Abstract
Misuse of prescription opioids, opioid addiction, and overdose underscore the urgent need for developing addiction-free effective medications for treating severe pain. Mu opioid peptide (MOP) receptor agonists provide very effective pain relief. However, severe side effects limit their use in the clinical setting. Agonists of the nociceptin/orphanin FQ peptide (NOP) receptor have been shown to modulate the antinociceptive and reinforcing effects of MOP agonists. Here, we report the discovery and development of a bifunctional NOP/MOP receptor agonist, AT-121, which possesses partial agonist activity for both NOP and MOP receptors. AT-121 suppressed oxycodone’s reinforcing effects and exerted morphine-like analgesic effects in nonhuman primates. AT-121 treatment did not induce side effects commonly associated with opioids, such as respiratory depression, abuse potential, opioid-induced hyperalgesia, and physical dependence. Our results in nonhuman primates suggest that bifunctional NOP/MOP agonists with the appropriate balance of NOP and MOP agonist activity may provide a dual therapeutic action for safe and effective pain relief and treating prescription opioid abuse.
One Sentence Summary:
The small molecule AT-121 is an agonist of nociceptin and mu opioid peptide receptors and mediates analgesia without opioid-associated side effects in non-human primates.
INTRODUCTION
The recent marked increase in misuse and abuse of prescription opioids and the epidemic of opioid overdose mortality have greatly affected our society (1–3). Although mu opioid peptide (MOP) receptor agonists remain the most effective and widely used analgesics, their abuse liability and possibility of causing respiratory arrest have contributed to escalating medical and economic burdens and fueled the opioid crisis in the global community (4, 5).
Multiple scientific strategies have been tested to develop safe and effective medications for pain and opioid addiction (6, 7). As finely articulated by Volkow and Collins (7), the crisis surrounding this dynamic epidemic of opioid use disorders fall into three critical needs that can be addressed with novel scientific approaches for (a) reversing opioid overdose, (b) treating opioid addiction, and (c), finding safe, new approaches for effective pain relief without the side effects of opioids. The lack of alternative analgesics that offer opioid-like pain relief without associated adverse effects is a major contributor to the current opioid crisis (2, 7, 8).
Kappa and delta opioid receptor agonist-based analgesics, although effective, possess different adverse effects, such as dysphoria and convulsion, and have a narrow therapeutic window (6, 9). Current efforts to modulate MOP agonist-induced adverse effects have centered on MOP agonists that favor intracellular G-protein over arrestin signaling, based on the rationale that arrestin signaling is associated with undesirable effects of opioids (10, 11). TRV-130 (oliceridine) was developed as a G-protein biased MOP agonist with potency and efficacy similar to morphine, but with fewer side effects (12). However, repeated administration of TRV-130 retained abuse liability in a rodent model (13). Clearly, new approaches are still needed to obtain effective opioid-like analgesic efficacy without MOP agonist-associated side effects.
Agonists targeted to the fourth opioid receptor subtype, the nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor, have been shown to modulate the pain-relieving and addictive properties of MOP agonists (14–17). The NOP receptor and its endogenous ligand N/OFQ are expressed in the same neuronal circuitry as the other opioid receptors and generally inhibit neuronal transmission (16, 18). In nonhuman primates, selective NOP receptor agonists produce antinociception and antihypersensitivity at the spinal, supraspinal, and systemic levels (19–21). More importantly, NOP agonists do not produce respiratory depression and reinforcing effects (abuse potential) in primates, and they enhance MOP agonist-induced analgesia synergistically at spinal and systemic levels (21–23). Given the inhibitory effects on dopamine release and neurotransmission by the N/OFQ-NOP receptor system (14, 24, 25), we hypothesized that bifunctional NOP/MOP agonists may be a viable approach to develop non-addictive analgesics (9, 26–28).
Several bifunctional NOP/MOP agonists have been discovered in the past few years (27–30). Although these compounds, such as AT-201 and AT-212, had antinociceptive effects in rodent models of acute and chronic pain, they exerted rewarding effects (27, 28, 31). In addition, some aspects of the antinociceptive functional profiles of NOP/MOP agonists in rodents did not generalize to rhesus monkeys (for instance, the opposing effects of NOP antagonists) (22, 30, 32, 33). The balance of NOP versus MOP agonist efficacy appears to be a key factor for developing a suitable non-addictive analgesic devoid of MOP agonist liabilities.
Differences between rodents and primates in the antinociceptive and pharmacological profiles of NOP- and MOP-targeted compounds have been extensively documented (22, 34, 35) and there is ample evidence of neuroanatomic, neurochemical, and neuropharmacological similarities between primates and humans (9, 36, 37). Thus, we conducted in vivo pharmacological studies in monkeys as a translational platform to determine the effects of a dual NOP/MOP agonist on a variety of behavioral and physiological responses.
Using receptor structure-guided drug design and rational structure-activity relationship (SAR) exploration, we developed a bifunctional NOP/MOP agonist, AT-121, that produced potent analgesic effects without inducing opioid-associated hyperalgesia or physical dependence in non-human primates. In addition, AT-121 attenuated the reinforcing effects of oxycodone as measured by drug self-administration in primates. Our results suggest that AT-121 might be an effective safer alternative to opioids for treating severe pain.
RESULTS
Bifunctional NOP/MOP receptor agonists were developed by structure-guided drug design and optimization starting from a NOP receptor-selective chemical scaffold
To design a bifunctional ligand with the desired spectrum of binding affinity and functional efficacy at the NOP and MOP receptors within a single chemical scaffold, we began with structure-activity optimization of a new chemical lead within our NOP ligand library, 1, which showed modest binding affinity for the NOP receptor but about 10-fold lower affinity for the MOP receptor (Fig. 1A, Table 1). This early lead had low partial agonist efficacy at the NOP receptor in the guanosine 5’-O-[gamma-thio]triphosphate (GTPγS) functional assay and no agonist efficacy at MOP (Table 1). To improve binding affinity and bifunctional NOP/MOP efficacy, we used structure-guided SAR optimization, starting by docking 1 in the active-state NOP receptor structure we previously developed (38). We hypothesized that substituting cationic groups for the non-ionic cyanomethyl group on the isoquinolinone nitrogen of 1 would improve NOP binding affinity by enabling interactions with the anionic amino acids in the second extracellular loop (EL2) of the NOP receptor towards which the ligand 1 was oriented. We synthesized analogs 2–6 (Fig. 1) to explore this SAR hypothesis. All analogs were synthesized from chemical modifications of the lead chemical scaffold 1, as shown in fig. S1, and described in Supplementary Methods.
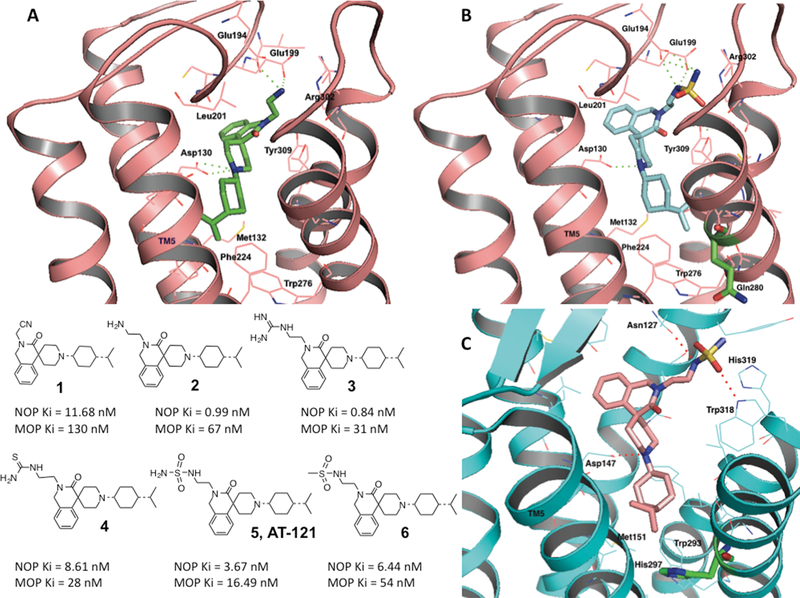
Figure 1. Structure-based design and optimization of bifunctional NOP-MOP ligands.
