from mucous membranes melanocytes and uveal melanoma from ocular stroma melanocytes (4). Melanoma is characterized by high invasion and metastasis capacity and remarkable genotypic and phenotypic heterogeneity (5). It is located mostly on the back of male and legs of female. Melanoma usually affects Caucasian in the fourth life decade. Men found to be more vulnerable to melanoma than women (6). Melanoma risk factors include pale skin, blond or red hair, numerous freckles and tendency to burn and tan poorly (7, 8), existence of more than 50 acquired naevi (9) or 5 dysplastic naevi, large congenital nevi (10), chemical exposures, immunosuppression, genetic factors, scars etc.
Malignant melanocyte transformation has been recognized to be associated with oxidative stress (OS) (11). Redox homeostasis impairment in living organisms is consequence of free radicals (FRs) overproduction and/or insufficient antioxidative defense. Oxidative injuries of biomolecules (including DNA, proteins and lipids) disrupt cell`s signalization, devastate reduction equivalent cell sources and energy and usually culminate with cell death (apoptosis). Noteworthy, changed cell signalization can trigger disease development.
Cell mitochondrial respiratory chain, inflammatory responses and oxidative metabolism of endogenous as well as exogenous compounds are the major sources of FRs generation in humans. Reactive oxygen/nitrogen/thiyl species (ROS/RNS/RSS) have been shown to be involved in all three stages of carcinogenesis (initiation-promotion-progression) (12, 13, 14). Extensive DNA damage induced by FRs can lead to mutation, alteration of phenotypic expression and cell death. Antioxidative defense system (ADS), composed of antioxidative enzymes and antioxidants, prevents biomolecules oxidative injury through FRs sequestration and reparation of already oxidatively damaged cell constituents (12, 13).
American Joint Committee on Cancer (AJCC) set up four melanoma stages based on the status of tumor thickness/size, ulceration, mitotic rate, presence of micrometastasis, tumor positive lymph nodes and distant metastasis (15).
Herein, we studied the association between OS development and melanoma stages by measuring OS parameters, including: superoxide anion radical (O2•-), total and mitochondrial superoxide dismutase (tSOD, Mn-SOD) and catalase (CAT) activities and lipid peroxidation (LPO) by measuring malondialdehyde (MDA).
Materials and Methods
Consented melanoma patients were recruited from the Clinic for Dermatology and Venereology and Melanoma Center of the Military Medical Academy, Belgrade, Serbia, while healthy controls referred to healthy persons (with no prior history of cancer) on periodical systematic examinations. The study was approved by the local Research Ethics Committee, Military Medical Academy (11-03/2014).
According to the 7th edition of AJCC there are four melanoma stages: IA stage- tumors not thicker than 1.0 mm, not ulcerated, and have a mitotic rate <1 mitosis/mm2; stage IB- tumors are >1.0 mm and either have at least 1 mitosis/mm2 or evidence of tumor ulceration; stage IIA-ulcerated, 1.01–2.0 mm sized tumors or no ulcerated, 2.01–4.0 mm sized tumors; stage IIB-ulcerated, 2.01–4.0 mm sized tumors or no ulcerated, thicker than 4.0 mm; stage IIC-ulcerated, thicker than 4.0 mm; stage III- isolated tumor cells or tumor deposits >0.1 mm (micrometastasis, tumor positive lymph nodes) detected histopathologically or immunohistochemically; stage IV-melanomas with distant metastasis (15).
Herein, 72 melanoma patients (33 men and 39 women, mean age 54.72 ± 16.50; total melanoma patients – TMP group) were classified into three stages: initial (joined patients with IA, IB, IIA, IIB, and IIC stages), middle (III melanoma stage) and final (IV melanoma stage), according to the 7th edition of AJCC melanoma classification (15). Thirty healthy controls (15 men and 15 women, mean age 50.10 ± 25.20) were recruited as control group – C group.
Samples
Venous blood from healthy controls and melanoma patients was collected in vacuettes with clot activator. After isolation (centrifugation at 3000 rpm for 10 minutes) serum samples were frozen at - 70 °C, until testing. The activity of CAT, tSOD and Mn-SOD and levels of O2•- and MDA were analyzed.
