Abstract
Introduction:
Using objectively-collected physical activity (PA) data from the Baltimore Longitudinal Study of Aging, we tested whether patterns of daily activity and sedentary time differed by cancer survivorship in older adults.
Methods:
659 participants (mean age 71 ± 10 years, 51% women) were instructed to wear an accelerometer for 7 consecutive days and had self-reported information on cancer history. Accelerometer data were summarized into: 1) PA volume and 2) activity fragmentation (interrupted activity), both expressed as continuous and as dichotomized (low and high) variables. Participants were categorized into four groups by cross-classification of dichotomous PA volume and fragmentation. Multiple regression models were used to estimate differences in PA patterns by cancer history.
Results:
Cancer survivors averaged 0.12 (SE=0.05, p=0.02) fewer log-transformed activity counts per day than those reporting no cancer history after adjusting for demographics, behavioral factors, and comorbidities. Although fragmentation did not differ by cancer survivorship in the continuous model (p=0.13), cancer survivorship was associated with a 77% (odds ratio (OR): 1.77, 95% confidence interval (CI): 1.11–2.82) higher odds of having high (versus low) fragmentation and a 94% (OR: 1.94, 95% CI: 1.13–3.33) higher odds of having combined low PA/high fragmentation (versus high PA/low fragmentation) relative to no cancer history.
Discussion:
Findings suggest that cancer survivors engage in lower total daily PA and that this activity is performed in a more fragmented manner. These results may reflect the onset and progression of a low activity phenotype that is more vulnerable to heightened levels of fatigue and functional decline with aging.
Keywords: Accelerometer, fragmentation, activity transition, sedentary, disease, older adult, fatigue
CONDENSED ABSTRACT
Total physical activity (PA) and participation in sustained PA throughout the day is reduced in cancer survivors compared to those not reporting cancer history, suggesting that cancer and its treatments have deleterious, long-term effects on both the amount and characteristic patterns of daily PA.
INTRODUCTION
Cancer is the second leading cause of death in the United States (1), but recent evidence indicates that the prevalence of cancer survival has been increasing due to more effective treatments and longer life expectancy (2). With improvements in early cancer detection/diagnosis and treatment, the prevalence of cancer survivors is projected to continue to rise to 20 million by 2026 (2). This rise poses a new public health challenge as cancer survivors live with higher levels of pain (3), neurocognitive dysfunction (4), anxiety (5), and are at higher risk of fatigue (6), cancer reoccurrence (2,7), and mortality (1). Thus, developing interventions to combat adverse health factors in this vulnerable population remains paramount to improving quality of life and extending lifespan in cancer survivors.
Moderate-to-vigorous physical activity (MVPA) is a frequent intervention target to compress morbidity (8,9) and benefit quality of life by reducing fatigue and pain (10,11). Additionally, MVPA engagement is strongly associated with decreased mortality risk (12). Yet, only 8% of US cancer survivors met Federally-recommended levels of MVPA participation from 2003–2006, and, on average, they spent over 8 waking hours/day in sedentary behaviors (13). However, studies examining MVPA and sedentary time may be insensitive to volumes of light intensity activities or modifications in patterns of activity accumulation that may be informative to evaluating the onset and/or severity of fatigue, pain, and subsequent poor health outcomes (6). Using objective, continuous assessment of minute-by-minute activity as well as sedentary cycles throughout the day, a measure of activity fragmentation can be extracted to determine how physical activity (PA) is accrued and hampered among those with cancer history. This measure characterizes the frequency with which one transitions into a sedentary state from an active state throughout the day, providing contextual relevance to the manner in which PA is accrued (14). To date, the differences in fragmentation and the combined assessment of total daily PA and fragmentation in cancer survivors remain unexplored.
The primary aim of this study was to assess differences in markers of PA patterns in a large cohort of well-functioning middle- and older-aged adults (50+ years old) by cancer history. We hypothesized that cancer survivors would engage in less total daily PA and exhibit more fragmented patterns of PA than those without cancer history.