(A) top: Docking and binding interactions of compound 2 (shown as green stick representation) into the active-state NOP homology model (38), shown in cartoon representation (pink). Amino acids in a 4 angstrom radius around the ligand are shown as pink sticks and are labeled. Polar interactions of the 2-aminoethyl moiety of 2 with Glu194 and Glu199 in the extracellular loop (EL) 2 of the receptor are shown as green dotted lines. Bottom: structures of the isoquinolinone-based bifunctional ligands and their binding affinities at NOP and MOP. (B) Docking and binding interactions of compound 5 (AT-121) (shown in cyan) in the active-state NOP structure (pink). Note the interactions of the nitrogens of the sulfamide group with the Glu194 and Glu199 of the EL2 loop of NOP. Gln280, the non-conserved amino acid between NOP and the opioid receptors (His, shown in Fig. 1C), shown as green sticks, does not interact with the ligand. (C) Docking and binding interactions of bifunctional compound 5 in the MOP active-state crystal structure (73), shown in cyan cartoon representation. The interacting amino acids are shown as cyan stick representations. The lipophilic isopropylcyclohexyl group binds to a nonpolar pocket lined with Met131, His 297 (green sticks) and Trp 293 in the MOP binding pocket.
Table 1:
In vitro pharmacological profile of NOP/MOP bifunctional agonists in binding and functional assays at the opioid receptors†
| Receptor Binding Ki (nM) | NOP Functional Assays# | MOP Functional Assays# | KOP Functional Assays# | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [35S] GTPyS NOP | [35S] GTPyS MOP | [35S] GTPyS KOP | ||||||||
| NOP | MOP | KOP | DOP | EC50 (nM) | % Stim | EC50 (nM) | % Stim | EC50 (nM) | % Stim | |
| N/OFQ | 0.08 ± 0.03 | 133 ± 30 | 247 ± 3.4 | 2846 ± 512 | 4.0 ± 0.1 | 100 | >10,000 | >10,000 | ||
| DAMGO | 2.96 ± 0.54 | 32.6 ± 4.06 | 100 | |||||||
| DPDPE$ | 1.11 ± 0.07 | |||||||||
| U69.593 | 1.05 ± 0.02 | 60.14 ± 7.45 | 100 | |||||||
| 1 | 11.7 ± 3.75 | 130.3 ± 0.7 | 500.3 ± 28 | 331.5 ± 1.6 | 181.7 ± 4.4 | 28.2 ± 0.6 | >10,000 | - | NT | |
| 2 | 0.99 ± 0.04 | 67.24 ± 2.5 | 260.3 ± 22 | 1603 ± 63 | 186 ± 45 | 42.1 ± 1.0 | >10,000 | - | NT | |
| 3 | 0.84 ± 0.06 | 30.8 ± 2.5 | 68.9 ± 3.2 | 834 ± 74 | 229.0 ± 22 | 34.1 ± 0.4 | 128 ± 12.8 | 9.3 ± 2.2 | 36.81 ± 11.7 | 21.35 ± 3.4 |
| 4 | 8.61 ± 0.56 | 28.08 ± 2.7 | 69.7 ± 22 | 43.09 ± 18.6 | 123.5 ± 50 | 27.5 ± 3.0 | 96.0 ± 38.7 | 8.2 ± 2.2 | >10,000 | - |
| 5(AT-121) | 3.67 ± 1.10 | 16.49 ± 2.1 | 301.3 ± 35.4 | 145.6 ± 25.5 | 34.7 ± 6.29 | 41.1 ± 0.3 | 19.6 ± 6.9 | 14.2 ± 0.40 | NT | |
| 6 | 6.44 ± 0.44 | 53.94 ± 2.0 | 455.5 ± 63 | 184.7 ± 63 | 173.4 ± 22 | 45.1 ± 12 | 115.6 ± 20 | 6.7 ± 0 | >10,000 | - |
| Morphine | >10,000 | 1.1 ± 0.1 | 46.9 ± 14.5 | 140.0 ± 1.5 | >10,000 | 5.4 ± 1 | 15.6 ± 0.5 | 93 ± 9 | 576 ± 81.5 | 25.0 ± 1.95 |
| Buprenorphine | 140 ± 11.2 | 0.15± 0.13 | 2.50 ± 1.2 | 6.13 ± 0.4 | >10,000 | 12.6± 6 | 7.2 ± 3.5 | 27.0 ± 4.8 | >10,000 | - |
Details of the experimental procedures are given in the Supplementary Information-Materials and Methods. Values are the Mean ± SEM of three independent experiments run in triplicate.
NT = Not tested. Compounds with binding affinity Ki >100 nM are not tested in the functional assay.
DPDPE = (D-Penicillamine2,D-Penicillamine5)-Enkephalin.
Functional activity was determined by stimulation of [35S]GTPγS binding to cell membranes, % stimulation was obtained as a percentage of stimulation of the standard full agonists, N/OFQ (for NOP), DAMGO (for MOP), and U69,593 (for KOP), which showed at least 2- to 5-fold stimulation over basal, indicative of a robust assay. The stimulation by the standard full agonists was taken as 100% when comparing stimulation by the test compound.
As shown in Table 1, an aminoethyl substitution on the isoquinolinone nitrogen significantly improved the affinity of analog 2 for the NOP receptor to a binding affinity constant (Ki) of 1 nM. As seen in Fig. 1A, the aminoethyl groups showed ionic polar interactions with both the Glu194 and Glu199 of the EL2 loop. Analog 2 had slightly improved affinity for the MOP receptor over compound 1; however, it still lacked agonist efficacy at the MOP receptor when tested in the GTPγS functional assay (Table 1). An amidinoethyl group in the same position (in analog 3) further improved NOP and MOP binding affinity. An improvement in MOP affinity was also observed with a polar but non-ionic thioureidoethyl group (analog 4), albeit with a slight decrease in NOP affinity, suggesting that binding of this chemical scaffold at the MOP receptor could be improved with larger polar substituents without significant loss in affinity at the NOP receptor (Fig. 1A, Table 1).
Further ligand optimization by introducing the larger polar ethylsulfamide group onto the isoquinolinone nitrogen significantly increased MOP receptor affinity (MOP Ki = 16.49 nM) compared to analogs 2 and 3, maintaining high affinity at the NOP receptor (Ki = 3.67 nM), resulting in the bifunctional NOP/MOP ligand, 5 (AT-121) (Fig. 1A, Table 1). The SAR showed that the ethylsulfamide group was important for high affinity at both receptors, because the closely related N-ethylmethanesulfonamide analog 6 had much lower affinity at MOP and also at NOP receptors (Table 1). Docking the bifunctional NOP/MOP ligand 5 into the active-state NOP receptor (Fig. 1B) and MOP receptor (Fig. 1C) showed that 5 interacted with conserved amino acids in both receptors (NOP Asp1303.32 and MOP Asp1473.32, NOP Met1323.36 and MOP Met1513.36, NOP Trp2766.48 and MOP Trp2936.48) (Fig.1 B and C respectively) (Ballesteros Weinstein numbering in superscript) leading to high affinity at both targets. Ligand 5 selectively interacted with a non-conserved amino acid His2976.52 in the MOP receptor (Fig. 1C), that is shown to confer binding selectivity to opiate ligands for MOP over NOP receptors (39). Compound 5 did not interact with the corresponding non-conserved residue in NOP, Gln2806.52 in transmembrane helix 6 (TM6) of the NOP receptor (shown in Fig. 1B). Selective interactions of ligand 5 with both conserved and non-conserved residues in both receptors possibly contributed to high affinity binding at both receptors. All analogs of this chemical scaffold showed significantly lower affinity for the kappa (KOP) and delta (DOP) opioid receptors compared to NOP and MOP receptors (Table 1).