Determination of O 2•-
Superoxide anion was determined by the reduction of nitroblue-tetrazolium (NBT) in alkaline nitrogen saturated medium (16). Kinetic analysis was performed at 550 nm on Ultrospec 2000 spectrophotometer. The results were expressed as mmol red NBT/min/L.
Determination of t-SOD
Superoxide dismutase (EC 1.15.1.1.; SOD) activity was measured spectrophotometrically as the inhibition of epinephrine spontaneous auto-oxidation at 480 nm (17). The kinetics of sample enzyme activity was followed in a carbonate buffer (50 mmol/L, pH 10.2) containing 0.1 mmol/L EDTA after the addition of 10 mmol/L epinephrine, on Ultrospec 2000 spectrophotometer. Data were expressed as U/mL.
Determination of Mn-SOD
Activity of Mn-SOD was measured at the same way as t-SOD (17) with the modification in sample amount and proceeded incubation with 25 μL of KCN (8 mmol/L) (to block Cu/Zn-SOD) for 20 min, on the room temperature.
Determination of CAT
Catalase (EC 1.11.1.6) activity was determined spectrophotometrically by using ammonium molybdate to produce yellow complex with H2O2 (18). Kinetic analysis was performed at 405 nm on Ultrospec 2000 spectrophotometer. CAT activity was defined as mmol H2O2 reduced per minute (mmol H2O2/min). Data were expressed as kU/L.
Determination of lipid peroxidation
Serum MDA level was measured by thiobarbituric acid reactive substances (TBARS) assay, as described by Girotti et al. (19). Two molecules of TBARS reagent (15% trichloroacetic acid + 0.375% thiobarbituric acid + 0.25 mol/L HCl) react with MDA, forming complex with absorbance measurable at 531 nm. The results were expressed as μmol/L.
Statistical analysis
Kolmogorov-Smirnov normality test followed by nonparametric one-way ANOVA (for multiple groups analysis) and Mann-Whitney (two groups analysis) tests were used in statistical data analysis. Spearman’s test was used to test correlation between OS parameters across melanoma stages. Statistically significant differences were considered at p<0.05. The values are expressed as means with standard error mean (SEM), since data did not follow Gaus distribution and standard deviation can not be used. Graph Pad Prism 5 software was used for data analysis. Power analysis and sample size were obtained using GPower statistical analysis program. It was calculated that total sample size is 66, based on effect size 0.4, a=0.05 (type 1 error probability), power analysis 0.8 and three groups.
Results
Superoxide anion in melanoma patients
The highest O2•- was measured in group III, though elevated values were documented in all groups: TMP (p<0.0001), I+II (p<0.0001), III (p<0.0001) and IV (p=0.0005) compared to C group (Figure 1). No significant differences were found across the groups.
Figure 1.
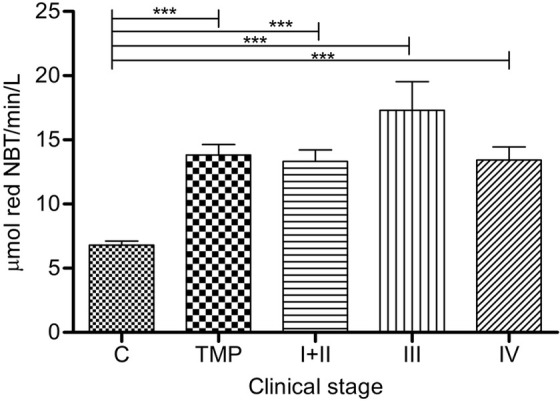
Superoxide anion radical in serum of melanoma patients: Serum O2•- levels (expressed as mmol red NBT/min/L) are presented as average (SEM). Statistically significant differences were considered at p<0.05. Labeling: ***p<0.001. Melanoma patients’ groups (according to AJCC): I+II (n=53), III (n=14) and IV (n=5). TMP- total melanoma patients (n=72), C-controls (n=30), NBT- nitroblue tetrazolium
Total superoxide dismutase activity in melanoma patients
Total SOD activity was significantly high only in III group compared to C group (p=0.0322) (Figure 2).
Figure 2.