METHODS
Study design and population
This study used data collected from the Baltimore Longitudinal Study of Aging (BLSA) between 2007 and 2015. The BLSA is an ongoing enrollment cohort study primarily focused on the study of normative human aging and is conducted by the National Institute on Aging (NIA) Intramural Research Program. BLSA enrollment criteria and sample details have been published (15). Concisely, enrollment into BLSA requires no cognitive impairment, functional limitation, and chronic disease (except for hypertension) within the past 10 years. When enrolled, participants are followed for life and attend periodically scheduled comprehensive health, cognitive, and functional assessments every 1–4 years depending on age. These assessments are completed over a 3-day visit in the NIA Clinical Research Unit located at Harbor Hospital in Baltimore, Maryland. Trained and certified study staff who follow standardized protocols administer all evaluations. All participants gave written informed consent and the National Institute for Environmental Health Sciences Internal Review Board approved the study protocol.
Analytic sample
A total of 673 BLSA participants aged 50–96 had at least 3 valid days of accelerometer data (Actiheart, CamNtech, Cambridge, United Kingdom). Non-valid days of accelerometry collection defined as > 5% of 24-hour/day data missing, were excluded from the analysis (536 days or 12% of 4,597 days). For valid days (≤ 5% of data missing), missing values were imputed as the average counts/minute over all available days for each participant (16). Participants were excluded if they did not have a measure of usual gait speed (n=10, mean age of 75 ± 11 years) or information on depressive symptoms (n=4, mean age of 71 ± 16 years). The final analytic sample consisted of 659 participants who answered cancer history questions from a medical interview and had at least 3 valid days of PA data.
Cancer history
Participants self-reported cancer history via a standardized medical interview conducted by a nurse practitioner. Cancer survivors included participants who enrolled 10 years after completion of treatment or developed cancer history during time under study. Participants were asked “Has a doctor or other health professional ever said you had cancer, a malignant growth, or malignant tumor? (except for uterine ‘fibroids’)” and given options to answer either “Yes”, “No”, “Don’t know” or “Refused”. The 119 participants who answered “Yes” were defined as having self-reported cancer history, and were subsequently asked about cancer type, age at diagnosis, and history of recurrence. Participants who reported only basal or squamous skin cancers were not considered cancer survivors (n=104).
Accelerometer variables
Participants were fitted with an Actiheart monitor on the last day of their BLSA clinic visit. The Actiheart is a lightweight device that utilizes a uniaxial accelerometer and a heart rate monitor to measure PA in non-laboratory, community-dwelling settings. The device was positioned horizontally on the chest at the third intercostal space using two standard electrocardiogram electrodes, and participants were instructed to wear the monitor continuously for 7 consecutive days. The Actiheart collects movement as acceleration in units of gravity (g) at a sampling rate of 32 Hz per second. Data are aggregated into 1-minute activity counts (unit-less quantities of overall movement). At the end of the accelerometer collection period, participants returned the Actiheart to the Clinical Research Unit via express mail and the data were downloaded using Actiheart Software (version 4.0.103).
Accelerometer data were summarized into two continuous metrics: 1) total daily activity and 2) activity fragmentation. To calculate total PA volume, activity counts were summed across all minutes for each valid day and averaged across all valid days (total activity counts/day) for each participant. Because the distribution of total activity counts/day is right-skewed at higher intensities, total PA volume was log-transformed (LTAC; log-transformed total activity counts). To calculate activity fragmentation, an active-to-sedentary transition probability was calculated as the number of PA bouts (consecutive minutes registering > 10 counts per minute) divided by the total sum of minutes spent in PA (17). Higher activity fragmentation (e.g., higher score) represents more interruptions in activity performed throughout the day, translating to shorter activity bouts and more sedentary time. Both total volume and activity fragmentation were also treated as categorical variables by dichotomizing each variable at their respective medians to derive “high” and “low” groups. To characterize PA patterns, participants were categorized into four groups: high PA/low fragmentation, low PA/low fragmentation, high PA/high fragmentation, and low PA/high fragmentation.