AT-121 is a bifunctional NOP/MOP partial agonist
Functional efficacy at both receptors was determined for all compounds using a guanosine-5’-O-(3-[35S]thio) triphosphate ([35S]GTPγS) binding assay in cell membranes from chinese hamster ovary cells (CHO-hNOP and CHO-hMOP) stably expressing the human NOP and MOP receptors as we have reported previously (40–42). Concentration-response curves were run in parallel with the prototypical receptor full agonists N/OFQ for the NOP receptor and DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) for the MOP receptor as reference agonists for the assay. Functional efficacies of the test compounds are reported as percent stimulation of [35S]GTPγS binding compared to a full response (100% stimulation) by the reference full agonists (Table 1). The potencies of the functional response are reported as EC50 values determined by nonlinear regression analysis and are presented in Table 1.
The initial lead compound 1 showed low efficacy and potency at the NOP receptor and no agonist efficacy at the MOP receptor in the GTPγS assay. Analogs 2–4 had nanomolar binding affinity at NOP but poor to low agonist potencies and efficacies at NOP and MOP in the GTPγS assay, and did not have a desirable bifunctional NOP/MOP agonist profile. Analog 5 (AT-121) on the other hand, showed high potency (EC50 = 35 nM) and partial agonist efficacy at the NOP receptor (Table 1) relative to N/OFQ, and high potency (EC50 = 20 nM) and partial agonist activity at the MOP receptor, relative to DAMGO. AT-121 showed significantly lower binding affinity at the DOP or KOP receptors (Ki >100 nM) (Table 1). Overall, these findings indicate that AT-121 has bifunctional NOP/MOP agonist activity, showing high potency and partial agonist efficacy at both NOP and MOP receptors. We selected AT-121 as a prototype to explore our hypothesis that bifunctional NOP/MOP agonists possessing an appropriate balance of NOP and MOP agonist efficacies may be a viable approach to develop analgesics with reduced side effects. Characterization of the pharmacokinetics and brain permeability of AT-121 in rats and assessment of stability of AT-121 in monkey plasma (shown in figs. S2 and S3 and tables S1, S2 and S3 respectively) showed that AT-121 had suitable pharmacokinetic properties for further in vivo studies.
AT-121 produces potent antinociceptive and antiallodynic effects
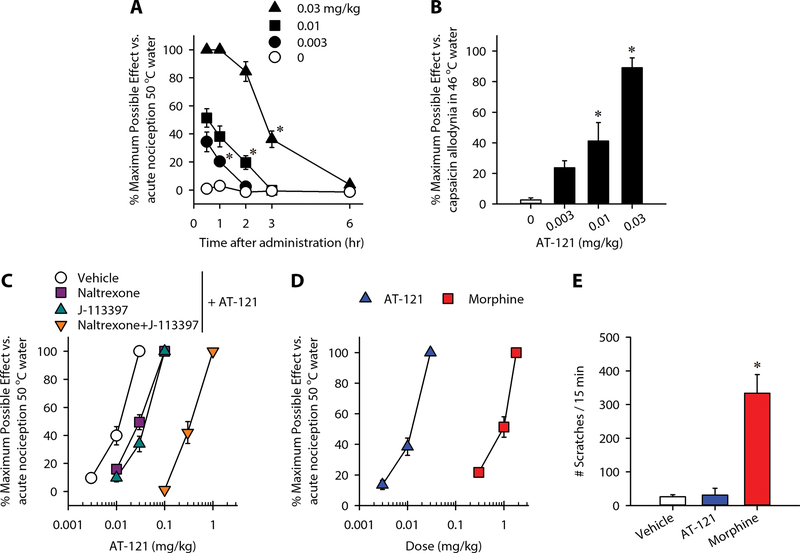
MOP receptor agonists change nociceptive thresholds and produce antinociception in nonhuman primates and humans (43–45). We used the warm water tail-withdrawal assay in primates to determine the functional efficacy of AT-121 for changing the nociceptive threshold. Subjects’ baselines values are presented in table S4. Following subcutaneous administration (0.003–0.03 mg/kg), AT-121 produced antinociceptive effects against an acute noxious stimulus, 50 °C water, in a dose-dependent [F(3, 9) = 163.9; p < 0.05] and time-dependent [F(4, 12) = 114; p < 0.05] manner (Fig. 2A). The minimum effective dose of AT-121 to produce full antinociception was 0.03 mg/kg. This dose had a 3-hour duration of action, which subsided by 6 hours (Fig. 2A). To determine the effects of AT-121 on pain sensitivity, we used a clinically relevant model, capsaicin-induced allodynia, which is widely applied to evaluate analgesics in humans (46, 47). Systemic AT-121 exerted a dose-dependent inhibitory effect on capsaicin-induced thermal allodynia in 46 °C water (F = 22.5; p < 0.05) (Fig. 2B).
Figure 2. Effects of systemic administration of AT-121 on modulating sensory processing in nonhuman primates.
(A) Effect of AT-121 administration on acute noxious stimulus, 50 °C water. (B) Bar graph showing the effect of AT-121 on antihypersensitivity against capsaicin-induced allodynia in 46 °C water. (C) Effects of NOP receptor antagonist J-113397 (0.1 mg/kg) and MOP receptor antagonist naltrexone (0.03 mg/kg) on AT-121-induced antinociception. (D) Comparison of antinociceptive potency of AT-121 and morphine. (E) Comparison of itch scratching responses elicited by AT-121 (0.03 mg/kg) and morphine (1 mg/kg) at antinociceptive doses. Each data point represents mean ± SEM (n = 4). All compounds were delivered subcutaneously. Data were analyzed by two-way ANOVA with repeated measures (panel A) or one-way ANOVA with repeated measures (panels B and E), followed by Bonferroni’s multiple comparisons test. ∗p < 0.05, significantly different from vehicle condition from the first time point to the corresponding time point.
Next, we conducted antagonist studies using the NOP receptor antagonist J-113397 and the MOP receptor-selective dose of the opioid receptor antagonist naltrexone (19, 23). Pretreatment with J-113397 (0.1 mg/kg) or naltrexone (0.03 mg/kg) produced small degrees (2–3-fold dose ratio) of rightward shift of the dose-response curve for AT-121-induced antinociception. Furthermore, combined pretreatment with both J-113397 and naltrexone provided a larger rightward shift (30-fold) of AT-121’s dose-response curve (Fig. 2C). These findings indicate that both NOP and MOP receptors contributed to antinociceptive effects of AT-121. Analysis of the dose-response curves showed that systemic AT-121 was more potent than morphine (ED50 = 0.01 mg/kg versus 1 mg/kg) (Fig. 2D). To examine whether AT-121 elicited an itch sensation, we compared its effects with morphine, which has been shown to elicit scratching responses in monkeys (48). AT-121 (0.03 mg/kg) did not significantly increase scratching responses (p > 0.9) (Fig. 2E). In contrast, morphine (1 mg/kg) increased the number of scratches in the same subjects (p < 0.05). Taken together, the data show that systemic AT-121 had a promising analgesic profile in primates distinct from typical mu opioid analgesics such as morphine.