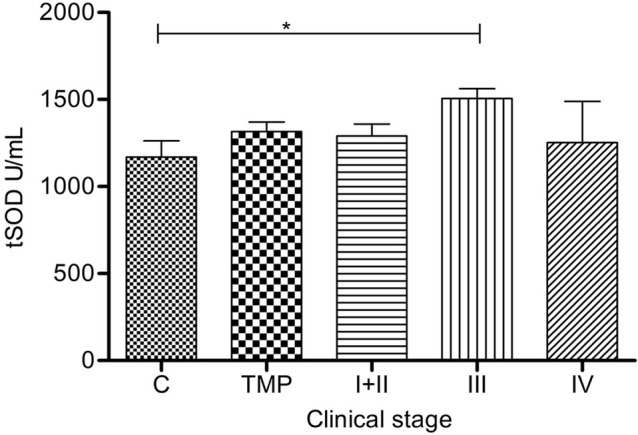
Total superoxide dismutase activity in serum of melanoma patients: Serum tSOD (sum of Cu/Zn-SOD and Mn-SOD) (U/mL) is presented as average (SEM). Statistically significant differences were considered at p<0.05, labeled as *. Melanoma patients’ groups (according to AJCC): I+II (n=53), III (n=14), IV (n=5). TMP- total melanoma patients (n=72) and C-controls (n=30)
Manganese superoxide dismutase activity in serum of melanoma patients
In group IV, Mn-SOD accomplished significantly higher activity than in all other groups: I+II (p= 0.0086), III (p=0.0201) and C group (p=0.0038) (Figure 3). Mn-SOD activity showed a clear increment with the disease progression.
Figure 3.
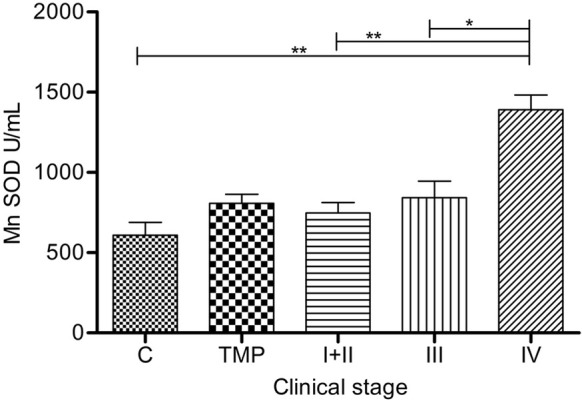
Manganese superoxide dismutase activity in serum of melanoma patients: Serum Mn-SOD (U/mL) is presented as average (SEM). Statistically significant differences were considered at p<0.05 Labeling: *p<0.05, **p<0.01. Melanoma patients’ groups (according to AJCC): I+II (n=46), III (n=13), IV (n=4). TMP- total melanoma patients (n=63) and C-controls (n=21)
Catalase activity in melanoma patients
Catalase activities in groups: TMP (p=0.0081), I+II (p=0.0269) and III (p=0.0018) were significantly higher than in C group (Figure 4). The highest CAT activity was in group III.
Figure 4.
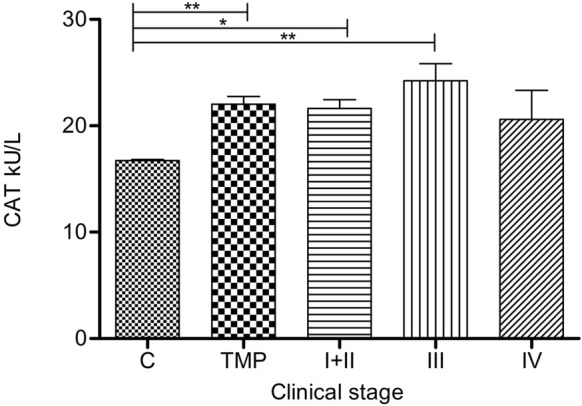
Catalase activity in serum of melanoma patients: Serum CAT (kU/L) is presented as average (SEM). Statistically significant differences were considered at p<0.05. Labeling: *p<0.05, **p<0.01. Melanoma patients’ groups (according to AJCC): I+ II (n=53), III (n=14) and IV (n=5). TMP- total melanoma patients (n=72) and C-controls (n=30)
Malondialdehyde values in melanoma patients
Lipid peroxidation, expressed as a MDA, was significantly elevated in TMP (p=0.0008), I+II (p=0.0058), III (p=0.0050) and IV (p=0.0033) compared to controls (Figure 5). Patients in group IV had significantly higher MDA than in group I+II (p=0.0282) and III (p=0.0299). The highest MDA was in group IV.