Covariates
Age, sex, race, employment status, and smoking history were self-reported via a standardized questionnaire administered by study staff. Body mass index (BMI) was calculated using measured weight and height (kg/m2). Usual gait speed was measured over a 6-m course, with the faster of two trials used for analysis. Depressive symptoms were measured using the 20-item Center for Epidemiologic Studies-Depression scale (ranging from 0–60 where a higher score represents higher depressive symptoms). Participants were asked whether they were ever told by a doctor or other health professional that they had any of the following conditions: cardiovascular disease including angina, myocardial infarction, congestive heart failure, peripheral arterial disease, and vascular-related procedures; hypertension or high blood pressure; diabetes, glucose intolerance, or high blood sugar; cerebrovascular disease including stroke and transient ischemic attack (TIA); chronic bronchitis, emphysema, chronic obstructive pulmonary disease, or asthma; arthritis or osteoarthritis. Responses were summed and categorized into a comorbidity index score (0, 1, and 2+ morbid conditions).
Statistical considerations
Analysis of variance and chi-squared tests were used to test differences in participant characteristics by cancer history for continuous and categorical variables, respectively. Using a cross-sectional design, multivariable regression models were created to estimate differences in LTAC and fragmentation between those with and without cancer history. Because this association has not been previously explored, linear regression models were constructed for continuous accelerometer variables and logistic regression models were created for categorical variables to understand both continuous and threshold effects. Multinomial logistic regression was utilized to estimate the differences in PA accumulation among cancer history groups. All models were successively adjusted for age, sex, race, BMI, employment, smoking history, usual gait speed, depressive symptoms, and two or more comorbidities. Statistical significance was determined using two-tailed hypothesis testing with an alpha level=0.05. All statistical analyses were performed using Stata software (version 14.2; Stata Corporation, College Station, TX).
RESULTS
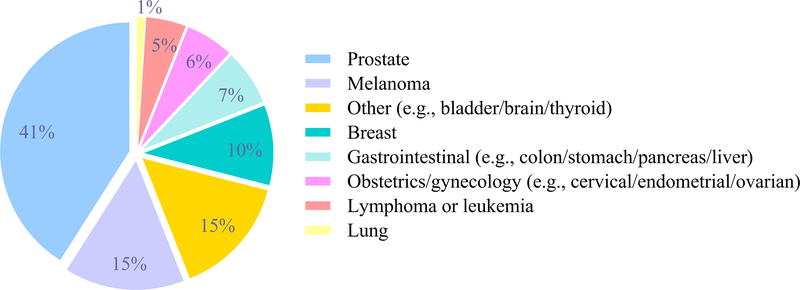
Those with cancer history tended to be older, men, not employed, and report a history of smoking when compared to those without cancer history (Table 1). Additionally, those with cancer history were more likely to have two or more comorbid conditions, particularly cardiovascular disease, hypertension, cerebrovascular disease, diabetes, and osteoarthritis. The average age at cancer diagnosis was 65 (SD=11) years; Figure 1 describes the major types of cancer reported. The highest prevalent cancer type was prostate (41%) and the lowest was lung cancer (1%).
Table 1.
Participant characteristics by cancer history (n=659)
| Cancer history (n=119) | No cancer (n=540) | p-value | |
|---|---|---|---|
| Age (years), mean (SD) | 75.0 (9.2) | 70.7 (9.9) | <0.001 |
| Female, n (%) | 37 (31.1) | 302 (55.9) | <0.001 |
| Black or African American, n (%) | 29 (24.4) | 137 (25.4) | 0.854 |
| Body mass index (kg/cm2), mean (SD) | 27.4 (4.5) | 27.5 (4.8) | 0.821 |
| Currently employed, n (%) | 34 (28.6) | 220 (40.7) | 0.014 |
| Cigarette smoking ever, n (%) | 57 (47.9) | 187 (34.4) | 0.006 |
| Usual gait speed (m/s), mean (SD) | 1.1 (0.2) | 1.1 (0.2) | 0.387 |
| CES-D, mean (SD) | 5.3 (5.6) | 4.8 (4.8) | 0.315 |
| 2 or more comorbiditiesa, n (%) | 98 (82.4) | 355 (65.7) | <0.001 |
| MI/CHF/angina/vascular procedure/PAD, n (%) | 26 (21.9) | 55 (10.0) | <0.001 |
| Hypertension, n (%) | 70 (58.8) | 250 (46.3) | 0.013 |
| Hyperlipidemia, n (%) | 75 (63.0) | 338 (62.6) | 0.930 |
| Stroke/TIA, n (%) | 16 (13.5) | 27 (5.0) | 0.001 |
| Pulmonary disease, n (%) | 18 (15.1) | 75 (13.9) | 0.726 |
| Diabetes, n (%) | 22 (27.7) | 97 (18.0) | 0.015 |
| Osteoarthritis, n (%) | 77 (64.7) | 290 (53.7) | 0.029 |
| Total activity counts/day, mean (SD) | 28,001 (16,861) | 34,727 (20,591) | 0.001 |
| LTAC/day, mean (SD) | 10.1 (0.6) | 10.3 (0.6) | <0.001 |
| Fragmentation index/day, mean (SD) | 0.29 (0.08) | 0.27 (0.07) | <0.001 |
| Accelerometer days, mean (SD) | 5.4 (1.2) | 5.1 (1.1) | 0.054 |
Self-reported history of being diagnosed by a doctor or other health professional
Notes: CES-D = Center for Epidemiological Studies-Depression scaled 0–60 where higher scores represent higher depressive-like symptoms; MI = myocardial infarction; CHF = congestive heart failure; PAD = peripheral arterial
Figure 1.