AT-121 lacks reinforcing effects and attenuates reinforcing effects of oxycodone
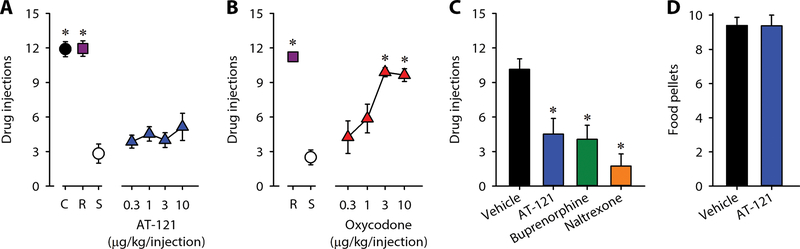
To examine and compare the reinforcing strengths of compounds, we used a progressive-ratio schedule of reinforcement, which is commonly used for evaluating abuse potential (49, 50). Monkeys were allowed to self-administer single doses of cocaine and remifentanil, and various doses of AT-121 and oxycodone (0.3–10 μg/kg/injection). Substitution of saline between test compounds resulted in a low number of reinforcers (three or fewer injections) (Fig. 3A). The reinforcing strength of remifentanil (0.3 μg/kg/injection) was similar to that of cocaine (0.03 mg/kg/injection) (Fig. 3A). There was no significant difference between the reinforcing strengths of saline and AT-121 (F = 1.2; p > 0.1) (Fig. 3A). In contrast, oxydocone produced reinforcing effects dose-dependently (F = 9.7; p <0.05) (Fig. 3B). In addition, similar to buprenorphine (0.1 mg/kg) and naltrexone (0.01 mg/kg), pretreatment with AT-121 (0.03 mg/kg) effectively attenuated the reinforcing effects of oxycodone (F = 25.5; p < 0.05) (Fig. 3C). This attenuation by AT-121 was selective against oxycodone, because AT-121 did not attenuate the reinforcing effects of food pellets (Fig. 3D). Collectively, AT-121 was devoid of reinforcing effects (abuse potential) and attenuated reinforcing effects of oxycodone, suggesting its therapeutic potential for opioid addiction.
Figure 3. Effects of AT-121 on reinforcing effects in nonhuman primates.
(A, B) Number of injections received as a function of dose in monkeys responding to cocaine (C, 0.03 mg/kg/injection), remifentanil (R, 0.3 μg/kg/injection), saline (S, ~0.14 mL/kg/injection), AT-121 (0.3‒10 μg/kg/injection) or oxycodone (0.3‒10 μg/kg/injection) under a progressive-ratio schedule of reinforcement. (C) Effects of the vehicle (0.1 mL/kg), AT-121 (0.03 mg/kg), buprenorphine (0.1 mg/kg) or naltrexone (0.01 mg/kg) on the reinforcing effects of oxycodone (3 μg/kg/injection). Each compound was administered intramuscularly 30 min before starting the progressive-ratio schedule of oxycodone. (D) Effect of the vehicle (0.1 mL/kg) or AT-121 (0.03 mg/kg) on the reinforcing effects of food pellets. AT-121 or its vehicle was administered intramuscularly 30 min before starting the fixed-ratio schedule of food pellets. Each data point represents mean ± SEM (n = 4). Data were analyzed by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test. ∗p < 0.05, a significant difference from saline or vehicle.
Higher doses of AT-121 do not compromise physiologic functions
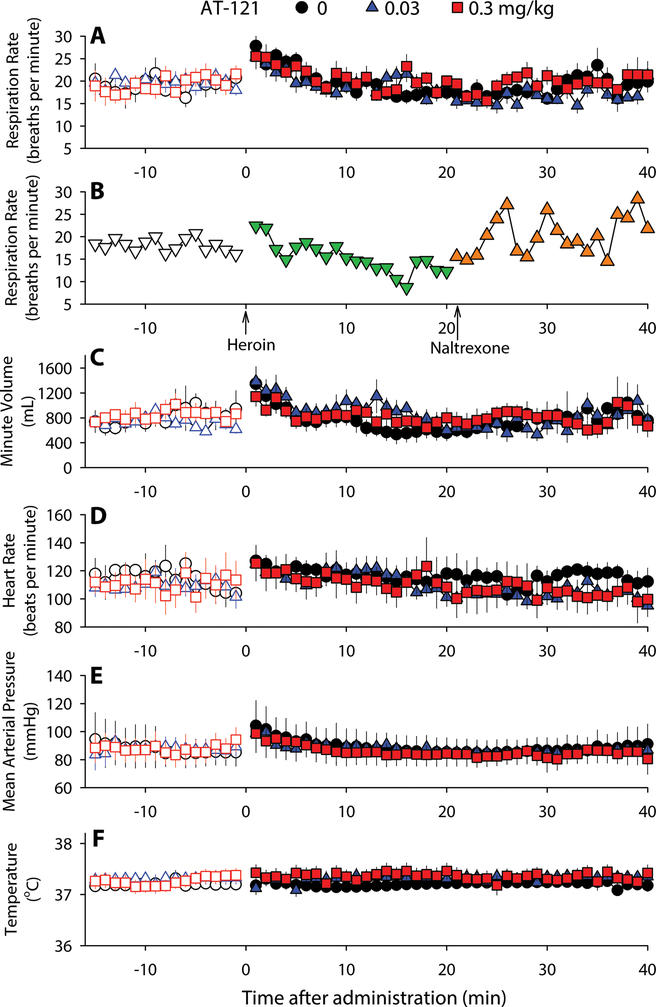
To characterize the appropriate dosage of AT-121, we measured physiologic parameters in monkeys implanted with radio-telemetric transmitters able to monitor real-time respiratory and cardiovascular activities (32). A systemic dose (0.03 mg/kg) of AT-121 that produced full antinociception did not affect respiratory function (respiration rate and minute volume), cardiovascular activity (heart rate and blood pressure), or body temperature of monkeys (Fig. 4) At a dose (0.3 mg/kg) approximately 10–30 times higher than its antinociceptive doses (0.01–0.03 mg/kg), AT-121 also did not significantly change any physiologic parameters (all F values: 1–4,p > 0.1) during the first 40 min and the 6-hour period (Fig. 4 and fig. S4). In contrast, heroin (1 mg/kg, intramuscular, i.m.) rapidly (<20 min) caused respiratory depression in a single monkey, which required naltrexone (0.01 mg/kg) to reverse it (Fig. 4B). For safety reasons, this particular experiment was only conducted in one monkey. These findings illustrate that unlike heroin, AT-121 did not cause respiratory and cardiovascular concerns in primates. Moreover, animals did not show sedation or motor impairment when they were tested with these doses of AT-121. Animals were alert and did not display eye closure while being tested with these doses of AT-121.
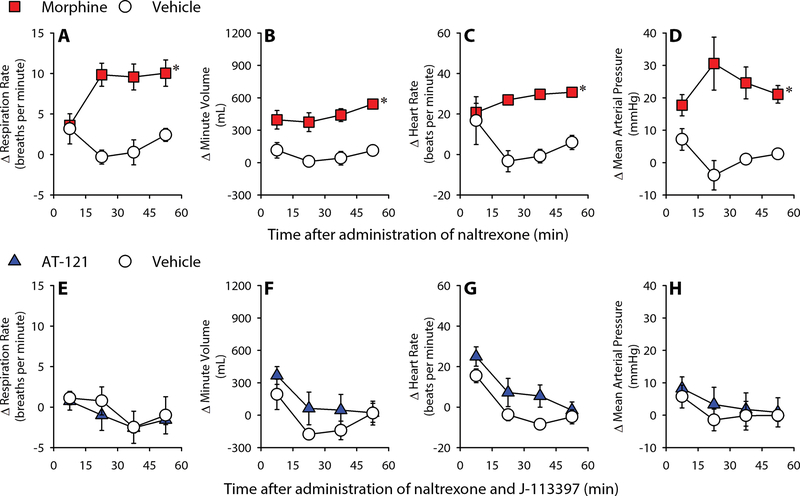
Figure 4. Acute effects of systemic administration of AT-121 on physiologic functions of freely moving nonhuman primates.
(A) Respiration rate before and after AT-121 administration. (B) Respiration rate before and after Heroin (1 mg/kg) and Naltrexone (0.01 mg/kg) administration. (C-F) Minute volume (C), Heart rate (D), Mean arterial pressure (E) and Body temperature (F) before and after different doses of AT-121 administration. Each data point represents mean ± SEM (n = 4–7) from each individual data averaged from a 1-min time block. All compounds were delivered intramuscularly. Open symbols represent baselines of different dosing conditions for the same monkeys before administration.