Figure 5.
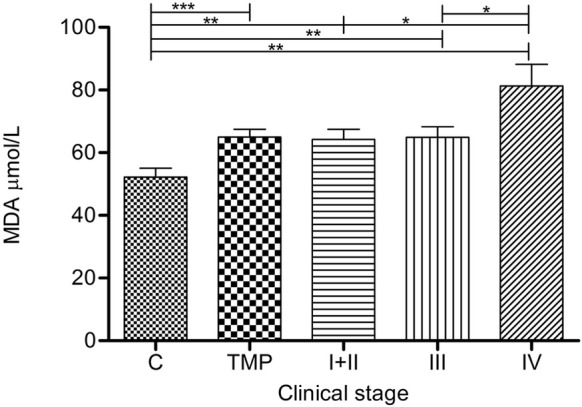
Lipid peroxidation in serum of melanoma patients: Lipid peroxidation (mmol MDA/L) is presented as average (SEM). Statistically significant differences were considered at p<0.05. Labeling: *p<0.05, **p<0.01, ***p<0.001. Melanoma patients’ groups (according to AJCC): I+II (n=53), III (n=14) and IV (n=5). TMP- total melanoma patients (n=72) and C-controls (n=30).
Correlations between parameters of OS
Negative correlation between O2•- and CAT, O2•- and tSOD, O2•- and Mn-SOD; and positive between CAT and tSOD, CAT and Mn-SOD, tSOD and Mn-SOD were obtained in early stage of disease (I+II) (Table I), while no correlation was obtained in the middle and later stages (III+IV).
Table I.
Correlation between OS parameters in early melanoma stage.
| Correlations: I+II stage | ||||||
|---|---|---|---|---|---|---|
| O2•- | MDA | CAT | t-SOD | Mn-SOD | ||
| O2•- | r | 1.000 | 0.177 | -0.491** | -0.359** | -0.292* |
| p | – | 0.206 | 0.000 | 0.008 | 0.049 | |
| N | 53 | 53 | 53 | 53 | 46 | |
| MDA | r | 0.177 | 1.000 | -0.134 | 0.037 | 0.044 |
| p | 0.206 | – | 0.338 | 0.792 | 0.772 | |
| N | 53 | 53 | 53 | 53 | 46 | |
| CAT | r | -0.491** | -0.134 | 1.000 | 0.570** | 0.366* |
| p | 0.000 | 0.338 | – | 0.000 | 0.012 | |
| N | 53 | 53 | 53 | 53 | 46 | |
| t-SOD | r | -0.359** | 0.037 | 0.570** | 1.000 | 0.393** |
| p | 0.008 | 0.792 | 0.000 | – | 0.007 | |
| N | 53 | 53 | 53 | 53 | 46 | |
| Mn-SOD | r | -0.292* | 0.044 | 0.366* | 0.393** | 1.000 |
| p | 0.049 | 0.772 | 0.012 | 0.007 | – | |
| N | 46 | 46 | 46 | 46 | 46 | |
Spearman's correlation was used to test OS parameters association with melanoma stage. Two tailed test Spearman’s correlation was performed. Labeling: number of patients – N; Correlation coefficient – r; Statistical significance – p (*p<0.05, **p<0.01)
Discussion
Cutaneous malignant melanoma develops in three different stages, from radial to vertical growth phases and metastatic disease. Clinically, radial growth phase presents as patches or plaques, this is an early melanoma stage (stage I+II, according to the AJCC). Melanoma cells show radial spread, usually confined to the intra-epidermal compartment, while melanoma’s mitosis are frequently seen in the epidermis but rarely in the dermis (20). Vertical growth phase of melanoma refers to gray-black, blue-black or even amelanotic nodules and is classified as an early and/or a late stage (stage III, according to the AJCC). In an early stage, a small papulonodule arises in a radial growth phase lesion and is usually darker than radial growth phase associated lesions, whereas in a late or developed vertical may be present and tumor aggregates may extend into the reticular dermis or even subcutaneous fat (20). The terminal phase of melanoma progression assumes distant metastasis expansion (stage IV, according to the AJCC).