Cancer types in those with cancer history, n=119
Descriptively, cancer survivors accrued fewer daily activity counts (e.g., lower total PA) and had higher fragmentation indices (e.g., more interruptions in activity), shown in Table 1. Table 2 presents the means and SDs for LTAC and fragmentation (index score) by total sample and stratified by the median of each metric. The sample median was 10.28 for LTAC and 0.27 for fragmentation. Medians were used as thresholds for both variables that categorized 330 into a low group and 329 into a high group.
Table 2.
Categorization of total daily physical activity and fragmentation index stratified at the median
| Cancer history | No cancer | ||||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Threshold | |
| Total daily physical activity (LTAC) | 119 | 10.1 (0.6) | 540 | 10.3 (0.6) | 10.285 |
| Low physical activity | 77 | 9.7 (0.4) | 253 | 9.8 (0.4) | |
| High physical activity | 42 | 10.7 (0.3) | 287 | 10.7 (0.3) | |
| Total fragmentation index score | 119 | 0.29 (0.08) | 540 | 0.27 (0.07) | 0.264 |
| Low fragmentation | 41 | 0.21 (0.03) | 289 | 0.22 (0.03) | |
| High fragmentation | 78 | 0.33 (0.06) | 251 | 0.32 (0.05) | |
Note: LTAC = log-transformed total activity counts; representing transformed total activity volume; threshold column indicates the median used to categorize the total sample into low and high groups
Table 3 shows the estimated differences in PA by daily volume and fragmentation by cancer history status across four models that represent successive covariate adjustment. In model 4 (full covariate adjustment), cancer survivors accrued less total PA (beta coefficient= −0.12 LTAC/day, SE=0.05, p=0.02) than those with no cancer history. Additionally, those with cancer history had a 63% greater odds (Model 3: odds ratio (OR): 1.63, 95% confidence interval (CI): 1.03–2.58) of being in the low PA group (versus high) when compared to those without cancer history after adjusting for demographics and behaviors; however this association was attenuated after including comorbidities (Model 4: OR: 1.57, 95% CI: 0.99–2.49). No difference in activity fragmentation by cancer status was detected in the fully adjusted continuous model (Model 4: p=0.132). However, in the fully adjusted categorical model, cancer survivors had a 77% greater odds (Model 4: OR: 1.77, 95% CI: 1.11–2.82) of accumulating PA throughout the day in a more fragmented manner than those without cancer history.
Table 3.
Estimated differences in daily physical activity volume (LTAC) and fragmentation between those with and without cancer history (n=659; reference is no cancer history)
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| LTAC beta coefficient (SE) | ||||
| LTAC | −0.23 (0.06)*** | −0.13 (0.05)* | −0.12 (0.05)* | −0.12 (0.05)* |
| Odds ratio (95% conference interval) | ||||
| Low physical activity | 2.08 (1.38–3.14)*** | 1.66 (1.08–2.56)* | 1.63 (1.03–2.58)* | 1.57 (0.99–2.49) |
| High physical activity | Reference | Reference | Reference | Reference |
| Fragmentation index beta coefficient (SE) | ||||
| Fragmentation | 0.02 (0.007)** | 0.01 (0.007)* | 0.01 (0.006) | 0.01 (0.006) |
| Odds ratio (95% conference interval) | ||||
| Low fragmentation | Reference | Reference | Reference | Reference |
| High fragmentation | 2.19 (1.45–3.31)*** | 1.79 (1.16–2.75)** | 1.74 (1.10–2.77)* | 1.77 (1.11–2.82)* |
Model 1: Unadjusted model.