Repeated exposure to AT-121 does not cause physical dependence
Following repeated exposure to mu opioid analgesics, primates and humans quickly develop physical dependence (35, 51, 52). In such conditions, antagonist-precipitated withdrawal is indicated as changes in respiratory and cardiovascular parameters in primates (32, 35). Compared to the vehicle-treated condition (0.1 mL/kg), naltrexone (0.01 mg/kg) precipitated withdrawal signs on Day 4 in morphine (2 injections of 1.8 mg/kg daily for 3 days)-treated monkeys. These withdrawal signs included increases in respiratory rate [F(1, 3) = 49; p < 0.05], minute volume [F(1, 3) = 28.1; p < 0.05], heart rate [F(1, 3) = 10.4; p < 0.05] and mean arterial pressure [F(1, 3) = 15.4; p < 0.05] (Fig. 5A–D). In contrast, following repeated administration of AT-121 (2 injections of 0.03 mg/kg daily for 3 days), a combination of naltrexone (0.01 mg/kg) and J-113397 (0.3 mg/kg) did not produce changes in the physiologic parameters measured (all F values: 0.1–3, p > 0.1) (Fig. 5E–H). Collectively, unlike morphine, AT-121 did not produce physical dependence after 3 days of repeated administration.
Figure 5. Development of physical dependence on morphine or AT-121 in nonhuman primates following short-term repeated administration.
(A-D) Effects of naltrexone on Respiration rate (A), minute volume (B) heart rate (C) and mean arterial pressure (D) in morphine- or vehicle-treated monkeys. (E-H) Effects of J-113397 (0.03 mg/kg) and naltrexone (0.01 mg/kg) on Respiration rate (E), minute volume (F) heart rate (G) and mean arterial pressure (H) in AT-121- or vehicle-treated monkeys. Data are shown as changes from the baselines (before antagonist treatment). Each data point represents mean ± SEM (n = 4) from each individual data averaged from a 15 min time block. All compounds were delivered intramuscularly. Data were analyzed by two-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test. ∗p < 0.05, significantly different from vehicle from 15–30 min to the corresponding time point.
Repeated exposure to AT-121 does not cause opioid-induced hyperalgesia
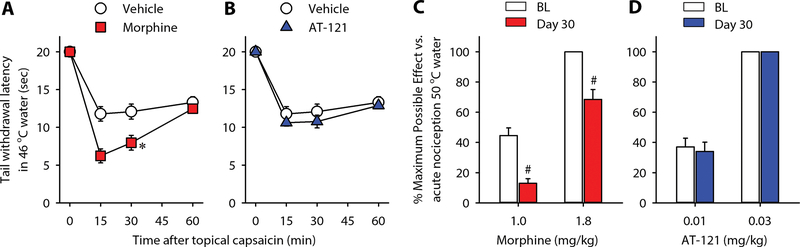
After repeated exposure to mu opioid analgesics, animals and humans develop opioid-induced hyperalgesia and tolerance (53–55). Following short-term exposure to morphine (2 injections of 1.8 mg/kg for 1 day), monkeys had a lower threshold to capsaicin-induced hypersensitivity on Day 2 [F(1, 5) = 10.2; p < 0.05], shown as decreased tail-withdrawal latencies in 46 °C water (Fig. 6A). In contrast, AT-121 (2 injections of 0.03 mg/kg for 1 day)-treated monkeys displayed similar hypersensitivity to capsaicin on Day 2 [F(1, 5) = 2.4; p > 0.1] (Fig. 6B). After long-term exposure to morphine (2 injections of 1.8 mg/kg daily for 4 weeks), morphine-treated monkeys showed a decrease in antinociceptive effects produced by 1 and 1.8 mg/kg of morphine (p < 0.05) (Fig. 6C), indicative of tolerance development. In contrast, following the same duration of repeated administration, AT-121 (0.03 mg/kg)-treated monkeys did not show tolerance to antinociceptive effects of AT-121 at 0.01 or 0.03 mg/kg (Fig. 6D). These results demonstrate that unlike morphine, repeated administration of AT-121 did not cause opioid-induced hyperalgesia and may have a slower development of analgesic tolerance compared to morphine.
Figure 6. Development of opioid-induced hyperalgesia and tolerance in nonhuman primates following repeated administration of morphine or AT-121.
(A, B) Tail-withdrawal latencies in 46 °C water following topical capsaicin (0.4 mg/mL × 0.3mL) in vehicle (A,B)-, morphine 1.8 mg/kg (A)- and AT-121 0.03 mg/kg (B)- treated animals (n=6). (C, D) Tail withdrawal latencies in 50 °C before (BL) and after (Day 30) repeated administration of morphine (1.8 mg/kg, n=4) (C), or AT-121 (0.03 mg/kg, n=5) (D). Each data point represents mean ± SEM. Data were analyzed by two-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test. ∗p < 0.05, significantly different from the vehicle at 15 and 30 min. #p < 0.05, significantly different from BL values.
DISCUSSION
This study provides four significant findings in non-human primates indicating the translational potential of AT-121, a bifunctional NOP/MOP agonist, as a safe, non-addictive analgesic and possible treatment for prescription opioid abuse (dual therapeutic action). First, AT-121 produced morphine-comparable antinociceptive and antihypersensitive effects mediated by NOP and MOP receptors. Second, unlike oxycodone, AT-121 lacked reinforcing effects, suggesting little or no abuse liability or apparently much less abuse liability than oxycodone. AT-121 selectively attenuated reinforcing effects of oxycodone, suggesting its treatment potential for prescription opioid abuse. Third, unlike heroin, AT-121 was safe and did not compromise respiratory and cardiovascular activities at 10 times the analgesic doses. Fourth, AT-121 produced less opioid-induced hyperalgesia, physical dependence, or tolerance than morphine following repeated administration.
AT-121 is approximately 100-fold more potent than morphine in producing antinociceptive effects. Since MOP agonists increased nociceptive threshold and inhibited capsaicin-induced allodynia in humans (43, 46, 47), the antinociceptive and antiallodynic effects of AT-121 suggest that its functional efficacy as an analgesic may be similar to MOP agonists. Although few pain models are established in primates, several rodent studies support the rationale for developing bifunctional NOP/MOP agonists as effective analgesics across diverse pain modalities (16, 18, 21). Previous studies in nonhuman primates showed that co-administration of NOP and MOP agonists produced synergistic antinociception without eliciting itch and respiratory depression (22). In addition, pretreatment with both NOP and MOP antagonists produced a much larger rightward shift of the AT-121 dose-response curve. Together, these findings with AT-121 support the enhanced potency and broad therapeutic window displayed by ligands with dual NOP/MOP agonist activity (9, 22).
Compared to highly abused drugs like oxycodone and cocaine, in this study AT-121 did not produce reinforcing effects in monkeys. In our intravenous drug self-administration procedure in primates, considered a gold standard to evaluate the abuse potential of drugs (56, 57), AT-121 showed little to no abuse liability. The MOP partial agonist buprenorphine, on the other hand, produced mild-to-moderate reinforcing effects under the same schedule (32). Even though AT-121 had comparable agonist efficacy as buprenorphine at MOP receptors (58), its higher agonist efficacy at NOP receptors (compared to that of buprenorphine) likely counteracts its MOP receptor-mediated reinforcing effects. More importantly, AT-121 attenuated reinforcing effects of oxycodone without affecting reinforcing effects of food pellets, indicating its selective attenuation of reinforcing effects of a prescription opioid. Because AT-121 was devoid of abuse liability compared to current medications such as buprenorphine, our findings suggest that bifunctional NOP/MOP agonists have potential as ‘replacement opioid therapy’ for patients prescribed opioids and at risk for opioid dependence. Given the medical burden associated with misuse and abuse of prescription opioids (1, 2, 59), these findings shed light on the future potential of bifunctional NOP/MOP agonists as having a dual therapeutic action as non-addictive analgesics and as medications to treat opioid use disorders (9, 16, 26). However, the bifunctional NOP/MOP partial agonist profile of AT-121 is distinguishable from that of cebranopadol (GRT6005), a NOP and opioid receptor agonist currently in clinical trials for acute and chronic pain (60). In contrast to AT-121, cebranopadol is a full agonist at the NOP and MOP receptors (29). Although cebranopadol had a wider therapeutic window than morphine and classical MOP agonists, it generalized to a morphine discriminative stimulus and showed a conditioned place preference in rodents (61, 62), suggestive of intrinsic rewarding properties and the potential for abuse liability.