Positive association between OS and clinical stages of melanoma progression is confirmed by our study. Oxidative stress-associated diseases, including melanoma, underline cross-reactions between overproduced FRs and immune responses, in humans (21, 22, 23, 24, 25). Regulatory mechanisms of OS on tumor growth and progression comprise genomic instability, oncogene activation and angiogenesis (26). It was shown that ROS alter proto-oncogene B-Raf that encodes B-Raf protein (BRAF), a known activator of mitogen-activated protein kinase (MAPK) pathways and suppress apoptosis (27). High ROS levels inactivate p53 (tumor suppressor gene and regulator of apoptosis) which leads to inhibition of apoptosis (28). Also, ROS can directly activate MAPK pathways leading to aberrant activation of nuclear factor-kappa B (NF-B), which in turn induces expression of protooncogenes such as c-fos, c-jun and c-myc, that leads to cell proliferation and blocking of apoptosis (29, 30). Notable, ROS promote many aspects of tumor development and progression including: (a) cellular proliferation e.g. extracellular-regulated kinase 1/2 (ERK1/2) activation; (b) evasion of apoptosis e.g. Src, NF-B and phosphatidylinositol-3 kinase (PI3K)/Akt activation; (c) tissue invasion and metastasis e.g. metalloproteinase(MMP) secretion into the extracellular matrix (ECM); and (d) angiogenesis e.g. release of vascular endothelial growth factor (VEGF) and angiopoietin (31).
Overproduction of ROS is necessary but not sufficient to induce malignancy. Free radicals readily attack all classes of biomolecules (proteins, DNA, unsaturated fatty acids) and cause toxic and/or mutagenic effects. In reaction with DNA, ROS induce base-oxidation and deamination, base loss, single and double-strand breaks, crosslinks, deletion, mutation, translocation. The consequences of oxidatively damaged DNA are transcription blockage, replication errors and genomic instability, which is the first step in process of mutagenesis, carcinogenesis and aging (32). Deteriorated protein's primary structure by ROS causes modification and loss of some amino acids, formation of S-S bridges and carbonyl groups, aggregation and fragmentation, increased proteolytic sensitivity, loss of catalytic function and changes in secondary and tertiary protein structure, affecting their viscosity and charge (33). Changed secondary and tertiary protein structure can induce cell death. Protonated O2•- form, perhydroxyl radical (HO2•, pKa=4.7) can abstract bis-allylic H+ from poly unsaturated fatty acids (PUFA) and triggers LPO, unlike O2•- itself. Hydrogen peroxide produced in the reaction of O2•-dismutation by SOD, easily diffuses through cellular membranes and precedes the production of the most potent hydroxyl radical (HO•) by its homolytic cleavage or through Fenton reaction. Conversion of H2O2 into water is catalyzed by CAT primarily and glutathione peroxidase (GPx). If the production of H2O2 overwhelms the activity of CAT and GPx, it can participate in Fenton-like reactions together with transitional metals, such as Fe2+ or Cu1+, giving rise to toxic HO• that imposes mutagenic effect in reaction with DNA (34). Initiation, propagation and termination of LPO comprise the formation of PUFA radical (PUFA•), alkyl peroxyl (PUFA-OO•), alkoxy (PUFA-O•) and alkyl hydroperoxides (PUFA-OOH), which undergo-scission reactions or intramolecular cyclisation, followed by the decomposition into carbonyls (including MDA) (35, 36). Malondialdehyde is highly cytotoxic and it has been confirmed as a potent enzymes inhibitor, tumor promoter and co-carcinogenic (37).
Increased activity of CAT in TMP suggests a pivotal role of this enzyme against OS. The higher SOD and CAT activities, seen in melanoma patients, corresponded to ROS overproduction (increased O2•-) and LPO (increased MDA), confirming that melanoma is OS- associated disease (38).