Model 2: Model 1 adjusted for age.
Model 3: Model 2 + sex, race, body mass index (kg/m2), employment, smoking history, usual gait speed (m/s)
Model 4: Model 3 + depressive-like symptoms and 2 or more comorbidities.
p<0.001
p<0.01
p<0.05
Note: LTAC – log-transformed total activity counts; representing transformed total activity volume
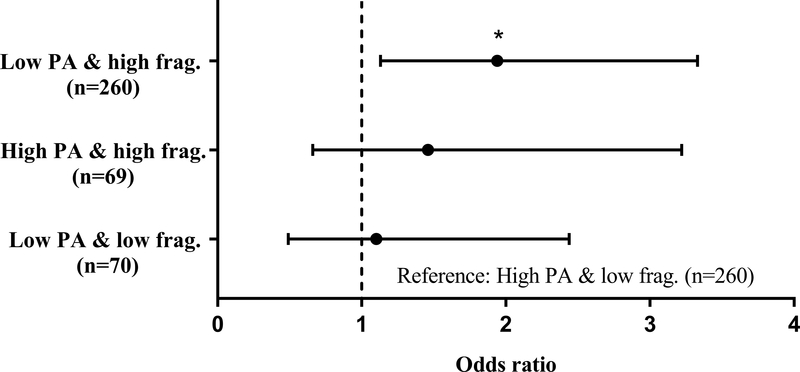
Differences in PA accumulation by cancer history groups are shown in Figure 2. Cancer survivorship was associated with a 94% greater odds (OR: 1.94, 95% CI: 1.13–3.33) of being low PA/high fragmentation (versus high PA/low fragmentation) than no cancer history after full covariate adjustment. No detectable differences were observed between cancer history status with either low PA/low fragmentation (p=0.824) or high PA/high fragmentation (p=0.346) when compared to high PA/low fragmentation, respectively.
Figure 2.
Adjusted odds ratios of physical activity accumulation in those with cancer history compared to those without (reference), n=659
Note: PA – physical activity; frag. – fragmentation
Model adjusted for age, sex, race, body mass index (kg/m^2), employment, smoking history, usual gait speed (m/s), depressive-like symptoms and 2 or more comorbidities. *p<0.05
DISCUSSION
In addition to differences in total daily PA between participants reporting no history of cancer and cancer survivors, we found that survivors tended to accumulate their daily PA in a more fragmented manner. For example, a 60-year old cancer survivor accumulates the same amount of PA in a nearly-identical fragmented pattern as a cancer-free, 65-year old adult when holding other demographics, behavioral factors, and comorbidities constant. These results suggest that cancer onset or medical interventions to treat it may have detrimental and lasting effects on both the time and the duration spent being active, possibly reducing the capacity to endure longer bouts of daily activity, with aging.
Our results suggest that cancer survivors not only engage in less daily PA but also accumulate daily activities in bouts of shorter duration by taking more sedentary breaks. Previous studies have primarily investigated differences in time spent at varying intensities of activity by cancer history, with a focus on meeting Federally-recommended PA guidelines (150 minutes/week in MVPA) (13,18,19). In general, these studies have suggested that cancer survivors largely do not reach recommended PA guidelines and are more likely to engage in light-intensity activities than moderate or vigorous intensity activities (13,18,20). Further, Thraen-Borowski and colleagues showed that total time spent in light-intensity activity was significantly lower while sedentary time was higher in those with cancer history than those with no cancer history (13). It is important to note that the accelerometer data from that study was collected from NHANES (National Health and Nutrition Examination Survey) whose analytic sample was comparatively younger (mean age of 62 years) with more women surviving cancer (58%) than the current BLSA sample (mean age 74 years, 31% women) (13). These differences are mainly driven by differences in the distribution of cancer types, but show that despite these differences, there are robust variations in quantities and patterns of daily PA by cancer history. Further, our results expand on these findings to show that these differences may perpetuate for years after treatment in well-functioning older adults.