AT-121 at antinociceptive doses and at 10-fold higher dose did not cause significant respiratory depression or affect cardiovascular activities immediately after administration or during and after its analgesic action. In contrast, a high dose of heroin quickly suppressed respiration rate. In contrast to the rapid respiratory depression/arrest by heroin or fentanyl (32) at analgesic doses, AT-121 demonstrates a wide safety window in primates. Overall, these in vivo findings in primates demonstrate that following acute administration, bifunctional NOP/MOP partial agonists such as AT-121 are safe, non-addictive analgesics with a wider therapeutic window than traditional opioids. Development of such bifunctional NOP/MOP agonists with a suitable profile of partial agonist efficacy at NOP and MOP receptors to treat pain and opioid addiction might help to reduce the burden related to opioid abuse (4, 5, 7).
Following repeated administration, opioid analgesics often cause adverse events, such as opioid-induced hyperalgesia, physical dependence, and tolerance (52, 53, 55). After short-term exposure (1 or 3 days), morphine-treated primates developed opioid-induced hyperalgesia (enhanced sensitivity to pain) and physical dependence (precipitated withdrawal signs). In a side-by-side comparison with morphine, AT-121-treated primates did not develop opioid-induced hyperalgesia or physical dependence. Moreover, after long-term exposure (4 weeks), morphine-treated primates developed tolerance to its antinociceptive effects. In contrast, AT-121-treated primates showed a slower development of tolerance to antinociceptive effects. Although more frequent dosing and longer durations of treatment could result in tolerance, our findings indicate that bifunctional NOP/MOP agonists like AT-121 may have advantages over morphine in repeated or chronic dosing regimens. Central NOP receptors possess an intriguing plasticity under chronic pain states (18, 21). Unlike downregulation of MOP receptors, mRNA expression of NOP receptors remained unchanged in the spinal cord of diabetic primates with neuropathy (63). Future studies are warranted to investigate whether bifunctional NOP/MOP agonists cause tolerance to develop more slowly compared to clinically used MOP agonists in patients with chronic pain.
There were some limitations of this study. The effect of potential sex differences could not be evaluated with the limited number of primates. It is important to characterize the behavioral responses to bifunctional NOP/MOP receptor agonists in male and female subjects. Although primate studies are labor-intensive, studies with larger sample sizes can address these limitations and are warranted to further validate our findings.
In conclusion, the rational design and modulation of the bifunctional profile of a new non-morphinan chemical structure (42, 64, 65) resulted in the identification of a potent NOP/MOP bifunctional agonist AT-121. The functional utility of AT-121 extends beyond that of the recently reported orvinol analog BU08028, a universal opioid ligand with NOP/MOP agonist activity (32) or the NOP/MOP full agonist cebranopadol (29). In vivo findings with AT-121 in primates support bifunctional NOP/MOP partial agonists as safe, non-addictive analgesics, and more importantly, suggest their potential as treatments for opioid use disorders. Generally, AT-121 was devoid of several adverse effects associated with clinically used MOP agonists following acute and repeated/chronic administration. It is pivotal to further investigate the interactions between NOP and MOP receptors with pharmacologic tools with varying efficacies at NOP versus MOP receptors (26, 66–68). Primate models will continue to be a translational bridge to facilitate research and development of bifunctional NOP/MOP agonists as treatment for pain and substance abuse.
MATERIALS AND METHODS
Study design
The main objective of this study was to design and optimize bifunctional NOP/MOP receptor agonists and establish the functional profile of a newly discovered agonist as a safe, non-addictive analgesic and potential treatment for opioid abuse (dual therapeutic action) in primate models. For behavioral and physiological measurements, primate subjects were trained by using the pole & collar procedure to be transported between their home cages and procedure rooms and habituated to different experimental conditions. These healthy subjects were randomly selected for each study component listed below. Based on our previous experience using power and statistical analysis for quantifying primate behavioral and physiological responses (34, 44, 69–71), the sample size of four subjects is sufficient to determine the magnitude, duration of action, and dose dependency of ligand-induced effects. All behavioral measurements were conducted during the day time (9:00 AM – 3:00 PM) by laboratory staff who were blinded to dosing conditions.
Subjects
All animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Wake Forest University. Fifteen adult male and female rhesus monkeys (Macaca mulatta), 10–18 years, 6.5–12.4 kg, were kept at an indoor facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animals were individually housed in species-specific rooms with environmental controls set to maintain 21–25 °C, 40–60% relative humidity and a 12-h light-dark cycle (light: 6:30 AM – 6:30 PM). The daily diet consisted of approximately 20–28 biscuits (Purina Monkey Chow; Ralston Purina Co.), fresh fruit, and water ad libitum. Small amounts of primate treats and various cage-enrichment devices were supplied as forms of environmental enrichment. Animals were not exposed to any opioid compound for 1 month prior to experiments. This study is reported in accordance with the Animal research: Reporting in vivo experiments (ARRIVE)) guidelines for reporting experiments involving animals (72).
Sensory assays
Acute nociception.
The warm water tail-withdrawal assay was used to evaluate thermal antinociceptive effects of AT-121 and morphine. Monkeys were seated in primate restraint chairs in a designated procedure room, and the lower parts of their shaved tails (~15 cm) were immersed in a thermal flask containing water maintained at 42, 46 or 50 °C. Water at 42 and 46 °C was used as non-noxious stimulus, and water at 50 °C was used as an acute noxious stimulus. All tail-withdrawal latencies were measured at each temperature using a computerized timer by individuals who were blinded to the experimental conditions. If a monkey did not remove its tail within 20 sec (cutoff), the flask was removed and a maximum time of 20 sec was recorded. Test sessions began with baseline measurements at each temperature. Subsequent tail-withdrawal latencies were measured at multiple time points after subcutaneous administration of the test compound. For dose-response curves, the test compound was administered by a cumulative dosing procedure with a 30 min inter-injection interval. Tail-withdrawal latencies were measured at 20 min after each injection. A single dose of NOP receptor-selective antagonist J-113397 (0.1 mg/kg) or MOP receptor-selective antagonist naltrexone (0.03 mg/kg) was administered subcutaneously 15 min before determination of dose-response curves to determine the NOP and MOP receptor components mediating AT-121-induced antinociception. The doses and pretreatment time for J-113397 and naltrexone were chosen based on previous studies (19, 22).
Capsaicin-induced thermal allodynia.
Antiallodynic effects of AT-121 were evaluated by using a 1 hr pretreatment time regimen (1 hr before capsaicin administration). Capsaicin (1.2 mg/mL × 0.3 mL) was administered topically via a bandage attached on the terminal 3–5 cm of the tail for 15 min (70). The allodynic response was manifested as reduced tail withdrawal latency from a maximum value of 20 sec to ~2–3 sec in 46 °C water. This allodynic effect peaked at 15 min after removal of the capsaicin bandage, which is when tail-withdrawal latency was measured in 46 °C water in order to determine the antiallodynic effects of the test compound (23, 70). For evaluating the potential development of opioid-induced hyperalgesia, a low dose (0.4 mg/mL × 0.3 mL) of capsaicin was used; this dosing condition elicited moderate, reduced tail-withdrawal latency (10–12 sec in 46 °C water), which allowed detection of enhanced hypersensitivity. Monkeys received AT-121 (0.03 mg/kg) or morphine (1.8 mg/kg) on Day 1 (2 injections: ~9:00 AM and 16:00 PM). Their responses to capsaicin were measured on the morning of Day 2.
Itch scratching responses.
Scratching activity as a behavioral response to itch sensation was recorded on videotapes when monkeys were in their home cages (48). A 15 min recording session was conducted at 1 hr after subcutaneous administration of AT-121 (0.03 mg/kg) or morphine (1 mg/kg). A scratch was defined as one brief (<1 sec) episode of scraping contact of the forepaw or hind paw on the skin surface of other body parts. Total scratches were counted by individuals who were unaware of the experimental conditions.