Redox status differs across body organs/tissues due to anatomical, blood supply status and other specificities (25). Sander et al. (39) reported on significantly elevated antioxidant enzymes (CAT, SOD) activity and MDA level in malignant tissues of melanoma patients. They were the first who found the correlation between melanoma and MDA in human skin in vivo.
Schadendorf et al. (40) reported on statistically elevated serum Mn-SOD activity in all clinical stages of melanoma, compared with controls (p < 0.005), while in our study Mn-SOD activity was significantly higher only in stage IV compared with control group (Figure 3).
In line with our results regarding OS development in melanoma patients are findings of other authors. Accordingly, Gadjeva et al. (22) documented significant increase of plasma MDA and CAT activity in melanoma patients, as we found too, but significantly low CuZn-SOD activity if compared with healthy controls. Interestingly, they showed that plasma MDA levels decreased after the surgery (removal of melanoma tissues) indicating melanoma tissue as a significant FRs producer, though activities of SOD and CAT remained the same, as before the surgery. Mantovani et al. (41) emphasized that OS development is associated with insufficient antioxidative capacity in different types of cancer, reporting on ROS overproduction, significantly elevated CuZn-SOD (but not affected tSOD activity) and reduced GPx activity (42).
Positive correlation between tSOD and CAT activity was confirmed in the early stage patients (stage I+II). It appears logical, because CAT follows SOD catalyzed production of H2O2 (during O2•- dismutation). Negative correlations between O2•-and antioxidative enzymes: tSOD, Mn-SOD and CAT allude to other O2•- sequestration pathways that predominately occurs, than dismutation by tSOD and Mn-SOD. This finding is in accordance with reports on O2•- and nitrogen monoxide radical reaction, when harmful peroxynitrite is generated, which is three times faster than O2•- dismutation by SOD (43).
The observed changes in MDA and O2•- levels as well as the altered serum activities of the antioxidant enzymes such as SOD and CAT in melanoma patients, confirmed that melanoma is OS associated disease. Deteriorated cell functioning at mitochondrial level was confirmed by elevated Mn-SOD activity in IV stage compared to early and middle stages (this may be explanation why despite the increase in enzymatic activity, the disease continues to develop). Taken together, the results of our study could be useful in assessing the defense system in melanoma patients and for better understanding the role of OS in melanoma progression.
The main limitation of this study include small number of melanoma patients in late melanoma stage (IV group), that is expected because of high mortality rate.
List of abbreviations
- ADS
antioxidative defense system;
- AJCC
American Joint Committee on Cancer;
- C
control group;
- CAT
catalase;
- FRs
free radicals;
- LPO
lipid peroxidation;
- MDA
malondialdehyde;
- Mn-SOD
manganese superoxide dismutase;
- NBT
nitroblue-tetrazolium;
- NF- B
nuclear factor kappa B;
- O2•-
superoxide anion radical;
- OS
oxidative stress;
- PUFA
poly unsaturated fatty acids;
- ROS
reactive oxygen species;
- rpm
revolutions per minute;
- TMP
total melanoma patients;
- tSOD
total superoxide dismutase;
- WHO
World Health Organization.
Footnotes
Conflict of Interest Statement:The authors declare that they have no conflicts of interest for this work.