Objective methods to describe transitions into and out of activity throughout the day are not well understood. To our knowledge, our findings are the first to define and describe activity fragmentation, using the probability to transition from an active to sedentary state, in cancer survivors. These findings suggest that long-term cancer survivors accrue PA in shorter bouts with a greater number of breaks in activity throughout the day. Taking more activity breaks translates to spending more time in sedentary behaviors and increasing likelihood of taking fewer breaks in sedentary time. These findings are consistent with previous work from NHANES, suggesting that cancer survivors tend to take fewer breaks from their sedentary behavior, despite accumulating similar total daily sedentary time, translating to longer bouts of sedentary behavior compared to those with no cancer history (13). Additionally, BLSA cancer survivors had markedly lower employment rates and higher rates of smoking and comorbidities compared to those without cancer history; factors that warrant further investigation as possible contributors to the degradation of daily PA accrual. Collectively, these results indicate that cancer survivors may be at elevated risk of deleterious effects of prolonged sedentary behavior, including adverse metabolic (21,22) and cardiovascular health (23), functional decline (24,25), and mortality (26).
Fragmented PA patterns observed in BLSA participants with cancer history are potentially explained through the likelihood of becoming fatigued faster when performing sustained PA. In healthy adults 50 years and older, self-reported fatigue in response to a standardized task, or perceived fatigability, has been shown to be negatively associated with PA (27). Further, time-of-day differences in PA accumulation were observed across fatigability levels—showing that those with higher fatigability had delayed activity peaks and earlier downshifting in PA participation later in the day. Using the same cohort, Gresham and colleagues showed that cancer history was associated with higher odds of being highly fatigable and presenting poor walking endurance (6). Additionally, those with cancer history had an increased risk of becoming highly fatigable with progressive aging. Potential explanations of fatigue-driven fragmentation include the burden of senescence cell accumulation as a side-effect from cancer treatment (28), or more clinical side effects such as muscle deconditioning and loss (29,30), weight gain (31,32), and pain (3). Collectively, these results suggest that fatigue and fatigability are likely lasting results of either cancer, cancer treatment, or a combination of both but more research is needed to examine fragmentation’s capability to indicate the onset and progression of fatigability, changes in body composition, pain, and reductions in energetic capacity and reserve experienced by cancer survivors.
We acknowledge there are limitations to this study. First, due to limited accelerometer data, we were not able to characterize a longitudinal relationship between cancer history and PA accumulation. Second, our study was not powered to determine whether cancer type, stage, and treatment played a role in the association between cancer history and daily PA. Third, cancer diagnoses occurring closer to accelerometer collection periods were not accounted and may explain some of the differences observed in cancer survivors. Fourth, activity was calculated as movement generated from the chest and the observed differences may be attenuated when compared to body locations that capture more movement such as the wrist (33). Fifth, BLSA participants are healthier than the general older adult population thus potentially underestimating the effects of cancer history on daily PA. More research is needed to replicate our findings, particularly in short-term cancer survivors and those actively receiving treatment. Strengths of our study include a large sample of older adults with objective PA data, utilization and combination of novel accelerometer metrics to phenotype PA accumulation in a cancer-related cohort, and using data from a study primarily meant to study normative aging and therefore reducing the potential of comorbid disease burden.
To our knowledge, this is the first study to utilize activity fragmentation, a novel biophysical marker of activity performed in free-living settings, in a cancer population. Our findings suggest that older adults with a history of cancer accumulate less daily activity, and that their activity is performed in a less continuous and more fragmented way. Further research is necessary to understand the biological underpinnings driving the fragmentation of daily activity—including physiological reserve and accelerated functional decline with aging—and develop interventions that promote recovery to normal activity levels in adults who experience cancer. Additionally, longitudinal studies are needed to define clinically important increases in activity fragmentation that may be indicative of the onset and progression of adverse health conditions commonly observed among cancer survivors.