Drug self-administration
Monkeys with indwelling intravenous catheters and subcutaneous vascular access ports were used to evaluate the reinforcing effects of the test compound under a progressive-ratio schedule as described previously (32). Briefly, lever pressing responses were maintained by injections of 0.3 μg/kg remifentanil until responding was stable (mean ± 3 injections for 3 consecutive sessions with no trend). Dose-response curves were determined in each monkey by substituting saline, cocaine (0.03 mg/kg/injection) or a range of doses of AT-121 (0.3‒10 μg/kg/injection) or oxycodone (0.3‒10 μg/kg/injection) for the maintenance dose in a randomized order. Doses were available for at least 5 consecutive sessions and until responding was deemed stable. AT-121 (0.03 mg/kg), buprenorphine (0.1 mg/kg), or naltrexone (0.01 mg/kg) was administered intramuscularly 30 min before starting the progressive-ratio schedule of oxycodone (3 μg/kg/injection) to evaluate each compound’s potential attenuation of reinforcing effects by oxycodone. In addition, vehicle (0.1 mL/kg) or AT-121 (0.03 mg/kg) was administered intramuscularly 30 min before starting the fixed ratio (FR10) schedule of banana-flavored food pellets to determine whether AT-121 alters the value of other reinforcers or affects general motor function.
Physiological responses
Freely moving monkeys implanted with the D70-PCTR telemetry transmitter were used to evaluate the effects of AT-121 and MOP receptor agonists on physiologic functions as described previously (32). Respiration, heart rate, blood pressure, and temperature were measured and analyzed with Ponemah software version 5.2 (Data Sciences International). For acute drug effects, data from the 30 min interval before drug administration were collected as baseline and then at each time point (1, 3 and 6 hr) after intramuscular administration of AT-121 (0, 0.03, 0.3 mg/kg) compared to a high dose of heroin (1 mg/kg). For detecting precipitated withdrawal signs following 3 days (2 injections per day; 1st injection at ~09:00 AM and 2nd injection at ~16:00 PM) of intramuscular administration of AT-121 (0.03 mg/kg) or morphine (1.8 mg/kg), data from the 30-min interval before administration of antagonist were collected and then continuously for 1 hr after antagonist administration on Day 4. The mean value of each 15 min time block was generated from each subject to represent the measure outcome for each single data point.
Drugs
1) The chemical synthesis procedures (fig. S1), 2) in vitro pharmacological characterization of compounds 1–6, 3) molecular docking and computational modeling, 4) in vivo rodent pharmacokinetic data (fig. S2 & tables S1–S2), and 5) in vitro primate plasma stability data (fig. S3 & Table S3) are described and presented in detail in the Supplementary Materials. AT-121 was dissolved in a solution of dimethyl sulfoxide/1% (mass/vol) hydroxypropyl cellulose in a ratio of 3:97. Morphine sulfate, buprenorphine HCl, heroin (diamorphine HCl), remifentanil HCl and naltrexone HCl (National Institute on Drug Abuse (NIDA)) were dissolved in sterile water. (‒)Cocaine HCl and oxycodone HCl (NIDA) were dissolved in sterile 0.9% saline. J-113397 (R&D Systems) was dissolved in a solution of dimethyl sulfoxide/Tween 80/sterile water in a ratio of 1:1:8. Capsaicin (Sigma-Aldrich) was dissolved in 70% (vol/vol) ethanol. For systemic administration, drugs were administered at a volume of 0.1 mL/kg. There was a minimum 1-week interval between drug administrations.
Statistical analysis
Mean values ± standard error of the mean (S.E.M.) were calculated from individual data for all study endpoints. Comparisons were made for the same monkeys across all test sessions in the same experiment. Data were analyzed by either two-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test, or one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test. The criterion for significance for all tests was set at p < 0.05. To analyze nociceptive responses, individual tail-withdrawal latencies were converted to the percentage of maximum possible effect by using the formula defined as [(test latency ‒ control latency)/(cutoff latency, 20 s ‒ control latency)] × 100. Raw data for all experiments are presented in table S5.
Supplementary Material
Fig. S1. Chemical synthesis scheme for compounds 1–6.
Fig. S2. AT-121 plasma and brain concentrations after a single subcutaneous administration at 3 mg/kg to male Sprague-Dawley rats.
Fig. S3. In vitro plasma stability assessment of AT-121 in monkey plasma.
Fig. S4. Effects of systemic administration of AT-121 on physiologic functions of freely moving monkeys implanted with telemetric probes.
Table S1. Brain sample records for AT-121 brain penetration after subcutaneous administration at 3 mg/kg to male Sprague-Dawley rats.
Table S2. Pharmacokinetic parameters (plasma and brain) for AT-121 after subcutaneous administration in male Sprague-Dawley rats.
Table S3. Plasma stability assessment of AT-121 in monkey plasma.
Table S4. Baseline values for the monkey tail withdrawal assay that are normalized across different dosing conditions.
Table S5. Raw data (Excel file).
Acknowledgments
We acknowledge the editorial assistance of Karen Klein, MA, in the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: D. McClain). We thank Ms. Jade Lackey, Kelsey Reynold, and Emily Whitaker for their technical assistance with training animals and data collection. We also thank Drs. Tyler Aycock and Quentin Wilson for their veterinary care for our animals.
Funding: The work was supported by grants from the National Institutes of Health, National Institute on Drug Abuse (R01DA032568, R01DA027811, R44DA042465, R21DA040104, R21DA044775, and P50DA006634) and the US Department of Defense (W81XWH-13-2-0045). The content is solely the responsibility of the authors and does not necessarily represent the official views of these U.S. federal agencies.
Footnotes
Competing interests: D.Y., W.E.P., J.J.L., and N.T.Z. are employees of Astraea Therapeutics. D.Y. and N.T.Z. are listed as inventors on a patent application (US patent application number 15/368,508, entitled: Piperidinyl nociceptin receptor compounds) submitted by Astraea Therapeutics that covers compounds in this manuscript. All other authors report no biomedical financial interests or potential conflicts of interest.
Data and materials availability:
All the data are included in the manuscript or in its supplementary materials.