References
- 1.Bandarchi B, Ma L, Navab R, Seth A, Rasty G. From melanocyte to metastatic malignant melanoma. Der - matol Res Pract. 2010;2010:583748. doi: 10.1155/2010/583748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanojević I, Gavević M, Jović M, Miju{ković Z, Zevević R, Zolotarevski L. Interferon alpha-induced re - duction in the values of myeloid-derived suppressor cells in melanoma patients. Vojnosanit Pregl. 2015;72(4):342. doi: 10.2298/vsp1504342s. et al. –. [DOI] [PubMed] [Google Scholar]
- 3.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20. doi: 10.1634/theoncologist.12-1-20. –. [DOI] [PubMed] [Google Scholar]
- 4.Ali Z, Yousaf N, Larkin J. Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013;11(2):81. doi: 10.1016/j.ejcsup.2013.07.012. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Zhu S, Yang Y, Ma X, Guo S. Matrix metalloproteinase- 12 expression is increased in cutaneous melanoma and associated with tumor aggressiveness. Tumour Biol. 2015;36(11):8593. doi: 10.1007/s13277-015-3622-9. –. [DOI] [PubMed] [Google Scholar]
- 6.Swetter SM, Clarke CA, Keegan THM. Why Do Men Have Worse Melanoma Survival than Women? Is It Behavior, Biology, or Both? The Melanoma Letter Summer. 2014;32(2):4. –. [Google Scholar]
- 7.Olsen CM, Carroll HJ, Whiteman DC. Estimating the attributable fraction for melanoma: a meta-analysis of pigmentary characteristics and freckling. Int J Cancer. 2010;127(10):2430. doi: 10.1002/ijc.25243. –. [DOI] [PubMed] [Google Scholar]
- 8.Williams PF, Olsen CM, Hayward NK, Whiteman DC. Melanocortin 1 receptor and risk of cutaneous mela - noma: a meta-analysis and estimates of population burden. Int J Cancer. 2011;129(7):1730. doi: 10.1002/ijc.25804. –. [DOI] [PubMed] [Google Scholar]
- 9.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28. doi: 10.1016/j.ejca.2004.10.015. et al. –. [DOI] [PubMed] [Google Scholar]
- 10.Olsen CM, Carroll HJ, Whiteman DC. Estimating the attributable fraction for cancer: A meta-analysis of nevi and melanoma. Cancer Prev Res (Phila) 2010;3(2):233. doi: 10.1158/1940-6207.CAPR-09-0108. –. [DOI] [PubMed] [Google Scholar]
- 11.Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel- Malek ZA. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol. 2014;134(6):1512. doi: 10.1038/jid.2014.65. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djukic M, Ninkovic M, Jovanovic M. Oxidative stress - clinical diagnostic significance. J Med Biochem. 2008;27(4):409. –. [Google Scholar]
- 13.Li YR, Jia Z, Trush MA. Defining ROS in biology and medicine. Reactive Oxygen Species. 2016;1(1):9. doi: 10.20455/ros.2016.803. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479. doi: 10.3109/10715761003667554. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR. AJCC melanoma staging and classification. J Clin Oncol 2009; 2009;27(36):6199. doi: 10.1200/JCO.2009.23.4799. et al. Final version of. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auclair C, Voisin E. Nitroblue tetrazolium reduction. In: Green wald RA, editor. Handbook of Methods for Oxygen Radical Research, 3th ed. Florida: CRC Press. 1985. p. 123-32.
- 17.Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;90(1):81. doi: 10.1016/0003-2697(78)90010-6. –. [DOI] [PubMed] [Google Scholar]
- 18.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143. doi: 10.1016/0009-8981(91)90067-m. –. [DOI] [PubMed] [Google Scholar]
- 19.Girotti MJ, Khan N, McLellan BA. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J Trauma. 1991;31(1):32. doi: 10.1097/00005373-199101000-00007. –. [DOI] [PubMed] [Google Scholar]
- 20.Slominski A, Wortsman J, Carlson A, Matsuoka L, Balch C, Mihm M. Malignant melanoma: An update. Arch Pathol Lab Med. 2001;125:1295. doi: 10.5858/2001-125-1295-MM. –. [DOI] [PubMed] [Google Scholar]
- 21.De Cavanagh EM, Honegger AE, Hofer E, Bordenave RH, Bullorsky EO, Chasseing NA. Higher oxidation and lower antioxidant levels in peripheral blood plasma and bone marrow plasma from advanced cancer patients. Cancer. 2002;94(12):3247. doi: 10.1002/cncr.10611. et al. –. [DOI] [PubMed] [Google Scholar]