Acknowledgments
FUNDING
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. Data used in the analyses were obtained from the Baltimore Longitudinal Study of Aging, a study of the National Institute on Aging Intramural Research Program. Accelerometer analyses were supported by R21AG053198 and P30AG021334. AW is supported by T32AG000247 and P30AG021334. JS is supported by R21AG053198, P30AG021334, and U01AG057545.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no disclosures.
INVITATION
This was not an invited paper to CANCER.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 3.Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32(16):1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deprez S, Kesler SR, Saykin AJ, Silverman DH, de Ruiter MB, McDonald BC. International cognition and cancer task force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. JNCI: Journal of the National Cancer Institute. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. The lancet oncology. 2013;14(8):721–732. [DOI] [PubMed] [Google Scholar]
- 6.Gresham G, Dy SM, Zipunnikov V, et al. Fatigability and endurance performance in cancer survivors: Analyses from the baltimore longitudinal study of aging. Cancer. 2018;124(6):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100(16):1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries JF. Physical activity, the compression of morbidity, and the health of the elderly. J R Soc Med. 1996;89(2):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. The Journals of Gerontology Series A: Biological sciences and medical sciences. 2001;56(suppl_2):23–35. [DOI] [PubMed] [Google Scholar]
- 11.Dimeo FC, Stieglitz R, Novelli‐Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85(10):2273–2277. [PubMed] [Google Scholar]
- 12.Fishman EI, Steeves JA, Zipunnikov V, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. 2016;48(7):1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the united states. PloS one. 2017;12(8):e0182554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chastin SFM, Ferriolli E, Stephens NA, Fearon KC, Greig C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing. 2011;41(1):111–114. [DOI] [PubMed] [Google Scholar]
- 15.Stone JL, Norris AH. Activities and attitudes of participants in the baltimore longitudinal study. J Gerontol. 1966;21(4):575–580. [DOI] [PubMed] [Google Scholar]
- 16.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: Detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrack JA, Leroux A, Fleg JL, et al. Using heart rate and accelerometry to define quantity and intensity of physical activity in older adults. The Journals of Gerontology: Series A. 2018;73(5):668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loprinzi PD, Lee H, Cardinal BJ. Objectively measured physical activity among US cancer survivors: Considerations by weight status. Journal of Cancer Survivorship. 2013;7(3):493–499. [DOI] [PubMed] [Google Scholar]
- 19.Smith WA, Nolan VG, Robison LL, Hudson MM, Ness KK. Physical activity among cancer survivors and those with no history of cancer- a report from the national health and nutrition examination survey 2003–2006. Am J Transl Res. 2011;3(4):342–350. [PMC free article] [PubMed] [Google Scholar]
- 20.Vallance JK, Boyle T, Courneya KS, Lynch BM. Accelerometer-assessed physical activity and sedentary time among colon cancer survivors: Associations with psychological health outcomes. Journal of Cancer Survivorship. 2015;9(3):404–411. [DOI] [PubMed] [Google Scholar]
- 21.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. [DOI] [PubMed] [Google Scholar]
- 22.Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58(8):1776–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seguin R, LaMonte M, Tinker L, et al. Sedentary behavior and physical function decline in older women: Findings from the women’s health initiative. Journal of aging research. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis MG, Fox KR, Stathi A, Trayers T, Thompson JL, Cooper AR. Objectively measured sedentary time and its association with physical function in older adults. J Aging Phys Act. 2014;22(4):474–481. [DOI] [PubMed] [Google Scholar]
- 26.Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in US middle-aged and older adults: A national cohort study. Ann Intern Med. 2017;167(7):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid-to late-life. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2017:glx181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaria M, O’Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin Nutr. 2014;33(5):737–748. [DOI] [PubMed] [Google Scholar]
- 30.Moylan JS. Preserving muscle health and wellbeing for long‐term cancer survivors. J Physiol (Lond). 2015;593(8):1767–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obesity reviews. 2011;12(4):282–294. [DOI] [PubMed] [Google Scholar]
- 32.Berg M, Winkels R, Kruif JTC, et al. Weight change during chemotherapy in breast cancer patients: A meta-analysis. BMC Cancer. 2017;17(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang JH, Macfarlane DJ, Sobko T. Feasibility of a chest-worn accelerometer for physical activity measurement. Journal of science and medicine in sport. 2016;19(12):1015–1019. [DOI] [PubMed] [Google Scholar]