REFERENCES AND NOTES
- 1.Brady KT, McCauley JL, Back SE, Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry 173, 18–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan AT, Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med 374, 1253–1263 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL, Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372, 241–248 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, Vos T, The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109, 1320–1333 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Rudd RA, Seth P, David F, Scholl L, Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65, 1445–1452 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Corbett AD, Henderson G, McKnight AT, Paterson SJ, 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147 Suppl 1, S153–162 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Collins FS, The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377, 391–394 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Gunther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S, Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol, DOI: 10.1111/bph.13809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin AP, Ko MC, The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4, 214–224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madariaga-Mazon A, Marmolejo-Valencia AF, Li Y, Toll L, Houghten RA, Martinez-Mayorga K, Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics? Drug Discov Today, doi: 10.1016/j.drudis.2017.1007.1002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siuda ER, Carr R 3rd, Rominger DH, Violin JD, Biased mu-opioid receptor ligands: a promising new generation of pain therapeutics. Curr Opin Pharmacol 32, 77–84 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F, A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 157, 264–272 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS, Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31, 730–739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaveri NT, The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem 11, 1151–1156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert DG, The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7, 694–710 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT, Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68, 419–457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calo G’, Guerrini R, in Research and Development of Opioid-Related Ligands, Ko MC, Husbands SM, Eds. (American Chemical Society, Washington, DC, USA, 2013), vol. ACS Symposium Series, chap. 15, pp. 275–325. DOI: 210.1021/bk-2013-1131.ch1015. [Google Scholar]
- 18.Schroder W, Lambert DG, Ko MC, Koch T, Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171, 3777–3800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP, Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34, 2088–2096 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC, Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172, 3302–3312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiguchi N, Ding H, Ko MC, Central N/OFQ-NOP Receptor System in Pain Modulation. Adv Pharmacol 75, 217–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremeans CM, Gruley E, Kyle DJ, Ko MC, Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343, 72–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu E, Calo G, Guerrini R, Ko MC, Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148, 107–113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Giannuario A, Pieretti S, Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides 21, 1125–1130 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Murphy NP, Lee Y, Maidment NT, Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res 832, 168–170 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Zaveri NT, Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J Med Chem 59, 7011–7028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT, Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther 331, 954–964 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L, SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320, 934–943 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schroder W, Kogel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S, Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349, 535–548 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L, The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-meth oxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther 336, 952–961 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC, Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/mu-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther 346, 11–22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC, A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113, E5511–5518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L, Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331, 946–953 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowen CA, Fischer BD, Mello NK, Negus SS, Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther 302, 264–273 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Ko MC, Divin MF, Lee H, Woods JH, Traynor JR, Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther 316, 772–779 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D, Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4, 2501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML, Why primate models matter. Am J Primatol 76, 801–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daga PR, Zaveri NT, Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins 80, 1948–1961 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mollereau C, Moisand C, Butour JL, Parmentier M, Meunier JC, Replacement of Gln280 by His in TM6 of the human ORL1 receptor increases affinity but reduces intrinsic activity of opioids. FEBS Lett 395, 17–21 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Zaveri N, Polgar WE, Olsen CM, Kelson AB, Grundt P, Lewis JW, Toll L, Characterization of opiates, neuroleptics, and synthetic analogs at ORL1 and opioid receptors. Eur J Pharmacol 428, 29–36 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaveri NT, Jiang F, Olsen CM, Deschamps JR, Parrish D, Polgar W, Toll L, A novel series of piperidin-4-yl-1,3-dihydroindol-2-ones as agonist and antagonist ligands at the nociceptin receptor. J Med Chem 47, 2973–2976 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Journigan VB, Polgar WE, Khroyan TV, Zaveri NT, Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP-receptor selective scaffolds. Part II. Bioorg Med Chem 22, 2508–2516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM, A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain 123, 28–36 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Sukhtankar DD, Lee H, Rice KC, Ko MC, Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 231, 1377–1387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H, Naughton NN, Woods JH, Ko MC, Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology 107, 478–485 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenach JC, Hood DD, Curry R, Tong C, Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology 86, 1279–1287 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ, Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain 63, 163–172 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Ko MC, Song MS, Edwards T, Lee H, Naughton NN, The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther 310, 169–176 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Richardson NR, Roberts DC, Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66, 1–11 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Rowlett JK, A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 153, 1–16 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Kishioka S, Paronis CA, Woods JH, Acute dependence on, but not tolerance to, heroin and morphine as measured by respiratory effects in rhesus monkeys. Eur J Pharmacol 398, 121–130 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Azolosa JL, Stitzer ML, Greenwald MK, Opioid physical dependence development: effects of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl) 114, 71–80 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L, A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14, 145–161 (2011). [PubMed] [Google Scholar]
- 54.Arout CA, Edens E, Petrakis IL, Sofuoglu M, Targeting Opioid-Induced Hyperalgesia in Clinical Treatment: Neurobiological Considerations. CNS Drugs 29, 465–486 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Hayhurst CJ, Durieux ME, Differential Opioid Tolerance and Opioid-induced Hyperalgesia: A Clinical Reality. Anesthesiology 124, 483–488 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Ator NA, Griffiths RR, Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70, S55–72 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Banks ML, Czoty PW, Negus SS, Utility of Nonhuman Primates in Substance Use Disorders Research. Ilar j, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, Zaveri NT, Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153, 609–619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Compton WM, Jones CM, Baldwin GT, Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med 374, 154–163 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Raffa RB, Burdge G, Gambrah J, Kinecki HE, Lin F, Lu B, Nguyen JT, Phan V, Ruan A, Sesay MA, Watkins TN, Cebranopadol: novel dual opioid/NOP receptor agonist analgesic. J Clin Pharm Ther 42, 8–17 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Tzschentke TM, Rutten K, Mu-opioid peptide (MOP) and nociceptin/orphanin FQ peptide (NOP) receptor activation both contribute to the discriminative stimulus properties of cebranopadol in the rat. Neuropharmacology 129, 100–108 (2018). [DOI] [PubMed] [Google Scholar]
- 62.de Guglielmo G, Matzeu A, Kononoff J, Mattioni J, Martin-Fardon R, George O, Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J Pharmacol Exp Ther 362, 378–384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiguchi N, Ding H, Peters CM, Kock ND, Kishioka S, Cline JM, Wagner JD, Ko MC, Altered expression of glial markers, chemokines, and opioid receptors in the spinal cord of type 2 diabetic monkeys. Biochim Biophys Acta 1863, 274–283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaveri NT, Jiang F, Olsen C, Polgar WE, Toll L, Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP receptor-selective scaffolds. Part I. Bioorg Med Chem Lett 23, 3308–3313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaveri NT, Yasuda D, Journigan BV, Daga PR, Jiang F, Olsen C, in Research and Development of Opioid-Related Ligands, Ko MC, Husbands SM, Eds. (American Chemical Society, Washington, DC, USA, 2013), vol. 1131, chap. 8, pp. 145–160. DOI: 110.1021/bk-2013-1131.ch1008. [Google Scholar]
- 66.Pacifico S, Carotenuto A, Brancaccio D, Novellino E, Marzola E, Ferrari F, Cerlesi MC, Trapella C, Preti D, Salvadori S, Calo G, Guerrini R, Structure- and conformation-activity studies of nociceptin/orphanin FQ receptor dimeric ligands. Sci Rep 7, 45817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Husbands SM, in Research and Development of Opioid-Related Ligands, Ko MC, Husbands SM, Eds. (American Chemical Society, Washington, DC, USA, 2013), vol. 1131, pp. 127–144. DOI: 110.1021/bk-2013-1131.ch1007. [Google Scholar]
- 68.Schunk S, Linz K, Hinze C, Frormann S, Oberborsch S, Sundermann B, Zemolka S, Englberger W, Germann T, Christoph T, Kogel BY, Schroder W, Harlfinger S, Saunders D, Kless A, Schick H, Sonnenschein H, Discovery of a Potent Analgesic NOP and Opioid Receptor Agonist: Cebranopadol. ACS Med Chem Lett 5, 857–862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH, Intracisternal norbinaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291, 1113–1120 (1999). [PMC free article] [PubMed] [Google Scholar]
- 70.Butelman ER, Harris TJ, Kreek MJ, Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther 311, 155–163 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Collins GT, Carey KA, Narasimhan D, Nichols J, Berlin AA, Lukacs NW, Sunahara RK, Woods JH, Ko MC, Amelioration of the cardiovascular effects of cocaine in rhesus monkeys by a long-acting mutant form of cocaine esterase. Neuropsychopharmacology 36, 1047–1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160, 1577–1579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mustazza C, Borioni A, Sestili I, Sbraccia M, Rodomonte A, Del Giudice MR, Synthesis and pharmacological evaluation of 1,2-dihydrospiro[isoquinoline-4(3H),4’-piperidin]-3-ones as nociceptin receptor agonists. J Med Chem 51, 1058–1062 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Cheng Y, Prusoff WH, Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22, 3099–3108 (1973). [DOI] [PubMed] [Google Scholar]
- 75.Daga PR, Polgar WE, Zaveri NT, Structure-based virtual screening of the nociceptin receptor: hybrid docking and shape-based approaches for improved hit identification. J Chem Inf Model 54, 2732–2743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Chemical synthesis scheme for compounds 1–6.
Fig. S2. AT-121 plasma and brain concentrations after a single subcutaneous administration at 3 mg/kg to male Sprague-Dawley rats.
Fig. S3. In vitro plasma stability assessment of AT-121 in monkey plasma.
Fig. S4. Effects of systemic administration of AT-121 on physiologic functions of freely moving monkeys implanted with telemetric probes.
Table S1. Brain sample records for AT-121 brain penetration after subcutaneous administration at 3 mg/kg to male Sprague-Dawley rats.
Table S2. Pharmacokinetic parameters (plasma and brain) for AT-121 after subcutaneous administration in male Sprague-Dawley rats.
Table S3. Plasma stability assessment of AT-121 in monkey plasma.
Table S4. Baseline values for the monkey tail withdrawal assay that are normalized across different dosing conditions.
Table S5. Raw data (Excel file).