- 22.Gadjeva V, Dimov A, Georgieva N. Influence of therapy on the antioxidant status in patients with melanoma. J Clin Pharm Ther. 2008;33(2):179. doi: 10.1111/j.1365-2710.2008.00909.x. –. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Bhatt ML, Misra MK. Lipid peroxidation and anti - oxidant status in head and neck squamous cell carcinoma patients. Oxid Med Cell Longev. 2009;2(2):68. doi: 10.4161/oxim.2.2.8160. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133(3):881. doi: 10.1007/s10549-011-1851-1. et al. –. [DOI] [PubMed] [Google Scholar]
- 25.Djukic M. Oksidativni stres - klini~ko-dijagnosti~ki zna~aj. Belgrade: Mono i Manjana. 2008 [Google Scholar]
- 26.Joosse A, De Vries E, van Eijck CH, Eggermont AM, Nijsten T, Coebergh JW. Reactive oxygen species and melanoma: an explanation for gender differences in survival? Pigment Cell Melanoma Res. 2010;23(3):352. doi: 10.1111/j.1755-148X.2010.00694.x. –. [DOI] [PubMed] [Google Scholar]
- 27.Hussain MR, Baig M, Mohamoud HS, Ulhaq Z, Hoessli DC, Khogeer GS. BRAF gene: From human cancers to developmental syndromes. Saudi J Biol Sci. 2015;22(4):359. doi: 10.1016/j.sjbs.2014.10.002. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1. doi: 10.1042/BJ20061131. –. [DOI] [PubMed] [Google Scholar]
- 29.Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20(2):183. doi: 10.1097/CCO.0b013e3282f5271c. –. [DOI] [PubMed] [Google Scholar]
- 30.Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24(2):301. doi: 10.1007/s10555-005-1579-7. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376. doi: 10.1016/j.arr.2012.10.004. –. [DOI] [PubMed] [Google Scholar]
- 32.Buonocore G, Perrone S, Tataranno ML. Oxygen toxicity: chemistry and biology of reactive oxygen species. Semin Fetal Neonatal Med. 2010;15(4):186. doi: 10.1016/j.siny.2010.04.003. –. [DOI] [PubMed] [Google Scholar]
- 33.Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38(7):995. doi: 10.1590/s0100-879x2005000700003. –. [DOI] [PubMed] [Google Scholar]
- 34.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44. doi: 10.1016/j.biocel.2006.07.001. –. [DOI] [PubMed] [Google Scholar]
- 35.Catalá A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int J Biochem Cell Biol. 2006;38(9):1482. doi: 10.1016/j.biocel.2006.02.010. –. [DOI] [PubMed] [Google Scholar]
- 36.Fagali N, Catalá A. Fe2+ and Fe3+ initiated peroxidation of sonicated and non-sonicated liposomes made of retinal lipids in different aqueous media. Chem Phys Lipids. 2009;159(2):88. doi: 10.1016/j.chemphyslip.2009.03.001. –. [DOI] [PubMed] [Google Scholar]
- 37.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venza M, Visalli M, Beninati C, De Gaetano GV, Teti D, Venza I. Cellular Mechanisms of Oxidative Stress and Action in Melanoma. Oxid Med Cell Longev. 2015;2015:481782. doi: 10.1155/2015/481782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sander CS, Hamm F, Elsner P, Thiele JJ. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol. 2003;148(5):913. doi: 10.1046/j.1365-2133.2003.05303.x. –. [DOI] [PubMed] [Google Scholar]
- 40.Schadendorf D, Zuberbier T, Diehl S, Schadendorf C, Czarnetzki BM. Serum manganese superoxide dismutase is a new tumour marker for malignant melanoma. Melanoma Res. 1995;5(5):351. doi: 10.1097/00008390-199510000-00008. –. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani G, Macciò A, Madeddu C, Mura L, Massa E, Gramignano G. Reactive oxygen species, antioxidant mechanisms and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J Cell Mol Med. 2002;6(4):570. doi: 10.1111/j.1582-4934.2002.tb00455.x. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuksel M, Ates I, Kaplan M, Arikan MF, Ozin YO, Kilic ZMY, Topcuoglu C, Kayacetin E. Is oxidative stress associated with activation and pathogenesis of inflammatory bowel disease? J Med Biochem. 2017;36:341-8. doi: 10.1515/jomb-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djukic M. Reaktivne hemijske vrste i oksidativni stress. In: Djukic M, editor. Oksidativni stres: Slobodni radikali, Prooksidansi, Antioksidansi. Belgrade: Mono i Manjana. 2008. p. 3-23.


